Pathological neovessels growing into the normally avascular photoreceptors cause vision loss in many eye diseases. Sun et al. find that a master inflammatory regulator, c-Fos, in photoreceptor controls retinal blood vessel growth into the photoreceptors through the inflammatory signal–induced STAT3/VEGFA pathway.
Abstract
Pathological neovessels growing into the normally avascular photoreceptors cause vision loss in many eye diseases, such as age-related macular degeneration and macular telangiectasia. Ocular neovascularization is strongly associated with inflammation, but the source of inflammatory signals and the mechanisms by which these signals regulate the disruption of avascular privilege in photoreceptors are unknown. In this study, we found that c-Fos, a master inflammatory regulator, was increased in photoreceptors in a model of pathological blood vessels invading photoreceptors: the very low-density lipoprotein receptor–deficient (Vldlr−/−) mouse. Increased c-Fos induced inflammatory cytokines interleukin 6 (IL-6) and tumor necrosis factor (TNF), leading to activation of signal transducer and activator of transcription 3 (STAT3) and increased TNFα–induced protein 3 (TNFAIP3) in Vldlr−/− photoreceptors. IL-6 activated the STAT3/vascular endothelial growth factor A (VEGFA) pathway directly, and elevated TNFAIP3 suppressed SOCS3 (suppressor of cytokine signaling 3)–activated STAT3/VEGFA indirectly. Inhibition of c-Fos using photoreceptor-specific AAV (adeno-associated virus)-hRK (human rhodopsin kinase)–sh_c-fos or a chemical inhibitor substantially reduced the pathological neovascularization and rescued visual function in Vldlr−/− mice. These findings suggested that the photoreceptor c-Fos controls blood vessel growth into the normally avascular photoreceptor layer through the inflammatory signal–induced STAT3/VEGFA pathway.
Introduction
Pathological retinal angiogenesis is a leading cause of vision loss. The photoreceptor layer is a privileged zone devoid of vasculature to allow light to access photosensitive receptors. Photoreceptors lie between the highly organized but open lattice of vessels in the inner retina, which allows light through to photoreceptors, and the dense sinusoidal vascular plexus, the choriocapillaris, which lies beneath the retinal pigment epithelium (RPE). Both vascular beds are sources of neovascularization extending into the normally avascular photoreceptor and subretinal space between the RPE and the photoreceptor outer segments. The mechanisms by which photoreceptors maintain their avascular state are not well understood, but they are key to understanding neovascular eye diseases, including neovascular age-related macular degeneration (AMD) and macular telangiectasia (Klein et al., 2004). Abnormal blood vessels that extend into this privileged zone not only block light, but also disrupt retinal function to cause blindness (Luo et al., 2013).
Invasion of vessels into photoreceptors has been associated generally with increased inflammatory signals (Espinosa-Heidmann et al., 2003; Sakurai et al., 2003), but the timing and the localization of these signals are not well understood, particularly with respect to the initiation signals for neovascularization. Adaptive immunity and inflammation are highly controlled in the photoreceptor/subretinal space (Streilein et al., 2002; Masli and Vega, 2011), maintained by expression of immunosuppressive factors and characterized by lack of immune cells and, thereby, tolerance of foreign antigens (Streilein, 1995; Masli and Vega, 2011). Macrophages and dendritic cells are normally absent in the outer retina and are limited to the underlying choroidal vessels.
Eye diseases with pathological angiogenesis have a slow parainflammatory response (Medzhitov, 2008; Perez and Caspi, 2015), with changes in adaptive immunity and macrophage infiltration violating the normal ocular environment of immune privilege. In AMD, the blood–retinal barrier breaks down, and immune/inflammatory cells are recruited from the systemic circulation through the choroid or retinal vessels (Ambati et al., 2013) and accumulate in the subretinal space. Here, under stress conditions, they eliminate visual byproducts (Xu et al., 2008). In degenerative diseases, this breakdown in the immune privilege may be a compensatory response to maintain vision because impaired microglial migration into or out of the subretinal space promotes photoreceptor cell death (Combadière et al., 2007; Chen et al., 2011b). Loss of immune privilege may correlate with and increase neovascularization (Apte et al., 2006; Roychoudhury et al., 2010). We hypothesized that inflammatory signals from photoreceptors initiate the process to break down their avascular privilege, with secondary alterations in aspects of immune privilege. This mechanism might suggest novel molecular targets for anti-neovascular therapies directed at the initiation step.
The very low-density lipoprotein receptor–deficient (Vldlr−/−) mouse is a model of pathological angiogenesis invading the avascular zone (Heckenlively et al., 2003; Li et al., 2007; Chen et al., 2009; Dorrell et al., 2009; Joyal et al., 2016). Vldlr−/− mice first develop retinal vascular lesions, invading photoreceptors from the retinal vessels, and then later develop choroidal neovascularization (also invading photoreceptors but from the choroidal vessels), associated with cone degeneration and then rod loss (Chen et al., 2009; Dorrell et al., 2009), retinal vascular leakage, and chronic inflammation, features similar to human neovascular AMD. Previous studies report that dysregulated photoreceptor energy metabolism (Joyal et al., 2016) and oxidative stress (Dorrell et al., 2009) contribute in part to increased levels of vascular endothelial growth factor A (VEGFA), which leads to neovascularization in Vldlr−/− retinas. Here, we used Vldlr−/− mice to explore the contribution of photoreceptor-specific inflammatory signals through c-Fos to control photoreceptor vascular privilege.
We postulated that c-Fos, a transcription factor that regulates many inflammatory signals, might control photoreceptor inflammation (Hoffman et al., 1993). c-fos encodes a nuclear DNA-binding phosphoprotein that forms heterodimeric complexes with members of the Jun family of proteins to constitute transcription factor complex activator protein 1 (AP-1; Curran and Franza, 1988; Hafezi et al., 1997). AP-1 regulates genes that affect the epidermal microenvironment, including cytokines such as IL-6, TNFα, and matrix proteins (Wagner and Eferl, 2005). c-fos is one of the immediate early genes (Hoffman et al., 1993) that are activated transiently and rapidly in response to a wide variety of cellular stimuli. c-Fos promotes inflammation and disease development in inflammatory diseases such as arthritis (Aikawa et al., 2008; Shiozawa and Tsumiyama, 2009), although it also acts as a negative regulator of inflammatory responses in myeloid and lymphoid cell lineages (Ray et al., 2006).
We found that photoreceptor c-Fos controlled vascular invasion into the photoreceptor layer in Vldlr−/− mice. Increased c-Fos in Vldlr−/− photoreceptors was associated with increased expression of its target genes including Il6 and Tnf, which led to activation of the STAT3/VEGFA pathway driven by both IL-6 and TNF/TNFα-induced protein 3 (TNFAIP3)/suppressor of cytokine signaling 3 (SOCS3) signals. Inhibition of c-Fos in photoreceptors using AAV (adeno-associated virus)-hRK (human rhodopsin kinase)–sh_c-fos blocked neovascularization in Vldlr−/− mice and rescued visual function. A chemical inhibitor of c-Fos also reduced neovascularization in Vldlr−/− mice. These findings suggested that photoreceptor c-Fos controls neovascularization through the neuronal STAT3/VEGFA pathway.
In summary, in the Vldlr−/− retina, in which vessels invade the avascular photoreceptor layer, c-Fos induced inflammatory signals in photoreceptors, leading to increased VEGF, which promoted neovascularization. Macrophage infiltration into the subretinal space occurred only in the late stage. c-Fos is hence a key factor that controls photoreceptor/subretinal space avascular and immune privilege and may be targeted to treat an initiating process in neovascular eye diseases.
Results
In the photoreceptor layer of Vldlr−/− retina, inflammation is seen in association with neovascularization
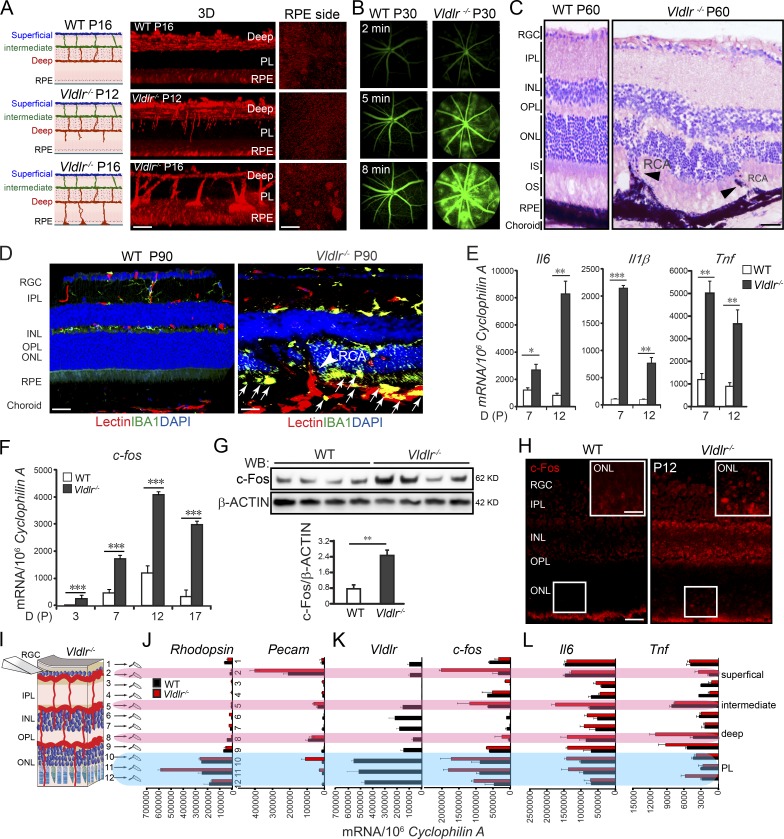
There are three retinal vascular layers (superficial, intermediate, and deep) in the normal mouse retina (Fig. 1 A, left). In the Vldlr−/− retina, neovascularization extends from the retinal vasculature into the normally avascular photoreceptor layer by postnatal day 12 (P12) and reaches the RPE by P16 (Fig. 1 A). Newly formed vessels in adult Vldlr−/− retinas were leaky, as shown with fundus fluorescein angiography (FFA) at P30 (Fig. 1 B). These ectopic vessels reached the RPE and merged with the choroidal vessels to form retinal–choroidal anastomoses and choroidal neovascularization at ∼6–8 wk of age, violating the avascular privilege of the photoreceptor layer (Fig. 1 C). Later, in 3-mo-old adult (P90) Vldlr−/− retinas, IBA1-positive activated macrophages were seen infiltrating into the photoreceptor layer and subretinal space, indicating secondary loss of immune privilege (Fig. 1 D).
