Abstract
Repetitive oscillations in cytoplasmic Ca2+ due to periodic Ca2+ release from the endoplasmic reticulum (ER) drive mammalian embryo development following fertilization. Influx of extracellular Ca2+ to support the refilling of ER stores is required for sustained Ca2+ oscillations, but the mechanisms underlying this Ca2+ influx are controversial. Although store-operated Ca2+ entry (SOCE) is an appealing candidate mechanism, several groups have arrived at contradictory conclusions regarding the importance of SOCE in oocytes and eggs. To definitively address this question, Ca2+ influx was assessed in oocytes and eggs lacking the major components of SOCE, the ER Ca2+ sensor STIM proteins, and the plasma membrane Ca2+ channel ORAI1. We generated oocyte-specific conditional knockout (cKO) mice for Stim1 and Stim2, and also generated Stim1/2 double cKO mice. Females lacking one or both STIM proteins were fertile and their ovulated eggs displayed normal patterns of Ca2+ oscillations following fertilization. In addition, no impairment was observed in ER Ca2+ stores or Ca2+ influx following store depletion. Similar studies were performed on eggs from mice globally lacking ORAI1; no abnormalities were observed. Furthermore, spontaneous Ca2+ influx was normal in oocytes from Stim1/2 cKO and ORAI1-null mice. Finally, we tested if TRPM7-like channels could support spontaneous Ca2+ influx, and found that it was largely prevented by NS8593, a TRPM7-specific inhibitor. Fertilization-induced Ca2+ oscillations were also impaired by NS8593. Combined, these data robustly show that SOCE is not required to support appropriate Ca2+ signaling in mouse oocytes and eggs, and that TRPM7-like channels may contribute to Ca2+ influx that was previously attributed to SOCE.
Keywords: oocyte, calcium, store-operated calcium entry, fertilization, egg activation
Graphical Abstract
1. INTRODUCTION
A large increase in the level of cytoplasmic Ca2+ is the key trigger for activation of development at fertilization in all animal species. In preparation for fertilization, mammalian germinal vesicle (GV)-intact, prophase I-arrested oocytes take up Ca2+ from the extracellular space and suppress Ca2+ leak from the endoplasmic reticulum (ER), resulting in a significant increase in ER Ca2+ stores as they resume meiosis and become arrested at metaphase II (MII eggs) [1–3]. In addition, they increase expression of the inositol 1,4,5-trisphosphate (IP3) receptor and alter ER localization such that it becomes enriched in the cortical region below the plasma membrane [4–6]. Together, these and other maturation-associated changes result in MII eggs that efficiently release Ca2+ in response to the fertilizing sperm. The initial Ca2+ release event at fertilization is induced by the activity of the sperm-specific phospholipase C zeta (PLCζ), which enters the egg’s cytoplasm following sperm-egg plasma membrane fusion [7, 8]. PLCζ catalyzes hydrolysis of phosphatidylinositol 4,5-bisphosphate to generate diacylglycerol and IP3; IP3 then activates the IP3 receptor to cause Ca2+ release from ER stores.
In mammals, periodic Ca2+ release from ER stores, known as Ca2+ oscillations, continue for several hours after fertilization. The persistence of these oscillations appears to be a critical driver of the success of embryo development [9]. In the absence of extracellular Ca2+, reductions in intraluminal ER Ca2+ levels that occur with each release event lead to cessation of the oscillations over time [10–12]. Hence, it is apparent that Ca2+ influx from the extracellular space, followed by accumulation into the ER, must occur during both oocyte maturation and following fertilization to support effective initial Ca2+ release, continued Ca2+ oscillations and optimal embryo development.
A common mechanism to replenish depleted ER Ca2+ stores via influx from extracellular sources is known as store-operated Ca2+ entry (SOCE) [13, 14]. The main molecular mediators of SOCE are STIM proteins, which sense ER Ca2+ levels, and ORAI proteins, which serve as plasma membrane Ca2+ channels. In mammals there are two STIM proteins, STIM1 and STIM2, and three ORAI proteins, ORAI1, ORAI2, and ORAI3. In response to a reduction in ER Ca2+, STIM proteins oligomerize and undergo redistribution within the ER to regions closely apposed to the plasma membrane (PM), known as ER-PM junctions. There, the STIM proteins interact directly with ORAI channels, inducing Ca2+ influx. This Ca2+ is subsequently pumped back into the ER by the action of sarco-endoplasmic reticulum Ca2+ ATPases (SERCA).
SOCE would be a logical mechanism to mediate Ca2+ entry following fertilization. Indeed, SOCE has been reported in both oocytes and MII eggs, though it appears more robust in oocytes [1, 15]. However, there is conflicting data in the literature regarding whether or not SOCE is an important physiological mediator of Ca2+ influx following fertilization. We and others demonstrated previously that chemical inhibitors of SOCE did not prevent Ca2+ influx following fertilization in the mouse [16, 17]. In contrast, several studies in both mouse and porcine oocytes suggest, instead, that SOCE is essential for this process [18–21].
To definitively determine whether SOCE is required to support Ca2+ influx at fertilization in the mouse, we generated and analyzed conditional knockout mouse lines in which the oocytes lacked the major SOCE components. Here we show that there is no requirement for either STIM1, STIM2, or ORAI1 to support Ca2+ influx during oocyte maturation or to support persistent Ca2+ oscillations following fertilization. Experiments to determine what alternative channels mediate Ca2+ influx in mouse oocytes uncovered compelling evidence that spontaneous Ca2+ influx in oocytes and post-fertilization Ca2+ influx, which were previously attributed to SOCE, are instead mediated by either TRPM7, the melastatin-related transient receptor potential channel, or a TRPM7-like channel.
2. MATERIALS AND METHODS
2.1. Mice
Stim1flox/flox and Stim2flox/flox mice [22] were crossed with a Zp3-cre transgenic line (Jackson Laboratory, Bar Harbor, ME, USA, Stock No. 003651) [23] to generate oocyte-specific conditional knockout (cKO) mice for Stim1 and Stim2. These mice were further intercrossed to obtain Stim1-Stim2 double cKO mice. All mice for these crosses were maintained on a predominantly C57Bl/6J genetic background, and the Zp3-cre transgene was bred through the male to avoid germline transmission of excised alleles. Orai1 full knockout mice [24] outbred with Institute of Cancer Research (ICR) strain mice [25] were also used. CF-1 strain female mice were obtained from Charles River (Wilmington, MA, USA). B6SJLF1 males for sperm collection and C57Bl/6J male breeders were from Jackson Laboratory. For analysis of fertility, littermate Stim1/2 cKO and Zp3-cre-negative Stim1flox/flox;Stim2flox/flox control females were paired with adult C57Bl/6J males at 7–12 weeks of age and were housed together as breeding pairs for 6 months. Pups were counted on the day of birth and sexed at weaning 21 days later. All mice were maintained under a standard 12 h light/dark cycle and provided food and water ad libitum. All studies and procedures were performed under an animal study protocol approved by the National Institutes of Health Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals.
2.2. Gamete Collection
For collection of GV stage oocytes, female mice 6–15 weeks of age were injected with 5 IU pregnant mare serum gonadotropin (PMSG, Calbiochem) and sacrificed by CO2 asphyxiation 42–48 h later. Ovaries were placed in Leibovitz L-15 medium (L-15, Life Technologies, Grand Island, NY, USA) containing 1% calf serum (Atlanta Biologicals, Norcross, GA, USA) and 10 μM milrinone, and antral follicles were punctured using 27 G needles. Cumulus cells were removed by gentle pipetting through a narrow bore capillary. For collection of metaphase II (MII)-arrested eggs, PMSG-primed mice were injected with 5 IU human chorionic gonadotropin (hCG) 46–48 h after PMSG injection and sacrificed 13–14 h later. Cumulus-oocyte masses were removed from oviducts and cumulus cells were removed by brief treatment with 0.03% hyaluronidase in L-15. Prior to imaging or collection, oocytes and eggs were held in Minimal Essential Medium Alpha (aMEM, Life Technologies) containing 5% calf serum or potassium simplex optimized medium (KSOM) with amino acids (EMD Millipore, Billerica, MA, USA) at 37° C/5% CO2 in drops of pre-equilibrated medium under light mineral oil.
