Abstract
Many of the best-studied neural sex differences relate to differences in cell number and are due to the hormonal control of developmental cell death. However, several prominent neural sex differences persist even if cell death is eliminated. We hypothesized that these may reflect cell phenotype “decisions” that depend on epigenetic mechanisms, such as DNA methylation. To test this, we treated newborn mice with the DNA methyltransferase (DNMT) inhibitor zebularine, or vehicle, and examined two sexually dimorphic markers at weaning. As expected, control males had more cells immunoreactive for calbindin-D28k (CALB) in the medial preoptic area (mPOA) and fewer cells immunoreactive for estrogen receptor α (ERα) in the ventrolateral portion of the ventromedial nucleus of the hypothalamus (VMHvl) and the mPOA than did females. Neonatal DNMT inhibition markedly increased CALB cell number in both sexes and ERα cell density in males; as a result, the sex differences in ERα in the VMHvl and mPOA were completely eliminated in zebularine-treated animals. Zebularine treatment did not affect developmental cell death or the total density of Nissl-stained cells at weaning. Thus, a neonatal disruption of DNA methylation apparently has long-term effects on the proportion of cells expressing CALB and ERα, and some of these effects are sex specific. We also found that sex differences in CALB in the mPOA and ERα in the VMHvl persist in mice with a neuron-specific depletion of either Dnmt1 or Dnmt3b, indicating that neither DNMT alone is likely to be required for the sexually dimorphic expression of these markers.
A DNA methyltransferase inhibitor administered during the neonatal critical period causes long-term increases in the proportion of cells expressing calbindin and estrogen receptor α in the brain.
Developmental cell death is a well-established mechanism for generating sex differences in the nervous system (1, 2). For example, differential cell death in males and females has been implicated in sex differences in total cell number in the spinal nucleus of the bulbocavernosus, principal nucleus of the bed nucleus of the stria terminalis, sexually dimorphic nucleus (SDN) of the preoptic area (POA), and anteroventral periventricular nucleus (3–7). Cell death does not underlie all sex differences, however. In particular, several sex differences in neurochemical phenotype persist after the elimination of developmental cell death (5, 8–10).
For example, male mice have more cells that are immunoreactive (ir) for calbindin-D28k (CALB) in the medial preoptic area (mPOA) than do females (11, 12). The sex difference in this cell group, known as the CALB-SDN, depends on perinatal exposure to gonadal steroids and is not altered by adult hormone manipulations (10, 13). In mice lacking the prodeath gene Bax, virtually all cell death is eliminated throughout the perinatal forebrain (7), but the number of CALB-SDN cells, and the magnitude of the sex difference in CALB-SDN cell number, is unchanged (10). Similarly, sex differences in the number of vasopressin-expressing neurons in the bed nucleus of the stria terminalis and kisspeptin- or tyrosine hydroxylase–expressing neurons in the anteroventral periventricular nucleus depend on perinatal exposure to testosterone and persist after the elimination of developmental cell death (5, 8, 9). This suggests that perinatal gonadal steroids cause sex differences in the number of CALB-, vasopressin-, tyrosine hydroxylase–, or kisspeptin-expressing cells by directing the differentiation of phenotype (i.e., the long-term “decision” of a cell to express a specific marker), rather than by controlling cell survival.
Two sex differences in estrogen receptor α (ERα) cell density may also reflect such phenotypic decisions. Female rats have more ERα protein and messenger RNA (mRNA), as well as greater estrogen binding, than do males in the ventrolateral portion of the ventromedial nucleus of the hypothalamus (VMHvl) and mPOA (14–16). These differences are seen throughout life, can be reversed by perinatal hormone treatments (17–19), and persist after adult gonadectomy (20). Thus, although hormones circulating in adulthood modulate ERα mRNA and protein expression (20–22), perinatal gonadal steroids may program the number of cells with the potential to express ERα. The programming effects of gonadal steroids on ERα cell density in mice has been less well studied, although we, and others, find sex differences in ERα expression in several brain regions prior to puberty that presumably are independent of differences in circulating steroids (22, 23).
Epigenetic modifications of chromatin are critically involved in determining cell type–specific gene expression throughout the developing organism (24, 25) and may also play a role in steroid hormone–dependent specification of neural cell phenotype. The methylation of cytosine residues of DNA is one of the most stable epigenetic marks. Increased DNA methylation is usually associated with the suppression of gene transcription, although there are exceptions, and is controlled by a family of enzymes known as DNA methyltransferases [(DNMTs) 26]. Sex differences, and effects of perinatal steroids, on DNA methylation patterns in regions of the ERα gene (Esr1) promoter have previously been reported in the rat POA (27, 28). The direction of the sex differences went in opposite directions in the two reports, however. As in all studies of this kind, the evidence is necessarily correlative, and determining whether any given difference in DNA methylation causes a sex difference in gene expression requires manipulating only those specific methyl sites, an undertaking that is currently technically prohibitive.
Here we have taken a different approach by examining the effects of neonatal inhibition of DNA methylation on the expression of CALB and ERα. We reasoned that if sex differences in CALB or ERα cell number depend on differential DNA methylation in males and females, then those sex differences might be disrupted by DNMT inhibition during the neonatal critical period for sexual differentiation. We also examined the neuron-specific requirement for two DNMTs, DNMT1 and DNMT3b, in the development of neural sex differences in CALB and ERα.
Materials and Methods
Animals
All procedures were in accordance with National Institutes of Health animal welfare guidelines and approved by the Georgia State University Institutional Animal Care and Use Committee. Wild-type C57BL/6J mice purchased from The Jackson Laboratory [Bar Harbor, ME; Research Resource Identifier (RRID): IMSR_JAX:000664] were housed in a 12:12 light:dark cycle (lights on at 0700 hours) at 22°C with food and water available ad libitum. Cages of breeding pairs were checked daily for births.
DNMT inhibitor treatment
Intracerebroventricular injections of the DNMT inhibitor zebularine were administered to cryoanesthetized wild-type C57BL/6J pups of both sexes. The dose and mode of administration were based on that used in Nugent et al. (29). Each hemisphere was injected on the day of birth [postnatal day (P)0] and on P1. Bright light illumination allowed for visualization of bregma through the skin of the scalp (i.e., no incision was required). Using a Micro4 microsyringe pump (World Precision Instruments, Sarasota, FL), a Hamilton syringe was lowered 2 mm beneath the skull at approximately 1 mm rostral and 1 mm lateral to bregma, and 0.5 µL of either zebularine (Calbiochem, San Diego, CA; 600 ng in 10% dimethyl sulfoxide/90% saline) or the same volume of vehicle alone was administered.
Brain collection
Although prepubertal female rats have more ERα cells than do males in the VMHvl and the mPOA (14, 15, 18, 27), this has not to our knowledge been examined in mice. Therefore, we first examined ERα cell density in the VMHvl and mPOA of untreated mice on P26. To test for long-term effects of neonatal zebularine treatment, animals were euthanized as juveniles on P25 to P27 and examined for CALB and ERα protein expression. This age was chosen because it allowed us to examine relatively long-lasting effects of neonatal inhibition of DNA methylation in the absence of effects of postpubertal gonadal hormones. Mice were euthanized with CO2 and decapitated, and their brains were removed and fixed overnight in 5% acrolein (Alfa Aesar, Ward Hill, MA) in 0.1 M phosphate buffer (PB). The tissue was then transferred to 30% sucrose in 0.1M PB and stored at 4°C. Three series of 30-µm coronal sections were collected, placed in cryoprotectant (30% sucrose, 1% polyvinylpyrrolidone, 30% ethylene glycol in 0.1 M PB), and stored at –20°C until use.
A second cohort of pups was injected with zebularine or vehicle, as described previously, and euthanized 6 hours after the last injection on P1 to determine whether zebularine treatment altered developmental cell death or affected ERα expression acutely. Brains were removed and fixed as described previously, and four series of 40-µm sections were collected and stored at –20°C until use.