Figure 1.
c-Fos was induced in Vldlr−/− retinas. (A) Vldlr deficiency led to neovascularization in the normally avascular photoreceptor layer shown by 3D reconstruction of representative confocal images. n = 6. (B) Vldlr deficiency led to retinal vascular leakage at 2, 5, and 8 min after intraperitoneal injection of fluorescent dye, as shown by FFA images from P30 WT and Vldlr−/− mice. n = 6. (C) H&E staining showed retinal layer disorganization and retinal-choroidal vascular anastomoses in P60 Vldlr−/− retinas. Black arrowheads indicate neovascularization. n = 6. (D) Macrophage marker IBA1 (green) costained with the endothelial cell marker isolectin (red) and nuclear marker DAPI (blue) in 3-mo Vldlr−/− retinas with retinal-choroidal vascular anastomoses (arrowhead); macrophages were seen in the subretinal space between the ONL and RPE (arrows). (E) Cytokine expression, including Il6, Il1β, and Tnf, was increased during development in Vldlr−/− retinas. n = 6. (F–L) c-fos mRNA and protein expression were markedly increased (F and G) in Vldlr−/− retinas during development (F), mainly in the P12 ONL (K) and colocalized with the expression of Vldlr in P12 WT (H–K) and its target genes including Il6 and Tnf (L). n = 6. (H) IHC staining showed increased c-Fos expression in the ONL of P12 Vldlr−/− retinas. INL, inner nuclear layer; IPL, inner plexiform layer; IS, photoreceptor inner segment; OPL, outer plexiform layer; OS, photoreceptor outer segment; PL, photoreceptor layer; RCA, retinal-choroidal anastomosis; RGC, retinal ganglion cell; WB, Western blot. Bars: (A, 3D) 100 µm; (A, RPE) 250 µm; (C, D, and H) 50 µm; (H, inset) 25 µm. All data are representative of at least three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Results are presented as mean ± SEM.
Elevated aqueous inflammatory cytokine levels are found in neovascular AMD patients (Roh et al., 2009). We examined inflammatory cytokine expression in WT and Vldlr−/− retinas at P12 as vessels began to invade photoreceptors. Total retinal mRNA of inflammatory cytokines Il6, Tnf, and Il1β were markedly increased at P12 in Vldlr−/− versus WT retinas (Fig. 1 E). Yet, at this point, there were no detectable macrophages (a potential source of inflammatory cytokines) recruited into the photoreceptor layer and subretinal space (Fig. S1, A–C), as seen in P90 adult retinas (Fig. 1 D). At P12, there was no difference in the level of mRNA expression of macrophage markers including Cd45, Cd11b, Cd68, and Cx3cr1 between Vldlr−/− and WT retinas (Fig. S1 D). These data suggested that Vldlr deficiency increased expression of inflammatory cytokines early (≤P12), before macrophage recruitment to the deep layer and the subretinal space, which was seen only at a later stage (3 mo). These results indicated that in Vldlr−/− retinas, neuronal cell inflammatory signals might initiate neovascularization, followed later by macrophage/immune cell recruitment (Fig. S1), which is consistent with our previous finding that there is no evidence for macrophage-induced onset of neovascularization (Joyal et al., 2016).
c-Fos was highly induced in Vldlr−/− retinas and si_c-fos reduced subretinal neovascularization
We examined inflammation as a possible mechanism underlying neovascularization (loss of immune/vascular privilege) in the photoreceptor layer in Vldlr−/− mice with microarray analyses. c-fos expression was markedly increased in P14 Vldlr−/− versus WT retinas (Table S1). We confirmed an increase in c-fos mRNA and protein levels in Vldlr−/− retinas before and during the development of pathological neovascularization (Fig. 1, F and G) with c-Fos immunohistochemistry staining (Fig. 1 H). c-Fos was highly induced in the outer nuclear layer (ONL; photoreceptors) in P12 Vldlr−/− retinas. To quantitatively localize c-fos, we developed a technique to tangentially section whole-mounted retinas and quantify mRNA in 12 individual cellular layers, from retinal ganglion cells to RPE (Fig. 1, I–L; and Fig. S2). The specificity and homogeneity of sectioned layers was confirmed by quantifying mRNA expression of rhodopsin (photoreceptor marker) and platelet endothelial cell adhesion molecule (Pecam; vascular endothelial cell marker) in each layer (Fig. 1 J). Increased c-fos expression was mainly seen in the rhodopsin-expressing layers where Vldlr was also highly expressed (Fig. 1 K). c-fos expression was also increased in layer 2, which mainly contained superficial vessels and retinal ganglion cells, suggesting that c-fos may also play a role in cells other than photoreceptors, which needs further study. Target inflammatory genes of c-fos, Il6, and Tnf were identified mainly in the same layer as Vldlr and c-fos, suggesting that VLDLR/c-Fos might mediate the disruption of angiostatic privilege in the retinal photoreceptor layer by controlling local photoreceptor inflammatory signals (Fig. 1 L). In addition, the expressions of Il6 and especially Tnf were also increased in layers 8 and 9, which contain the deep layer of retinal vessels. This increase might arise from proliferating endothelial cells, as the deep layer of vessels form at that time.
To suppress increased c-fos expression, we used pooled si_c-fos comprising three siRNA sequences. The knockdown efficiency was confirmed to be ∼65% in photoreceptor 661W cells (Fig. S3 A). Subretinal injection of the si_c-fos pool suppressed both mRNA and protein expression of c-fos in the P12 Vldlr−/− photoreceptor layer by ∼40–50% (Fig. S3, B and C), associated with a reduction in neovascularization in the photoreceptor layer (Fig. S3 D). Three-dimensional (3D) reconstruction of representative confocal images of retinal whole mounts stained with the endothelial cell marker isolectin IB4 showed 45% fewer vascular lesions reaching the RPE, which lies beneath the photoreceptors (Fig. S3, D and E). The mean lesion size was also reduced (Fig. S3 F). Thus, knockdown of c-fos inhibited neovascularization in Vldlr−/− retinas.
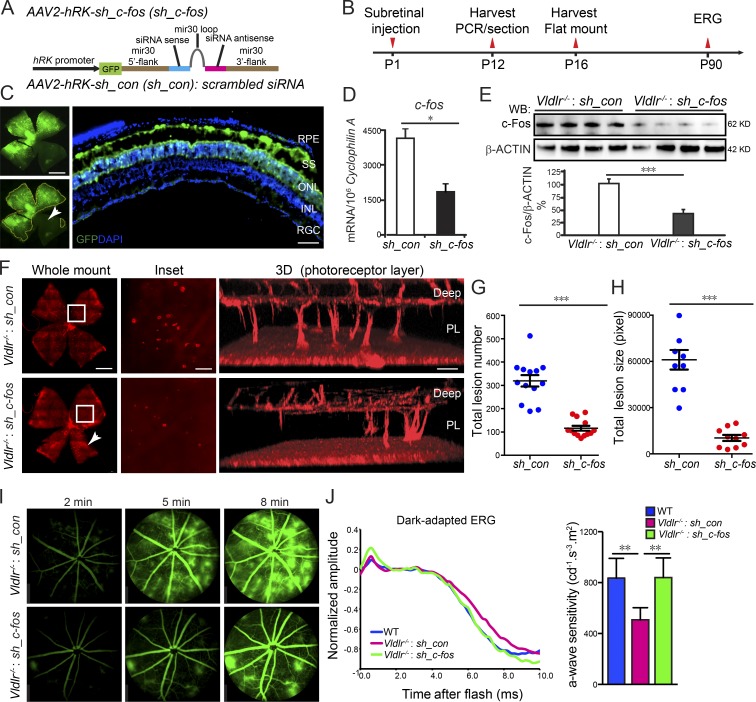
AAV expressing sh_c-fos in the photoreceptor layer inhibited neovascularization and preserved retinal function
Because of nonspecific targeting and the transient effect of siRNA, si_c-fos could neither specifically nor efficiently target photoreceptor cells (Fig. S3). Therefore, we generated a photoreceptor-specific AAV2 carrying shRNA of c-fos with the hRK promoter (Fig. 2 A; Khani et al., 2007). Three shRNA sequences were cloned, and the knockdown efficiency was validated using photoreceptor 661W cells (Fig. S3 G). We injected AAV2-hRK–sh_c-fos subretinally at P1 and evaluated retinas at P16 (Fig. 2 B). The infection was confirmed with anti-GFP antibody staining in the photoreceptor layer and subretinal space (Fig. 2 C), showing that 75–80% of the retina was successfully transfected with AAV (Fig. 2 C). Consequently, both mRNA and protein expression of c-fos were reduced by ∼60% (Fig. 2, D and E), and neovascularization was reduced by >70% at P16 (Fig. 2, F and G), as measurements included total retinal areas, which may not be completely affected by AAV2-hRK–sh_c-fos (∼25% not transfected) as shown (Fig. 2, C and F, white arrowhead). The mean lesion size was reduced by 83% (Fig. 2, F–H). AAV2-hRK–sh_c-fos treatment also prevented vessel leakage (Fig. 2 I). These results suggest that c-Fos and VLDLR functioned synergistically in maintaining photoreceptor avascular privilege. Moreover, AAV2-hRK–sh_c-fos significantly rescued visual dysfunction in Vldlr−/− mice. Untreated Vldlr−/− mice were characterized by lower electroretinographic photoreceptor sensitivity (a-waves) than age-matched WT mice. AAV2-hRK–sh_c-fos injection improved photoreceptor sensitivity at 3 mo after injection compared with AAV2-hRK–sh_control–injected Vldlr−/− mice (Fig. 2 J), indicating protection of photoreceptor function.
Figure 2.
