Abstract
Hyperuricemia is characterized by the high uric acid (UA) level in serum (or plasma) and has been considered to be an important risk factor for gout. In the present study, we have attempted to construct an assay system for UA production in vitro employing cultured AML12 hepatocytes. UA levels in balanced salt solution (BSS) in the presence of UA precursor nucleosides, adenosine, inosine, guanosine and xanthine, at 12.5, 25, and 100 µM were significantly higher than BSS alone and their effects were dose-dependent, while all the UA precursors did not significantly increase intracellular UA levels. Hence, UA levels in BSS were thereafter adopted as an index of UA production. UA production from nucleosides was significantly higher than that from nucleotides (GMP, IMP and AMP). UA production from guanosine and inosine in combination (GI mixture) as well as nucleosides increased time-dependently and almost linearly up to 2 h. Selecting GI mixture, effects of allopurinol, a widely used anti-hyperuricemic agent, and quercetin, a well-known polyphenol in onion and strawberry, on UA production were examined. Both allopurinol and quercetin dose-dependently (0.1, 0.3 and 1 μM for allopurinol and 10, 30, and 100 μM for quercetin) and significantly reduced UA production in the hepatocytes. They also significantly reduced hyperuricemia induced by intraperitoneal injection of UA precursor purine bodies to mice at a single oral dose of 10 (allopurinol) or 200 (quercetin) mg/kg body weight. This assay system for UA production in cultured hepatocytes is considered to be useful to search for novel anti-hyperuricemic compounds in foods and natural resources with possibility to have human health benefits.
Keywords: AML12 hepatocyte, Hyperuricemia, Purine body, Uric acid
Introduction
Hyperuricemia is characterized by the high uric acid (UA) level in serum (or plasma) and has been considered to be an important risk factor for gout (Zhao et al. 2006; Richette and Bardin 2012; Bardin and Richette 2014). In addition to gout, hyperuricemia is suggested to increase the risk of other diseases including hypertension, kidney disease and metabolic syndrome (Choi et al. 2005). The association of chronic intake of purine-rich foods, mainly of animal origin, and gout incident is well established (Choi et al. 2004). The serum levels of UA depend on the balance between synthesis of UA in the liver and excretion of UA from the kidneys (Ishikawa et al. 2013). In the metabolic pathways of purine catabolism and uric acid formation, multiple steps of reactions catalyzed by various enzymes such as adenosine monophosphate (AMP) deaminase, adenosine deaminase, 5′-nucleotidase, purine nucleoside phosphorylase, guanine deaminase and xanthine oxidase (XO), a key enzyme for producing UA at the final stage (Ishikawa et al. 2013). Adenosine, inosine and guanosine are finally converted to xanthine, which is converted to UA by XO. Adenosine is converted by adenosine deaminase to inosine, which is in turn converted to hypoxanthine and then to xanthine by XO (adenosine–inosine–hypoxanthine–xanthine–UA). Guanosine is converted to guanine, which is converted by guanine deaminase to xanthine (guanosine–guanine–xanthine–UA). Thus, the pathway of guanosine conversion to xanthine is simple and different in comparison with those of adenosine and inosine. In contrast, the pathway of adenosine and inosine are common (Ishikawa et al. 2013). So far, to find new medicinal and natural compounds, their direct inhibitory effects on XO activity were widely measured in vitro (Noro et al. 1983; Nguyen et al. 2004; Kondo et al. 2013). The findings obtained in the in vitro assay have greatly contributed towards examining in vivo efficacy of candidate compounds (Mo et al. 2007; Kondo et al. 2013). As multiple steps of reactions catalyzed by various enzymes are involved in purine metabolism, XO assay system has a possibility to overlook compounds that have their targets other than XO. If we could measure UA production from various precursors in cultured hepatocytes, natural and food compounds possessing targets other than XO would be also screened. Recently, mouse-derived hepatocytes are reported to produce sufficient UA in culture (Petrie et al. 2013). In general, assay systems employing cultured cells are advantageous as first screening for effective extracts and compounds, because amounts of test compounds required are very small and applicable to ex vivo examination before animal experiments in some cases as described previously (Miura et al. 1997). They are also useful to know detailed mechanisms of effective compounds (Miura et al. 2004; Minakawa et al. 2012).