Immunohistochemistry
One series of sections from all juvenile animals were immunohistochemically stained for CALB, and a second for ERα. Free-floating sections were extensively rinsed in 1× tris(hydroxymethyl)aminomethane-buffered saline [(TBS) pH 7.6] and immersed in 0.1M glycine in 1× TBS for 30 minutes. Sections were then rinsed, incubated in a concentrated blocking solution (1× TBS, 10% normal goat serum, 1% hydrogen peroxide, and 0.4% Triton-X), and incubated overnight in a primary antibody solution [1× TBS, 0.4% Triton-X, and 2% normal goat serum (TTG)] against either CALB (Anti-Calbindin-D28k; Sigma, St. Louis, MO; RRID: AB_476894; 1:20,000) or ERα (Anti-Estrogen Receptor α; EMD Millipore, Billerica, MA; RRID: AB_310305; 1:20,000). The next day, sections were rinsed in TTG, incubated in goat anti-mouse or goat anti-rabbit secondary antibody solution (1:500 and 1:250 in TTG, respectively; Vector Laboratories, Burlingame, CA), rinsed in 1× TBS with 0.2% Triton-X, and then incubated for 1 hour in an avidin-biotin complex (ABC) solution (Vectastain Elite ABC Kit, Vector Laboratories). Sections were incubated in diaminobenzidine-nickel solution (Vector Laboratories) until the reaction plateaued, followed by rinses in 1× TBS. Sections were then mounted onto microscope slides and coverslipped.
To determine whether zebularine altered developmental cell death, alternate sections from animals euthanized on P1 were immunohistochemically stained for activated caspase-3 (AC3), as previously described (30). Briefly, sections were rinsed and prepared for an overnight incubation in primary antibody solution (Cleaved Caspase-3; Cell Signaling, Danvers, MA; RRID: AB_2341188; 1:20,000 in 1× TBS, 2% normal goat serum, 0.3% Triton-X). The next day, sections were washed in a dilute blocking solution (1× TBS, 1% normal goat serum, 0.02% Triton-X) and incubated in secondary goat anti-rabbit antibody solution (Vector Laboratories; 1:250 in 1× TBS, 2% normal goat serum, 0.3% Triton-X). They were then rinsed, incubated in ABC solution (Vectastain Elite ABC Kit), and immersed for 2 to 5 minutes in diaminobenzidine-nickel. Sections were mounted onto slides and counterstained with thionin.
The remaining brain sections of neonates were used to determine whether sex differences and effects of zebularine on ERα cell density were present on P1, or emerged later. These sections were processed for detection of ERα as described previously. A similar analysis could not be performed for the CALB-SDN because this cell group is not discernable until the second postnatal week (31; our own unpublished observations in mice).
Analysis of sexually dimorphic markers
To analyze the effects of zebularine on CALB-ir cell number, sections through the mPOA were analyzed with the aid of StereoInvestigator software (MicroBrightfield, Williston, VT) using the method of Gilmore et al. (10). Briefly, an ellipsoidal contour (300-μm major axis, 178-μm minor axis) was superimposed on the CALB-SDN, and all CALB-ir cells within the contour were counted. Counts were performed on the left and right sides of the relevant sections, and the two highest counts were summed for each animal. In the majority of cases, the maximal counts came from the left and right sides of a single section. In the remaining cases (presumably due to asymmetrical sectioning), the two maximal counts came from adjacent sections.
Next, we moved the contour just lateral to the CALB-SDN and counted all CALB-ir cells in what we refer to here as the lateral mPOA. Finally, the contour was moved further laterally in the same sections, and we counted the number of CALB-ir cells in the piriform cortex as a control region. The third ventricle and optic chiasm were used as landmarks to achieve consistency in contour placement across animals, as in Gilmore et al. (10). All counts in this and subsequent analyses were performed by an observer blind to treatment group, and the number of animals per group is indicated in the figure legends.
For quantification of ERα immunoreactivity, we first performed direct manual counts of all ERα-ir cells in the VMHvl of weanling animals. Briefly, a contour was drawn around the VMHvl bilaterally using video microscopy and StereoInvestigator software (MicroBrightfield). Volume of the area was determined, and all ERα-ir cells were counted. We then compared these counts to those obtained with a less time-intensive method using the particle counting function of ImageJ (Version 1.47; National Institutes of Health, Bethesda, MD; RRID: SCR_003070). Images of the VMHvl were captured, and a threshold value, based on background staining of an adjacent brain area with no ERα-ir cells, was determined for each animal. A contour was drawn around the region of interest, and the particle counting function recorded the number of cells above threshold within the contour. This number was divided by the volume sampled to calculate density of ERα-ir cells (expressed as cells per mm3). This semiautomated method gave very similar results to our direct cell counts and was therefore used for the ERα data presented below for weanling animals.
On P1, ERα-ir–positive cells were packed too closely together for the particle-counting function of ImageJ to accurately delineate separate cells. We therefore report ERα staining density on P1 as the number of pixels above threshold per mm3.
Analysis of cell death
Neuronal cell death is at its peak in many mouse forebrain regions, including the ventromedial nucleus of the hypothalamus (VMH) and mPOA, during the first 72 hours of life (7, 30). To determine whether zebularine treatment influenced naturally occurring developmental cell death, brains from vehicle- and zebularine-treated pups were collected 6 hours after the last injection on P1 and immunoreacted for AC3 as previously (7, 30). In the relevant brain sections, we then made bilateral counts of the number of AC3-ir cells in the VMHvl and mPOA. Cell death density was obtained by dividing the number of AC3-ir cells by the volume sampled and is expressed as AC3 cells per mm3.
Total cell density
As described below, neonatal zebularine treatment increased the density of CALB and ERα cells. To determine whether total cell density was affected, one series of brain sections from animals euthanized on P25 to P27 and neonatally treated with vehicle or zebularine were thionin stained, and stereological cell counts were made of the mPOA. This region was chosen because it housed both the sexually dimorphic CALB cell group and one of the two ERα cell groups analyzed here. We used StereoInvestigator software (MicroBrightfield) running the optical fractionator probe and used a counting frame of 25 × 25 μm2 and sampling grid of 90 × 90 μm2. Only cells with a neuronal morphology (large, multipolar) were included in counts, and counts were divided by volume measured to determine cells per mm3.
Dnmt1- and Dnmt3b-deficient mice
Three DNMTs are expressed in the brain and are presumably responsible for all DNA methylation events: the maintenance methyltransferase DNMT1 and the de novo methyltransferases DNMT3a and DNMT3b. Neuronal Dnmt1- or Dnmt3b-deficient mice were generated in-house by mating C57BL/6J Dnmt1fl/fl or Dnmt3bfl/fl mice (32) with mice expressing Synapsin1Cre+/– (33). SynCre+/– mice express Cre recombinase enzyme in brain, spinal cord, and dorsal root ganglion neurons beginning at embryonic day 12.5 (34). Male and female pups were either Dnmt1fl/flSynCre+/– (neuronal Dnmt1 knockout), Dnmt3bfl/flSynCre+/– (neuronal Dnmt3b knockout), or Dnmt1fl/flSynCre–/– or Dnmt3bfl/flSynCre–/– (controls; no knockout), with genotypes verified from tail snips. We also attempted to generate a cohort of Dnmt3a neuronal knockouts using the same breeding strategy, but Dnmt3a homozygous knockouts were born at a frequency much lower than expected and did not thrive. Brains from Dnmt1- and Dnmt3b-deficient mice were collected on P25, stained for CALB and ERα, and analyzed as described previously.
Statistical analysis
Two-way analysis of variance (sex by treatment) was used to assess effects of sex, zebularine treatment, and their interaction on CALB-ir cell number, ERα cell density, and total cell density at weaning, as well as AC3 and ERα cell density on P1. Planned comparisons were performed using Fisher’s least significant difference. ERα cell density in untreated males and females was compared using independent two-tailed t tests.