Attenuation of c-Fos in the photoreceptor layer extending to the subretinal space reduced neovascularization. (A) Schematic diagram illustrating AAV2 carrying sh_c-fos within a mir30 cassette under the control of an hRK promoter (hRK-sh_c-fos). sh_con, sh_control. (B) Time line for AAV subretinal injection and retina collection. (C) The infection of AAV2 in the subretinal space (SS) and photoreceptor layer was confirmed by AAV2-hRK-eGFP. n = 6. INL, inner nuclear layer; RGC, retinal ganglion cell. The white arrowhead indicates the retinal areas were not affected by AAV2-hRK-eGFP because of the limitation of subretinal injection. (D and E) The knockdown efficiency of AAV2-hRK–sh_c-fos via subretinal injection into Vldlr−/− retinas was confirmed by comparison with AAV2-hRK-sh_control–injected retinas at both mRNA (D) and protein (E) levels. (D) *, P < 0.05. n = 6. (E) ***, P < 0.001. n = 4. WB, Western blot. (F) Knocking down c-fos in the photoreceptor layer (PL) extending to the subretinal space using AAV2-hRK–sh_c-fos–inhibited neovascularization shown by whole-mounted images of AAV2-hRK-sh_control– or AAV2-hRK–sh_c-fos–treated Vldlr−/− retinas stained with isolectin IB4 to label endothelial cells and representative 3D reconstruction images for neovascularization in photoreceptor layers, including the retinal areas, which may not be completely affected by AAV2-hRK–sh_c-fos (about 25% not transfected) as shown in C (white arrowhead in C and F). (G and H) Both total lesion number and total lesion size (pixels) were reduced in AAV-hRK–sh_c-fos–treated compared with AAV2-hRK-sh_control–treated retinas. ***, P < 0.001. n = 8–13. (I) AAV2-hRK–sh_c-fos injected in the subretinal space at P1 rescued Vldlr deficiency–induced retinal vascular leakage shown by FFA images at P60 in Vldlr−/−-treated mice. n = 6–8. (J) Representative dark-adapted (scotopic) ERG graph (left) and quantification of photoreceptor a-wave sensitivity (right) showed that photoreceptor function was attenuated in 3-mo-old Vldlr−/− retinas and was particularly rescued by AAV-hRK–sh_c-fos (one-time treatment on P1) in 3-mo-old Vldlr−/− retinas. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Bars: (C and F, whole-mount images) 1,000 µm; (C, cross section) 50 µm; (F, inset) 250 µm; (F, 3D) 100 µm. All data are representative of at least three independent experiments. Results are presented as mean ± SEM.
c-Fos promoted retinal angiogenesis via the neuronal STAT3/VEGF pathway
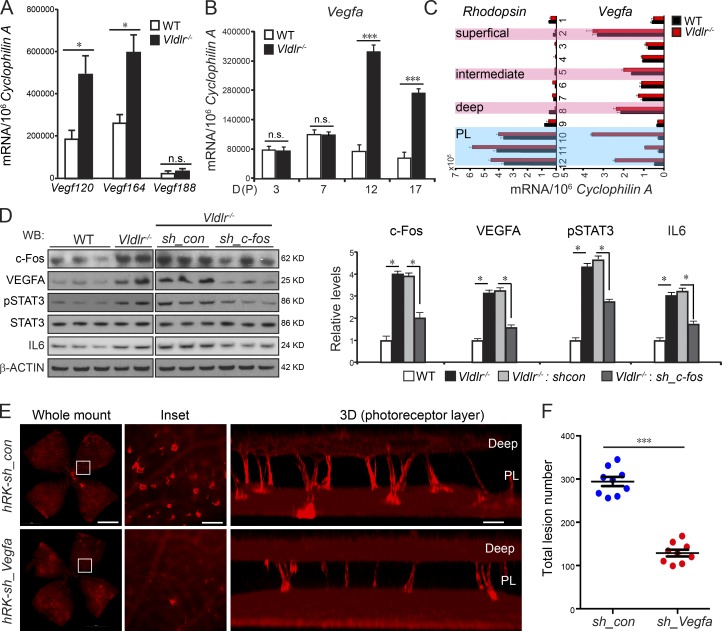
IL-1β and TNF increase the expression of VEGF (Margetts et al., 2002); c-Fos transcriptionally regulates VEGF production in peritoneal inflammation via IL-1β and TNF (Catar et al., 2013). We found that increased levels of Il6 (Fig. 1 E), a transcriptional target of c-Fos, activated STAT3 and increased expression of Vegfa isoforms Vegf120 and Vegf164 (Fig. 3 A), which likely contributed to the neovascularization in Vldlr−/− retinas. Vegfa expression was markedly increased in Vldlr−/− mice at P12 and P17, as well as in the P12 photoreceptor layer of Vldlr−/− retinas (Fig. 3, B and C). VEGFA was reduced by AAV2-hRK–sh_c-fos in Vldlr−/− retinas (Fig. 3 D), which was associated with activated (phosphorylated) STAT3 and increased IL-6. We also knocked down VEGFA expression in photoreceptors using AAV2-hRK-sh_Vegfa, which strongly suppressed neovascularization in Vldlr−/− retinas (Fig. 3, E and F), suggesting that VEGFA from photoreceptors directly caused neovascularization, consistent with previous studies (Usui et al., 2015; Joyal et al., 2016). Therefore, it is likely that c-Fos promoted neovascularization in the photoreceptor layer via VEGFA signaling, which may be driven by IL-6/STAT3.
Figure 3.
c-Fos promoted retinal angiogenesis via VEGFA signaling modulated by neuronal IL-6/STAT3. (A) Vegf120 and Vegf164 but not Vegf188 expression was increased in P12 Vldlr−/− retinas. n = 6. (B and C) Vegfa expression was markedly increased in Vldlr−/− whole retinas at P12 and P17 (B) as well as in the photoreceptor layer (PL) of P12 Vldlr−/− retinas (C). n = 6. (D) The protein levels of c-Fos, pSTAT3, total STAT3, IL-6, and VEGFA in P12 WT, Vldlr−/−, and Vldlr−/− treated with AAV2-hRK–sh_c-fos or AAV2-hRK–sh_control (sh_con). n = 4–6. WB, Western blot. (E and F) Representative P16 whole mount images stained with isolectin IB4 and quantification showed that knocking down VEGFA in Vldlr−/− retinas using AAV2-hRK-sh_Vegfa reduced total lesion number by 55% compared with AAV2-hRK-sh_control–treated Vldlr−/− retinas. (E) Bars: (whole-mount images) 1,000 µm; (inset) 250 µm; (3D) 100 µm. PL, photoreceptor layer. All data are representative of at least three independent experiments. *, P < 0.05; ***, P < 0.001. Results are presented as mean ± SEM.
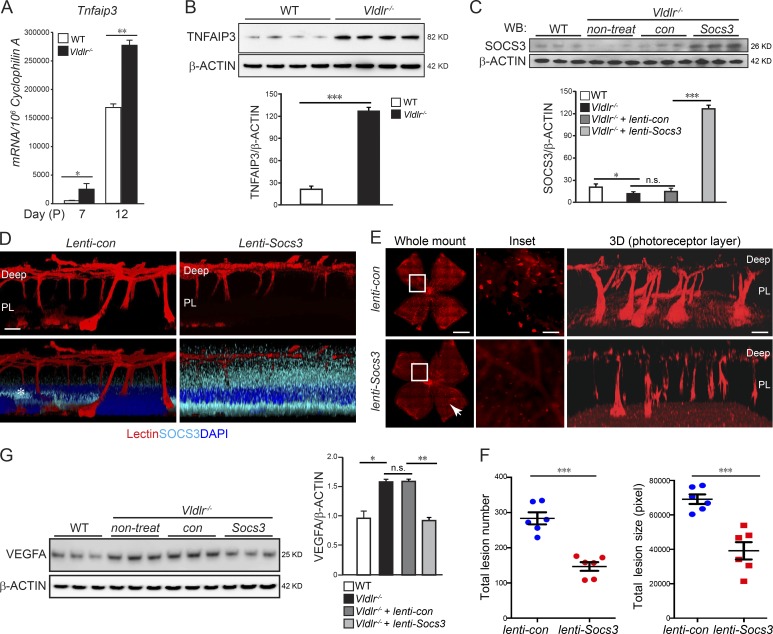
TNFAIP3, a gene important in cytokine-mediated immune and inflammatory responses, is induced by TNF, another c-Fos target gene. We found that TNFAIP3 was induced at both mRNA and protein levels in Vldlr−/− retinas (Fig. 4, A and B), concomitant with increased Tnf. Interestingly, TNFAIP3 is reported to suppress SOCS3 expression to enhance STAT3 proliferative signals (da Silva et al., 2013). We found that Vldlr−/− retinas have less SOCS3 expression than WT (Sun et al., 2015b). These findings suggested that STAT3 could also be activated by increased Tnf/Tnfaip3 signals that further led to reduced Socs3 level in Vldlr−/− retinas. Here, we showed that overexpression of Socs3 mainly in the photoreceptor layer reduced neovascularization in Vldlr−/− retinas (Fig. 4, C–F). These results suggested that SOCS3 is critical to neovascularization and that reduction of SOCS3 expression in Vldlr−/− versus WT retinas may be secondary to up-regulation of Tnf/Tnfaip3 induced by c-fos. SOCS3 inhibits activation of STAT3 by binding to the kinase JAK and the IL-6 receptor (Wei et al., 2014), which induces the expression of VEGF (Wei et al., 2003). Consistently, we found that, in Vldlr−/− retinas, overexpression of Socs3 suppressed the increased VEGFA level by ∼45% (Fig. 4 G).
Figure 4.
c-Fos promoted retinal angiogenesis via TNF/TNFAIP3/SOCS3. (A and B) Tnfaip3 mRNA (A; n = 6) and protein levels (B; n = 4) were increased in the developing Vldlr−/− retinas compared with WT controls. (C) Lenti-Socs3 was injected subretinally into Vldlr−/− eyes, and SOCS3 protein levels were confirmed by Western blotting (WB). n = 3. con, control; non-treat, nontreated. (D) The infection of lenti-Socs3 in the photoreceptor layer (PL) was confirmed by anti-SOCS3 antibody staining (cyan). 3D reconstructed representative images show that SOCS3 was mainly expressed in the photoreceptor layer after subretinal injection. n = 4. The asterisk in the lenti-control image indicates nonspecific staining. (E and F) Overexpression of Socs3 using lenti-Socs3 inhibited neovascularization, including the retinal areas, which may not be completely affected by lenti-Socs3 (∼25% not transfected; white arrowhead). (E) The representative whole-mount images of lenti-control– or lenti-Socs3–treated Vldlr−/− retinas stained with isolectin IB4 to label endothelial cells. 3D reconstruction images show neovascularization in the photoreceptor layer. n = 6. (F) Quantification shows a decrease in both total lesion number and total lesion size in lenti-Socs3–treated Vldlr−/− retinas compared with lenti-control–treated Vldlr−/− retinas. n = 6. (G) Increased protein level of VEGFA in Vldlr−/− retinas was suppressed by lenti-Socs3 treatment. n = 3. Bars: (E, whole-mount images) 1,000 µm; (E, inset) 250 µm; (D and E, 3D) 100 µm. All data are representative of at least three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Results are presented as mean ± SEM.
