Abstract
Matrix metalloproteinases (MMPs) are a family of zinc dependent endopeptidases whose main function is to degrade and deposit structural proteins within the extracellular matrix (ECM). A dysregulation of MMPs is linked to vascular diseases. MMPs are classified into collagenases, gelatinases, membrane-type, metalloelastase, stromelysins, matrilysins, enamelysins, and unclassified subgroups. The production of MMPs is stimulated by factors such as oxidative stress, growth factors and inflammation which lead to its up- or down-regulation with subsequent ECM remodeling. Normally, excess activation of MMPs is controlled by their endogenous inhibitors, tissue inhibitors of metalloproteinases (TIMPs). An imbalance of MMPs and TIMPs has been implicated in hypertension, atherosclerotic plaque formation and instability, aortic aneurysms and varicose vein wall remodeling. Also, recent evidence suggests epigenetic regulation of some MMPs in angiogenesis and atherosclerosis. Over the years, pharmacological inhibitors of MMPs have been used to modify or prevent the development of the disease with some success. In this review, we discuss recent advances in MMP biology, and their involvement in the manifestation of vascular disease.
Keywords: MMPs, TIMPs, miRNA, Epigenetics, Extracellular Matrix, Atherosclerosis, Cardiovascular, Review
2. INTRODUCTION
Vascular diseases are the leading cause of morbidity and mortality worldwide. In general, vascular disease relates to pathological states of the circulatory system including arteries, vein, capillaries, lymph vessels, and blood disorders which affects circulation. One of the major manifestation of vascular disease is atherosclerosis, a plaque build-up containing fat, cholesterol, and other substances within the arterial walls. Over time, the plaque hardens leading to narrowing of the arterial lumen which ultimately decreases blood flow to the tissue or organ. The consequence of atherosclerosis can progress to a spectrum of serious health problems including hypertension, stroke, heart attack and death.
The inner lining of blood vessels is composed of endothelial cells (ECs) and damage to ECs by pathogens or oxidative radicals lead to inflammatory response which triggers leukocyte adhesion and transmigration into the vessel wall. As a result, extracellular matrix regulatory peptidases, matrix metalloproteinases (MMPs) become activated leading to vascular smooth muscle cells (VSMCs) proliferation, cell-to-cell tight junction protein alteration and vascular leakage, elastin and collagen derangement, and vascular dysfunction. This review, summarizes the impact of uncontrolled MMP activation in the vasculature and highlights their regulatory mechanisms involved in maintaining physiological function and deviations leading to pathophysiology.
3. GENERAL CONCEPTS OF MMPS
In 1962, Gross and Lapiere discovered an enzyme capable of degrading collagen during the metamorphosis of the anuran tadpole tail (1). The enzyme, a collagenase was found to play a significant role in the normal development and growth in amphibians thus maintaining a balance between tissue synthesis and degradation (1). From this conclusion arose the general concept of MMPs which has been applied continuously in various studies including homeostasis and tissue remodeling under pathological conditions. The MMPs in turn are regulated by a group of enzymes, tissue inhibitors of metalloproteases (TIMPs). The general concept dictates that a balance between TIMPs and MMPs regulates tissue remodeling, repair, and resorption. A dysregulation of the MMP/TIMP ratio may lead to unchecked MMP activity leading to adverse events in tissue homeostasis (2). There are currently 25 MMPs with substrate specificity for a broad spectrum of fibrous structural proteins (2). Many of these substrates are found within the extracellular matrix (ECM) surrounding the blood vessels (3). Indeed, dysregulated MMP activity has been observed in a number of diseases including atherosclerosis, fibrosis, heart failure, emphysema, and chronic obstructive pulmonary disease (COPD) where ECM alteration is a predominant feature (2,4). Since MMP activity is known to play a prominent role in vascular diseases a potential strategy counter would be to reduce MMP activity to ameliorate ECM remodeling.
4. STRUCTURE AND BIOSYNTHESIS
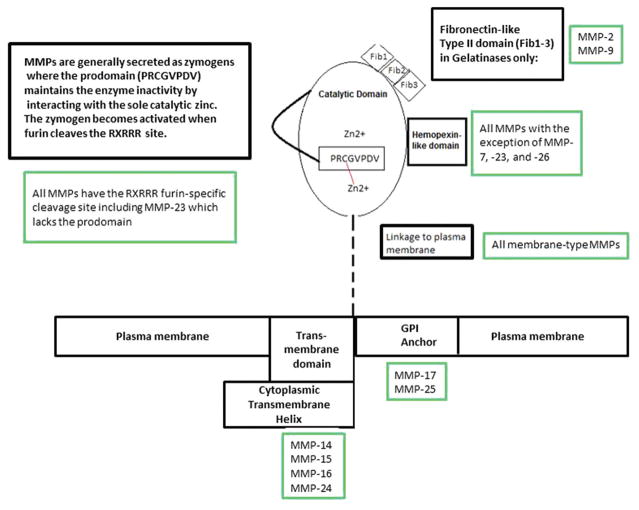
X-ray crystallography and NMR spectroscopy have been used for elucidation of MMP structure in the last 20 years. Prior to this, thermolysin, a bacterial endopeptidase, and astacin, a crayfish endopeptidase, were used to design MMP inhibitors and subsequent structure elucidation. However, the models lacked the information necessary to carry out rational drug design, and the catalytic domain of MMP-1 became the first of the group to undergo structural studies using multiple isomorphous replacements at a resolution of 2.4 Angstroms (5). MMPs have three conserved domains; (i) zinc-containing catalytic domain; (ii) pro-peptide on the amino terminus; (iii) hemopexin-like domain at the C-terminus (Figure 1). The catalytic domain has two zinc ions and at least one calcium ion. Of the zinc ions, only one ion (Z1) is catalytic (2). MMP-23 does not have a catalytic domain (2). The hemopexin-like domain is called as such because of its sequence homology to hemopexin, a plasma protein involved in heme binding and transport (2). MMPs are a family of zinc endopeptidases present in the extracellular matrix (ECM) or membrane (2). Sequence alignment has confirmed that they belong to a superfamily of zinc peptidases known as metzincins. Among all the MMPs described so far, 25 MMPs have been discovered where MMP-4,-5, and -6 are encoded by MMPs can be either membrane-bound or secreted (2,6,7). Membrane-type MMP is often referred to as simply MT-MMPs (2).
Figure 1.
Schematic of generalized structure of MMPs. The Prodomain has a cleavage site at RxRRR which activates the subsequent autocatalysis of the pro-enzyme. Although MMP-23 has the cleavage site, it is the only one that lacks a prodomain. The catalytic domain is common across all MMPs consisting of two Zn2+ ions where one is purely structural, while the other is involved in catalytic activity. The catalytic zinc interacts with a cysteine residue (cysteine switch) within the prodomain to remain inactive. The Hemopexin-like domain and catalytic domain are connect by a linker peptide, and is observed at the C-terminus of all MMPs except for MMP-7,-23, and -26. Its sequence homology resembles that of hemopexin, a protein with heme-binding capabilities. It enhances substrate recognition and specificity in stromelysins and collagenases, but not required for elastase activity in metalloelastase. Lastly, TIMP: proenzyme interactions are facilitated by this domain in gelatinases. The Fib1-3s are fibronectin-like type II domains found only in gelatinases.
All MMPs are secreted and cleavage of the signal sequence yields a zymogen inactivated by a highly conserved prodomain displaying the consensus sequence PRCGVPDV (Figure 1). This prodomain is often called a switch loop as it contains Cys92 whose thiol coordinates with Z1 in the proenzyme (2,8). The polypeptide linker connecting the catalytic domain to the prodomain can undergo furin cleavage, proconvertase action, sheddase action, or auto-activation to yield the active enzyme (2).
5. TYPES OF MMPS AND THEIR INVOLVEMENT IN DISEASES
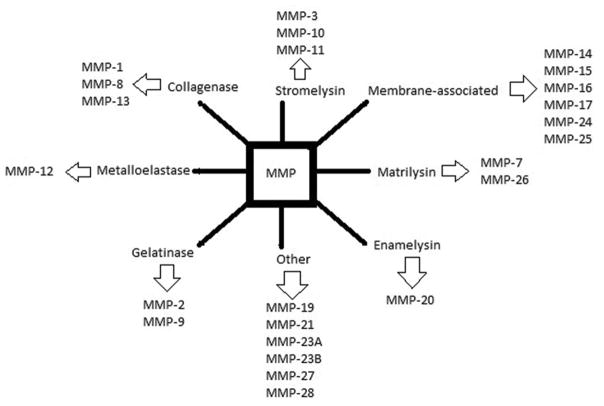
MMPs are further divided into subgroups based on their substrate preference (Figure 2). Blood vessels are known to be under the control of hormonal, neuronal, and hemodynamic input. Since the blood vessels are surrounded by ECM, dysregulation of MMP activity may lead to chronic vascular remodeling and vascular disease (9). Table 1 displays the vascular events associated with specific MMPs.
Figure 2.
Subgroup classification of MMPs.
Table 1.
MMPs in vascular health and disease
| Type | Enzyme | Other name (s) | Associated vascular events (Ref.) |
|---|---|---|---|
| Collagenases | MMP-1 | Interstitial collagenase Fibroblast collagenase |
Venus hypertension (10) Carotid atheroma (14) Insulin resistance (13) |
| MMP-8 | Neutrophil collagenase | Carotid atheroma (14) | |
| MMP-13 | CLG3; MANDP1; Collagenase-3 | Neointima formation (198) Restenosis/atherothrombosis (16) Renal Disease (17, 18) |
|
| MMP-18 | Collagenase-4 Xenopus collagenase |
Not known | |
| Gelatinases | MMP-2 | Gelatinase A | ICAS, thrombosis, heart and renal disease (12,36,39,197) |
| MMP-9 | Gelatinase B | Hypertension (12) Carotid stenosis (25) Cardiac and renal disease (31,197) |
|
| Stromelysins | MMP-3 | Stromelysin-1 | Inflammatory bowel disease (44) |
| MMP-10 | Stromelysin-2 | Inflammatory bowel disease (44) Tumor growth and progression (46) Microvascular disease in diabetes (47) Muscular dystrophy (48) |
|
| MMP-11 | Stromelysin-3 | Mammary gland development (49) | |
| Membrane type | MMP-14 | MT1-MMP | Lung tumor (51) Neuroinflammation (53) Neointima formation (54) Pressure overload (59) Macular degeneration (64) Hypertension (66) |
| MMP-15 | MT2-MMP | Inflammatory disease (71) | |
| MMP-16 | MT3-MMP | HSP and deep vein thrombosis (72,73) | |
| MMP-17 | MT4-MMP | Tumor growth and metastasis (74) | |
| MMP-24 | MT5-MMP | Deep vein thrombosis (73) | |
| MMP-25 | MT6-MMP | Abdominal aortic aneurysm (75) | |
| Matrilysins | MMP-7 | PUMP1 | Myocardial infarction (77) Kawasaki disease (80) Coronary artery disease (81) Hypertension (83,87) |
| MMP-26 | Matrilysin-2 Endometase |
Wound healing (88) cerebral amyloid angiopathy (90) |
|
| Enamelysin | MMP-20 | Enamel metalloproteinase | No report |
| Metalloelastase | MMP-12 | Macrophage metallo-/elastase (MME or ME) | Atherosclerosis (92,99) Aortic dissection (96) Retinopathy (101) Intracerebral hemorrhage (102) Peripheral vascular damage (104) COPD (108) Deep vein thrombosis (73) |
| Unclassified | MMP-19 | RASI-1 | Thoracic aortic aneurysms (110) Cerebral amyloid angiopathy (90) Rheumatoid arthritis (112) |
| MMP-21 | Xenopus MMP (XMMP) | Melanoma development (113) Tissue remodeling/embryogenesis (114,115,117) |
|
| MMP-23 | MMP-23A/B | Thrombosis (73) Reproductive system (11) |
|
| MMP-27 | Epilysin | Not known | |
| MMP-28 | Epilysin | Left ventricular remodeling (125) Soft tissue edema (72) |
Abbreviations: CLG3, collagenase-3; MANDP1, Metaphyseal anadysplasia 1; ICAS, Intracranial atherosclerosis; HSP, Henoch–Schönlein purpura; COPD, Chronic Obstructive Pulmonary Disease
5.1. Collagenases
MMP-1, -8 -13 and -18 are collagenases which mainly cleave fibrillar collagen types I, II and III. In addition, these collagenases exhibit specificity for other substrates such as gelatin, casein, aggrecan, laminin, versican, perlecan, fibronectin, and tenascin (10,11). High levels of MMP-1 have been reported in varicose veins (12). In spontaneously hypertensive rats, DeLano and Schmid-Schonbein showed that cell membrane receptor cleavage by MMP-1 was associated with insulin resistance (13). Elevated MMP-1 and -8 activity in the mesentery were observed in rat models of acute venous hypertension (12). In the same study, upstream of the venous pressure increased vascular endothelial growth factor receptor-2 (VEGRF2) expression was observed, and became more elevated with MMP inhibition (12). In human carotid atheroma, angiotensin II receptor 1 blockage significantly reduced MMP-1 and -8 concentrations within atheroma suggesting the involvement of these MMPs in plaque stability (14). Recent findings suggest that M1 macrophage-mediated MMP-13 expression induced neointima formation after vascular injury in eNOS knockout mice, and that MMP-13 is involved in vascular disease (15). Protease-activated receptor-1 (PAR1) is a G protein-coupled receptor and MMP-13 has been demonstrated to cleave PAR1 resulting in the activation of G-protein signaling pathway in restenosis and atherothrombotic diseases (16). In addition, MMP-13 is an interstitial collagenase and has been shown to have significant renal expression related to ECM remodeling during progressive renal diseases (17,18). In a recent study, we reported that increased expression of MMP-13 in mouse glomerular ECs were mediated by homocysteine, a non-protein amino acid and vascular risk factor (19). Using pharmacological MMP-13 inhibitor, Quillard et al showed that the accumulation of collagen in the plaque is associated with resistance to rupture (20). Although the expression of MMP-18 has been reported in a variety of human cell lines, it has not been associated with any disease (21,22).
