Abstract
Primary aldosteronism is characterized by excess aldosterone (ALDO) secretion independent of the renin–angiotensin system and accounts for approximately 10% of hypertension cases. Excess ALDO that is inappropriate for salt intake status causes cardiac hypertrophy, inflammation, fibrosis, and hypertension. The molecular mechanisms that trigger the onset and progression of ALDO-mediated cardiac injury are poorly understood. MicroRNAs (miRNAs) are endogenous, small, noncoding RNAs that have been implicated in diverse cardiac abnormalities, yet very little is known about their regulation and role in ALDO-mediated cardiac injury. To elucidate the regulation of miRNAs in ALDO-mediated cardiac injury, we performed a time-series analysis of left ventricle (LV) miRNA expression. Uninephrectomized male Sprague–Dawley rats were treated with ALDO (0.75 µg/h) infusion and SALT (1.0% NaCl/0.3% KCl) in the drinking water for up to 8 weeks. ALDO/SALT time dependently modulated the expression of multiple miRNAs in the LV. miR-21 was the most upregulated miRNA after 2 weeks of treatment and remained elevated until the end of the study. To elucidate the role of miR-21 in ALDO/SALT-mediated cardiac injury, miR-21 was downregulated by using antagomirs in ALDO/SALT-treated rats. miR-21 downregulation exacerbated ALDO/SALT-mediated cardiac hypertrophy, expression of fibrosis marker genes, interstitial and perivascular fibrosis, OH-proline content, and cardiac dysfunction. These results suggest that ALDO/SALT-mediated cardiac miR-21 upregulation may be a compensatory mechanism that mitigates ALDO/SALT-mediated cardiac deleterious effects. We speculate that miR-21 supplementation would have beneficial effects in reverting or mitigating cardiac injury and dysfunction in patients with primary aldosteronism.
MicroRNA-21 is upregulated in the left ventricle by aldosterone treatment in vivo, and its downregulation exacerbates aldosterone-mediated cardiac injury and dysfunction.
Excess aldosterone (ALDO) that is inappropriate for the salt intake status causes hypertension and cardiac hypertrophy, inflammation, fibrosis, and dysfunction (1–7). Primary aldosteronism is a human pathologic condition characterized by the excess autonomous secretion of ALDO by the adrenal gland, independent of the renin–angiotensin system, and is associated with severe cardiorenal damage and hypertension (8–13). Primary aldosteronism is the most common cause of secondary hypertension, accounting for approximately 10% of hypertension cases (8–10). Furthermore, excess ALDO is not only associated with hypertension. Patients with primary aldosteronism have a higher incidence of cardiorenal and cerebrovascular events than those with essential hypertension with similarly elevated blood pressure (14–17). Despite the prevalence of primary aldosteronism and its deleterious consequences to human health, the molecular mechanisms that mediate the onset and progression of ALDO-mediated cardiac injury and dysfunction remain poorly understood.
MicroRNAs (miRNAs) are short endogenous single-stranded noncoding RNAs that are generated by sequential processing from longer transcripts that contain a stem-loop (18–22). miRNAs exert their biological effects by downregulating the expression levels of specific genes by translational repression and/or messenger RNA (mRNA) degradation (23–26). miRNAs have been implicated in a variety of biological functions, including development, proliferation, apoptosis, metabolism, and immune response (24, 27–29). Furthermore, several miRNAs have been implicated in cardiovascular disease (19, 29–36). However, the role of miRNAs in ALDO-mediated cardiac injury and dysfunction remains largely unknown.
miR-21 is weakly expressed in normal hearts, but it is the most consistently upregulated miRNA in most models of cardiac hypertrophy and failure (37–39) and in human end-stage heart failure (39–42). However, although miR-21 is one of the most studied miRNAs in cardiac tissue, its role in cardiovascular pathophysiology remains highly controversial. miR-21 has been reported to have deleterious, beneficial, or neutral effects in multiple models of cardiac injury, suggesting a cardiac disease–specific role for this miRNA (37–39, 43–59).
Chronic ALDO infusion in the presence of high salt intake (ALDO/SALT) is a well-established animal experimental model to study the pathophysiologic consequences of excess ALDO (1, 2). Using this experimental model, which closely resembles the cardiac findings from humans with primary aldosteronism, we screened for miRNAs regulated by ALDO in cardiac tissue. We found that miR-21 is the miRNA most upregulated by ALDO in the left ventricle (LV). We performed further studies to characterize ALDO-mediated miR-21 regulation in the heart as well as its role in ALDO-mediated cardiac injury and dysfunction.
Materials and Methods
Animals
Eight-week old male Sprague–Dawley rats (Harlan Laboratories) were uninephrectomized and the next day randomly assigned to the following experimental groups: control, ALDO, SALT, ALDO/SALT, ALDO/SALT + eplerenone, or ALDO/SALT + antihypertensive therapy. ALDO (0.75 µg/h; Steraloids) was administered in polyethylene glycol 300 (vehicle; Fluka) by subcutaneously implanted osmotic mini-pumps (Alzet 2004; Durect). SALT (1.0% NaCl) was administered in the drinking fluid instead of tap water. ALDO-treated animals were supplemented with 0.3% KCl in the drinking fluid to avoid hypokalemia. Eplerenone (Pfizer) was administered in the rodent diet at a concentration of 1.66 g/kg chow (daily dose: approximately 112 mg/kg body weight). Triple antihypertensive therapy was administered in the rodent diet containing 240 mg/kg hydralazine (Sigma-Aldrich), 75 mg/kg hydrochlorothiazide (Sigma-Aldrich), and 1.5 mg/kg reserpine (Sigma-Aldrich). This diet delivers an approximate daily dose of 18.3 mg/kg body weight hydralazine, 5.7 mg/kg body weight hydrochlorothiazide, and 1.1 mg/kg body weight reserpine. All animals were maintained on standard rodent chow (Teklad #8640; Harlan Laboratories). Animals were treated for increasing periods up to 8 weeks, as indicated for each experiment.
All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center, and studies were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, 8th edition, 2011.
Blood pressure determination
Blood pressure was measured by radiotelemetry (Data Sciences International). Transmitters (TA11PA-C40) were implanted in the abdominal aorta under isoflurane anesthesia as previously reported (60). Animals were allowed to recover for 2 weeks before treatment initiation, and blood pressure was determined every 5 minutes in 10-second sampling periods. Mean arterial pressure is expressed as 24-hour averages.
Echocardiography
Echocardiographic assessment of cardiac function was conducted by using a Vevo 770 High Resolution In Vivo Imaging System (VisualSonics) as previously described (61) according to the American Society of Echocardiography guidelines by using the leading edge technique (62). Cardiac output (mL/min) and fractional shortening (percentage) were analyzed and calculated with the VisualSonics Advanced Cardiovascular Measurements Package using the mean of 12 to 15 cardiac cycles derived from three separate M-mode images during each session. LV dimensions, including anterior wall thickness in diastole (millimeters) and posterior wall thickness in diastole (millimeters) were determined from the M-mode images.
Antagomir administration
To downregulate miR-21, animals were treated with miR-21 antagomir, a cell-permeable, chemically modified, highly specific antisense oligonucleotide against miR-21. On the basis of previously reported sequence and modifications (55, 63, 64), miR-21 antagomir or mismatched control was synthesized by Midland Certified Reagent Co. with the following structures, respectively: 5′-GsUsCAACAUCAGUCUGAUAAGCsUsAsCs-Chol-3′ and 5′-GsUsCUACAACAAUCUGAUAGGCsUsAsCs-Chol-3′. All bases are 2′-OMe-modified ribonucleotides; the subscript "s" represents a phosphorothioate linkage; "Chol" represents cholesterol linked through a hydroxyprolinol linkage.
Eight-week old male Sprague–Dawley rats were uninephrectomized and treated with ALDO/SALT for 14 days. Starting at day 5, after ALDO/SALT treatment initiation, animals were injected daily for 3 consecutive days with 40 mg antagomir-21/kg body weight in warm saline (0.5 mL) by the tail vein (ALDO/SALT + Antag). All other experimental groups were treated identically with mistmatched control antagomirs.
RNA extraction
Total RNA (including miRNAs) was extracted with TRI-Reagent (MRC Gene), resuspended in nuclease free water and subjected to further purification by using the RNeasy mini kit (Qiagen) following an Exiqon modified protocol to preserve miRNAs. Purified total RNA was quantified by ultraviolet absorbance by using the Nanodrop spectrophotometer (Nanodrop Technologies) and quality checked by using a 2100 Bioanalyzer (Agilent Technologies).
miRNA microarrays
RNA quality control, labeling, hybridization, and scanning were performed by Exiqon. One microgram total RNA from sample and reference (a pool of all tested samples) was labeled with Hy3 and Hy5 fluorescent labels, respectively, using the miRCURY locked nucleic acid (LNA) array power labeling kit (Exiqon), following the procedure described by the manufacturer. The Hy3-labeled samples and a Hy5-labeled reference RNA sample were mixed pairwise and hybridized to the miRCURY LNA array, version 11.0 (Exiqon), which contains capture probes targeting all miRNAs for human, mouse, or rat registered in the miRBASE, version 13.0, at the Sanger Institute (65). The hybridization was performed according to the miRCURY LNA array manual by using an HS4800 hybridization station (Tecan). After hybridization, the microarray slides were scanned by using the Agilent G2565BA Microarray Scanner System and the image analysis was carried out by using ImaGene 8.0 software (BioDiscovery). The quantified signals were background corrected (Normexp with offset value 10) (66) and normalized by using the global Lowess (LOcally WEighted Scatterplot Smoothing) regression algorithm. Data were log2 transformed and analyzed by two-way analysis of variance with Benjamini–Hochberg false discovery rate multiple testing correction, followed by Student–Newman–Keuls post hoc tests using GeneSpring GX, version 12.0 (Agilent Technologies). Heat maps were generated by using Heatmap Builder software (67). Microarray hybridization data were submitted to the Gene Expression Omnibus database (National Center for Biotechnology Information) under accession number GSE87122.
miRNA reverse transcriptase quantitative polymerase chain reaction
Twenty-five nanograms of total RNA were reverse transcribed with the miRCURY LNA Universal cDNA Synthesis Kit (Exiqon), following the manufacturer’s suggested protocol. miRNA reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) was performed by using the Universal RT SYBR Green master mix (Exiqon) and highly specific LNA primers (Exiqon), following the manufacturer’s suggested protocol. Primer details are shown in Supplemental Table 1 (85.7KB, pdf) . PCR product quantification was performed by the relative quantification method and expressed as arbitrary units (68, 69).
For LV miRNA quantification in time-series experiments, data variation and expression level were analyzed with the miRNA microarray expression data, filtering for miRNAs expressed across all samples. We selected rno-let-7f as the reference gene because it presented the lowest coefficient of variation (percentage) across all time-series samples, was expressed in all samples, and had a high average expression signal [Supplemental Fig. 1(a) (4.6MB, pdf) ]. For other miRNA expression experiments, a series of reference genes (RNU1A1, RNU5G, and U6 snRNA), as well as let-7f, were tested. The most suitable one with the highest P value by one- or two-way analysis of variance was selected [Supplemental Fig. 1(b)–1(d) (4.6MB, pdf) ]. Different reference genes were selected for different experiments because it has been previously reported that the stability of reference genes may be affected, among many other factors, by origin of tissue sample, sex, or treatment (70, 71).