In the present study, we have attempted to construct an easy and rapid assay system for UA production employing cultured AML12 hepatocytes. Following the cell culture assay, we have tried to confirm in vivo anti-hyperuricemic effects of known compounds that reduced UA production in the cultured hepatocytes, using mice administered with purine precursors.
Materials and methods
Culture of AML12 cells
AML12 cells were provided by American Type Culture Collection (ATCC® CRL-2254, Manassas, VA, USA). AML12 cells were cultured according to the cell supplier’s instruction with slight modifications in DMEM/F-12 (Life Technologies, Grand Island, NY, USA) supplemented with 10 % fetal bovine serum (FBS, Hyclone, Logan, UT, USA), 5 µg/ml recombinant human insulin (Wako Pure Chemical Industries, Ltd., Osaka, Japan), 5 µg/ml transferrin from human blood (Wako Pure Chemical Industries, Ltd.), 3 ng/ml selenium (Sigma-Aldrich Chemical Co., St. Louis, MO, USA), 40 ng/ml dexamethasone (Wako Pure Chemical Industries, Ltd.), penicillin (100 IU/ml), and streptomycin (100 µg/ml) (Nacalai Tesque, Inc., Kyoto, Japan) (10 % FBS/DMEM/F-12) under atmosphere of 5 % CO2/95 % humidified air at 37 °C. The cells (1.0 × 105 cells/well) were subcultured into 24-place multiwell plates (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) and grown for 72 h in 10 % FBS/DMEM/F-12, and then kept for 24 h in serum-free DMEM/F-12 with other factors.
Effect of various uric acid precursors on uric acid production by AML12 cells
After 24 h culture in serum-free DMEM/F-12, AML12 cells were washed once with calcium- and magnesium-free phosphate buffered saline (PBS(−), Wako Pure Chemical Industries, Ltd.) and incubated for 2 h in 300 µl of balanced salt solution (BSS), containing 188 mM NaCl, 5 mM KCl, 1 mM MgCl2, 0.8 mM CaCl2, 25 mM NaHCO3, 1 mM NaH2PO4, 10 mM HEPES, 5 mM glucose (Petrie et al. 2013), with various concentrations of uric acid (UA) precursors (12.5, 25, 100 µM adenosine, inosine, guanosine, and xanthine). All reagents for BSS were purchased from Wako Pure Chemical Industries, Ltd. All UA precursors were purchased from Sigma-Aldrich Chemical Co. These UA precursors were dissolved in dimethyl sulfoxide (DMSO, Wako Pure Chemical Industries, Ltd.) and added to BSS at the final DMSO concentration of 0.05 %. Wells without UA precursors (0 µM) contained 0.05 % DMSO alone. After 2 h incubation, 200 µl of BSS was collected for determination of UA. After washed once with PBS(−), the cells were scraped out in 300 µl of buffer containing 50 mM Tris (pH 7.5, Sigma-Aldrich Chemical Co.) and 1 mM sodium phosphate (Wako Pure Chemical Industries, Ltd.), sonicated, and centrifuged at 12,000×g and 4 °C for 5 min. UA levels in the BSS and the cell homogenates were determined by the uricase method (Uric acid C-test Wako, Wako Pure Chemical Industries, Ltd.). Protein concentrations in the cell homogenates were determined using a BCA protein assay kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). UA production in BSS and cell homogenates was expressed as nanomoles (nmol) per 2 h per mg cellular protein.
Effect of various purine nucleotides on uric acid production by AML12 cells
Purine nucletides, i.e., adenosine-5′-monophosphate (AMP), inosine-5′-monophosphate (IMP), and guanosine-5′-monophosphate (GMP), were purchased from Tokyo Chemical Industry Co., Tokyo, Japan. These purine nucleotides were dissolved in DMSO and added to BSS at the final DMSO concentration of 0.05 %. After 24 h culture in serum-free DMEM/F-12, AML12 cells were washed once with PBS(−) and incubated for 2 h in 300 µl of BSS with 100 µM AMP, 100 µM xanthine, 100 µM adenosine, and BSS alone (control). Similarly, the cells were incubated in 300 µl of BSS with 100 µM IMP, 100 µM xanthine, 100 µM inosine, and BSS alone. Moreover, the cells were incubated in 300 µl of BSS with 100 µM GMP, 100 µM xanthine, 100 µM guanosine, and BSS alone. On the termination of the 2 h incubation, the BSS was collected for determination of UA production.