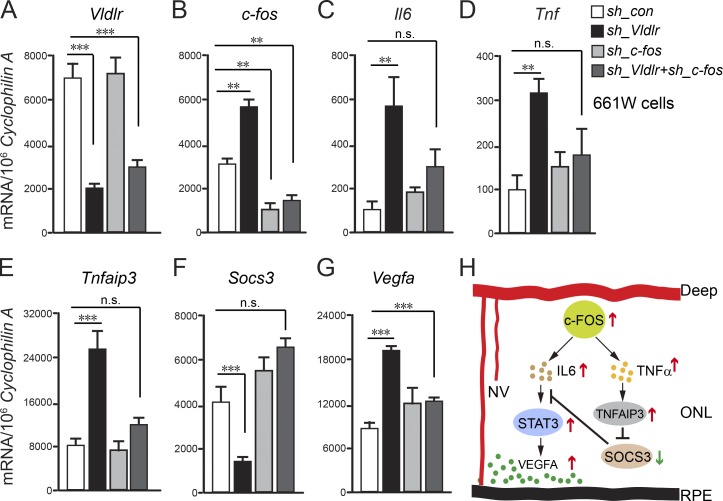
To confirm that c-Fos activates the STAT3/VEGFA pathway via IL-6 and TNF/TNFAIP3/SOCS3 signals in retinal photoreceptors, we examined Vldlr-deficient 661W photoreceptor cells (treated with AAV2-hRK-shRNA targeting Vldlr). Knocking down Vldlr increased mRNA expression of c-fos, Il6, Tnf, Tnfaip3, and Vegfa, also decreasing Socs3. In Vldlr−/− retinas, AAV2-hRK–sh_c-fos treatment restored Il6, Tnf, Tnfaip3, Vegfa, and Socs3 expression to normal levels (Fig. 5, A–G). Thus, c-Fos in photoreceptors promoted retinal neovascularization via the neuronal STAT3/VEGF pathway induced by IL-6 and TNF/TNFAIP3/SOCS3 signals (Fig. 5 H).
Figure 5.
c-Fosregulated Vegfa via TNF/TNFAIP3/SOCS3 in photoreceptor cells. (A–G) The mRNA levels of Vldlr, c-fos, Il6, Tnf, Tnfaip3, Socs3, and Vegfa in 661W photoreceptor cells treated with AAV2-hRK-sh_Vldlr and/or AAV2-hRK–sh_c-fos or AAV2-hRK-sh_Control (sh_con). **, P < 0.01; ***, P < 0.001. n = 6. (H) A schematic diagram illustrates pathological neovascularization (NV) in the normal avascular photoreceptor layer controlled by transcriptional factor c-FOS through both IL-6/STAT3/VEGFA and TNF/TNFAIP3/SOCS3/STAT3/VEGFA pathways. All data are representative of at least three independent experiments. Results are presented as mean ± SEM.
As c-Fos and c-Jun proteins form the AP-1 complex (Chinenov and Kerppola, 2001), we also assessed the expression of c-jun, the levels of which were comparable between WT and Vldlr−/− retinas (Fig. S4). In addition, mRNA expression of urokinase-type plasminogen activator (uPA) was markedly increased in Vldlr−/− retinas (Fig. S4 B), suggesting that it might be a potential factor underlying high levels of transcription factor c-fos expression.
We previously found that energy deficiency in photoreceptors, which is exacerbated in the dark because of increased energy demand of the dark current, also causes an increase in photoreceptor VEGF and increased neovascularization in Vldlr−/− mice (Joyal et al., 2016). c-fos mRNA expression increased ∼10-fold at P8 and 20-fold at P12 in dark-reared (vs. 12 h dark/light control) Vldlr−/− mice (Fig. S4 C), suggesting that c-fos expression may also be mediated by increased photoreceptor energy demands of dark rearing in Vldlr−/− mice.
Pharmacologic inhibition of c-Fos suppressed retinal neovascularization
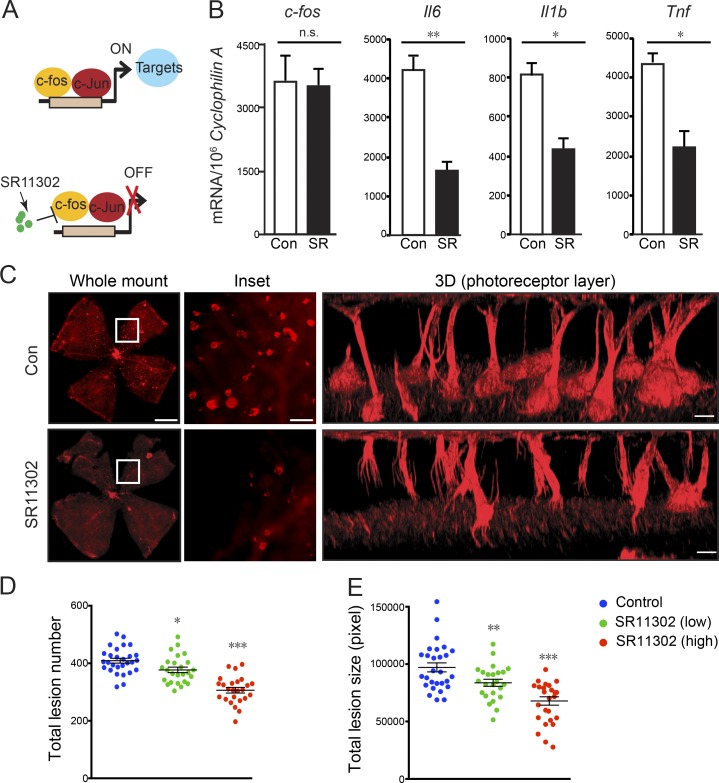
SR11302 is a retinoid that specifically inhibits AP-1 activity without activating the transcription of retinoic acid response element (Fig. 6 A; Fanjul et al., 1994). AP-1 consists of a family of c-Jun/c-Fos dimers that include different Jun proteins (c-Jun, JunB, and JunD) and Fos proteins (c-Fos, FosB, FosL1, FosL2, and FosB2; Angel and Karin, 1991). The efficiency of SR11302 on AP-1 inhibition in SR11302-treated retinas was confirmed with the reduction of c-Fos target gene expression (Il6, Il1β, and Tnf), without a change in c-fos expression itself (Fig. 6 B). SR11302 treatment reduced the total vascular lesion number and lesion size in Vldlr−/− mice in a dose-dependent manner. High-dose SR11302 from P5 to P15 in Vldlr−/− mice reduced the total vascular lesion number by 48% and decreased the lesion size by 40% (Fig. 6, C–E), without detectable signs of toxicity in mice, including no change in body weight (Fig. S5).
Figure 6.
c-Fos inhibitor SR11302 prevented retinal neovascularization. (A) Schematic diagram illustrating how c-Fos inhibitor SR11302 may block c-Fos binding to its target promoters to inhibit target gene expression. (B) The mRNA levels of c-fos and its target genes (Il6, Il1β, and Tnf) in SR11302 (SR)-treated and control (con) Vldlr−/− retinas confirmed the efficiency of the c-Fos inhibitor. n = 6. (C) SR11302 inhibited neovascularization. Representative whole-mount images of control or SR11302-treated Vldlr−/− retinas stained with isolectin IB4 to label endothelial cells. Enlarged images show reduced total lesion number and lesion size in the SR11302-treated retinas. 3D reconstruction images show neovascularization in the photoreceptor cell layer. Bars: (whole-mount images) 1000 µm; (inset) 250 µm; (3D) 100 µm. (D and E) Quantification shows reduced total lesion number and lesion size (pixels) in SR11302-treated retinas compared with controls in a dose-dependent manner. n = 22–29. All data are representative of at least three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Results are presented as mean ± SEM.
Discussion
Here, we identify photoreceptors as the source of inflammatory signals that regulate the disruption of avascular privilege and later the disruption of immune privilege in photoreceptors in Vldlr−/− mice. We show that increased c-fos and inflammatory signals in the Vldlr−/− photoreceptor layer were associated with neovascularization invading a normally avascular-privileged zone, followed later by macrophage migration into the photoreceptor/subretinal space. Reducing c-fos expression suppressed local photoreceptor-produced proinflammatory cytokines and prevented neovascularization. Thus, photoreceptor inflammatory signals initiated neovessel growth into the avascular zone. It is plausible that c-fos could promote and c-fos inhibitors could suppress neovascularization in many eye diseases. The theoretical advantage in suppressing c-Fos (or AP-1) to suppress downstream targets (which include VEGF) rather than suppressing VEGF directly is that many AMD patients do not respond to anti-VEGF treatment and that inflammation is also a target in pathological retinal angiogenesis in neovascular eye diseases.
Although retinal inflammation is clearly involved in AMD, the relevant cells and pathways have not been fully defined. In Vldlr−/− mice, increased leukostasis and elevated levels of proinflammatory factors, such as intercellular adhesion molecule 1, TNF, endothelial nitric oxide synthase, and cyclooxygenase 2, indicate the presence of inflammation (Chen et al., 2009). We showed a direct role for photoreceptor c-Fos (or AP-1) in promoting pathological vasculature in the photoreceptor avascular-privileged zone through photoreceptor-derived inflammatory mediators. AP-1 positively regulates the expression of inflammatory mediators such as IL-6 and TNF in a variety of cells (Lee et al., 2004). The AP-1 complex is prototypically a c-Fos and c-Jun protein dimer (Chinenov and Kerppola, 2001). In contrast to c-Jun proteins, c-Fos subunits cannot form homodimers but require combination with other binding proteins. In Vldlr−/− retinas, we found increased expression of c-fos but not of c-jun, which is likely to enlarge the pool of c-Fos available for heterodimerization and increase the fraction of AP-1 complexes composed of c-Fos and c-Jun (Catar et al., 2013). This may directly affect proinflammatory cytokines gene expression, as complexes of c-Fos and c-Jun bind more effectively to target DNA sequences than c-Jun homodimers (Halazonetis et al., 1988). However, inflammatory cytokines including IL-6, TNF, and IL-1β can induce a rapid and transient expression of mRNA encoding c-Fos (McKay et al., 2001). In Vldlr−/− retinas, the positive feedback loop of c-fos and cytokine-mediated inflammatory signals induced neovascularization. To explore the importance of this feedback loop in neovascularization, we broke down the positive feedback loop through subretinal injection of lentivirus expressing SOCS3, which inhibited STAT3 activation in Vldlr−/− retinas. Overexpression of SOCS3 led to reduced cytokine production and a 50% reduction of neovascularization in Vldlr−/− retinas. These findings suggest that the feedback loop of c-fos/inflammatory cytokines plays a critical role in Vldlr deficiency–induced neovascularization. This is consistent with our previous study (Sun et al., 2015b) showing that induction of retinal Socs3 expression with a synthetic inverse agonist of retinoic acid receptor–related orphan receptor α (RORα) inhibited subretinal neovascular lesions in Vldlr−/− retinas.