5.2. Gelatinases
MMP-2 and MMP-9 are gelatinases highly expressed in varicose veins (9). Increased MMP-9 activity has been demonstrated in the mesenteric veins of acutely hypertensive rats (12). In a meta-analysis focusing on characterizing the expression profile of low grade inflammation in patients with coronary artery disease, MMP-9 levels were found to be elevated suggesting its use as a potential biomarker, however, further studies are required to conclude the causality of low grade inflammation in vascular diseases (23).
The receptor activation of NF-kB was studied by using receptor activator of nuclear factor kappa-B ligand (RANKL) /Osteoprotegerin ratio in a cohort study consisting of patients with severe carotid artery stenosis and was observed to correlate directly with MMP-9 activity (24). Hydroxytyrol is commonly found in olive oil and is associated with cardioprotection. It has been suggested that this molecule inhibits monocyte activation of cyclooxygenase 2 which generates eicosanoids capable of suppressing MMP-9 activity (25). The proposed mechanism of action is inhibition of the protein kinase C alpha and beta 1 pathways conferring anti-atherosclerotic and anti-inflammatory properties (25). High serum MMP-9 is associated with increased intimal thickness and plaque instability (26). Tumor necrosis factor-induced activation of eNOS along with MMP-9 and protein kinase B activation are markers for endothelial dysfunction which is mitigated by treatment with apigenin, an estrogen receptor agonist (27). The formation of calcified aortic valve is influenced by a number of cardiac risk factors, molecular signaling pathways, and hemodynamics (28,29). A model for fluid shear stress (FSS) showed increased ECM degradation due to elevated MMP-9 and MMP-2 activity was associated with FSS (30). In patients suffering from cardiac disease, increased expression and binding of CD40 ligand/receptor has been associated with activation of MMP-9 and pro-angiogenesis (31). The upregulation of soluble CD40 ligand (sCD40L) occurs via CD40/CD40L/ TRAF axis and the stimulation of sCD40L has been shown to induce the phosphorylation of p38 MAPK and to increase the release of MMP-9 in endothelial progenitor cells (31). In a recent study, vitamin C deficiency directly correlated with stress-induced cardiac damage which was associated with increased activity of MMP-2 and -9 (32).
The cardiac matrix was shown to regulate physiological processes involved in angiogenesis, hypertrophy, and cardiac fibrosis through the differential microRNA profile (33). A recent review article reports the current mechanistic understanding on the effects of reactive oxygen/nitrogen species effect on MMP-2 expression (34).
MMP-2 was also shown to induce VSM relaxation in the inferior vena cava of rats leading to venous dilatation, varicose veins and insufficiency (35). Treatment with L-NG-nitroarginine methyl ester (L-NAME) and indomethacin showed that elevated levels of nitric oxide (NO) or prostacyclin was not involved because inhibition of nitric oxide synthase (NOS) and cyclooxygenase did not stop vascular smooth muscle (VSM) relaxation (8,35). Iberiotoxin treatment was successful in stopping VSM relaxation through the action of blocking large conductance Ca2+-activated K+ channels, suggesting a role in the VSM hyperpolarization pathway (8).
In patients with chronic ischemic stroke it has been demonstrated that decreased plasma concentrations of MMP-2 and homocysteine were more closely associated with intracranial atherosclerosis (ICAS) than extracranial atherosclerosis (ECAS) suggesting that MMP-2 may play a role in the development of ICAS (36). In another clinical study, Shimizu et al reported the progression of intracranial large artery atherosclerosis (ILA) in 12.5% patients with acute ischemic stroke, and these patients exhibited decreased MMP-2 and increased MMP-9 along with increased IL-6 in their serum (37). Although the authors did not conclude on the role of MMPs in this study, it is possible that both MMPs may have role in ILA progression along with IL-6. It is also known that carotid artery atherosclerotic plaque rupture can lead to thromboembolism and stroke. In fact, Heo et al conducted a study to determine the relationship between the expression of MMP-2 and -9 in patients with atherosclerotic plaque instability. Their findings demonstrated a significant correlation between increased expression of MMP-2 and -9 and cap rupture suggesting their involvement in plaque instability (38).
Cell-to-cell gap junction proteins play a pivotal role in vascular remodeling and disease progression. In a pressure overload rodent model, our group demonstrated that gap junction proteins, connexins-37 and -43 were decreased in TIMP-2 knockout mice with ascending aortic banding (AB), a model of pressure overload-induced left ventricular dysfunction and heart failure (39). In addition, decreased expression of pro-angiogenic MMP-2, VEGF and increase in anti-angiogenic factors exacerbated abnormal left ventricular remodeling in these animals suggesting a possible relationship between MMP-2, connexins-37 and -43 in heart failure (39).
Unlike gap junction proteins, caveolae are lipid rafts within plasma membrane and contains caveolins which are involved in endocytosis, transcytosis and signal transduction in vascular health and disease. In bleomycin-induced caveolin-1 knockout mice, Shivshankar et al demonstrated that MMP-2 and -9 expression was reduced and this was associated with attenuated pulmonary injury and collagen deposition in caveolin-1 knockout mice (40). On the other hand, loss of caveolin-1 has been reported to upregulate MMP-2 and -9, tight junction proteins, and enhanced blood brain barrier (BBB) permeability in focal cerebral ischemia and reperfusion injury (41). These above experimental evidences suggest that gap junction proteins and collagenases (MMP-2 and -9) may have a role in BBB permeability as observed in caveolin-1 knockout mice where one modulates another and vice versa leading to vascular pathogenesis. Furthermore, our group has demonstrated that hyperhomocysteinemia enhanced cerebrovascular permeability through activation of MMP-9 and increased the formation of fibrinogen-β-amyloid complex within the brain (42). Additionally, increased cerebrovascular permeability and memory loss was also observed with increased MMP-9 activity which was ameliorated by MMP-9 ablation (43).
5.3. Stromelysins
Stromelysin-1, -2, and -3 subcategorize MMP-3, MMP-10, and MMP-11 respectively. Among them, MMP-3 and -10 are intestinal proteases, and are established key players in the development of ulcers in inflammatory bowel disease (44). In addition, although in chronic human hypertension elevated activity of MMP-3 has been reported (45), MMP-10 experimentally has been shown to regulate tumor cell migration, invasion and EC tube formation (46). In a recent report, Toni et al documented that increased circulating MMP-10 was associated with increased microvascular disease risks including diabetic retinopathy in type 1 diabetes (47). In addition, MMP-10 has been reported to play a critical role in muscle regeneration during injury and muscular dystrophy (48). Using a MMP-11-deficient mice model, Tan et al showed that during postnatal mammary gland development ductal tree, alveolar structure and milk production were reduced suggesting a paracrine function of MMP-11 (49). MMP-11 is a negative regulator of adipogenesis which reduces and even reverts adipocyte differentiation, and has been shown to require correct collagen IV folding for fat tissue cohesion and adipocyte function (50).
5.4. Membrane-type MMPs
MMP-14, -15, -16, -17, -24 and -25 are membrane type or membrane bound enzymes. MMP-14 was the first MMP discovered possessing a transmembrane domain cloned from lung tumor cells conferring their invasiveness and metastasis (51). A study focusing on ischemia-reperfusion in the spinal cord injury of rats reported that TIMP-2 binding to MMP-2 activates MMP-14 (52). Furthermore, MMP-14 has been found to be involved in neuroinflammation (53). In another study, elevation of MMP-14 and MMP-2 correlated with abnormal aortic remodeling in sheep fetus development under hypoxic conditions. Furthermore, aortic intimal proliferation was associated with increased expression of cell adhesion molecule, E-Selectin suggesting interaction between endothelial cells and leukocytes (54).
In diabetic retinopathy, MMP-14 induced MMP-2 activation has been associated MMP-2 interaction with integrin and promotion of apoptosis leading to impaired retinal pericyte survival (55). In an in vitro study using VSMCs, MMP-14 induced ApoE degradation was associated with migration of VSMCs in both lipid-free and lipid-loaded treatment (56). Further, proteomic studies has shown that ApoA1, a lipid-free lipoprotein is a substrate of MMP-14 and more susceptible to cleavage than the lipid-loaded lipoprotein suggesting that degradation of high-density lipoprotein (HDL) can severely hamper lipid metabolism and possibly induce vascular disease (57). In salt-sensitive rats, elevation of MMP-14 was seen in late phase of chronic hypertension and left ventricular remodeling (59).
The ischemia-reperfusion (IR) model was applied to the left rat lung, and showed upregulation of several MMPs, including MMP-2 and MMP-9. Uninhibited protease activity was observed during the early phase of reperfusion which was demonstrated to arise from plasma via protein extravasation instead of de novo synthesis within the pulmonary tissue. Preconditioning rats with NO was shown to mitigate protease activity suggesting the mitigation IR injury (58). In another study, IL-6 induced upregulation of MMP-14 has been reported to contribute in carotid artery plaque formation involving Raf-MEK-ERK1/2-AP-1 pathway leading to ECM collagen degradation (61). In group X secretory phospholipase A2-deficient double knockout mice treatment with angiotensin II failed to induce MMP-2, -13,-14 and was associated with decreased progression of abdominal aortic aneurysms in this model suggesting a pathogenic role for MMP-14 (62). Elevated MMP-14 has been observed in early phase venous thrombosis. However, its role in thrombus formation or resolution has not been defined (63). In age-related macular degeneration, MMP-14 and basigin mediated increase in MMP-2 activity was associated with ECM dysregulation and sub-retinal pigment epithelium deposits (64).
MMP-14 has been shown to cleave brain-specific angiogenic inhibitor-1 to yield vasculostatin-40 a potent angiogenesis inhibitor (65). Early stage hypertension is associated with increased MMP activities including MMP-14 and enhanced NAPDH oxidase activity suggesting that tissue remodeling occurs from the onset of hypertension and is linked to oxidative stress (66). This study further suggests that vascular remodeling associated with the early phase of hypertension may be mitigated through the administration of MMP inhibitors or antioxidants (66).
In hepatic ischemia reperfusion injury (IRI), fibronectin- α4β1 integrin interaction with macrophages leads to increased MMP-9 and -14 levels worsening the injury (67). Using a peptide, connecting segment-1, CS-1 the authors showed that the levels of both MMPs decreased offering protection to the liver (67). Furthermore, the pharmacological inhibition of p38 MAPK pathway via SB203590 also mitigated the MMP-14 expression suggesting the involvement of this pathway in hepatic IRI (67).