Time-course of UA production by AML12 cells after addition of its precursors
After 24 h culture in serum-free DMEM/F-12, AML12 cells were washed once with PBS(−) and incubated in 300 µl of BSS with 100 µM adenosine, 100 µM inosine, 100 µM guanosine, 100 µM xanthine, guanosine + inosine (100 µM each), and BSS alone (control). Following incubation in BSS for 30, 60, 90, and 120 min, the BSS was collected by 35 µl every time for determination of UA production.
Effect of allopurinol and quercetin on UA production by AML 12 cells
After 24 h culture in serum-free DMEM/F-12, AML12 cells were washed once with PBS(−) and incubated in 200 µl of BSS with guanosine + inosine (100 µM each), containing 0, 0.1, 0.3, 1 µM allopurinol (Wako Pure Chemical Industries, Ltd.) at the final DMSO concentration of 0.15 %. Similarly, the cells were incubated in 200 µl of BSS with guanosine + inosine (100 µM each), containing 10, 30, 100 µM quercetin (Wako Pure Chemical Industries, Ltd.) at the final DMSO concentration of 0.15 %. At the end of 2 h incubations, the BSS samples were collected for determination of UA production.
Animal experiment
This experiment was carried out in accordance with the guideline for Animal Experiments of Utsunomiya University Animal Research Committee (ethic approval number: A14-0017). Male ICR mice (Charles River Japan, Inc., Yokohama, Japan) at 4 weeks of age were housed in plastic cages in a room with a 12 h light and dark cycle (dark phase of 18:00–6:00) and constant temperature (22 °C). They were housed in groups of five for 7 days for acclimatization to the environment. The mice were provided with tap water and regular pellet diet (CRF-1, Oriental Yeast Co., Tokyo, Japan) ad libitum. After acclimatization to the environment for 7 days, the mice were divided into five groups with similar body weights: normal group (n = 8), hyperuricemic model control group (n = 12), allopurinol group (n = 8), low-dose of quercetin group (n = 8) and high-dose of quercetin group (n = 8). Allopurinol and quercetin were suspended in 0.5 % sodium carboxymethyl cellulose (CMC-Na, Wako Pure Chemical Industries, Ltd.). After 4 h fasting, allopurinol at 10 mg/kg body weight and quercetin at 100 mg/kg body weight (low-dose group) and 200 mg/kg body weight (high-dose group) were orally given to the mice. Normal and hyperuricemic control groups were orally given 0.5 % CMC-Na alone. The mice were intraperitoneally injected with both IMP and GMP (400 mg each/kg body weight) to induce hyperuricemia 1 h after allopurinol or quercetin administration. IMP and GMP were dissolved in PBS(−). The normal group was injected with the PBS(−) alone as vehicle. The blood was collected under isoflurane anesthesia from the inferior vena cava 1 h after IMP and GMP injection, left to clot at room temperature for 2 h, and centrifuged at 5000×g for 10 min at 4 °C to obtain the serum. Serum samples were stored at −80 °C until analyzed. The serum uric acid level was determined by the uricase method (Uric acid C-test Wako, Wako Pure Chemical Industries, Ltd.).
Statistical analyses
Data are expressed as mean ± SEM. Data on UA production in AML12 cells were analyzed by paired t test or one-way ANOVA followed by Tukey’s multiple-comparisons test as a post hoc test. The results on the animal experiments were analyzed by one-way ANOVA and Dunnett’s multiple-comparisons test as a post hoc test. P values < 0.05 were considered statistically significant. Statistical analyses were conducted by using the Prism 6 software package (GraphPad, San Diego, CA, USA).
Results
Effects of various uric acid precursors on UA production
UA levels in BSS in the presence of adenosine, inosine, guanosine and xanthine at 12.5, 25, and 100 µM were significantly higher than that in BSS alone and the effect was dose-dependent (Fig. 1a–d). On the other hand, all the UA precursors did not significantly increase intracellular UA levels. Furthermore, UA levels in BSS with UA precursors at 12.5, 25, and 100 µM were significantly higher than those in intracellular UA levels at the same concentration of UA precursors. Judging from the results shown in Fig. 1, UA produced by hepatocytes was considered to be released into BSS. This phenomenon might be explanatory of the constant intracellular UA levels in cultured hepatocytes. These results demonstrated that UA detected in BSS was considered to be a reliable and simple index for UA productivity. Significant differences between extracellular (BSS) and intracellular (cell) UA levels also strengthened the validity of UA detected in BSS as an index of its productivity. Hereafter, we measured UA levels in BSS to estimate UA productivity by hepatocytes in culture.
