Abstract
Background
Being diagnosed with human immunodeficiency virus (HIV), and labelled with a chronic, life‐threatening, and often stigmatizing disease, can impact on a person's well‐being. Psychosocial group interventions aim to improve life‐functioning and coping as individuals adjust to the diagnosis.
Objectives
To examine the effectiveness of psychosocial group interventions for improving the psychological well‐being of adults living with HIV/AIDS.
Search methods
We searched the following electronic databases up to 14 March 2016: the Cochrane Central Register of Controlled Trials (CENTRAL) published in the Cochrane Library (Issue 2, 2016), PubMed (MEDLINE) (1996 to 14 March 2016), Embase (1996 to 14 March 2016), and Clinical Trials.gov.
Selection criteria
Randomized controlled trials (RCTs) or quasi‐RCTs that compared psychosocial group interventions with versus control (standard care or brief educational interventions), with at least three months follow‐up post‐intervention. We included trials that reported measures of depression, anxiety, stress, or coping using standardized scales.
Data collection and analysis
Two review authors independently screened abstracts, applied the inclusion criteria, and extracted data. We compared continuous outcomes using mean differences (MD) with 95% confidence intervals (95% CIs), and pooled data using a random‐effects model. When the included trials used different measurement scales, we pooled data using standardized mean difference (SMD) values. We reported trials that we could not include in the meta analysis narratively in the text. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 16 trials (19 articles) that enrolled 2520 adults living with HIV. All the interventions were multifaceted and included a mix of psychotherapy, relaxation, group support, and education. The included trials were conducted in the USA (12 trials), Canada (one trial), Switzerland (one trial), Uganda (one trial), and South Africa (one trial), and published between 1996 and 2016. Ten trials recruited men and women, four trials recruited homosexual men, and two trials recruited women only. Interventions were conducted with groups of four to 15 people, for 90 to 135 minutes, every week for up to 12 weeks. All interventions were conducted face‐to‐face except two, which were delivered by telephone. All were delivered by graduate or postgraduate trained health, psychology, or social care professionals except one that used a lay community health worker and two that used trained mindfulness practitioners.
Group‐based psychosocial interventions based on cognitive behavioural therapy (CBT) may have a small effect on measures of depression, and this effect may last for up to 15 months after participation in the group sessions (SMD −0.26, 95% CI −0.42 to −0.10; 1139 participants, 10 trials, low certainty evidence). Most trials used the Beck Depression Inventory (BDI), which has a maximum score of 63, and the mean score in the intervention groups was around 1.4 points lower at the end of follow‐up. This small benefit was consistent across five trials where participants had a mean depression score in the normal range at baseline, but trials where the mean score was in the depression range at baseline effects were less consistent. Fewer trials reported measures of anxiety, where there may be little or no effect (four trials, 471 participants, low certainty evidence), stress, where there may be little or no effect (five trials, 507 participants, low certainty evidence), and coping (five trials, 697 participants, low certainty evidence).
Group‐based interventions based on mindfulness have not demonstrated effects on measures of depression (SMD −0.23, 95% CI −0.49 to 0.03; 233 participants, 2 trials, very low certainty evidence), anxiety (SMD −0.16, 95% CI −0.47 to 0.15; 62 participants, 2 trials, very low certainty evidence), or stress (MD −2.02, 95% CI −4.23 to 0.19; 137 participants, 2 trials, very low certainty evidence). No mindfulness based interventions included in the studies had any valid measurements of coping.
Authors' conclusions
Group‐based psychosocial interventions may have a small effect on measures of depression, but the clinical importance of this is unclear. More high quality evidence is needed to assess whether group psychosocial intervention improve psychological well‐being in HIV positive adults.
2 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (14 Mar, 2016) were included
Plain language summary
Does group therapy improve well‐being in people living with HIV?
Cochrane researchers conducted a review of the effects of group therapy for people living with human immunodeficiency virus (HIV). After searching for relevant trials up to 14 March 2016, they included 16 trials reported in 19 articles that enrolled 2520 adults living with HIV. The included trials were conducted in the USA (12 trials), Canada (one trial), Switzerland (one trial), Uganda (one trial), and South Africa (one trial), and published between 1996 and 2016. Ten trials recruited men and women, four trials recruited homosexual men, and two trials recruited women only.
What is group therapy and how might if benefit people with HIV?
Group therapy aims to improve the well‐being of individuals by delivering psychological therapy in a group format, which can encourage the development of peer support and social networks. Group therapy often also incorporates training in relaxation techniques and coping skills, and education on the illness and its management.
Human immunodeficiency virus (HIV) causes a chronic, life threatening, and often stigmatising disease, which can impact on a person's well‐being. Group therapy could help people living with HIV to adapt to knowing they have HIV, or recover from depression, anxiety, and stress.
What the research says
Group‐based therapy based on cognitive behavioural therapy may have a small effect on measures of depression, and this effect may last for up to 15 months after participation in the group sessions (low certainty evidence). This effect was apparent in groups who did not appear to be depressed on clinical scoring systems before the therapy started. The research also showed there may be little or no effect on measures of anxiety, stress, and coping (low certainty evidence).
Group‐based interventions based on mindfulness have been studied in two small trials, and have not demonstrated effects on measures of depression, anxiety or stress (all very low certainty evidence). No mindfulness based interventions included in the studies had any valid measurements of coping.
Overall, the review suggests that existing interventions have little to no effect in increasing psychological adjustment to living with HIV. More good quality studies are required to inform good practice and evidence.
Summary of findings
Background
Description of the condition
Infection with the human immunodeficiency virus (HIV) causes a chronic, life‐threatening disease, characterized by progressive destruction of the immune system and increasing susceptibility to infection and malignancy. Consequently, despite the availability of highly active antiretroviral therapy (HAART), which has revolutionized treatment, a new diagnosis of HIV carries multiple threats to a person's psychological well‐being (Lawless 1996; Hudson 2001; Colbert 2010). Being labelled with a chronic illness, especially one associated with the stigma of HIV, and the accompanying need to take multiple daily medications with unpleasant side effects can lead to uncertainty about the future, relationship difficulties, social isolation, and loss of self esteem. Failure to adjust can in turn lead to clinical depression, anxiety, stress, and poor coping (Vanable 2006). Particularly, the prevalence of depression and suicide in people living with HIV/AIDS is very high (Cooperman 2005; Rezaee 2013; Bhatia 2014; Anagnostopoulos 2015).
Description of the intervention
Psychosocial group interventions, by definition, include some form of psychological therapy such as cognitive behavioural therapy (CBT) delivered in a group format. However, many will also include additional components that may also have effects on psychological well‐being, such as: relaxation techniques and stress management; problem solving and coping skills; social or peer support; and education and empowerment (see Figure 1).
1.
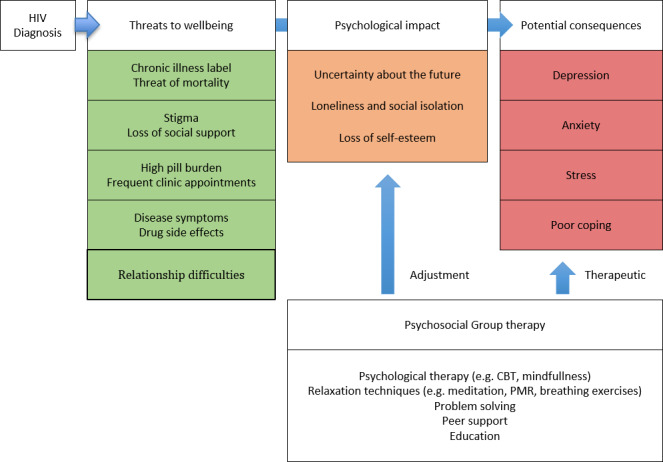
Conceptual framework
How the intervention might work
Psychological well‐being is usually conceptualized as some combination of positive affective states such as happiness and functioning, with optimal effectiveness in individual and social life (Deci 2008). As summarized by Huppert 2009 (p.137): “Psychological well‐being is about lives going well. It is the combination of feeling good and functioning effectively”. By definition therefore, people with high psychological well‐being report feeling happy, capable, well‐supported, and satisfied with life.
Fundamentals to psychological well‐being for HIV‐positive people are positive coping strategies and perceived social support (Friedland 1996; Côté 2002; Turner‐Cobb 2002). As compared to individual therapy, group therapy is believed to confer a wider range of psychosocial benefits. In a well‐functioning group, members may give and receive motivational support and encouragement for self‐efficacy, and through shared experiences can empower each other to access services, adhere to treatment, and cope with stigma and stress (Kelly 1998; Metcalfe 1998; Moneyham 1998; Gielen 2001; Walker 2002; Peterson 2003). This is contrasted with the problem of stigma inherent in joining groups defined by HIV‐status (Roopnaraine 2012). Being in a group helps participants to feel they are not alone in dealing with their problems and also encourages relating to yourself and others in healthier ways. Group formats are also cost effective and resource effective, reaching more patients than individual or one‐on‐one therapies. This is advantageous, particularly in resource‐poor settings.
Why it is important to do this review
A positive diagnosis of HIV means a lifetime of medical treatment, but also dealing with the psychological effects of living with a chronic disease. Psychosocial interventions focus on stress management, coping, and self efficacy and have the potential to have a positive effect on peoples' mental health and treatment adherence. However, the evidence base of what works to improve the psychological well‐being of people living with HIV, particularly those in high‐risk groups and those living in resource‐poor settings, is lacking. In order to inform practice and research, this Cochrane Review can contribute to evidence on what types of psychosocial interventions are most effective to improve psychological well‐being for HIV‐positive adults. Certainty of the evidence is also important to define future studies, and whether findings are consistent and can be generalized across populations and settings.
Objectives
To examine the effectiveness of psychosocial group interventions for improving the psychological well‐being of adults living with HIV/AIDS.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) or quasi‐RCTs with at least three months follow‐up post‐intervention.
Types of participants
HIV‐positive adults with and without current psychological illness.
Types of interventions
Any psychosocial intervention delivered in a group format that aims to improve the psychological well‐being of people living with HIV. This might include the following types of interventions.
Interventions conducted in hospitals, clinics, or community settings.
Interventions delivered face‐to‐face or via telephone or video link.
Interventions focused on providing information and psychoeducation, cognitive restructuring, stress appraisal and management, relaxation and mindfulness, adaptive and productive coping, assertiveness training and social support.
Interventions based on any theoretical approach.
The control intervention may be standard care, a waiting list for future intervention, or a brief educational/psychosocial intervention delivered in‐group or individual format.
Types of outcome measures
Primary outcomes
Improved psychological well‐being of HIV‐positive people measured by decreases in depression scores using validated scales.
Secondary outcomes
Measures of anxiety using validated scales.
Measures of stress using validated scales.
Measures of coping using validated scales.
All outcomes must be measures at baseline, post‐intervention and at a time point at least three months after the intervention.
Search methods for identification of studies
The HIV/AIDS Information Specialist, Joy Oliver, assisted the review author team to identify trials for inclusion in the review.
Electronic searches
We searched the following electronic databases up to 14 March 2016 using the search strategy presented in Appendix 1: the Cochrane Central Register of Controlled Trials (CENTRAL) published in the Cochrane Library (Issue 2, 2016), PubMed (MEDLINE) (1996 to 14 March 2016), and Embase (1996 to 14 March 2016). We also checked Clinical Trials.gov using the search terms in Appendix 2.
Searching other resources
We searched the reference list of all papers that matched our inclusion criteria and contacted the first authors to identify any other trial analyses that were published.
Data collection and analysis
Selection of studies
Two review authors (IVDH and NA) independently screened the abstracts of all citations identified by the literature search to see if any met the inclusion criteria. If an abstract was potentially relevant, or it was unclear whether it was relevant or not, we accessed the full‐text paper and read it carefully to see if it the trial met the inclusion criteria. Regarding multiple reports of the same trial, we linked these, collated the data available in the reports, and presented these as one trial. We listed all excluded studies and their reasons for exclusion in the 'Characteristics of excluded studies' table. We constructed a PRISMA study flow diagram to illustrate the study selection process.
Data extraction and management
Two review authors (IVDH and NA) independently extracted data from the included trials using a standardized data extraction form that included the following characteristics: citation of authors and year, the type of trial design, population characteristics and trial setting, eligibility criteria, type of intervention, treatment and control group duration and components, all relevant outcomes with measures (scales), time assessment points, percentage lost to follow‐up and results, see Appendix 3.
Assessment of risk of bias in included studies
Two review authors (IVDH and NA) independently assessed the risk of bias of all trials included in the review using the Cochrane 'Risk of bias' assessment tool (Higgins 2008; Higgins 2011). We resolved any discrepancies through discussion and by consulting the third review author, and if necessary we contacted the trial authors for clarification.
We followed the guidance to assess whether the included trials took adequate steps to reduce the risk of bias across the following six domains: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective outcome reporting; and other sources of bias.
For sequence generation and allocation concealment, we reported the methods that the included trials used. For blinding, we described who was blinded and the blinding method. For incomplete outcome data, we reported the percentage of participants lost to follow‐up. For selective outcome reporting, we stated any discrepancies between the methods used and the results in terms of the outcomes measured or the outcomes reported. For other biases, we described any other trial features that could have affected the trial result (for example, if the trial was stopped early, or how the use of a wait‐list group may bias results because waiting‐list controls might be biased because of resentment at not receiving an intervention, changes in disease state over time, and an absence of engagement in care. However, blinding and randomization will balance out this bias risk.
We categorized our risk of bias judgements as either 'low', 'high', or 'unclear'. Where risk of bias was unclear, we attempted to contact the trial authors for clarification and resolved any differences of opinion through discussion.
Measures of treatment effect
We reported results using mean difference (MD) with 95% confidence intervals (95% CI), and used standardized mean difference (SMD) values when we pooled trials using different scales.
Unit of analysis issues
The unit of analysis in all trials was the individual (HIV‐positive adult). For complicated designs, such as cluster‐RCTs, we planned to use the cluster as the unit of analysis, and planned to account for clustering by adjusting the analysis to the same level as the individual following the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008; Higgins 2011).
When trials had more than two treatment arms we took care to avoid double counting of participants in the control group.
Dealing with missing data
Our primary analysis was a complete‐case analysis, which included only the participants with data at each time point.
We considered the potential for bias due to missing data in our GRADE evaluation of the certainty of the evidence, by considering the size of losses to follow‐up, and any differential losses between groups.
We contacted trial authors for clarification regarding missing data.
Assessment of heterogeneity
We described clinical heterogeneity between trials by summarizing key characteristics of the trial design, intervention, population, and outcome measures in tables.
We assessed statistical heterogeneity by looking at forest plots to examine the presence of overlapping CIs and using the Chi² test using a P value of 0.10 to determine statistical significance. We quantified heterogeneity using the I² statistic, which describes the percentage of variability in the effect estimates and we applied the standard categorization of heterogeneity to interpret the statistic i.e. low heterogeneity = I² statistic value of 0% to 25%; moderate heterogeneity = I² statistic value of 26% to 50% and high heterogeneity = I² statistic value of greater than 50%.
Assessment of reporting biases
We planned to use funnel plots to look for evidence of publication bias. However, there were few included trials to facilitate this.
Data synthesis
We summarized and analysed the included trials in Review Manager 5 (RevMan 5) (Review Manager 2014).
We analysed trials with different underlying psychological theories separately, and stratified the primary analysis by time point: baseline, end of the group session, and longest follow‐up.
When pooling studies was considered appropriate we used a random‐effects approach due to the clinical heterogeneity between trials.
We created a 'Summary of findings' for each comparison including the four main outcomes: depression, anxiety, stress, and coping. For each effect estimate we evaluated our confidence in the effect by considering each of the five GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. When we found sufficient problems to downgrade the overall certainty of the evidence we justified these decisions using footnotes.
Subgroup analysis and investigation of heterogeneity
We explored possible causes of statistical heterogeneity by conducting sub group analyses by: primary focus of the intervention, outcome scale used, the control intervention, and gender. We only performed these subgroup analyses when there were sufficient trials to make it meaningful.
Sensitivity analysis
We did not plan to perform any sensitivity analysis.
Results
Description of studies
Results of the search
We searched the available literature up to 14 March 2016 and identified 2414 citations from the electronic database searches. After discarding 1376 duplicates, we screened 1038 articles by title and abstract. We selected abstracts that potentially matched our inclusion criteria, and also articles where it was unclear whether or not they fulfilled the inclusion criteria, for full‐text assessment. We excluded 942 articles and identified 96 full‐text articles for further assessment. After full‐text assessment of these articles. we excluded 77 articles. These corresponded to 70 studies after we collated them, which we listed in the 'Characteristics of excluded studies' table. Nineteen articles met the inclusion criteria. We collated multiple reports on the same trial, which gave 16 included trials. We have illustrated the study selection process in Figure 2.
2.
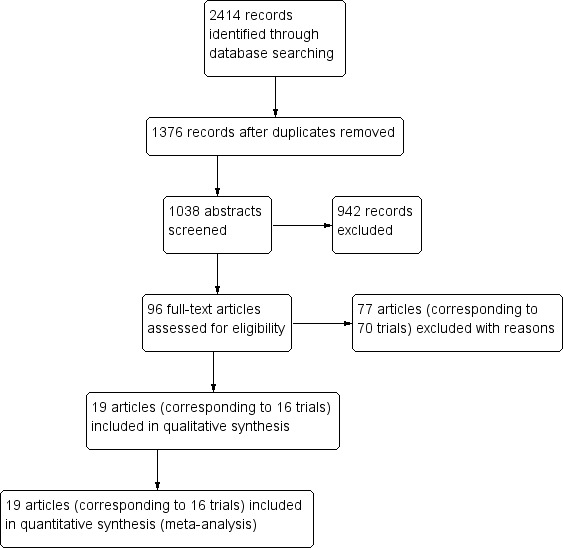
Study flow diagram
Included studies
Sixteen randomized controlled trials (RCTs) that included 2520 HIV‐positive adults met the inclusion criteria of this Cochrane Review. Four trials exclusively recruited homosexual men (Chesney 2003; Carrico 2005; Antoni 2006; Gayner 2012), and two trials recruited women only (Antoni 2008; Jones 2010). The remaining trials recruited both men and women, with four specifying they were all on antiretroviral therapy (ART) (Berger 2008; Duncan 2012; Peltzer 2012; Safren 2012), one trial recruited only those with a history of childhood sexual abuse (Sikkema 2013), one recruited injection drug users (Safren 2012), one recruited those over 50 years old (Heckman 2011), and one recruited participants with major depression (Nakimuli‐Mpungu 2015). All trials excluded pregnant women (see Table 3).
1. Description of populations.
| Trial | Country | Age (years) | On antiretroviral therapy (ART) | General population or subgroup | Mood disorders | |
| Inclusion criteria | Exclusion criteria | |||||
| Antoni 2006 | USA | 18 to 65 | Yes | Homosexual men | None stated | Current psychosis or panic disorder |
| Antoni 2008 | USA | 18 to 60 | Not stated | Women with evidence of CIN1 | None stated | Current major psychiatric illness |
| Berger 2008 | Switzerland | 18 to 65 | Yes | General population | None stated | Current major psychiatric disorder |
| Carrico 2005 | USA | Not stated | No (recruited largely during the era prior to highly active antiretroviral therapy (HAART; 1992 to 1997) |
Homosexual men | None stated | Current major psychiatric illness |
| Jones 2010 | USA | > 18 | Not stated | Minority women | None stated | Untreated major psychiatric illness |
| McCain 2003 | USA | > 18 | Yes | General population | None stated | Significant psychiatric illness |
| Safren 2012 | USA | 18 to 65 | Yes | Prior intravenous drug users | Current depressive mood disorder | Untreated or unstable major mental illness |
| Heckman 2007 | USA | Not stated | Not stated | Rural population | No inclusion or exclusion criteria related to psychological functioning were employed | No inclusion or exclusion criteria related to psychological functioning were employed |
| Heckman 2011 | USA | > 50 | Not stated | Individuals over the age of 50 | BDI‐II score is minimum 10 | Exclude severe depression or cognitive impairment |
| Sikkema 2013 | USA | Not stated | Not stated | History of child sexual abuse | Not stated | Not stated |
| Peltzer 2012 | South Africa | > 18 | Yes | Individuals with ART adherence problem/new antiretroviral (ARV) medication users (6 to 24 months of ARV use) | Not stated | Not stated |
| Chesney 2003 | USA | 21 to 60 | No | Homosexual men | Reported depressed mood 10 or higher on CES‐D scale | Major depressive disorder or other psychotic disorders |
| Bormann 2006 | USA | 18 to 65 | Not stated | Adult men and women (50% homosexual) | Not stated | Cognitive impairment of active psychosis |
| Duncan 2012 | USA | Not specified | Yes | Adult men and women who reported distress associated with side effects from ART treatment | Reporting a level of side effect‐related bother for the previous 30 days at or above eight (corresponding to the 40th percentile in another sample) on the side effect and symptom distress scale | Severe cognitive impairment, active psychosis, or active substance abuse |
| Gayner 2012 | Canada | Not specified | Not stated | Homosexual men | None stated | Active current major depression, substance abuse, or significant cognitive deficit |
| Nakimuli‐Mpungu 2015 | Uganda | > 19 | Not stated | Both men and women | People with major depression on Mini Psychiatric Interview Scale | The trial excluded individuals with a severe medical disorder such as pneumonia or active tuberculosis, psychotic symptoms, and hearing or visual impairment. |
Abbreviations: ART: antiretroviral therapy; ARV: antiretroviral.