Results
Neonatal zebularine treatment increased CALB-ir cell number in the mPOA but not in the piriform cortex at weaning
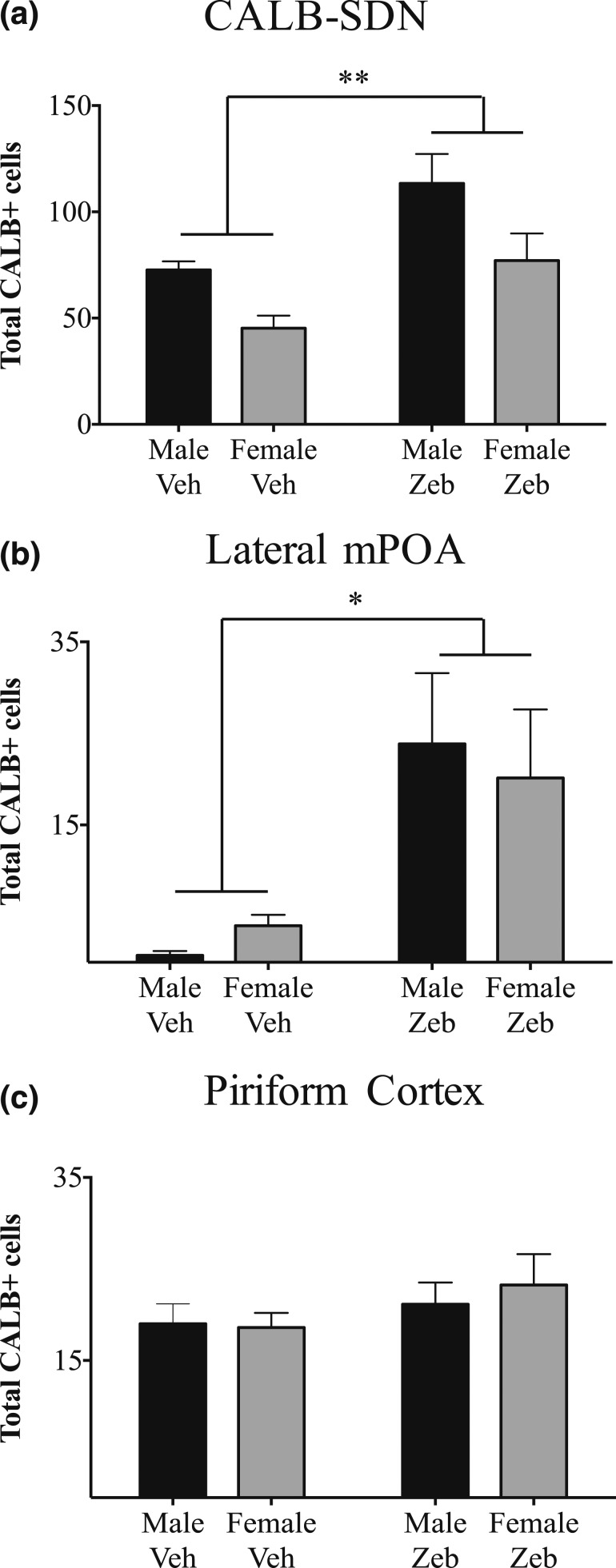
As expected, males had more CALB-ir cells than females in the CALB-SDN (main effect of sex: F1,20 = 6.67; P < 0.02; Figs. 1 and 2). Neonatal zebularine treatment caused a 58% increase in the number of CALB-SDN cells 3.5 weeks later (effect of treatment: F1,20 = 8.04; P = 0.01), with no sex-by-treatment interaction [F1,20 = 0.27; P > 0.6; Fig. 2(a)]. As a result, zebularine-treated females had as many CALB-SDN cells as control males, although a trend for a sex difference remained among zebularine-treated animals (P = 0.075).
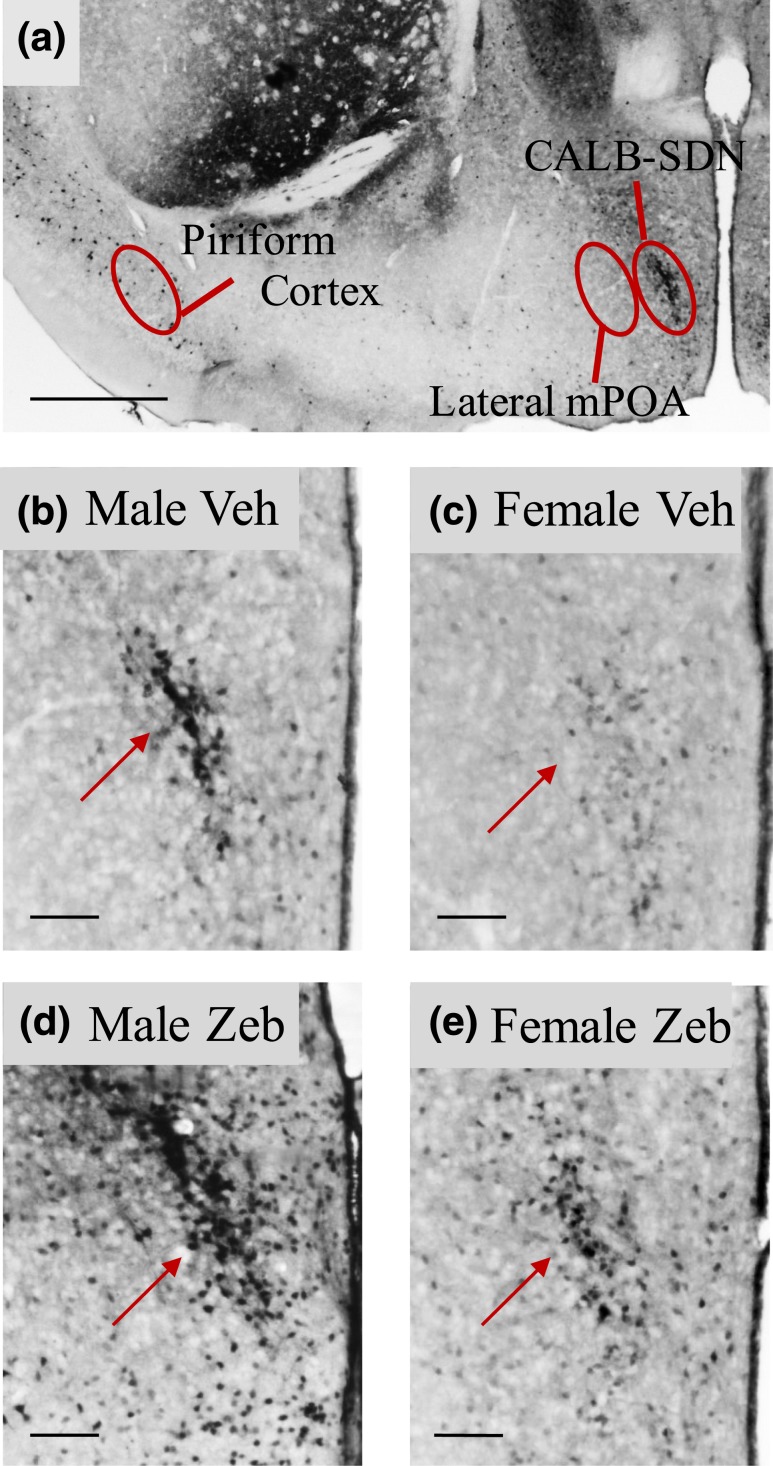
Figure 1.
Neonatal zebularine treatment increased CALB cell number in the mPOA at weaning. (a) Low-power photomicrograph of CALB-ir in the CALB-SDN, lateral mPOA, and piriform cortex of a male on P26. Contours indicate approximate areas quantified. (b–e) Higher-magnification photomicrographs of the CALB-SDN (arrows) in a vehicle-treated male (Male Veh), vehicle-treated female (Female Veh), zebularine-treated male (Male Zeb), and zebularine-treated female (Female Zeb). Scale bars = 200 µm in (a) and 50 µm in (b–e).
Figure 2.
Quantification of CALB cell number at weaning in animals treated neonatally with zebularine. (a) As expected, vehicle-treated males had more immunoreactive cells than did females in the CALB-SDN. Neonatal zebularine treatment increased CALB-SDN cell number in both sexes, with no significant sex-by-treatment interaction. (b) CALB-ir cells in the lateral mPOA did not differ between sexes, but neonatal zebularine treatment significantly increased calbindin expression. (c) CALB-ir in the piriform cortex did not differ between the sexes and was not affected by neonatal zebularine treatment. The number of animals per group was n = 4 vehicle males (Male Veh), 5 vehicle females (Female Veh), 7 zebularine males (Male Zeb), and 8 zebularine females (Female Zeb). *P < 0.05, **P < 0.01.