RT-qPCR
Five micrograms of total RNA were reverse transcribed with 0.5 μg T20VN and Superscript III (Invitrogen), following the manufacturer's suggested protocol. Primer pairs were designed with Primer3 software (72) (Supplemental Table 2 (20.1KB, xlsx) ). qPCR was performed as previously reported by using 1 μL RT product, 1 μL Titanium Taq DNA polymerase (Clontech), 1:20,000 dilution SYBR Green I (Molecular Probes), 0.2 mM deoxynucleotide triphosphates, and 0.1 μM each primer (73). Cycling conditions were 1 minute at 95°C, 50 cycles of 15 seconds at 95°C, 15 seconds at 60°C, and 1 minute at 72°C. Fluorescent data were obtained during the extension phase, and threshold cycle values were obtained at the log phase of each gene amplification. After PCR amplification, the specificity of the PCR was confirmed by melting temperature determination of the PCR product and electrophoretic analysis in 4% NuSieve 3:1 agarose gels (Cambrex). PCR product was quantified by the relative quantification method and expressed as arbitrary units standardized against glyceraldehyde 3-phosphate dehydrogenase (68, 69).
miRNA Northern blot
miRNA Northern blot was performed, following previously reported protocols (74). Briefly, 10 µg total RNA were separated in 15% Tris/borate/EDTA–urea polyacrylamide gel, transferred to Nytran SPC nylon membrane (Whatman), crosslinked, hybridized with 32P-labeled locked nucleic acid probes (Exiqon) (Supplemental Table 3 (20.1KB, xlsx) ), exposed to an Imaging Screen-K and scanned with a Personal Molecular Imager System (Bio-Rad).
Fibrosis analysis and quantification
Formalin-fixed, paraffin-embedded 5-µm heart sections were stained with trichrome-Masson. Digital images (original magnification, ×20) were acquired with a NanoZoomer 2.0-HT Digital slide scanner (Hamamatsu Photonics). Digital images were segmented with NDP.view, version 2.2, software (Hamamatsu Photonics), and interstitial and perivascular fibrosis volumetric areas were analyzed by using ImageJ software (National Institutes of Health), as reported (2). For scar analysis, digital images were processed without segmentation, as described above.
OH-proline quantification
Total collagen content was quantified as OH-proline concentration after acid hydrolysis and a colorimetric assay, as reported (2, 75). OH-proline content was expressed as milligrams of OH-proline per gram of dry tissue, with samples assayed in triplicate.
Plasma biochemistry assays
Plasma ALDO concentration (PAC) was quantified by radioimmunoassay (Coat-A-Count, Siemens) and expressed as ng/dL. Plasma renin activity (PRA) was quantified by PRA enzyme-linked immunosorbent assay kit (Crystal Chem) and expressed as nanograms of angiotensin I per milliliter per hour. The aldosterone-to-renin ratio (ARR) was calculated as PAC/PRA. For the calculation of the ARR, PRA values less than 0.2 ng angiotensin I per milliliter per hour were arbitrarily set to 0.2 to avoid false-positive results due to extremely low PRA values (76).
Statistical analysis
All results were expressed as mean ± standard error of the mean. Multiple experimental groups were analyzed by one- or two-way analysis of variance, followed by Newman–Keuls post hoc contrasts. Statistical calculations were performed with GraphPad Prism package, version 6.07 (GraphPad Software). All experiments were repeated at least three times. Differences were considered significant at P < 0.05.
Results
Screening of ALDO-regulated LV miRNAs
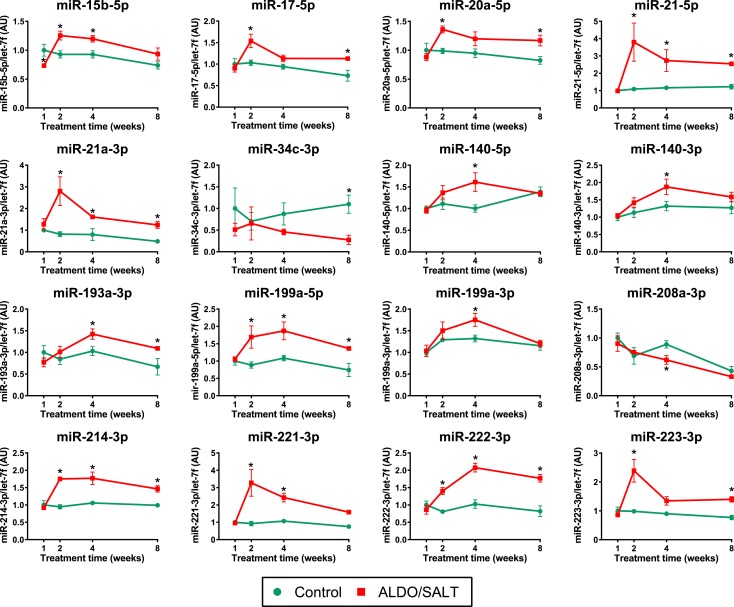
To study ALDO-mediated regulation of cardiac miRNAs, we performed a time-series screening of differentially regulated miRNAs in the LV of ALDO/SALT-treated rats. Animals were treated with ALDO/SALT for up to 8 weeks, and LV miRNA expression levels were analyzed by microarrays. We analyzed 347 miRNAs and observed 96 miRNAs to be differentially regulated at one or more time points (Supplemental Table 4 (20.1KB, xlsx) ).Twenty miRNAs were regulated more than 1.5-fold by ALDO/SALT in the miRNA microarray screening, as shown in the heat map (Supplemental Fig. 2 (4.6MB, pdf) ), and 16 of these were validated by RT-qPCR [Fig. 1 and Supplemental Fig. 1(a) (4.6MB, pdf) ].
Figure 1.
miRNAs regulated by ALDO/SALT in the LV. Rats were treated with ALDO/SALT or vehicle for up to 8 weeks. Selected prescreened miRNAS were quantified by RT-qPCR. Results are expressed as mean ± standard error of the mean (n = 6). *P < 0.05 versus control. AU, arbitary unit.
To validate the animal experimental model, we measured PAC and PRA and calculated ARR in the time-series analysis samples. After 7 days of treatment, ALDO/SALT-treated animals tested positive in a preliminary screening for primary aldosteronism with use of the most commonly adopted cutoff values (ARR > 30 [PAC (ng/dL)/PRA (ng/mL per hour)] and a PAC ≥ 15 ng/dL) according to the latest clinical guidelines for the management of primary aldosteronism (77) [Supplemental Fig. 3(a)–3(c) (4.6MB, pdf) ]. Furthermore, average PAC in ALDO/SALT-treated animals (mean ± standard error of the mean, 58.2 ± 3.8 pg/dL; range, 33.4 to 115.9 pg/dL) is in the range of PAC values (median, 29.7 pg/dL; range, 17.0 to 226.0 pg/dL; n = 126) observed in a large prospective study of the prevalence of primary aldosteronism in hypertensive patients (PAPY study) (76).
Although miR-21 is the most consistently upregulated miRNA in most models of cardiac hypertrophy and failure (37–39) and in human end-stage heart failure (39–42), the role of miR-21 in ALDO-mediated hypertension and cardiac injury remains unknown. For these reasons and because miR-21 was the most upregulated miRNA in the LV of ALDO/SALT-treated animals, we focused on the role and regulation of miR-21 in ALDO-mediated hypertension and cardiac injury.
miR-21 is upregulated by ALDO/SALT in the LV
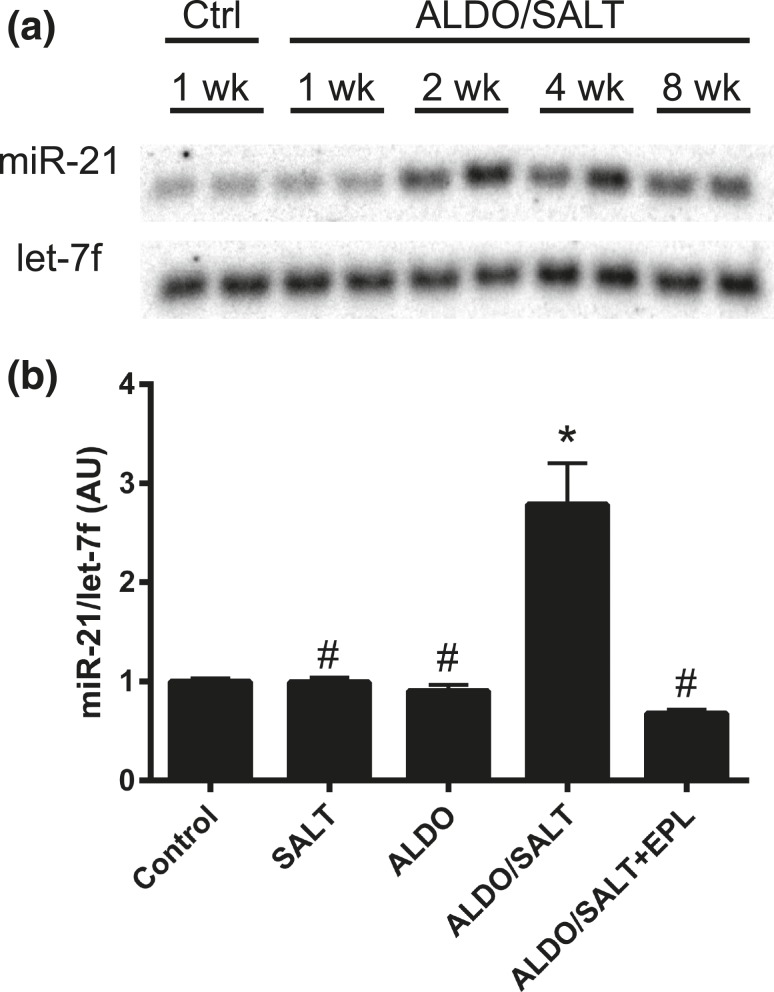
After validating our screening results by RT-qPCR as mentioned above (Fig. 1), we further confirmed by Northern blot that miR-21 is upregulated by ALDO/SALT in the LV after 2 weeks of treatment and remains elevated until the end of the 8-week experimental period [Fig. 2(a)]. ALDO/SALT-mediated miR-21 upregulation requires both excess ALDO and SALT administration because these treatments alone failed to regulate LV miR-21 expression after 2 weeks of treatment [Fig. 2(b)]. Furthermore, ALDO/SALT-mediated upregulation was completely abolished by coadministration of the mineralocorticoid receptor blocker eplerenone, suggesting that these effects are indeed mediated by the mineralocorticoid receptor [Fig. 2(b)].