Twelve trials were conducted in the USA (Chesney 2003; McCain 2003; Carrico 2005; Antoni 2006; Bormann 2006; Heckman 2007; Antoni 2008; Jones 2010; Heckman 2011; Duncan 2012; Safren 2012; Sikkema 2013), Canada (Gayner 2012), and one in Switzerland (Berger 2008), one in Uganda (Nakimuli‐Mpungu 2015) and one in South Africa (Peltzer 2012). Recruitment was from hospitals, clinics, drop‐in centres, or community settings.
Therapy was conducted with groups of four to 15 people every week for up to 12 weeks. All were face‐to‐face interventions except in Heckman 2007 and Heckman 2011 in which they were delivered by telephone. All interventions were delivered by postgraduate trained health, psychology, or social care professionals, except one which used lay health workers (Peltzer 2012). Sessions lasted 90 to 135 minutes, and the period of follow‐up varied from three to 15 months. The control groups ranged from wait‐list, standard care or treatment as usual, information or education only, or a brief form of the intervention (30 minutes or one day).
We subgrouped the interventions by their underlying psychological theory, although all the interventions were multifaceted.
Cognitive behavioural therapy (CBT) (13 trials): a psychotherapeutic approach that addresses dysfunctional emotions, maladaptive behaviours, and cognitive processes through a number of goal‐oriented, problem‐focused, action‐orientated procedures. The name refers to therapy based upon a combination of basic behavioral and cognitive principles and research. Most therapists working with patients dealing with anxiety and depression use a blend of cognitive and behavioral therapy. Six trials focused particularly on stress management (McCain 2003; Carrico 2005; Antoni 2006; Antoni 2008; Berger 2008; Sikkema 2013), three on coping (Chesney 2003; Heckman 2007; Heckman 2011), one on self‐efficacy (Jones 2010), one on adherence and depression (Safren 2012), one on depression (Nakimuli‐Mpungu 2015), and one on adherence (Peltzer 2012).
Mindfulness Based Stress Reduction (three trials): a structured group programme that employs mindfulness meditation to enhance awareness of irrational thoughts and negative affect (Duncan 2012; Gayner 2012), and includes a spirituality approach that involves repeating a mantram‐sacred word or phrase (Bormann 2006).
The trials reported a variety of scales for depression, anxiety, stress, and coping (Table 4; Table 5; Table 6). We pooled these data using standardized mean differences, before presenting a subgroup analysis using the same assessment scale.
2. Depression scales reported by trials.
| Scale | Number of items (depression) | Scale for each item1 | Total score (depression) | Interpretation | Description2 | Trials |
| Beck Depression Inventory (BDI/BDI‐II) (Beck 1988) | 21 | 0 (I do not) to 3 (I do and I can't stand it) | 0 to 63 | 0 to 13: minimal 14 to 19: mild 20 to 28: moderate 29 to 63: severe |
Participants rate the intensity of depressive feelings over the preceding 1 or 2 weeks | Carrico 2005; Antoni 2006; Heckman 2007; Jones 2010; Duncan 2012; Peltzer 2012; Safren 2012 |
| Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983) | 7 | 0 (not at all) to 3 (very often) | 0 to 21 | 0 to 7: normal 8 to 10: borderline 11 to 21: depression |
Participants rate the frequency of depressive thoughts and behaviours over the preceding 4 weeks | Berger 2008; Gayner 2012 |
| Geriatric Depression Scale (GDS) (Yesavage 1982) | 30 | 0 (no) to 1 (yes) | 0 to 30 | 0 to 9: normal 10 to 19: mild 20 to 30: severe |
Participants report the presence or absence of depressive thoughts and behaviours | Heckman 2011 |
| The Centre for Epidemiological Studies‐Depression Scale (CES‐D) (Radloff 1977) | 20 | 0 (not at all) to 3 (all of the time) | 0 to 60 | 0 to 15: normal 16 to 60: depression Cut off is 16 |
Participants rate the frequency of depressive symptoms, feelings, and behaviours over the past week | Chesney 2003; Bormann 2006 |
| Self Reported Questionnaire (SRQ‐20) (Sheehan 1997) | 20 | 0 (no) to 1 (yes) | 0 to 20 | 0 to 5: normal 6 to 20: depression |
Participants report the presence or absence of depressive symptoms, thoughts, and behaviours | Nakimuli‐Mpungu 2015 |
| Profile of Mood States (POMS) Depression (D) (McNair 1971) |
15 | 0 (not at all) to 4 (extremely) | 0 to 60 | Higher scores indicate worsening depression | Participants rate adjectives describing their mood states over the past week | Antoni 20063 |
| Montgomery‐Asberg Depression Rating Scale (MADRS) (Montgomery 1979) | 10 | 0 (normal) to 6 (severe) | 0 to 60 | 0 to 6: normal 7 to 19: mild 20 to 34: moderate > 34: severe |
A physician assessment based on a clinical interview covering depression symptoms | Safren 20123 |
1The exact responses may vary between items. 2The descriptions in this table represent our best understanding of the scale derived from the description provided by the included studies and other information available through Internet searches. The exact scale used in the trials may have differed. 3These papers presented more than one measure of depression. In the main analysis only Beck Depression Inventory was presented. The additional measures are presented in Analysis 1.2 and had similar findings.
3. Anxiety scales reported by the included trials.
| Scale | Number of items (anxiety) | Score for each item1 | Total score (anxiety) | Interpretation | Description2 | Trials |
| Profile of Mood States
(POMS) Anxiety (A) (McNair 1971) |
9 | 0 (not at all) to 4 (extremely) | 0 to 36 | Higher scores indicate worsening anxiety | Participants rate adjectives describing their mood states over the past week | Antoni 2006 |
| Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983) | 7 | 0 (not at all) to 3 (very often) | 0 to 21 | 0 to 7: normal 8 to 10: borderline 11 to 21: anxiety |
Participants rate the frequency of anxiety feelings and behaviours over the preceding 4 weeks | Berger 2008; Gayner 2012 |
| State Trait‐Anxiety Inventory (STAI) (Spielberger 2010) | 20 | 1 (not at all) to 4 (very much so) | 20 to 80 | A cut point of 39 to 40 has been suggested to detect clinically significant symptoms | Participants rate the frequency and intensity of anxiety feelings at this moment (state), and more generally (trait) | Chesney 2003; Bormann 2006; Jones 2010 |
1The exact responses may vary between items. 2The descriptions in this table represent our best understanding of the scale derived from the description provided by the included studies and other information available through Internet searches. The exact scale used in the trials may have differed.
4. Stress scales reported by trials.
| Scale | Number of items | Score for each item1 | Total score | Interpretation | Description2 | Trials |
| Perceived Stress Scale (PSS) (Cohen 1983) | 10 | 0 (never) to 4 (very often) | 0 to 40 | Higher scores indicate higher perceived stress | Participants rate the frequency of stress related thoughts and feelings in the preceding 4 weeks | Chesney 2003; Bormann 2006; Duncan 2012 |
| Dealing with Illness Scale ‐ Stress subscale (DIS) (McCain 1992) | Unclear | Unclear | Unclear | Higher scores reflect higher stress | Participants rate the desirability or undesirability and personal impact of experienced events | McCain 2003 |
| HIV‐Related Life‐Stress Scale (Sikkema 2000) | 19 | 1 (not a problem) to 5 (most serious problem) | 1 to 5 | Higher scores indicate higher stress | Participants rate the severity of each HIV‐related potential stressor | Heckman 2007 |
| Life Experiences Survey (LES) (Sarason 1978) | 10 | 0 (not at all stressed) to 3 (extremely stressful) | 0 to 3 | 0 = not at all stressed 1 = mildly stressed 2 = moderately stressed 3 = extremely stressed |
Participants rate the extent to which an event commonly experienced by HIV‐positive women had been stressful | Antoni 2008 (a 10‐item abbreviated version) |
| Impact of Event Scale (IES) (Horowitz 1979) |
15 | 0 (not at all) to 4 (often) | 0 to 75 | Higher scores indicate higher impact | Participants rate the frequency of intrusive or avoidant thoughts and experiences over the preceding 7 to 28 days (the authors describe this scale as measuring 'psychological distress' or 'traumatic stress') | McCain 2003; Gayner 2012; Sikkema 2013 |
1The exact responses may vary between items. 2The descriptions in this table represent our best understanding of the scale derived from the description provided by the included studies and other information available through Internet searches. The exact scale used in the trials may have differed.
Excluded studies
We excluded a total of 70 trials (77 articles) after full‐text assessment and listed the reasons for exclusion in the 'Characteristics of excluded studies' table. Some studies were not RCTs or did not report on RCT data, and most studies did not include group interventions; this was not apparent when we only assessed the abstract. Other trials did not have at least a three‐month post‐intervention follow‐up assessment. Safren 2009 invited the control to cross‐over to the intervention after three months but had no post‐intervention three‐month follow‐up. For example, we excluded Wyatt 2004 as we did not receive any response from the study author about what data they included in the post‐intervention period, that is whether the data presented in this trial was the three‐month or six‐month follow‐up data; it remains unclear. Latkin 2003 and Yu 2014 did not have HIV‐positive populations. Six trials did not measure our specified outcomes. One trial, Laperriere 2005, presented a subgroup analysis from a larger trial. Prado 2012 had a sample of adolescents and not adults. Sacks 2011 did not report on effects for the group intervention.
We excluded seven trials conducted in Africa: Papas 2011 in Western Kenya measured reductions in alcohol use in people on antiretroviral (ARV) treatment using a CBT intervention so did not include a specified outcomes, Olley 2006 in Nigeria measured psychological distress, risky sexual behaviour, and self‐disclosure of HIV using an individual psychoeducation intervention rather than a group intervention, Mundell 2011 measured rates of disclosure and active and avoidant coping in pregnant and recently diagnosed HIV‐positive women in South Africa participating in structured up support groups, but this was not an RCT. Petersen 2014's trial on a group‐based counselling intervention for depression in South Africa did not have a three‐month follow‐up postintervention. Petersen 2014 also had a very small sample size. Kunutsor 2011's trial was based in Uganda but was a treatment supporter intervention and not a group intervention, and Jones 2005's trial among HIV positive women in Zambia did not have a control group. Kaaya 2013's trial in Tanzania measured prenatal HIV disclosure and depression, however we excluded it because there was no three‐month post‐intervention follow‐up.
Risk of bias in included studies
For a summary of the 'Risk of bias' assessments see Figure 3 and Figure 4.
3.
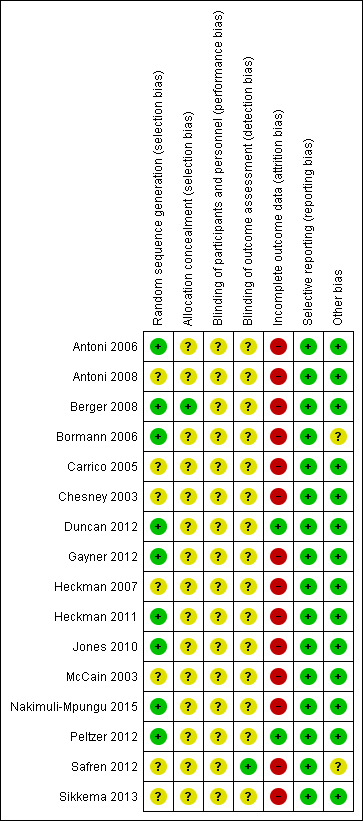
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial
4.
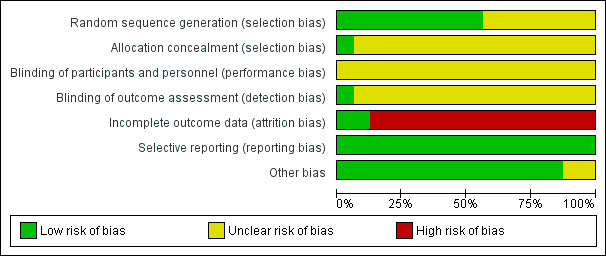
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included trials
Allocation
We only included 'randomized' trials. However, only Berger 2008 provided enough detail of the methods to be considered at low risk of selection bias. All other included trials were at unclear risk of selection bias.
Blinding
True blinding of participants to the types of interventions evaluated in this review is rarely possible. Trial authors did not describe blinding sufficiently. However, it is possible to blind those conducting the analysis but only one trial described the methods to achieve this and we judged it to be at low risk of detection bias (Safren 2012). All other trials were at unclear risk of detection bias.
Incomplete outcome data
Fourteen trials reported high levels of loss to follow‐up in at least one treatment arm over the duration of the trial (greater than 20%), or differential losses between groups, which had the potential to bias the results. Consequently we only judged two included trials to be at low risk of attrition bias (Duncan 2012; Peltzer 2012).
Selective reporting
We found no evidence of selective reporting and we considered all included trials as at low risk of reporting bias.
Other potential sources of bias
Bormann 2006 and Safren 2012 noted some imbalances in potential confounders between the intervention and control groups at baseline. We judged these trials to be at unclear risk of bias. All other included trials were at low risk of other sources of bias.
Effects of interventions
Summary of findings for the main comparison. 'Summary of findings' table 1.
| Group therapy (cognitive behavioural therapy (CBT)) versus control for improving psychological well‐being in adults living with HIV | |||||
| Patient or population: adults living with HIV Settings: any setting Intervention: group therapy based on CBT | |||||
| Outcomes | Illustrative comparative risks (95% CI)* | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Control | Group therapy (CBT) | ||||
| Depression score Follow‐up: 6 to 15 months | The mean scores in the control groups at the end of follow‐up ranged from normal to moderately depressed | The mean score in the intervention groups was: 0.26 standard deviations (SDs) lower (0.42 lower to 0.10 lower) | 1139 (10 trials) | ⊕⊕⊝⊝
low1,2,3,4 due to indirectness and risk of bias |
There may be a small benefit which lasts for up to 15 months |
|
Anxiety score Follow‐up: 6 to 15 months |
The mean scores in the control groups at the end of follow‐up ranged from normal to clinically anxious | The mean score in the intervention groups was: 0.12 SDs lower (0.31 lower to 0.06 higher) | 471 (4 trials) | ⊕⊕⊝⊝
low2,5,6,7 due to indirectness and risk of bias |
There may be little or no effect on mean anxiety scores |
|
Stress score Follow‐up: 6 to 15 months |
The mean score in the control groups at the end of follow‐up were variable | The mean score in the intervention groups was 0.04 SDs lower (0.23 lower to 0.15 higher) | 507 (5 trials) | ⊕⊕⊝⊝
low2,5,6,7 due to indirectness and risk of bias |
There may be little or no effect on mean stress scores |
|
Coping score Follow‐up: 6 to 15 months |
The mean score in the control groups at the end of follow‐up were variable | The mean score in the intervention groups was 0.04 SDs higher (0.11 lower to 0.19 higher) | 697 (5 trials) | ⊕⊕⊝⊝
low2,5,6,7 due to indirectness and risk of bias |
There may be little or no effect on mean coping scores |
| Studies used a variety of different scales to measure depression, anxiety and stress. Consequently, trials were pooled using a standardized mean difference. Examples of how large this effect would be on standardized measurement scales are given in the review main text and abstract. Abbreviations: CBT: cognitive behavioural therapy; CI: confidence interval; SD: standard deviation. | |||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||
1Downgraded by 1 for serious risk of bias: most of the trials did not adequately described a method of allocation concealment, and so trials are at unclear or high risk of selection bias. Loss of follow‐up was generally more than 20% and attrition bias may be present. 2No serious inconsistency: statistical heterogeneity between trials was low. 3Downgraded by 1 for serious indirectness: most trials were from high‐income settings (USA, Canada, and Switzerland), and in five trials the mean depression score at baseline was in the normal (not depressed) range. Only five trials evaluated groups with measurable levels of depression and in these trials the effects were inconsistent. 4No serious imprecision: the effect is small but statistically significant. The clinical significance is unclear. 5Downgraded by 1 for serious risk of bias: most of the trials did not adequately described methods to prevent selection bias. 6Downgraded by 1 for serious indirectness: although effects were not seen in these few trials, we cannot exclude the possibility of effects in some populations. 7No serious imprecision: the effect size is close to zero with a narrow 95% CI.
Summary of findings 2. 'Summary of findings' table 2.
| Group therapy (mindfulness) compared to control for improving psychological well‐being in adults living with HIV | |||||
| Patient or population: adults living with HIV Settings: any setting Intervention: group therapy based on mindfulness | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Control | Group therapy (Mindfulness) | ||||
|
Depression score Follow‐up: 4 to 6 months |
The mean scores in the control groups at the end of follow‐up were in the range of normal to mild depression | The mean score in the intervention groups was 0.23 standard deviations (SDs) lower (0.49 lower to 0.03 higher) | 233 (3 trials) | ⊕⊝⊝⊝
very low1,2,3,4 due to risk of bias, indirectness, and imprecision |
We don't know if there is a benefit on depression scores |
|
Anxiety score Follow‐up: 4 to 6 months |
The mean scores in the control group at the end of follow‐up were in the range of normal to mild anxiety | The mean score in the intervention groups was 0.16 SDs lower (0.47 lower to 0.15 higher) | 162 (2 trials) | ⊕⊝⊝⊝
very low1,3,4 due to risk of bias, indirectness, and imprecision |
We don't know if there is an effect on mean anxiety scores |
|
Stress score Follow‐up: 4 to 6 months |
The mean scores in the control group at the end of follow‐up were in the range of mild stress | The mean score in the intervention groups was 2.02 points lower (4.23 lower to 0.19 higher) | 137 (2 trials) | ⊕⊝⊝⊝
very low1,3,4 due to risk of bias, indirectness, and imprecision |
We don't know if there is an effect on mean stress scores |
|
Coping score Follow‐up: no coping was measured by mindfulness intervention trials |
— | — | 0 (0 trials) |
— | — |
| Studies used a variety of different scales to measure depression, anxiety and stress. Consequently, trials were pooled using a standardized mean difference. Examples of how large this effect would be on standardized measurement scales are given in the review main text and abstract. Abbreviations: CI: confidence interval; OR: odds ratio; SD: standard deviation. | |||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||
1Downgraded by 1 for serious risk of bias: none of the trials adequately described a method of allocation concealment, and so trials are at unclear or high risk of selection bias. Loss of follow‐up was generally more than 20% and attrition bias may be present. 2No serious inconsistency: statistical heterogeneity between trials was low. 3Downgraded by 1 for serious indirectness: these three trials were conducted in the USA and Canada in people with scores in the range of mild to moderate depression. The results are not easily generalized to other settings or populations. 4Downgraded by 1 for serious imprecision: the 95% CI are wide and includes both potentially important effects and no effect.
See 'Summary of findings' table 1 (Table 1) and 'Summary of findings' table 2 (Table 2). We have presented the findings by intervention type.
Comparison 1: Group therapy (CBT) versus control
See 'Summary of findings' table 1 (Table 1).