In tumor pathology, angiostatin mediated inhibition of angiogenesis was found to be dependent on MMP-2 and MMP-14 expression (68). This was associated with an increase in hypoxia-induced VEGF which was sufficient in protecting against angiostatin-induced apoptosis (68).
In another study, osteopontin upregulation directly correlated with MMP-14 expression in a rat model of balloon-injured carotid artery suggesting a pathogenic role. One study showed that MMP-14 expression levels increased with respect to osteopontin upregulation in balloon-injured rat carotid arteries suggesting that osteopontin may be involved in the signaling leading to MMP-14 expression (69). The study suggested that osteopontin may be a potential target for avoiding arterial restenosis (69). Sirtuin-1, deficiency in mice was shown to decrease MMP-14 expression promoting a fibrotic phenotype which was associated with vascular dysfunction and diminished angiogenesis (70).
There is limited literature on the role of MMP-15 in vascular diseases. One study reported the upregulation of MMP-15 in chronic inflammatory diseases and malignant tumors (71). It is reported that VEGF-A upregulates ADAMSTS-1 (a Disintegrin And Metalloproteinase with Thrombospondin motifs-1) and increases the development of large ‘mother’ vessels in human cancers (71). In the above study, VEGF-A was found to upregulate MMP-15 and ADAMTS-1 which together contributed to pathological angiogenesis (71).
MMP-16 levels were reported to be upregulated in patients with Henoch–Schönlein purpura (72). Another study focusing on the MMP expression profile in traumatic deep vein thrombosis (TDVT) in rats reported low expression of MMP-16 and -24 in early thrombotic stage but was upregulated during the resolution stage (73). The role of MMP-17 in vascular disease has not been described so far, however, it has been reported to stimulate tumor growth and facilitate a permissive microenvironment for metastsis (74).
MMP-25 expression was shown to be downregulated in the aortic wall of patients suffering from abdominal aortic aneurysm after the administration of doxycycline (75). Furthermore, increased MMP-25 expression was observed in classically activated macrophages, and increased mRNA expression was observed in alternate activation of macrophages (76).
5.5. Matrilysins
MMPs-7 and -26 are matrilysins. MMP-7 has been shown to be involved in adverse left ventricular remodeling post-myocardial infarction and proteomic analysis revealed fibronectin and tenascin-C as its substrate (77). Thyroid cancer-1 gene was suggested to activate MMP-7 and endothelial growth factor downstream of the Wnt/β-catenin signaling pathway to enhance non-small-cell lung cancer in patient (79).
In patients with Kawasaki’s disease, increased expression of integrins α4 and αM on macrophages and myofibroblasts were associated with increased MMP-7 activation and collagen type 1 α1 expression in coronary artery stenosis (80).
In another study involving coronary artery disease patients, the expression of MMP-7, osteopontin, IFN-gamma, and osteopontin were found to be increased suggesting its potential use as biomarkers (81). MMP-7 has been reported to be increased by over 100-fold in patients with atherosclerotic carotid plaques and associated with plaque instability (82). In addition the upregulation of MMP-7 correlated with overexpression of inflammatory genes, RANKL and CD68 (82). In spontaneously hypertensive rats, the increase in MMP-7,-9 and elastase expressions has been demonstrated to cleave ICAM-1 in the glomeruli leading to renal inflammation (83). Using a protease-activated receptor 1 antagonist, F16688, Chieng-Yane et al demonstrated that downregulation of MMP-7 and TNF-alpha levels mitigated post angioplasty restenosis of carotid artery in rats (84). Similarly, using RNA interference against MMP-7 and TACE, Odenbach et al demonstrated mitigation of MMP-2 mediated angiotensin-II-induced cardiac pathology (85). Taken together, the above studies suggest a significant role for MMP-7 in various vascular pathologies. It is known that β2 adrenergic receptor (β2 AR) agonists mediate vasodilatation of blood vessels (87). In rats, when MMP-7 and -9 were injected into the superior mesenteric artery the diameter of arterioles and venules in the mesentery were reduced and these effects have been attributed to MMP-7 and -9 mediated cleavage of β2 AR (87).
A study focusing on endothelium regeneration post-vascular injury reported a decrease loss of pertussis toxin-induced endothelial derived relaxation factor mechanisms as well as an increase in MMP-7 expression (86).
In wound healing, MMP-26 expression is associated with chronic wound healing (88). In an in vitro study using human macrophages, Krishnatry et al observed that acute nitroglycerin exposure decreased MMP-26 expression along with testican-1, integrin α-1, thrombospondin-3, fibronectin-1 suggesting that nitroglycerin modulates ECM by changes in the activities of proteases (89). Furthermore, MMP-26 was found to be associated in patients with cerebral amyloid angiopathy (90).
5.6. Enamelysin
MMP-20 is a protease secreted during teeth development and functions mainly in the deposition of minerals in the dental matrix. There is not reported literature on its vascular effects.
5.7. Metalloelastase
MMP-12 is a metalloelastase originally discovered in alveolar macrophages of cigarette smokers (91). In a mouse model of brachicephalic artery atherosclerosis, MMP-12 was found to be detrimental to plaque stability (230). Using a MMP-12 inhibitor, RXP470.1, Johnson et al demonstrated a significant reduction in plaque formation, necrosis, calcification and macrophage apoptosis. Furthermore, this was associated with increased smooth muscle cell: macrophage ratio leading to a loss of thickness of fibrous plaque and slowing of atherosclerosis (92).
Henoch-Schönlein purpura (HSP) is a IgA mediated immune complex disease characterized by small vessel vasculitis of the skin, gastrointestinal tract, kidneys, joints and rarely the respiratory and central nervous system. Increased MMP activity has been implicated in the pathogenesis of HSP (93). In a recent study, patients with HSP receiving steroids showed negative correlation of MMP-12 suggesting a role of this MMP-12 in HSP (72). Further, a recent study identified MMP-12 as a potential biomarker in patients with Stanford-A Acute Aortic Dissection (94). In a mice model of angiotensin-II induced abdominal aortic aneurysm, 3, 4-Benzopyrene, a compound present in cigarette was found to increase macrophage infiltration, upregulation of MMP-2, -9 and -12 and apoptosis of vascular smooth muscle cells (95). Further, MMP-12 levels were found to be elevated in patients with aortic dissection and coronary artery disease suggesting its use as a potential biomarker and effect of treatment (96).
In patients with abdominal aortic aneurysm (AAA), elevated leptin, a hormone involved in ECM degradation was associated with increased MMP-12 (97). Further, when ApoE−/− mice were treated with local leptin, the expression of MMP-12 and MMP-9 increased by several fold which was associated with medial degeneration of aorta suggesting a link between leptin and AAA (97). MMP-12 ApoE−/− mice increased levels of MMP-12 and exacerbated degeneration of the aortic wall in Ang-II-induced abdominal aortic aneurysm was detected which further demonstrated enhanced degeneration upon endogenous leptin induction (97).
Osteopontin, an atherosclerotic factor, can be cleaved by MMP-12 into an N-terminal and C-terminal fragment where the former is associated with high-risk carotid plaque formation in hypertensive patients (98). In another clinical study carotid atherosclerotic plaques were found to be associated with MMP-12-expressing foam cells and increased MMP-12 is linked to plaque instability (99,100).
Using MMP-12 knockout mice, Li et al demonstrated decreased macrophage infiltration in oxygen-induced retinopathy (101). Furthermore, it reduced adverse neovascularization and promoted non pathological revascularization of the retina (101). Increased MMP-12 has been associated with disruption of blood brain barrier following intracerebral hemorrhage (102). Furthermore, in a neonatal hypoxic-ischemic brain injury, increased MMP-12 expression was observed in neurons suggesting its participation in immature brain injury (103).
Elevated MMP-12 was observed in systemic sclerosis patients and associated with the classical symptoms of scleroderma including peripheral vascular damage (104). Aortic dilation induced by congenital bicuspid aortic valves is associated with increased MMP-12 levels among other MMP and TIMP levels (105). Spleen tyrosine kinase leads to JNK and p38 signaling leading to glomerular injury which enhances pro-inflammatory expression of MMP-12 (106). Certain MMP-12 haplotype are associated with elevated risk of aneurysm in patients suffering from Kawasaki disease (107). Mice susceptible to atherosclerosis showed cigarette smoke-induced COPD vascular effects as well as increased activity of MMP-12 (108). Furthermore, pentraxin-3, a modulator of inflammation, was upregulated upon cigarette smoke as well as an increase in MMP-12/TIMP-1 ratio (109). Expression of MMP-12 was found to be elevated in deep vein thrombosis in rats (73).
5.8. Unclassified MMPs
Some MMPs such as, MMPs-19, -21, -23, -27 and – 28 remain uncategorized. In patients with tricuspid aortic valves, elevated MMP-19 has been associated with thoracic aortic aneurysms (110). Furthermore, a human study has revealed immunoreactivity against MMP-12 in cerebral blood vessels in cerebral amyloid angiopathy (90). A study focusing on the acute and chronic manifestations of myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalitis in mice showed that VEGF levels directly correlated with demyelination of lesions which was associated with increased MMP-19 (111). MMP-19 has been extracted from the synovium of patient suffering from rheumatoid arthritis and was found to be localized to smooth muscle cells in the media of blood vessels (112).
Since its original description in ovarian serous tumors, the role of MMP-21 has now expanded to include invasiveness in breast cancer cells and early melanoma development (113). Apart from tumor progression, MMP-21 activity is associated with embryogenesis (114,115,117). Also, MMP-21 may be a marker for keratinocytes as demonstrated by a study which showed increased MMP-21 upon keratinocyte differentiation in cell culture (116). Increased MMP-21 expression was suggested to play a role in invasiveness of human colorectal cancer and progression in human gastric cancer (118, 119).
MMP-23 is encoded by two genes, MMP23A and MMP23B (120, 121). MMP23A is a pseudogene which is duplicated on chromosomal region 1p36.3, while MMP23B is the protein encoding gene. In a study involving DVT in rats, the expression of MMP-12 was found to be low during thrombus formation but increased during resolution suggesting a wound healing role than pathological (73). MMP-23 expression has been described in reproductive tissues such as, testis, prostate and ovaries however, their exact role remains unknown (11).
MMP-27 was first discovered as epilysin expressed in response to injury, and in testis and keratinocytes (122). The role of MMP-27 in vascular disease has currently not been identified. MMP-27 is expressed in B-cells, bone and kidney tissues of rats (123). In humans, MMP-27 has been implicated in osteogenesis (231). Although mutations in MMP-27 have been identified in thyroid cancers, it has not been suggested to play a role in tumorigenesis (124).
The role of MMP-28 in vascular diseases is yet to be defined. However, in a recent study MMP-28-deficient mice worsened left ventricular remodeling to the point of rupture as a result of inhibiting the type 2 subpopulation of macrophages (125). In a clinical study focusing of MMP/ TIMP profile in children with Henoch-Schönlein purpura, decreased MMP-28 expression was observed in soft tissue edema and the levels of MMP-19 was decreased during convalescent stage compared to its level in the acute stage (72). Treatment with steroid mitigated the changes in MMP and TIMP expression in these patients (72).
6. REGULATION OF MMPS IN VASCULAR DISEASES
MMP expression and their biological function are regulated by a variety of factors that include biological effectors, endogenous inhibitors, epigenetic regulators, miRNAs and pharmacological agents. A summarized chart is presented in Table 2. Below, we discuss how these factors regulate MMPs in normal and pathophysiological conditions.
Table 2.