Figure 2.
miR-21 is regulated by ALDO/SALT combination through the mineralocorticoid receptor. (a) Rats were treated with ALDO/SALT or vehicle for up to 8 weeks and LV miR-21 was analyzed by Northern blot. (b) Rats were treated with SALT, ALDO, ALDO/SALT, ALDO/SALT + eplerenone (EPL) or vehicle for 2 weeks and LV miR-21 was quantified by RT-qPCR. Results are expressed as mean ± standard error of the mean (n = 6). *P < 0.05 versus control; #P < 0.05 versus ALDO/SALT. AU, arbitary unit.
miR-21 regulation is blood pressure dependent
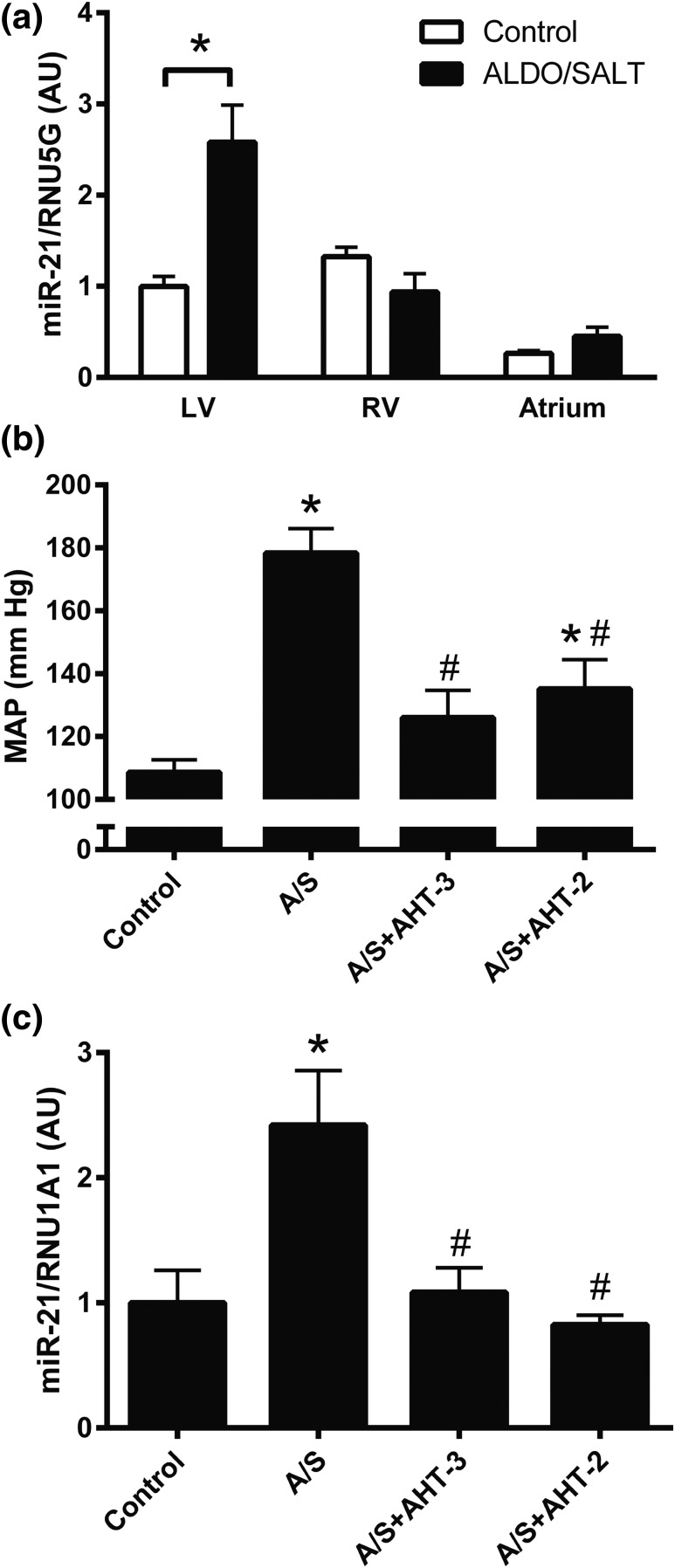
miR-21 is expressed in the atrium as well as in the right and left ventricles in the adult rat but is upregulated by ALDO/SALT only in the LV after 2 weeks of ALDO/SALT treatment [Fig. 3(a) and Supplemental Fig. 1(b) (4.6MB, pdf) ]. Administration of a triple antihypertensive therapy (hydralazine, hydrochlorothiazide, and reserpine) significantly attenuated ALDO/SALT-mediated blood pressure increase [Fig. 3(b)] and abolished ALDO/SALT-mediated LV miR-21 upregulation [Fig. 3(c) and Supplemental Fig. 1(c) (4.6MB, pdf) ]. Similar results were obtained when animals were treated with a double antihypertensive therapy (hydralazine and reserpine) to avoid the confounding effect of the diuretic drug hydrochlorothiazide [Fig. 3(b) and 3(c)]. These results suggest that ALDO/SALT-mediated miR-21 upregulation is blood pressure dependent.
Figure 3.
miR-21 is blood pressure dependently regulated by ALDO/SALT exclusively in the LV. (a) Rats were treated with ALDO/SALT or vehicle for 2 weeks and miR-21 was quantified by RT-qPCR in the different cardiac chambers. Rats were treated with ALDO/SALT (A/S) and triple (AHT-3: hydralazine + hydrochlorothiazide + reserpine) or double (AHT-2: hydralazine + reserpine) antihypertensive therapy or vehicle for 2 weeks. Mean arterial pressure (MAP) during the last day of treatment was measured by (b) radiotelemetry and (c) LV miR-21 was quantified by RT-qPCR. Results are expressed as mean ± standard error of the mean (n = 6). *P < 0.05 versus control. AU, arbitary unit.
miR-21 downregulation exacerbates ALDO/SALT-mediated cardiac injury and dysfunction
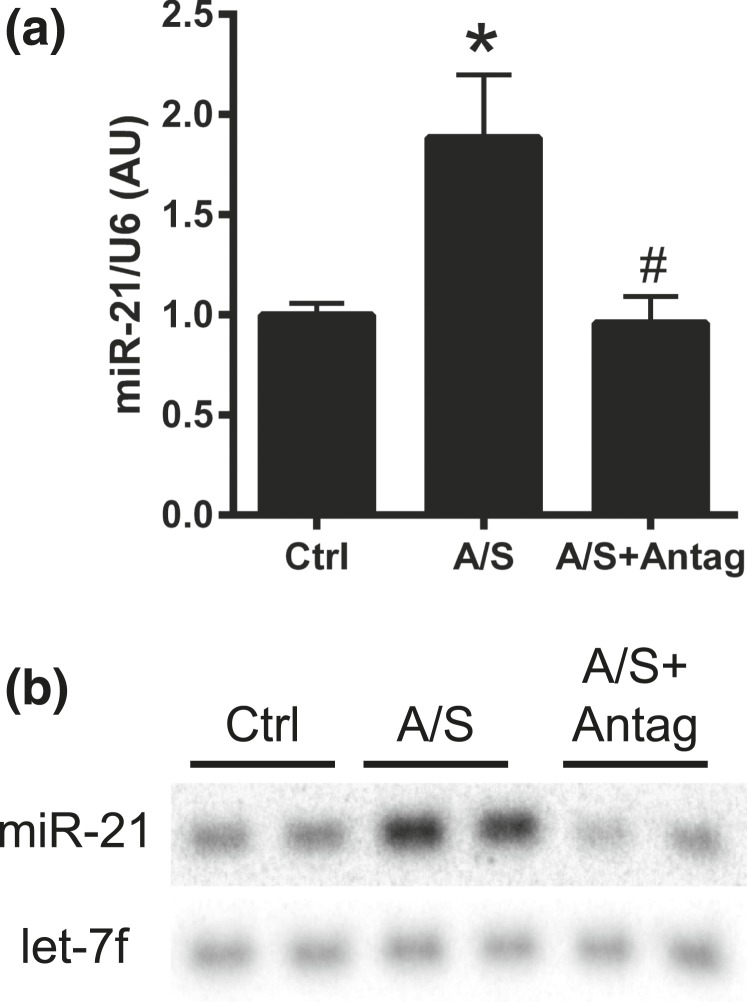
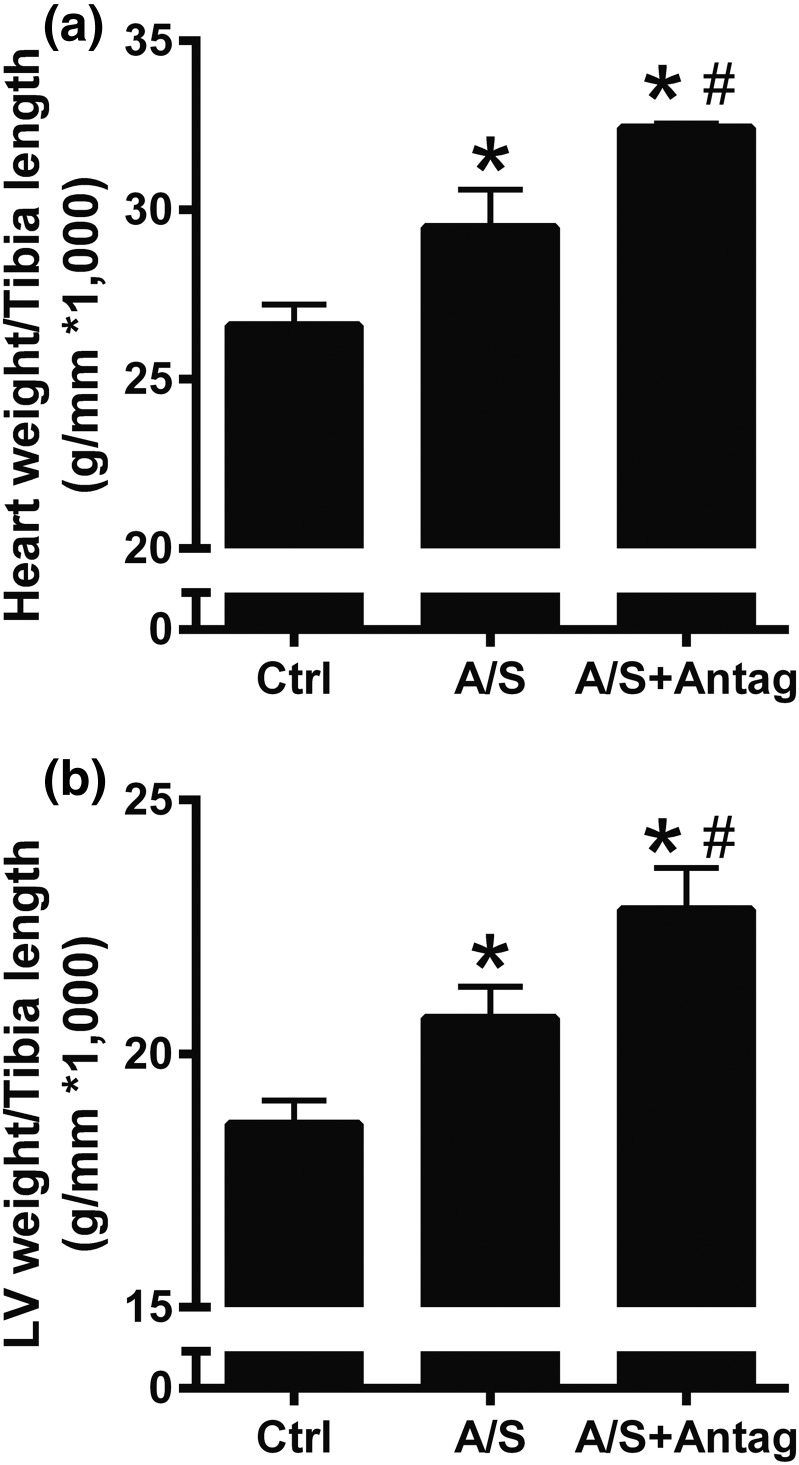
To analyze the role of ALDO/SALT-mediated miR-21 upregulation on cardiac injury and function, ALDO/SALT-treated rats were administered a miR-21 antagomir to specifically downregulate miR-21. A 2-week treatment period with ALDO/SALT was selected because this is when cardiac injury and dysfunction are incipient and worsening of these parameters is easier to detect. miR-21 antagomir administration successfully abolished ALDO/SALT-mediated LV miR-21 upregulation, as evidenced by RT-qPCR and Northern blot [Fig. 4(a) and 4(b) and Supplemental Fig. 1(d) (4.6MB, pdf) ]. miR-21 downregulation exacerbated ALDO/SALT-mediated cardiac and LV hypertrophy [Fig. 5(a) and 5(b)], with no significant change in kidney or body weight [Supplemental Fig. 4(a) and 4(b) (4.6MB, pdf) ]. Cardiac dimensions determined by echocardiography showed that miR-21 downregulation caused a tendency to exacerbate ALDO/SALT-mediated diastolic anterior and posterior wall thickness [Supplemental Fig. 4(c) and 4(d) (4.6MB, pdf) ].