Visual inspection of the mPOA suggested that zebularine-treated animals also had more CALB-ir cells in regions immediately surrounding the CALB-SDN (Fig. 1). To test this, we counted CALB-ir cells within a contour placed just lateral to the CALB-SDN, but still within the mPOA (referred to here as the lateral mPOA). The number of CALB-ir cells was much lower in this region than in the CALB-SDN and did not differ by sex [Fig. 2(b); F1,20 = 0.08; P > 0.7]. However, neonatal zebularine treatment caused a fivefold increase in CALB-ir cells in the lateral mPOA (F1,20 = 5.46; P < 0.05), with no sex-by-treatment interaction (F1,20 = 0.01; P > 0.9).
There is also a prominent population of large CALB-ir neurons in the piriform cortex at the same rostro-caudal level of the brain as the CALB-SDN [Fig. 1(A)]. We found no effect of sex (F1,20 = 0.08; P > 0.7) or treatment (F1,20 = 1.31; P > 0.25) and no sex-by-treatment interaction (F1,20 = 0.18; P > 0.6) on CALB-ir cell number in the piriform cortex [Fig. 2(c)], indicating regionally specific effects of zebularine treatment. In summary, neonatal treatment with a DNMT inhibitor led to a long-term increase in CALB-SDN cell number and caused ectopic expression of CALB in cells immediately surrounding the CALB-SDN but had no effect on a major calbindin-expressing cell group in the piriform cortex within the same brain sections.
Neonatal zebularine treatment sex-specifically increased ERα cell density and eliminated sex differences in ERα in the VMHvl and mPOA
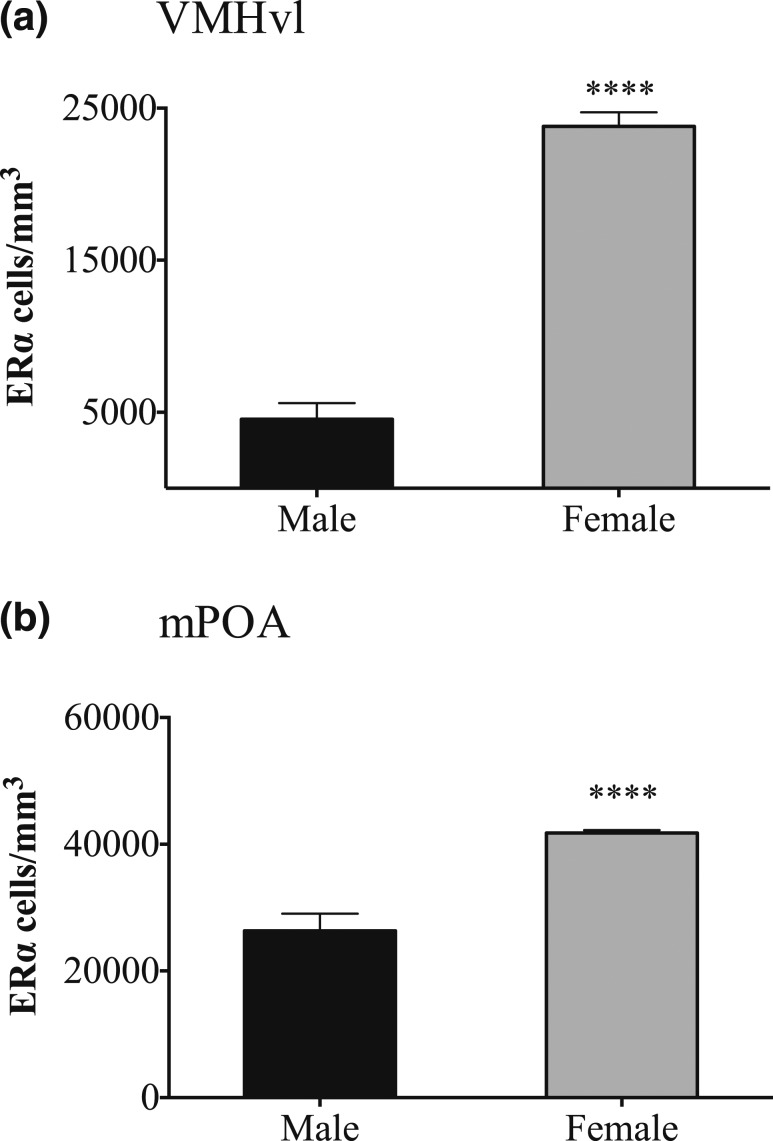
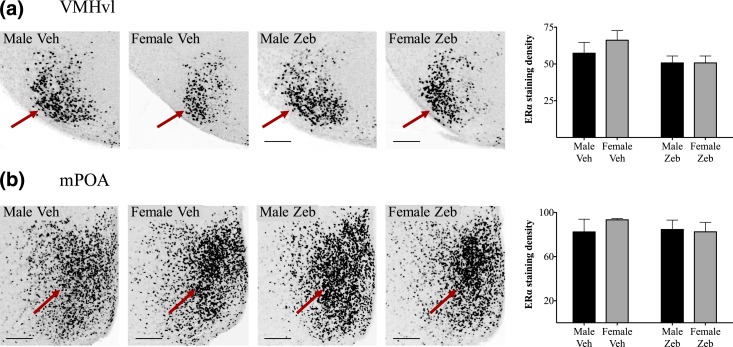
We first examined untreated mice at weaning to determine whether the sex differences in ERα cell density in the VMHvl and mPOA previously reported in prepubertal rats were are also present in mice. Indeed, sex differences were robust: Untreated females had more ERα-ir cells in both regions on P26 than did males, with no overlap between the sexes (Fig. 3; P < 0.0001 for both the VMHvl and mPOA). Next we determined the effects of neonatal treatment with zebularine. Although sections from fewer animals were available for analyses of ERα (n = 15) than for CALB (n = 21), highly significant effects of sex and zebularine treatment were found in both cell groups examined.
Figure 3.
ERα-ir cell density in the (a) VMHvl and the (b) mPOA is significantly higher in untreated females than in males on P26. n = 8 males and 8 females for the VMHvl; n = 9 males and 10 females for the mPOA. ****P < 0.0001.
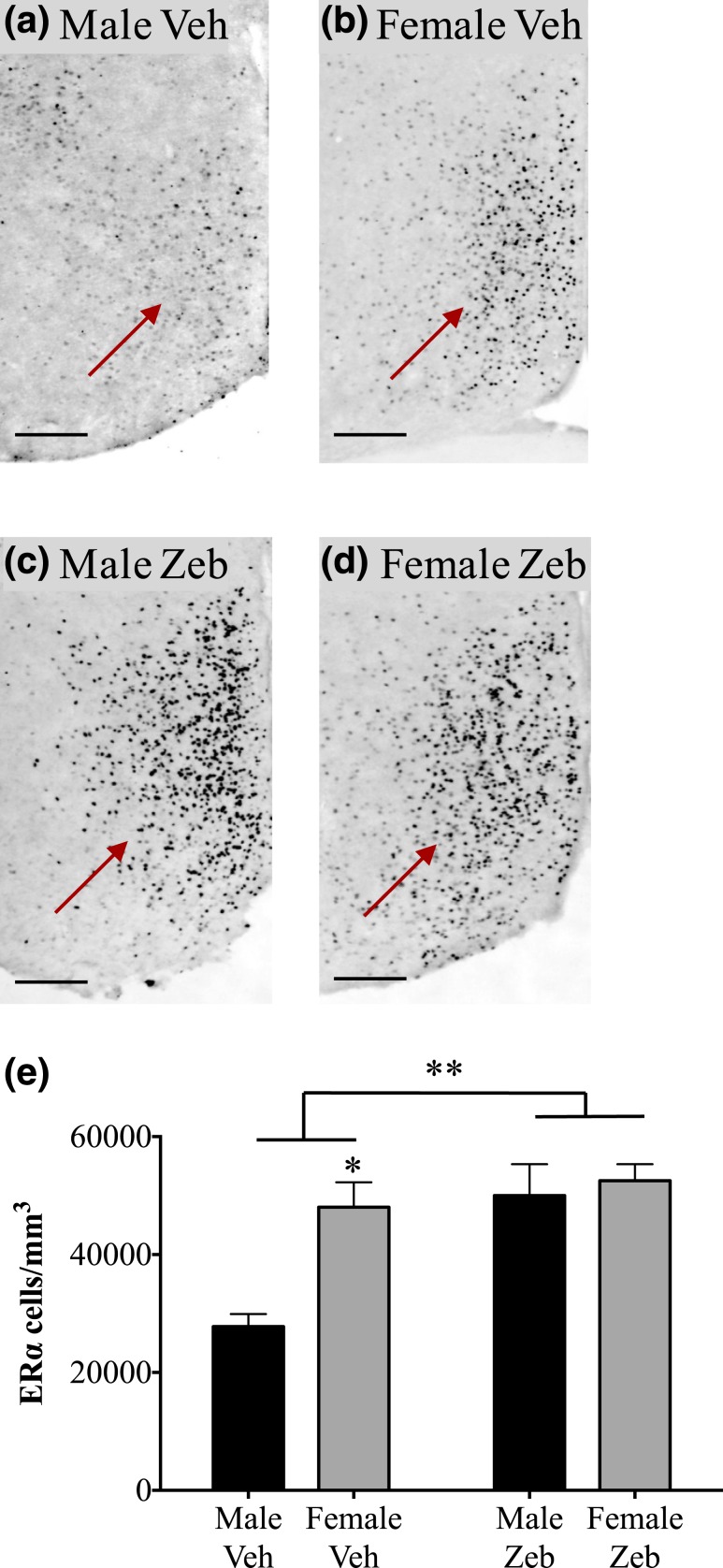
In the VMHvl, we found a main effect of sex (F1,14 = 23.09; P < 0.0005), a main effect of neonatal zebularine treatment (F1,14 = 41.64; P < 0.0001), and a sex-by-treatment interaction (F1,14 = 18.70; P < 0.001) on ERα-ir cell density (Fig. 4). Vehicle-treated females had about 7 times more ERα-ir cells than males (P < 0.001). Neonatal zebularine treatment increased ERα cell density eightfold in males (P < 0.0001) but had no effect in females. As a result, the sex difference in ERα-ir cell density in the VMHvl was eliminated by neonatal treatment with the DNMT inhibitor.