Regulators of MMPs and examples of their targets
| Category | Type | Class | Name | Target MMPs (Ref.) |
|---|---|---|---|---|
| Biological | Activator | Oxidized derivatives of cholesterol | Oxysterols | MMP-9 (126) |
| Inducer | Transcription factor | E2F1 | MMP-9, -16 (127) | |
| Natural | Inhibitor | TIMPs | TIMP-1 | MMP-8, -9 (135,144) |
| TIMP-2 | MMP-2, -14 (199–201) | |||
| TIMP-3 | MMP-9 (202) | |||
| TIMP-4 | MMP-2, -9 (139–143) | |||
| Epigenetic | Modulator | Methylation | Dystrophin | MMP-2 (150) |
| miRNAs | Gene regulator | - | - | MMP-2, -9, -11, -13 (158,162,164–167,169) |
| Pharmacological | Inhibitor | Anti-cancer drug | Batimastat | MMP-2, -14 (203–205) |
| Marimastat | MMP-1, -2, -3, -7, -9 (172,206) | |||
| Ilomastat | MMP-2, -9 (207, 208) | |||
| Cipemastat | MMP-2, -9 (205) | |||
| Inhibitor | Statins (cholesterol lowering drug) | Cerivastatin | MMP-1, -2, -3, -9 (209–211) | |
| Simvastatin | MMP-1, -3, -9 (209,212–216) | |||
| Lovastatin | MMP-1, -3, -9 (209,216) | |||
| Fluvastatin | MMP-9 (217–219) | |||
| Inhibitor | Hydroxamates | R-phosphonate | MMP-8 (220) | |
| Sulfone hydroxamates | MMP-1, -2, -9, 13 (221) | |||
| Inhibitor | Sulfa drugs | Sulphonamides | MMP-8, -12, -13 (222) | |
| Unclassified | Inhibitor | Recombinant | C-TIMP-2 | MMP-2 (182) |
| Inhibitor | Pyrimidine | 5-hydroxy, 5-substitute- pyrimidine-2,4,6,-triones | MMP-2, -9 (183) | |
| Inhibitor | Methyl rosmarinate derivatives | (R)-10n | MMP-1 (223) | |
| Inhibitor | Divalent cation chelators | Alendronate and EDTA | MMP-1, -3, -9, -13 (187,224) | |
| Quinolinone-derivative | Cilostazol | MMP-1, -9 (188,225–227) | ||
| Inhibitor | Anti-hypertension drug | Captopril | MMP-2, -9 (189,190) | |
| Candesartan | MMP-9 (191) | |||
| Inhibitor | Herbal | Hydroxysafflor yellow A | MMP-2, -9 (193) | |
| Inhibitor | Flavonoid | Kaempferol | MMP-2 (194) | |
| Inhibitor | Gasotransmitter | Hydrogen sulfide | MMP-2, -9 (195–197) | |
| Inhibitor | Phosphinic peptide | RXP470.1. | MMP-1, -2,-3,-8,-9, -10,-12, -13, -14 (228) | |
| RXP470, Compound 4 | MMP-1, -2, -3,-7, -8, -9, -10, -12, -13, -14 (229) |
Abbreviations: R-phosphonate, (1-(4′-methoxybiphenyl-4-sulfonylamino)-2-methylpropyl) phosphonate; EDTA, Ethylenediaminetetraacetic acid.
6.1. Biological activators of MMPs
Oxysterols, a promotor of oxidative stress through Nox2 activation, has been shown to enhance the effects of MMP-9 (126). On the other hand, E2F1 is a transcription factor that modulates cell cycle regulation, proliferation and apoptosis, and has shown to enhance MMP expression (127).
6.2. Natural inhibitors of MMPs: tissue inhibitor of metalloproteinases (TIMPs)
A number of inhibitors for MMPs have been described over the years. The most common include endogenous inhibitors known as TIMPs. This family consists of four protease inhibitors TIMP 1–4 which exist as glycosylated or unglycosylated form. TIMPs are 21 to 29 kDa protein consisting of an N-terminal oligosaccharide/oligonucleotide binding (OB)-fold of ~125 amino acids, 5 beta-barrels, and a C-terminal portion ~60 amino acids long forming a beta-turn (128–130). The N-terminal domain interacts strongly with the catalytic domains of MMPs (129). In some cases, however, the C-terminal domain can further enhance the MMP/TIMP interaction (131). Furthermore, TIMPs can also interact to form complexes with pro-gelatinases suggesting that the C-terminal domain interacts with the hemopexin-like domain (131).
TIMP-2: proMMP-2 complex has an important role in both activation and inhibition of MMP-2 (132). A ternary model for TIMP-2: proMMP-2 interacting with MT1-MMP has been proposed (2). Thus, studying MMP/ TIMP ratios enable to define the collective role of TIMPs in vascular disease. A time course study performed in rats demonstrated that besides the expression of MMP-9 protein and mRNA the MMP-9: TIMP-1 ratio correlated with brain water content (BWC) following acute cerebral infarction (133). TIMP-1 concentration alone did not correlate BWC in this cerebral edema model suggesting that there was no disenable relationship between TIMP-1 concentration and edema (133).
An epidemiology study concluded that certain polymorphism within the promoter regions of MMP-9 and TIMP-2 is statistically associated with varicose vein development within a subset of the Chinese population (134). In another study focusing on obesity increased plasma concentrations of MMP-9 and MMP-9: TIMP-1 ratio and MMP-8 and MMP-8: TIMP-1 ratio was observed in obese women versus lean women suggesting their association with obesity (135). In addition, MMP-9 levels and MMP-9:TIMP-1 ratio was associated with healing in diabetic foot ulcers (136). However, there was only a slight variation in TIMP-1, TIMP-2, and MMP-2 when comparing good vs. poor healing outcomes (136).
TIMP-1 has been used in gene therapy by viral and plasmid delivery systems. Hybridization of TIMP-1 to urokinase receptor decreased neointimal formation in vein cultures (137). Furthermore, in an in vivo model of venous graft interposition to carotid artery, TIMP-1 urokinase protein reduced graft thickening suggesting its potential as a therapeutic agent (138). TIMP-4 was shown to mitigate MMP-2 expression in an ischemia-reperfusion injury rat model of myocardial infarction (139). In addition, monocytes isolated from abdominal aortic aneurysm (AAA) were shown to have a greater capacity for transmigration and EC adhesion along with increased MMP-9 and decreased TIMP- 4 levels (140). Furthermore, intermittent hypobaric hypoxia has been shown to ameliorate the effects of ischemia-reperfusion by decreasing MMP-2 activation, and increasing TIMP-4 (141).
A study focusing on ataxia telangiectasia, mutated heterozygous kinase knockout (ATM hKO) mice showed decreased levels of TIMP-4 within the infarct tissue which was associated with increased MMP-9 expression and fibrosis (142). In another study, fibrinogen induced caveolae formation as well as caveolin protein 1 expression, and its phosphorylation was mitigated in presence of the TIMP-4 and MMP-9 modulation (143).
Sirtuin1 (SIRT1) is a protein/histone deacetylase known for its protective effects against development of pulmonary emphysema in smokers (144). In a study by Yao et al, SIRT1 deficiency was associated with decreased TIMP-1 and increased MMP-9 activity in human subjects suffering from COPD and in the lungs of mice exposed to cigarette smoke, whereas increased SIRT1 in transgenic mice was associated with decreased MMP-9 activity (144). The above study did not demonstrate changes in the levels of MMP-2, -12 and TIMP-2, -3 or -4 were observed (144). Additionally, in an in vitro rat cardiac fibroblast model, El Hajj et al demonstrated that ethanol exposure up-regulated TIMP-1, -3, and -4 levels with no significant changes in MMP profile (145). They concluded that MMP/TIMP imbalance favors collagen accumulation in the development of myocardial fibrosis and cardiac dysfunction in chronic alcohol abuse (145).
6.3. Epigenetic regulation of MMPs
Epigenetics is defined as reversible events and consists of gene silencing mechanisms which stabilize gene expression without modifying the DNA sequence. In mammalian cells, the major epigenetic mechanism includes, DNA methylation, histone modification, and small non-coding RNA (146). DNA methylation is carried out by three DNA methyltransferases (DMNT): DNMT 1, DNMT3, and DNMT 3b. Although epigenetic regulation of MMPs have not been extensively studied in vascular diseases much of what is known has been uncovered through cancer research. Several cancers have overlapping vascular pathology which we discuss in the following paragraphs. In a recent study, methylation of cytosine within the promoter region of dystrophin was examined by using Duchenne muscular dystrophy (DMD) knockout mice, and this deficiency was shown to increase MMP-2 expression levels (150). In breast cancer cells MD-MB-231, high glucose led to the phosphorylation of histone 3 at the Ser10 residue while dephosphorylating the Ser9 residue (151). This event correlated with the increase in DNMT1 expression (151). Silencing GSK-3β by siRNA inhibited the phosphorylation of H3, and thus decreasing DNMT1 expression (151). Furthermore, phospho-H3 levels were directly correlated with MMP-7 expression (151).
MMP-14 was suggested to be a regulator of H3K9ac in adipogenic collagenolysis (152). Another study suggested that proMMP-1 expression is influenced by epigenetics in human fibrosarcoma cells even though its promoter is not believed to possess CpG islands, yet proMMP-1 expression increased under 5-aza-dC treatment which was mitigated with cycloheximide suggesting epigenetic regulation through some other intermediate product (153). Enzymes involved in the pathology, mainly MMP-9 and superoxide dismutase 2, of diabetic retinopathy have been reported to be under the epigenetic regulation of histone lysine demethylase 1, (LSD1) and DNMT (154). Our lab has recently demonstrated that changes in the levels of MMP-9, TIMP-1 are regulated by DNMT1, 3a, Methyl CpG-Binding Domain Protein 2 (MBD2), and H3K9 contributing to aortic remodeling during hyperhomocysteinemia (156).
The expression of MMP9 is inhibited by trichostatin A (TSA), a HDAC inhibitor, by its action on thrombospondin-1 (TSP-1), an adhesive glycoprotein (147,148). TSP-1 stabilizes the ECM and also suppresses VEGF release thus regulating angiogenesis (148).
Proto-oncogene c-Fos was also shown to be linked to MMP-2/MMP-9 expression (160). Treatment of cells with OxLDL caused c-Fos activation to decreased histone deacetylase 1 (HDAC1) expression levels (160). Also, shRNA-mediated knockdown of c-Fos restored HDAC1 mRNA and protein expression (160). The increased acetylation and decreased methylation of H3K9 had disappeared in the knockdown of c-Fos treated with OxLDL suggesting that c-Fos was necessary for histone modification and lead to MMP-2/MMP-9 overexpression further downstream of the signaling pathway (160). Furthermore, since c-Fos can activate transcription factor AP-1, a link between OxLDL, AP-1, miRNA-29b, and cardiovascular disease had been suggested (160). MMP-9 has also been shown to be regulated epigenetically (161).
6.4. miRNA regulation of MMPs
MicroRNAs (miRs) are small non-coding RNAs which regulate transcriptional and post-transcriptional gene expression (157). MicroRNA-29b (miRNA-29b) has been shown to be an epigenetic regulator of pro-atherogenic genes (158). OxLDL was shown to up-regulate miRNA-29b expression in a dose-dependent and time-dependent manner within human aortic smooth muscle cells (HASMCs) (158). In vivo studies suggest that increased miRNA-29b levels reduced the expression of DNA methyltransferase 3b in primary human aortic smooth muscle cells, and subsequently lead to reduced MMP-2/MMP-9 silencing (158). These results suggested a mechanism for OxLDL to induce HASMC migration via miRNA-29b/DNMT 3b/MMP-2 and MMP-9 pathway (158). These findings reveal that miRNA-29b may generate atherosclerosis (158). Furthermore, increased miRNA-29b levels were shown to dysregulate ECM homeostasis by impacting the expression of collagen (Col1A1 & Col3A1), and elastin (159).
Among miRs, miR133a, miR133b, and miR145 target SP-1 transcription factor which in turn decreases MMP-9 expression in renal cancer (162). In oral squamous cell carcinoma, miR2 targeted STAT3 which in turn decreased MMP-2 in WP1066 treated cells (163). Propofol administration was shown to induce miR-143 which targeted MMP-13 in an osteosarcoma cell line (164). This was shown to inhibit the cell proliferation and invasion which facilitated apoptosis (164). Moreover, miR-143 levels indirectly correlated with MMP-13 levels, which were restored with the application of anti-miR-143 (164). Under hypoxic conditions, neovascularization and therefore MMP-9 and VEGF were suggested to be under the control of miR-126 in monkey chorioretinal vessel ECs (RF/6A) (165). The miR21 expression was suggested to be induced by Ang II which induced MMP-2 expression in mouse cardiofibroblasts (166). TNF- α signaling leading to IL-6, iNOS, and MMP-9 expression and endothelial dysfunction was suggested to occur when miR-149 was down-regulated in a p38MAPK-dependent manner (167). MMP-14 was suggested to be downregulated by miR-9 expression (168). Finally, miR-98 is reported to inhibit tumor angiogenesis and invasion in part by targeting MMP-11 (169).