Figure 4.
miR-21 antagomir abolished ALDO/SALT-mediated LV miR-21 upregulation. Rats were treated with ALDO/SALT (A/S) or vehicle for 2 weeks. miR-21 antagomir (Antag) was administered starting on day 5 for 3 consecutive days. miR-21 was quantified by (a) RT-qPCR or (b) Northern blot. Results are expressed as mean ± standard error of the mean (n = 5). *P < 0.05 versus control (Ctrl); #P < 0.05 versus A/S. AU, arbitary unit.
Figure 5.
miR-21 downregulation exacerbated ALDO/SALT-mediated cardiac hypertrophy. Rats were treated with ALDO/SALT (A/S) or vehicle for 2 weeks. miR-21 antagomir (Antag) was administered starting on day 5 for 3 consecutive days. (a) Heart or (b) LV weights were determined by gravimetry and corrected by tibia length. Results are expressed as mean ± standard error of the mean (n = 5). *P < 0.05 versus control (Ctrl); #P < 0.05 versus A/S.
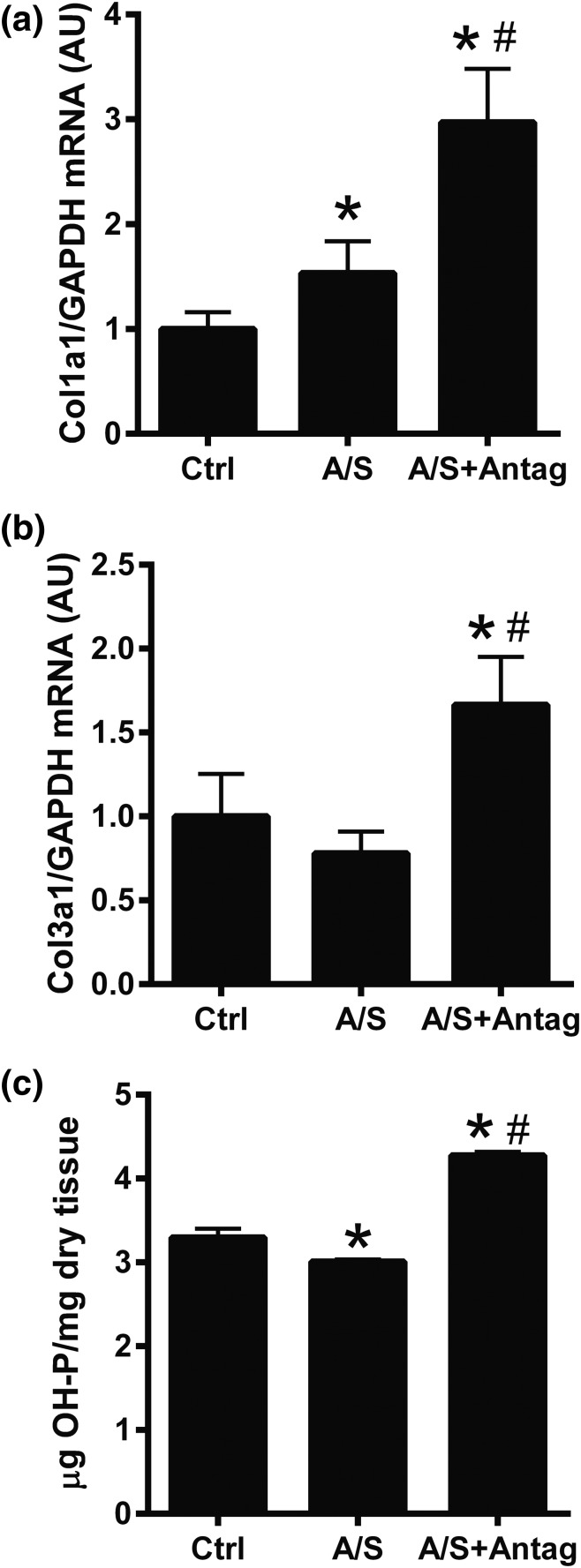
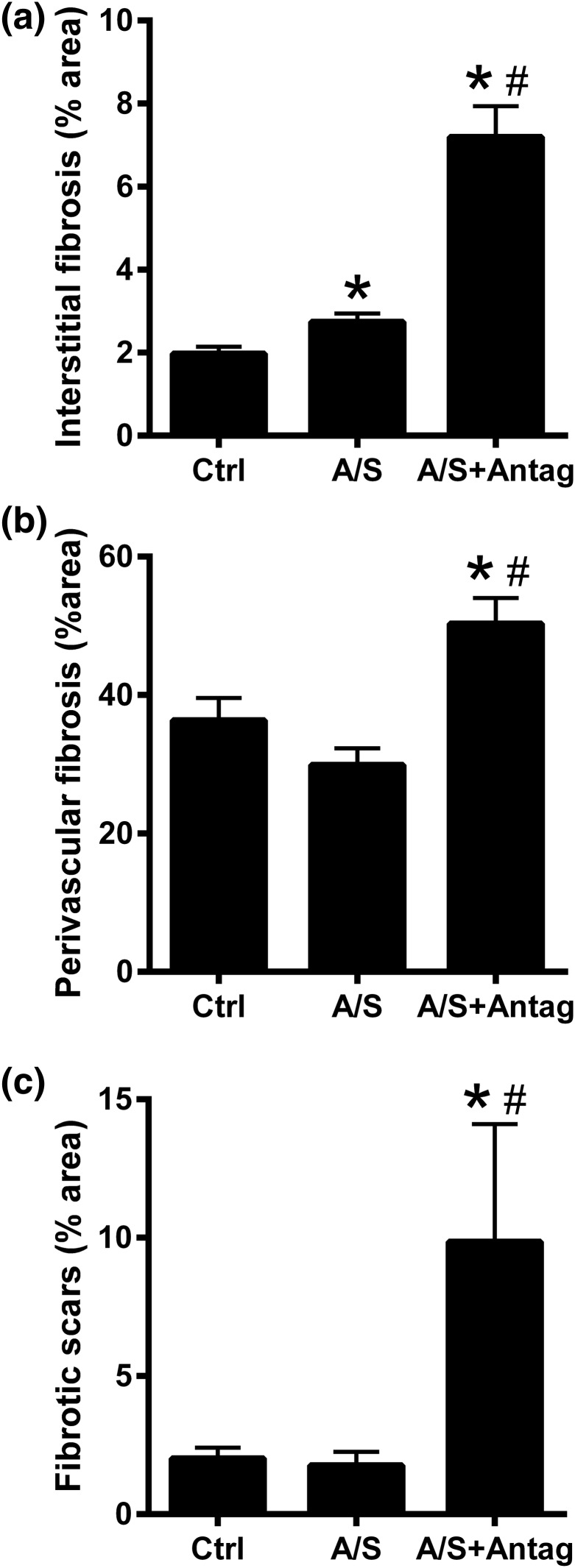
To analyze the role of miR-21 downregulation in ALDO/SALT-mediated cardiac fibrosis, we analyzed the expression of fibrosis markers and tissular collagen deposition. miR-21 downregulation exacerbated ALDO/SALT-mediated LV fibrosis marker mRNA expression upregulation, such as collagen I (Col1a1) and collagen III (Col3a1) [Fig. 6(a) and 6(b)], whereas there was no significant change in fibronectin (Fn1), connective tissue growth factor (Ctgf), or tissue inhibitor of metalloproteinase metallopeptidase inhibitor 1 (Timp1) expression [Supplemental Fig. 5(a)–5(c) (4.6MB, pdf) ]. The increase in collagen expression translated into an increase in LV OH-proline content [Fig. 6(c)]. Histologic analysis showed that miR-21 downregulation exacerbated ALDO/SALT-mediated cardiac interstitial fibrosis [Fig. 7(a)]. Furthermore, the effect of miR-21 downregulation on interstitial fibrosis was restricted to the LV and interventricular septum walls with negligible effect on the right ventricle wall [Supplemental Fig. 6(a)–6(c) (4.6MB, pdf) ]. miR-21 downregulation also caused significant perivascular fibrosis [Fig. 7(b) and Supplemental Fig. 7 (4.6MB, pdf) ], which was also restricted to the LV and interventricular septum walls [Supplemental Fig. 8(a)–8(c) (4.6MB, pdf) ]. Furthermore, miR-21 downregulation promoted scar formation, as evidenced by extensive collagen deposition by histologic analysis [Fig. 7(c) and Supplemental Fig. 9 (4.6MB, pdf) ].
Figure 6.
miR-21 downregulation exacerbated ALDO/SALT-mediated LV fibrosis. Rats were treated with ALDO/SALT (A/S) or vehicle for 2 weeks. miR-21 antagomir (Antag) was administered starting on day 5 for 3 consecutive days. (a) LV mRNA levels of collagen I (Col1a1) and (b) collagen III (Col3a1) were quantified by RT-qPCR. (c) LV collagen was determined by quantifying OH-proline. Results are expressed as mean ± standard error of the mean (n = 5). *P < 0.05 versus control (Ctrl); #P < 0.05 versus A/S. AU, arbitary unit; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Figure 7.
miR-21 downregulation exacerbated ALDO/SALT-mediated cardiac interstitial and perivascular fibrosis and scar formation. Rats were treated with ALDO/SALT (A/S) or vehicle for 2 weeks. miR-21 antagomir (Antag) was administered starting on day 5 for 3 consecutive days. (a) Interstitial and (b) perivascular fibrosis and (c) scars were quantified in trichrome-Masson–stained cardiac sections. Results are expressed as mean ± standard error of the mean (n = 5). *P < 0.05 versus control (Ctrl); #P < 0.05 versus A/S.
Despite the increase in fibrosis in ALDO/SALT-treated animals with decreased miR-21 levels, miR-21 downregulation did not modify the following ALDO/SALT-mediated cardiac fibroblasts marker expression: periostin (Postn), vimentin (Vim), Thy-1 cell surface antigen (Thy1, CD90), fibroblast specific protein 1 (Fsp1, S100a4), and discoidin domain receptor tyrosine kinase 2 (Ddr2) [Supplemental Fig. 10(a)–10(e) (4.6MB, pdf) ]. In addition, miR-21 downregulation had a tendency to decrease ALDO/SALT-mediated smooth muscle α actin (Acta2) upregulation [Supplemental Fig. 10(f) (4.6MB, pdf) ]. These results suggest that miR-21 downregulation activates the cardiac fibroblasts fibrotic response without significant changes in proliferation.
