Abstract
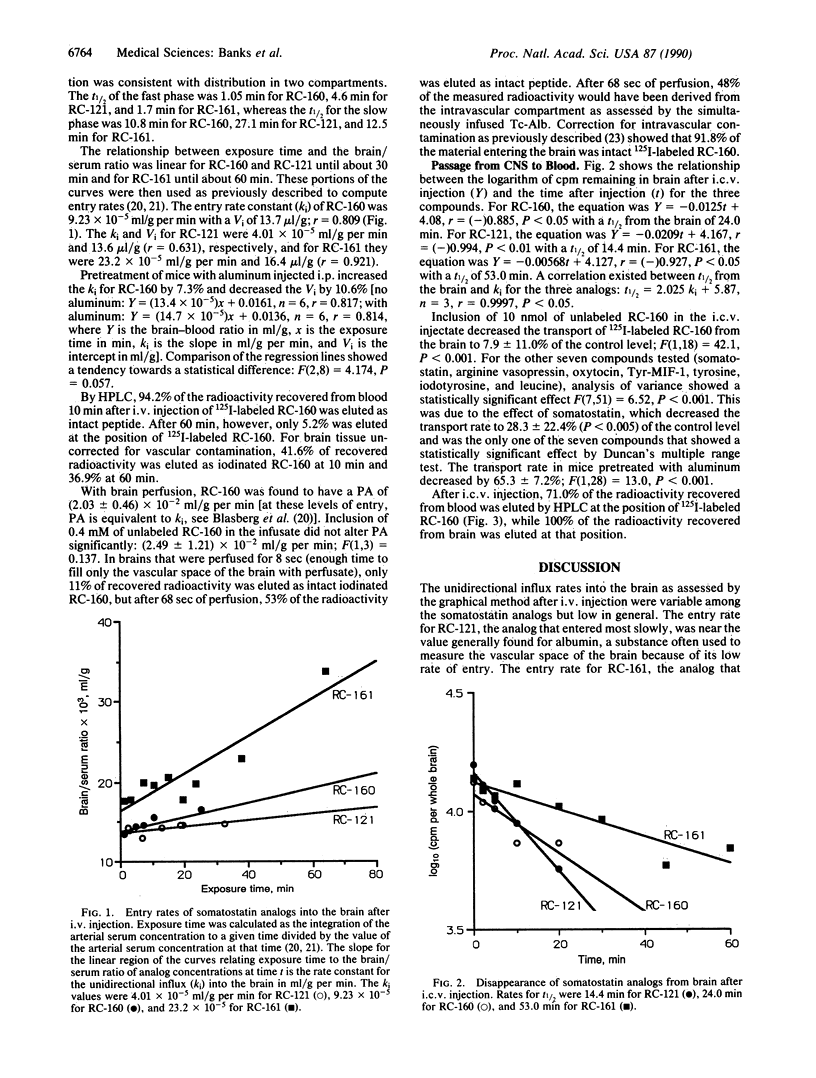
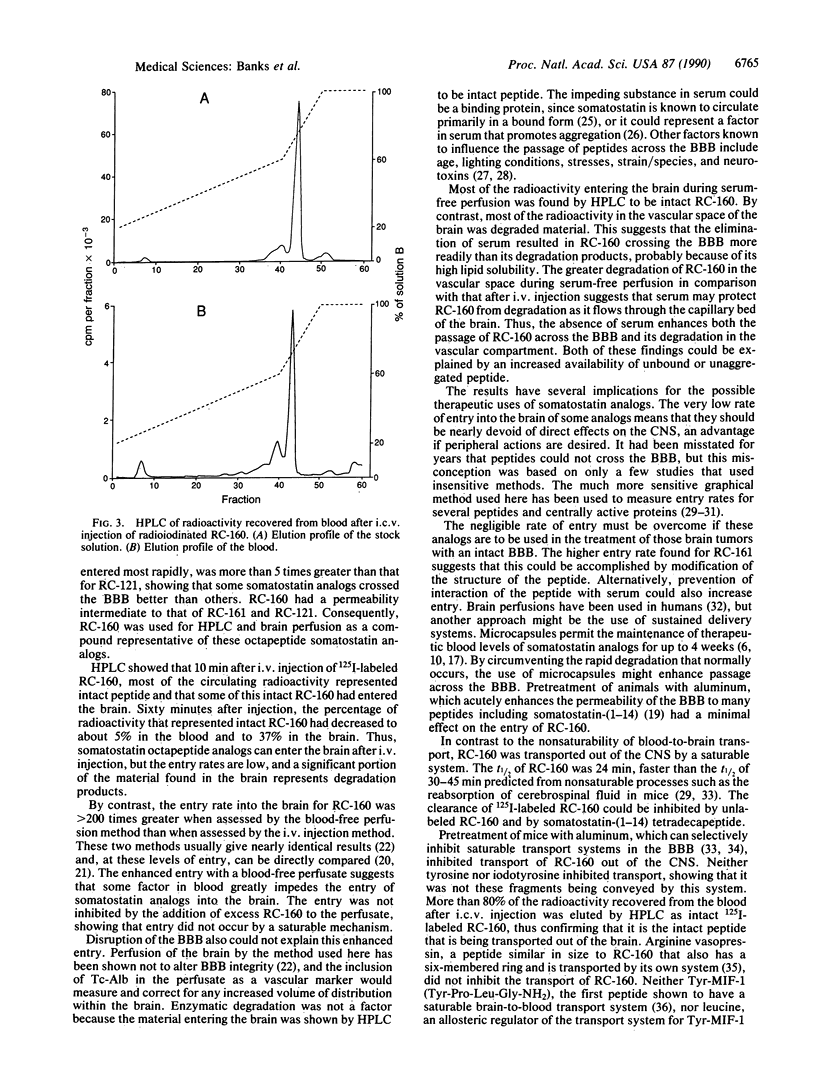
Analogs of somatostatin are being investigated clinically for the treatment of various malignancies, including brain tumors. We studied the ability of three therapeutically promising radioactively labeled somatostatin octapeptide analogs, RC-160, RC-121, and RC-161, to cross the blood-brain barrier (BBB) after peripheral or central injection. After i.v. injection, intact RC-160 was recovered from the blood and the brain. The entry rates were different from each compound but were generally low. By contrast, entry across the intact BBB increased 220 times when RC-160 was given in a serum-free perfusate. This suggests that some serum-related factor, probably the previously described protein binding or an aggregation-promoting factor, is the main determinant in limiting the blood-to-brain passage of somatostatin analogs. Entry into the brain was not inhibited by the addition of unlabeled analog to the perfusate, showing that passage was probably by diffusion across the membranes that comprise the BBB rather than by saturable transport. By contrast, a saturable system was found to transport peptide out of the central nervous system (CNS). The clearance from the CNS of RC-160 and RC-121, but not RC-161, was faster than could be accounted for by reabsorption of cerebrospinal fluid. Transport of radioactively labeled RC-160 out of the CNS was inhibited by unlabeled RC-160 or somatostatin but was not affected by some other peptide known to cross the BBB by their own transport systems. More than 80% of the radioactivity recovered from the blood after intracerebroventricular injection of RC-160 was eluted by HPLC at the position of the labeled analog, showing that the peptide had crossed the BBB in intact form. Our results indicate the presence of a saturable transport system in one direction across the BBB for some superactive analogs of somatostatin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks W. A., Kastin A. J. Aluminum-induced neurotoxicity: alterations in membrane function at the blood-brain barrier. Neurosci Biobehav Rev. 1989 Spring;13(1):47–53. doi: 10.1016/s0149-7634(89)80051-x. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Durham D. A. Bidirectional transport of interleukin-1 alpha across the blood-brain barrier. Brain Res Bull. 1989 Dec;23(6):433–437. doi: 10.1016/0361-9230(89)90185-8. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Fasold M. B., Barrera C. M., Augereau G. Studies of the slow bidirectional transport of iron and transferrin across the blood-brain barrier. Brain Res Bull. 1988 Dec;21(6):881–885. doi: 10.1016/0361-9230(88)90021-4. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Fasold M. B. Differential effect of aluminum on the blood-brain barrier transport of peptides, technetium and albumin. J Pharmacol Exp Ther. 1988 Feb;244(2):579–585. [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Fischman A. J., Coy D. H., Strauss S. L. Carrier-mediated transport of enkephalins and N-Tyr-MIF-1 across blood-brain barrier. Am J Physiol. 1986 Oct;251(4 Pt 1):E477–E482. doi: 10.1152/ajpendo.1986.251.4.E477. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Horvath A., Michals E. A. Carrier-mediated transport of vasopressin across the blood-brain barrier of the mouse. J Neurosci Res. 1987;18(2):326–332. doi: 10.1002/jnr.490180209. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J. Inhibition of the brain to blood transport system for enkephalins and Tyr-MIF-1 in mice addicted or genetically predisposed to drinking ethanol. Alcohol. 1989 Jan-Feb;6(1):53–57. doi: 10.1016/0741-8329(89)90073-6. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J. Modulation of the carrier-mediated transport of the Tyr-MIF-1 across the blood-brain barrier by essential amino acids. J Pharmacol Exp Ther. 1986 Dec;239(3):668–672. [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J. Peptide transport systems for opiates across the blood-brain barrier. Am J Physiol. 1990 Jul;259(1 Pt 1):E1–10. doi: 10.1152/ajpendo.1990.259.1.E1. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J. Peptides and the blood-brain barrier: lipophilicity as a predictor of permeability. Brain Res Bull. 1985 Sep;15(3):287–292. doi: 10.1016/0361-9230(85)90153-4. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J. Quantifying carrier-mediated transport of peptides from the brain to the blood. Methods Enzymol. 1989;168:652–660. doi: 10.1016/0076-6879(89)68047-0. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J. Saturable transport of peptides across the blood-brain barrier. Life Sci. 1987 Sep 14;41(11):1319–1338. doi: 10.1016/0024-3205(87)90606-0. [DOI] [PubMed] [Google Scholar]
- Barrera C. M., Banks W. A., Kastin A. J. Passage of Tyr-MIF-1 from blood to brain. Brain Res Bull. 1989 Dec;23(6):439–442. doi: 10.1016/0361-9230(89)90186-x. [DOI] [PubMed] [Google Scholar]
- Barrera C. M., Kastin A. J., Banks W. A. D-[Ala1]-peptide T-amide is transported from blood to brain by a saturable system. Brain Res Bull. 1987 Dec;19(6):629–633. doi: 10.1016/0361-9230(87)90048-7. [DOI] [PubMed] [Google Scholar]