6.5. Pharmacological inhibitors of MMPs
Pharmacological inhibitors of MMP fit into two classes: non-specific and specific. The non-specific inhibitors act by chelating the catalytic Zn2+ ion (170,171). These inhibitors include but are not limited to batimastat, marimastat, ilomastat, PD-166793, and ONO-4817 (172,173). Peptide inhibitors containing motifs HWGF, CRRHWGFEFC and CTTHWGFTLC provide targeted inhibition of MMP-2 and MMP-9 (174). Antibiotics have also demonstrated the ability to inhibit MMPs, such as the tetracycline class (175). Furthermore, chemically modified tetracyclines maintain MMP inhibition in the absence of antimicrobial activity (33).
Statins are a class of drugs used to lower cholesterol levels and have been shown to inhibit MMP-2, MMP-7, and MMP-9 (11). Although cerivastatin was withdrawn from the market due to cases of muscle breakdown, it has exhibited dose-dependent inhibition properties for MMP-1, MMP-3, and MMP-9 secretion in human VSMC (176). Lovastatin inhibited the inducible activity of MMP-1, -3 and -9, while it blocked the MMP-2 constituent activity in rabbit SMC whereas, TIMP-1 and -2 secretions remained uninhibited (176). Both cerivastatin and lovastatin inhibited the secretion of MMP-1, -3, and -9 in rabbit foam cells with cerivastatin demonstrating greater inhibition (176). In a study, aortic mRNA expression demonstrated elevated levels of MMP-1, MMP-9, and MMP-12 in rabbits that were fed a high cholesterol diet which was decreased but not normalized upon fluvastatin treatment (177). Although MMP-2 level remained the same for normal diet, and cholesterol diet with or without fluvastatin, MMP-12 showed a pronounced decrease with fluvastatin treatment (177).
Interestingly, a recent study focused on a series of MMP inhibitors known as α-sulfone hydroxamates which were conjugated to various dyes for visualizing cells that express MMPs for as a way to measure their synthesis and potency in disease conditions (178). In particular, N-O-Isopropyl sulfonamido-based hydroxamates have shown to be selective inhibitors of MMP-12 and -13 (179,180).
Sulphonamides are another class of inhibitors which have been shown to inhibit MMP activity in vitro (181). However, their inhibitory effect in vivo remains known as it may be dependent on intracellular signaling and crosstalk between different compounds (181).
In order for proMMP-2 to become activated, the hemopexin domain has to interact with the C-terminal domain of TIMP-2 (182). Using recombinant technology, the activation of proMMP-2 was successfully blocked by TIMP-2 (C-TIMP-2) (182). The C-TIMP-2 was shown to interact with the inactive MMP-2E404A and hemopexin domain of MMP-2 in a dose-dependent manner (182). Furthermore, C-TIMP-2 was shown to compete with TIMP-2 in its interaction with proMMP-2 (182).5-hydroxy,5-substitute-pyrimidine-2,4,6,-triones demonstrate high potency in their IC50 values for gelatinases (183). Also, 5-hydroxy moiety may enhance the pharmacokinetics of this inhibitor (183).
A new class of inhibitors has been identified with the ability to selectively inhibit MMP-13 (184). This class of pharmacological inhibitors is unique because they are non-zinc chelating (184). The structure-activity relationships (SAR) studies on this lead compound revealed that reducing the lipophilic property conferred metabolic stability, and lowered clearance rate in vivo (185).
Methyl rosmarinate derivatives have also been identified as a potential class of inhibitors for MMP-1 (186). A molecular scaffold has been identified and tested with SAR studies identifying potential lead compounds with IC50 on micro molar scale (186). Alendronate and EDTA, divalent chelators, have been shown to inhibit MMP-9 activity irreversibly via plasmin-mediated inactivation (187). Cryptic plasmin degradation sites within the catalytic domain of MMP-9 become accessible to form hemopexin-domain fragments which have the inhibitory property (187).
Cilostazol inhibits MMP-9 in a dose-dependent manner and on the transcriptional level by suppressing its promoter and blocking the translocation of NF-κB (188). Treatment with Cilostazol, reduced MMP-9 activity on and decreased transcript and protein expression (188). The study above presents a mechanism for anti-atherosclerosis by inhibiting monocyte invasion and further differentiation by modulating MMP-9 and TIMP-1 (188).
Anti-hypertensive drug captopril has been shown to inhibit MMP-2 and -9 in a rat model of right ventricular hypertrophy (189), and to decrease plasma MMP-9 in acute myocardial infarction patients (190). Similarly, serum MMP-9 levels were found to decrease in patients with candesartan treatment (191). However, a recent study focusing on the inhibitory action of captopril and lisinopril using purified MMP-2 concluded that these two ACE inhibitors do not act as pharmacological inhibitors of MMP-2 (192).
Hydroxysafflor yellow A, an active ingredients of Chinese herb Carthamus tinctorius L, was also shown to inhibit MMP-2 and MMP-9 activity, and also reduces associated apoptosis which helps to mitigate the effects of left ventricular remodeling within the hypertensive heart (193). In addition, kaempferol has shown to act as a pharmacological inhibitor of MMP-2 through the inhibition of the ERK1/2 as well as activator protein-1 pathway making it a good candidate for cardiac disease and cancer (194). Recently, we have shown that hydrogen sulfide treatment confers vascular protection within the kidney and neuronal tissues of hyperhomocysteinemic mice as observed through the decrease of MMP-2 and MMP-9 levels (195–197). Furthermore, phosphinic peptide RXP470.1. is a selective inhibitor of MMP-12 which was shown to mitigate plaque instability in ApoE KO mice (92).
7. SUMMARY AND CONCLUSION
MMPs in general have a fundamental role in matrix homeostasis of vascular health. In disease conditions, MMP activity is altered causing a dysregulation in ECM synthesis and degradation. As a result, vessels become weaker in performing their normal physiological function, such as maintaining blood pressure. In order to keep up with the increased metabolic demand of tumor cells, angiogenesis is facilitated by MMPs in cancer. Similarly, cardiovascular diseases including atherosclerosis, inflammation and ischemia are influenced by MMPs activity. The endogenous inhibitors, TIMPs regulate the expression of MMPs however, they lack specificity. Our current knowledge on interventions to modify MMP activity by pharmacological agents, genetic manipulation and/or by biological agents for prevention or even cure of vascular diseases are limited. Therefore, more research is needed to develop strategies to regulate specific MMPs in a particular disease without affecting other MMPs that are vital for maintaining normal physiological functions.
Acknowledgments
This work was supported, in part, by National Institutes of Health grants HL-104103 (to Utpal Sen) and HL-108621 (to Suresh C. Tyagi).
Abbreviations
- AAA
Abdominal aortic aneurysm
- AT1
Angiotensin II receptor type1
- BBB
Blood brain barrier
- BWC
Brain water content
- COPD
Chronic obstructive pulmonary disease
- DNMT1
DNA methyltransferase 1
- DVT
Deep-vein thrombosis
- E2F1
is a transcription factor
- ECAS
Extracranial atherosclerosis
- ECM
Extracellular matrix
- ECs
Endothelial cells
- FRET
Fluorescence resonance energy transfer
- FSS
Fluid shear stress
- H3K9
Histone 3 tri-methylated at lysine
- 9H3K9ac
H3 lysine 9 acetylation
- HDAC1
Histone deacetylase 1
- HDL
High-density lipoprotein
- HSP
Henoch-Schönlein purpura
- ICAS
Intracranial atherosclerosis
- ILA
intracranial large artery atherosclerosis
- L-NAME
L-NG-Nitroarginine Methyl Ester
- LSD1
Lysine-specific demethylase 1
- MBD2
Methyl binding domain 2
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- Nox2
NADPH oxidase 2
- OxLDL
Oxidized low-density lipoprotein
- PLA2
Phospholipase A2
- RANKL
Receptor activator of nuclear factor kappa-B ligand
- siRNA
silencing of GSK-3β inhibited the phosphorylation of H3SIRT1: Deacetylase Sirtuin-1
- TACE
Tumor necrosis factor-a-converting enzyme (also known as ADAM17)
- TFPI2
Tissue factor pathway inhibitor 2
- TIMPs
Tissue inhibitor of metalloproteinases
- TSA
Trichostatin A
- TSP-1
Thrombospondin-1
- VEGF
Vascular endothelial growth factor
- VEGRF2
Vascular endothelial growth factor receptor-2
- VSMC(s)
Vascular smooth muscle cell(s)
References
- 1.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962;48:1014–22. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maskos K. Crystal structures of MMPs in complex with physiological and pharmacological inhibitors. Biochimie. 2005;87(3–4):249–263. doi: 10.1016/j.biochi.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Razzaque MS, Kumari S, Foster CS, Ahmed AR. Expression profiles of collagens, HSP47, TGF-beta1, MMPs and TIMPs in epidermolysis bullosa acquisita. Cytokine. 2003;21(5):207–13. doi: 10.1016/S1043-4666(03)00034-6. [DOI] [PubMed] [Google Scholar]
- 4.Demedts IK, Morel-Montero A, Lebecque S, Pacheco Y, Cataldo D, Joos GF, Pauwels RA, Brusselle GG. Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax. 2006;61(3):196–201. doi: 10.1136/thx.2005.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovejoy B, Cleasby A, Hassell AM, Longley K, Luther MA, Weigl D, McGeehan G, McElroy AB, Drewry D, Lambert MH, et al. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science. 1994;263(5145):375–7. doi: 10.1126/science.8278810. [DOI] [PubMed] [Google Scholar]
- 6.Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 1998;12(12):1075–95. [PubMed] [Google Scholar]
- 7.Overall CM. Molecular determinants of metalloproteinase substrate specificity: matrix metalloproteinase substrate binding domains, modules, and exosites. Mol Biotechnol. 2002;22(1):51–86. doi: 10.1385/MB:22:1:051. [DOI] [PubMed] [Google Scholar]
- 8.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–59. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bujan J, Jurado F, Gimeno MJ, Garcia-Honduvilla N, Pascual G, Jimenez J, Bellon JM. Changes in metalloproteinase (MMP- 1, MMP-2) expression in the proximal region of the varicose saphenous vein wall in young subjects. Phlebology. 2000;15(2):64–70. doi: 10.1007/s005230070024. [DOI] [Google Scholar]
- 10.Newby AC. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006;69(3):614–24. doi: 10.1016/j.cardiores.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Back M, Ketelhuth DF, Agewall S. Matrix metalloproteinases in atherothrombosis. Prog Cardiovasc Dis. 2010;52(5):410–28. doi: 10.1016/j.pcad.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Alsaigh T, Pocock ES, Bergan JJ, Schmid-Schonbein GW. Acute venous occlusion enhances matrix metalloprotease activity: Implications on endothelial dysfunction. Microvasc Res. 2011;81(1):108–16. doi: 10.1016/j.mvr.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLano FA, Schmid-Schonbein GW. Proteinase activity and receptor cleavage: mechanism for insulin resistance in the spontaneouslyhypertensiverat. Hypertension. 2008;52(2):415–23. doi: 10.1161/HYPERTENSIONAHA.107.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clancy P, Seto SW, Koblar SA, Golledge J. Role of the angiotensin converting enzyme 1/angiotensin II/angiotensin receptor 1 axis in interstitial collagenase expression in human carotid atheroma. Atherosclerosis. 2013;229(2):331–7. doi: 10.1016/j.atherosclerosis.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Lavin B, Gomez M, Pello OM, Castejon B, Piedras MJ, Saura M, Zaragoza C. Nitric Oxide Prevents Aortic Neointimal Hyperplasia by Controlling Macrophage Polarization. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.114.303866. [DOI] [PubMed] [Google Scholar]