The complex of uPA and plasminogen activator inhibitor 1 is one of the VLDLR ligands. It can promote cell proliferation and migration by activating the extracellular signal–regulated kinase pathway (Webb et al., 2001). The plasminogen and plasminogen activator system consists of serine proteases that regulate migratory and tissue-remodeling events, such as those observed in neovascular AMD (Pepper, 2001). uPA mRNA is expressed in both choroidal neovascularization membranes extracted from patients with neovascular AMD and in experimentally induced choroidal neovascularization in mice (Rakic et al., 2003; Balasubramanian et al., 2014). Increases in uPA are reported in patients with neovascular AMD (Sung et al., 2012). However, the role of uPA in neovascular AMD is largely unknown. Binding of uPA to its receptor uPAR induces the rapid but transient expression of c-fos in human ovarian cancer cells (Dumler et al., 1994). This signal generates protein tyrosine kinase activity feeding into a signal transduction pathway that activates nuclear transcription factors (Dumler et al., 1994). Our data suggest that increases in free uPA caused by Vldlr deficiency (Fig. S4 B) might potentially induce expression of high levels of c-fos.
Our previous study (Joyal et al., 2016) found that dark-reared Vldlr−/− mice develop 1.5-fold more vascular lesions than 12-h light and dark cycle–raised mice, suggesting that energy metabolism influences neovascular disease, as dark rearing increases the energy demand of the dark current in photoreceptors (Ames et al., 1992). In this study, we found that c-fos mRNA expression increased >10-fold in dark-reared (vs. 12 h dark/light control) Vldlr−/− mice (Fig. S4 C), which suggests that c-fos expression is also mediated by increased photoreceptor energy demands of dark rearing in Vldlr−/− mice. There is a strong correlation between mRNA levels of c-fos in the ONL (photoreceptors) and the genes coding for the phototransduction proteins related to diurnal changes (Nir and Agarwal, 1993). In rat retinas, c-fos mRNA is transiently expressed in the inner nuclear layer and ganglion cell layer after the onset of the light period and expressed continuously in the ONL throughout the dark period under a light/dark (12/12 h) cycle (Yoshida et al., 1993). This relationship suggests that c-fos may also play a role in the transcriptional regulation of the genes encoding these light/dark cycle proteins (Ohki et al., 1996).
Our previous study (Joyal et al., 2016) shows that activation of the free fatty acid receptor 1 (Ffar1) impairs glucose entry into photoreceptors resulting in a dual (lipid and glucose) fuel shortage in Vldlr−/− mice that have impaired lipid uptake. This energy shortage results in a reduction in the levels of the Krebs cycle intermediate α-ketoglutarate, which promotes stabilization of hypoxia-induced factor 1α and secretion of VEGF by starved Vldlr−/− photoreceptors, leading to neovascularization. However, the double knockout Vldlr−/−/Ffar1−/− did not completely suppress the lesions, indicating that there are other pathways involved in this process. This study showed that c-fos–driven inflammation signals might also be induced during this energy-deprived process to control photoreceptor VEGF secretion.
Anti-VEGF drugs are widely used clinically to control the proliferation of pathological blood vessels driven by high levels of VEGF. However, basal levels of VEGF are needed for neuron and normal vessel survival. Under such circumstances, blocking only excess but not all VEGF will be very helpful. Targeting c-Fos or SOCS3 may prevent photoreceptor cells from releasing excessive VEGF without affecting its basal level.
c-fos−/− mice were reported to have reduced brain size because of apoptosis at the ventricular zone (Velazquez et al., 2015), and retina from c-fos−/− mice have 23% fewer rod photoreceptor cells with 25% lower rhodopsin content (Kueng-Hitz et al., 2000), suggesting c-fos is required for protection against cell apoptosis and that complete suppression of c-fos may be detrimental. We suppressed increased c-Fos using si_c-fos, AAV2-hRK–sh_c-fos, and SR11302 to control neovascularization in Vldlr−/− photoreceptors. Based on Fig. 3 D, the protein level of c-Fos after AAV2-hRK–sh_c-fos treatment for 3 mo was little higher than but comparable with that in WT, suggesting that normalization of the large increase in c-Fos seen with pathological neovascularization rather than complete suppression would be of benefit.
In summary, this study demonstrated that photoreceptor inflammatory signals initiate the vision-threatening invasion of blood vessels into an avascular-privileged zone in the retina. Retinal photoreceptor transcription factor c-Fos controls local inflammatory signaling to mediate neovascularization. Targeting the transcription factor c-Fos might be a novel way to prevent vision loss secondary to pathological retinal angiogenesis and may have broad therapeutic value for other vascular disorders involving photoreceptor microenvironmental inflammation.
Materials and methods
Animals
All animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee at the Boston Children’s Hospital. Mice were housed under specific pathogen–free conditions. Vldlr+/− (heterozygous) mice from The Jackson Laboratory (stock no. 002529) were crossed to generate homozygous and WT littermates. SR11302 (cat. 2476; Tocris Bioscience) was dissolved in corn oil. Vldlr−/− pups were orally gavaged with SR11302 or vehicle control (corn oil) at two doses (low dose 0.5 mg/kg body weight and high dose 1 mg/kg body weight) daily from P5 to P15. P16 retinas were collected for PCR and neovascularization analysis.
Neovascularization analysis
Vldlr−/− and WT retina whole mounts were stained with isolectin IB4 and imaged with a microscope (AxioObserver.Z1; ZEISS) with a monochrome digital camera (AxioCam MRm; ZEISS) focusing on the terminal end of lesions on the RPE layer (usually at P16), and individual images were merged to create one retinal image using the automated merge function (mosaiX; ZEISS) in the software (AxioVision 4.6.3.0; ZEISS). ImageJ (National Institutes of Health) was used for quantification of subretinal neovascularization lesion number and areas in Vldlr−/− retinas with designed plugins adapted from the method used to measure retinal neovascularization (SWIFT_ MACTEL) in the oxygen-induced retinopathy model (Stahl et al., 2009; Joyal et al., 2016). In brief, SWIFT_MACTEL isolates the red channel from a lectin-stained retinal whole mount, divides the image into four quadrants, and removes background fluorescence to allow for the neovascularization structures to stand out clearly against the background fluorescence of normal vessels. Using a slide bar to either increase or decrease a particular quadrant’s fluorescence threshold, the SWIFT_MACTEL user designates a threshold that marks neovascularization structures but not normal vessels in each quadrant. After setting the appropriate threshold, artifacts like cellular debris or hyperfluorescent retinal edges can be manually removed and excluded from quantification. Then, SWIFT_MACTEL analyzes all pixels in the image that lie above the chosen intensity threshold and that are part of an object that has a minimum size of 100 pixels. By setting this cut off in object size, small artifacts like vessel branch points are automatically removed. After measuring all four quadrants, SWIFT_MACTEL creates a composite from all four neovascularization quadrants and calculates the total neovascularization pixel number. The n number is the number of eyes quantified. Lesion numbers and areas were quantified with researchers masked to the identity of samples.
Confocal imaging and 3D reconstruction
At P16, mice were anesthetized, and the eyes were enucleated and fixed in 4% paraformaldehyde followed by dissection and staining of the retinas with fluoresceinated isolectin IB4 (Invitrogen) to visualize subretinal neovascularization in whole-mounted retinas. 3D reconstructed images were taken with confocal microscopy (TCS SP2 AOBS; Leica Biosystems), and z stacks were 3D reconstructed using Volocity software (PerkinElmer).
FFA
Mice were anesthetized and injected intraperitoneally with fluorescein AK-FLUOR (Akorn) at 5 µg/g body weight. Fluorescent fundus images with dilated pupils were taken with a retinal-imaging microscope (Micron IV; Phoenix Research Laboratories) at 5 and 8 min after fluorescein injection.
Expression array
Illumina mouse gene microarray analysis of P14 WT and Vldlr−/− retinas was performed in biological triplicate (Mouse-WG6 expression BeadChip; Illumina). The chip contains 45,000 probe sets representing 34,000 genes. Microarray studies, from cDNA synthesis to raw data normalization, were performed by the Molecular Genetics Core Facility at Boston Children’s Hospital. In brief, total RNA (1 µg each) was reverse transcribed, followed by a single in vitro transcription amplification to incorporate biotin-labeled nucleotide, and subsequent hybridization and staining with strepatavidin-Cy3 was performed according to the manufacturer’s instructions. The chip was scanned with a BeadArray Reader (Illumina) to measure the signal intensity of the labeled target. Raw data were analyzed with microarray software (Bead Studio Gene Expression; version 3.4.0; Illumina) for quality control, background analysis, and normalization with the rank-invariant algorithm. Normalized data were further analyzed for comparative molecular and cellular pathway regulation using Ingenuity Pathway Analysis (P = 0.05 and Δ of 0.19; QIAGEN; Calvano et al., 2005). The microarray data are available in the NCBI Gene Expression Omnibus database under accession no. GSE78831.
Retina layer sectioning
Retina layer sectioning of fresh prepared retinas was performed according to the following steps (Fig. S2). In brief, a fresh retina was dissected, and eight evenly spaced cuts were made radially to flatten the retina before placement on a parafilm-wrapped slide. A drop of optimal cutting temperature (OCT) medium was placed on top of the retina, and then the parafilm-wrapped slide with the retina was flipped upside down, gently placed on the flat surface of a pretrimmed frozen block of OCT, and held for 1–2 min to adhere to the OCT block. The slide and parafilm were gently removed, and the flattened retina was transferred onto frozen OCT medium. OCT medium was applied to cover the flattened retina, and the frozen block with the retina was placed in a cryostat. Each horizontal retinal section was cut at 20-µm thickness, and each of ∼12 sections per retina was collected into a separate RNase-free tube for RNA extraction.