2.3. Immunoblotting
Immunoblot analysis was carried out essentially as previously described [26]. LF PVDF membranes (BioRad, Atlanta, GA, USA) were blocked in 5% non-fat dry milk in Tris-buffered saline with 0.1% Tween-20 (TBS-T). Primary antibodies directed against STIM1 and STIM2 were obtained from Cell Signaling (Danvers, MA, USA; AntibodyRegistry: AB_2271287 and AB_2198021) and were diluted 1:250 in blocking solution. Peroxidase-conjugated anti-rabbit secondary antibody from Jackson ImmunoResearch (West Grove, PA, USA; #711-035-152) was used at 1:10,000 dilution, and SuperSignal West Femto Chemiluminescent Substrate (ThermoFisher Scientific, Grand Island, NY, USA) was used for detection.
2.4. Real-time PCR
Quantitative real time PCR was performed essentially as previously described [27]. Samples were spiked with EGFP cRNA as an internal reference, as described in [16], and RNA was isolated from three groups of 30 GV oocytes each from Stim2flox/flox (control) and Stim2flox/flox;Zp3-cre+ (cKO) mice using an Arcturus PicoPure RNA Isolation Kit (Life Technologies). Reverse transcription and amplification were performed as previously described [26]. Primer sequences for detection of Stim2 were as follows: [Exon3–4 Forward: GGATTTGTGGAAACAGTGGAA; Reverse: GGGAGTGTTGTTCCCTTCAC], [Exon4–5 Forward: TGTGAAGGGAACAACACTCC; Reverse: GTCAGAGGCCCAAACAGAAC].
2.5. In vitro fertilization (IVF) and Ca2+ Imaging
Imaging of Ca2+ flux following fertilization was performed essentially as previously described [27]. Briefly, zona pellucida-free fura-2 AM (Life Technologies)-loaded eggs were adhered to Cell Tak (EMD Millipore)-treated glass-bottom dishes, and 510 nm fluorescence was imaged with excitation at 340 nm and 380 nm as 5 × 104 capacitated sperm/mL was added to the imaging drop. Change in F340/F380 ratio was measured 8 times per minute and is used to reflect changes in intracellular Ca2+ ([Ca2+]i). For all experiments using genetic mouse models, control and KO eggs were placed side-by-side in the same media drop and were imaged simultaneously. For experiments using inhibitor treatment, eggs were pre-incubated in 20 uM NS8593 for 15–30 min prior to sperm addition; parallel control experiments were performed in the same manner immediately before or after inhibitor studies using gametes from the same mice and vehicle (DMSO) treatment. Analysis of Ca2+ oscillation parameters was performed as previously described [27].
2.6. ER Ca2+ Store, Store-operated Ca2+ Entry, and Spontaneous Ca2+ Influx Assays
Measurement of Ca2+ release following addition of thapsigargin, reflecting ER Ca2+ store content, was performed essentially as described [27]. Briefly, zona pellucida-intact oocytes or eggs were adhered to glass-bottom dishes in 1.85 mL Ca2+-, Mg2+-, and bovine serum albumin (BSA)-free CZB medium [28] containing 2 mM EGTA. Solutions of thapsigargin, CaCl2, or MgCl2 diluted in 150 μL medium were added drop-wise to dishes during imaging. Cells were imaged for 3–5 min in Ca2+-free medium before adding thapsigargin at a final concentration of 10 μM; 35–40 min later, 5 mM CaCl2 was added and imaging continued for at least 15 min. For spontaneous Ca2+ influx assays, Ca2+ influx was measured for GV oocytes without prior depletion of ER Ca2+ using thapsigargin [1]. GV oocytes were placed in Ca2+-, Mg2+-, and BSA-free CZB and imaged as CaCl2 was added to a final concentration of 2 mM or 5 mM. Ten μM milrinone was included in medium used for GV oocytes to maintain meiotic arrest. Area under the curve for 10 minutes following each addition was calculated for individual oocytes or eggs by computing trapezoidal area for each curve following subtraction of the average baseline measurement for 2–3 min prior to the response.
2.7. Additional Chemicals and Reagents
If not otherwise specified, all chemicals, reagents, and inhibitors were obtained from Sigma Aldrich (St. Louis, MO, USA).
2.8. Statistical Analysis
GraphPad Prism, version 7.0a was used to perform all statistical tests. Student’s t-test was performed for normally distributed data, as determined by D’Agostino & Pearson omnibus normality test. For datasets lacking normal distribution, the Mann-Whitney U-test was used. Contingency data was analyzed using Fisher’s exact test. Asterisks indicate p < 0.05 and ns indicates non-significant differences in all graphs, and all error bars show SEM.
3. RESULTS
3.1. STIM1 and STIM2 are dispensable for Ca2+ influx in mouse oocytes and eggs
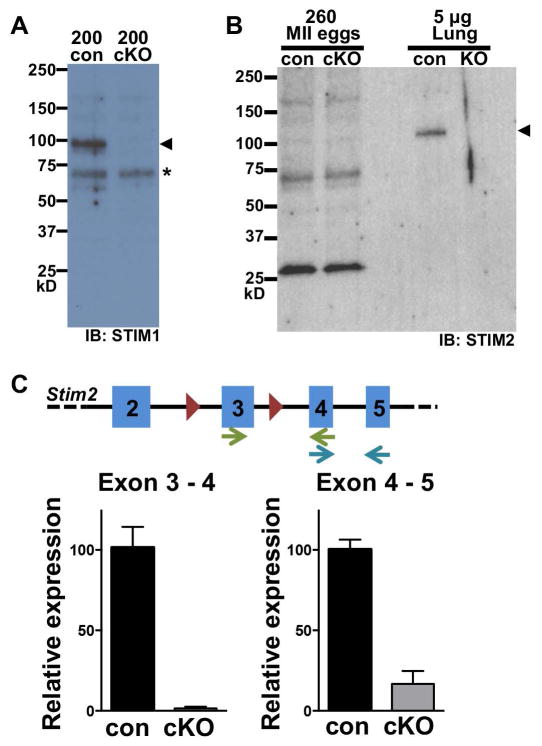
Oocyte-specific conditional knockout (cKO) mice for Stim1 and Stim2 were generated by crossing lines carrying loxP-flanked (flox) alleles with a Zp3-cre transgenic line [22, 23]. Eggs were collected from superovulated females for immunoblot analysis to confirm loss of STIM1 and STIM2 proteins. STIM1 was detected in a lysate of 200 eggs from Stim1flox/flox (control) mice but was absent from eggs of Stim1flox/flox;Zp3-cre+ (cKO) mice, confirming that Zp3-cre causes efficient loss of STIM1 protein in Stim1 cKO eggs (Fig. 1A). Although a specific band for STIM2 was observed at approximately 115 kD in a lysate of control neonatal lung tissue and was absent from Stim2 knockout tissue, we were unable to detect STIM2 protein by immunoblot using 260 eggs (Fig. 1B). However, using real time PCR, we found that Stim2 transcript levels were greatly reduced in Stim2 cKO eggs, with transcripts containing the loxP-flanked exon 3 reduced by 68-fold, becoming nearly undetectable (Fig. 1C). We conclude that efficient loss of both STIM1 and STIM2 was achieved using this method.
Figure 1. STIM1 and STIM2 expression in control and cKO eggs.