- 16.Austin KM, Covic L, Kuliopulos A. Matrix metalloproteases and PAR1 activation. Blood. 2013;121(3):431–9. doi: 10.1182/blood-2012-09-355958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed AK, Haylor JL, El Nahas AM, Johnson TS. Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int. 2007;71(8):755–763. doi: 10.1038/sj.ki.5002108. [DOI] [PubMed] [Google Scholar]
- 18.Lenz O, Elliot SJ, Stetler-Stevenson WG. Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol. 2000;11(3):574–81. doi: 10.1681/ASN.V113574. [DOI] [PubMed] [Google Scholar]
- 19.Sen U, Sathnur PB, Kundu S, Givvimani S, Coley DM, Mishra PK, Qipshidze N, Tyagi N, Metreveli N, Tyagi SC. Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. Am J Physiol Cell Physiol. 2012;303(1):C41–51. doi: 10.1152/ajpcell.00398.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quillard T, Tesmenitsky Y, Croce K, Travers R, Shvartz E, Koskinas KC, Sukhova GK, Aikawa E, Aikawa M, Libby P. Selective inhibition of matrix metalloproteinase-13 increases collagen content of established mouse atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(11):2464–72. doi: 10.1161/ATVBAHA.111.231563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant GM, Giambernardi TA, Grant AM, Klebe RJ. Overview of expression of matrix metalloproteinases (MMP-17, MMP-18, and MMP-20) in cultured human cells. Matrix Biol. 1999;18(2):145–148. doi: 10.1016/S0945-053X(99)00003-7. [DOI] [PubMed] [Google Scholar]
- 22.Foos MJ, Hickox JR, Mansour PG, Slauterbeck JR, Hardy DM. Expression of matrix metalloprotease and tissue inhibitor of metalloprotease genes in human anterior cruciate ligament. J Ortho Res. 2001;19(4):642–649. doi: 10.1016/S0736-0266(00)00071-1. [DOI] [PubMed] [Google Scholar]
- 23.Kaptoge S, Seshasai SRK, Gao P, Freitag DF, Butterworth AS, Borglykke A, Di Angelantonio E, Gudnason V, Rumley A, Lowe GDO, Jorgensen T, Danesh J. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35(9):578-U35. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenglet S, Quercioli A, Fabre M, Galan K, Pelli G, Nencioni A, Bauer I, Pende A, Python M, Bertolotto M, Spinella G, Pane B, Palombo D, Dallegri F, Mach F, Vuilleumier N, Montecucco F. Statin Treatment Is Associated with Reduction in Serum Levels of Receptor Activator of NF-kappa B Ligand and Neutrophil Activation in Patients with Severe Carotid Stenosis. Mediators Inflamm. 2014 doi: 10.1155/2014/720987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scoditti E, Nestola A, Massaro M, Calabriso N, Storelli C, De Caterina R, Carluccio MA. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKC alpha and PKC beta 1 inhibition. Atherosclerosis. 2014;232(1):17–24. doi: 10.1016/j.atherosclerosis.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Tan CH, Liu Y, Li WN, Deng F, Liu X, Wang X, Gui YJ, Qin L, Hu CL, Chen LF. Associations of matrix metalloproteinase-9 and monocyte chemoattractant protein-1 concentrations with carotid atherosclerosis, based on measurements of plaque and intima-media thickness. Atherosclerosis. 2014;232(1):199–203. doi: 10.1016/j.atherosclerosis.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 27.Palmieri D, Perego P, Palombo D. Estrogen Receptor Activation Protects Against TNF-alpha-Induced Endothelial Dysfunction. Angiology. 2014;65(1):17–21. doi: 10.1177/0003319713477909. [DOI] [PubMed] [Google Scholar]
- 28.Sun L, Chandra S, Sucosky P. Ex vivo evidence for the contribution of hemodynamic shear stress abnormalities to the early pathogenesis of calcific bicuspid aortic valve disease. PLoS One. 2012;7(10):e48843. doi: 10.1371/journal.pone.0048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould ST, Srigunapalan S, Simmons CA, Anseth KS. Hemodynamic and cellular response feedback in calcific aortic valve disease. Circ Res. 2013;113(2):186–97. doi: 10.1161/CIRCRESAHA.112.300154. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, Rajamannan NM, Sucosky P. Defining the Role of Fluid Shear Stress in the Expression of Early Signaling Markers for Calcific Aortic Valve Disease. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0084433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khzam LB, Boulahya R, Abou-Saleh H, Hachem A, Zaid Y, Merhi Y. Soluble CD40 Ligand Stimulates the Pro-Angiogenic Function of Peripheral Blood Angiogenic Outgrowth Cells via Increased Release of Matrix Metalloproteinase-9. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0084289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H, Bae S, Kim Y, Cho CH, Kim SJ, Kim YJ, Lee SP, Kim HR, Hwang YI, Kang JS, Lee WJ. Vitamin C prevents stress-induced damage on the heart caused by the death of cardiomyocytes, through down-regulation of the excessive production of catecholamine, TNF-alpha, and ROS production in Gulo (−/−)(Vit C-Insufficient) mice. Free Radic Biol Med. 2013;65:573–583. doi: 10.1016/j.freeradbiomed.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Mishra PK, Givvimani S, Chavali V, Tyagi SC. Cardiac matrix: A clue for future therapy. Biochim Biophys Acta. 2013;1832(12):2271–2276. doi: 10.1016/j.bbadis.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacob-Ferreira AL, Schulz R. Activation of intracellular matrix metalloproteinase-2 by reactive oxygen nitrogen species: Consequences and therapeutic strategies in the heart. Arch Biochem Biophys. 2013;540(1–2):82–93. doi: 10.1016/j.abb.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg. 2007;45(2):373–80. doi: 10.1016/j.jvs.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeon SB, Chun S, Choi-Kwon S, Chi HS, Nah HW, Kwon SU, Kim WK, Kim JS. Biomarkers and location of atherosclerosis: Matrix metalloproteinase-2 may be related to intracranial atherosclerosis. Atherosclerosis. 2012;223(2):442–447. doi: 10.1016/j.atherosclerosis.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu K, Shimomura K, Tokuyama Y, Sakurai K, Isahaya K, Takaishi S, Kato B, Usuki N, Shimizu T, Yamada K, Hasegawa Y. Association between Inflammatory Biomarkers and Progression of Intracranial Large Artery Stenosis after Ischemic Stroke. J Stroke Cerebrovasc Dis. 2013;22(3):211–217. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Heo SH, Cho CH, Kim HO, Jo YH, Yoon KS, Lee JH, Park JC, Park KC, Ahn TB, Chung KC, Yoon SS, Chang DI. Plaque Rupture is a Determinant of Vascular Events in Carotid Artery Atherosclerotic Disease: Involvement of Matrix Metalloproteinases 2 and 9. J Clin Neurol. 2011;7(2):69–76. doi: 10.3988/jcn.2011.7.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Givvimani S, Kundu S, Narayanan N, Armaghan F, Qipshidze N, Pushpakumar S, Vacek TP, Tyagi SC. TIMP-2 mutant decreases MMP-2 activity and augments pressure overload induced LV dysfunction and heart failure. Arch Physiol Biochem. 2013;119(2):65–74. doi: 10.3109/13813455.2012.755548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shivshankar P, Brampton C, Miyasato S, Kasper M, Thannickal VJ, Le Saux CJ. Caveolin-1 Deficiency Protects from Pulmonary Fibrosis by Modulating Epithelial Cell Senescence in Mice. Am J Respir Cell Mol Biol. 2012;47(1):28–36. doi: 10.1165/rcmb.2011-0349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu Y, Zheng GQ, Xu MJ, Li Y, Chen XM, Zhu WZ, Tong Y, Chung SK, Liu KJ, Shen JG. Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J Neurochem. 2012;120(1):147–156. doi: 10.1111/j.1471-4159.2011.07542.x. [DOI] [PubMed] [Google Scholar]
- 42.Muradashvili N, Tyagi R, Metreveli N, Tyagi SC, Lominadze D. Ablation of MMP9 gene ameliorates paracellular permeability and fibrinogen-amyloid beta complex formation during hyperhomocysteinemia. J Cereb Blood Flow Metab. 2014;34(9):1472–82. doi: 10.1038/jcbfm.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muradashvili N, Benton RL, Saatman KE, Tyagi SC, Lominadze D. Ablation of matrix metalloproteinase-9 gene decreases cerebrovascular permeability and fibrinogen deposition post traumatic brain injury in mice. Metab Brain Dis. 2014 doi: 10.1007/s11011-014-9550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giuffrida P, Biancheri P, MacDonald TT. Proteases and small intestinal barrier function in health and disease. Curr Opin Gastroenterol. 2014;30(2):147–53. doi: 10.1097/MOG.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 45.SanSilvestri-Morel P, Fioretti F, Rupin A, Senni K, Fabiani JN, Godeau G, Verbeuren TJ. Comparison of extracellular matrix in skin and saphenous veins from patients with varicose veins: does the skin reflect venous matrix changes? Clinical Science. 2007;112(3–4):229–239. doi: 10.1042/CS20060170. [DOI] [PubMed] [Google Scholar]
- 46.Zhang G, Miyake M, Lawton A, Goodison S, Rosser CJ. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer. 2014;14(1):310. doi: 10.1186/1471-2407-14-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boucher BJ. Matrix metalloproteinase-10 and microvascular complications of type 1 diabetes: might vitamin D status be relevant? Diabetologia. 2014;57(5):1081–1081. doi: 10.1007/s00125-014-3189-9. [DOI] [PubMed] [Google Scholar]
- 48.Bobadilla M, Sainz N, Rodriguez JA, Abizanda G, Orbe J, de Martino A, Verdugo JMG, Paramo JA, Prosper F, Perez-Ruiz A. MMP-10 Is Required for Efficient Muscle Regeneration in Mouse Models of Injury and Muscular Dystrophy. Stem Cells. 2014;32(2):447–461. doi: 10.1002/stem.1553. [DOI] [PubMed] [Google Scholar]
- 49.Tan J, Buache E, Alpy F, Daguenet E, Tomasetto CL, Ren GS, Rio MC. Stromal matrix metalloproteinase-11 is involved in the mammary gland postnatal development. Oncogene. 2013;33(31):4050–59. doi: 10.1038/onc.2013.434. [DOI] [PubMed] [Google Scholar]
- 50.Motrescu ER, Blaise S, Etique N, Messaddeq N, Chenard MP, Stoll I, Tomasetto C, Rio MC. Matrix metalloproteinase-11/stromelysin-3 exhibits collagenolytic function against collagen VI under normal and malignant conditions. Oncogene. 2008;27(49):6347–55. doi: 10.1038/onc.2008.218. [DOI] [PubMed] [Google Scholar]
- 51.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A Matrix Metalloproteinase Expressed on the Surface of Invasive Tumor-Cells. Nature. 1994;370(6484):61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 52.Anik I, Kokturk S, Genc H, Cabuk B, Koc K, Yavuz S, Ceylan S, Ceylan S, Kamaci L, Anik Y. Immunohistochemical analysis of TIMP-2 and collagen types I and IV in experimental spinal cord ischemia-reperfusion injury in rats. J Spin Cord Med. 2011;34(3):257–264. doi: 10.1179/107902611X12972448729648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse Roles of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Neuroinflammation and Cerebral Ischemia. Neuroscience. 2009;158(3):983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson JA, Richardson BS, Gagnon R, Regnault TRH. Chronic intrauterine hypoxia interferes with aortic development in the late gestation ovine fetus. J Physio-London. 2011;589(13):3319–3332. doi: 10.1113/jphysiol.2011.210625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang R, Liu H, Williams I, Chaqour B. Matrix metalloproteinase-2 expression and apoptogenic activity in retinal pericytes: implications in diabetic retinopathy. Ann N Y Acad Sci. 2007;1103:196–201. doi: 10.1196/annals.1394.000. [DOI] [PubMed] [Google Scholar]