Thirteen trials evaluated group therapy sessions based on cognitive behavioural therapy (CBT) techniques. Ten trials were delivered face‐to‐face (Chesney 2003; McCain 2003; Carrico 2005; Antoni 2006; Antoni 2008; Berger 2008; Jones 2010; Peltzer 2012; Nakimuli‐Mpungu 2015) and two were delivered by telephone (Heckman 2007; Heckman 2011). The primary focus of CBT was stress reduction or coping (nine trials), self‐efficacy (one trial), depression (one trial), adherence (one trial), and adherence and depression (one trial). Eight trials described additional relaxation components such as progressive muscle relaxation (PMR) or meditation, four explicitly described methods to facilitate peer/group support, and six described specific educational components. Most sessions lasted between 50 and 135 minutes, were conducted weekly for between eight and 12 weeks, and included between four and 12 participants (Table 7).
5. Description of cognitive‐behavioural interventions.
| Trial | Group size | Session duration (mins) | Session frequency | Intervention | Comparison | |||||
| Underlying theory | Primary focus | Components | ||||||||
| Skills training | Relaxation techniques | Peer support2 | Education2 | |||||||
| McCain 2003 | 6 to 10 | 90 | Weekly for 8 weeks | Cognitive‐behavioural | Stress management | Cognitive restructuring coping skills |
Progressive muscle relaxation (PMR)/meditation/yoga | Not described | Not described | No intervention ‐ waitlist group |
| Carrico 2005 | 4 to 9 | 135 | Weekly for 10 weeks | Cognitive‐behavioural | Stress management | Cognitive restructuring, coping skills, anger management, use of social network | PMR/meditation | Not described | Not described | No intervention ‐ waitlist group |
| Antoni 2006 | 4 to 9 | 135 | Weekly for 10 weeks | Cognitive‐behavioural | Stress management | Cognitive restructuring, coping skills | PMR/meditation | Not described | Medication adherence | 1 hour medication adherence training (MAT) |
| Antoni 2008 | 4 to 6 | 135 | Weekly for 10 weeks | Cognitive‐behavioural | Stress management | Cognitive restructuring, coping skills, assertiveness, anger management, use of social network | PMR/meditation | Not described | Not described | Condensed 1 day CBSM workshop |
| Berger 2008 | 4 to 10 | 120 | Weekly for 12 weeks | Cognitive‐behavioural | Stress management | Cognitive strategies | PMR | Group dynamic exercises | HIV related topics | No intervention ‐ 30‐minute health check by physician |
| Sikkema 2013 | 10 | 90 | Weekly for 15 weeks | Cognitive theory of stress and coping | Traumatic stress | Cognitive appraisal, coping skills | 'relaxation strategies' | Not described | Not described | Attention control ‐ HIV standard therapeutic support group |
| Chesney 2003 | 8 to 10 | 90 | Weekly for 10 weeks | Cognitive theory of stress and coping | Coping/stress | Appraising stressors, coping skills, stress management | 'relaxation guidance' | Skill building group exercises | Psychoeducation around models of coping | No intervention ‐ waitlist group |
| Heckman 2007 | 6 to 8 | 90 | Weekly for 8 weeks | Transactional model of stress and coping | Coping | Appraising stressors, coping skills, use of personal and social resources | No | Not described | Not described | No intervention ‐ usual care |
| Heckman 2011 | 6 to 810 | 90 | Weekly for 12 weeks | Transactional model of stress and coping | Coping | Appraising stressors, coping skills, use of personal and social resources | No | Not described | Not described | No intervention ‐ individual therapy upon request (ITUR) |
| Jones 2010 | Not stated | 120 | Weekly for 10 weeks | Cognitive‐behavioural | Self‐efficacy | Cognitive restructuring, stress management, coping skills, anger management, use of social network | 'relaxation' | Expressive supportive therapy | HIV/mental health topics | Attention control group |
| Nakimuli‐Mpungu 2015 | 10 to 12 | 120 to 180 | Weekly for 8 weeks | Cognitive‐behavioural, social learning theory, and the sustainable livelihoods framework | Depression | Coping skills, problem solving skills, dealing with stigma, income‐generation | Not described | Group rituals | Triggers, symptoms, and treatment of depression. | Active control group |
| Safren 2012 | Not stated | 50 mins | Weekly for 9 weeks | Cognitive‐behavioural | Adherence/ depression | Cognitive restructuring, problem solving, activity scheduling | PMR/diaphragmatic breathing | Not described | Adherence, depression | Enhanced treatment as usual (ETAU) |
| Peltzer 2012 | 10 | 60 mins | Monthly for 3 months | Cognitive‐behavioural | Adherence | Not specifically described | Not described | Buddy system to increase social support | Knowledge of HIV and HIV‐related medication | No intervention ‐ standard care |
Abbreviations: HIV: human immunodeficiency virus; PMR: progressive muscle relaxation. 1The interventions used in McCain 2003, Carrico 2005, Antoni 2006, Antoni 2008, Berger 2008, and Jones 2010 appear to be very similar. 2Although peer support and education were often not well described in these papers, these aspects are inevitable with group therapy and are likely to be an important factor in any observed effect regardless of the therapeutic theory.
Fourteen trials were from high‐income settings (USA, Canada, and Switzerland). Two trials were from low‐ or middle‐income settings: South Africa (Peltzer 2012), and Uganda (Nakimuli‐Mpungu 2015). Five trials were conducted among general populations, three trials with homosexual men, one trial with women from minority groups, one trial with women with an abnormal cervical smear, one trial among prior intravenous drug users, one trial among people with a history of being abused, and one trial among people with adherence problems (see Table 3).
Depression scores
Overall, there was a small reduction in mean depression scores at the end of the group sessions (standardized mean difference (SMD) −0.17, 95% confidence interval (CI) −0.29 to −0.05; 1142 participants, 9 trials; low certainty evidence; Analysis 1.1), and this appeared to be maintained for up to 15 months after randomization (SMD −0.26, 95% CI −0.42 to −0.10; 1139 participants, 10 trials, low certainty evidence; Analysis 1.1).
1.1. Analysis.
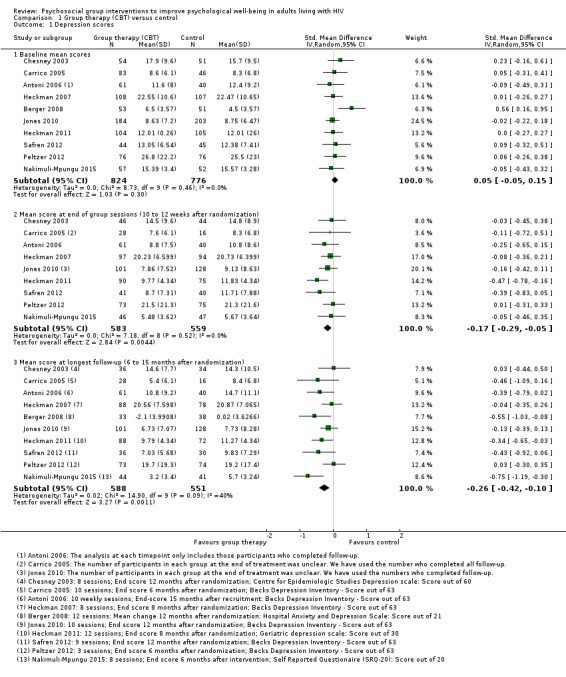
Comparison 1 Group therapy (CBT) versus control, Outcome 1 Depression scores.
The most commonly used depression score was Beck Depression Inventory (BDI) (used by six trials), which scores depression out of 63. The overall effect was a reduction of around 1.4 points (mean difference (MD) −1.41, 95% CI −2.61 to −0.21; 753 participants, 6 trials, Analysis 1.2). See Table 4 for a description of the different depression scores that the included trials used.
1.2. Analysis.
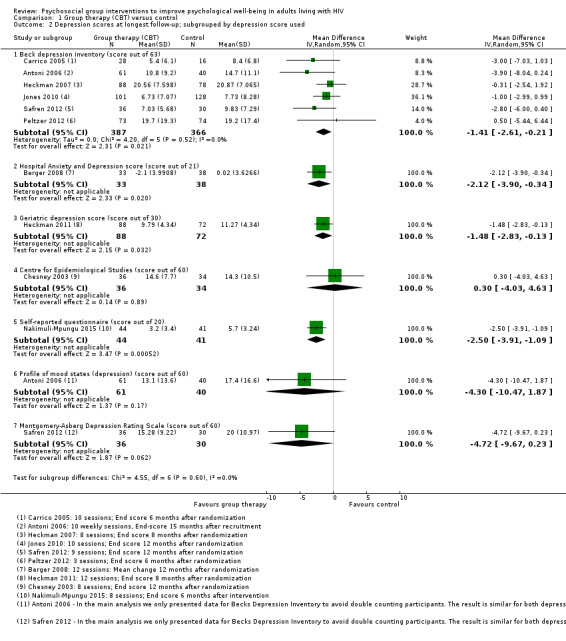
Comparison 1 Group therapy (CBT) versus control, Outcome 2 Depression scores at longest follow‐up; subgrouped by depression score used.
Mean depression scores at baseline were in the depressive range in five trials. Over the duration of these trials, there was a modest improvement in mean score in both the intervention and control groups, but only two trials showed increased benefit with the intervention (Analysis 1.3). In the remaining five trials mean depression scores were in the normal range at baseline, and there was a small but consistent reduction in mean score with the intervention (Analysis 1.5).
1.3. Analysis.
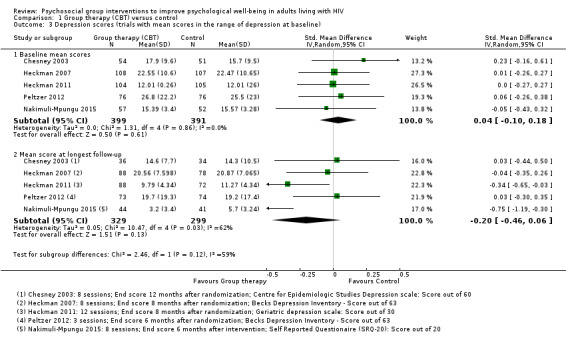
Comparison 1 Group therapy (CBT) versus control, Outcome 3 Depression scores (trials with mean scores in the range of depression at baseline).
1.5. Analysis.
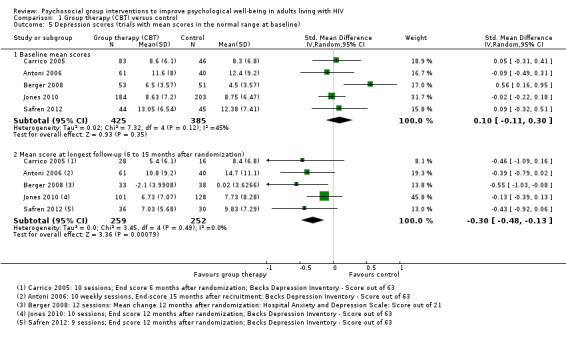
Comparison 1 Group therapy (CBT) versus control, Outcome 5 Depression scores (trials with mean scores in the normal range at baseline).
We conducted additional subgrouping by the type of control group used (Analysis 1.4), the primary focus of the intervention (Analysis 1.6), and gender (Analysis 1.7). The most consistent effects on depression were from three trials that focused on stress management interventions (SMD −0.46, 95% CI −0.73 to −0.18; 216 participants, 3 trials).
1.4. Analysis.
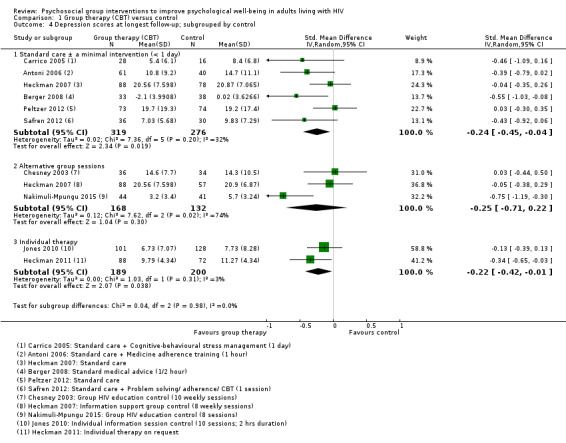
Comparison 1 Group therapy (CBT) versus control, Outcome 4 Depression scores at longest follow‐up; subgrouped by control.
1.6. Analysis.
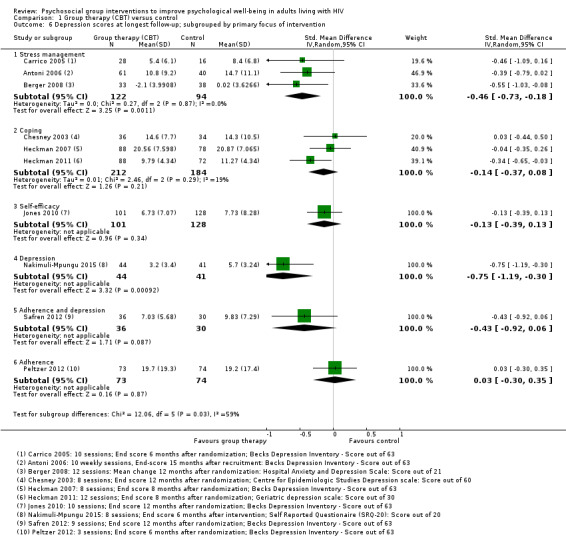
Comparison 1 Group therapy (CBT) versus control, Outcome 6 Depression scores at longest follow‐up; subgrouped by primary focus of intervention.
1.7. Analysis.
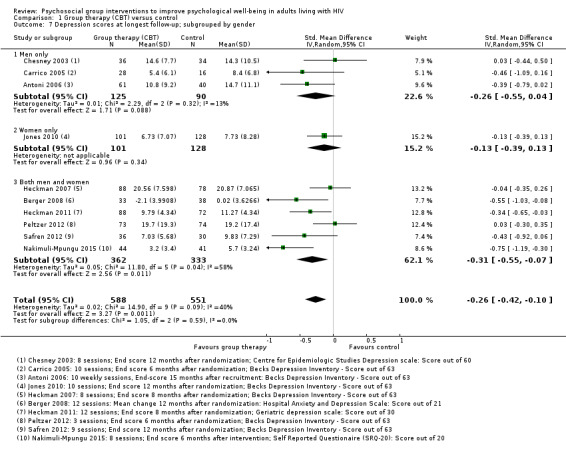
Comparison 1 Group therapy (CBT) versus control, Outcome 7 Depression scores at longest follow‐up; subgrouped by gender.
Anxiety scores
Four trials assessed measures of anxiety, and overall there was no effect apparent at the end of the group sessions (SMD −0.01, 95% CI −0.25 to 0.22; 420 participants, 3 trials, low certainty evidence), or at 12 to 15 months after randomization (SMD −0.12, 95% CI −0.31 to 0.06; 471 participants, 4 trials, low certainty evidence; Analysis 1.8).
1.8. Analysis.
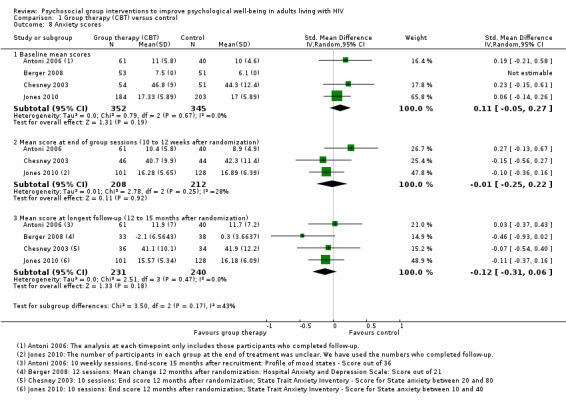
Comparison 1 Group therapy (CBT) versus control, Outcome 8 Anxiety scores.
All four trials used different anxiety scales, and only one trial (using the Hospital Anxiety and Depression Scale) reported a statistically significant effect (see Table 5 and Analysis 1.9). This study was conducted in Switzerland among the general population, and baseline and end anxiety scores were in the normal range (Berger 2008). Only the participants recruited by Chesney 2003 appeared to have substantial anxiety at baseline, and although there was a small improvement in both groups over the course of the trial, there were no differences between groups at any time point.
1.9. Analysis.
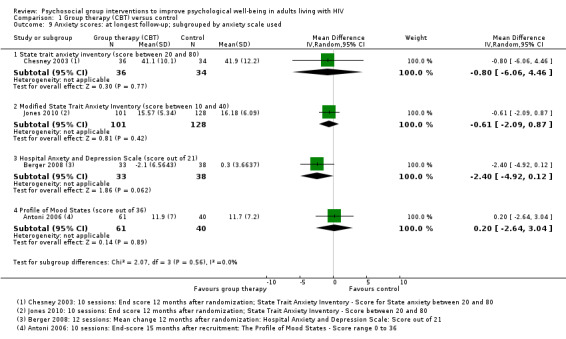
Comparison 1 Group therapy (CBT) versus control, Outcome 9 Anxiety scores: at longest follow‐up; subgrouped by anxiety scale used.
Stress scores
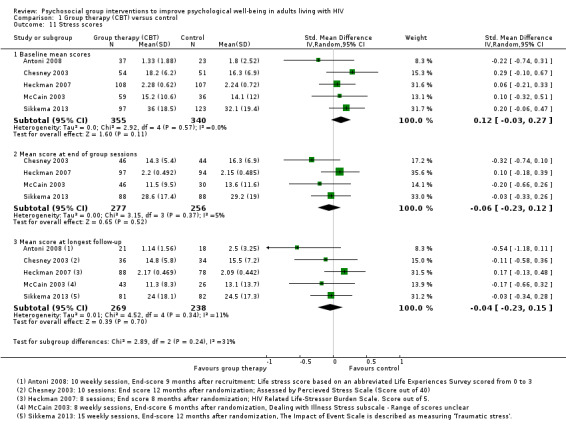
Five trials assessed measures of stress, and overall there was no effect apparent at the end of group sessions (SMD −0.06, 95% CI −0.23 to 0.12; four trials, 533 participants, low certainty evidence, Analysis 1.11), or at the end of follow‐up (SMD −0.04, 95% CI −0.23 to 0.15; five trials, 507 participants, low certainty evidence, Analysis 1.11). Only Antoni 2008, which was conducted among HIV‐positive women with a recent abnormal cervical smear, found a statistically significant effect at any time point. For a description of the stress scores used see Table 6 and Analysis 1.12.
1.11. Analysis.
Comparison 1 Group therapy (CBT) versus control, Outcome 11 Stress scores.
1.12. Analysis.
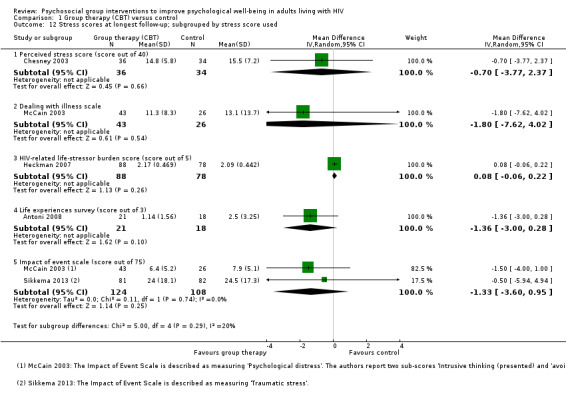
Comparison 1 Group therapy (CBT) versus control, Outcome 12 Stress scores at longest follow‐up; subgrouped by stress score used.
Coping scores
Five trials reported some measure of coping with no effects seen at the end of group therapy (SMD 0.02, 95% CI −0.16 to 0.19; 762 participants, 5 trials; low certainty evidence, Analysis 1.14), or at the end of follow‐up (SMD 0.04, 95% CI −0.11 to 0.19; 697 participants, 5 trials; low certainty evidence; Analysis 1.14). In all measures of coping, higher scores reflected increased coping (see Table 8 and Analysis 1.15).