(A) Representative immunoblot of STIM1 protein in Stim1 cKO and control (con) eggs. 200 eggs per lane; blot is one of three independent replicates. Arrowhead indicates STIM1 protein band; asterisk indicates non-specific band. (B) Representative immunoblot of STIM2 protein in control and Stim2 cKO eggs; 260 eggs/lane. Lung protein extract (5 μg) from either control or Stim2−/− postnatal day 1 pups was used for positive and negative controls. Arrowhead indicates STIM2 band. (C) Real time qPCR of Stim2 mRNA in control and Stim2 cKO eggs. Schematic shows Stim2 exons (blue), loxP sites (red triangles), and primer pairs (arrows). Graphs show expression relative to that in control eggs for the indicated primer pair.
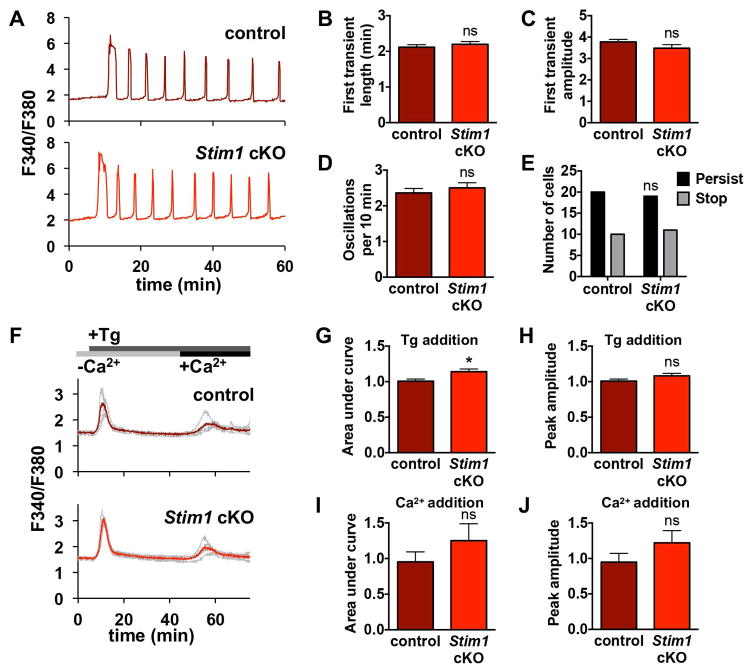
To determine whether STIM1 is necessary for normal Ca2+ signaling following fertilization, we performed live-cell ratiometric Ca2+ imaging on control eggs from Stim1flox/flox females alongside cKO eggs lacking STIM1 from Stim1flox/flox;Zp3-cre+ females during in vitro fertilization. Representative traces for 60 minutes of imaging are shown, along with analysis of data from 5 replicate experiments (Fig. 2A–E). None of the parameters measured, including first transient length, maximum amplitude, oscillation frequency, and persistence of oscillations to 60 minutes, differed between control and Stim1 cKO eggs. Of note, the oscillation frequency we observe is slightly faster than that reported in some previous studies (E.g., [16, 29]), but is consistent with our previous data using the same method [27]. We attribute this variance primarily to differences in media composition, use of intracytoplasmic sperm injection vs. IVF, and genetic background of mice used in these studies. Based on our analysis of Ca2+ oscillation parameters, STIM1 is not required for appropriate Ca2+ signaling following fertilization.
Figure 2. Ca2+ oscillation patterns following IVF and ER Ca2+ stores in eggs lacking STIM1.
Ratiometric Ca2+ imaging of control and Stim1 cKO eggs was performed during IVF. (A) Representative traces are shown. (B–E) Analysis of Ca2+ oscillation patterns, n=30 eggs per genotype over 5 replicate experiments. (B) Length of first transient. (C) Amplitude of first transient. (D) Oscillation frequency. (E) Persistence of oscillations to the end of the 60 min imaging period. (F–J) Measurements of ER Ca2+ stores and Ca2+ influx 35–40 min after thapsigargin (Tg)-induced ER store depletion in Stim1 cKO and control eggs. (F) Representative traces (gray) and mean curve (red) are shown for one experiment. Timing of Tg and Ca2+ addition indicated in gray and black bars. (G–J) Analysis of Ca2+ stores and influx, n=24–33 eggs per genotype over 4 replicate experiments. (G,H) Graphs of ER Ca2+ stores indicated by area under the curve (G) and peak amplitude (H). * p < 0.05, unpaired t test. (I,J) Graphs of Ca2+ entry following store depletion indicated by area under the curve (I) and peak amplitude (J).
We next investigated whether loss of STIM1 impacts Ca2+ stores or Ca2+ influx following store depletion. Ca2+ imaging was performed on control and Stim1 cKO eggs placed side-by-side in Ca2+-free medium, and thapsigargin was added to inhibit SERCA-mediated refilling of Ca2+ stores and allow leak of Ca2+ from the ER (Fig. 2F). Area under the curve and peak amplitude were measured as indicators of ER Ca2+ content. Peak amplitude was similar and area under the curve was slightly higher for Stim1 cKO eggs compared to control (Fig. 2G,H), indicating that absence of STIM1 did not impair accumulation of ER Ca2+ in eggs. Thapsigargin treatment was continued for at least 30 min to deplete ER stores, then 5 mM CaCl2 was added and the increase in [Ca2+]i was measured for 10 minutes as an indication of Ca2+ influx following store depletion. Neither peak amplitude nor area under the curve was significantly different for Stim1 cKO eggs compared to controls (Fig. 2I,J). Overall, these results indicate that STIM1 is dispensable for normal Ca2+ oscillations and homeostasis in mouse eggs.
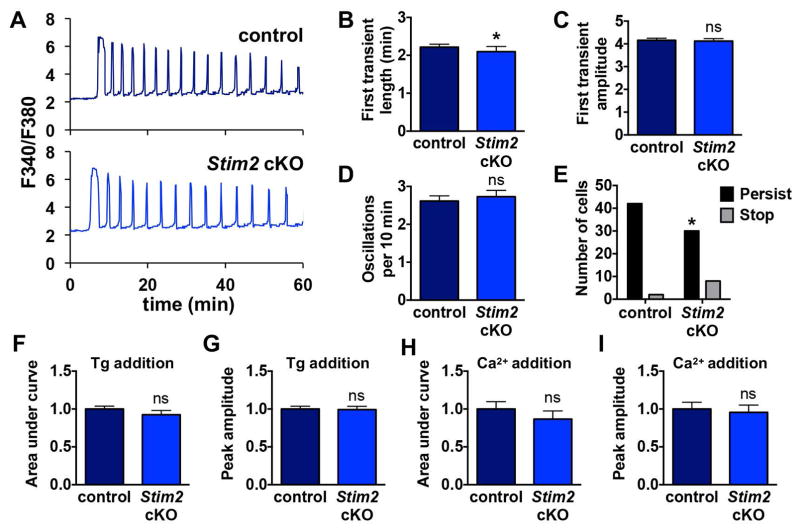
Whereas STIM1 is activated by substantial release of Ca2+ from ER stores, STIM2 can be activated by alterations in ER Ca2+ closer to the resting level [30, 31]. We therefore also sought to determine whether STIM2 was necessary for Ca2+ signaling in mouse eggs. As for the Stim1 cKO model, we performed Ca2+ imaging during in vitro fertilization for control and Stim2 cKO eggs. Representative traces and summary analyses are shown in Fig. 3A–E. Ca2+ oscillations of Stim2 cKO eggs appeared largely normal. Oscillation frequency and amplitude were unaltered, and only very subtle decreases were observed in length of the first Ca2+ transient and in the proportion of eggs with oscillations persisting throughout the 60 min imaging period. There was no significant difference in either ER Ca2+ store content or Ca2+ influx following store depletion (Fig. 3F–I). Based on these data, loss of STIM2 did not impair Ca2+ store accumulation or influx and had at most a very minor impact on fertilization-induced oscillations.
Figure 3. Ca2+ oscillation patterns following IVF and ER Ca2+ stores in eggs lacking STIM2.