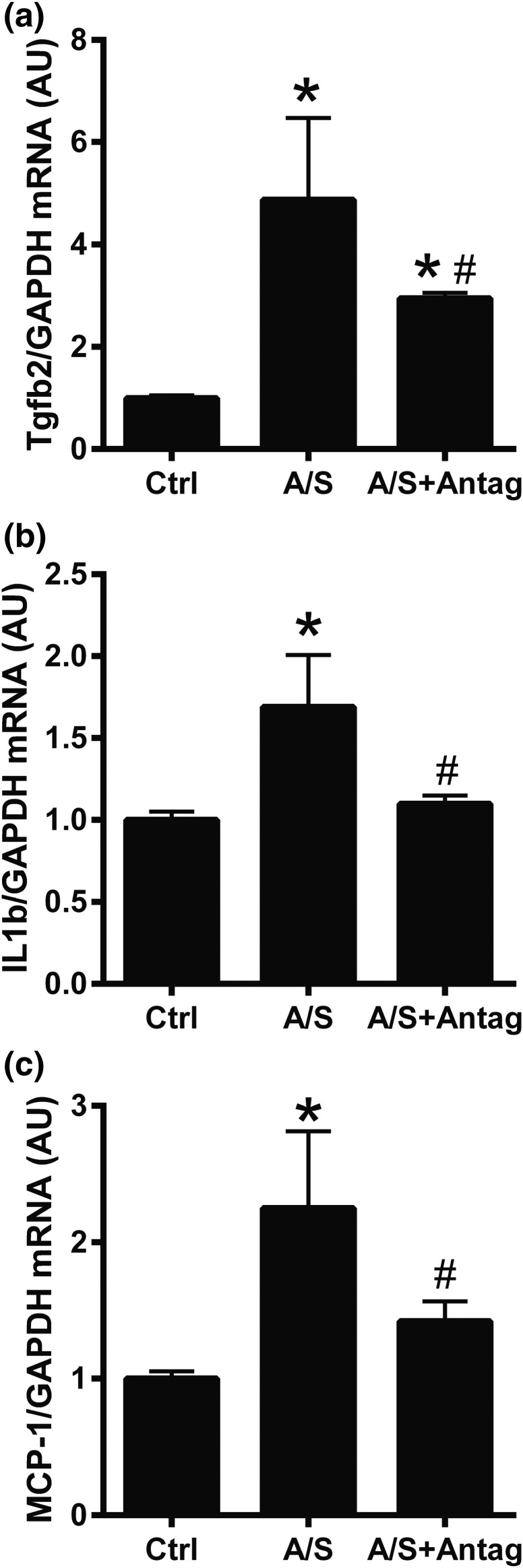
To analyze the role of miR-21 downregulation in ALDO/SALT-mediated cardiac inflammation, we analyzed the expression of inflammation markers. miR-21 downregulation attenuated ALDO/SALT-mediated LV inflammation marker mRNA expression, such as transforming growth factor β-2 (Tgfb2), interleukin-1β (Il1b), and monocyte chemoattractant protein-1 (MCP-1, Ccl2) [Fig. 8(a)–8(c)]. However, miR-21 downregulation did not significantly modify transforming growth factor-β1 (Tgfb1), vascular cell adhesion molecule 1 (Vcam1), plasminogen activator inhibitor type 1 (PAI-1, Serpine1), or osteopontin (OPN, Spp1) expression [Supplemental Fig. 11(a)–11(d) (4.6MB, pdf) ].
Figure 8.
miR-21 downregulation attenuated ALDO/SALT-mediated LV inflammation marker mRNA expression. Rats were treated with ALDO/SALT (A/S) or vehicle for 2 weeks. miR-21 antagomir (Antag) was administered starting on day 5 for 3 consecutive days. (a) LV mRNA levels of transforming growth factor β-2 (Tgfb2), (b) interleukin-1β (Il1b), and (c) monocyte chemoattractant protein-1 (MCP-1, Ccl2) were quantified by RT-qPCR. Results are expressed as mean ± standard error of the mean (n = 5). *P < 0.05 versus control (Ctrl); #P < 0.05 versus A/S. AU, arbitary unit; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
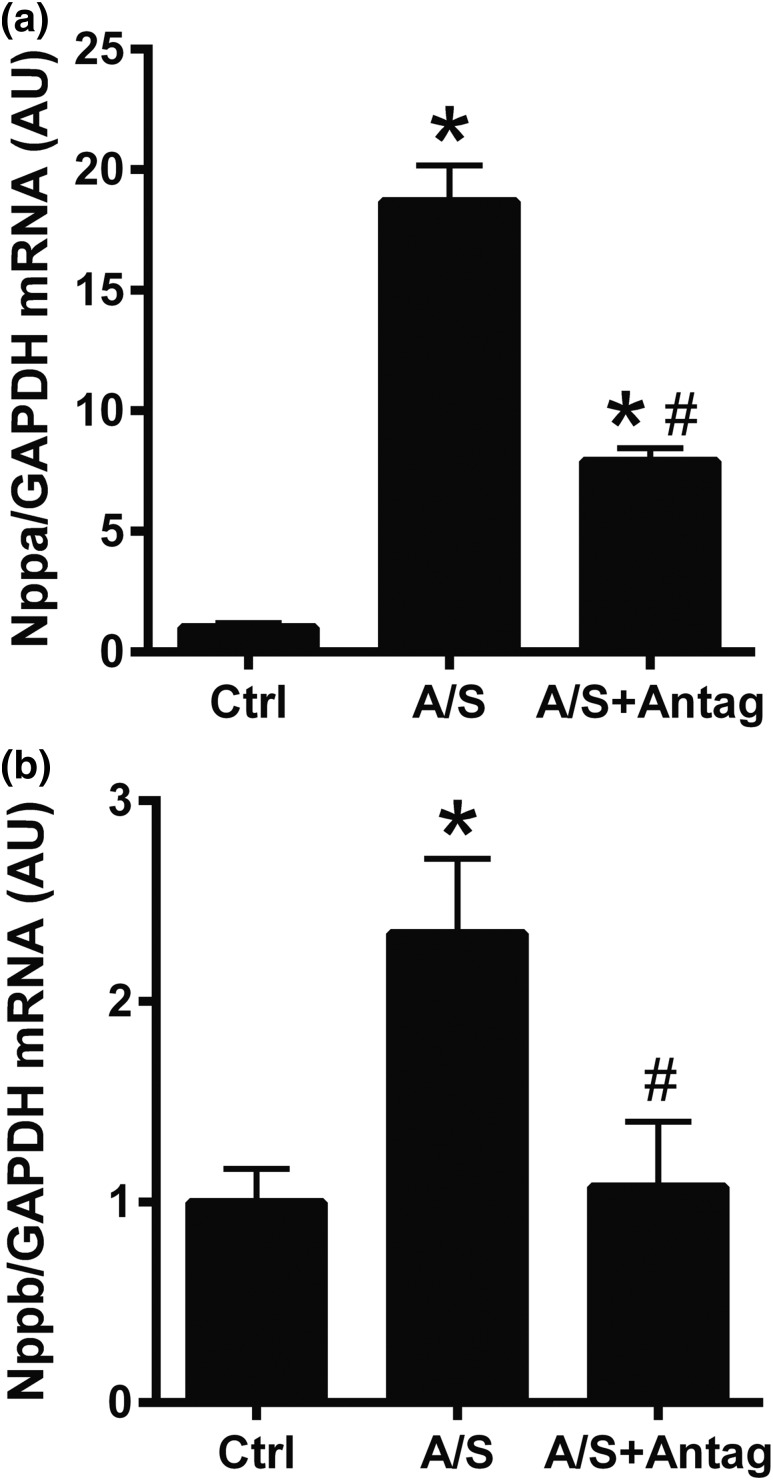
To analyze the role of miR-21 downregulation in ALDO/SALT-mediated cardiac function, we analyzed the expression of cardiac injury markers and cardiac function. miR-21 downregulation attenuated ALDO/SALT-mediated LV cardiac injury marker mRNA expression, such as natriuretic peptide precursor A (Nppa) and natriuretic peptide precursor B (Nppb) [Fig. 9(a) and 9(b)] but did not significantly modify myosin heavy chain α (Myh6), myosin heavy chain β (Myh7), or heme oxigenase-1 (Hmox1) [Supplemental Fig. 12(a)–12(c) (4.6MB, pdf) ]. miR-21 downregulation exacerbated ALDO/SALT-mediated cardiac dysfunction as observed by a further decrease in cardiac output and fractional shortening [Fig. 10(a) and 10(b)]. However, none of the cardiac effects of miR-21 downregulation on ALDO/SALT-mediated cardiac injury and dysfunction seem to be mediated by effects on LV mineralocorticoid receptor (N3rc2) expression or plasma ALDO concentration because none of these seem to be affected [Supplemental Fig. 13(a) and 13(b) (4.6MB, pdf) ].
Figure 9.
miR-21 downregulation attenuated ALDO/SALT-mediated LV cardiac injury marker mRNA expression. Rats were treated with ALDO/SALT (A/S) or vehicle for 2 weeks. miR-21 antagomir (Antag) was administered starting on day 5 for 3 consecutive days. (a) LV mRNA levels of natriuretic peptide precursor A (Nppa) and (b) natriuretic peptide precursor B (Nppb) were quantified by RT-qPCR. Results are expressed as mean ± standard error of the mean (n = 5). *P < 0.05 versus control (Ctrl); #P < 0.05 versus A/S. AU, arbitary unit; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
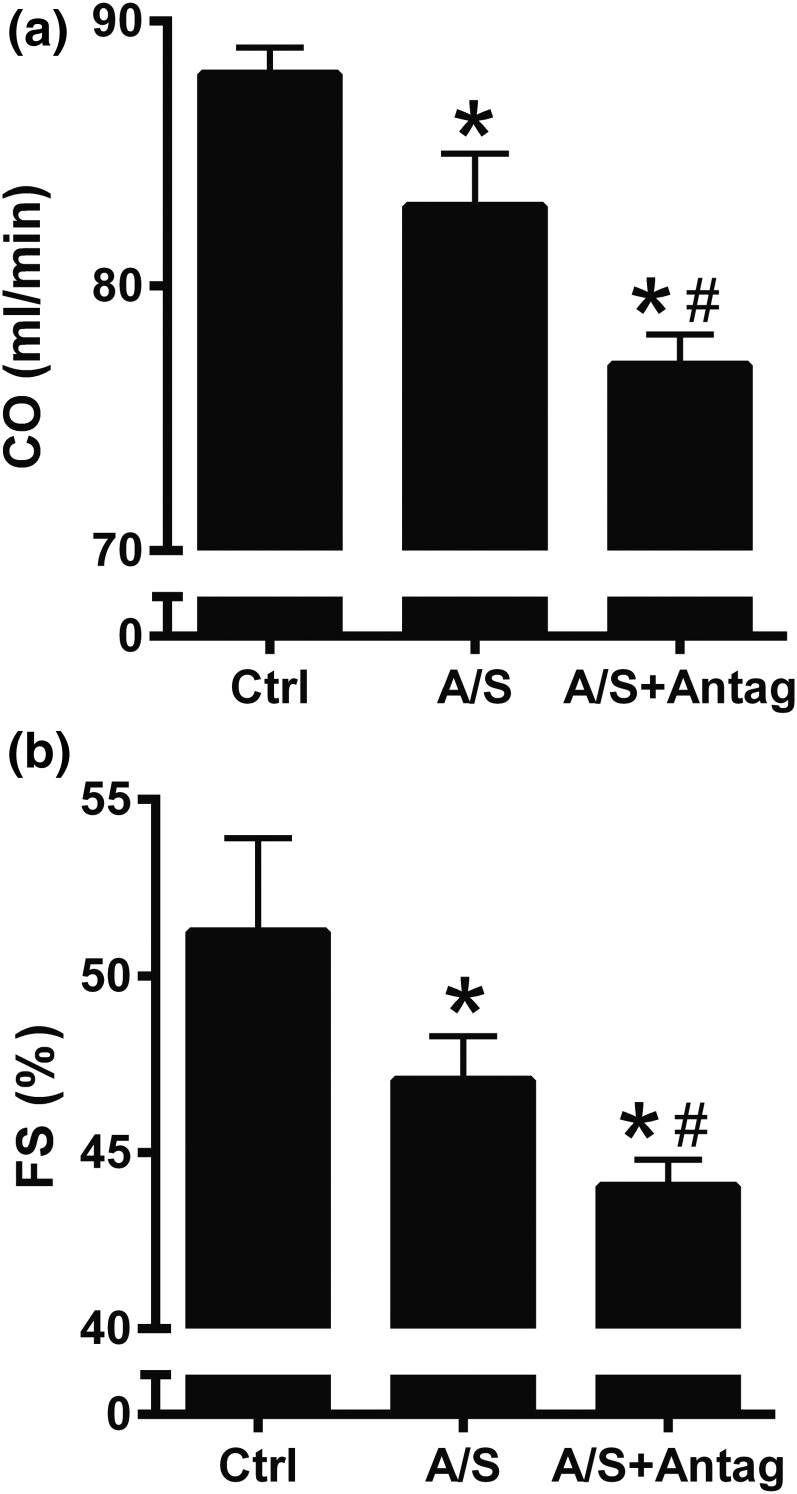
Figure 10.
miR-21 downregulation exacerbated ALDO/SALT-mediated cardiac dysfunction. Rats were treated with ALDO/SALT (A/S) or vehicle for 2 weeks. miR-21 antagomir (Antag) was administered starting on day 5 for 3 consecutive days. (a) Cardiac output (CO) and (b) fractional shortening (FS) were measured by echocardiography. Each determination is the mean of 12 to 15 cardiac cycles derived from three separate M-mode images during each session. Results are expressed as mean ± standard error of the mean (n = 5). *P < 0.05 versus control (Crtl); #P < 0.05 versus A/S.