Figure 4.
Neonatal treatment with zebularine eliminates the sex difference in ERα-ir in the VMHvl. (a–d) Photomicrographs of the VMHvl (arrows) in a (a) vehicle-treated male (Male Veh), (b) vehicle-treated female (Female Veh), (c) zebularine-treated male (Male Zeb), and (d) zebularine-treated female (Female Zeb). (e) There was a main effect of neonatal zebularine treatment and a sex-by-treatment interaction on ERα cells/mm3. ERα cell density was significantly greater in vehicle-treated females than in vehicle males. Neonatal zebularine increased ERα expression only in males and eliminated the sex difference. The number of animals per group was: n = 3 vehicle males, 5 vehicle females, 5 zebularine males, and 7 zebularine females. One vehicle-treated male had almost no ERα staining in the VMH or mPOA, and the data from this animal is omitted (inclusion increases the magnitude of the sex differences and zebularine effects in both brain regions but does not change the pattern of statistical results). Scale bars = 100 µm in (a–d). ***P < 0.001, ****P < 0.0001.
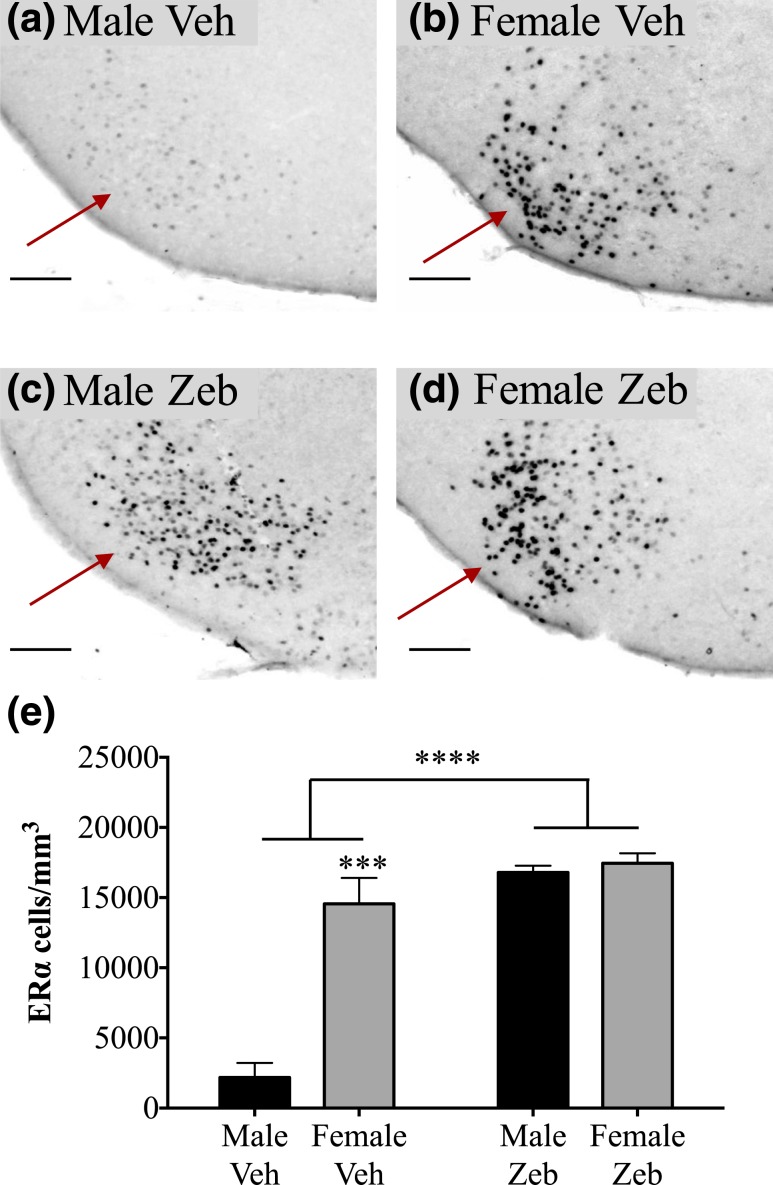
In the mPOA, vehicle-treated females had almost twice the density of ERα-ir cells, as did control males (Fig. 5; P < 0.03), and there was also a main effect of sex in the overall analysis of variance (F1,13 = 5.90; P < 0.04). Neonatal zebularine treatment increased ERα-ir cell density approximately 30% (F1,13 = 8.16; P < 0.02). There was a trend for a sex-by-treatment interaction, due to a larger effect in males than in females, but this did not reach significance (F1,13 = 3.58; P = 0.08). Nonetheless, ERα-ir cell number in zebularine-treated males was equivalent to that in control and zebularine-treated females (P > 0.6 in both cases; Fig. 5).
Figure 5.
Neonatal treatment with zebularine eliminates the sex difference in ERα-ir in the mPOA. (a–d) Photomicrographs of the mPOA (arrows) in a (a) vehicle-treated male (Male Veh), (b) vehicle-treated female (Female Veh), (c) zebularine-treated male (Male Zeb), and (d) zebularine-treated female (Female Zeb). (e) There was a main effect of neonatal zebularine treatment and a main effect of sex on ERα cells/mm3. Zebularine increased ERα expression, but only in males, and the sex difference was eliminated in zebularine-treated animals. n = 3 vehicle males, 4 vehicle females, 5 zebularine males, and 6 zebularine females. Scale bars = 100 µm in (a–d). *P < 0.05, **P < 0.01.
Sex differences and effects of neonatal DNMT inhibition on ERα emerge after P1
It was not known whether zebularine acutely affected ERα labeling, or whether effects emerged over time. To test this, we examined ERα cell density on P1, 30 hours after the first zebularine injection and 6 hours after the last zebularine injection. We found strong labeling for ERα in the VMHvl and mPOA of both male and female mice, but no sex differences in ERα cell density at this age (F1,19 = 0.5539; P > 0.4 and F1,13 = 0.1853; P > 0.6 in the VMHvl and mPOA, respectively; Fig. 6). We also found no acute effect of zebularine treatment on ERα labeling in either the VMHvl (F1,19 = 3.447; P > 0.07) or mPOA (F1,13 = 0.1778; P > 0.6; Fig. 6). Thus, the sex differences and effects of zebularine on ERα both emerged after P1. Similarly, sex differences in ERα-ir and mRNA in the mPOA and VMHvl of rats are absent at birth and emerge several days later (15, 16).
Figure 6.