Fig. 1.
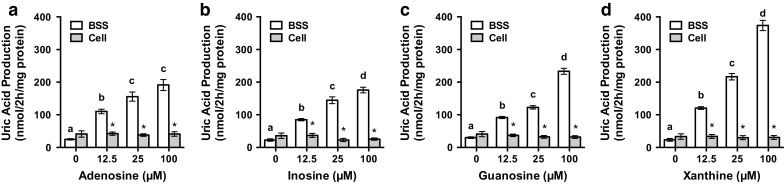
Effects of adenosine, inosine, guanosine and xanthine on uric acid production in AML12 cells. AML12 cells were treated with various concentrations of adenosine (a), inosine (b), guanosine (c) and xanthine (d) for 2 h in balanced salt solution (BSS). Uric acid levels in the BSS (white bars) and the cells (gray bars) were measured by the uricase method. Each value represents mean ± SEM for six wells (duplicate measurements per well). Values not sharing a common letter are significantly different at P < 0.05 among the BSS groups (Tukey test). *Significantly different at P < 0.05 from UA levels in the BSS at the same UA precursor concentrations (paired Student t test)
Effects of various purine nucleotides on uric acid production by AML12 cells
UA production induced by the purine nucleotides, i.e., AMP, IMP and GMP, were significantly higher than that in BSS alone but lower than UA production induced by the purine nucleosides, i.e., adenosine, inosine, and guanosine, respectively (Fig. 2a–c). UA production induced by the purine nucleosides were lower than that induced by xanthine which showed the highest UA productivity.
Fig. 2.
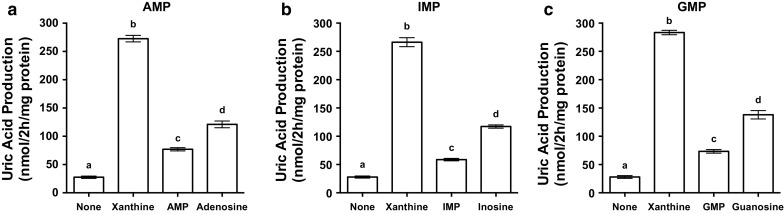
Effects of AMP, IMP and GMP on uric acid production in AML12 cells. AML12 cells were treated with 100 µM AMP (a), 100 µM IMP (b) and 100 µM GMP (c) for 2 h in balanced salt solution (BSS). Uric acid production was measured by the uricase method. Each value represents mean ± SEM for six wells (duplicate measurements per well). Values not sharing a common letter are significantly different at P < 0.05 (Tukey test)
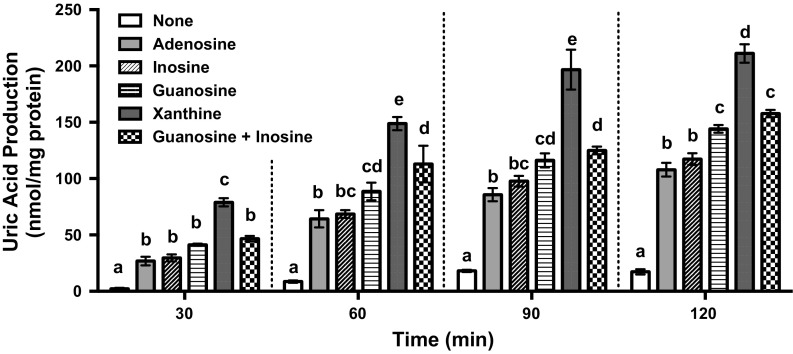
Time-course of UA production by AML12 cells after addition of its precursors
In AML12 cells, the rates of UA production from its precursors were approximately linear. UA production from xanthine 30, 60, 90, and 120 min later was significantly higher than that in BSS alone and the production from adenosine, inosine, guanosine, and guanosine + inosine (Fig. 3). At 120 min time point, UA production from guanosine alone and guanosine + inosine was significantly higher than that in BSS alone and those from adenosine alone and inosine alone. Thus, we thereafter employed guanosine and inosine (100 μM each) in combination (GI mixture) as UA precursors. The pathway of UA production via xanthine from guanosine is different from those of the other nucleosides (adenosine and inosine) (Ishikawa et al. 2013). Hence, the treatment of hepatocytes with GI mixture was considered to be efficient for searching novel anti-hyperuricemic compounds, because we would be able to find a certain test compound possessing action target in either pathway. The reason why the UA productivity from guanosine alone or guanosine + inosine was higher than that from adenosine alone and inosine alone is not clear from the present results, but the differences in pathways of UA production from guanosine and other nucleosides might be involved at least partly. Further intensive studies are required to explain the reason precisely.