In summary, miR-21 downregulation exacerbated ALDO/SALT-mediated cardiac fibrosis and dysfunction.
Discussion
We report a comprehensive time-series analysis of LV miRNA expression regulation by ALDO/SALT in vivo. We identified miR-21 as the most upregulated miRNA by ALDO/SALT in the LV. We report that miR-21 is upregulated by a combination of ALDO/SALT exclusively in the LV in a blood pressure–dependent manner. Furthermore, pharmacologic downregulation of miR-21 exacerbated ALDO/SALT-mediated cardiac fibrosis and dysfunction, suggesting that miR-21 plays a protective role in ALDO/SALT-mediated cardiac injury and dysfunction.
ALDO plays a critical role in sodium and potassium balance under physiologic conditions. However, excess ALDO in the presence of high salt intake causes target organ damage mainly in the heart, kidney, and vessels (1–7). Despite primary aldosteronism accounting for approximately 10% of cases of secondary hypertension, the molecular basis of ALDO-mediated cardiac injury remains poorly understood.
We report that LV miRNAs are time dependently dynamically regulated by ALDO/SALT treatment. Although the fold change for each miRNA is discrete, as has been reported in many experimental systems, the highly time-dependent expression regulation pattern for each miRNA suggests that multiple miRNAs may exert different actions through the course of ALDO-mediated cardiac pathologic abnormality. Many of the top validated ALDO/SALT-regulated miRNAs have been reported to have a role in different aspects of cardiovascular pathophysiology (19, 29–36, 78–80). However, few studies, as shown below, have addressed the regulation or role of miRNAs in the context of ALDO/SALT-mediated cardiac injury and dysfunction. Therefore, our study provides a comprehensive and unbiased analysis of miRNAs regulated by ALDO/SALT in the LV.
Calcineurin/NFAT3c/myocardin constitutes an intracellular signaling pathway that leads to cardiomyocyte hypertrophy. ALDO upregulates the expression of the miR-23≈27a≈24-2 cluster in neonatal rat cardiomyocytes in vitro through the calcineurin/NFAT3c signaling pathway (81). Furthermore, miR-23a, but not miR-27a or miR-24, inhibition abolished ALDO-mediated cardiomyocyte hypertrophy. On the other hand, in vitro treatment of neonatal rat cardiomyocytes with ALDO causes downregulation of miR-9 and upregulation of the miR-9 target myocardin, leading to cardiomyocyte hypertrophy (82). Interestingly, our studies show that miR-9, miR-24, and miR-27a are subjected to a regulation in the LV by ALDO/SALT in vivo similar to that observed in neonatal rat cardiomyocytes in vitro.
The MyomirRs, miR-208a and miR-208b, are intronic miRNAs encoded in the α-myosin heavy chain (Myh6) and the β-myosin heavy chain (Myh7) genes, respectively (83). The α-myosin heavy chain (Myh6) and its intronic miR-208a are highly expressed in the normal adult rodent heart, whereas the β-myosin heavy chain (Myh7) and its intronic miR-208b are upregulated by stress in adult hearts. Transgenic mice with liver-specific renin overexpression develop hypertension as well as cardiac hypertrophy and have significantly increased cardiac levels of miR-208b, a cardiac injury marker, despite normal cardiac and circulating ALDO levels (84). On the other hand, double transgenic mice with liver-specific renin overexpression and cardiomyocyte-specific aldosterone synthase (CYP11B2) overexpression are hypertensive, develop exacerbated cardiac hypertrophy, and present elevated cardiac and circulating ALDO levels. Surprisingly, despite the exacerbated deleterious phenotype observed in the double transgenic mice, these animals have a modest miR-208b upregulation but a substantial miR-208a downregulation (84). The surprising results of the double transgenic mice may be explained by ALDO-mediated inhibition of natriuretic peptides transcription because atrial natriuretic peptide infusion reverses cardiac hypertrophy in the double transgenic animals but not in the animals overexpressing renin (84).
Cardiac miR-1 expression is transiently downregulated after myocardial infarction (85, 86). Treatment with the mineralocorticoid receptor antagonist spironolactone abolishes this decrease (85). Because miR-1 has been implicated in cardiac hypertrophy and arrhythmogenic potential (87, 88), it may be possible that miR-1 mediates the beneficial effects of mineralocorticoid receptor antagonists.
Plasma circulating miRNAs have been studied as potential biomarkers in the DOCA/SALT rat model (89). A subset of 11 miRNAs found to be regulated by a high-salt diet in the Dahl Salt Sensitive rats were analyzed. Seven miRNAs were found to be upregulated in the circulation, three (miR-16, miR-19b, miR-223) of which we found to be elevated in the LV of ALDO/SALT-treated animals. On the other hand, three (miR-125a, mir-143, miR-199a-3p) of the four miRNAs that showed a tendency to be downregulated in the circulation were upregulated in our experimental model. This discrepancy may be explained by the fact that correlations between circulating miRNAs and tissues are not always straightforward, as in many cases particular miRNAs can present opposite regulation in the tissues versus circulation. Also it must be considered that the circulating pool is subjected to the input of multiple organs. These reasons may also explain why we were unsuccessful in detecting an ALDO/SALT treatment effect in circulating miR-21 in our experimental model (data not shown).
We focused our studies on the regulation and role of miR-21 in ALDO/SALT-mediated cardiac injury and dysfunction because it was the most upregulated miRNA in the LV of ALDO/SALT-treated animals. miR-21 is weakly expressed in normal human hearts, but it is consistently upregulated in human end-stage heart failure (39–42). In animal experimental models, miR-21 has been shown to be upregulated by thoracic aortic banding (37, 38, 49, 53, 90, 91), ischemia/reperfusion (92), ischemic preconditioning (55, 93), and heat shock (52) as well as in transgenic mice expressing activated calcineurin A (CnA), in the heart (90) and in the border zone of myocardial infarct (51, 94, 95). Our studies show that miR-21 is upregulated almost fourfold after two weeks of treatment and remains elevated for at least 8 weeks of ALDO/SALT treatment. ALDO/SALT-mediated LV miR-21 upregulation seems to be blood pressure dependent for the following reasons: (a) ALDO treatment alone, which does not elevate the blood pressure, does not affect miR-21 levels; (b) ALDO/SALT treatment increases miR-21 only in the LV, a cardiac chamber classically associated with blood pressure increase; and (c) coadministration of a double or triple antihypertensive therapy, which effectively decreases blood pressure, abolishes the ALDO/SALT-mediated increase in LV miR-21. These results suggest that the ALDO/SALT-mediated increase in blood pressure drives or is permissive for ALDO to increase miR-21 levels in the LV.
Despite multiple studies, the role of miR-21 in cardiac pathophysiology remains highly controversial with a plethora of studies suggesting that this particular microRNA can be deleterious (37, 39, 43–48) or beneficial (38, 49–59) in cardiac injury.
Thum et al. (39) reported that miR-21 is upregulated in cardiac fibroblasts of transverse aortic constriction (TAC) mouse hearts and in human end-stage failing hearts. Furthermore, administration of a chemically modified antisense oligonucleotide specific for miR-21 (antagomir-21) to mice subjected to TAC decreased collagen mRNA expression, interstitial fibrosis, cardiomyocyte size, cardiac hypertrophy, and normalized cardiac function (39), suggesting a critical role of miR-21 in cardiac fibroblasts stress response.
On the other hand, Patrick et al. (54) reported that miR-21 null mice showed no effect on cardiac hypertrophy and stress marker gene expression in response to TAC, cardiomyocyte-specific calcineurin overexpression, or myocardial infarction compared with WT mice, suggesting that miR-21 may not be essential for pathologic cardiac remodeling. Furthermore, the same group reported that treatment of animals with a tiny locked nucleic acid (LNA)-modified antimiR-21 failed to block cardiac remodeling due to the above mentioned cardiac stressors, despite significant miR-21 downregulation (54) in clear contrast with Thum et al. (39).
However, Patrick et al. (54) showed that miR-21 ablation had a remarkable potentiating effect on Angiotensin II-mediated cardiac hypertrophy, although this effect was not observed when miR-21 was knocked-down using antimiR-21. It is worth mentioning that angiotensin II is the most potent hormonal stimulator of ALDO secretion and these results are in agreement with our studies where miR-21 downregulation worsens the ALDO/SALT-mediated cardiac injury and dysfunction phenotype.
More recently, Bang et al. (46) reported that angiotensin II induces the cardiac fibroblasts to secrete exosomes preferentially loaded with miR-21 passenger strands (miR-21-3p or miR-21*) into the cardiac pericardial fluid and induce cardiomyocyte hypertrophy. We doubt that this is the case in our experimental model because although miR-21-3p is upregulated by ALDO/SALT, its expression is more than 500 times lower than the expression level of miR-21 (data not shown).
As shown above, contradictory results have been the rule and not the exception in miR-21 cardiac functional studies. These differences may be due to the use of miR-21 inhibitors with different chemistries, which may translate to varying degrees of miR-21 inhibition across tissues and cell types, off-target effects, and targeting multiple cell types and tissues. On the other hand, although transgenic animals may be seen as a superior experimental model, the use of ubiquitous noninducible ablation or overexpression systems may cause developmental and compensatory mechanisms that reduce the advantage of this system (19, 32, 33, 96).
A few more examples of the role of miR-21 in cardiac pathophysiology show how widespread its role depends on different experimental models of cardiac pathology. miR-21 has been reported to have protective or beneficial effects in other models of cardiac injury. For example, miR-21 is upregulated in the border zone of infarcted hearts, and infarct size miR-21 has been shown to be significantly decreased by upregulation by overexpression using adenoviruses (51), transgenic animals with cardiomyocyte-specific miR-21 overexpression (53) as well as synthetic microRNA mimics (52). Supporting a protective effect of miR-21 are studies showing that cardiac miR-21 is upregulated during ischemic preconditioning, and cardiomyocyte miR-21 overexpression significantly attenuates the infarct size (53, 55, 56). Moreover, miR-21 is upregulated in multiple animal experimental models and human samples of pulmonary hypertension. Furthermore, miR-21 downregulation or ablation exacerbates, whereas miR-21 overexpression protects from, the deleterious phenotype suggesting a protective role for miR-21 (57, 97, 98).
On the other hand, miR-21 has also been reported to be a mediator of cardiac injury as highlighted in these few examples. The left atria of patients with atrial fibrillation show increased levels of miR-21 and fibrosis markers, which are associated with increased levels of the small Rho-GTPase Rac1 (44). Interestingly, Rac1 has been implicated in ligand-independent mineralocorticoid receptor activation in cardiomyocytes in vitro and in vivo leading to cardiac injury (99, 100). Furthermore, left atrium–specific miR-21 downregulation attenuates myocardial infarction–induced atrial fibrillation duration and fibrosis (45). Similarly, miR-21 is upregulated in sterile pericarditis through a positive reciprocal feedback loop with STAT3, and left atrium–specific miR-21 downregulation reduces fibrosis and atrial fibrillation promotion (48). Recently, osteopontin has been shown to upregulate cardiac fibroblast miR-21 expression, which leads to increased cardiac fibroblast survival and extracellular matrix production in vitro and exacerbates angiotensin II–induced cardiac fibrosis in vivo, suggesting that miR-21 is a mediator of osteopontin-induced cardiac injury (47).