There was robust ERα immunoreactivity in the (a) VMHvl and (b) mPOA of both sexes on P1. Scale bars = 50 µm. Quantification indicated no sex difference and no effect of zebularine treatment on ERα cell density (×107 pixels/mm3) in either the VMHvl or mPOA. The number of animals per group were: VMHvl, n = 6 vehicle males (Male Veh), 5 vehicle females (Female Veh), 6 zebularine males (Male Zeb), and 4 zebularine females (Female Zeb); mPOA, n = 5 vehicle males, 3 vehicle females, 6 zebularine males, and 3 zebularine females.
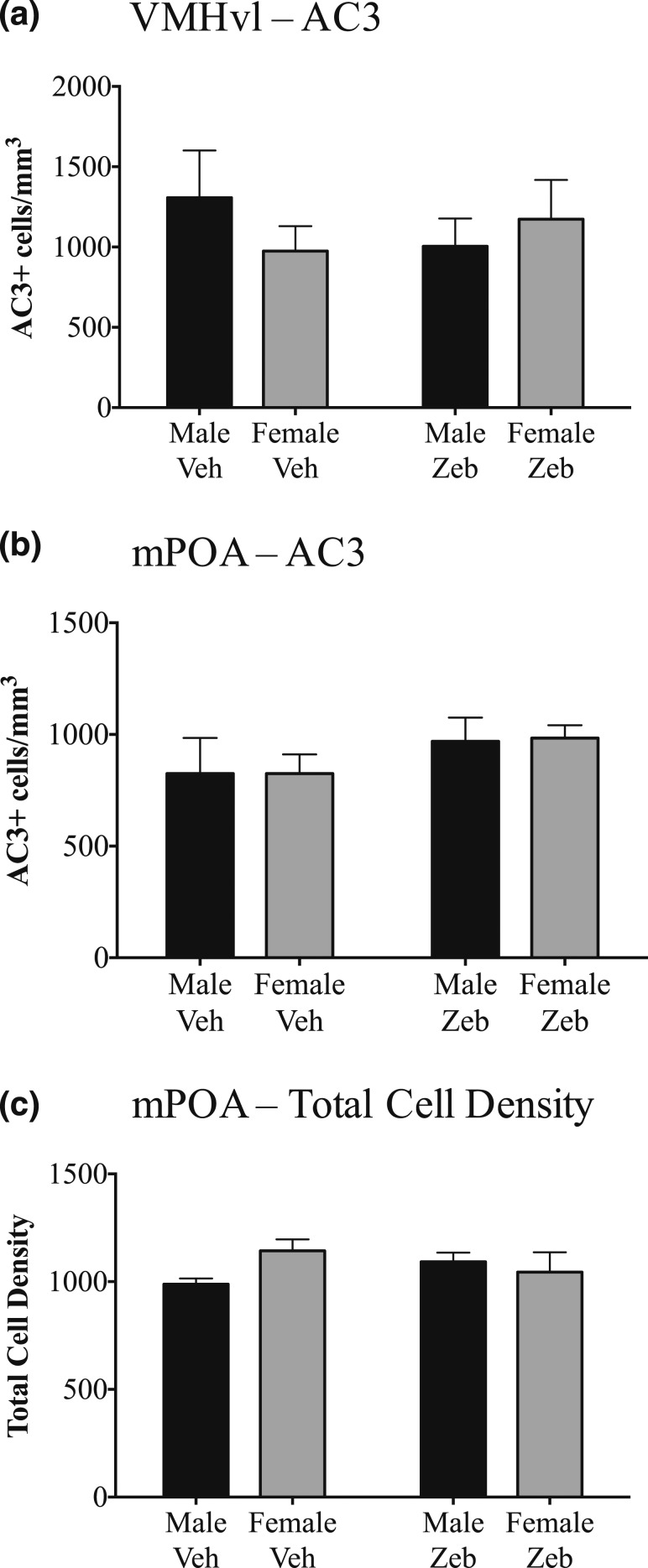
Neonatal zebularine treatment does not alter developmental cell death or total cell density
Neonatal zebularine treatment could have increased CALB and ERα cell number at weaning by influencing cell phenotype or by decreasing developmental cell death. To test the latter possibility, we treated a separate cohort of mice with zebularine or vehicle on P0 and P1, as before, and examined the VMHvl and mPOA for the cell death marker AC3 6 hours after the last injection. We found no sex difference in cell death in the VMH (F1,16 = 0.4976; P > 0.49) or mPOA (F1,17 = 0.003175; P > 0.95) on P1. There also was no effect of zebularine treatment [VMHvl, F1,16 = 0.699; P > 0.69; mPOA, F1,17 = 1.40; P > 0.25; Fig. 7(a) and 7(b)] and no sex-by-treatment interaction on cell death in either region. We also performed stereological cell counts in thionin-stained material to determine whether neonatal zebularine treatment influenced total cell density in the mPOA at weaning. We found no effect of sex (F1,13 = 0.702; P > 0.4) and no effect of zebularine treatment [F1,13 = 0.001; P > 0.9; Fig. 7(c)] on total cell density. Thus, neonatal zebularine treatment apparently increased the number of cells expressing CALB and ERα, without decreasing cell death or increasing total number of cells in the mPOA. Presumably, cells that normally would not express CALB or ERα now do so after neonatal DNMT inhibition.
Figure 7.
(a, b) Zebularine treatment on P0 and P1 did not alter cell death in the VMHvl or the mPOA, as measured by the density of AC3+ cells 6 hours after the last injection on P1. The number of animals per group was: VMHvl, n = 11 vehicle (6 female) and 9 zebularine (4 female); mPOA, n = 13 vehicle (7 female) and 9 zebularine (3 female). (c) Total cell density in the mPOA on P26 also was not altered by neonatal zebularine treatment. n = 10 vehicle (7 females) and 8 zebularine (4 females). Female Veh, vehicle-treated female; Female Zeb, zebularine-treated female; Male Veh, vehicle-treated male; Male Zeb, zebularine-treated male.
Sex differences in CALB and ERα persist in neuron-specific Dnmt1 or Dnmt3b knockout mice
To determine whether sex differences in CALB or ERα cell number could be linked to a single DNMT, we euthanized mice with a neuron-specific depletion of DNMT1 or DNMT3b on P25 and processed their brains for the detection of CALB and ERα as described previously. We confirm that males had more CALB-SDN cells than females (main effect of sex; P < 0.0001), whereas ERα cell density in the VMHvl was higher in females than in males (P < 0.005) among the control mice (Dnmt1fl/flSynCre–/– and Dnmt3bfl/flSynCre–/–; Supplemental Fig. 1 (104.4KB, pdf) ). Females also had more ERα in the mPOA of Dnmt1 and Dnmt3b (P < 0.01 and P < 0.02, respectively, for main effects of sex), although the magnitude of the sex differences was smaller than that seen previously in C57Bl/6 mice. Importantly, there was no effect of neuron-specific knockout of Dnmt1 or Dnmt3b and no sex-by-genotype interaction on either the CALB-SDN or ERα in the mPOA (Supplemental Fig. 1 (104.4KB, pdf) ). In the VMHvl, ERα cell density was slightly lower in Dnmt1 knockouts than in controls (P < 0.003), but again, there was no sex-by-genotype interaction, and a sex difference in ERα persisted in the knockouts (Supplemental Fig. 1 (104.4KB, pdf) ).
Discussion
Neonatal treatment with a DNMT inhibitor increased the number of CALB-SDN cells and the density of ERα cells in the VMHvl and mPOA. This suggests that DNA methylation normally suppresses the number of cells immunoreactive for both CALB and ERα. Developmental cell death as well as total cell density at weaning were unchanged by neonatal zebularine treatment. Therefore, zebularine apparently increased the proportion of cells in the mPOA and VMH positive for CALB and ERα at weaning by causing cells that would not normally express CALB or ERα to do so. This effect of neonatal zebularine treatment on cell phenotype was at least somewhat regionally specific, because no increase in CALB-ir cells was seen in the piriform cortex.
The effects of zebularine on CALB and ERα cell number were in the expected direction for a DNMT inhibitor (i.e., reduced DNA methylation correlates with increased gene expression), and it is possible that neonatal zebularine treatment directly affected methylation of the CALB and/or ERα gene promoters. There are several previous reports of differences in DNA methylation in Esr1 depending on age, sex, or hormone exposure (27, 28, 35, 36), although decreased DNA methylation did not always correspond to increased expression in these studies. It is also possible, however, that zebularine acted indirectly, via methylation changes to upstream genes that control the expression of the CALB gene (Calb1) and Esr1. This could be resolved by directly examining the DNA methylation state of Calb1 and Esr1 following neonatal zebularine treatment. Even if differences are found, however, several technical hurdles would stand in the way of demonstrating a causal link, which would require manipulating a particular DNA methylation site(s) in the gene and specific cell type of interest in vivo.