1.14. Analysis.
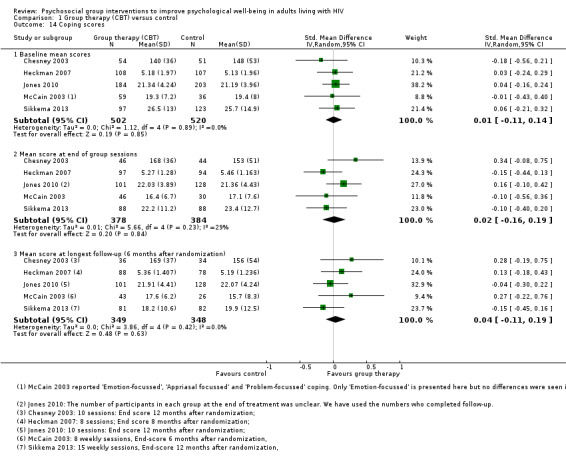
Comparison 1 Group therapy (CBT) versus control, Outcome 14 Coping scores.
6. Coping scales reported by trials.
| Scale | Number of items | Score for each item1 | Total score | Interpretation | Description2 | Trials |
| Coping Self‐Efficacy Scale (CSES) (Chesney 2006) | 26 | 0 (cannot do at all) to 10 (certain can do) | 0 to 260 | Higher scores indicate better coping skills | Participants rate the extent to which they believe they could perform behaviours important to adaptive coping | Chesney 2003; Heckman 2007 |
| Cognitive Behavioral Self Efficacy (CB‐SE) (Ironson 1987) | 7 | 0 (not at all) to 4 (all of the time) | 0 to 28 | Higher scores indicate higher self‐efficacy | Participants rate their certainty that they could perform certain skills related to AIDS, and antiretroviral medication adherence | Jones 2010 |
| Dealing with Illness Scale ‐ coping subscale (DIS) (McCain 1992) | 40 | 0 (never used) to 3 (regularly used) | 0 to 120 | Higher scores reflect more frequent use of the various coping strategies. | The DIS is a 40‐item coping subscale modelled on the Revised Ways of Coping Checklist. Participants rate the frequency that thoughts or behaviours have been used to deal with problems and stresses over the past month. | McCain 2003 |
| 'Avoidant Coping Scale' Created from 23 items taken from 'The ways of Coping Questionnaire' and 'the Coping with AIDS Scale' (Sikkema 2013) |
23 | 0 (not at all) to 3 (used a great deal) | 0 to 69 | Higher scores reflect more frequent use of coping strategies | Participants rate how often they have used avoidant strategies for coping | Sikkema 2013 |
1The exact responses may vary between items. 2The descriptions in this table represent our best understanding of the scale derived from the description provided by the included studies and other information available through Internet searches. The exact scale used in the trials may have differed.
1.15. Analysis.
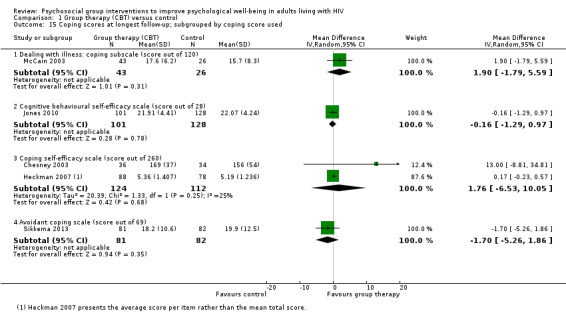
Comparison 1 Group therapy (CBT) versus control, Outcome 15 Coping scores at longest follow‐up; subgrouped by coping score used.
Comparison 2: Group therapy (mindfulness) versus control
Three trials evaluated face‐to‐face group therapy sessions based on mindfulness stress reduction (Bormann 2006; Duncan 2012; Gayner 2012). The interventions included mindfulness meditation, with one intervention including mantram repetition and one described additional educational components. Group sessions lasted between 135 and 180 minutes, and were conducted weekly for eight to 10 weeks, plus one day retreat, The group size was eight to 18 participants across trials (see Table 9).
7. Description of mindfulness interventions.
| Trial | Group size | Session duration (mins) | Session frequency | Intervention | Comparison | |||
| Primary focus | Secondary components | |||||||
| Relaxation techniques | Peer support | Education | ||||||
| Duncan 2012 | Not stated | 150 to 180 | Weekly for 8 weeks plus 1 day retreat | Mindfulness‐stress reduction | Mindfulness meditation | Not described | Stress physiology and reactivity | No intervention ‐ waitlist group |
| Gayner 2012 | 14 to 18 | 180 | Weekly for 8 weeks plus 1 day retreat | Mindfulness‐stress reduction | Mindfulness meditation | Not described | Not described | No intervention ‐ treatment as usual (TAU) group offered at end of intervention |
| Bormann 2006 | 8 to 15 | 90 | Weekly for 5 weeks, then 4 automated phone calls, then a final session in week 10 | Mindfulness‐stress reduction | Mantram repetition | Not described | Not described | Attention control |
All trials were conducted in high‐income settings. One trial was conducted among homosexual men in Canada (Gayner 2012), one trial was among men and women experiencing some side effects or distress in the USA (Duncan 2012), and the other included trial was among men and women (50%) who were homosexual (Bormann 2006) (see Table 3).
Depression scores
Individually, none of these trials reported statistically significant effects on mean depression scores. The pooled effect suggests a modest reduction in mean scores at the end of the group sessions (SMD −0.22, 95% CI −0.48 to 0.04; 242 participants, 3 trials; very low certainty evidence, Analysis 2.1), and at six months postrandomization (SMD −0.23, 95% CI −0.49 to 0.03; 233 participants, 3 trials;very low certainty evidence; Analysis 2.1), but the 95% CIs were wide and included no effect.
2.1. Analysis.
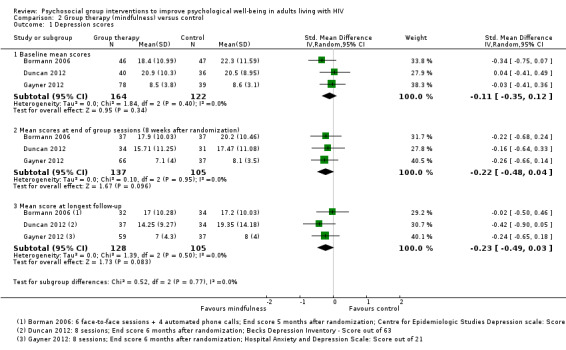
Comparison 2 Group therapy (mindfulness) versus control, Outcome 1 Depression scores.
Anxiety scores
Bormann 2006 and Gayner 2012 measured anxiety scores using different measurement scales. There were no statistically significant differences in the mean scores at the end of the group sessions (SMD −0.23, 95% CI −0.53 to 0.07; 178 participants, 2 trials, very low certainty evidence; Analysis 2.2), or at six months (SMD −0.16, 95% CI −0.47 to 0.15; 162 participants, 2 trials, very low certainty evidence; Analysis 2.2).
2.2. Analysis.
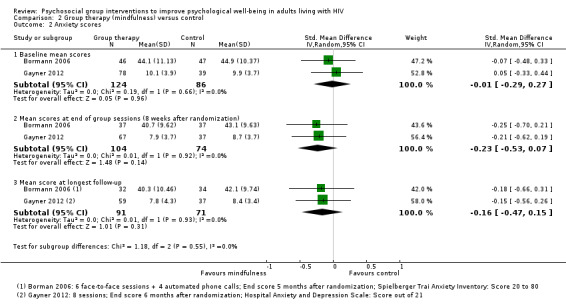
Comparison 2 Group therapy (mindfulness) versus control, Outcome 2 Anxiety scores.
Stress scores
Bormann 2006 and Duncan 2012 measured stress scores using the perceived stress score, A small statistically significant difference was present at the end of group sessions (MD −2.29, 95% CI −4.46 to −0.11; 139 participants, 2 trials; very low certainty evidence), but not at six months postrandomization (MD −2.02, 95% CI −4.23 to 0.19; 137 participants, 2 trials, very low certainty evidence; Analysis 2.3). The effect size was around 2 points on a 40‐point scale.
2.3. Analysis.
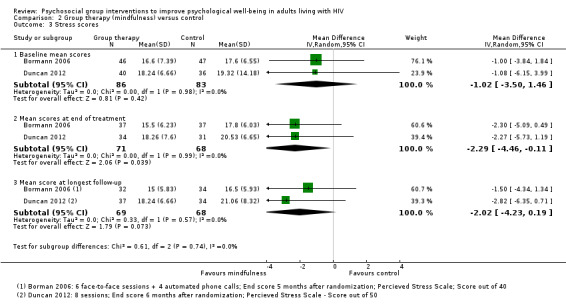
Comparison 2 Group therapy (mindfulness) versus control, Outcome 3 Stress scores.
Discussion
Summary of main results
Group‐based psychosocial interventions based on cognitive behavioural therapy (CBT) may have a small effect on measures of depression, and this effect may last for up to 15 months after participation in the group sessions (low certainty evidence). Most trials used the Beck Depression Inventory (BDI) which has a maximum score of 63, and the mean score in the intervention groups was around 1.4 points lower at the end of follow‐up. This small benefit was consistent across five trials where participants had a mean depression score in the normal range at baseline, but trials where the mean score was in the depression range at baseline effects were less consistent. Fewer trials reported measures of anxiety (low certainty evidence), stress (low certainty evidence), and coping (low certainty evidence), and there was no clear evidence of effects.
Group‐based interventions based on mindfulness have not demonstrated effects on measures of depression (very low certainty evidence), anxiety (very low certainty evidence), or stress (very low certainty evidence).
Overall completeness and applicability of evidence
Most of the trial interventions were based on cognitive‐behavioural approaches, which were delivered in similar ways (with similar group sizes, similar trained facilitators, and similar numbers of sessions). Most included trials were conducted in high‐income settings (USA, Canada, and Switzerland). Two trials of CBT were conducted in low‐ and middle‐income countries, which is where the prevalence of HIV is highest; over 95% of HIV infections are in low‐ and middle‐income countries, two‐thirds of them in sub‐Saharan Africa (Boyle 2016). One trial was conducted among an urban population in Uganda and measures of depression improved significantly in both the intervention and control groups over the course of the study (greater than 10 points on a 20‐point scale) with only small differences between groups (2.5 points). The small effects in the Uganda trial are unlikely to be cost‐effective where resources and trained staff are scarce. The trial conducted among depressed adults on ART in South Africa showed no effects between the two groups for depression.
One of the main aims of this Cochrane Review was to evaluate whether effects seen at the end of group sessions persisted, or were lost, and our findings do seem to suggest that improvements at the end of group session are sustained for up to 15 months. However, as over half the included trials had mean scores at baseline within the normal range (not depressed), the clinical significance of this effect is unclear. In trials that did contain people with measurable depression the effects were inconsistent and there were too few trials to explore why some interventions seemed to have a benefit and some did not.
The included trials used a variety of measurement scales to assess each outcome which made pooling of studies and comparison across population groups difficult.
Quality of the evidence
We rated the certainty of the evidence for a small effect on measures of depression with CBT group therapy as 'low' meaning we can only have low confidence in the observed effect. We downgraded the evidence from high to low due to 'risk of bias': as most studies were at unclear risk of selection bias and high risk of attrition bias, and due to 'indirectness' of the evidence (with most trials being from high income settings), and the difficulty in generalising the findings to other settings and population groups. We also rated the evidence of no effect on anxiety, stress, and coping as 'low' for the same reasons.
For mindfulness‐based group interventions, we rated all outcomes as 'very low' meaning we are very uncertain about the observed effect. We downgraded the evidence for 'risk of bias' due to deficiencies in the study methods, 'indirectness' due to the difficulty generalising the findings to other populations and settings, and 'imprecision' as the effect is towards benefit but the 95% CI are wide and include no effect.
Potential biases in the review process
The inclusion criteria of this Cochrane Review may increase the possibility of poor reproducibility due to many subjective decisions regarding which studies and populations to include. We included all HIV‐positive populations (both genders) and we had no exclusion criteria regarding settings when we selected studies for inclusion. However, we minimized this potential bias by including stringent follow‐up time criteria and only RCTS.
We only assessed peer‐reviewed published trials, which may have led to exclusion of trials in grey literature and presented at conferences. Having no knowledge of unpublished studies may have limited the assessment of the strength of the body of evidence in the review. Another limitation is that we only included papers that reported in English, thus disregarding high quality trials that may have been published in other languages.
We selected only trials that used validated scales, which is a strength of the review. We amended specified outcomes by only including psychological outcomes and no longer behavioural outcomes after we realized that there were poor or invalidated assessments for behavioural outcomes in the first set of literature search results (June 2013). Our decision to change specified outcomes after seeing the results for included studies could have lead to biased and misleading interpretation if the importance of the outcome (primary or secondary) is changed on the basis of those results. We have provided transparency in the reporting of changes in outcome specification to reduce this risk of bias.
Even when review authors have a common understanding of the selection criteria, random error or mistakes may result from individual errors in reading and reviewing studies. We reduced this potential bias by ensuring that all review authors read the papers and extracted data independently, and then came to mutual agreements.
We noted issues regarding poor reporting in the papers and we were unable to obtain all relevant data due to the lack of responses from study authors about missing data or clarification of data.
Agreements and disagreements with other studies or reviews
To our knowledge, no published systematic review has assessed the psychological impact of psychosocial group interventions for HIV positive adults.
The findings of this Cochrane Review are in contrast to a narrative review (Brown 2011), as well as older meta‐analysis syntheses (Crepaz 2008; Scott‐Sheldon 2008), of the effect of various group interventions on outcomes for improved mental health. These report on how stress management and cognitive behavioural interventions and mindfulness‐based interventions are effective in improving various psychological states in people living with HIV. However, Crepaz 2008 did not assess the randomization method, allocation concealment, and blinding used by the trials, thus making data extraction and quality assessment less rigorous. Scott‐Sheldon 2008's meta‐analytic review of RCTs of stress‐management interventions showed significant overall impact on mental health, whereas Brown 2011's narrative critique of the effectiveness of mindfulness based intervention is the only review of the effectiveness of mindfulness‐based interventions on mental health that we found. However, the Brown 2011 review is undermined by inclusion of trials that did not fit our stringent inclusion criteria that trials must have had longer than three months follow‐up in order to show sustained effects, and did not include a meta‐analysis. Similar to our Cochrane Review, current published reviews on this subject mainly include trials in high‐income settings, which makes a strong case for high quality research to be funded and motivated for marginalized populations where HIV is most prevalent.
Authors' conclusions
Implications for practice.
Group‐based psychosocial interventions may have a small but sustained effect on measures of depression. However, the clinical importance of this is unclear as we only consistently observed the small effect in trials where the mean score of participants in both the intervention and control groups was in the normal range (not depressed) for the duration of the trial.
Implications for research.
Further trials that include people with signs of depression, stress, or poor coping at baseline will provide evidence of improving psychological adjustment and coping for those who need it most.
Further randomized studies should also meet current standards in reporting of methods as outlined in the CONSORT statement and take appropriate steps to reduce the risk of bias.
To enable a clear understanding of the results, and facilitate future meta‐analyses, it would also be useful if this research field utilized a common set of outcome scales rather than the ad‐hoc selection that is seen across the included studies.
Additionally, evidence is lacking where HIV prevalence is the highest. This indicates a need for strengthening trial research capacity in low‐ and middle‐income settings.
Acknowledgements
We are grateful to Anne‐Marie Stephani and Paul Garner of the Cochrane Infectious Diseases Group (CIDG), and Elizabeth Pienaar and Tamara Kredo of Cochrane South Africa for their valuable encouragement and help with this review.