Preparation of AAV2-hRK–sh_c-fos vector and AAV2
Four independent shRNAs against mouse c-fos were designed using a published algorithm (Park et al., 2008). The sequences of the mouse c-fos shRNAs are as follows. sh_c-fos no. 1: 5′-ACCTGGTGCTGGATTGTATCTA-3′; sh_c-fos no. 2: 5′-GGACCTTACCTGTTCGTGAAAC-3′; sh_c-fos no. 3: 5′-GGTAGTTAGTAGAGCATGTGAG-3′; sh_Vegfa no. 1: 5′-AACCTCACCAAAGCCAGCACAT-3′; sh_Vegfa no. 2: 5′-AGGACCTTGTGTGATCAGACCA-3′; sh_Vldlr no. 1: 5′-GGAAAGTTCAAGTGCAGAAGCG-3′; sh_Vldlr no. 2: 5′-GGAATGCCATATCAACGAATGC-3′; sh_Vldlr no. 3: 5′-GGGATCTGCAGTCAAATTTGTA-3′; and scramble shRNA control: 5′-GATTTAAGACAAGCGTATAACA-3′. The shRNAs were cloned into a CAGmiR30-GFP plasmid to test the knockdown efficiency in pup retinas and photoreceptor 661W cells. T. Li (National Eye Institute, Bethesda, MD) and C. Cepko (Harvard Medical School, Boston, MA) provided AAV constructs containing the hRK promoter. Recombinant AAV2 vectors were produced as previously described (Grieger et al., 2006; Vandenberghe et al., 2010). In brief, AAV vector, rep/cap packaging plasmid, and adenoviral helper plasmid were mixed with polyethylenimine (Sigma-Aldrich) and transfected into HEK293T cells (catalog no. HCL4517; Thermo Fisher Scientific). 72 h after transfection, cells were harvested, and the cell pellet was resuspended in virus buffer, followed by three cycles of freeze thaw and homogenization (Dounce). Cell debris was pelleted at 5,000 g for 20 min, and the supernatant was run on an iodixanol gradient. Recovered AAV vectors were washed three times with PBS using Amicon 100K columns (EMD Millipore). Real-time PCR was used to determine genome titers of the recombinant AAV. This protocol also was used to prepare a control (AAV2-shControl). Viruses were diluted to various concentrations to test infection, and a concentration of ∼2 × 1012 genome copies/ml was used for the experiments.
Subretinal injection
Subretinal injection into P1 neonate eyes was performed as previously described (Matsuda and Cepko, 2004; Wang et al., 2014) under a dissection microscope. P1 Vldlr−/− or WT pups were anesthetized on ice for several minutes. The eyelid was prepared with Betadine, followed by water and then 70% ethanol using cotton swabs. A blade was used to gently cut open the eyelid. The injection needle was inserted into the eyeball through the incision until slight resistance was felt. Approximately 0.5 µl of solution containing siRNA/siControl or AAV2/control (1012–1013 genome copies/ml) was introduced into the subretinal space using a pulled angled glass pipette controlled by a FemtoJet microinjector (Eppendorf). si_c-fos was a pool of three sequences of siRNA (cat. 4390771; Ambion), containing three siRNA sequences (siRNA nos. s66197, s66198, and s66199). The left eyes were uninjected for within-animal controls. Curved forceps were used to slowly close the eyelid. Mice were placed on a circulating water blanket for warmth. The retinas were collected at P12 for PCR assay and P16 for whole mount analysis.
Immunohistochemistry
Immunohistochemistry in retinas was performed as described previously (Chen et al., 2011a; Sun et al., 2015a,b). In brief, eyes were isolated from Vldlr−/− and littermate WT mice and fixed in 4% paraformaldehyde for 1 h. Dissected retinas or frozen sections were placed in cold methanol for 20 min, blocked, and permeabilized in PBS containing 5% bovine serum albumin and 0.1% Triton X-100 for 1 h. The following antibodies were used: isolectin IB4 (121413; Invitrogen), CX3CR1 (bs-1728R; Bioss), IBA1 (019-19741; Wako Pure Chemical Industries), DAPI (D3571; Invitrogen), and SOCS3 (2923; Cell Signaling Technology). For morphological studies, mouse eyes were enucleated and fixed in 4% paraformaldehyde in PBS, cryoprotected in 30% sucrose in PBS, embedded in OCT compound (Tissue-Tek), and snap frozen. 16 µm–thick sections were taken on a cryostat (Leica Biosystems) and stained with hematoxylin and eosin (Sigma-Aldrich) followed by standard protocol.
RNA isolation and quantitative RT-PCR
Total RNA was extracted from mouse retinas using an RNeasy kit (QIAGEN) and reverse transcribed with SuperScript III reverse transcription (Thermo Fisher Scientific) to generate cDNA. Quantitative PCR was performed using a 7300 system (Applied Biosystems) with SYBR FAST qPCR kits (Kapa Biosystems) using Rhodopsin, Pecam, Vldlr, CD68, CD11β, CD45, c-fos, Il6, Il1β, Tnf, mmp10, Socs3, uPA, c-jun, Vegf120, Vegf164, and Vegf188 primers. Primer sequences for RT-PCR were as follows. c-fos: 5′-AACAGATCCGAGCAGCTTCTA-3′ (forward) and 5′-TTTTGAGCTTCAACCGGCATC-3′ (reverse); c-jun: 5′-GTCCCCTGTCTGATTTGTAGGAA-3′ (forward) and 5′-CCACACCATCTTCTGGTGTACAGT-3′ (reverse); Il1b: 5′-GCCCATCCTCTGTGACTCATG-3′ (forward) and 5′-GGAGCCTGTAGTGCAGCTGTCT-3′ (reverse); Il6: 5′-TGGAGTCACAGAAGGAGTGGCTAAG-3′ (forward) and 5′-TCTGACCACAGTGAGGAATGTCCAC-3′ (reverse); Pecam1: 5′-GAGCCCAATCACGTTTCAGTTT-3′ (forward) and 5′-TCCTTCCTGCTTCTTGCTAGCT-3′ (reverse); Rhodopsin: 5′-TCATGGTCTTCGGAGGATTCAC-3′ (forward) and 5′-TCACCTCCAAGTGTGGCAAAG-3′ (reverse); Socs3: 5′-GTTGAGCGTCAAGACCCAGT-3′ (forward) and 5′-GGGTGGCAAAGAAAAGGAG-3′ (reverse); Tnf: 5′-TCCAGTAGAATCCGCTCTCCT (forward) and 5′-GCCACAAGCAGGAATGAGAAG-3′ (reverse); Tnfaip3: 5′-ACCATGCACCGATACACGC-3′ (forward) and 5′-AGCCACGAGCTTCCTGACT-3′ (reverse); uPA: 5′-GAAGTCCTCCCTCCTTTAAATGTG-3′ (forward) and 5′-TGGGAGTTGAATGAAGCAGTGT-3′ (reverse); Vegf120: 5′-AACGATGAAGCCCTGGAGTG-3′ (forward) and 5′-TGAGAGGTCTGGTTCCCGA-3′ (reverse); Vegf164: 5′-AACGATGAAGCCCTGGAGTG-3′ (forward) and 5′-GACAAACAAATGCTTTCTCCG-3′ (reverse); Vegf188: 5′-AACGATGAAGCCCTGGAGTG-3′ (forward) and 5′-AACAAGGCTCACAGTGAACG-3′ (reverse); Vegfa: 5′-GGAGATCCTTCGAGGAGCACTT-3′ (forward) and 5′-GGCGATTTAGCAGCAGATATAAGAA-3′ (reverse); and Vldlr: 5′-TCTCTTGCTCTTAGTGATGG-3′ (forward) and 5′-CTTACAACTGATATTGCTGGG-3′ (reverse).
Western blotting
A standard Western blot protocol was used with minor modifications. In brief, radioimmunoprecipitation assay buffer (89900; Thermo Fisher Scientific) was used to lyse cells. Proteinase inhibitor cocktail (P8340; Sigma-Aldrich) was added. Proteins were separated by electrophoresis using 4–12% NuPAGE Novex bis-Tris gels (NP0321BOX; Invitrogen). Mouse β-actin (A1978; Sigma-Aldrich) antibody was used for control.
Preparation of lenti-Socs3 vector and virus
Mouse Socs3 cDNA was inserted into plentiCMV/TO (775-1) with CMV promoter using a Gateway LR clonase kit (Invitrogen) according to the manufacturer’s instruction, and sequences were confirmed by sequencing at the Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center Molecular Genetics Core Facility. Socs3-carrying lentiviruses were produced as follows. In brief, Lenti-Socs3 or lenti-control, REV, VSVG, and pMDL (gas-pol) packaging plasmids were mixed with polyethylenimine and transfected into HEK293T cells (cat. no. HCL4517; Thermo Fisher Scientific). 72 h after transfection, the cell medium was harvested, and cellular debris was removed by centrifugation at 1,500 rpm before filtration over a 0.22-µm low-protein binding filter. Then, the filtered medium was centrifuged using a SW28 rotor (37 ml) for 2 h at 19,500 rpm (50,000 g) at 4°C. The supernatant was removed, and 100 ml PBS was added to the pellet and transferred to a new tube after 5–10 min. The virus was aliquotted and stored at −80°C.
Electroretinography (ERG)
Retinal function was assessed by ERG as previously described (Zhang et al., 2013) in 3-mo untreated WT mice and littermate Vldlr−/− mice treated with AAV-hRK-shRNA targeting c-fos or Vegfa or AAV-hRK–sh_control. In brief, dark-adapted, anesthetized (ketamine/xylazine) mouse pupils were dilated (Cyclomydril; Alcon), and their corneas were anesthetized (proparacaine). A Burian–Allen bipolar electrode designed for the mouse eye (Hansen Laboratories) was placed on the cornea, and the ground electrode was placed on a foot. The stimuli consisted of a series of green LED flashes of doubling intensity from ∼0.0064–2.05 cd.s.m−2 and then white xenon arc flashes from ∼8.2−1,050 cd.s.m−2. The equivalent light for the green and white stimuli was determined from the shift of the stimulus/response curves. ERG stimuli were delivered using a Colordome Ganzfeld stimulator (Diagnosys LLC). The saturating sensitivity of the rod photoresponse was estimated by fitting a model of the biochemical processes involved in the activation of phototransduction to the ERG a-waves (Lamb and Pugh, 1992; Pugh and Lamb, 1993; Hood and Birch, 1994).
Photoreceptor (661W) cell culture
Cone photoreceptor cells (al-Ubaidi et al., 1992; Tan et al., 2004) were cultured as monolayers at 37°C and 5% CO2 in a humidified atmosphere in DMEM with FBS 10% supplemented with 20 µg/500 ml hydrocortisone (H-2270; Sigma-Aldrich), 20 µg/500 ml progesterone (P-8783; Sigma-Aldrich), 0.016 g/500 ml putrescine (P-7505; Sigma-Aldrich), and 20 µl/500 ml β-mercaptoethanol (M-6250; Sigma-Aldrich). No mycoplasma contamination of the cells was detected. An equal number of 661W cells (3 × 105) was plated in each well of 6-well dishes. Cells were treated with AAV2-hRK-sh_control or AAV2-hRK–sh_Vldlr for overnight. On day 2, the cell medium was changed and treated with AAV2-hRK-sh_control or AAV2-hRK–sh_c-fos for overnight. On day 3, the medium was changed with fresh culture medium. After 48 h, photoreceptors were collected to determine gene expression by real time PCR.