Neonatal zebularine treatment increased ERα cell density only in males and completely eliminated the normal sex differences in ERα in the VMHvl and mPOA. This indicates that differential DNA methylation underlies the sex differences in ERα cell density. Because zebularine treatment did not further increase ERα cell density in females, the number of cells expressing ERα in females may be at a maximum, at least in terms of regulation by DNA methylation. In contrast, CALB-SDN cell number was increased in both males and females by neonatal zebularine treatment. One interpretation of this outcome is that some mechanism other than DNA methylation is responsible for the sex difference. Alternatively, DNA methylation differences may underlie the sex difference in the CALB-SDN if, for example, promoter regions of Calb1 (or of genes that upregulate Calb1) are methylated in both sexes, but hypermethylated in females. The current study does not allow us to discriminate between these possibilities.
Zebularine treatment overlapped with the critical period for testosterone action in the current study. Previous reports indicate that genes undergoing active regulation at the time of treatment may be particularly susceptible to drugs inhibiting specific epigenetic processes (37, 38). Thus, it is possible that testosterone normally orchestrates sex differences in DNA methylation in the ERα gene and that DNMT inhibition disrupted this process. Similarly, a transient reduction in the DNA methyl-binding protein MeCP2 in the amygdala of neonatal male rats permanently eliminates the normal sex difference in vasopressin expression, demonstrating an enduring effect of a neonatal epigenetic perturbation (39).
Ghahramani and colleagues (40) recently reported that sex differences, and the effects of neonatal testosterone treatment, on the methylome of mice are late emerging. In both the mPOA/bed nucleus of the stria terminalis and striatum, testosterone treatment of females on P0 alters the DNA methylation of only a small number of genes on P4, but many more genes are affected several weeks later (40). Similarly, at least one effect of neonatal estradiol treatment on DNA methylation of the Esr1 gene in rats does not emerge until adulthood (28). The molecular underpinnings of these delayed effects on the epigenome are unclear but have been aptly referred to as an “epigenetic echo” of neonatal hormone exposure (41). Here, we found robust labeling for ERα in the VMHvl and mPOA on P1, but no sex difference in either cell group and no effect of zebularine treatment at that time point. Thus, both the sex differences and the effects of zebularine emerge over the next 3.5 weeks and appear to be primarily due to a downregulation of ERα cell density in males.
We examined the effects of neonatal DNMT inhibition at weaning to minimize any effects of circulating gonadal steroids, and it is not known if the effects of zebularine seen here would persist into adulthood. A study of similar design found that neonatal DNMT inhibition of female rats masculinized their sexual behavior and mPOA dendritic spine density in adulthood (29). Based on their findings, Nugent and colleagues (29) concluded that female neural development is actively inhibited by DNA methylation. Our results suggest a more nuanced view, because DNMT inhibition increased CALB-SDN cell number (a change in the masculine direction), but also increased ERα cell number in the mPOA and VMHvl (changes in the feminine direction). This suggests that whether an early disruption of DNA methylation masculinizes or feminizes depends on brain region, the end point(s) measured, and/or species examined. Nonetheless, taken together with Nugent et al. (29) and other recent findings (42), the current results demonstrate that the transient disruption of DNA methylation at birth has long-lasting effects on sex differences in gene expression, neural morphology, and behavior.
DNA methylation depends on the maintenance methyltransferase DNMT1 and the de novo methyltransferases DNMT3a and DNMT3b. We found no effect of neuron-specific deletion of Dnmt1 or Dnmt3b on CALB or ERα cell number in the mPOA or VMHvl, suggesting that, individually, these DNMTs may be dispensable for development of the sex differences in CALB and ERα cell number. Because we examined neuron-specific knockouts, it is possible that Dnmt1 or Dnmt3b expressed by nonneuronal cells is sufficient to support the development of sex differences in neuronal CALB and ERα. Other possible explanations include the compensation that can occur in life-long gene knockouts and/or redundancy among the DNMTs (43, 44). For example, DNMT3b compensates for loss of DNMT1 in the intestinal epithelium (44). Similarly, there is no effect on learning and memory in conditional mutant mice lacking either Dnmt1 or Dnmt3a alone, but knocking out both genes within forebrain excitatory neurons causes significant deficits (43). However, it is also possible that DNMT3a alone is crucial for determining CALB or ERα cell number. In our hands, synapsin-Cre neuron-specific Dnmt3a knockouts failed to thrive, so we were not able to examine effects of the gene deletion. However, regional depletion of Dnmt3a restricted to the nucleus accumbens alters the rewarding effects of cocaine (45), and Dnmt3a depletion specifically in the POA masculinizes some measures of sexual behavior in female rats (29).
Conclusion and Perspective
Almost 30 years ago, Brown and colleagues (46) wrestled with the mystery of how perinatal testosterone exposure might cause an essentially permanent downregulation of estrogen receptor expression in the male rat brain. The present results suggest an answer; namely, that changes in DNA methylation are programmed by the early hormone exposure. To the extent that such hormonal effects are long term, or even permanent, they may be considered gonadal steroid control of cell fate and may fruitfully be considered in the broader context of neural cell differentiation. The brain contains hundreds, if not thousands, of different cell types (47, 48), with “type” often defined by gene expression. The differentiation of cell type depends on extracellular signaling molecules, as well as cell-intrinsic determinants (49), and the long-term stabilization of gene expression requires epigenetic mechanisms such as DNA methylation (24, 25, 50). Even small DNA methylation changes during development can determine cell fate (51), and such processes may also contribute to sexual differentiation of the brain. In the context of the present results, perinatal testosterone may be thought of as a cell-extrinsic signal that activates ligand-dependent transcription factors (androgen and estrogen receptors), which then orchestrate changes in DNA methylation to determine the “decision” (or, at least, propensity) of a cell to express specific markers (e.g., CALB or ERα). Although terms such as “cell fate,” “cell specification,” or “differentiation of phenotype” have not been used extensively in the sexual differentiation literature, this perspective may be useful because substantial work has been done in other systems that could direct future studies of sex differences in the diversification of neuronal cell type.
Acknowledgments
Acknowledgments
This work was supported by National Institutes of Health Grant R01 MH068482 (to N.G.F.), National Institutes of Health Grant R01 DK107544 (to B.X.), and a Georgia State University Brains and Behavior Seed Grant (to N.G.F.).
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|
| Calbindin-D-28k | Mouse anti-bovine calbindin-D-28K Monoclonal antibody, unconjugated, clone CB-955 | Sigma-Aldrich, C9848 | Mouse; monoclonal | 1:20,000 | AB_476894 |
| Anti-estrogen receptor α antibody | ERα (C1355) human, mouse, and rat | Millipore, 06-935 | Rabbit; polyclonal | 1:20,000 | AB_310305 |
| Cleaved caspase-3 (Asp175) canine, rat, porcine, bovine, mouse, human, and nonhuman primate | Cleaved caspase-3 (Asp175) antibody | Cell Signaling Technology, 9661 | Rabbit; polyclonal | 1:20,000 | AB_2341188 |
Footnotes
- AC3
- activated caspase-3
- CALB
- calbindin-D28k
- DNMT
- DNA methyltransferase
- ERα
- estrogen receptor α
- ir
- immunoreactive
- mPOA
- medial preoptic area
- mRNA
- messenger RNA
- P
- postnatal day
- PB
- phosphate buffer
- POA
- preoptic area
- RRID
- Research Resource Identifier
- SDN
- sexually dimorphic nucleus
- TBS
- tris(hydroxymethyl)aminomethane-buffered saline
- TTG
- 1× tris(hydroxymethyl)aminomethane-buffered saline, 0.4% Triton-X, and 2% normal goat serum
- VMH
- ventromedial nucleus of the hypothalamus
- VMHvl
- ventrolateral portion of the ventromedial nucleus of the hypothalamus.