David Sinclair is supported by the Effective Health Care Research Consortium. This Consortium and the editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
Appendices
Appendix 1. Search strategy
| Search set | CENTRAL | MEDLINE | Embase |
| 1 | MeSH descriptor: [HIV Infections] explode all trees | (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp])) | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR 'human immuno+deficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune+deficiency virus':ab,ti OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno+deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti OR 'acquired immune+deficiency syndrome':ab,ti |
| 2 | MeSH descriptor: [HIV] explode all trees | (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | (psychosocial NEXT/1 intervention*):ab,ti OR 'social support'/syn OR 'social support':ab,ti OR (social NEXT/1 network*):ab,ti OR (support NEXT/1 system*):ab,ti OR 'self help'/syn OR ('self‐help' NEXT/1 group*):ab,ti OR (support NEXT/1 group*):ab,ti OR 'educational therapy':ab,ti OR 'psychotherapy'/syn OR psychotherapy:ab,ti OR 'group therapy'/syn OR 'group therapy':ab,ti OR 'behavior therapy':ab,ti OR 'behaviour therapy':ab,ti OR 'family therapy':ab,ti OR 'group interventions':ab,ti OR 'cognitive therapy':ab,ti OR 'cognition therapy':ab,ti OR (psychological NEXT/1 adjustment*):ab,ti OR 'psychological adaptation':ab,ti OR (adaptive NEXT/1 behavior*):ab,ti OR (adaptive NEXT/1 behaviour*):ab,ti OR (coping NEXT/1 behavio*):ab,ti OR (coping NEXT/1 intervention*):ab,ti OR (coping NEXT/1 strateg*):ab,ti OR (coping NEXT/1 skill*):ab,ti |
| 3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or HIV INFECT* or HUMAN IMMUNODEFICIENCY VIRUS or HUMAN IMMUNEDEFICIENCY VIRUS or HUMAN IMMUNE‐DEFICIENCY VIRUS or HUMAN IMMUNO‐DEFICIENCY VIRUS or HUMAN IMMUN* DEFICIENCY VIRUS or ACQUIRED IMMUNODEFICIENCY SYNDROME or ACQUIRED IMMUNEDEFICIENCY SYNDROME or ACQUIRED IMMUNO‐DEFICIENCY SYNDROME or ACQUIRED IMMUNE‐DEFICIENCY SYNDROME or ACQUIRED IMMUN* DEFICIENCY SYNDROME | (psychosocial intervention*[tiab] OR social support[mh] OR social network*[tiab] OR social support[tiab] OR support system[tiab] OR support systems[tiab] OR self‐help groups[mh] OR self‐help group[tiab] OR self‐help groups[tiab] OR support group[tiab] OR support groups[tiab] OR educational therapy[tiab] OR psychotherapy[mh] OR psychotherapy[tiab] OR behavior therapy[tiab] OR behaviour therapy[tiab] OR family therapy[tiab] OR group therapy[tiab] OR group interventions[tiab] OR cognitive therapy[tiab] OR cognition therapy[tiab] OR adaptation, psychological[mh] OR psychological adjustment[tiab] OR psychological adjustments[tiab] OR psychological adaptation[tiab] OR adaptive behavior[tiab] OR adaptive behaviors[tiab] OR adaptive behaviour[tiab] OR adaptive behaviours[tiab] OR coping behavio* [tiab] OR coping intervention*[tiab] OR coping strateg*[tiab] OR coping skill*[tiab]) | #1 AND #2 |
| 4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | (#1 AND #2 AND #3) | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl* NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*):ab,ti |
| 5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only[DW1] | Search (((#1 AND #2 AND #3))) | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de |
| 6 | #1 or #2 or #3 or #4 or #5 | — | 'human'/de OR 'normal human'/de OR 'human cell'/de |
| 7 | MeSH descriptor: [Social Support] explode all trees | — | #5 AND #6 |
| 8 | MeSH descriptor: [Self‐Help Groups] explode all trees | — | #5 NOT #7 |
| 9 | MeSH descriptor: [Psychotherapy] explode all trees | — | #4 NOT #8 |
| 10 | MeSH descriptor: [Adaptation, Psychological] explode all trees | — | #3 AND #9 |
| 11 | "psychosocial intervention*":ti,ab,kw or "social support":ti,ab,kw or (social next network*):ti,ab,kw or (support next system*):ti,ab,kw or (self‐help next group*):ti,ab,kw or (support next group*):ti,ab,kw or educational therapy:ti,ab,kw or psychotherapy:ti,ab,kw or "behavior therapy":ti,ab,kw or "behaviour therapy":ti,ab,kw or "family therapy":ti,ab,kw or "group therapy":ti,ab,kw or "group interventions":ti,ab,kw or "cognitive therapy":ti,ab,kw or "cognition therapy":ti,ab,kw or (psychological next adjustment*):ti,ab,kw or "psychological adaptation":ti,ab,kw or "adaptive behavior":ti,ab,kw or "adaptive behaviour":ti,ab,kw or "coping behavior":ti,ab,kw or "coping behaviour":ti,ab,kw or "coping skills":ti,ab,kw or "coping strategies":ti,ab,kw (Word variations have been searched) | — | #3 AND #9 |
| 12 | #7 or #8 or #9 or #10 or #11 | — | — |
| 13 | #6 and #12 | — | — |
Appendix 2. ClinicalTrials.gov search strategy
Search strategy: (psychosocial OR "social support" OR "coping skills" OR "self‐help groups" OR "cognitive therapy") AND hiv | Interventional Studies | received from 01/01/1996 to 03/14/2016
Appendix 3. Data extraction form
| Authors and year | Sample size and population characteristics | Trialsetting | Trialdesign | Trialobjectives | Methodological criteria | Eligibility criteria | Intervention components | Outcome measures | Results and effect size |
Data and analyses
Comparison 1. Group therapy (CBT) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression scores | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Baseline mean scores | 10 | 1600 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.05, 0.15] |
| 1.2 Mean score at end of group sessions (10 to 12 weeks after randomization) | 9 | 1142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.29, ‐0.05] |
| 1.3 Mean score at longest follow‐up (6 to 15 months after randomization) | 10 | 1139 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.42, ‐0.10] |
| 2 Depression scores at longest follow‐up; subgrouped by depression score used | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Beck depression inventory (score out of 63) | 6 | 753 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐2.61, ‐0.21] |
| 2.2 Hospital Anxiety and Depression score (score out of 21) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐3.90, ‐0.34] |
| 2.3 Geriatric depression score (score out of 30) | 1 | 160 | Mean Difference (IV, Random, 95% CI) | ‐1.48 [‐2.83, ‐0.13] |
| 2.4 Centre for Epidemiological Studies (score out of 60) | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐4.03, 4.63] |
| 2.5 Self‐reported questionnaire (score out of 20) | 1 | 85 | Mean Difference (IV, Random, 95% CI) | ‐2.5 [‐3.91, ‐1.09] |
| 2.6 Profile of mood states (depression) (score out of 60) | 1 | 101 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐10.47, 1.87] |
| 2.7 Montgomery‐Asberg Depression Rating Scale (score out of 60) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐4.72 [‐9.67, 0.23] |
| 3 Depression scores (trials with mean scores in the range of depression at baseline) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Baseline mean scores | 5 | 790 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.10, 0.18] |
| 3.2 Mean score at longest follow‐up | 5 | 628 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.46, 0.06] |
| 4 Depression scores at longest follow‐up; subgrouped by control | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Standard care ± a minimal intervention (< 1 day) | 6 | 595 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.45, ‐0.04] |
| 4.2 Alternative group sessions | 3 | 300 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.71, 0.22] |
| 4.3 Individual therapy | 2 | 389 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.42, ‐0.01] |
| 5 Depression scores (trials with mean scores in the normal range at baseline) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Baseline mean scores | 5 | 810 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.11, 0.30] |
| 5.2 Mean score at longest follow‐up (6 to 15 months after randomization) | 5 | 511 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.48, ‐0.13] |
| 6 Depression scores at longest follow‐up; subgrouped by primary focus of intervention | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Stress management | 3 | 216 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.73, ‐0.18] |
| 6.2 Coping | 3 | 396 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.37, 0.08] |
| 6.3 Self‐efficacy | 1 | 229 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.39, 0.13] |
| 6.4 Depression | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.19, ‐0.30] |
| 6.5 Adherence and depression | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.92, 0.06] |
| 6.6 Adherence | 1 | 147 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.30, 0.35] |
| 7 Depression scores at longest follow‐up; subgrouped by gender | 10 | 1139 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.42, ‐0.10] |
| 7.1 Men only | 3 | 215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.55, 0.04] |
| 7.2 Women only | 1 | 229 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.39, 0.13] |
| 7.3 Both men and women | 6 | 695 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.55, ‐0.07] |
| 8 Anxiety scores | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Baseline mean scores | 4 | 697 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.05, 0.27] |
| 8.2 Mean score at end of group sessions (10 to 12 weeks after randomization) | 3 | 420 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.25, 0.22] |
| 8.3 Mean score at longest follow‐up (12 to 15 months after randomization) | 4 | 471 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.31, 0.06] |
| 9 Anxiety scores: at longest follow‐up; subgrouped by anxiety scale used | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 State trait anxiety inventory (score between 20 and 80) | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐6.06, 4.46] |
| 9.2 Modified State Trait Anxiety Inventory (score between 10 and 40) | 1 | 229 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐2.09, 0.87] |
| 9.3 Hospital Anxety and Depression Scale (score out of 21) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐2.4 [‐4.92, 0.12] |
| 9.4 Profile of Mood States (score out of 36) | 1 | 101 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐2.64, 3.04] |
| 10 Anxiety scores: at longest follow‐up; subgrouped by control | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Standard care ± a minimal intervention (< 1 day) | 2 | 172 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐3.73, 1.36] |
| 10.2 Alternative group therapy | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐6.06, 4.46] |
| 10.3 Individual therapy | 1 | 229 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐2.09, 0.87] |
| 11 Stress scores | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Baseline mean scores | 5 | 695 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.03, 0.27] |
| 11.2 Mean score at end of group sessions | 4 | 533 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.23, 0.12] |
| 11.3 Mean score at longest follow‐up | 5 | 507 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.23, 0.15] |
| 12 Stress scores at longest follow‐up; subgrouped by stress score used | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Perceived stress score (score out of 40) | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐3.77, 2.37] |
| 12.2 Dealing with illness scale | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐7.62, 4.02] |
| 12.3 HIV‐related life‐stressor burden score (score out of 5) | 1 | 166 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.06, 0.22] |
| 12.4 Life experiences survey (score out of 3) | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐3.00, 0.28] |
| 12.5 Impact of event scale (score out of 75) | 2 | 232 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐3.60, 0.95] |
| 13 Stress scores at longest follow‐up; subgrouped by control | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Standard care ± a minimal intervention (< 1 day) | 3 | 274 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.46, 0.71] |
| 13.2 Alternative group therapy | 4 | 454 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.05, 0.22] |
| 13.3 Individual therapy | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Coping scores | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Baseline mean scores | 5 | 1022 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.11, 0.14] |
| 14.2 Mean score at end of group sessions | 5 | 762 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.16, 0.19] |
| 14.3 Mean score at longest follow‐up (6 months after randomization) | 5 | 697 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.11, 0.19] |
| 15 Coping scores at longest follow‐up; subgrouped by coping score used | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Dealing with illness: coping subscale (score out of 120) | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐1.79, 5.59] |
| 15.2 Cognitive behavioural self‐efficacy scale (score out of 28) | 1 | 229 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐1.29, 0.97] |
| 15.3 Coping self‐efficacy scale (score out of 260) | 2 | 236 | Mean Difference (IV, Random, 95% CI) | 1.76 [‐6.53, 10.05] |
| 15.4 Avoidant coping scale (score out of 69) | 1 | 163 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐5.26, 1.86] |
| 16 Coping scores at longest follow‐up; subgrouped by control | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Standard care ± a minimal intervention (< 1 day) | 2 | 235 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.21, 0.59] |
| 16.2 Alternative group therapy | 4 | 454 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.67, 0.17] |
| 16.3 Individual therapy | 1 | 229 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐1.29, 0.97] |
1.10. Analysis.
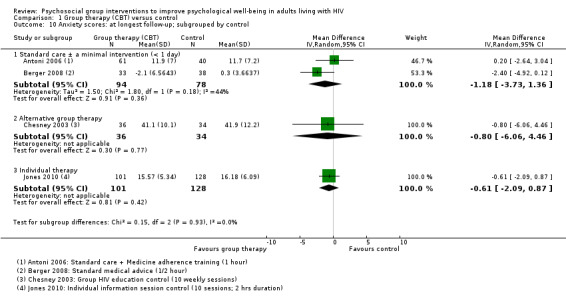
Comparison 1 Group therapy (CBT) versus control, Outcome 10 Anxiety scores: at longest follow‐up; subgrouped by control.
1.13. Analysis.
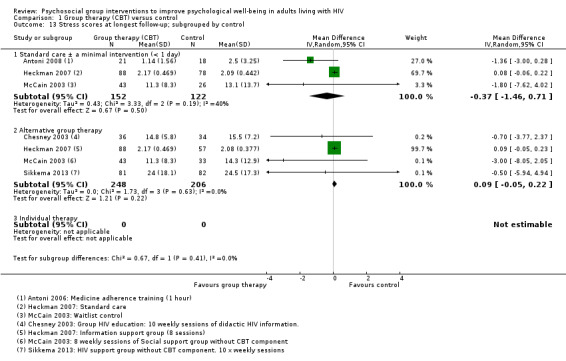
Comparison 1 Group therapy (CBT) versus control, Outcome 13 Stress scores at longest follow‐up; subgrouped by control.
1.16. Analysis.
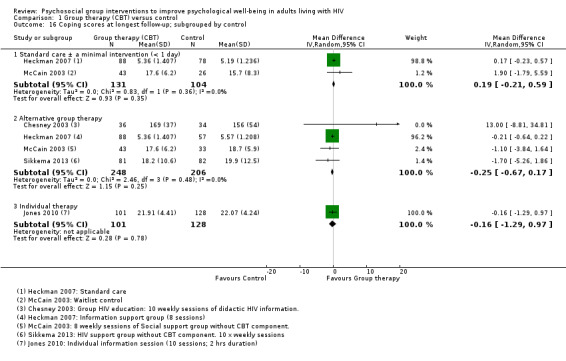
Comparison 1 Group therapy (CBT) versus control, Outcome 16 Coping scores at longest follow‐up; subgrouped by control.
Comparison 2. Group therapy (mindfulness) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression scores | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Baseline mean scores | 3 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.35, 0.12] |
| 1.2 Mean scores at end of group sessions (8 weeks after randomization) | 3 | 242 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.48, 0.04] |
| 1.3 Mean score at longest follow‐up | 3 | 233 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.49, 0.03] |
| 2 Anxiety scores | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Baseline mean scores | 2 | 210 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.29, 0.27] |
| 2.2 Mean scores at end of group sessions (8 weeks after randomization) | 2 | 178 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.53, 0.07] |
| 2.3 Mean score at longest follow‐up | 2 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.47, 0.15] |
| 3 Stress scores | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Baseline mean scores | 2 | 169 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐3.50, 1.46] |
| 3.2 Mean scores at end of treatment | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐2.29 [‐4.46, ‐0.11] |
| 3.3 Mean score at longest follow‐up | 2 | 137 | Mean Difference (IV, Random, 95% CI) | ‐2.02 [‐4.23, 0.19] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antoni 2006.
| Methods |
Trial design: 2‐arm randomized controlled trial (RCT) Follow‐up: 3, 9, and 15 months follow up Loss to follow‐up: 22 in treatment group, 23 in control group |
|
| Participants |
Population: 130 HIV‐positive homosexual men receiving highly active antiretroviral therapy (HAART); intervention = 76, control = 54 Inclusion criteria: 18 to 65 years old, no changes in their HAART regime during past month Exclusion criteria: prescribed medications with immunomodulatory effects, history of chemotherapy or whole‐body radiation for cancer that was not AIDS‐related, history of chronic illness associated with permanent changes in immune system |
|
| Interventions |
Intervention: N = 76. Cognitive Behavioural Stress Management (CBSM): focused on eliciting participant experiences with adherence and medication side effects using cognitive restructuring exercises, managing stressors related to adherence, using productive coping responses.
Control: N = 54. Medication adherence training (MAT): aimed to increase knowledge about HIV and HAART, including how medications work, why they must be taken on time and at the proper dose, and how to recognize possible side effects
|
|
| Outcomes |
Included in this review
Not included in this review
|
|
| Notes | Used 2 scales to measure depression POMS‐D and BDI; we chose BDI Setting: not reported Country: USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants identification numbers were drawn randomly from a box for assignment to trial conditions." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization procedures were conducted by a master’s level project manager and overseen by the principal investigator. Participants identification numbers were drawn randomly from a box for assignment to trial conditions." No further details. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 19.7% intervention group versus 25.9% control. Intention‐to‐treat (ITT) analysis done. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We did not detect any other sources of bias. |
Antoni 2008.
| Methods |
Trial design: 2‐arm RCT Follow‐up: 9‐month follow‐up Loss to follow‐up: Cognitive Behavioral Stess Management (CBSM) group: 47% completed follow‐up; control participants: 69% completed follow‐up |
|
| Participants |
Population: 39 HIV‐positive African American, Hispanic, or Caribbean women. Intervention = 21, control = 18. Inclusion criteria: HIV‐positive women aged 18 to 60 years old; at least 2 Papanicolaou smears indicating low grade squamous intraepithelial lesions (LSIL) or al least 2 cervical biopsies indication CIN 1 (mild or grade 1 neoplasia) in the 2 years prior to trial entry; fluency in spoken English. Exclusion criteria: no exclusion criteria were listed. |
|
| Interventions |
Treatment: N = 21. Cognitive behavioural training (CBT) that increased awareness of the effects of stress, identifying and reframing automatic thoughts, improving productive coping skills, anger management, assertiveness training, productive use of one's social network, and safer sex negotiation.
Control: N = 18. 5‐hour 1‐day CBSM workshop with four 20‐minute relaxation modules, four 40‐minute CBSM modules, and two 30‐minute breaks. |
|
| Outcomes |
Included in this review
Not included in this review
|
|
| Notes |
Jensen 2013 did secondary analysis on psychological well‐being but only used a subset of Beck Depression Inventory (BDI) Setting: not reported Country: USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned to either the 10‐week CBSM group intervention or the one‐day CBSM workshop at a 2:1 ratio (experimental:control)". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Participants and assessors were blinded to experimental condition during completion of all study entry procedures". No further details. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 43.2% intervention group versus 21.7% control. Trial authors conducted an ITT analysis. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We did not detect any other sources of bias. |
Berger 2008.
| Methods |
Trial design: 2‐arm RCT Follow‐up: 12 months Loss to follow‐up: intervention loss n = 2; treatment loss n = 4 |
|
| Participants |
Population: 104 HIV‐positive people taking combination antiretroviral therapy (ART), intervention = 53, control = 51. Inclusion criteria: adults between 18 to 65 years, German speaking, received combination Antiretroviral Therapy (cART) within the 3 months prior to screening, had a CD4 lymphocyte count > 100 cells/µL and no opportunistic infection at baseline. Exclusion criteria: received psychotherapy in past 3 months, intravenous drug users or on stable methadone maintenance, diagnosis of psychiatric disorder as determined by standardized interview. |
|
| Interventions |
Treatment: N = 53. Psychoeducation, group dynamics exercises, homework, cognitive strategies, and PMR
Control: N = 51. 30‐minute health check by physician. |
|
| Outcomes |
Included in review
Not included in review
|
|
| Notes | Ethics approval. Setting: outpatient clinics Country: Switzerland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation sequences included randomly permuted block sizes of two and four and were generated using the computer program RANCODE V3.0". |
| Allocation concealment (selection bias) | Low risk | Quote: "Individual assignment codes were properly concealed between black sheets, stored in sequentially numbered envelopes and opened in the presence of study participants". |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 37.7% intervention group versus 25.5% control group.Trial authors conducted an ITT analysis. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We did not detect any other sources of bias. |
Bormann 2006.
| Methods |
Trial design: RCT 2 group by 4 time repeated measures design Follow‐up: baseline, 10 weeks, 3 months follow‐up, 22 weeks post follow‐up Loss to follow‐up: 71% completed all trial points |
|
| Participants |
Population: 93 HIV‐positive adults, intervention = 46, control = 47 Inclusion criteria: HIV‐positive for ≥ 6 months; 18 to 65 years old; no drug or substance abuse for ≥ 6 months; able to read, write, and comprehend English Exclusion criteria: Trial authors did not list any exclusion criteria. |
|
| Interventions |
Treatment: N = 46. Information on choosing and using a mantram, attention to mindfulness, phone calls to encourage mantram practice.
Control: N = 47. Videotapes on HIV topics including medications, treatment issues, wasting syndrome, and nutrition. Attention control group the same. |
|
| Outcomes |
Included in review
Not included in review
|
|
| Notes | 50% homosexual participants Setting: not reported Country: USA University Institutional Review Board approval |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment was done by the project coordinator using a table of random numbers and stratifying on CD4 count". |
| Allocation concealment (selection bias) | Unclear risk | Trial authors did not describe selection bias. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | Trial authors conducted an ITT analysis. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 30.4% intervention group versus 27.7% control group. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Unclear risk | Groups differed on 4 baseline variables; "baseline imbalance". |
Carrico 2005.
| Methods |
Trial design: 2‐arm RCT Follow‐up: pre, post, 6 months, and 12 months Loss to follow‐up: 50% followed up in intervention and 47% in control. |
|
| Participants |
Population: 129 HIV seropositive homosexual men. Intervention = 31, control = 18 Inclusion criteria: have at least 1 non‐AIDS HIV‐related symptom occurring within 3 years of trial entry of laboratory signs of mildly progressed HIV infection, have at least an 8th grade education and ability to read and write in fluent English, CD4 counts > 200 cells/mm³, those on AZT or ART had to have maintained current dosage without change in regimen for at least 2 months before trial entry, those concurrently engaged in psychotherapy or support groups were asked not to change their involvement during the trial Exclusion criteria: individuals with AIDS symptomology, a prior diagnosis of AIDS (CD4 count < 200 cells/mm³), those who had been hospitalized in previous 3 months, those who had a chronic immune system‐related physical condition other than HIV and regular use of medications (other than ARVs) with substantial known effects on the endocrine or immune systems, those with psychiatric or neuropsychological conditions, alcohol or substance abuse dependency, or and major psychiatric and personality disorder, who were cognitively impaired, patients with concurrent clinical levels of depression, individuals who had been bereaved of a significant other within the previous 6 months, risk factors for HIV transmission other than sexual orientation (for example, past or present intravenous drug use, blood transfusion), initiating a new psychotherapy or exercise training programme in past 3 months |
|
| Interventions |
Treatment: N = 31. CBSM (GET SMART) included increasing awareness of physiological effects of stress, cognitive behavioural theory of stress and emotions, identification of cognitive distortions and automatic thoughts, rational thought replacement, coping skills training, assertiveness training, anger management, and identification and use of social supports. Homework was assigned and participants were taught a variety of relaxation techniques including PMR, autogenic training, meditation and breathing exercises
Control: N = 18. Waitlist, 2 weeks post treatment they were offered a 1 day seminar consisting of condensed CBSM components |
|
| Outcomes |
Included in review
Not included in review
|
|
| Notes |
Setting: not reported Country: USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors described the trial as "randomized" but did not provide any further details. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe selection biases, if any. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 66.3% intervention group versus 65.2% control group. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We did not detect any other sources of bias. |
Chesney 2003.
| Methods |
Trial design: 3‐arm RCT Follow‐up: 6 and 12 months Loss to follow‐up: 86% retained |
|
| Participants |
Population: 149 HIV‐positive men who have sex with men (MSM) and who have depression Inclusion criteria: (1) self‐identified as homosexual or bisexual, (2) 21 to 60 years of age, (3) self‐reported CD4 levels between 200 and 700 cells/mm³ Exclusion criteria: (1) individuals with major depressive or psychotic disorders, (2) history of drug or alcohol dependency in past year, (3) currently in psychotherapy, (4) using psychoactive medication |
|
| Interventions |
Treatment: N = 54. Coping Effectiveness Training (CET) comprising psychoeducation: appraisal of stressful situations, problem‐focused and emotion‐focused coping, use of social support; skills‐building group activities, relaxation guidance.