Ratiometric Ca2+ imaging of control and Stim2 cKO eggs was performed during IVF. (A) Representative traces are shown. (B–E) Analysis of Ca2+ oscillation patterns, n=36–45 eggs per genotype over 6 replicate experiments. (B) Length of first transient. * p < 0.05, Mann Whitney. (C) Amplitude of first transient. (D) Oscillation frequency. (E) Persistence of oscillations to the end of the 60 min imaging period. * p < 0.05, Fisher’s exact test. (F–I) Measurements of ER Ca2+ stores and Ca2+ influx 35–40 min after thapsigargin (Tg)-induced ER store depletion in Stim2 cKO and control eggs, n=23 eggs per genotype over 4 replicate experiments. (F,G) Graphs of ER Ca2+ stores indicated by area under the curve (F) and peak amplitude (G). (H,I) Graphs of Ca2+ entry following store depletion indicated by area under the curve (I) and peak amplitude (J).
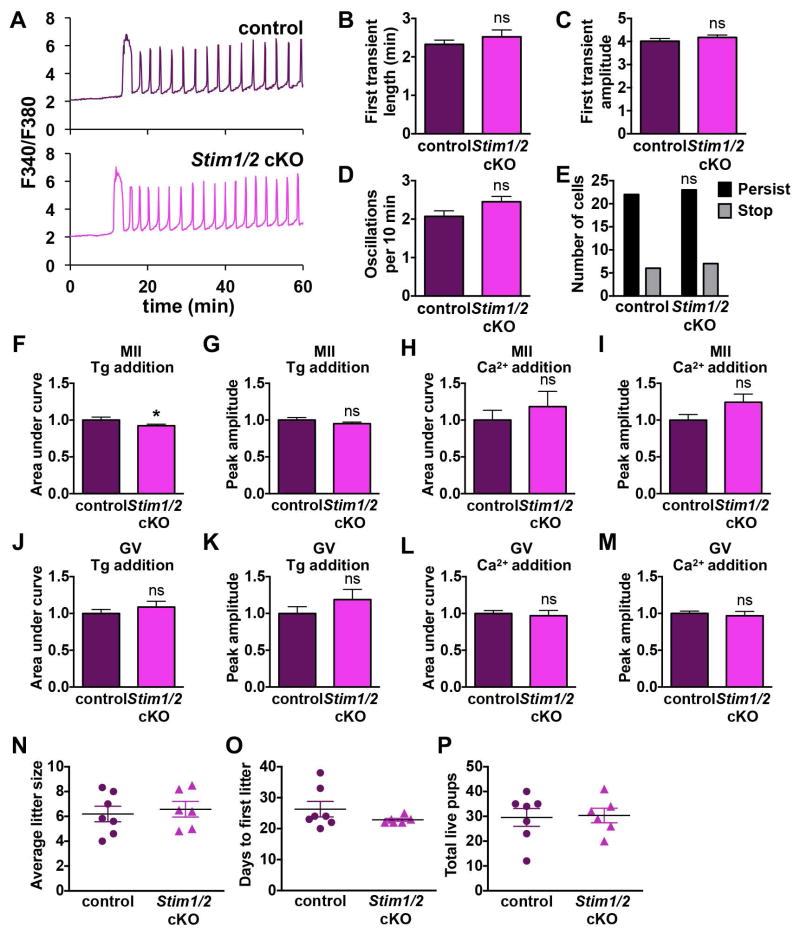
Although loss of STIM1 and STIM2 individually had very little impact on oocyte physiology and Ca2+ signaling, we also wanted to address the possibility of compensation between STIM1 and STIM2. Stim1 and Stim2 single cKO lines were intercrossed to produce a Stim1-Stim2 oocyte-specific double conditional knockout line (Stim1/2 cKO). Fertilization-induced Ca2+ oscillations of Stim1/2 cKO eggs were no different from controls (Fig. 4A–E) and ER Ca2+ stores measured by thapsigargin-mediated Ca2+ release were essentially identical to controls (Fig. 4F,G). Likewise, Ca2+ influx following store depletion was not significantly altered in Stim1/2 cKO eggs (Fig. 4H,I). It was previously shown that Ca2+ influx following store depletion is greater in GV oocytes than MII eggs [1]; therefore, we also assayed ER Ca2+ stores and Ca2+ influx in Stim1/2 cKO GV oocytes. Following thapsigargin addition, similar levels of Ca2+ release were observed in control and Stim1/2 cKO GV oocytes (Fig. 4J,K), and Ca2+ influx following store depletion was also unaltered (Fig. 4L,M). Taken together, these data indicate that in mouse oocytes and eggs neither STIM1 nor STIM2 is required for Ca2+ influx following ER store depletion.
Figure 4. Analysis of MII eggs and GV oocytes lacking both STIM1 and STIM2.
Ratiometric Ca2+ imaging of control and Stim1/2 cKO eggs was performed during IVF. (A) Representative traces are shown. (B–E) Analysis of Ca2+ oscillation patterns, n=28–31 eggs per genotype over 4 replicate experiments. (B) Length of first transient. (C) Amplitude of first transient. (D) Oscillation frequency. (E) Persistence of oscillations to the end of the 60 min imaging period. (F–I) Measurements of ER Ca2+ stores and Ca2+ influx 35–40 min after thapsigargin (Tg)-induced ER store depletion in Stim1/2 cKO and control eggs, n=23 eggs per genotype over 4 replicate experiments. (F,G) Graphs of ER Ca2+ stores indicated by area under the curve (F) and peak amplitude (G). * p < 0.05, Mann Whitney. (H,I) Graphs of Ca2+ entry following store depletion indicated by area under the curve (H) and peak amplitude (I). (J–M) Measurements of ER Ca2+ stores and Ca2+ influx 35–40 min after thapsigargin (Tg)-induced ER store depletion in Stim1/2 cKO and control GV oocytes, n=23–24 oocytes per genotype over 4 replicate experiments. (J,K) Graphs of ER Ca2+ stores indicated by area under the curve (J) and peak amplitude (K). (L,M) Graphs of Ca2+ entry following store depletion indicated by area under the curve (L) and peak amplitude (M). (N–P) Fertility of female Stim1/2 cKO mice. (N) Average litter size. (O) Days to first litter. (P) Total number of live pups.
To determine whether oocyte-specific loss of STIM1 and STIM2 had any impact on fertility, we conducted a formal breeding study for Stim1/2 double cKO females. Stim1/2 cKO females were fertile, and fertility parameters including average litter size, days to first litter, and total live pups per breeding pair were indistinguishable from those of littermate controls (Fig. 4N–P). One Stim1/2 cKO female did not produce pups during the study and was omitted from analysis because male infertility could not be excluded. We also housed several Stim1 and Stim2 single cKO females with males to confirm their fertility; all produced pups (4/4 females for each genotype), but a detailed breeding study was not carried out because this was done with the Stim1/2 cKO. Altogether we conclude that STIM1 and STIM2 are dispensable for normal fertility in female mice.