These studies may suggest that the effects of miR-21 are cardiac stressor and cell type–specific and generalizations about beneficial or deleterious effects of miR-21 in cardiac pathologic abnormalities should always be considered in the context of the disease model under study.
Few studies have analyzed the regulation or role of cardiac miR-21 by ALDO. Left atrium miR-21 levels have been reported to be positively correlated with fibrosis in patients with permanent atrial fibrillation or sinus rhythm (101). Furthermore, in vitro treatment of neonatal rat cardiac fibroblasts with ALDO upregulates miR-21 expression and downregulates the protein levels of the miR-21 target protein Sprouty-1. Those effects are abolished by cotreatment with mineralocorticoid receptor antagonists, suggesting that these are mineralocorticoid receptor–mediated events. More recently, the same group reported that the aldosterone synthase inhibitor SL242 abolishes angiotensin II–mediated miR-21 upregulation in neonatal rat cardiac fibroblasts (102). These results may suggest an autocrine effect of ALDO that regulates miR-21 expression in cardiac fibroblasts; however, the expression of aldosterone synthase and ALDO secretion by cardiac cells is questionable at best (103, 104). On the other hand, transgenic mice with cardiomyocyte-specific overexpression of a constitutively active Rac-1 presents increased fibrosis, miR-21 expression, and atrial fibrillation, most likely through ligand-independent activation of the mineralocorticoid receptor (102). These and our results suggest that the activated mineralocorticoid receptor upregulates miR-21 expression in a ligand-dependent or -independent manner.
In particular, our studies have shown that miR-21 downregulation exacerbates ALDO/SALT-induced cardiac fibrosis and dysfunction suggesting a protective role of miR-21 in the cardiac pathologic abnormalities induced in primary aldosteronism. Because miR-21 is upregulated by ALDO/SALT treatment in cardiac tissue and appears to play a protective role, it may be possible to speculate that this increase is a physiologic compensatory mechanism to attenuate ALDO/SALT-mediated cardiac injury and dysfunction. Furthermore, because miR-21 downregulation exacerbates the ALDO/SALT-mediated cardiac deleterious phenotype, it is possible to speculate that increasing miR-21 levels would be beneficial under such pathologic conditions. Although pharmacologic approaches to supplement miRNAs have lagged behind similar approaches to inhibit miRNA action, recent reports show that these therapeutics may be heading to the clinic in the near future. MRX34 (Mirna Therapeutics) is a chemically modified double-stranded RNA encapsulated in a liposomal formulation that mimics microRNA-34 to supplement decreased levels of that particular miRNA in patients with a variety of advanced solid tumors. Recent reports from a multicenter open-labeled dose-finding phase 1 clinical trial (ClinicalTrials.gov identifier: NCT01829971) suggest that MRX34 presents a manageable safety profile, dose-dependent repression of miR-34 target oncogenes, and, even more importantly, partial response in patients with hepatocellular carcinoma, renal cell carcinoma, or acral melanoma (105). These promising results suggest that miRNA supplementation therapies have stepped up from transgenic or viral delivery approaches and are already in the field of pharmaceuticals (106, 107).
Despite the advantages of systemic miRNA modulation therapies, one limitation of our study is that miR-21 antagomirs may downregulate miR-21 in other tissues besides the heart. This limitation may be overcome by the use of cell type–specific or tissue-specific miRNA modulation therapies that downregulate or supplement miRNAs in particular cell types by pharmacologic or genetic means.
In summary, we report a comprehensive and unbiased screening of LV miRNAs regulated by ALDO/SALT in vivo. Furthermore, we report that miR-21 is the most upregulated of such modulated miRNAs, using a well-established animal experimental model that mimics the deleterious consequences of excess ALDO as observed in patients with primary aldosteronism. Moreover, miR-21 downregulation exacerbates ALDO/SALT-mediated cardiac deleterious phenotype, suggesting that miR-21 plays a protective role on ALDO/SALT-mediated cardiac injury and dysfunction under these experimental conditions. Finally, it may be possible to speculate that miR-21 supplementation would have beneficial effects in reverting or mitigating cardiac injury and dysfunction in patients with primary aldosteronism.
Acknowledgments
We thank the University of Mississippi Medical Center (UMMC) Center for Psychiatric Neuroscience Imaging Core (supported by National Institutes of Health COBRE P30GM103328), the UMMC Molecular and Genomics Facility (supported, in part, by the National Institutes of Health, including Mississippi INBRE P20GM103476, Center for Psychiatric Neuroscience COBRE P30GM103328 and Obesity, Cardiorenal and Metabolic Diseases COBRE P20GM104357), and UMMC Pathology Research Histology Core for outstanding service.
Acknowledgments
This work was supported by American Heart Association grants 12SDG8980032 (D.G.R.) and 0830239N (L.L.Y.C.), Endocrine Fellows Foundation Endocrine Research Grant (L.L.Y.C.), National Institutes of Health grants K08DK099415 (M.E.H.), R01HL66072 (J.F.R.) and P01HL51971 (J.F.R.), and American Heart Association Postdoctoral Fellowship 14POST18640015 (R.O.M.).
Disclosure summary: The authors have nothing to disclose.
Footnotes
- ALDO
- aldosterone
- ARR
- aldosterone-to-renin ratio
- LNA
- locked nucleic acid
- LV
- left ventricle
- miRNA
- microRNA
- mRNA
- messenger RNA
- PAC
- plasma aldosterone concentration
- PRA
- plasma renin activity
- RT-qPCR
- reverse transcriptase quantitative polymerase chain reaction
- TAC
- transverse aortic constriction.
References
- 1.Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res. 1990;67(6):1355–1364. [DOI] [PubMed] [Google Scholar]
- 2.Young M, Fullerton M, Dilley R, Funder J. Mineralocorticoids, hypertension, and cardiac fibrosis. J Clin Invest. 1994;93(6):2578–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283(5):H1802–H1810. [DOI] [PubMed] [Google Scholar]
- 4.Rocha R, Stier CT Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141(10):3871–3878. [DOI] [PubMed] [Google Scholar]
- 5.Young MJ, Lam EY, Rickard AJ. Mineralocorticoid receptor activation and cardiac fibrosis. Clin Sci (Lond). 2007;112(9):467–475. [DOI] [PubMed] [Google Scholar]
- 6.Marney AM, Brown NJ. Aldosterone and end-organ damage. Clin Sci (Lond). 2007;113(6):267–278. [DOI] [PubMed] [Google Scholar]
- 7.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345(23):1689–1697. [DOI] [PubMed] [Google Scholar]
- 8.Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF Jr. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89(3):1045–1050. [DOI] [PubMed] [Google Scholar]
- 9.Mattsson C, Young WF Jr. Primary aldosteronism: diagnostic and treatment strategies. Nat Clin Pract Nephrol. 2006;2(4):198–208. [DOI] [PubMed] [Google Scholar]
- 10.Calhoun DA. Is there an unrecognized epidemic of primary aldosteronism? Pro. Hypertension. 2007;50(3):447–453, discussion 447–453. [DOI] [PubMed] [Google Scholar]
- 11.Young WF., Jr Minireview: primary aldosteronism--changing concepts in diagnosis and treatment. Endocrinology. 2003;144(6):2208–2213. [DOI] [PubMed] [Google Scholar]
- 12.Stowasser M. Update in primary aldosteronism. J Clin Endocrinol Metab. 2009;94(10):3623–3630. [DOI] [PubMed] [Google Scholar]
- 13.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF Jr, Montori VM; Endocrine Society . Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(9):3266–3281. [DOI] [PubMed] [Google Scholar]
- 14.Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98(12):4826–4833. [DOI] [PubMed] [Google Scholar]
- 15.Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62(2):331–336. [DOI] [PubMed] [Google Scholar]
- 16.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–1248. [DOI] [PubMed] [Google Scholar]
- 17.Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, März W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J. 2010;31(10):1237–1247. [DOI] [PubMed] [Google Scholar]
- 18.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469(7330):336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11(3):228–234. [DOI] [PubMed] [Google Scholar]
- 21.Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development. 2005;132(21):4645–4652. [DOI] [PubMed] [Google Scholar]
- 22.Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol. 2013;14(8):475–488. [DOI] [PubMed] [Google Scholar]
- 23.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. [DOI] [PubMed] [Google Scholar]
- 24.Flynt AS, Lai EC. Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nat Rev Genet. 2008;9(11):831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huntzinger E, Izaurralde E.. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12(2):99–110 [DOI] [PubMed] [Google Scholar]
- 26.Fabian MR, Sonenberg N. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol. 2012;19(6):586–593. [DOI] [PubMed] [Google Scholar]
- 27.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. [DOI] [PubMed] [Google Scholar]
- 28.Sun K, Lai EC. Adult-specific functions of animal microRNAs. Nat Rev Genet 2013;14(8):535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91(3):827–887. [DOI] [PubMed] [Google Scholar]
- 30.Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121(8):1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latronico MV, Catalucci D, Condorelli G. MicroRNA and cardiac pathologies. Physiol Genomics. 2008;34(3):239–242. [DOI] [PubMed] [Google Scholar]
- 32.Bauersachs J. Regulation of myocardial fibrosis by MicroRNAs. J Cardiovasc Pharmacol. 2010;56(5):454–459. [DOI] [PubMed] [Google Scholar]
- 33.Han M, Toli J, Abdellatif M. MicroRNAs in the cardiovascular system. Curr Opin Cardiol. 2011;26(3):181–189. [DOI] [PubMed] [Google Scholar]
- 34.Dai Y, Khaidakov M, Wang X, Ding Z, Su W, Price E, Palade P, Chen M, Mehta JL. MicroRNAs involved in the regulation of postischemic cardiac fibrosis. Hypertension. 2013;61(4):751–756. [DOI] [PubMed] [Google Scholar]
- 35.Tijsen AJ, Pinto YM, Creemers EE. Non-cardiomyocyte microRNAs in heart failure. Cardiovasc Res. 2012;93(4):573–582. [DOI] [PubMed] [Google Scholar]
- 36.Condorelli G, Latronico MV, Cavarretta E. microRNAs in cardiovascular diseases: current knowledge and the road ahead. J Am Coll Cardiol. 2014;63(21):2177–2187. [DOI] [PubMed] [Google Scholar]
- 37.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170(6):1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, Rojas M, Hammond SM, Wang DZ. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42(6):1137–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–984. [DOI] [PubMed] [Google Scholar]
- 40.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116(3):258–267. [DOI] [PubMed] [Google Scholar]
- 41.Matkovich SJ, Van Booven DJ, Youker KA, Torre-Amione G, Diwan A, Eschenbacher WH, Dorn LE, Watson MA, Margulies KB, Dorn GW II. Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation. 2009;119(9):1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang KC, Yamada KA, Patel AY, Topkara VK, George I, Cheema FH, Ewald GA, Mann DL, Nerbonne JM. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation. 2014;129(9):1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang H, Zhang C, Ban T, Liu Y, Mei L, Piao X, Zhao D, Lu Y, Chu W, Yang B. A novel reciprocal loop between microRNA-21 and TGFβRIII is involved in cardiac fibrosis. Int J Biochem Cell Biol. 2012;44(12):2152–2160. [DOI] [PubMed] [Google Scholar]
- 44.Adam O, Löhfelm B, Thum T, Gupta SK, Puhl SL, Schäfers HJ, Böhm M, Laufs U. Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res Cardiol. 2012;107(5):278. [DOI] [PubMed] [Google Scholar]