Fig. 3.
Time-course of uric acid production in AML12 cells after addition of its precursors. AML12 cells were treated with various UA precursors in 300 µl of BSS with 100 µM adenosine, 100 µM inosine, 100 µM guanosine, 100 µM xanthine, guanosine + inosine (100 µM each), and BSS alone (control). Uric acid production was measured by the uricase method at 30, 60, 90 and 120 min after treatment of the precursors, Each value represents mean ± SEM four wells (single measurement per well). Values not sharing a common letter are significantly different at P < 0.05 (Tukey test)
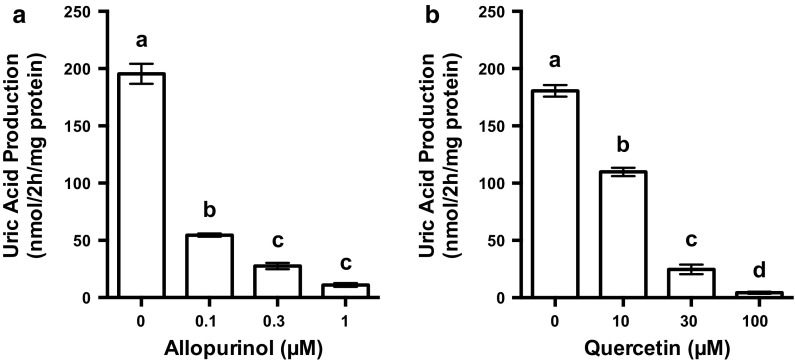
Effects of allopurinol and quercetin on UA production by AML 12 cells
UA production from GI mixture by AML12 hepatocytes treated with 0.1, 0.3 and 1 µM allopurinol was dose-dependently and significantly lower than that by the hepatocytes treated with no allopurinol (0 µM) (Fig. 4a). Likewise, the addition of quercetin dose-dependently and significantly decreased the UA production by the hepatocytes (Fig. 4b).
Fig. 4.
Effects of allopurinol and quercetin on UA production in AML12 cells. AML12 cells were treated with 0.1, 0.3 and 1 µM allopurinol or 10, 30 and 100 µM quercetin for 2 h in balanced salt solution containing guanosine + inosine (100 µM each). Uric acid production was measured by the uricase method. Each value represents mean ± SEM for six wells (duplicate measurements per well). Values not sharing a common letter are significantly different at P < 0.05 (Tukey test)
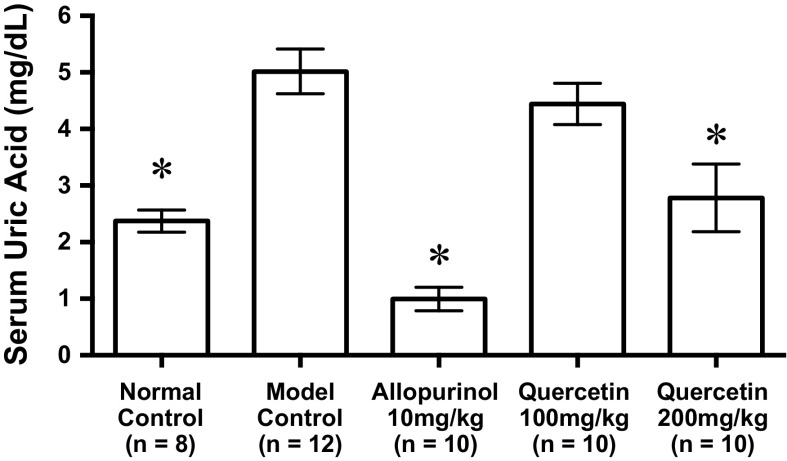
Animal experiment
The intraperitoneal injection of GMP + IMP into mice significantly increased the serum UA level (model control group) in comparison with that of mice injected with PBS(−) alone (normal control group, Fig. 5). The oral administration of allopurinol at 10 mg/kg body weight and quercetin at 200 mg/kg body weight significantly suppressed the increase in the serum UA levels as compared with that of mice orally received CMC-Na (model control group). In the animal experiment, we adopted a concurrent administration of GMP and IMP (nucleotides) as UA precursors instead of guanosine and inosine (nucleosides) in combination, because of insufficient solubility of these nucleosides in PBS(−). In addition, GMP and IMP are known to be involved in food palatability and ingested in our everyday life from meat, seafood and purine-rich vegetable like shiitake mushroom (Yamaguchi and Ninomiya 2000).