References
- 1.Forger NG. The organizational hypothesis and final common pathways: sexual differentiation of the spinal cord and peripheral nervous system. Horm Behav. 2009;55(5):605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138(3):929–938. [DOI] [PubMed] [Google Scholar]
- 3.Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229(4714):671–673. [DOI] [PubMed] [Google Scholar]
- 4.Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734(1-2):10–18. [PubMed] [Google Scholar]
- 5.Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci USA. 2004;101(37):13666–13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob DA, Bengston CL, Forger NG. Effects of Bax gene deletion on muscle and motoneuron degeneration in a sexually dimorphic neuromuscular system. J Neurosci. 2005;25(23):5638–5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahern TH, Krug S, Carr AV, Murray EK, Fitzpatrick E, Bengston L, McCutcheon J, De Vries GJ, Forger NG. Cell death atlas of the postnatal mouse ventral forebrain and hypothalamus: effects of age and sex. J Comp Neurol. 2013;521(11):2551–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vries GJ, Jardon M, Reza M, Rosen GJ, Immerman E, Forger NG. Sexual differentiation of vasopressin innervation of the brain: cell death versus phenotypic differentiation. Endocrinology. 2008;149(9):4632–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151(12):5807–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilmore RF, Varnum MM, Forger NG. Effects of blocking developmental cell death on sexually dimorphic calbindin cell groups in the preoptic area and bed nucleus of the stria terminalis. Biol Sex Differ. 2012;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelmann M, Wolfe C, Scordalakes EM, Rissman EF, Tobet S. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev Neurobiol. 2007;67(10):1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Büdefeld T, Grgurevic N, Tobet SA, Majdic G. Sex differences in brain developing in the presence or absence of gonads. Dev Neurobiol. 2008;68(7):981–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orikasa C, Sakuma Y. Estrogen configures sexual dimorphism in the preoptic area of C57BL/6J and ddN strains of mice. J Comp Neurol. 2010;518(17):3618–3629. [DOI] [PubMed] [Google Scholar]
- 14.Kühnemann S, Brown TJ, Hochberg RB, MacLusky NJ. Sex differences in the development of estrogen receptors in the rat brain. Horm Behav. 1994;28(4):483–491. [DOI] [PubMed] [Google Scholar]
- 15.Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-α immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389(1):81–93. [DOI] [PubMed] [Google Scholar]
- 16.Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors α and β and Kiss1 in neonatal male and female rats. J Comp Neurol. 2011;519(15):2954–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kühnemann S, Brown TJ, Hochberg RB, MacLusky NJ. Sexual differentiation of estrogen receptor concentrations in the rat brain: effects of neonatal testosterone exposure. Brain Res. 1995;691(1-2):229–234. [DOI] [PubMed] [Google Scholar]
- 18.DonCarlos LL, Handa RJ. Developmental profile of estrogen receptor mRNA in the preoptic area of male and female neonatal rats. Brain Res Dev Brain Res. 1994;79(2):283–289. [DOI] [PubMed] [Google Scholar]
- 19.DonCarlos LL, Malik K, Morrell JI. Region-specific effects of ovarian hormones on estrogen receptor immunoreactivity. Neuroreport. 1995;6(15):2054–2058. [DOI] [PubMed] [Google Scholar]
- 20.Lauber AH, Mobbs CV, Muramatsu M, Pfaff DW. Estrogen receptor messenger RNA expression in rat hypothalamus as a function of genetic sex and estrogen dose. Endocrinology. 1991;129(6):3180–3186. [DOI] [PubMed] [Google Scholar]
- 21.Shughrue PJ, Bushnell CD, Dorsa DM. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology. 1992;131(1):381–388. [DOI] [PubMed] [Google Scholar]
- 22.Kelly DA, Varnum MM, Krentzel AA, Krug S, Forger NG. Differential control of sex differences in estrogen receptor α in the bed nucleus of the stria terminalis and anteroventral periventricular nucleus. Endocrinology. 2013;154(10):3836–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brock O, De Mees C, Bakker J. Hypothalamic expression of oestrogen receptor α and androgen receptor is sex-, age- and region-dependent in mice. J Neuroendocrinol. 2015;27(4):264–276. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh J, Gage FH. Epigenetic control of neural stem cell fate. Curr Opin Genet Dev. 2004;14(5):461–469. [DOI] [PubMed] [Google Scholar]
- 25.Zhang F, Pomerantz JH, Sen G, Palermo AT, Blau HM. Active tissue-specific DNA demethylation conferred by somatic cell nuclei in stable heterokaryons. Proc Natl Acad Sci USA. 2007;104(11):4395–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li E, Zhang Y. DNA methylation in mammals. Cold Spring Harb Perspect Biol. 2014;6(5):a019133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-α promoter within the developing preoptic area. Endocrinology. 2010;151(5):2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151(10):4871–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18(5):690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosley M, Shah C, Morse KA, Miloro SA, Holmes MM, Ahern TH, Forger NG. Patterns of cell death in the perinatal mouse forebrain. J Comp Neurol. 2017;525(1):47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sickel MJ, McCarthy MM. Calbindin-D28k immunoreactivity is a marker for a subdivision of the sexually dimorphic nucleus of the preoptic area of the rat: developmental profile and gonadal steroid modulation. J Neuroendocrinol. 2000;12(5):397–402. [DOI] [PubMed] [Google Scholar]
- 32.Jackson-Grusby L, Beard C, Possemato R, Tudor M, Fambrough D, Csankovszki G, Dausman J, Lee P, Wilson C, Lander E, Jaenisch R. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat Genet. 2001;27(1):31–39. [DOI] [PubMed] [Google Scholar]
- 33.Hoesche C, Sauerwald A, Veh RW, Krippl B, Kilimann MW. The 5′-flanking region of the rat synapsin I gene directs neuron-specific and developmentally regulated reporter gene expression in transgenic mice. J Biol Chem. 1993;268(35):26494–26502. [PubMed] [Google Scholar]
- 34.Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15(7):859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010;151(2):731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endocrinol. 2011;25(12):2157–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Cancer Ther. 2003;2(2):151–163. [PubMed] [Google Scholar]
- 38.Menegola E, Di Renzo F, Broccia ML, Giavini E. Inhibition of histone deacetylase as a new mechanism of teratogenesis. Birth Defects Res C Embryo Today. 2006;78(4):345–353. [DOI] [PubMed] [Google Scholar]
- 39.Forbes-Lorman RM, Rautio JJ, Kurian JR, Auger AP, Auger CJ. Neonatal MeCP2 is important for the organization of sex differences in vasopressin expression. Epigenetics. 2012;7(3):230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, Muir S, Rubbi L, Arnold AP, de Vries GJ, Forger NG, Pellegrini M, Vilain E. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol Sex Differ. 2014;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarthy MM. Multifaceted origins of sex differences in the brain. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forger NG. Epigenetic mechanisms in sexual differentiation of the brain and behaviour. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13(4):423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elliott EN, Sheaffer KL, Kaestner KH. The “de novo” DNA methyltransferase Dnmt3b compensates the Dnmt1-deficient intestinal epithelium. eLife. 2016;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaPlant Q, Vialou V, Covington HE III, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iñiguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolaños CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH, Nestler EJ. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13(9):1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown TJ, Hochberg RB, Zielinski JE, MacLusky NJ. Regional sex differences in cell nuclear estrogen-binding capacity in the rat hypothalamus and preoptic area. Endocrinology. 1988;123(4):1761–1770. [DOI] [PubMed] [Google Scholar]
- 47.Stevens CF. Neuronal diversity: too many cell types for comfort? Curr Biol. 1998;8(20):R708–R710. [DOI] [PubMed] [Google Scholar]
- 48.Nelson SB, Hempel C, Sugino K. Probing the transcriptome of neuronal cell types. Curr Opin Neurobiol. 2006;16(5):571–576. [DOI] [PubMed] [Google Scholar]
- 49.Edlund T, Jessell TM. Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell. 1999;96(2):211–224. [DOI] [PubMed] [Google Scholar]
- 50.Holmberg J, Perlmann T. Maintaining differentiated cellular identity. Nat Rev Genet. 2012;13(6):429–439. [DOI] [PubMed] [Google Scholar]
- 51.Takizawa T, Nakashima K, Namihira M, Ochiai W, Uemura A, Yanagisawa M, Fujita N, Nakao M, Taga T. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell. 2001;1(6):749–758. [DOI] [PubMed] [Google Scholar]