- Blasberg R. G., Fenstermacher J. D., Patlak C. S. Transport of alpha-aminoisobutyric acid across brain capillary and cellular membranes. J Cereb Blood Flow Metab. 1983 Mar;3(1):8–32. doi: 10.1038/jcbfm.1983.2. [DOI] [PubMed] [Google Scholar]
- Cai R. Z., Karashima T., Guoth J., Szoke B., Olsen D., Schally A. V. Superactive octapeptide somatostatin analogs containing tryptophan at position 1. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2502–2506. doi: 10.1073/pnas.84.8.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R. Z., Szoke B., Lu R., Fu D., Redding T. W., Schally A. V. Synthesis and biological activity of highly potent octapeptide analogs of somatostatin. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1896–1900. doi: 10.1073/pnas.83.6.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima T., Cai R. Z., Schally A. V. Effects of highly potent octapeptide analogs of somatostatin on growth hormone, insulin and glucagon release. Life Sci. 1987 Aug 24;41(8):1011–1019. doi: 10.1016/0024-3205(87)90690-4. [DOI] [PubMed] [Google Scholar]
- Karashima T., Schally A. V. Superactive somatostatin analog decreases plasma glucose and glucagon levels in diabetic rats. Peptides. 1988 May-Jun;9(3):561–565. doi: 10.1016/0196-9781(88)90164-7. [DOI] [PubMed] [Google Scholar]
- Kastin A. J., Castellanos P. F., Fischman A. J., Proffitt J. K., Graf M. V. Evidence for peptide aggregation. Pharmacol Biochem Behav. 1984 Dec;21(6):969–973. doi: 10.1016/s0091-3057(84)80082-9. [DOI] [PubMed] [Google Scholar]
- Krenning E. P., Bakker W. H., Breeman W. A., Koper J. W., Kooij P. P., Ausema L., Lameris J. S., Reubi J. C., Lamberts S. W. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet. 1989 Feb 4;1(8632):242–244. doi: 10.1016/s0140-6736(89)91258-0. [DOI] [PubMed] [Google Scholar]
- Kronheim S., Sheppard M. C., Shapiro B., Pimstone B. L. Ultracentrifugation evidence for a somatostatin-binding protein in serum. Biochim Biophys Acta. 1979 Sep 3;586(3):568–573. doi: 10.1016/0304-4165(79)90047-3. [DOI] [PubMed] [Google Scholar]
- László F., Pávó I., Penke B., Bálint G. A. Protective effect of an orally administered, highly potent somatostatin analog (RC-121) against absolute ethanol-induced hemorrhagic erosions of the rat gastric mucosa. Life Sci. 1989;44(21):1573–1578. doi: 10.1016/0024-3205(89)90451-7. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Blasberg R. G., Fenstermacher J. D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983 Mar;3(1):1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Paz-Bouza J. I., Redding T. W., Schally A. V. Treatment of nitrosamine-induced pancreatic tumors in hamsters with analogs of somatostatin and luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1112–1116. doi: 10.1073/pnas.84.