3.2 ORAI1 is dispensable for Ca2+ signaling in oocytes and eggs
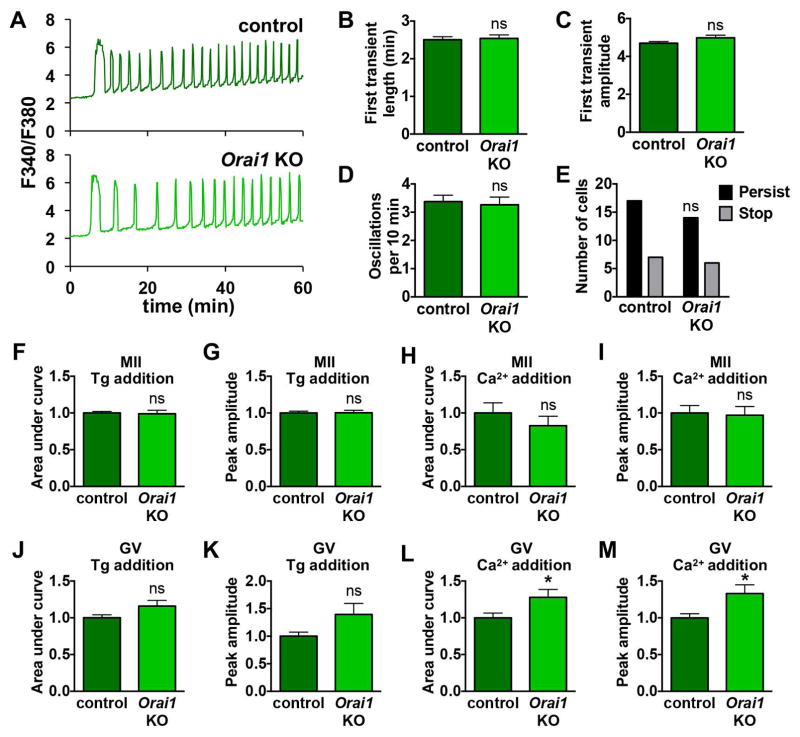
Female Orai1 knockout mice are fertile [32]; however, Ca2+ signaling following fertilization has not been studied in eggs lacking ORAI1. As for Stim1 and Stim2 cKO mice, we performed Ca2+ imaging during in vitro fertilization and found no significant difference in the pattern of Ca2+ oscillations between Orai1 KO and wild-type control eggs (Fig. 5A–E). Similarly, there was no difference in ER Ca2+ stores (Fig. 5F,G) or Ca2+ influx following store depletion (Fig. 5H,I) for Orai1 KO MII eggs compared to controls. Likewise, ER Ca2+ stores in Orai1 KO GV oocytes were not significantly different from controls (Fig. 5J,K). Ca2+ influx following store depletion was increased in Orai1 KO eggs (Fig. 5L,M), in contrast to the expected decrease in influx that would be anticipated if ORAI1 channels were critical for mediating this influx. Thus, ORAI1 channels are also dispensable for normal Ca2+ signaling in oocytes and eggs.
Figure 5. Analysis of MII eggs and GV oocytes lacking ORAI1.
Ratiometric Ca2+ imaging of control and Orai1 KO eggs was performed during IVF. (A) Representative traces are shown. (BE) Analysis of Ca2+ oscillation patterns, n=20–24 eggs per genotype over 4 replicate experiments. (B) Length of first transient. (C) Amplitude of first transient. (D) Oscillation frequency. (E) Persistence of oscillations to the end of the 60 min imaging period. (F–I) Measurements of ER Ca2+ stores and Ca2+ influx 35–40 min after thapsigargin (Tg)-induced ER store depletion in Orai1 KO and control eggs, n=18–25 eggs per genotype over 3–4 replicate experiments. (F,G) Graphs of ER Ca2+ stores indicated by area under the curve (F) and peak amplitude (G). (H,I) Graphs of Ca2+ entry following store depletion indicated by area under the curve (H) and peak amplitude (I). (J–M) Measurements of ER Ca2+ stores and Ca2+ influx 40 min after thapsigargin (Tg)-induced ER store depletion in Orai1 KO and control GV oocytes, n=22–23 oocytes per genotype over 4 replicate experiments. (J,K) Graphs of ER Ca2+ stores indicated by area under the curve (J) and peak amplitude (K). (L,M) Graphs of Ca2+ entry following store depletion indicated by area under the curve (L) and peak amplitude (M). * p < 0.05, unpaired t test.
3.3. TRPM7-like channels, not SOCE components, support spontaneous Ca2+ influx in mouse oocytes
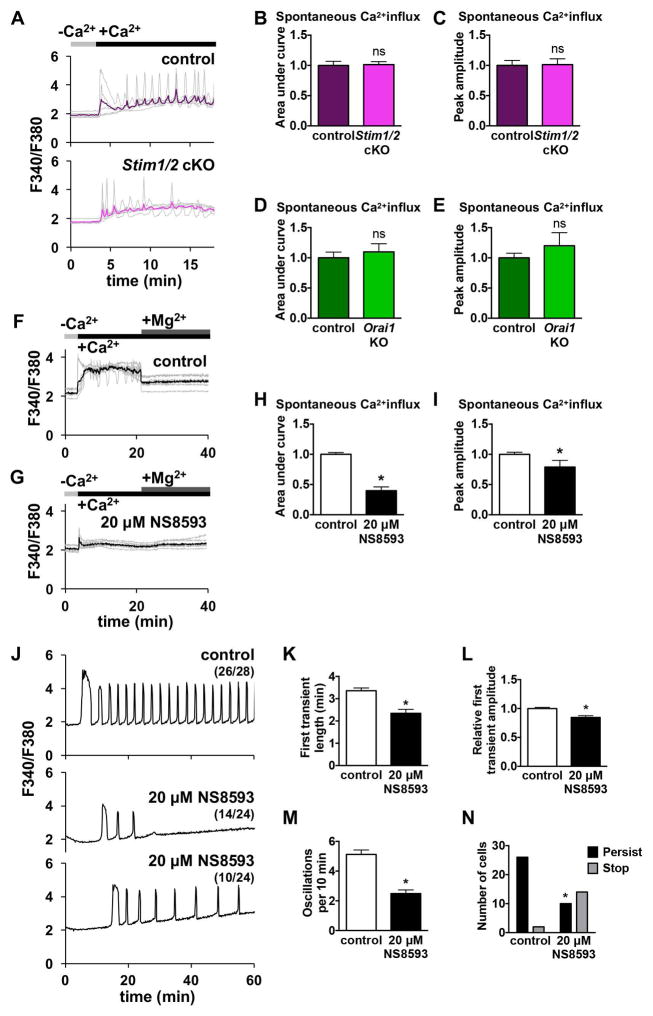
When placed in nominal Ca2+-free medium, GV oocytes respond to addition of 2–5 mM Ca2+ by showing a rise in [Ca2+]i, reflecting measurable spontaneous Ca2+ influx, while MII eggs do not show such a response [1]. To investigate whether SOCE mediators STIM1, STIM2, or ORAI1 contribute to this spontaneous Ca2+ influx in GV oocytes, we placed Stim1/2 cKO and control oocytes in Ca2+-free medium and added 2 mM CaCl2 during Ca2+ imaging. Both control and Stim1/2 cKO oocytes displayed increased [Ca2+]i in response to Ca2+ addition (Fig. 6A), and no significant difference was observed in their response when peak amplitude and area under the curve for 10 min following Ca2+ addition were measured (Fig. 6B,C). Orai1 KO oocytes, as compared to wild-type controls, also had no difference in peak amplitude or area under the curve following Ca2+ addition (Fig. 6D,E). Based on these results, spontaneous Ca2+ influx in GV stage oocytes does not require STIM1, STIM2, or ORAI1.
Figure 6. Spontaneous Ca2+ influx in mouse GV oocytes and post-fertilization Ca2+ influx in mouse eggs are mediated by TRPM7-like channels.