Fig. 5.
Effects of allopurinol and quercetin on serum uric acid levels in hyperuricemic mice. The mice were orally administered with allopurinol or quercetin at the different doses indicated. The mice were then intraperitoneally injected with both IMP and GMP (400 mg each/kg body weight) to induce hyperuricemia. Normal control and model control groups were treated with vehicles instead of test samples and nucleotides, respectively. Each value represents mean ± SEM for eight to twelve mice (duplicate measurements per mouse) *significantly different from the model control groups at P < 0.05 (Dunnet test)
Discussion
In the present study, the productivity of UA from purine nucleosides (0, 12.5, 25 and 100 μM) was first estimated in cultured AML12 hepatocytes. UA production from all the nucleosides detected in BSS dose-dependently and significantly increased, while the UA present within AML12 hepatocytes was almost constant (Fig. 1). Thus, UA detected in BSS was considered to be a reliable and simple index for UA productivity as already mentioned. Employing UA in BSS as the index, we next compared differences in UA productivity from precursor purine bodies, namely, nucleosides (adenosine, inosine and guanosine) and nucleotides (AMP, IMP and GMP). In this experiment, we used xanthine as the positive control precursor, because xanthine, immediate proximate substrate of UA production, gave the highest UA productivity in the hepatocytes (Fig. 1). Results showed that nucleosides were better precursors of UA production than nucleotides in AML12 hepatocytes in culture (Fig. 2). Nucleotides, unlike nucleosides, might be unable to enter the hepatocytes in culture system. Using various nucleosides, time-course experiment of UA productivity was conducted in AML12 cells (Fig. 3). All nucleosides caused time-dependent increases in UA productions. At 120 min time point, UA production from inosine + guanosine was significantly higher than that from adenosine alone and inosine alone. Thus, we thereafter employed inosine and guanosine (100 μM each) in combination (GI mixture) as UA precursors in hepatocytes.
We examined effects of allopurinol, the most common prescribed agent (Becker et al. 2005), and quercetin present in onion and strawberry, on UA production, employing the above-mentioned assay system (Fig. 4). Allopurinol clearly decreased UA production at very low concentrations of 0.1, 0.3 and 1 μM, demonstrating that the assay system constructed here worked precisely. Quercetin also dose-dependently and significantly suppressed UA production in hepatocytes, although effective doses were higher than those of allopurinol.
There has been a report on the induction of hyperuricemia by intraperitoneal injection of IMP (600 mg/kg body weight) to mice (Kondo et al. 2013). As mentioned above, inosine and guanosine in combination were added to cell culture system as UA precursors. In the present study, therefore, IMP and GMP in combination were injected into mice to induce hyperuricemia. Both allopurinol and quercetin significantly suppressed the rises in serum UA levels in this purine bodies-induced hyperuricemic animal model (Fig. 5), confirming the previous findings in oxonate-induced hyperuricemic model mice (Mo et al. 2007). Very recently, quercetin has been reported to lower plasma uric acid levels in pre-hyperuricemic humans (Shi and Williamson 2016).
As above mentioned, screening in XO assay system has a possibility to overlook compounds that has their targets other than XO. Thus, the assay system in cultured hepatocytes would be of use to search novel natural and food compounds including those to possess targets other than XO.
In summary, the assay systems in cultured AML12 hepatocytes (in vitro) and purine bodies-induced hyperuricemic model mice (in vivo) in combination seem to be useful for searching novel anti-hyperuricemic compounds in foods and natural resources with possibility to have human health benefit. Studies on searching for novel factors in foods and natural resources using the combined systems are in progress.
Acknowledgments
This work was supported by the Regional Innovation Strategy Support Program, MEXT, Japan. Authors are grateful to Shinichiro Koike, Yuki Takami and Miku Toyozaki for their excellent technical assistance.