- 45.Cardin S, Guasch E, Luo X, Naud P, Le Quang K, Shi Y, Tardif JC, Comtois P, Nattel S. Role for MicroRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ Arrhythm Electrophysiol. 2012;5(5):1027–1035. [DOI] [PubMed] [Google Scholar]
- 46.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124(5):2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lorenzen JM, Schauerte C, Hübner A, Kölling M, Martino F, Scherf K, Batkai S, Zimmer K, Foinquinos A, Kaucsar T, Fiedler J, Kumarswamy R, Bang C, Hartmann D, Gupta SK, Kielstein J, Jungmann A, Katus HA, Weidemann F, Müller OJ, Haller H, Thum T. Osteopontin is indispensible for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis. Eur Heart J. 2015;36(32):2184–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Z, Chen XJ, Qian C, Dong Q, Ding D, Wu QF, Li J, Wang HF, Li WH, Xie Q, Cheng X, Zhao N, Du YM, Liao YH. Signal transducer and activator of transcription 3/MicroRNA-21 feedback loop contributes to atrial fibrillation by promoting atrial fibrosis in a rat sterile pericarditis model. Circ Arrhythm Electrophysiol. 2016;9(7):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sayed D, Rane S, Lypowy J, He M, Chen IY, Vashistha H, Yan L, Malhotra A, Vatner D, Abdellatif M. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Mol Biol Cell. 2008;19(8):3272–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol. 2009;47(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284(43):29514–29525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin C, Wang X, Kukreja RC. Endogenous microRNAs induced by heat-shock reduce myocardial infarction following ischemia-reperfusion in mice. FEBS Lett. 2008;582(30):4137–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, Abdellatif M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem. 2010;285(26):20281–20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patrick DM, Montgomery RL, Qi X, Obad S, Kauppinen S, Hill JA, van Rooij E, Olson EN. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120(11):3912–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X, Chun B, Zhuang J, Zhang C. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res. 2010;87(3):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin Y, Yu Y, Dong H, Bian X, Guo X, Dong S. MicroRNA 21 inhibits left ventricular remodeling in the early phase of rat model with ischemia-reperfusion injury by suppressing cell apoptosis. Int J Med Sci. 2012;9(6):413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, Cottrill KA, Shaik RS, Waxman AB, Zhang YY, Maron BA, Hartner JC, Fujiwara Y, Orkin SH, Haley KJ, Barabási AL, Loscalzo J, Chan SY. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125(12):1520–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toldo S, Das A, Mezzaroma E, Chau VQ, Marchetti C, Durrant D, Samidurai A, Van Tassell BW, Yin C, Ockaili RA, Vigneshwar N, Mukhopadhyay ND, Kukreja RC, Abbate A, Salloum FN. Induction of microRNA-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circ Cardiovasc Genet. 2014;7(3):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH, Jiang L, Feng J, Yu XY. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016;7(6):e2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2009;296(4):F771–F779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall ME, Maready MW, Hall JE, Stec DE. Rescue of cardiac leptin receptors in db/db mice prevents myocardial triglyceride accumulation. Am J Physiol Endocrinol Metab. 2014;307(3):E316–E325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. [DOI] [PubMed] [Google Scholar]
- 63.Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438(7068):685–689. [DOI] [PubMed] [Google Scholar]
- 64.Krützfeldt J, Kuwajima S, Braich R, Rajeev KG, Pena J, Tuschl T, Manoharan M, Stoffel M. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 2007;35(9):2885–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42(Database issue):D68–D73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ritchie ME, Silver J, Oshlack A, Holmes M, Diyagama D, Holloway A, Smyth GK. A comparison of background correction methods for two-colour microarrays. Bioinformatics. 2007;23(20):2700–2707. [DOI] [PubMed] [Google Scholar]
- 67.King JY, Ferrara R, Tabibiazar R, Spin JM, Chen MM, Kuchinsky A, Vailaya A, Kincaid R, Tsalenko A, Deng DX, Connolly A, Zhang P, Yang E, Watt C, Yakhini Z, Ben-Dor A, Adler A, Bruhn L, Tsao P, Quertermous T, Ashley EA. Pathway analysis of coronary atherosclerosis. Physiol Genomics. 2005;23(1):103–118. [DOI] [PubMed] [Google Scholar]
- 68.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 69.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 70.Derks NM, Müller M, Gaszner B, Tilburg-Ouwens DT, Roubos EW, Kozicz LT. Housekeeping genes revisited: different expressions depending on gender, brain area and stressor. Neuroscience. 2008;156(2):305–309. [DOI] [PubMed] [Google Scholar]
- 71.Radonić A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313(4):856–862. [DOI] [PubMed] [Google Scholar]
- 72.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. [DOI] [PubMed] [Google Scholar]
- 73.Romero DG, Yanes LL, de Rodriguez AF, Plonczynski MW, Welsh BL, Reckelhoff JF, Gomez-Sanchez EP, Gomez-Sanchez CE. Disabled-2 is expressed in adrenal zona glomerulosa and is involved in aldosterone secretion. Endocrinology. 2007;148(6):2644–2652. [DOI] [PubMed] [Google Scholar]
- 74.Várallyay E, Burgyán J, Havelda Z. MicroRNA detection by northern blotting using locked nucleic acid probes. Nat Protoc. 2008;3(2):190–196. [DOI] [PubMed] [Google Scholar]
- 75.Chiariello M, Ambrosio G, Cappelli-Bigazzi M, Perrone-Filardi P, Brigante F, Sifola C. A biochemical method for the quantitation of myocardial scarring after experimental coronary artery occlusion. J Mol Cell Cardiol. 1986;18(3):283–290. [DOI] [PubMed] [Google Scholar]
- 76.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F; PAPY Study Investigators . A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48(11):2293–2300. [DOI] [PubMed] [Google Scholar]
- 77.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. [DOI] [PubMed] [Google Scholar]
- 78.Creemers EE, van Rooij E. Function and therapeutic potential of noncoding RNAs in cardiac fibrosis. Circ Res. 2016;118(1):108–118. [DOI] [PubMed] [Google Scholar]
- 79.Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol. 2015;12(3):135–142. [DOI] [PubMed] [Google Scholar]
- 80.Luo X, Yang B, Nattel S. MicroRNAs and atrial fibrillation: mechanisms and translational potential. Nat Rev Cardiol. 2015;12(2):80–90. [DOI] [PubMed] [Google Scholar]
- 81.Lin Z, Murtaza I, Wang K, Jiao J, Gao J, Li PF. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci USA. 2009;106(29):12103–12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang K, Long B, Zhou J, Li PF. miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy. J Biol Chem. 2010;285(16):11903–11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17(5):662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Azibani F, Devaux Y, Coutance G, Schlossarek S, Polidano E, Fazal L, Merval R, Carrier L, Solal AC, Chatziantoniou C, Launay JM, Samuel JL, Delcayre C. Aldosterone inhibits the fetal program and increases hypertrophy in the heart of hypertensive mice. PLoS One. 2012;7(5):e38197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu HD, Xia S, Zha CQ, Deng SB, Du JL, She Q. Spironolactone regulates HCN protein expression through micro-RNA-1 in rats with myocardial infarction. J Cardiovasc Pharmacol. 2015;65(6):587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bostjancic E, Zidar N, Stajer D, Glavac D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology. 2010;115(3):163–169. [DOI] [PubMed] [Google Scholar]
- 87.Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW II, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13(5):613–618. [DOI] [PubMed] [Google Scholar]
- 88.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13(4):486–491. [DOI] [PubMed] [Google Scholar]
- 89.Dickinson BA, Semus HM, Montgomery RL, Stack C, Latimer PA, Lewton SM, Lynch JM, Hullinger TG, Seto AG, van Rooij E. Plasma microRNAs serve as biomarkers of therapeutic efficacy and disease progression in hypertension-induced heart failure. Eur J Heart Fail. 2013;15(6):650–659. [DOI] [PubMed] [Google Scholar]
- 90.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA. 2006;103(48):18255–18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100(3):416–424. [DOI] [PubMed] [Google Scholar]
- 92.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duan X, Ji B, Wang X, Liu J, Zheng Z, Long C, Tang Y, Hu S. Expression of microRNA-1 and microRNA-21 in different protocols of ischemic conditioning in an isolated rat heart model. Cardiology. 2012;122(1):36–43. [DOI] [PubMed] [Google Scholar]
- 94.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105(35):13027–13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Port JD, Walker LA, Polk J, Nunley K, Buttrick PM, Sucharov CC. Temporal expression of miRNAs and mRNAs in a mouse model of myocardial infarction. Physiol Genomics. 2011;43(19):1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davis J, Maillet M, Miano JM, Molkentin JD. Lost in transgenesis: a user’s guide for genetically manipulating the mouse in cardiac research. Circ Res. 2012;111(6):761–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pullamsetti SS, Doebele C, Fischer A, Savai R, Kojonazarov B, Dahal BK, Ghofrani HA, Weissmann N, Grimminger F, Bonauer A, Seeger W, Zeiher AM, Dimmeler S, Schermuly RT. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2012;185(4):409–419. [DOI] [PubMed] [Google Scholar]
- 98.White K, Dempsie Y, Caruso P, Wallace E, McDonald RA, Stevens H, Hatley ME, Van Rooij E, Morrell NW, MacLean MR, Baker AH. Endothelial apoptosis in pulmonary hypertension is controlled by a microRNA/programmed cell death 4/caspase-3 axis. Hypertension. 2014;64(1):185–194. [DOI] [PubMed] [Google Scholar]
- 99.Nagase M, Ayuzawa N, Kawarazaki W, Ishizawa K, Ueda K, Yoshida S, Fujita T. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: role of small GTPase Rac1. Hypertension. 2012;59(2):500–506. [DOI] [PubMed] [Google Scholar]
- 100.Ayuzawa N, Nagase M, Ueda K, Nishimoto M, Kawarazaki W, Marumo T, Aiba A, Sakurai T, Shindo T, Fujita T. Rac1-mediated activation of mineralocorticoid receptor in pressure overload-induced cardiac injury. Hypertension. 2016;67(1):99–106. [DOI] [PubMed] [Google Scholar]
- 101.Lavall D, Selzer C, Schuster P, Lenski M, Adam O, Schäfers HJ, Böhm M, Laufs U. The mineralocorticoid receptor promotes fibrotic remodeling in atrial fibrillation. J Biol Chem. 2014;289(10):6656–6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adam O, Zimmer C, Hanke N, Hartmann RW, Klemmer B, Böhm M, Laufs U. Inhibition of aldosterone synthase (CYP11B2) by torasemide prevents atrial fibrosis and atrial fibrillation in mice. J Mol Cell Cardiol. 2015;85:140–150. [DOI] [PubMed] [Google Scholar]
- 103.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Origin of aldosterone in the rat heart. Endocrinology. 2004;145(11):4796–4802. [DOI] [PubMed] [Google Scholar]
- 104.Ye P, Kenyon CJ, MacKenzie SM, Jong AS, Miller C, Gray GA, Wallace A, Ryding AS, Mullins JJ, McBride MW, Graham D, Fraser R, Connell JM, Davies E. The aldosterone synthase (CYP11B2) and 11beta-hydroxylase (CYP11B1) genes are not expressed in the rat heart. Endocrinology. 2005;146(12):5287–5293. [DOI] [PubMed] [Google Scholar]
- 105.Hong DS, Kang Y-K, Brenner AJ, Sachdev JC, Ejadi S, Borad MJ, Kim T-Y, Lim HY, Park K, Becerra C, Bader AG, Stoudemire J, Smith S, Kim S, Beg MS. MRX34, a liposomal miR-34 mimic, in patients with advanced solid tumors: final dose-escalation results from a first-in-human phase I trial of microRNA therapy. J Clin Oncol. 2016;34 (Abstract 2508). [Google Scholar]
- 106.Dangwal S, Thum T. microRNA therapeutics in cardiovascular disease models. Annu Rev Pharmacol Toxicol. 2014;54:185–203. [DOI] [PubMed] [Google Scholar]
- 107.van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med. 2014;6(7):851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]