(A–E) Ratiometric Ca2+ imaging of control and Stim1/2 cKO or Orai1 KO GV oocytes treated with 2 mM Ca2+ 3 min after placement into Ca2+-free medium. (A) Representative traces (gray) and mean curve (red) are shown for one experiment. Timing of Ca2+ addition indicated by black bars. (B–E) Analysis of spontaneous Ca2+ influx in control and Stim1/2 cKO or Orai1 KO oocytes, n=18–26 oocytes per genotype over 3–4 replicate experiments. (B) Stim1/2 cKO, area under the curve. (C) Stim1/2 cKO, peak amplitude. (D) Orai1 KO, area under the curve. (E) Orai1 KO, peak amplitude. (F–I) Effect of TRPM7 channel inhibition on spontaneous Ca2+ influx in control GV oocytes. (F,G) Ratiometric Ca2+ imaging of control oocytes treated with either vehicle (DMSO) or 20 μM NS8593 and then tested for spontaneous Ca2+ influx by addition of 5 mM Ca2+. 10 mM Mg2+ was added 18 min following Ca2+ addition. (H,I) Graphs of spontaneous Ca2+ entry, n=39–46 oocytes per group over 4 replicate experiments. (H) Area under the curve. (I) Peak amplitude. * p < 0.05, Mann Whitney. (J–N) Ratiometric Ca2+ imaging of wild-type CF-1 eggs during IVF in medium containing vehicle (DMSO) or 20 μM NS8593. J) Representative traces are shown. (K–N) Analysis of Ca2+ oscillation patterns, n=24–28 eggs per genotype over 3 replicate experiments. (K) Length of first transient. * p < 0.05, Mann Whitney. (L) Amplitude of first transient. * p < 0.05, Mann Whitney. (M) Oscillation frequency. * p < 0.05, unpaired t test. (N) Persistence of oscillations to the end of the 60 min imaging period. * p < 0.05, Fisher’s exact test.
We next addressed the question of what SOCE-independent mechanisms of Ca2+ influx are at work in mouse oocytes. We recently demonstrated that CaV3.2 channels contribute to the increase in ER Ca2+ stores during oocyte maturation and support Ca2+ oscillations following fertilization; however, it is evident that additional channels also support Ca2+ influx in oocytes and eggs [27]. We hypothesized that transient receptor potential (TRP) channels were likely candidates for this role because these cation conducting channels are expressed in all types of cells and can contribute to a wide array of cellular responses (reviewed in [33]). TRPV3 current has been demonstrated in mouse eggs, but this current was not detected in GV oocytes [34]; therefore, TRPV3 is unlikely to mediate spontaneous Ca2+ influx in GV oocytes. Mice lacking all seven TRPC channels have been generated and shown to be viable and fertile [35]. In a limited set of experiments, we also observed normal Ca2+ oscillations in eggs from Trpc hepta-KO mice (not shown), indicating that TRPC channels are unlikely to contribute significantly to Ca2+ influx. Through surveying publicly available microarray data ([36], GEO Accession GDS3295), we found that in mouse oocytes, Trpm7 was highly expressed relative to other Trp channels, and there is electrophysiological evidence for the presence of functional TRPM7-like channels in oocytes [37]. To test whether TRPM7-like channels support spontaneous Ca2+ influx in mouse oocytes, we used two different TRPM7 inhibitors, Mg2+ and NS8593, which was initially characterized as a small conductance Ca2+ activated K+ channel inhibitor [38–40]. GV oocytes from wild-type CF-1 females were placed in Ca2+- and Mg2+-free medium, and 5 mM CaCl2 was added, causing an increase in [Ca2+]i (Fig. 6F). After 15 minutes, 10 mM MgCl2 was added to the medium; this treatment caused a substantial drop in [Ca2+]i (Fig. 6F). In parallel experiments, GV oocytes were placed in Ca2+- and Mg2+-free medium containing 20 μM NS8593 for 15 min prior to CaCl2 addition. In NS8593-treated oocytes, Ca2+ addition caused a substantially lower increase in [Ca2+]i relative to controls, and subsequent Mg2+ addition did not further decrease [Ca2+]i (Fig. 6G). Both area under the curve and peak amplitude following Ca2+ addition were significantly decreased in the presence of NS8593 (Fig. 6H,I). These results indicate that TRPM7-like channels contribute significantly to spontaneous Ca2+ influx in GV oocytes.
3.4. TRPM7-like channels contribute to Ca2+ influx following fertilization in mouse eggs
To test whether TRPM7-like channels also contribute to Ca2+ influx necessary to sustain post-fertilization Ca2+ oscillations, wild-type CF-1 eggs were treated with 20 μM NS8593 and fertilized in vitro (Fig. 6J). Compared to controls, NS8593-treated eggs had impaired Ca2+ oscillations, with significantly shorter first Ca2+ transients, reduced peak amplitude, lower frequency, and reduced persistence (Fig. 6K–N). These results suggest that TRPM7 or TRPM7-like channels also contribute to Ca2+ influx needed to maintain robust Ca2+ oscillations following fertilization.
4. DISCUSSION
A rise in intracellular Ca2+ is a universal activator of embryo development, and Ca2+ influx is required to sustain Ca2+ oscillations following mammalian fertilization [10, 11, 41]; however, the question of how this influx occurs is not fully resolved. We have recently shown that CaV3.2 channels contribute to Ca2+ influx in mouse oocytes and eggs, but additional Ca2+ influx mechanisms must also be functional [27]. Because Ca2+ content of the ER decreases and Ca2+ influx increases with each Ca2+ oscillation [12, 42], SOCE is a plausible candidate mechanism for mediating Ca2+ influx following fertilization. In this study we show that three SOCE mediators expressed in oocytes, STIM1, STIM2, and ORAI1, are dispensable for proper Ca2+ signaling and homeostasis in mouse oocytes and eggs. Our results are consistent with previous data showing that pharmacological inhibition of SOCE or expression of dominant negative SOCE components does not inhibit fertilization-induced oscillations in mouse eggs [16, 17].
Despite evidence to the contrary, there is a persistent view that SOCE is the primary mechanism of oocyte Ca2+influx. The idea that SOCE is critical in oocyte physiology has even appeared in review literature [43–45]. This may not be surprising because SOCE participation in fertilization seems logical, and because roles for SOCE in mouse and pig oocytes have been suggested through experiments using overexpression, knockdown, and inhibitor strategies [18–21]. However, our current data show that critical mediators of SOCE are expendable for fertilization and Ca2+ signaling in mouse eggs. We show that the only two STIM proteins characterized to date, STIM1 and STIM2, are both dispensable in mouse oocytes and eggs. We also show that loss of ORAI1 does not impair Ca2+ signaling in mouse oocytes and eggs. Based on microarray data, Orai1 is by far the most highly expressed transcript of the three Orai genes in mouse oocytes [36]; however, ORAI1 protein is internalized during meiosis so it is unlikely to function as a Ca2+ influx channel in eggs [15]. Results of prior studies supporting the actions of SOCE in mammalian eggs could have arisen in part from off-target effects of siRNA or morpholino oligonucleotides; however, it is likely that important differences between species also exist. For example, porcine oocytes may regulate ER Ca2+ differently than mouse oocytes and could therefore rely more heavily on SOCE. This interpretation is supported by experiments showing that the same SOCE inhibitors that halt Ca2+ oscillations in porcine eggs do not impair oscillations in mouse eggs [16, 17, 21]. Mouse eggs also have so-called “thapsigargin-insensitive” Ca2+ stores, capable of release by IP3 or Ca2+ ionophore but not thapsigargin, whereas these stores are absent in porcine eggs [11, 21].
SOCE supports Ca2+ oscillations in somatic cells [46]; therefore, it seems somewhat unusual that eggs use alternate means to refill ER stores following fertilization. Meiotic cell cycle arrest is unique to oocytes, and Ca2+ is a critical signal that regulates meiosis, prompting the MII-arrested egg to reenter the cell cycle and switch to a mitotic program. Therefore, it is logical that mature eggs must control Ca2+ signaling very tightly and have multiple mechanisms to ensure that meiotic arrest is robust [47]. Perhaps down-regulating SOCE at this time serves as an additional means of insulating the egg from extraneous Ca2+ signals. SOCE is downregulated in Xenopus oocytes during meiosis [48, 49], and although we show it is not reliant on SOCE components, Ca2+ influx following store depletion decreases during oocyte maturation in mice [1, 15]. This finding is also consistent with the down-regulation of SOCE that occurs as somatic cells enter mitotic M phase, which may help ensure that cell stage-specific needs for ion fluxes are met [50, 51].