Control: N = 51. HIV‐Informational Control comprising Didactic information on HIV‐related topics and resources, workbooks, fact sheets, and reading material. 10 weekly 90‐minute group sessions of 8 to 10 men plus 6 maintenance sessions Waiting list: N = 44. Waiting list control |
|
| Outcomes |
Included in review
Not included in review
Mediating variables
|
|
| Notes |
Setting: not reported Country: San Francisco, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors described the trial as "randomized" but did not provide any further details. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe selection bias, if any. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 33.3% intervention versus 33.3% control. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We did not detect any other sources of bias. |
Duncan 2012.
| Methods |
Trial design: 2‐arm RCT Follow‐up: baseline, 3 month and 6 month follow‐up Loss to follow‐up: 93% 6‐month follow‐up |
|
| Participants |
Population: 76 participants actively taking ART, intervention = 40, control = 36 Inclusion criteria: (1) documentation of HIV test results, (2) currently taking a recognized ART regimen and (3) reporting a side effect bother in last 30 days of 8 or a bother on the side effect and symptom distress scale. Exclusion criteria: (1) if enrolled in another behavioural coping or HIV adherence intervention research trial or MBSR |
|
| Interventions |
Treatment: N = 40. Mindfulness‐based stress reduction consisting of daily homework, sitting meditation with mindfulness of breath, thoughts, and emotions, including deliberate awareness of routine activities such as eating and interpersonal communication, and yoga postures
Control: N = 36. Waitlist control group offered the MBSR subsequent to 6 month follow‐up |
|
| Outcomes |
Included in the review
Not included in the review
|
|
| Notes |
Setting: not reported Country: USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in blocks of six using the SAS system’s PLAN procedure". |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe any selection bias. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up: 7.5% intervention group versus 5.6% control. |
| Selective reporting (reporting bias) | Low risk | We did not detect any other potential sources of selection reporting bias. |
| Other bias | Low risk | No differences between groups at baseline except for viral load and this was controlled for. |
Gayner 2012.
| Methods |
Trial design: 2‐arm follow‐up Follow‐up: baseline, 2 months post, and 6 months follow‐up Loss to follow‐up: 21 participants discontinued the intervention |
|
| Participants |
Population: 117 homosexual male participants, intervention =78, control = 39 Inclusion criteria: male, aged 18 to 70 years, living within 1 hour of the hospital, and having a diagnosis of HIV Exclusion criteria: (1) subjects with active current major depression, substance abuse, or significant cognitive deficit. |
|
| Interventions |
Treatment: N = 78. Mindfulness‐Based Stress Reduction (MBSR): participants were taught mindfulness skills geared towards enhancing their awareness of and relation to current experience rather than focusing on the content and reappraisal of thoughts and interpretations of experiences.
Control: N = 39. Treatment as Usual (TAU) group offered at end of intervention |
|
| Outcomes |
Included in review
Not included in review
|
|
| Notes | Homosexual population Ethics reviewed. Setting: hospital Country: Toronto, Canada |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomization free‐ware, software program (Network, 1997) was utilized to generate a random allocation sequence 2:1 in favour of the group intervention for each cohort of up to 30 eligible participants". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "study staff were not aware of a potential participant’s group member‐ ship until the whole cohort was assigned at the same time". The trial authors did not provide any further details. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 24.4% intervention group versus 5.1% control. ITT analysis done. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We did not detect any other sources of bias. |
Heckman 2007.
| Methods |
Trial design: 3‐arm RCT Follow‐up: baseline, post, 4 months, and 8 months follow‐up Loss to follow‐up: 73%; 68%, 82% |
|
| Participants |
Population: 299 adults, intervention = 84, control = 107 Inclusion: 18 years or older, provision of written consent, a self reported diagnosis of HIV AIDS, and residence in a community of 50,000 people or fewer that was at least 20 miles from a city of 100,000 or more. Exclusion: there were no exclusion related to psychological functioning. |
|
| Interventions |
Treatment: N = 108. Coping improvement group intervention used cognitive behavioural principles to appraise stressor severity, develop adaptive problem‐ and emotion‐focused coping skills, and optimize coping through the appropriate use of personal and social resources.
Treatment: N = 84. Information Support Group provided information on HIV symptom management, nutrition and HIV, exercise and HIV, and discussions of personal topics.
Control: N = 107. Usual care condition received no intervention but had access to usual services |
|
| Outcomes |
Included in review
Not included in review
|
|
| Notes |
Setting: telephone‐delivered Country: USA Groups conducted separately for MSM, heterosexual men, and women 71% reporting moderate to severe depression at baseline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors described the trial as "randomized" but did not provide any further details. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe selection bias, if any. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 18.5 intervention group versus 27.1% control. ITT done. A last‐observation‐carried‐forward (LOCF) approach was used to input missing data. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We did not detect any other sources of bias. |
Heckman 2011.
| Methods |
Trial design: 3‐arm RCT Follow‐up: baseline, post, 4 months, and 8 months follow‐up Loss to follow‐up: coping group 79% loss, interpersonal support group 69% loss, individual therapy group 86% loss. |
|
| Participants |
Population: 295 men and women over the age of 50, intervention = 104, control = 105. Inclusion: 50 years or older, a diagnosis of HIV infection or AIDS, a BDI‐II score of 10 or higher, a score of 75 or more on the 3MS, a minimum value of 10 on the BDI‐II. (A minimum value of 10 on the BDI‐II was used to ensure that participants had a minimally elevated number of depressive symptoms that had the potential to be reduced by the interventions). Exclusion: the project did not exclude individuals with alcohol or substance use disorders, active bipolar disorder, psychotic symptoms, or individuals receiving psychotherapy. |
|
| Interventions |
Treatment: N = 104. Coping improvement group intervention addressed introductions and participants' sharing of personal histories (Session 1 and 2) appraisal and changeability of stressors related to one's HIV infection and stressors related to normal ageing (Sessions 3 and 4); developing and implementing adaptive problem‐ and emotion‐focused coping skills (Sessions 5 through 9); optimizing coping efforts through the use of interpersonal supports (Session 10 and 11); and termination issues and voluntary sharing of personal contact information (Session 12)
Treatment: N = 105. Interpersonal Support Group of 12 x 90‐minute group sessions (5 minutes spent on viewing and discussing videotapes on HIV‐related topics of nutrition, treatment, adherence, sexual risk reduction, 45 minutes spent discussing how the sessions' topic pertained to their personal lives). Facilitators: 2 Masters‐level clinicians Control: N = 86. Individual Therapy Upon Request (ITUR) Group had access to standard psychosocial community‐based services. |
|
| Outcomes |
Included in review
Not included in the review
|
|
| Notes |
Setting: community Country: Ohio and New York, USA. Each 90‐minute group conducted separately for MSM, heterosexual men, and women. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors described the trial as "randomized" but did not provide any further details. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe selection bias, if any. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 15.4% intervention group versus 31.4% control. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We did not detect any other sources of bias. |
Jones 2010.
| Methods |
Trial design: 2‐arm RCT Follow‐up: pre, post, 12 months Loss to follow‐up: not reported. |
|
| Participants |
Population: 451 minority women, intervention = 212, control = 239 Inclusion: 18 years and older, meet the CDC classification for case‐defined AIDS (i.e. CD4+ cell count below 200/mm² and/or one opportunistic infection, CDC 1993), have at least 6th grade education Exclusion: women with psychiatric, neuropsychiatric or medical conditions were temporarily excluded pending treatment. |
|
| Interventions |
Treatment: N = 212. Cognitive Behavioural Stress Management: sessions included didactic components on the physiological effects of stress, cognitive behavioural interpretation of stress and emotions, identification of cognitive distortions and automatic thoughts, rational thought replacement, coping skills training, assertiveness training, anger management, and identification of social support.
Control: N = 239. Individual 120‐minute information education sessions delivered by videotape covering stress management, relaxation, and coping with HIV. 10 sessions |
|
| Outcomes |
Included in this review
Not included in this review
|
|
| Notes |
Setting: medical school setting and community health centres Country: USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used a table of random numbers to randomize participants to treatment. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe selection bias, if any. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 45.1% intervention group versus 36.9% control. |
| Selective reporting (reporting bias) | Low risk | We did not detect any potential source of selective reporting bias. |
| Other bias | Low risk | We did not detect any other sources of bias. |
McCain 2003.
| Methods |
Trial design: 3‐arm RCT Follow‐up: pre, post, and 6 months Loss to follow‐up: 69% follow‐up at 6 months |
|
| Participants |
Population: 148 individuals diagnosed with HIV disease (119 men, 29 women). Intervention = 59, support group = 3, Waitlist = 36 Inclusion: (1) 18 years of age, (2) able to read and speak English, (3) previously aware of their HIV diagnosis, (4) deemed capable of attending intervention sessions and completing 6 months follow‐up. Exclusion: (1) no significant psychiatric illness, (2) no cognitive impairment, (3) not pregnant or taking steroids. |
|
| Interventions |
Treatment 1: N = 59. CBSM focused on breathing, PMR, yoga‐form stretching, guided imagery, and beginning meditation, along with cognitive restructuring techniques and active coping skills.
Treatment 2: N = 43. SSG focused on facilitating communication related emotional issues, problem‐solving and cognitive‐reframing techniques, and individual and group empowerment
Control: N = 36. Waitlist group |
|
| Outcomes |
Included in this review
Not included in this review
|
|
| Notes |
Setting: Trial authors did not describe the setting. Country: USA Separate gender groups, interaction terms included by group by gender |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Quota sampling was used to achieve appropriate sample representation by gender, at a ratio of 4 males:1 female (20%)". The trial authors did not provide any further details. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe selection bias, if any. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 27.1% intervention group versus 27.8% control. |
| Selective reporting (reporting bias) | Low risk | We did not detect any potential source of selective reporting bias. |
| Other bias | Low risk | We did not detect any other sources of bias. |
Nakimuli‐Mpungu 2015.
| Methods |
Trial design: 2‐arm RCT Follow‐up: baseline, immediately post, 6 months follow‐up Loss to follow‐up: 23% in intervention group, 21% lost in control group |
|
| Participants |
Population: 109 HIV‐positive individuals (peasant farmers) with major depression, intervention = 57, control = 52 Inclusion: 19 years or older, met the MINI International Neuropsychiatric Interview criteria for major depression, from an urban HIV care centre, were antidepressant naive Exclusion: individuals with severe medical disorder such as pneumonia or active tuberculosis, psychotic symptoms, and hearing or visual impairment |
|
| Interventions |
Treatment: N = 57. Group Support Psychotherapy (GSP) is a culturally sensitive intervention that aims to treat depression by enhancing social support, teaching coping skills and income generating skills
Control: N=52. Active treatment group receives Group HIV education (GHE) immediately post intervention and 6 months later. |
|
| Outcomes |
Included in the review
Not included in review
|
|
| Notes |
Setting: HIV care centre, urban Country: Uganda Gender‐specific groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors performed randomization by urn with a ratio of 1:1. The trial authors did not provide any further details or properties of this method. |
| Allocation concealment (selection bias) | Unclear risk | Men and women separately picked a paper containing the intervention allocation from a basket, ratio. The trial authors did not provide further details on allocation concealment. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 22.8% intervention group versus 21.2% control. |
| Selective reporting (reporting bias) | Low risk | We did not detect any potential source of selective reporting bias. |
| Other bias | Low risk | We did not detect any other sources of bias. |
Peltzer 2012.
| Methods |
Trial design: 2 arm RCT Follow‐up: baseline, 1 month, 4 months Loss to follow‐up: 147 follow‐up (96.7% follow‐up); 3.9% attrition in MAI and 2.6% attrition in SC |
|
| Participants |
Population: 152 HIV‐positive individuals on ART with an adherence problem, intervention = 76, control = 72 Inclusion: 18 years or older, new ARV medication users (2 to 24 months of ARV use) Exclusion: Trial authors did not describe any exclusion criteria. |
|
| Interventions |
Treatment: N = 76. Medication adherence intervention (MAI) is medication information combined with problem‐solving skills in an experiential/interactive group format.
Control: N = 76. Practitioner medical directive (standard of care; 20 min) led by medical physician. Patients individually attended monthly 1 visit to review their health status with their medical practitioner. |
|
| Outcomes |
Included in this review
Not included in this review
|
|
| Notes |
Setting: hospital Country: South Africa. Short follow‐up of 3 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomized into study condition using a table of random numbers following their baseline assessment". |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe any selection bias, if any. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up: 3.9% intervention group versus 2.6% control. Trial authors performed an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | We did not detect any potential source of selective reporting bias. |
| Other bias | Low risk | We did not detect any other sources of bias. |
Safren 2012.
| Methods |
Trial design: 2‐arm RCT Follow‐up: 3 months, 6 months and 12 months Loss to follow‐up: 84% follow‐up to at least one follow‐up assessment |
|
| Participants |
Population: 89 HIV individuals prescribed ARVS and with a history of injection drug use, intervention = 44, control = 45 Inclusion: aged 18 to 65 years, HIV‐positive, prescribed ARVs, endorsed history of injection drug use, currently enrolled in opoid treatment for at least one month, and met criteria for a diagnosis of current or subsyndromal depressive mood disorder Exclusion: individuals with any active untreated or unstable major mental illness, inability or unwillingness to provide informed consent, or current participation in a CBT for depression. |
|
| Interventions |
Treatment: N = 44. CBT‐AD included Life Steps, psychoeducation about HIV and depression, motivational interviewing for behaviour change, behavioural activation to increase pleasurable activities, training in adaptive thinking, problem solving, PMR, and diaphragmatic breathing.
Control: N = 45. Enhanced Treatment As Usual (ETAU) with a letter to medical provider documenting participants depression and suggesting continued assessment and treatment. Both treatment conditions received a single‐session intervention on medication adherence (Life Steps) with involved 11 informational, problem‐solving and cognitive‐behavioural steps. |
|
| Outcomes |
Included in this review
Not included in this review
|
|
| Notes |
Setting: methadone clinics Country: Boston, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "Random assignment in block of 2 ‐ stratified by sex, depression severity and adherence". The trial authors did not provide any further details. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Assignment to study condition (CBT‐AD or ETAU) was concealed from both study therapists and participants until the conclusion of the first counseling visit (see below)". The trial authors did not provide any further details. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent assessor who was blinded to the trial conditions assessed depression. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 18.2% intervention group versus 33.3% control. |
| Selective reporting (reporting bias) | Low risk | We did not detect any potential sources of selective reporting bias. |
| Other bias | Unclear risk | Small sample size of 89. CD4 count differed at baseline but controlled for in analysis |
Sikkema 2013.
| Methods |
Trial design: 3‐arm RCT Follow‐up: baseline, post, 4, 8, 12 months Loss to follow‐up: minimal loss to follow‐up, ITT analysis done |
|
| Participants |
Population: 247 HIV‐positive men (N = 117) and women (N = 130), intervention = 124, control = 123 Inclusion: sexual abuse as a child (age 12 and under) and/or adolescent (age 13 to 17 years); current age of 18 or older; HIV serostatus Exclusion: acute distress due to sexual revictimization experienced within past month; presence of impaired mental status; extreme distress or depressive symptomatology |
|
| Interventions |
Treatment 1: N= 124. HIV and Trauma Coping Group Intervention (LIFT) where participants Identify stressors that they perceived to be related to their sexual abuse experiences and those related to their HIV diagnosis, learn adaptive coping and risk reduction skills related to both sexual abuse and HIV infection.
Control: N = 123. HIV standard therapeutic support group. The comparison intervention paralleled a standard therapeutic support group and was led by experienced co‐therapists not trained on the coping intervention model. The purpose of the group was to provide a supportive environment for participants to address issues of HIV and trauma. |
|
| Outcomes |
Included in this review
Not included in this review
|
|
| Notes |
Setting: Community Health Centre Country: USA Different populations in each report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors described the trial as 'randomized' but did not provide any further details. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment, if any. |
| Blinding of participants and personnel (performance bias) Anxiety | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial authors did not describe the method of blinding, if any. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: 16.5% Intervention group versus 33.3% control. The trial authors conducted an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | We did not detect any other sources of selective reporting bias. |
| Other bias | Low risk | We did not detect any other potential sources of bias. |
Abbreviations: HAART: highly active antiretroviral therapy; PMR: progressive muscle relaxation; RCT: randomized controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Antoni 2000 | No 3‐month follow‐up post intervention. |
| Balfour 2006 | Individual psychoeducational intervention, not a group intervention. |
| Bormann 2009 | No specified outcomes reported. The study measured salivary cortisol as a biomarker of immune function. |
| Carrico 2009 | Individual counselling sessions, not a group intervention. |
| Chan 2005 | No 3‐month follow‐up postintervention. |
| Chhatre 2013 | Not a group‐based intervention. |
| Chiou 2004 | No specified outcomes measured. |
| Creswell 2009 | No 3‐month follow‐up postintervention. |
| Cruess 2000 | No 3‐month follow‐up postintervention. |
| Côté 2002 | Nurse‐patient intervention, not a group intervention. |
| Davies 2006 | Overview of intervention, not a randomized controlled trial (RCT). |
| Davies 2009 | Only formative qualitative data presented, not a RCT. |
| Evans 2003 | We contacted the study author. This was not a group intervention. |
| Fife 2008 | Individual therapy for patient‐partner dyads. This was not a group intervention. |
| Gifford 1998 | No 3‐month follow‐up postintervention, only 3‐month follow‐up after baseline. |
| Golin 2006 | Individual, not a group intervention. |
| Goodkin 1998 | No specified outcomes. |
| Goodkin 1999 | No 3‐month postintervention follow‐up. |
| Hansen 2009 | No specified outcomes included. |
| Heckman 2004 | No RCT result reported, only baseline data reported. |
| Heckman 2006 | No post 3‐month follow‐up. |
| Ingersoll 2011 | Not a group intervention. |
| Jensen 2013 | Measured positive affect and positive mood. These are not specified outcomes. |
| Jones 2005 | Not a RCT as no control group. |
| Kaaya 2013 | No 3‐month follow‐up postintervention. |
| Kalichman 2005 | Describes intervention development and components, not a RCT. |
| Koenig 2008 | Nurse‐patient intervention, not a group intervention. |
| Kunutsor 2011 | Treatment supporter intervention, not a group intervention. |
| Laperriere 2005 | The study author presented a subgroup analysis only, a subgroup from the larger Jones 2010 study. |
| Latkin 2003 | Not a HIV‐positive population. |
| Lechner 2003 | No 3‐month follow‐up postintervention. |
| Lee 1999 | Not a RCT and no control group. |
| Lehavot 2011 | Not a group intervention. |
| MacNeil 1999 | Not a group intervention. |
| Marhefka 2014 | No specified outcomes reported. |
| Markowitz 1998 | Interpersonal therapy, not a group intervention. |
| Molassiotis 2002 | Not truly randomized. |
| Mundell 2011 | Not a RCT, quasi‐experimental. |
| Nakimuli‐Mpungu 2014 | No 3‐month follow‐up postintervention. |
| Nokes 2003 | No RCT reported. |
| Olley 2006 | Individual therapy, not a group intervention. |
| Pacella 2012 | Not a group intervention. |
| Papas 2011 | No specified outcomes reported. |
| Petersen 2014 | No 3‐month follow‐up postintervention. |
| Prado 2012 | Adolescents 12 to 17 years old. |
| Proeschold‐Bell 2011 | No RCT reported. |
| Rao 2009 | Individual art therapy sessions, not a group intervention. |
| Rao 2012 | No RCT reported. |
| Ravaei 2013 | No 3‐month follow‐up postintervention, small sample size (N = 30), and unsure whether a group intervention. |
| Remien 2005 | Not a group intervention. The intervention was individually administered to each couple. |
| Robins 2006 | No 3‐months follow‐up postintervention. |
| Roth 2012 | One‐to‐one intervention by lay health workers, not a group intervention. |
| Rotheram‐Borus 2011 | Study protocol only. No RCT data reported. |
| Rotheram‐Borus 2012 | No specified outcomes. |
| Sacks 2011 | Both individual and group formats to intervention. No effects reported for group components of intervention. |
| Safren 2009 | Control invited to cross over to intervention at 3‐months postintervention. No postintervention follow‐up of 3 months for control group. |
| Saleh‐Onoya 2009 | Measured coping using 3 subscales of Coping Scale. |
| SeyedAlinaghi 2012 | No specified outcomes. Used global score of broad range of psychological symptoms, did not measure depression and anxiety outcomes separately. |
| Sikkema 2004 | No 3‐month follow‐up postintervention. |
| Simoni 2013 | Not a group‐based intervention. |
| Stewart 2001 | Content analysis, no RCT reported. |
| Szapocznik 2004 | Therapy sessions with family at home. |
| Wagner 2006 | Individually administered intervention, not a group intervention. |
| Williams 2014 | Not a group intervention but home‐based individual intervention. |
| Wingood 2004 | Inconsistencies in reporting of data. |
| Wong 2008 | Individual counselling sessions, not a group intervention. |
| Wyatt 2004 | There was unclear presentation of follow‐up data and it was unclear whether it was 3‐month or 6‐month follow‐up data. We contacted the study author but received no response. |
| Yu 2014 | Not a RCT, no control group, HIV‐positive and HIV‐negative mixed sample. |
| Zisook 1998 | Individual psychotherapy, not a group intervention. |
| Znoj 2010 | Not a group intervention. |
Abbreviations: RCT: randomized controlled trial.