Abbreviations
- AMP
Adenosine-5′-monophosphate
- BSS
Balanced salt solution
- GMP
Guanosine-5′-monophosphate
- IMP
Inosine-5′-monophosphate
- UA
Uric acid
- XO
Xanthine oxidase
Compliance with ethical standards
Ethical Approval
This study was conducted on the basis of general ethical norms indicated by the Japan Society for the Promotion of Science (JSPS).
Conflict of interest
Authors declare that they have no conflict of interest.
References
- Bardin T, Richette P. Definition of hyperuricemia and gouty conditions. Curr Opin Rheumatol. 2014;26:186–191. doi: 10.1097/BOR.0000000000000028. [DOI] [PubMed] [Google Scholar]
- Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, daily and protein intake, and the risk of gout in men. N Engl J Med. 2004;350:1093–1103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med. 2005;143:499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Aw W, Kaneko K. Metabolic interactions of purine derivatives with human ABC transporter ABCG2: genetic testing to assess gout risk. Pharmaceuticals (Basel) 2013;6:1347–1360. doi: 10.3390/ph6111347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Hirano Y, Nishio M, Furuya Y, Nakamura H, Watanabe T. Xanthine oxidase inhibitory activity and hypouricemic effect of aspalathin from unfermented rooibos. J Food Sci. 2013;78:H1935–H1939. doi: 10.1111/1750-3841.12304. [DOI] [PubMed] [Google Scholar]
- Minakawa M, Miura Y, Yagasaki K. Piceatannol, a resveratrol derivative, promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and suppresses blood glucose levels in type 2 diabetic model db/db mice. Biochem Biophys Res Commun. 2012;422:469–475. doi: 10.1016/j.bbrc.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Miura Y, Shiomi H, Sakai F, Yagasaki K. Assay systems for screening food components that have anti-proliferative and anti-invasive activity to rat ascites hepatoma cells: in vitro and ex vivo effects of green tea extract. Cytotechnology. 1997;23:127–132. doi: 10.1023/A:1007951231617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura D, Miura Y, Yagasaki K. Resveratrol inhibits hepatoma cell invasion by suppressing gene expression of hepatocyte growth factor via its reactive oxygen species-scavenging property. Clin Exp Metastasis. 2004;21:445–451. doi: 10.1007/s10585-004-2698-1. [DOI] [PubMed] [Google Scholar]
- Mo SF, Zhou F, Lv YZ, Hu QH, Zhang DM, Kong LD. Hypouricemic action of selected flavonoids in mice: structure-activity relationships. Biol Pharm Bull. 2007;30:1551–1556. doi: 10.1248/bpb.30.1551. [DOI] [PubMed] [Google Scholar]
- Nguyen MT, Awale S, Tezuka Y, Tran QL, Watanabe H, Kadota S. Xanthine oxidase inhibitory activity of Vietnamese medicinal plants. Biol Pharm Bull. 2004;27:1414–1421. doi: 10.1248/bpb.27.1414. [DOI] [PubMed] [Google Scholar]
- Noro T, Oda Y, Miyase T, Ueno A, Fukushima S. Inhibitors of xanthine oxidase from the flowers and buds of Daphne genkwa. Chem Pharm Bull. 1983;31:3984–3987. doi: 10.1248/cpb.31.3984. [DOI] [PubMed] [Google Scholar]
- Petrie JL, Patman GL, Sinha I, Alexander TD, Reeves HL, Agius L. The rate of production of uric acid by hepatocytes is a sensitive index of compromised cell ATP homeostasis. Am J Physiol Endocrinol Metab. 2013;305:E1255–E1265. doi: 10.1152/ajpendo.00214.2013. [DOI] [PubMed] [Google Scholar]
- Richette P, Bardin T. Purine-rich foods: an innocent bystander of gout attacks? Ann Rheum Dis. 2012;71:1435–1436. doi: 10.1136/annrheumdis-2012-201838. [DOI] [PubMed] [Google Scholar]
- Shi Y, Williamson G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: a randomised, double-blinded, placebo-controlled, cross-over trial. Br J Nutr. 2016;115:800–806. doi: 10.1017/S0007114515005310. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Ninomiya K. Umami and food palatability. J Nutr. 2000;130:921S–926S. doi: 10.1093/jn/130.4.921S. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhu JX, Mo SF, Pan Y, Kong LD. Effects of cassia oil on serum and hepatic uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J Ethnopharmacol. 2006;103:357–365. doi: 10.1016/j.jep.2005.08.040. [DOI] [PubMed] [Google Scholar]