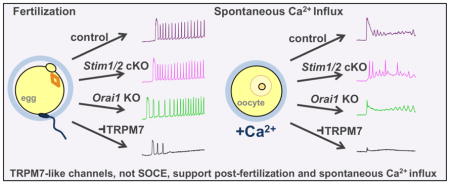
Given that SOCE is dispensable for appropriate Ca2+ signaling in mouse oocytes, the question of what pathways support Ca2+ influx remains. The data reported here and that of others suggest that TRPM7-like channels contribute, at least in part, to Ca2+ influx in oocytes. Functional expression of TRPM7-like channels was recently shown in mouse oocytes [37]. Similar to our observation that TRPM7-like channels support spontaneous Ca2+ influx in GV oocytes, NS8593 reduces oocyte Ca2+ oscillations that occur in response to raising extracellular Ca2+ from 2 mM to 5mM [37]. Spontaneous influx in GV oocytes was previously attributed to SOCE because this response could be inhibited by 2-APB [1], but 2-APB is a notoriously non-specific inhibitor [33]. In addition to inhibiting Ca2+ influx mediated by SOCE [52], 2-APB also acts on a number of other channels and pathways, including acting as a TRPM7 inhibitor at concentrations below 1 mM [39]. Therefore, our hypothesis that spontaneous Ca2+ influx is mediated by TRPM7 is further supported by past experiments showing that 2-APB blocks Ca2+ influx in GV oocytes [1]. Furthermore, we show that inhibition of TRPM7-like channels during IVF impairs Ca2+ oscillations, suggesting that these channels also contribute to Ca2+ influx following fertilization. This conclusion is further supported by recent data demonstrating that NS8593 effectively blocks TRPM7-like current in mouse eggs [37]. TRPM7 is a promising candidate for mediating SOCE-like responses in mouse oocytes and eggs; however, as we have seen with SOCE, knowledge that can be gained using solely pharmacological inhibition is inherently limited. Therefore, a genetic approach will be needed to definitively determine the contribution of TRPM7.
We postulate that TRPM7 contributes to the Ca2+ influx that had previously been attributed to SOCE, but TRPM7 is unlikely to act alone in supporting Ca2+ influx in oocytes and eggs. We previously demonstrated that CaV3.2 contributes to Ca2+ influx to support ER store accrual and robust Ca2+ oscillations, but females lacking CaV3.2 are merely subfertile, indicating that this channel does not function alone either [27]. TRPV3 channels are present in mouse eggs and contribute to Sr2+-mediated parthenogenesis; however, Trpv3−/− females are fertile and their eggs have normal Ca2+ oscillations following fertilization, indicating that these channels do not have a required physiological function in eggs [34]. It will be interesting to begin investigating the effects of knocking out these channels in combination to overcome inherent limitations of studies using pharmacological inhibitors and ultimately determine the mechanisms of Ca2+ influx that support egg activation.
We show that genetic knockout of SOCE components causes no measurable disruption of Ca2+ signaling in mouse oocytes and eggs; however, these cells display several behaviors reminiscent of SOCE, and explanations for these SOCE-like effects are still undetermined. Introduction of extracellular Ca2+ causes measurable Ca2+ influx in eggs that have depleted ER stores, but not in those with intact stores [1, 11, 15]. Membrane permeability to Ca2+ appears to rise following fertilization, and influx spikes occur concomitant with reduction in ER Ca2+ during initial Ca2+ oscillations [11, 42]. Overexpression of SOCE components in oocytes and eggs enhances the magnitude of SOCE-like responses and impacts Ca2+ oscillations and influx, indicating that SOCE components can function in eggs when expressed at supra-physiological levels [1, 15]. Furthermore, while some effects of 2-APB on oocyte and egg responses can be explained by modulation of TRPM7 or TRPV3 channels, it is possible that this inhibitor also impacts SOCE-like responses in other ways in these cells [1, 15, 34, 37]. Thus, it is apparent that the status of ER Ca2+ stores influences Ca2+ influx regulation in mouse oocytes and eggs, but this effect is not mediated by STIM-ORAI interaction. Studies to define the pathways connecting ER Ca2+ stores to channels other than ORAI and TRPC in oocytes and eggs and may lead to discovery of a yet undescribed cellular mechanism linking Ca2+ store regulation and influx in these specialized cells.
Highlights.
SOCE is not required for Ca2+ influx in mouse oocytes and eggs
Eggs lacking STIM1, STIM2, or ORAI1 have normal Ca2+ oscillations after fertilization
Oocyte spontaneous Ca2+ influx does not require STIM1, STIM2, or ORAI1
Trpm7-like channels support Ca2+ influx in mouse oocytes and eggs
Acknowledgments
We thank Drs. Jim Putney and Gary Bird for helpful discussions and critical reading of the manuscript. We thank Masatsugu Oh-Hora for providing the Stim1-floxed mice, Stefan Feske for providing the Stim2-floxed mice, and Lutz Birnbaumer for providing Trpc-hepta knockout mice. We also thank Jean-Pierre Kinet and James Putney for providing access to Orai1 knockout mice on an ICR background. This work was supported by the Intramural Research Program of the NIH, National Institutes of Environmental Health Sciences, 1ZIAES102985.
Footnotes
AUTHOR CONTRIBUTIONS
MLB and CJW designed the experiments. MLB, EPB, and PS performed the experiments. MLB, PS, and YZ analyzed the data. MLB and CJW wrote the paper, and all authors edited the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheon B, Lee HC, Wakai T, Fissore RA. Ca2+ influx and the store-operated Ca2+ entry pathway undergo regulation during mouse oocyte maturation. Molecular Biology of the Cell. 2013;24:1396–1410. doi: 10.1091/mbc.E13-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KT, Carroll J, Whittingham DG. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem. 1995;270:6671–6677. doi: 10.1074/jbc.270.12.6671. [DOI] [PubMed] [Google Scholar]
- 3.Kline D, Kline JT. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol. 1992;149:80–89. doi: 10.1016/0012-1606(92)90265-i. [DOI] [PubMed] [Google Scholar]
- 4.Fissore RA, Longo FJ, Anderson E, Parys JB, Ducibella T. Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol Reprod. 1999;60:49–57. doi: 10.1095/biolreprod60.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Mehlmann LM, Mikoshiba K, Kline D. Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev Biol. 1996;180:489–498. doi: 10.1006/dbio.1996.0322. [DOI] [PubMed] [Google Scholar]
- 6.Mehlmann LM, Terasaki M, Jaffe LA, Kline D. Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev Biol. 1995;170:607–615. doi: 10.1006/dbio.1995.1240. [DOI] [PubMed] [Google Scholar]
- 7.Knott JG, Kurokawa M, Fissore RA, Schultz RM, Williams CJ. Transgenic RNA interference reveals role for mouse sperm phospholipase Czeta in triggering Ca2+ oscillations during fertilization. Biol Reprod. 2005;72:992–996. doi: 10.1095/biolreprod.104.036244. [DOI] [PubMed] [Google Scholar]
- 8.Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 9.Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol. 2006;17:324–332. doi: 10.1016/j.semcdb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Igusa Y, Miyazaki S. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J Physiol. 1983;340:611–632. doi: 10.1113/jphysiol.1983.sp014783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem. 1992;267:17624–17630. [PubMed] [Google Scholar]
- 12.Wakai T, Fissore RA. Ca(2+) homeostasis and regulation of ER Ca(2+) in mammalian oocytes/eggs. Cell Calcium. 2013;53:63–67. doi: 10.1016/j.ceca.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiol Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Lee B, Palermo G, Machaca K. Downregulation of store-operated Ca2+ entry during mammalian meiosis is required for the egg-to-embryo transition. J Cell Sci. 2013;126:1672–1681. doi: 10.1242/jcs.121335. [DOI] [PubMed] [Google Scholar]