Differences between protocol and review
We excluded behavioural outcomes.
We only included the outcomes for meta‐analysis if the trials measured them for at least three months post‐intervention.
We changed the overall outcome of effectiveness to 'psychological well‐being' instead of 'Quality of Life' (QOL) as QOL was too broad.
Contributions of authors
Ingrid van der Heijden worked on developing and testing a gender‐specific coping intervention for people living with HIV in South Africa which prompted the importance of doing a review on what works to improve the lives of people living with HIV. As first author, Ingrid van der Heijden was responsible for the overall leadership in this review and received Cochrane‐based training and support to lead the review. She was responsible for reviewing abstracts and full papers, and extracting and collating data for the review, assessing risk of bias, and interpreting findings, and putting the review together.
Professor Naeemah Abrahams works in HIV‐related stigma research and post‐rape and HIV and mental health services in South Africa and helped with reviewing abstracts and full papers, and extracting and collating data for the review, assessing risk of bias, and interpreting findings.
David Sinclair is a UK qualified general practitioner (GP), and has worked within primary healthcare in the UK, Kenya, and North and South Sudan. Over the past seven years he has worked as an author and editor with the CIDG, and served as a temporary advisor to the World Health Organization’s Technical Guidelines Development Groups on Malaria Treatment, nutritional care for people with TB and HIV, and screening for active tuberculosis. David's contribution to this review proved invaluable for statistical analysis, interpretation of results, and guidance for Cochrane‐style edits and outputs.
Sources of support
Internal sources
Gender and Health Unit, Medical Research Council, South Africa.
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Grant: 5242
Declarations of interest
All review authors have no present or past affiliations in any organization or entity with an interest in the review that might lead to real or perceived conflict of interest.
All review authors have not been involved in any trials included in the review or in authoring a systematic review included in the Background.
All review authors do not know of any financial or commercial sources that may have or be perceived to have an interest in the outcome of the review.
All review authors will report any secondary conflicts of interest that may concern, for example, the inclusion or exclusion of trials, assessment of validity of included trials, or interpretation of results to the editors of the CIDG and they and the review authors will jointly decide on the management of such conflicts whether the conflicts of interest warrant being disclosed in the review (Higgins 2008; Higgins 2011).
Unchanged
References
References to studies included in this review
Antoni 2006 {published data only}
- Antoni MH, Carrico AW, Durán RE, Spitzer S, Penedo F, Ironson G, et al. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in gay men treated with highly active antiretroviral therapy. Psychosomatic Medicine 2006;68(1):143‐51. [DOI] [PubMed] [Google Scholar]
- Carrico AW, Antoni MH, Duran RE, Ironson G, Penedo F, Fletcher MA, et al. Reductions in depressed mood and denial coping during cognitive behavioral stress management with HIV‐positive gay men treated with HAART. Annals of Behavioral Medicine 2006;31(2):155‐64. [DOI] [PubMed] [Google Scholar]
Antoni 2008 {published data only}
- Antoni MH, Pereira DB, Marion I, Ennis N, Andrasik MP, Rose R, et al. Stress management effects on perceived stress and cervical neoplasia in low‐income HIV‐infected women. Journal of Psychosomatic Research 2008;65(4):389‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2008 {published data only}
- Berger S, Schad T, Wyl V, Ehlert U, Zellweger C, Furrer H, et al. Effects of cognitive behavioral stress management on HIV‐1 RNA, CD4 cell counts and psychosocial parameters of HIV‐infected persons. AIDS (London, England) 2008;22(6):767‐75. [DOI] [PubMed] [Google Scholar]
Bormann 2006 {published data only}
- Bormann JE, Carrico AW. Increases in positive reappraisal coping during a group‐based mantram intervention mediate sustained reductions in anger in HIV‐positive persons. International Journal of Behavioral Medicine 2009;16(1):74‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann JE, Gifford AL, Shively M, Smith TL, Redwine L, Kelly A, et al. Effects of spiritual mantram repetition on HIV outcomes: a randomized controlled trial. Journal of Behavioral Medicine 2006;29(4):359‐76. [DOI] [PubMed] [Google Scholar]
Carrico 2005 {published data only}
- Carrico AW, Antoni MH, Pereira DB, Fletcher MA, Klimas N, Lechner SC, et al. Cognitive behavioral stress management effects on mood, social support, and a marker of antiviral immunity are maintained up to 1 year in HIV‐infected gay men. International Journal of Behavioral Medicine 2005;12(4):218‐26. [DOI] [PubMed] [Google Scholar]
Chesney 2003 {published data only}
- Chesney MA, Chambers DB, Taylor JM, Johnson LM, Folkman S. Coping effectiveness training for men living with HIV: results from a randomized clinical trial testing a group‐based intervention. Psychosomatic Medicine 2003;65(6):1038‐46. [DOI] [PubMed] [Google Scholar]
Duncan 2012 {published data only}
- Duncan LG, Moskowitz JT, Neilands TB, Dilworth SE, Hecht FM, Johnson MO. Mindfulness‐based stress reduction for HIV treatment side effects: a randomised, wait‐list controlled trial. Journal of Pain and Symptom Management 2012;43(2):161‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gayner 2012 {published data only}
- Gayner B, Esplen MJ, DeRoche P, Wong J, Bishop S, Kavanagh L, et al. A randomized controlled trial of mindfulness‐based stress reduction to manage affective symptoms and improve quality of life in gay men living with HIV. Journal of Behavioral Medicine 2012;35(3):272‐85. [DOI] [PubMed] [Google Scholar]
Heckman 2007 {published data only}
- Heckman TG, Carlson B. A randomized clinical trial of two telephone‐delivered, mental health interventions for HIV‐infected persons in rural areas of the United States. AIDS and Behavior 2007;11(1):5‐14. [DOI] [PubMed] [Google Scholar]
Heckman 2011 {published data only}
- Heckman TG, Sikkema KJ, Hansen N, Kochman A, Heh V, Neufeld S, AIDS and Aging Research Group. A randomized clinical trial of a coping improvement group intervention for HIV‐infected older adults. Journal of Behavioral Medicine 2011;34(2):102‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2010 {published data only}
- Jones DL, Ishii Owens M, Lydston D, Tobin JN, Brondolo E, Weiss SM. Self‐efficacy and distress in women with AIDS: the SMART/EST women's project. AIDS Care 2010;22(12):1499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCain 2003 {published data only}
- McCain NL, Munjas BA, Munro CL, Elswick RK Jr, Robins JL, Ferreira‐Gonzalez A, et al. Effects of stress management on PNI‐based outcomes in persons with HIV disease. Research in Nursing & Health 2003;26(2):102‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakimuli‐Mpungu 2015 {published data only}
- Nakimuli‐Mpungu E, Wamala K, Okello J, Alderman S, Odokonyero R, Mojtabai R, et al. Group support psychotherapy for depression treatment in people with HIV/AIDS in northern Uganda: a single‐centre randomised controlled trial. Lancet HIV 2015;2(5):e190‐9. [DOI] [PubMed] [Google Scholar]
Peltzer 2012 {published data only}
- Peltzer K, Ramlagan S, Jones D, Weiss SM, Fomundam H, Chanetsa L. Efficacy of a lay health worker led group antiretroviral medication adherence training among non‐adherent HIV‐positive patients in KwaZulu‐Natal, South Africa: results from a randomized trial. SAHARA J 2012;9(4):218‐26. [DOI] [PubMed] [Google Scholar]
Safren 2012 {published data only}
- Safren SA, O'Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT‐AD) in HIV‐infected injection drug users: a randomized controlled trial. Journal of Consulting and Clinical Psychology 2012;80(3):404‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sikkema 2013 {published data only}
- Sikkema KJ, Ranby KW, Meade CS, Hansen NB, Wilson PA, Kochman A. Reductions in traumatic stress following a coping intervention were mediated by decreases in avoidant coping for people living with HIV/AIDS and childhood sexual abuse. Journal of Consulting and Clinical Psychology 2013;81(2):274‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema KJ, Wilson PA, Hansen NB, Kochman A, Neufeld S, Ghebremichael MS, et al. Effects of a coping intervention on transmission risk behavior among people living with HIV/AIDS and a history of childhood sexual abuse. Journal of Acquired Immune Deficiency Syndromes 2008;47(4):506‐13. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Antoni 2000 {published data only}
- Antoni MH, Cruess DG, Cruess S, Lutgendorf S, Kumar M, Ironson G, et al. Cognitive‐behavioral stress management intervention effects on anxiety, 24‐hr urinary norepinephrine output, and T‐cytotoxic/suppressor cells over time among symptomatic HIV‐infected gay men. Journal of Consulting and Clinical Psychology 2000;68(1):31‐45. [DOI] [PubMed] [Google Scholar]
Balfour 2006 {published data only}
- Balfour L, Kowal J, Silverman A, Tasca GA, Angel JB, Macpherson PA, et al. A randomized controlled psycho‐education intervention trial: Improving psychological readiness for successful HIV medication adherence and reducing depression before initiating HAART. AIDS Care 2006;18(7):830‐8. [DOI] [PubMed] [Google Scholar]
Bormann 2009 {published data only}
- Bormann JE, Aschbacher K, Wetherell JL, Roesch S, Redwine L. Effects of faith/assurance on cortisol levels are enhanced by a spiritual mantram intervention in adults with HIV: a randomized trial. Journal of Psychosomatic Research 2009;66(2):161‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrico 2009 {published data only}
- Carrico AW, Chesney MA, Johnson MO, Morin SF, Neilands TB, Remien RH, et al. Randomized controlled trial of a cognitive‐behavioral intervention for HIV‐positive persons: an investigation of treatment effects on psychosocial adjustment. AIDS and Behavior 2009;13(3):555‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2005 {published data only}
- Chan I, Kong P, Leung P, Au A, Li P, Chung R, et al. Cognitive‐behavioral group program for Chinese heterosexual HIV‐infected men in Hong Kong. Patient Education and Counseling 2005;56(1):78‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chhatre 2013 {published data only}
- Chhatre S, Metzger DS, Frank I, Boyer J, Thompson E, Nidich S, Montaner LJ, Jayadevappa R. Effects of behavioral stress reduction Transcendental Meditation intervention in persons with HIV. AIDS Care 2013;25(10):1291‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiou 2004 {published data only}
- Chiou PY, Kuo BIT, Lee MB, Chen YM, Wu SI, Lin LC. A program of symptom management for improving self‐care for patients with HIV/AIDS. AIDS Patient Care and STDs 2004;18(9):539‐47. [DOI] [PubMed] [Google Scholar]
Côté 2002 {published data only}
- Côté JK, Pepler C. A randomized trial of a cognitive coping intervention for acutely ill HIV‐positive men. Nursing Research 2002;51(4):237‐44. [DOI] [PubMed] [Google Scholar]
Creswell 2009 {published data only}
- Creswell JD, Myers HF, Cole SW, Irwin MR. Mindfulness meditation training effects on CD4+ T lymphocytes in HIV‐1 infected adults: a small randomized controlled trial. Brain, Behavior, and Immunity 2009;23(2):184‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cruess 2000 {published data only}
- Cruess DG, Antoni MH, Schneiderman N, Ironson G, McCabe P, Fernandez JB, et al. Cognitive‐behavioral stress management increases free testosterone and decreases psychological distress in HIV‐seropositive men. Health Psychology 2000;19(1):12‐20. [DOI] [PubMed] [Google Scholar]
Davies 2006 {published data only}
- Davies G, Koenig LJ, Stratford D, Palmore M, Bush T, Golde M, et al. Overview and implementation of an intervention to prevent adherence failure among HIV‐infected adults initiating antiretroviral therapy: lessons learned from Project HEART. AIDS Care 2006;18(8):895‐903. [DOI] [PubMed] [Google Scholar]
Davies 2009 {published data only}
- Davies SL, Horton TV, Williams AG, Martin MY, Stewart KE. MOMS: formative evaluation and subsequent intervention for mothers living with HIV. AIDS Care 2009;21(5):552‐60. [DOI] [PubMed] [Google Scholar]
Evans 2003 {published data only}
- Evans S, Fishman B, Spielman L, Haley A. Randomized trial of cognitive behavior therapy versus supportive psychotherapy for HIV‐related peripheral neuropathic pain. Psychosomatics 2003;44(1):44‐50. [DOI] [PubMed] [Google Scholar]
Fife 2008 {published data only}
- Fife BL, Scott LL, Fineberg NS, Zwickl BE. Promoting adaptive coping by persons with HIV disease: evaluation of a patient/partner intervention model. Journal of the Association of Nurses in AIDS Care 2008;19(1):75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gifford 1998 {published data only}
- Gifford AL, Laurent DD, Gonzales VM, Chesney MA, Lorig KR. Pilot randomized trial of education to improve self‐management skills of men with symptomatic HIV/AIDS. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 1998;18(2):136‐44. [DOI] [PubMed] [Google Scholar]
Golin 2006 {published data only}
- Golin CE, Earp J, Tien HC, Stewart P, Porter C, Howie L. A 2‐arm, randomized, controlled trial of a motivational interviewing‐based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. Journal of Acquired Immune Deficiency Syndromes 2006;42(1):42‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodkin 1998 {published data only}
- Goodkin K, Feaster DJ, Asthana D, Blaney NT, Kumar M, Baldewicz T, et al. A bereavement support group intervention is longitudinally associated with salutary effects on the CD4 cell count and number of physician visits. Clinical and Diagnostic Laboratory Immunology 1998;5(3):382‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodkin 1999 {published data only}
- Goodkin K, Blaney NT, Feaster DJ, Baldewicz T, Burkhalter JE, Leeds B. A randomized controlled clinical trial of a bereavement support group intervention in human immunodeficiency virus type 1‐seropositive and ‐seronegative homosexual men. Archives of General Psychiatry 1999;56(1):52‐9. [DOI] [PubMed] [Google Scholar]
Hansen 2009 {published data only}
- Hansen N, Tarakeshwar N, Ghebremichael M, Zhang H, Kochman A, Sikkema K. Longitudinal effects of coping on outcome in a randomized controlled trial of a group intervention for HIV‐positive adults with AIDS‐related bereavement. Death Studies 2006;30(7):609‐36. [DOI] [PubMed] [Google Scholar]
Heckman 2004 {published data only}
- Heckman TG, Anderson ES, Sikkema KJ, Kochman A, Kalichman SC, Anderson T. Emotional distress in nonmetropolitan persons living with HIV disease enrolled in a telephone‐delivered, coping improvement group intervention. Health Psychology 2004;23(1):94‐100. [DOI] [PubMed] [Google Scholar]
Heckman 2006 {published data only}
- Heckman TG, Barcikowski R, Ogles B, Suhr J, Carlson B, Holroyd K, et al. A telephone‐delivered coping improvement group intervention for middle‐aged and older adults living with HIV/AIDS. Annals of Behavioral Medicine 2006;32(1):27‐38. [DOI] [PubMed] [Google Scholar]
Ingersoll 2011 {published data only}
- Ingersoll KS, Farrell‐Carnahan L, Cohen‐Filipic J, Heckman CJ, Ceperich SD, Hettema J, et al. A pilot randomized clinical trial of two medication adherence and drug use interventions for HIV+ crack cocaine users. Drug and Alcohol Dependence 2011;116(1‐3):177‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2013 {published data only}
- Jensen SE, Pereira DB, Whitehead N, Buscher I, McCalla J, Andrasik M, et al. Cognitive‐behavioral stress management and psychological well‐being in HIV + racial/ethnic minority women with human papillomavirus. Health Psychology 2013;32(2):227‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005 {published data only}
- Jones DL, Ross D, Weiss SM, Bhat G, Chitalu N. Influence of partner participation on sexual risk behavior reduction among HIV‐positive Zambian women. Journal of Urban Health 2005;82(3 Suppl 4):iv92‐100. [DOI] [PubMed] [Google Scholar]
Kaaya 2013 {published data only}
- Kaaya SF, Blander J, Antelman G, Cyprian F, Emmons KM, Matsumoto K, et al. Randomized controlled trial evaluating the effect of an interactive group counseling intervention for HIV‐positive women on prenatal depression and disclosure of HIV status. AIDS Care 2013;25(7):854‐62. [DOI] [PubMed] [Google Scholar]
Kalichman 2005 {published data only}
- Kalichman SC, Rompa D, Cage M. Group intervention to reduce HIV transmission risk behavior among persons living with HIV/AIDS. Behavior Modification 2005;29(2):256‐85. [DOI] [PubMed] [Google Scholar]
Koenig 2008 {published data only}
- Koenig LJ, Pals SL, Bush T, Pratt Palmore M, Stratford D, Ellerbrock TV. Randomized controlled trial of an intervention to prevent adherence failure among HIV‐infected patients initiating antiretroviral therapy. Health Psychology 2008;27(2):159‐69. [DOI] [PubMed] [Google Scholar]
Kunutsor 2011 {published data only}
- Kunutsor S, Walley J, Katabira E, Muchuro S, Balidawa H, Namagala E, et al. Improving clinic attendance and adherence to antiretroviral therapy through a treatment supporter intervention in Uganda: a randomized controlled trial. AIDS and Behavior 2011;15(8):1795‐802. [DOI] [PubMed] [Google Scholar]
Laperriere 2005 {published data only}
- Laperriere A, Ironson GH, Antoni MH, Pomm H, Jones D, Ishii M, et al. Decreased depression up to one year following CBSM+ intervention in depressed women with AIDS: the smart/EST women's project. Journal of Health Psychology 2005;10(2):223‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Latkin 2003 {published data only}
- Latkin CA, Sherman S, Knowlton A. HIV prevention among drug users: outcome of a network‐oriented peer outreach intervention. Health Psychology 2003;22(4):332‐9. [DOI] [PubMed] [Google Scholar]
Lechner 2003 {published data only}
- Lechner SC, Antoni MH, Lydston D, LaPerriere A, Ishii M, Devieux J, et al. Cognitive–behavioral interventions improve quality of life in women with AIDS. Journal of Psychosomatic Research 2003;54(3):253‐61. [DOI] [PubMed] [Google Scholar]
Lee 1999 {published data only}
- Lee MR, Cohen L, Hadley SW, Goodwin FK. Cognitive‐behavioral group therapy with medication for depressed gay men with AIDS or symptomatic HIV infection. Psychiatric Services 1999;50(7):948‐52. [DOI] [PubMed] [Google Scholar]
Lehavot 2011 {published data only}
- Lehavot K, Huh D, Walters KL, King KM, Andrasik MP, Simoni JM. Buffering effects of general and medication‐specific social support on the association between substance use and HIV medication adherence. AIDS Patient Care and STDs. 2011;25(3):181‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacNeil 1999 {published data only}
- MacNeil JM, Mberesero F, Kilonzo G. Is care and support associated with preventive behaviour among people with HIV?. AIDS Care 1999;11(5):537‐46. [DOI] [PubMed] [Google Scholar]
Marhefka 2014 {published data only}
- Marhefka SL, Buhi ER, Baldwin J, Chen H, Johnson A, Lynn V, et al. Effectiveness of healthy relationships video‐Group—A videoconferencing group intervention for women living with HIV: preliminary findings from a randomized controlled trial. Telemedicine Journal and e‐Health 2014;20(2):128‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Markowitz 1998 {published data only}
- Markowitz JC, Kocsis JH, Fishman B, Spielman LA, Jacobsberg LB, Frances AJ, et al. Treatment of depressive symptoms in human immunodeficiency virus‐positive patients. Archives of General Psychiatry 1998;55(5):452‐7. [DOI] [PubMed] [Google Scholar]
Molassiotis 2002 {published data only}