- 56.Park JH, Park SM, Park SH, Cho KH, Lee ST. Cleavage and functional loss of human apolipoprotein E by digestion of matrix metalloproteinase-14. Proteomics. 2008;8(14):2926–2935. doi: 10.1002/pmic.200700487. [DOI] [PubMed] [Google Scholar]
- 57.Park JH, Park SM, Park KH, Cho KH, Lee ST. Analysis of apolipoprotein A-I as a substrate for matrix metalloproteinase-14. Biochem Biophys Res Comm. 2011;409(1):58–63. doi: 10.1016/j.bbrc.2011.04.105. [DOI] [PubMed] [Google Scholar]
- 58.Waldow T, Witt W, Buzin A, Ulmer A, Matschke K. Prevention of ischemia/ reperfusion-induced accumulation of matrix metalloproteinases in rat lung by preconditioning with nitric oxide. J Surg Res. 2009;152(2):198–208. doi: 10.1016/j.jss.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 59.Lin J, Davis HB, Dai QX, Chou YM, Craig T, Hinojosa-Laborde C, Lindsey ML. Effects of early and late chronic pressure overload on extracellular matrix remodeling. Hypertens Res. 2008;31(6):1225–1231. doi: 10.1291/hypres.31.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Straat K, de Klark R, Gredmark-Russ S, Eriksson P, Soderberg-Naucler C. Infection with Human Cytomegalovirus Alters the MMP-9/TIMP-1 Balance in Human Macrophages. J Virol. 2009;83(2):830–835. doi: 10.1128/JVI.01363-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng M, Cai XJ, Zhang W, Liu XL, Chen L, Zhang Y, Zhang MX, Zhang M. Interleukin-6 enhances matrix metalloproteinase-14 expression via the RAF-mitogen-activated protein kinase kinase-extracellular signal-regulated kinase 1/2-activator protein-1 pathway. Clin Exp Pharmacol Physiol. 2010;37(2):162–166. doi: 10.1111/j.1440-1681.2009.05246.x. [DOI] [PubMed] [Google Scholar]
- 62.Zack M, Boyanovsky BB, Shridas P, Bailey W, Forrest K, Howatt DA, Gelb MH, de Beer FC, Daugherty A, Webb NR. Group X secretory phospholipase A(2) augments angiotensin II-induced inflammatory responses and abdominal aortic aneurysm formation in apoE-deficient mice. Atherosclerosis. 2011;214(1):58–64. doi: 10.1016/j.atherosclerosis.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sood V, Luke CE, Deatrick KB, Baldwin J, Miller EM, Elfline M, Upchurch GR, Wakefield TW, Henke PK. Urokinase plasminogen activator independent early experimental thrombus resolution: MMP2 as an alternative mechanism. Thromb Haemost. 2010;104(6):1174–1183. doi: 10.1160/TH10-03-0184. [DOI] [PubMed] [Google Scholar]
- 64.Pons M, Cousins SW, Alcazar O, Striker GE, Marin-Castano ME. Angiotensin II-induced MMP-2 activity and MMP-14 and basigin protein expression are mediated via the angiotensin II receptor type 1-mitogen-activated protein kinase 1 pathway in retinal pigment epithelium: implications for age-related macular degeneration. Am J Pathol. 2011;178(6):2665–81. doi: 10.1016/j.ajpath.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cork SM, Kaur B, Devi NS, Cooper L, Saltz JH, Sandberg EM, Kaluz S, Van Meir EG. A proprotein convertase/MMP-14 proteolytic cascade releases a novel 40 kDa vasculostatin from tumor suppressor BAI1. Oncogene. 2012;31(50):5144–52. doi: 10.1038/onc.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ceron CS, Rizzi E, Guimaraes DA, Martins-Oliveira A, Cau SB, Ramos J, Gerlach RF, Tanus-Santos JE. Time course involvement of matrix metalloproteinases in the vascular alterations of renovascular hypertension. Matrix Biol. 2012;31(4):261–70. doi: 10.1016/j.matbio.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Duarte S, Shen XD, Fondevila C, Busuttil RW, Coito AJ. Fibronectin-alpha4beta1 interactions in hepatic cold ischemia and reperfusion injury: regulation of MMP-9 and MT1-MMP via the p38 MAPK pathway. Am J Transplant. 2012;12(10):2689–99. doi: 10.1111/j.1600-6143.2012.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radziwon-Balicka A, Ramer C, Moncada de la Rosa C, Zielnik-Drabik B, Jurasz P. Angiostatin inhibits endothelial MMP-2 and MMP-14 expression: a hypoxia specific mechanism of action. Vascul Pharmacol. 2013;58(4):280–91. doi: 10.1016/j.vph.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Xu J, Sun Y, Wang T, Liu G. Prevention of neointimal hyperplasia in balloon-injured rat carotid artery via small interference RNA mediated downregulation of osteopontin gene. Mol Cell Biochem. 2013;377(1–2):1–10. doi: 10.1007/s11010-012-1554-x. [DOI] [PubMed] [Google Scholar]
- 70.Vasko RXS, Chen J, Lin CH, Ratliff B, Rabadi M, Maizel J, Tanokuchi R, Zhang F, Cao J, Goligorsky MS. Endothelial sirtuin 1 deficiency perpetrates nephrosclerosis through downregulation of matrix metalloproteinase-14: relevance to fibrosis of vascular senescence. J Am Soc Nephrol. 2014;25(2):276–91. doi: 10.1681/ASN.2013010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fu YN, Nagy JA, Brown LF, Shih SC, Johnson PY, Chan CK, Dvorak HF, Wight TN. Proteolytic Cleavage of Versican and Involvement of ADAMTS-1 in VEGF-A/ VPF-Induced Pathological Angiogenesis. J Histochem Cytochem. 2011;59(5):463–473. doi: 10.1369/0022155411401748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shin JISK, Kim H, Cho NH, Kim J, Kim HS, Lee JS. The gene expression profile of matrix metalloproteinases and their inhibitors in children with Henoch-Schönlein purpura. Br J Dermatol. 2011;164(2):1348–55. doi: 10.1111/j.1365-2133.2011.10295.x. [DOI] [PubMed] [Google Scholar]
- 73.Zhang YB, Li W, Yao LQ, Zhao XL, Wang B, Li HK, Ning Y, Song E, Zhang XX. Expression changes and roles of matrix metalloproteinases in a rat model of traumatic deep vein thrombosis. Chin J Traumatol. 2010;13(3):188–92. [PubMed] [Google Scholar]
- 74.Host L, Paye A, Detry B, Blacher S, Munaut C, Foidart JM, Seiki M, Sounni NE, Noel A. The proteolytic activity of MT4-MMP is required for its pro-angiogenic and pro-metastatic promoting effects. Int J Canc. 2012;131(7):1537–1548. doi: 10.1002/ijc.27436. [DOI] [PubMed] [Google Scholar]
- 75.Abdul-Hussien H, Hanemaaijer R, Verheijen JH, van Bockel JH, Geelkerken RH, Lindeman JHN. Doxycycline therapy for abdominal aneurysm: Improved proteolytic balance through reduced neutrophil content. J Vasc Surg. 2009;49(3):741–749. doi: 10.1016/j.jvs.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 76.Huang WCS-NG, Susana A, Johnson JL, Newby AC. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chiao YA, Zamilpa R, Lopez EF, Dai QX, Escobar GP, Hakala K, Weintraub ST, Lindsey ML. In vivo Matrix Metalloproteinase-7 Substrates Identified in the Left Ventricle Post-Myocardial Infarction Using Proteomics. J Proteome Res. 2010;9(5):2649–2657. doi: 10.1021/pr100147r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yabluchanskiy A, Li Y, de Castro Bras LE, Hakala K, Weintraub ST, Lindsey ML. Proteomic analysis of the left ventricle post-myocardial infarction to identify in vivo candidate matrix metalloproteinase substrates. Methods Mol Biol. 2013;1066:185–99. doi: 10.1007/978-1-62703-604-7_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su KHL, Li W, Yan X, Li X, Zhang Z, Jin F, Lei J, Ba G, Liu B, Wang X, Wang Y. TC-1 (c8orf4) enhances aggressive biologic behavior in lung cancer through the Wnt/β-catenin pathway. J Surg Res. 2012;185(1):255–63. doi: 10.1016/j.jss.2013.05.075. [DOI] [PubMed] [Google Scholar]
- 80.Reindel R, Baker SC, Kim KY, Rowley CA, Shulman ST, Orenstein JM, Perlman EJ, Lingen MW, Rowley AH. Integrins alpha4 and alpha M, collagen1A1, and matrix metalloproteinase 7 are upregulated in acute Kawasaki disease vasculopathy. Pediatr Res. 2013;73(3):332–6. doi: 10.1038/pr.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.LaFramboise WA, Dhir R, Kelly LA, Petrosko P, Krill-Burger JM, Sciulli CM, Lyons-Weiler MA, Chandran UR, Lomakin A, Masterson RV, Marroquin OC, Mulukutla SR, McNamara DM. Serum protein profiles predict coronary artery disease in symptomatic patients referred for coronary angiography. BMC Med. 2012;10:157. doi: 10.1186/1741-7015-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Razuvaev A, Ekstrand J, Folkersen L, Agardh H, Markus D, Swedenborg J, Hansson GK, Gabrielsen A, Paulsson-Berne G, Roy J, Hedin U. Correlations Between Clinical Variables and Gene-expression Profiles in Carotid Plaque Instability. Eur J Vasc Endovasc Surg. 2011;42(6):722–730. doi: 10.1016/j.ejvs.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 83.Tong S, Neboori HJ, Tran ED, Schmid-Schonbein GW. Constitutive Expression and Enzymatic Cleavage of ICAM-1 in the Spontaneously Hypertensive Rat. J Vasc Res. 2011;48(5):386–396. doi: 10.1159/000323474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chieng-Yane P, Bocquet A, Letienne R, Bourbon T, Sablayrolles S, Perez M, Hatem SN, Lompre AM, Le Grand B, David-Dufilho M. Protease-activated receptor-1 antagonist F 16618 reduces arterial restenosis by down-regulation of tumor necrosis factor alpha and matrix metalloproteinase 7 expression, migration, and proliferation of vascular smooth muscle cells. J Pharmacol Exp Ther. 2011;336(3):643–51. doi: 10.1124/jpet.110.175182. [DOI] [PubMed] [Google Scholar]
- 85.Odenbach J, Wang X, Cooper S, Chow FL, Oka T, Lopaschuk G, Kassiri Z, Fernandez-Patron C. MMP-2 mediates angiotensin II-induced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension. 2011;57(1):123–30. doi: 10.1161/HYPERTENSIONAHA.110.159525. [DOI] [PubMed] [Google Scholar]
- 86.Vanhoutte PM. Regeneration of the endothelium in vascular injury. Cardiovasc Drugs Ther. 2010;24(4):299–303. doi: 10.1007/s10557-010-6257-5. [DOI] [PubMed] [Google Scholar]
- 87.Rodrigues SF, Tran ED, Fortes ZB, Schmid-Schonbein GW. Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299(1):H25–35. doi: 10.1152/ajpheart.00620.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pirila E, Korpi JT, Korkiamaki T, Jahkola T, Gutierrez-Fernandez A, Lopez-Otin C, Saarialho-Kere U, Salo T, Sorsa T. Collagenase-2 (MMP-8) and matrilysin-2 (MMP-26) expression in human wounds of different etiologies. Wound Repair Regen. 2007;15(1):47–57. doi: 10.1111/j.1524-475X.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 89.Krishnatry AS, Brazeau DA, Fung HL. Broad regulation of matrix and adhesion molecules in THP-1 human macrophages by nitroglycerin. Nitric Oxide. 2010;22(1):11–7. doi: 10.1016/j.niox.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tanskanen M, Myllykangas L, Saarialho-Kere U, Paetau A. Matrix metalloproteinase-beta19 expressed in cerebral amyloid angiopathy. Amyloid. 2011;18(1):3–9. doi: 10.3109/13506129.2010.541960. [DOI] [PubMed] [Google Scholar]
- 91.Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem. 1993;268(32):23824–9. [PubMed] [Google Scholar]
- 92.Johnson JL, Devel L, Czarny B, George SJ, Jackson CL, Rogakos V, Beau F, Yiotakis A, Newby AC, Dive V. A selective matrix metalloproteinase-12 inhibitor retards atherosclerotic plaque development in apolipoprotein E-knockout mice. Arterioscler Thromb Vasc Biol. 2011;31(3):528–35. doi: 10.1161/ATVBAHA.110.219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jithpratuck W, Elshenawy Y, Saleh H, Youngberg G, Chi DS, Krishnaswamy G. The clinical implications of adult-onset henoch-schonelin purpura. Clin Mol Allergy. 2011;9(1):9. doi: 10.1186/1476-7961-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Proietta M, Tritapepe L, Cifani N, Ferri L, Taurino M, Del Porto F. MMP-12 as a new marker of Stanford-A acute aortic dissection. Ann Med. 2014;46(1):44–48. doi: 10.3109/07853890.2013.876728. [DOI] [PubMed] [Google Scholar]