- 16.Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4169–4174. doi: 10.1073/pnas.1112333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi T, Kikuchi T, Kidokoro Y, Shirakawa H. Ca(2)(+) influx-dependent refilling of intracellular Ca(2)(+) stores determines the frequency of Ca(2)(+) oscillations in fertilized mouse eggs. Biochem Biophys Res Commun. 2013;430:60–65. doi: 10.1016/j.bbrc.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Fernandez C, Pozo-Guisado E, Ganan-Parra M, Perianes MJ, Alvarez IS, Martin-Romero FJ. Relocalization of STIM1 in mouse oocytes at fertilization: early involvement of store-operated calcium entry. Reproduction. 2009;138:211–221. doi: 10.1530/REP-09-0126. [DOI] [PubMed] [Google Scholar]
- 19.Lee K, Wang C, Machaty Z. STIM1 is required for Ca2+ signaling during mammalian fertilization. Dev Biol. 2012;367:154–162. doi: 10.1016/j.ydbio.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Lee K, Gajdocsi E, Papp AB, Machaty Z. Orai1 mediates store-operated Ca2+ entry during fertilization in mammalian oocytes. Dev Biol. 2012;365:414–423. doi: 10.1016/j.ydbio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Zhang L, Jaeger LA, Machaty Z. Store-Operated Ca2+ Entry Sustains the Fertilization Ca2+ Signal in Pig Eggs. Biol Reprod. 2015;93:25. doi: 10.1095/biolreprod.114.126151. [DOI] [PubMed] [Google Scholar]
- 22.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Vries WN, Binns LT, Fancher KS, Dean J, Moore R, Kemler R, Knowles BB. Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes. Genesis. 2000;26:110–112. [PubMed] [Google Scholar]
- 24.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing J, Petranka JG, Davis FM, Desai PN, Putney JW, Bird GS. Role of Orai1 and store-operated calcium entry in mouse lacrimal gland signalling and function. J Physiol. 2014;592:927–939. doi: 10.1113/jphysiol.2013.267740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jefferson WN, Chevalier DM, Phelps JY, Cantor AM, Padilla-Banks E, Newbold RR, Archer TK, Kinyamu HK, Williams CJ. Persistently altered epigenetic marks in the mouse uterus after neonatal estrogen exposure. Mol Endocrinol. 2013;27:1666–1677. doi: 10.1210/me.2013-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernhardt ML, Zhang Y, Erxleben CF, Padilla-Banks E, McDonough CE, Miao YL, Armstrong DL, Williams CJ. CaV3.2 T-type channels mediate Ca2+ entry during oocyte maturation and following fertilization. J Cell Sci. 2015;128:4442–4452. doi: 10.1242/jcs.180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- 29.Sanusi R, Yu Y, Nomikos M, Lai FA, Swann K. Rescue of failed oocyte activation after ICSI in a mouse model of male factor infertility by recombinant phospholipase Czeta. Mol Hum Reprod. 2015;21:783–791. doi: 10.1093/molehr/gav042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bird GS, Hwang SY, Smyth JT, Fukushima M, Boyles RR, Putney JW., Jr STIM1 is a calcium sensor specialized for digital signaling. Curr Biol. 2009;19:1724–1729. doi: 10.1016/j.cub.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis FM, Janoshazi A, Janardhan KS, Steinckwich N, D’Agostin DM, Petranka JG, Desai PN, Roberts-Thomson SJ, Bird GS, Tucker DK, Fenton SE, Feske S, Monteith GR, Putney JW., Jr Essential role of Orai1 store-operated calcium channels in lactation. Proc Natl Acad Sci U S A. 2015;112:5827–5832. doi: 10.1073/pnas.1502264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu LJ, Sweet TB, Clapham DE International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carvacho I, Lee HC, Fissore RA, Clapham DE. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep. 2013;5:1375–1386. doi: 10.1016/j.celrep.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birnbaumer L. From GTP and G proteins to TRPC channels: a personal account. Journal of molecular medicine (Berlin, Germany) 2015;93:941–953. doi: 10.1007/s00109-015-1328-5. [DOI] [PubMed] [Google Scholar]
- 36.Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol. 2008;316:397–407. doi: 10.1016/j.ydbio.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carvacho I, Ardestani G, Lee HC, McGarvey K, Fissore RA, Lykke-Hartmann K. TRPM7-like channels are functionally expressed in oocytes and modulate post-fertilization embryo development in mouse. Sci Rep. 2016;6:34236. doi: 10.1038/srep34236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strobaek D, Hougaard C, Johansen TH, Sorensen US, Nielsen EO, Nielsen KS, Taylor RD, Pedarzani P, Christophersen P. Inhibitory gating modulation of small conductance Ca2+-activated K+ channels by the synthetic compound (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphtylamine (NS8593) reduces afterhyperpolarizing current in hippocampal CA1 neurons. Mol Pharmacol. 2006;70:1771–1782. doi: 10.1124/mol.106.027110. [DOI] [PubMed] [Google Scholar]
- 39.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chubanov V, Mederos y Schnitzler M, Meissner M, Schafer S, Abstiens K, Hofmann T, Gudermann T. Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br J Pharmacol. 2012;166:1357–1376. doi: 10.1111/j.1476-5381.2012.01855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 42.McGuinness OM, Moreton RB, Johnson MH, Berridge MJ. A direct measurement of increased divalent cation influx in fertilised mouse oocytes. Development. 1996;122:2199–2206. doi: 10.1242/dev.122.7.2199. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Machaty Z. Calcium influx in mammalian eggs. Reproduction. 2013;145:R97–r105. doi: 10.1530/REP-12-0496. [DOI] [PubMed] [Google Scholar]
- 44.Amdani SN, Yeste M, Jones C, Coward K. Sperm Factors and Oocyte Activation: Current Controversies and Considerations. Biol Reprod. 2015;93:50. doi: 10.1095/biolreprod.115.130609. [DOI] [PubMed] [Google Scholar]
- 45.Dupont G, Combettes L. Fine tuning of cytosolic Ca (2+) oscillations. F1000Research. 2016:5. doi: 10.12688/f1000research.8438.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bird GS, Putney JW., Jr Capacitative calcium entry supports calcium oscillations in human embryonic kidney cells. J Physiol. 2005;562:697–706. doi: 10.1113/jphysiol.2004.077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernhardt ML, Lowther KM, Padilla-Banks E, McDonough CE, Lee KN, Evsikov AV, Uliasz TF, Chidiac P, Williams CJ, Mehlmann LM. Regulator of G-protein signaling 2 (RGS2) suppresses premature calcium release in mouse eggs. Development. 2015;142:2633–2640. doi: 10.1242/dev.121707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Machaca K, Haun S. Store-operated calcium entry inactivates at the germinal vesicle breakdown stage of Xenopus meiosis. J Biol Chem. 2000;275:38710–38715. doi: 10.1074/jbc.M007887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Machaca K, Haun S. Induction of maturation-promoting factor during Xenopus oocyte maturation uncouples Ca(2+) store depletion from store-operated Ca(2+) entry. J Cell Biol. 2002;156:75–85. doi: 10.1083/jcb.200110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tani D, Monteilh-Zoller MK, Fleig A, Penner R. Cell cycle-dependent regulation of store-operated I(CRAC) and Mg2+-nucleotide-regulated MagNuM (TRPM7) currents. Cell Calcium. 2007;41:249–260. doi: 10.1016/j.ceca.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Preston SF, Sha’afi RI, Berlin RD. Regulation of Ca2+ influx during mitosis: Ca2+ influx and depletion of intracellular Ca2+ stores are coupled in interphase but not mitosis. Cell regulation. 1991;2:915–925. doi: 10.1091/mbc.2.11.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gregory RB, Rychkov G, Barritt GJ. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem J. 2001;354:285–290. doi: 10.1042/0264-6021:3540285. [DOI] [PMC free article] [PubMed] [Google Scholar]