Abstract
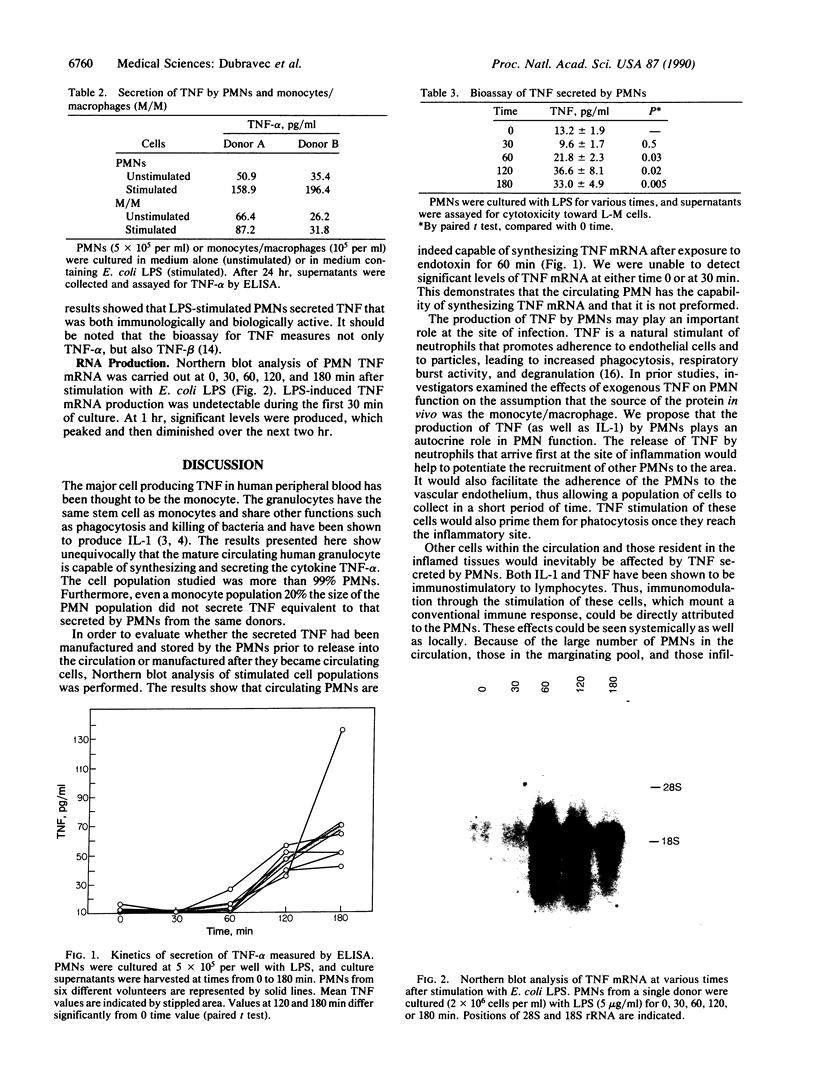
Circulating peripheral blood polymorphonuclear neutrophils (PMNs) have long been considered terminally differentiated cells that do not synthesize or secrete protein. However, work of others and ourselves has shown that PMNs can secrete the cytokine interleukin 1. In the present study we investigated whether circulating PMNs are capable of synthesizing and secreting another cytokine, tumor necrosis factor alpha (TNF-alpha). Highly purified (greater than 99% granulocytes) PMNs were isolated from normal human volunteer blood and cultured with or without bacterial lipopolysaccharide (LPS) for up to 24 hr. Cell culture supernatants were collected and tested for TNF-alpha, and total RNA was isolated from cells at various times after stimulation and assessed for TNF-alpha mRNA by Northern blot techniques. The results showed that message for TNF-alpha was produced after 60 min of in vitro stimulation with LPS and was maximal at about 4 hr. TNF-alpha was secreted into the supernatant of unstimulated PMNs from two different donors during 24 hr of culture (35-50 pg/ml), but significantly more (160-190 pg/ml) was secreted by PMNs when stimulated with LPS. PMNs from six other normal volunteers showed significant LPS-stimulated secretion of TNF at 60-180 min of culture. The secreted product also had biological activity against the TNF-sensitive L-M cell line, confirming that PMNs can make and secrete immunologically and biologically active TNF. Since it is also possible for monocytes to synthesize and secrete TNF, the amount of TNF secreted by a monocyte population equal to 20% of the PMNs cultured was measured. The results showed that monocytes at a concentration 20 times that potentially contaminating the PMN populations cultured could not produce as much TNF (unstimulated, 26-65 pg/ml; stimulated, 32-87 pg/ml). The PMN must now be considered a cell capable of altering the acute inflammatory response and modulating the immune response through the synthesis and release of cytokines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachwich P. R., Chensue S. W., Larrick J. W., Kunkel S. L. Tumor necrosis factor stimulates interleukin-1 and prostaglandin E2 production in resting macrophages. Biochem Biophys Res Commun. 1986 Apr 14;136(1):94–101. doi: 10.1016/0006-291x(86)90881-8. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Bertoglio J., Blay J. Y., Marchiol-Fournigault C., Fradelizi D. Generation of lymphokine-activated killer cells: synergy between tumor necrosis factor and interleukin 2. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6875–6879. doi: 10.1073/pnas.85.18.6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J. A., Gustilo K., Baeder W., Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol. 1987 Jun 1;138(11):3812–3816. [PubMed] [Google Scholar]
- Jack R. M., Fearon D. T. Selective synthesis of mRNA and proteins by human peripheral blood neutrophils. J Immunol. 1988 Jun 15;140(12):4286–4293. [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Kramer S. M., Carver M. E. Serum-free in vitro bioassay for the detection of tumor necrosis factor. J Immunol Methods. 1986 Nov 6;93(2):201–206. doi: 10.1016/0022-1759(86)90189-4. [DOI] [PubMed] [Google Scholar]
- Meager A., Leung H., Woolley J. Assays for tumour necrosis factor and related cytokines. J Immunol Methods. 1989 Jan 6;116(1):1–17. doi: 10.1016/0022-1759(89)90306-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sariban E., Mitchell T., Kufe D. Expression of the c-fms proto-oncogene during human monocytic differentiation. Nature. 1985 Jul 4;316(6023):64–66. doi: 10.1038/316064a0. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Dewhirst F. E., Peros W. J., Kent R. L., Ago J. M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987 Mar 1;138(5):1464–1468. [PubMed] [Google Scholar]
- Tiku K., Tiku M. L., Skosey J. L. Interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1986 May 15;136(10):3677–3685. [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]