Statistics
Results are presented as mean ± SEM and were compared using two-tailed unpaired Student’s t test. Statistical analyses were performed with Prism (v5.0; GraphPad Software). A p-value <0.05 was considered to be statistically significant.
Online supplemental material
Fig. S1 shows there was no macrophage recruitment in P12 Vldlr−/− retinas. Fig. S2 shows a diagram of retinal layer sectioning. Fig. S3 shows knocking down c-fos reduced neovascularization in Vldlr−/− retinas. Fig. S4 shows the mRNA expression of c-Jun and uPA in WT and Vldlr−/− mice and the mRNA expression of c-fos in dark-adapted Vldlr−/− retinas. Fig. S5 shows the body weight of mice treated with a high dose of SR11302 or control from P5 to P15. Table S1 is included as an Excel file and shows upstream regulator prediction of the microarray data.
Supplementary Material
Acknowledgments
We thank Zhuo Shao, Lucy P. Evans, Katherine T. Tian, Nicholas J. Saba, and Peyton C. Morss for their excellent technical assistance and thank Drs. Tiansen Li and Connie Cepko for kindly providing reagents.
This work was supported by the National Institutes of Health/National Eye Institute (grants EY024864, EY017017, and P01 HD18655), Lowy Medical Research Institute (project 84134), and European Commission Seventh Framework Programme (project 305485 for L.E.H. Smith) and by the National Institutes of Health/National Eye Institute (grant R01 EY024963) for J. Chen and the American Heart Association (Scientist Development Grant 15SDG25590001) for Z. Lin.
The authors declare no competing financial interests.
Author contributions: This study was designed by Y. Sun and L.E.H. Smith. Y. Sun, Z. Lin, C-H. Liu, Y. Gong, T.W. Fredrick, S.S. Meng, S.B. Burnim, R. Liegl, Z. Wang, and J.D. Akula collected data, which were analyzed and interpreted by Y. Sun, Z. Lin, J.D. Akula, W.T. Pu, and L.E.H. Smith. The manuscript was drafted by Y. Sun and revised by L.E.H. Smith, Y. Sun, J. Chen, Z. Lin, C-H. Liu, Y. Gong, T.W. Fredrick, S.S. Meng, and J.D. Akula.
Footnotes
Abbreviations used:
- 3D
- three-dimensional
- AAV
- adeno-associated virus
- AMD
- age-related macular degeneration
- AP-1
- activator protein 1
- ERG
- electroretinography
- FFA
- fundus fluorescein angiography
- hRK
- human rhodopsin kinase
- OCT
- optimal cutting temperature
- Pecam
- platelet endothelial cell adhesion molecule
- RPE
- retinal pigment epithelium
- SOCS3
- suppressor of cytokine signaling 3
- TNFAIP3
- TNFα-induced protein 3
- uPa
- urokinase-type plasminogen activator
- VEGF
- vascular endothelial growth factor
- Vldlr−/−
- very low-density lipoprotein receptor deficient
References
- Aikawa Y., Morimoto K., Yamamoto T., Chaki H., Hashiramoto A., Narita H., Hirono S., and Shiozawa S.. 2008. Treatment of arthritis with a selective inhibitor of c-Fos/activator protein-1. Nat. Biotechnol. 26:817–823. 10.1038/nbt1412 [DOI] [PubMed] [Google Scholar]
- al-Ubaidi M.R., Font R.L., Quiambao A.B., Keener M.J., Liou G.I., Overbeek P.A., and Baehr W.. 1992. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J. Cell Biol. 119:1681–1687. 10.1083/jcb.119.6.1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati J., Atkinson J.P., and Gelfand B.D.. 2013. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 13:438–451. 10.1038/nri3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A. III, Li Y.Y., Heher E.C., and Kimble C.R.. 1992. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J. Neurosci. 12:840–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., and Karin M.. 1991. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1072:129–157. [DOI] [PubMed] [Google Scholar]
- Apte R.S., Richter J., Herndon J., and Ferguson T.A.. 2006. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 3:e310 10.1371/journal.pmed.0030310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S.A., Krishna Kumar K., and Baird P.N.. 2014. The role of proteases and inflammatory molecules in triggering neovascular age-related macular degeneration: basic science to clinical relevance. Transl. Res. 164:179–192. 10.1016/j.trsl.2014.04.005 [DOI] [PubMed] [Google Scholar]
- Calvano S.E., Xiao W., Richards D.R., Felciano R.M., Baker H.V., Cho R.J., Chen R.O., Brownstein B.H., Cobb J.P., Tschoeke S.K., et al. Inflamm and Host Response to Injury Large Scale Collab. Res. Program . 2005. A network-based analysis of systemic inflammation in humans. Nature. 437:1032–1037. 10.1038/nature03985 [DOI] [PubMed] [Google Scholar]
- Catar R., Witowski J., Wagner P., Annett Schramm I., Kawka E., Philippe A., Dragun D., and Jörres A.. 2013. The proto-oncogene c-Fos transcriptionally regulates VEGF production during peritoneal inflammation. Kidney Int. 84:1119–1128. 10.1038/ki.2013.217 [DOI] [PubMed] [Google Scholar]
- Chen J., Stahl A., Krah N.M., Seaward M.R., Dennison R.J., Sapieha P., Hua J., Hatton C.J., Juan A.M., Aderman C.M., et al. . 2011a Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation. 124:1871–1881. 10.1161/CIRCULATIONAHA.111.040337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Forrester J.V., and Xu H.. 2011b Dysregulation in retinal para-inflammation and age-related retinal degeneration in CCL2 or CCR2 deficient mice. PLoS One. 6:e22818 10.1371/journal.pone.0022818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Hu Y., Moiseyev G., Zhou K.K., Chen D., and Ma J.X.. 2009. Photoreceptor degeneration and retinal inflammation induced by very low-density lipoprotein receptor deficiency. Microvasc. Res. 78:119–127. 10.1016/j.mvr.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinenov Y., and Kerppola T.K.. 2001. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene. 20:2438–2452. 10.1038/sj.onc.1204385 [DOI] [PubMed] [Google Scholar]
- Combadière C., Feumi C., Raoul W., Keller N., Rodéro M., Pézard A., Lavalette S., Houssier M., Jonet L., Picard E., et al. . 2007. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Invest. 117:2920–2928. 10.1172/JCI31692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., and Franza B.R. Jr. 1988. Fos and Jun: the AP-1 connection. Cell. 55:395–397. 10.1016/0092-8674(88)90024-4 [DOI] [PubMed] [Google Scholar]
- da Silva C.G., Studer P., Skroch M., Mahiou J., Minussi D.C., Peterson C.R., Wilson S.W., Patel V.I., Ma A., Csizmadia E., and Ferran C.. 2013. A20 promotes liver regeneration by decreasing SOCS3 expression to enhance IL-6/STAT3 proliferative signals. Hepatology. 57:2014–2025. 10.1002/hep.26197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell M.I., Aguilar E., Jacobson R., Yanes O., Gariano R., Heckenlively J., Banin E., Ramirez G.A., Gasmi M., Bird A., et al. . 2009. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. J. Clin. Invest. 119:611–623. 10.1172/JCI35977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler I., Petri T., and Schleuning W.D.. 1994. Induction of c-fos gene expression by urokinase-type plasminogen activator in human ovarian cancer cells. FEBS Lett. 343:103–106. 10.1016/0014-5793(94)80298-X [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann D.G., Suner I.J., Hernandez E.P., Monroy D., Csaky K.G., and Cousins S.W.. 2003. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 44:3586–3592. 10.1167/iovs.03-0038 [DOI] [PubMed] [Google Scholar]
- Fanjul A., Dawson M.I., Hobbs P.D., Jong L., Cameron J.F., Harlev E., Graupner G., Lu X.P., and Pfahl M.. 1994. A new class of retinoids with selective inhibition of AP-1 inhibits proliferation. Nature. 372:107–111. 10.1038/372107a0 [DOI] [PubMed] [Google Scholar]
- Grieger J.C., Choi V.W., and Samulski R.J.. 2006. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 1:1412–1428. 10.1038/nprot.2006.207 [DOI] [PubMed] [Google Scholar]
- Hafezi F., Steinbach J.P., Marti A., Munz K., Wang Z.Q., Wagner E.F., Aguzzi A., and Remé C.E.. 1997. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat. Med. 3:346–349. 10.1038/nm0397-346 [DOI] [PubMed] [Google Scholar]
- Halazonetis T.D., Georgopoulos K., Greenberg M.E., and Leder P.. 1988. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 55:917–924. 10.1016/0092-8674(88)90147-X [DOI] [PubMed] [Google Scholar]
- Heckenlively J.R., Hawes N.L., Friedlander M., Nusinowitz S., Hurd R., Davisson M., and Chang B.. 2003. Mouse model of subretinal neovascularization with choroidal anastomosis. Retina. 23:518–522. 10.1097/00006982-200308000-00012 [DOI] [PubMed] [Google Scholar]
- Hoffman G.E., Smith M.S., and Verbalis J.G.. 1993. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front. Neuroendocrinol. 14:173–213. 10.1006/frne.1993.1006 [DOI] [PubMed] [Google Scholar]
- Hood D.C., and Birch D.G.. 1994. Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest. Ophthalmol. Vis. Sci. 35:2948–2961. [PubMed] [Google Scholar]
- Joyal J.S., Sun Y., Gantner M.L., Shao Z., Evans L.P., Saba N., Fredrick T., Burnim S., Kim J.S., Patel G., et al. . 2016. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med. 22:439–445. 10.1038/nm.4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khani S.C., Pawlyk B.S., Bulgakov O.V., Kasperek E., Young J.E., Adamian M., Sun X., Smith A.J., Ali R.R., and Li T.. 2007. AAV-mediated expression targeting of rod and cone photoreceptors with a human rhodopsin kinase promoter. Invest. Ophthalmol. Vis. Sci. 48:3954–3961. 10.1167/iovs.07-0257 [DOI] [PubMed] [Google Scholar]
- Klein R., Klein B.E., Moss S.E., Wong T.Y., Hubbard L., Cruickshanks K.J., and Palta M.. 2004. The relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy: XIX: the Wisconsin epidemiologic study of diabetic retinopathy. Arch. Ophthalmol. 122:76–83. 10.1001/archopht.122.1.76 [DOI] [PubMed] [Google Scholar]