- Molassiotis A, Callaghan P, Twinn SF, Lam SW, Chung WY, Li CK. A pilot study of the effects of cognitive‐behavioral group therapy and peer support/counseling in decreasing psychologic distress and improving quality of life in Chinese patients with symptomatic HIV disease. AIDS Patient Care and STDs 2002;16(2):83‐96. [DOI] [PubMed] [Google Scholar]
Mundell 2011 {published data only}
- Mundell JP, Visser MJ, Makin JD, Kershaw TS, Forsyth BW, Jeffery B, et al. The impact of structured support groups for pregnant South African women recently diagnosed HIV positive. Women & Health 2011;51(6):546‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakimuli‐Mpungu 2014 {published data only}
- Nakimuli‐Mpungu E, Wamala K, Okello J, Alderman S, Odokonyero R, Musisi S, et al. Outcomes, feasibility and acceptability of a group support psychotherapeutic intervention for depressed HIV affected Ugandan adults: a pilot study. Journal of Affective Disorders 2014;166:144‐50. [DOI] [PubMed] [Google Scholar]
Nokes 2003 {published data only}
- Nokes KM, Chew L, Altman C. Using a telephone support group for HIV‐positive persons aged 50+ to increase social support and health‐related knowledge. AIDS Patient Care and STDs 2003;17(7):345‐51. [DOI] [PubMed] [Google Scholar]
Olley 2006 {published data only}
- Olley BO. Improving well‐being through psycho‐education among voluntary counseling and testing seekers in Nigeria: a controlled outcome study. AIDS Care 2006;18(8):1025‐31. [DOI] [PubMed] [Google Scholar]
Pacella 2012 {published data only}
- Pacella ML, Armelie A, Boarts J, Wagner G, Jones T, Feeny N, et al. The impact of prolonged exposure on PTSD symptoms and associated psychopathology in people living with HIV: a randomized test of concept. AIDS and Behavior 2012;16(5):1327‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papas 2011 {published data only}
- Papas RK, Sidle JE, Gakinya BN, Baliddawa JB, Martino S, Mwaniki MM, et al. Treatment outcomes of a stage 1 cognitive‐behavioral trial to reduce alcohol use among human immunodeficiency virus‐infected out‐patients in western Kenya. Addiction (Abingdon, England) 2011;106(12):2156‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petersen 2014 {published data only}
- Petersen I, Hanass Hancock J, Bhana A, Govender K. A group‐based counselling intervention for depression comorbid with HIV/AIDS using a task shifting approach in South Africa: a randomised controlled pilot study. Journal of Affective Disorders 2014;158:78‐84. [DOI] [PubMed] [Google Scholar]
Prado 2012 {published data only}
- Prado G, Pantin H, Huang S, Cordova D, Tapia MI, Velazquez M‐R, et al. Effects of a family intervention in reducing HIV risk behaviors among high‐risk Hispanic adolescents: a randomized controlled trial. Archives of Pediatrics & Adolescent Medicine 2012;166(2):127‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Proeschold‐Bell 2011 {published data only}
- Proeschold‐Bell RJ, Hoeppner B, Taylor B, Cohen S, Blouin R, Stringfield B, et al. An interrupted time series evaluation of a hepatitis C intervention for persons with HIV. AIDS and Behavior 2011;15(8):1721‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2009 {published data only}
- Rao D, Nainis N, Williams L, Langner D, Eisin A, Paice J. Art therapy for relief of symptoms associated with HIV/AIDS. AIDS Care 2009;21(1):64‐9. [DOI] [PubMed] [Google Scholar]
Rao 2012 {published data only}
- Rao D, Desmond M, Andrasik M, Rasberry T, Lambert N, Cohn SE, et al. Feasibility, acceptability, and preliminary efficacy of the unity workshop: an internalized stigma reduction intervention for African American women living with HIV. AIDS Patient Care and STDs 2012;26(10):614‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ravaei 2013 {published data only}
- Ravaei F, Hosseinian S, Tabatabaei S. Effectiveness of cognitive behavioral and spiritual trainings on improving mental health of HIV positive drug addicts. Archives of Clinical Infectious Diseases 2013;8(1):23‐6. [Google Scholar]
Remien 2005 {published data only}
- Remien RH, Stirratt MJ, Dolezal C, Dognin JS, Wagner GJ, Carballo‐Dieguez A, et al. Couple‐focused support to improve HIV medication adherence: a randomized controlled trial. AIDS (London, England) 2005;19(8):807‐14. [DOI] [PubMed] [Google Scholar]
Robins 2006 {published data only}
- Robins JL, McCain NL, Gray DP, Elswick RK Jr, Walter JM, McDade E. Research on psychoneuroimmunology: tai chi as a stress management approach for individuals with HIV disease. Applied Nursing Research 2006;19(1):2‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 2012 {published data only}
- Roth AM, Holmes AM, Stump TE, Aalsma MC, Ackermann RT, Carney TS, et al. Can lay health workers promote better medical self‐management by persons living with HIV? An evaluation of the Positive Choices program. Patient Education and Counseling 2012;89(1):184‐90. [DOI] [PubMed] [Google Scholar]
Rotheram‐Borus 2011 {published data only}
- Rotheram‐Borus MJ, Richter L, Rooyen H, Heerden A, Tomlinson M, Stein A, et al. Project Masihambisane: a cluster randomised controlled trial with peer mentors to improve outcomes for pregnant mothers living with HIV. Trials 2011;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotheram‐Borus 2012 {published data only}
- Rotheram‐Borus MJ, Rice E, Comulada WS, Best K, Elia C, Peters K, et al. Intervention outcomes among HIV‐affected families over 18 months. AIDS and Behavior 2012;16(5):1265‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sacks 2011 {published data only}
- Sacks S, McKendrick K, Vazan P, Sacks JY, Cleland CM. Modified therapeutic community aftercare for clients triply diagnosed with HIV/AIDS and co‐occurring mental and substance use disorders. AIDS Care 2011;23(12):1676‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Safren 2009 {published data only}
- Safren SA, O'Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT‐AD) in HIV‐infected individuals. Health Psychology 2009;28(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, O'Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT‐AD) in HIV‐infected injection drug users: a randomized controlled trial. Journal of Consulting and Clinical Psychology 2012;80(3):404‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saleh‐Onoya 2009 {published data only}
- Saleh‐Onoya D, Reddy PS, Ruiter RAC, Sifunda S, Wingood G, Borne B. Condom use promotion among isiXhosa speaking women living with HIV in the Western Cape Province, South Africa: a pilot study. AIDS Care 2009;21(7):817‐25. [DOI] [PubMed] [Google Scholar]
SeyedAlinaghi 2012 {published data only}
- SeyedAlinaghi S, Jam S, Foroughi M, Imani A, Mohraz M, Djavid GE, et al. Randomized controlled trial of mindfulness‐based stress reduction delivered to human immunodeficiency virus‐positive patients in Iran: effects on CD4(+) T lymphocyte count and medical and psychological symptoms. Psychosomatic Medicine 2012;74(6):620‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sikkema 2004 {published data only}
- Hansen NB, Tarakeshwar N, Ghebremichael M, Zhang H, Kochman A, Sikkema KJ. Longitudinal effects of coping on outcome in a randomized controlled trial of a group intervention for HIV‐positive adults with AIDS‐related bereavement. Death Studies 2006;30(7):609‐36. [DOI] [PubMed] [Google Scholar]
- Sikkema KJ, Hansen NB, Ghebremichael M, Kochman A, Tarakeshwar N, Meade CS, et al. A randomized controlled trial of a coping group intervention for adults with HIV who are AIDS bereaved: longitudinal effects on grief. Health Psychology 2006;25(5):563‐70. [DOI] [PubMed] [Google Scholar]
- Sikkema KJ, Hansen NB, Kochman A, Tate DC, DiFranceisco W. Outcomes from a randomised controlled trail of a group intervention for HIV positive men and women coping with HIV‐related loss and bereavement. Death Studies 2004;28(3):187‐209. [DOI] [PubMed] [Google Scholar]
- Sikkema, KJ, Hansen, NB, Meade, CS, Kochman, A, Lee. RS. Improvements in health‐related quality of life following a group intervention for coping with AIDS‐bereavement among HIV‐infected men and women. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation 2005;14(4):991‐1005. [DOI] [PubMed] [Google Scholar]
- Smith NG, Tarakeshwar N, Hansen NB, Kochman A, Sikkema KJ. Coping mediates outcome following a randomized group intervention for HIV‐positive bereaved individuals. Journal of Clinical Psychology 2009;65(3):319‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simoni 2013 {published data only}
- Simoni JM, Wiebe JS, Sauceda JA, Huh D, Sanchez G, Longoria V, et al. A preliminary RCT of CBT‐AD for adherence and depression among HIV‐positive Latinos on the U.S.‐Mexico border: the Nuevo Dia study. AIDS and Behavior 2013;17(8):2816‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2001 {published data only}
- Stewart MJ, Hart G, Mann K, Jackson S, Langille L, Reidy M. Telephone support group intervention for persons with haemophilia and HIV/AIDS and family caregivers. International Journal of Nursing Studies 2001;38(2):209‐25. [DOI] [PubMed] [Google Scholar]
Szapocznik 2004 {published data only}
- Feaster DJ, Brincks AM, Mitrani VB, Prado G, Schwartz SJ, Szapocznik J. The efficacy of Structural Ecosystems Therapy for HIV medication adherence with African American women. Journal of Family Psychology 2010;24(1):51‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaster DJ, Burns MJ, Brincks AM, Prado G, Mitrani VB, Mauer MH, et al. Structural Ecosystems Therapy for HIV+ African‐American women and drug abuse relapse. Family Process 2010;49(2):204‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapocznik J, Feaster DJ, Mitrani VB, Prado G, Smith L, Robinson‐Batista C, et al. Structural ecosystems therapy for HIV‐seropositive African American women: effects on psychological distress, family hassles, and family support. Journal of Consulting and Clinical Psychology 2004;72(2):288‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2006 {published data only}
- Wagner GJ, Kanouse DE, Golinelli D, Miller LG, Daar ES, Witt MD, et al. Cognitive‐behavioral intervention to enhance adherence to antiretroviral therapy: a randomized controlled trial (CCTG 578). AIDS (London, England) 2006;20(9):1295‐302. [DOI] [PubMed] [Google Scholar]
Williams 2014 {published data only}
- Williams AB, Wang H, Li X, Chen J, Li L, Fennie K. Efficacy of an evidence‐based ARV adherence intervention in China. AIDS Patient Care and STDs 2014;28(8):411‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wingood 2004 {published data only}
- Wingood GM, DiClemente RJ, Mikhail I, Lang DL, McCree DH, Davies SL, et al. A randomized controlled trial to reduce HIV transmission risk behaviors and sexually transmitted diseases among women living with HIV: the WILLOW Program. Journal of Acquired Immune Deficiency Syndromes 2004;37(Suppl 2):S58‐67. [DOI] [PubMed] [Google Scholar]
Wong 2008 {published data only}
- Wong FL, Rotheram‐Borus MJ, Lightfoot M, Pequegnat W, Comulada WS, Cumberland W, et al. Effects of behavioral intervention on substance use among people living with HIV: the Healthy Living Project randomized controlled study. Addiction (Abingdon, England) 2008;103(7):1206‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wyatt 2004 {published data only}
- Wyatt GE, Longshore D, Chin D, Carmona JV, Loeb TB, Myers HF, et al. The efficacy of an integrated risk reduction intervention for HIV‐positive women with child sexual abuse histories. AIDS and Behavior 2004;8(4):453‐62. [DOI] [PubMed] [Google Scholar]
Yu 2014 {published data only}
- Yu X, Lau JTF, Mak WWS, Cheng Y, Lv Y, Zhang J. A pilot theory‐based intervention to improve resilience, psychosocial well‐being, and quality of life among people living with HIV in rural China. Journal of Sex & Marital Therapy 2014;40(1):1‐16. [DOI] [PubMed] [Google Scholar]
Zisook 1998 {published data only}
- Zisook S, Peterkin J, Goggin KJ, Sledge P, Atkinson JH, Grant I. Treatment of major depression in HIV‐seropositive men. Journal of Clinical Psychiatry 1998;59(5):217‐24. [DOI] [PubMed] [Google Scholar]
Znoj 2010 {published data only}
- Znoj HJ, Messerli‐Burgy N, Tschopp S, Weber R, Christen L, Christen S, et al. Psychotherapeutic process of cognitive‐behavioral intervention in HIV‐infected persons: results from a controlled, randomized prospective clinical trial. Psychotherapy Research 2010;20(2):203‐13. [DOI] [PubMed] [Google Scholar]
Additional references
Anagnostopoulos 2015
- Anagnostopoulos A, Ledergerber B, Jaccard R, Shaw SA, Stoeckle M, Bernasconi E, et al. Frequency of and risk factors for depression among participants in the Swiss HIV Cohort Study (SHCS). PLoS ONE 2015;10(10):e0140943. [DOI: 10.1371/journal.pone.0140943] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty‐five years of evaluation. Clinical Psychology Review 1988;8(1):77‐100. [Google Scholar]
Bhatia 2014
- Bhatia MS, Munjal S. Prevalence of depression in people living with HIV/AIDS undergoing ART and factors associated with it. Journal of Clinical and Diagnostic Research 2014;8(10):WC‐01‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyle 2016
- Boyle BA. HIV in developing countries: a tragedy only starting to unfold. AIDS Reader 2016;10(2):77‐9. [Google Scholar]
Brown 2011
- Brown JL, Vanable PA. Stress management interventions for HIV‐infected individuals: review of recent intervention approaches and directions for future research. Neurobehavioral HIV Medicine 2011;3(1):95‐106. [Google Scholar]
Chesney 2006
- Chesney MA, Neilands TB, Chambers DB, Taylor JM, et al. A validity and reliability study of the coping self‐efficacy scale. British Journal of Health Psychology 2006;11:421‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1983
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior 1983;24(4):385‐96. [PubMed] [Google Scholar]
Colbert 2010
- Colbert AM, Kim KH, Sereika SM, Erlen JA. An examination of the relationships among gender, health status, social support, and HIV‐related stigma. Journal of the Association of Nurses in AIDS Care 2010;21(4):302‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooperman 2005
- Cooperman N, Simoni JM. Suicidal ideation and attempted suicide among women living with HIV/AIDS. Journal of Behavioral Medicine 2005;28(2):149‐56. [DOI] [PubMed] [Google Scholar]
Crepaz 2008
- Crepaz N, Passin WF, Herbst JH, Rama SM, Malow RM, Purcell DW, et al. Meta‐analysis of cognitive‐behavioral interventions on HIV‐positive persons' mental health and immune functioning. Health Psychology 2008;27(1):4‐14. [DOI] [PubMed] [Google Scholar]
Deci 2008
- Deci EL, Ryan RM. Facilitating optimal motivation and psychological wellbeing across life’s domains. Canadian Psychology 2008;49(1):14‐23. [Google Scholar]
Friedland 1996
- Friedland J, Renwick R, McColl M. Coping and social support as determinants of quality of life in HIV/AIDS. AIDS Care 1996;8(1):15‐31. [DOI] [PubMed] [Google Scholar]
Gielen 2001
- Gielen AC, McDonnell KA, Wu AW, O'Campo P, Faden R. Quality of life among women living with HIV: the importance violence, social support, and self care behaviors. Social Science & Medicine 2001;52(2):315‐22. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horowitz 1979
- Horowitz M, Wilner M, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic Medicine 1979;41(3):209‐18. [DOI] [PubMed] [Google Scholar]
Hudson 2001
- Hudson AL, Lee KA, Miramontes H, Portillo CJ. Social interactions, perceived support, and level of distress in HIV‐positive women. Journal of the Association of Nurses in AIDS Care 2001;12(4):68‐76. [DOI] [PubMed] [Google Scholar]
Huppert 2009
- Huppert FA. Psychological well‐being: Evidence regarding its causes and consequences. Applied Psychology: Health and Well‐Being 2009;1(2):137‐64. [Google Scholar]
Ironson 1987
- Ironson G, Antoni M, August S, Baggett HL. Coping Self efficacy Scale. University of Miami; Coral Gables, Florida 1987.
Kelly 1998
- Kelly JA. Group psychotherapy for persons with HIV and AIDS‐related illnesses. International Journal of Group Psychotherapy 1998;48(2):143‐62. [DOI] [PubMed] [Google Scholar]
Lawless 1996
- Lawless S, Kippax S, Crawford J. Dirty, diseased and undeserving: the positioning of HIV positive women. Social Science & Medicine 1996;43(9):1371‐7. [DOI] [PubMed] [Google Scholar]
McCain 1992
- McCain NL, Gramling LF. Living with dying: coping with HIV‐disease. Issues in Mental Health Nursing 1992;13(3):271‐84. [DOI] [PubMed] [Google Scholar]
McNair 1971
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services, 1971. [Google Scholar]
Metcalfe 1998
- Metcalfe KA, Langstaff JE, Evans SJ, Paterson HM, Reid JL. Meeting the needs of women living with HIV. Public Health Nursing 1998;15(1):30‐4. [DOI] [PubMed] [Google Scholar]
Moneyham 1998
- Moneyham L, Hennessy M, Sowell R, Demi A, Seals B, Mizuno Y. The effectiveness of coping strategies used by HIV‐seropositive women. Research in Nursing and Health 1998;21(4):351‐62. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134(4):382–9. [DOI] [PubMed] [Google Scholar]
Peterson 2003
- Peterson JL. The Development of a Normative Model of Social Support for Women Living with HIV [PhD thesis]. University of Illinois: Urbana‐Champaign, 2003. [Google Scholar]
Radloff 1977
- Radloff LS. The CES‐D Scale: a self‐report depression scale for research in the general population. Applied Psychological Measurement 1977;1(3):385‐401. [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rezaee 2013
- Rezaee H, Khalili H, Hatamkhani S, Dashti‐Khavidaki S, Khazaeipour Z. Frequency of depression and its correlation with serum carnitine level in HIV/AIDS patients. Current HIV Research 2013;11(3):226‐30. [DOI] [PubMed] [Google Scholar]
Roopnaraine 2012
- Roopnaraine T, Rawat R, Babirye F, Ochai R, Kadiyala S. "The group" in integrated HIV and livelihoods programming: opportunity or challenge?. AIDS Care 2012;24(5):649‐57. [DOI] [PubMed] [Google Scholar]
Sarason 1978
- Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the life experiences survey. Journal of Consulting and Clinical Psychology 1978;46(5):932‐46. [DOI] [PubMed] [Google Scholar]
Scott‐Sheldon 2008
- Scott‐Sheldon LA, Kalichman SC, Carey MP, Fielder RL. Stress management interventions for HIV+ adults: a meta‐analysis of randomised controlled trials, 1989 to 2006. Health Psychology 2008;27(2):129‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheehan 1997
- Sheehan DV, Lecrubier Y, Harnett‐Sheehan K, Janavs J, Weiller E, Keskiner A, et al. The validity of the MINI International Neuropsychiatric Interview (M.I.N.I.) according to the SCID‐P and its reliability. European Psychiatry 1997;12(5):232‐41. [Google Scholar]
Sikkema 2000
- Sikkema, KJ, Kalichman, SC, Hoffmann, R, Koob, JJ, Kelly, JA, Heckman. TG. Coping strategies and emotional well‐being among HIV‐infected men and women experiencing AIDS‐related bereavement. AIDS Care 2000;12:613–24. [DOI] [PubMed] [Google Scholar]
Spielberger 2010
- Spielberger, CD State‐Trait anxiety inventory. State‐Trait Anxiety Inventory. John Wiley & Sons, Inc. Palo Alto, California: Consulting Psychologists Press, Inc, 2010:1‐36. [Google Scholar]
Turner‐Cobb 2002
- Turner‐Cobb JM, Gore‐Felton C, Marouf F, Koopman C, Kim P, Israelski D, et al. Coping, social support, and attachment style as psychosocial correlates of adjustment in men and women with HIV/AIDS. Journal of Behavioral Medicine 2002;25(4):337‐53. [DOI] [PubMed] [Google Scholar]
Vanable 2006
- Vanable PA, Carey MP, Blair DC, Littlewood RA. Impact of HIV‐related stigma on health behaviors and psychological adjustment among HIV‐positive men and women. AIDS and Behavior 2006;10(5):473‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2002
- Walker J. Rural women with HIV and AIDS: perceptions of service accessibility, psychosocial, and mental health counseling needs. Journal of Mental Health Counseling 2002;24(4):299‐407. [Google Scholar]
Yesavage 1982
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research 1982‐1983;17(1):37‐49. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
van der Heijden 2013
- Heijden I, Abrahams N. Psychosocial group interventions for improving quality of life in adults living with HIV. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010806] [DOI] [PMC free article] [PubMed] [Google Scholar]