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi J. C., Lamberts S. W., Maurer R. Somatostatin receptors in normal and tumoral tissue. Horm Res. 1988;29(2-3):65–69. doi: 10.1159/000180970. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Lang W., Maurer R., Koper J. W., Lamberts S. W. Distribution and biochemical characterization of somatostatin receptors in tumors of the human central nervous system. Cancer Res. 1987 Nov 1;47(21):5758–5764. [PubMed] [Google Scholar]
- Reubi J. C., Maurer R., Klijn J. G., Stefanko S. Z., Foekens J. A., Blaauw G., Blankenstein M. A., Lamberts S. W. High incidence of somatostatin receptors in human meningiomas: biochemical characterization. J Clin Endocrinol Metab. 1986 Aug;63(2):433–438. doi: 10.1210/jcem-63-2-433. [DOI] [PubMed] [Google Scholar]
- Rose D. P., Gottardis M., Noonan J. J. Rat mammary carcinoma regressions during suppression of serum growth hormone and prolactin. Anticancer Res. 1983 Sep-Oct;3(5):323–325. [PubMed] [Google Scholar]
- Schally A. V., Comaru-Schally A. M., Redding T. W. Antitumor effects of analogs of hypothalamic hormones in endocrine-dependent cancers. Proc Soc Exp Biol Med. 1984 Mar;175(3):259–281. doi: 10.3181/00379727-175-41797. [DOI] [PubMed] [Google Scholar]
- Schally A. V. Oncological applications of somatostatin analogues. Cancer Res. 1988 Dec 15;48(24 Pt 1):6977–6985. [PubMed] [Google Scholar]
- Schally A. V., Redding T. W., Comaru-Schally A. M. Potential use of analogs of luteinizing hormone-releasing hormones in the treatment of hormone-sensitive neoplasms. Cancer Treat Rep. 1984 Jan;68(1):281–289. [PubMed] [Google Scholar]
- Schally A. V., Redding T. W. Somatostatin analogs as adjuncts to agonists of luteinizing hormone-releasing hormone in the treatment of experimental prostate cancer. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7275–7279. doi: 10.1073/pnas.84.20.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srkalovic G., Cai R. Z., Schally A. V. Evaluation of receptors for somatostatin in various tumors using different analogs. J Clin Endocrinol Metab. 1990 Mar;70(3):661–669. doi: 10.1210/jcem-70-3-661. [DOI] [PubMed] [Google Scholar]
- Takasato Y., Rapoport S. I., Smith Q. R. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am J Physiol. 1984 Sep;247(3 Pt 2):H484–H493. doi: 10.1152/ajpheart.1984.247.3.H484. [DOI] [PubMed] [Google Scholar]
- Zalatnai A., Paz-Bouza J. I., Redding T. W., Schally A. V. Histologic changes in the rat prostate cancer model after treatment with somatostatin analogs and D-Trp-6-LH-RH. Prostate. 1988;12(1):85–98. doi: 10.1002/pros.2990120111. [DOI] [PubMed] [Google Scholar]