- Kueng-Hitz N., Grimm C., Lansel N., Hafezi F., He L., Fox D.A., Remé C.E., Niemeyer G., and Wenzel A.. 2000. The retina of c-fos−/− mice: electrophysiologic, morphologic and biochemical aspects. Invest. Ophthalmol. Vis. Sci. 41:909–916. [PubMed] [Google Scholar]
- Lamb T.D., and Pugh E.N. Jr. 1992. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J. Physiol. 449:719–758. 10.1113/jphysiol.1992.sp019111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.N., Tuckerman J., Nechushtan H., Schutz G., Razin E., and Angel P.. 2004. c-Fos as a regulator of degranulation and cytokine production in FcεRI-activated mast cells. J. Immunol. 173:2571–2577. 10.4049/jimmunol.173.4.2571 [DOI] [PubMed] [Google Scholar]
- Li C., Huang Z., Kingsley R., Zhou X., Li F., Parke D.W. II, and Cao W.. 2007. Biochemical alterations in the retinas of very low-density lipoprotein receptor knockout mice: an animal model of retinal angiomatous proliferation. Arch. Ophthalmol. 125:795–803. 10.1001/archopht.125.6.795 [DOI] [PubMed] [Google Scholar]
- Luo L., Uehara H., Zhang X., Das S.K., Olsen T., Holt D., Simonis J.M., Jackman K., Singh N., Miya T.R., et al. . 2013. Photoreceptor avascular privilege is shielded by soluble VEGF receptor-1. eLife. 2:e00324 10.7554/eLife.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margetts P.J., Kolb M., Yu L., Hoff C.M., Holmes C.J., Anthony D.C., and Gauldie J.. 2002. Inflammatory cytokines, angiogenesis, and fibrosis in the rat peritoneum. Am. J. Pathol. 160:2285–2294. 10.1016/S0002-9440(10)61176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masli S., and Vega J.L.. 2011. Ocular immune privilege sites. Methods Mol. Biol. 677:449–458. 10.1007/978-1-60761-869-0_28 [DOI] [PubMed] [Google Scholar]
- Matsuda T., and Cepko C.L.. 2004. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 101:16–22. 10.1073/pnas.2235688100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay S., Bromhaar M.M., de Jongste J.C., Hoogsteden H.C., Saxena P.R., and Sharma H.S.. 2001. Pro-inflammatory cytokines induce c-fos expression followed by IL-6 release in human airway smooth muscle cells. Mediators Inflamm. 10:135–142. 10.1080/09629350124155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. 2008. Origin and physiological roles of inflammation. Nature. 454:428–435. 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- Nir I., and Agarwal N.. 1993. Diurnal expression of c-fos in the mouse retina. Brain Res. Mol. Brain Res. 19:47–54. 10.1016/0169-328X(93)90147-H [DOI] [PubMed] [Google Scholar]
- Ohki K., Yoshida K., Harada T., Takamura M., Matsuda H., and Imaki J.. 1996. c-fos gene expression in postnatal rat retinas with light/dark cycle. Vision Res. 36:1883–1886. 10.1016/0042-6989(95)00284-7 [DOI] [PubMed] [Google Scholar]
- Park Y.K., Park S.M., Choi Y.C., Lee D., Won M., and Kim Y.J.. 2008. AsiDesigner: exon-based siRNA design server considering alternative splicing. Nucleic Acids Res. 36:W97–W103. 10.1093/nar/gkn280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M.S. 2001. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler. Thromb. Vasc. Biol. 21:1104–1117. 10.1161/hq0701.093685 [DOI] [PubMed] [Google Scholar]
- Perez V.L., and Caspi R.R.. 2015. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 36:354–363. 10.1016/j.it.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E.N. Jr., and Lamb T.D.. 1993. Amplification and kinetics of the activation steps in phototransduction. Biochim. Biophys. Acta. 1141:111–149. 10.1016/0005-2728(93)90038-H [DOI] [PubMed] [Google Scholar]
- Rakic J.M., Lambert V., Munaut C., Bajou K., Peyrollier K., Alvarez-Gonzalez M.L., Carmeliet P., Foidart J.M., and Noël A.. 2003. Mice without uPA, tPA, or plasminogen genes are resistant to experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 44:1732–1739. 10.1167/iovs.02-0809 [DOI] [PubMed] [Google Scholar]
- Ray N., Kuwahara M., Takada Y., Maruyama K., Kawaguchi T., Tsubone H., Ishikawa H., and Matsuo K.. 2006. c-Fos suppresses systemic inflammatory response to endotoxin. Int. Immunol. 18:671–677. 10.1093/intimm/dxl004 [DOI] [PubMed] [Google Scholar]
- Roh M.I., Kim H.S., Song J.H., Lim J.B., Koh H.J., and Kwon O.W.. 2009. Concentration of cytokines in the aqueous humor of patients with naive, recurrent and regressed CNV associated with amd after bevacizumab treatment. Retina. 29:523–529. 10.1097/IAE.0b013e318195cb15 [DOI] [PubMed] [Google Scholar]
- Roychoudhury J., Herndon J.M., Yin J., Apte R.S., and Ferguson T.A.. 2010. Targeting immune privilege to prevent pathogenic neovascularization. Invest. Ophthalmol. Vis. Sci. 51:3560–3566. 10.1167/iovs.09-3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai E., Anand A., Ambati B.K., van Rooijen N., and Ambati J.. 2003. Macrophage depletion inhibits experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 44:3578–3585. 10.1167/iovs.03-0097 [DOI] [PubMed] [Google Scholar]
- Shiozawa S., and Tsumiyama K.. 2009. Pathogenesis of rheumatoid arthritis and c-Fos/AP-1. Cell Cycle. 8:1539–1543. 10.4161/cc.8.10.8411 [DOI] [PubMed] [Google Scholar]
- Stahl A., Connor K.M., Sapieha P., Willett K.L., Krah N.M., Dennison R.J., Chen J., Guerin K.I., and Smith L.E.. 2009. Computer-aided quantification of retinal neovascularization. Angiogenesis. 12:297–301. 10.1007/s10456-009-9155-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein J.W. 1995. Immunological non-responsiveness and acquisition of tolerance in relation to immune privilege in the eye. Eye (Lond.). 9:236–240. 10.1038/eye.1995.46 [DOI] [PubMed] [Google Scholar]
- Streilein J.W., Ohta K., Mo J.S., and Taylor A.W.. 2002. Ocular immune privilege and the impact of intraocular inflammation. DNA Cell Biol. 21:453–459. 10.1089/10445490260099746 [DOI] [PubMed] [Google Scholar]
- Sun Y., Ju M., Lin Z., Fredrick T.W., Evans L.P., Tian K.T., Saba N.J., Morss P.C., Pu W.T., Chen J., et al. . 2015a SOCS3 in retinal neurons and glial cells suppresses VEGF signaling to prevent pathological neovascular growth. Sci. Signal. 8:ra94 10.1126/scisignal.aaa8695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Liu C.H., SanGiovanni J.P., Evans L.P., Tian K.T., Zhang B., Stahl A., Pu W.T., Kamenecka T.M., Solt L.A., and Chen J.. 2015b Nuclear receptor RORα regulates pathologic retinal angiogenesis by modulating SOCS3-dependent inflammation. Proc. Natl. Acad. Sci. USA. 112:10401–10406. 10.1073/pnas.1504387112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H.J., Han J.I., Lee J.W., Uhm K.B., and Heo K.. 2012. TCCR/WSX-1 is a novel angiogenic factor in age-related macular degeneration. Mol. Vis. 18:234–240. [PMC free article] [PubMed] [Google Scholar]
- Tan E., Ding X.Q., Saadi A., Agarwal N., Naash M.I., and Al-Ubaidi M.R.. 2004. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest. Ophthalmol. Vis. Sci. 45:764–768. 10.1167/iovs.03-1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui Y., Westenskow P.D., Kurihara T., Aguilar E., Sakimoto S., Paris L.P., Wittgrove C., Feitelberg D., Friedlander M.S., Moreno S.K., et al. . 2015. Neurovascular crosstalk between interneurons and capillaries is required for vision. J. Clin. Invest. 125:2335–2346. 10.1172/JCI80297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L.H., Xiao R., Lock M., Lin J., Korn M., and Wilson J.M.. 2010. Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum. Gene Ther. 21:1251–1257. 10.1089/hum.2010.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez F.N., Prucca C.G., Etienne O., D’Astolfo D.S., Silvestre D.C., Boussin F.D., and Caputto B.L.. 2015. Brain development is impaired in c-fos−/− mice. Oncotarget. 6:16883–16901. 10.18632/oncotarget.4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E.F., and Eferl R.. 2005. Fos/AP-1 proteins in bone and the immune system. Immunol. Rev. 208:126–140. 10.1111/j.0105-2896.2005.00332.x [DOI] [PubMed] [Google Scholar]
- Wang S., Sengel C., Emerson M.M., and Cepko C.L.. 2014. A gene regulatory network controls the binary fate decision of rod and bipolar cells in the vertebrate retina. Dev. Cell. 30:513–527. 10.1016/j.devcel.2014.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D.J., Thomas K.S., and Gonias S.L.. 2001. Plasminogen activator inhibitor 1 functions as a urokinase response modifier at the level of cell signaling and thereby promotes MCF-7 cell growth. J. Cell Biol. 152:741–752. 10.1083/jcb.152.4.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D., Le X., Zheng L., Wang L., Frey J.A., Gao A.C., Peng Z., Huang S., Xiong H.Q., Abbruzzese J.L., and Xie K.. 2003. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 22:319–329. 10.1038/sj.onc.1206122 [DOI] [PubMed] [Google Scholar]
- Wei X., Wang G., Li W., Hu X., Huang Q., Xu K., Lou W., Wu J., Liang C., Lou Q., et al. . 2014. Activation of the JAK-STAT3 pathway is associated with the growth of colorectal carcinoma cells. Oncol. Rep. 31:335–341. 10.3892/or.2013.2858 [DOI] [PubMed] [Google Scholar]
- Xu H., Chen M., Manivannan A., Lois N., and Forrester J.V.. 2008. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 7:58–68. 10.1111/j.1474-9726.2007.00351.x [DOI] [PubMed] [Google Scholar]
- Yoshida K., Kawamura K., and Imaki J.. 1993. Differential expression of c-fos mRNA in rat retinal cells: regulation by light/dark cycle. Neuron. 10:1049–1054. 10.1016/0896-6273(93)90053-T [DOI] [PubMed] [Google Scholar]
- Zhang N., Favazza T.L., Baglieri A.M., Benador I.Y., Noonan E.R., Fulton A.B., Hansen R.M., Iuvone P.M., and Akula J.D.. 2013. The rat with oxygen-induced retinopathy is myopic with low retinal dopamine. Invest. Ophthalmol. Vis. Sci. 54:8275–8284. 10.1167/iovs.13-12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.