- 95.Ji KT, Zhang Y, Jiang FC, Qian L, Guo HH, Hu JJ, Liao LM, Tang JF. Exploration of the mechanisms by which 3,4-benzopyrene promotes angiotensin II-induced abdominal aortic aneurysm formation in mice. J Vasc Surg. 2014;59(2):492–499. doi: 10.1016/j.jvs.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 96.Song Y, Xie YH, Liu F, Zhao C, Yu R, Ban S, Ye QF, Wen JX, Wan HB, Li X, Ma RW, Meng ZH. Expression of matrix metalloproteinase-12 in aortic dissection. BMC Cardiovasc Disord. 2013:13. doi: 10.1186/1471-2261-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tao M, Yu P, Nguyen BT, Mizrahi B, Savion N, Kolodgie FD, Virmani R, Hao S, Ozaki CK, Schneiderman J. Locally Applied Leptin Induces Regional Aortic Wall Degeneration Preceding Aneurysm Formation in Apolipoprotein E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2013;33(2):311. doi: 10.1161/ATVBAHA.112.300543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolak T, Sion-Vardi N, Novack V, Greenberg G, Szendro G, Tarnovscki T, Nov O, Shelef I, Paran E, Rudich A. N-Terminal Rather Than Full-Length Osteopontin or Its C-Terminal Fragment Is Associated With Carotid-Plaque Inflammation in Hypertensive Patients. Am J Hypertens. 2013;26(3):326–333. doi: 10.1093/ajh/hps043. [DOI] [PubMed] [Google Scholar]
- 99.Scholtes VPW, Johnson JL, Jenkins N, Sala-Newby GB, de Vries JPPM, de Borst GJ, de Kleijn DPV, Moll FL, Pasterkamp G, Newby AC. Carotid Atherosclerotic Plaque Matrix Metalloproteinase-12-Positive Macrophage Subpopulation Predicts Adverse Outcome After Endarterectomy. J Am Heart Assoc. 2012;1(6) doi: 10.1161/JAHA.112.001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Korol RM, Canham PB, Liu L, Viswanathan K, Ferguson GG, Hammond RR, Finlay HM, Baker HV, Lopez C, Lucas AR. Detection of Altered Extracellular Matrix in Surface Layers of Unstable Carotid Plaque: An Optical Spectroscopy, Birefringence and Microarray Genetic Analysis. Photochem Photobiol. 2011;87(5):1164–1172. doi: 10.1111/j.1751-1097.2011.00960.x. [DOI] [PubMed] [Google Scholar]
- 101.Li JM, Wang JJ, Peng QS, Chen C, Humphrey MB, Heinecke J, Zhang SX. Macrophage Metalloelastase (MMP-12) Deficiency Mitigates Retinal Inflammation and Pathological Angiogenesis in Ischemic Retinopathy. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shin JW, Kang HC, Shim J, Sohn NW. Scutellaria baicalensis Attenuates Blood-Brain Barrier Disruption after Intracerebral Hemorrhage in Rats. Am J Chin Med. 2012;40(1):85–96. doi: 10.1142/S0192415X12500073. [DOI] [PubMed] [Google Scholar]
- 103.Svedin P, Hagberg H, Mallard C. Expression of MMP-12 after Neonatal Hypoxic-Ischemic Brain Injury in Mice. Dev Neurosci. 2009;31(5):427–436. doi: 10.1159/000232561. [DOI] [PubMed] [Google Scholar]
- 104.Manetti M, Guiducci S, Romano E, Bellando-Randone S, Conforti ML, Ibba-Manneschi L, Matucci-Cerinic M. Increased serum levels and tissue expression of matrix metalloproteinase-12 in patients with systemic sclerosis: correlation with severity of skin and pulmonary fibrosis and vascular damage. Ann Rheum Dis. 2012;71(6):1064–1072. doi: 10.1136/annrheumdis-2011-200837. [DOI] [PubMed] [Google Scholar]
- 105.Ikonomidis JS, Ruddy JM, Benton SM, Arroyo J, Brinsa TA, Stroud RE, Zeeshan A, Bavaria JE, Gorman JH, Gorman RC, Spinale FG, Jones JA. Aortic Dilatation With Bicuspid Aortic Valves: Cusp Fusion Correlates to Matrix Metalloproteinases and Inhibitors INVITED COMMENTARY. Ann Thorac Surg. 2012;93(2):457–464. doi: 10.1016/j.athoracsur.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ryan J, Ma FY, Kanellis J, Delgado M, Blease K, Nikolic-Paterson DJ. Spleen tyrosine kinase promotes acute neutrophil-mediated glomerular injury via activation of JNK and p38 MAPK in rat nephrotoxic serum nephritis. Lab Invest. 2011;91(12):1727–1738. doi: 10.1038/labinvest.2011.137. [DOI] [PubMed] [Google Scholar]
- 107.Shimizu C, Matsubara T, Onouchi Y, Jain S, Sun S, Nievergelt CM, Shike H, Brophy VH, Takegawa T, Furukawa S, Akagi T, Newburger JW, Baker AL, Burgner D, Hibberd ML, Davila S, Levin M, Mamtani M, He WJ, Ahuja SK, Burns JC. Matrix metalloproteinase haplotypes associated with coronary artery aneurysm formation in patients with Kawasaki disease. J Hum Genet. 2010;55(12):779–784. doi: 10.1038/jhg.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arunachalam G, Sundar IK, Hwang JW, Yao HW, Rahman I. Emphysema is associated with increased inflammation in lungs of atherosclerosis-prone mice by cigarette smoke: implications in comorbidities of COPD. J Inflamm-London. 2010:7. doi: 10.1186/1476-9255-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pauwels NS, Bracke KR, Maes T, Van Pottelberge GR, Garlanda C, Mantovani A, Joos GF, Brusselle GG. Cigarette smoke induces PTX3 expression in pulmonary veins of mice in an IL-1 dependent manner. Respir Res. 2010:11. doi: 10.1186/1465-9921-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jackson V, Olsson T, Kurtovic S, Folkersen L, Paloschi V, Wagsater D, Franco-Cereceda A, Eriksson P. Matrix metalloproteinase 14 and 19 expression is associated with thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2012;144(2):459–466. doi: 10.1016/j.jtcvs.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 111.Roscoe WA, Welsh ME, Carter DE, Karlik SJ. VEGF and angiogenesis in acute and chronic MOG((35–55)) peptide induced EAE. J Neuroimmunol. 2009;209(1–2):6–15. doi: 10.1016/j.jneuroim.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 112.Kolb CMS, Krawinkel U, Sedlacek R. Matrix metalloproteinase-19 in capillary endothelial cells: expression in acutely, but not in chronically, inflamed synovium. Exp Cell Res. 1999;250(1):122–30. doi: 10.1006/excr.1999.4493. [DOI] [PubMed] [Google Scholar]
- 113.Hegedus L, Cho H, Xie X, Eliceiri GL. Additional MDA-MB-231 breast cancer cell matrix metalloproteinases promote invasiveness. J Cell Physiol. 2008;216(2):480–5. doi: 10.1002/jcp.21417. [DOI] [PubMed] [Google Scholar]
- 114.Ahokas K, Lohi J, Lohi H, Elomaa O, Karjalainen-Lindsberg ML, Kere J, Saarialho-Kere U. Matrix metalloproteinase-21, the human orthologue for XMMP, is expressed during fetal development and in cancer. Gene. 2002;301(1–2):31–41. doi: 10.1016/S0378-1119(02)01088-0. [DOI] [PubMed] [Google Scholar]
- 115.Kuivanen T, Ahokas K, Virolainen S, Jahkola T, Holtta E, Saksela O, Saarialho-Kere U. MMP-21 is upregulated at early stages of melanoma progression but disappears with more aggressive phenotype. Virchows Archiv. 2005;447(6):954–960. doi: 10.1007/s00428-005-0046-8. [DOI] [PubMed] [Google Scholar]
- 116.Skoog T, Elomaa O, Pasonen-Seppanen SM, Forsberg S, Ahokas K, Jeskanen L, Parssinen J, Tiala I, Rollman O, Lohi J, Saarialho-Kere U. Matrix Metalloproteinase-21 Expression Is Associated with Keratinocyte Differentiation and Upregulated by Retinoic Acid in HaCaT Cells. J Invest Dermatol. 2009;129(1):119–130. doi: 10.1038/jid.2008.206. [DOI] [PubMed] [Google Scholar]
- 117.Marchenko GN, Marchenko ND, Strongin AY. The structure and regulation of the human and mouse matrix metalloproteinase-21 gene and protein. Biochem J. 2003;372(Pt 2):503–15. doi: 10.1042/BJ20030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang Y, Li WH, Chu DK, Zheng JY, Ji G, Li MB, Zhang HW, Wang WZ, Du JJ, Li JP. Overexpression of Matrix Metalloproteinase-21 is Associated with Poor Overall Survival of Patients with Colorectal Cancer. J Gastrointest Surg. 2011;15(7):1188–1194. doi: 10.1007/s11605-011-1519-5. [DOI] [PubMed] [Google Scholar]
- 119.Wu T, Li Y, Lu JG, Qiao Q, Bao GQ, Wang N, He XL, Du XL. Increased MMP-21 expression is associated with poor overall survival of patients with gastric cancer. Med Oncol. 2013;30(1) doi: 10.1007/s12032-012-0323-8. [DOI] [PubMed] [Google Scholar]
- 120.Gururajan R, Grenet J, Lahti JM, Kidd VJ. Isolation and characterization of two novel metalloproteinase genes linked to the Cdc2L locus on human chromosome 1p36.3. Genomics. 1998;52(1):101–6. doi: 10.1006/geno.1998.5401. [DOI] [PubMed] [Google Scholar]
- 121.Gajecka M, Yu W, Ballif BC, Glotzbach CD, Bailey KA, Shaw CA, Kashork CD, Heilstedt HA, Ansel DA, Theisen A, Rice R, Rice DP, Shaffer LG. Delineation of mechanisms and regions of dosage imbalance in complex rearrangements of 1p36 leads to a putative gene for regulation of cranial suture closure. Eur J Hum Genet. 2005;13(2):139–49. doi: 10.1038/sj.ejhg.5201302. [DOI] [PubMed] [Google Scholar]
- 122.Lohi J, Wilson CL, Roby JD, Parks WC. Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J Biol Chem. 2001;276(13):10134–10144. doi: 10.1074/jbc.M001599200. [DOI] [PubMed] [Google Scholar]
- 123.Bernal F, Hartung HP, Kieseier BC. Tissue mRNA expression in rat of newly described matrix metalloproteinases. Biol Res. 2005;38(2–3):267–271. doi: 10.4067/S0716-97602005000200016. [DOI] [PubMed] [Google Scholar]
- 124.Murugan AK, Yang CF, Xing MZ. Mutational analysis of the GNA11, MMP27, FGD1, TRRAP and GRM3 genes in thyroid cancer. Oncol Lett. 2013;6(2):437–441. doi: 10.3892/ol.2013.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ma YG, Halade GV, Zhang JH, Ramirez TA, Levin D, Voorhees A, Jin YF, Han HC, Manicone AM, Lindsey ML. Matrix Metalloproteinase-28 Deletion Exacerbates Cardiac Dysfunction and Rupture After Myocardial Infarction in Mice by Inhibiting M2 Macrophage Activation. Circ Res. 2013;112(4):675. doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Poli G, Biasi F, Leonarduzzi G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013;1(1):125–130. doi: 10.1016/j.redox.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Z, Guo Y, Jiang H, Zhang T, Jin C, Young CY, Yuan H. Differential regulation of MMPs by E2F1, Sp1 and NF-kappa B controls the small cell lung cancer invasive phenotype. BMC Cancer. 2014;14:276. doi: 10.1186/1471-2407-14-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Williamson RA, Martorell G, Carr MD, Murphy G, Docherty AJ, Freedman RB, Feeney J. Solution structure of the active domain of tissue inhibitor of metalloproteinases-2, A new member of the OB fold protein family. Biochemistry. 1994;33(39):11745–59. doi: 10.1021/bi00205a010. [DOI] [PubMed] [Google Scholar]
- 129.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477(1–2):267–83. doi: 10.1016/S0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 130.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 131.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 132.Lafleur MA, Hollenberg MD, Atkinson SJ, KV, Murphy G, Edwards DR. Activation of pro-(matrix metalloproteinase-2) (pro-MMP-2) by thrombin is membrane-type-MMP-dependent in human umbilical vein endothelial cells and generates a distinct 63 kDa active species. Biochem J. 2001;357:107–115. doi: 10.1042/0264-6021:3570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li DD, Song JN, Huang H, Guo XY, An JY, Zhang M, Li Y, Sun P, Pang HG, Zhao YL, Wang JF. The roles of MMP-9/TIMP-1 in cerebral edema following experimental acute cerebral infarction in rats. Neurosci Lett. 2013;550:168–72. doi: 10.1016/j.neulet.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 134.Xu HM, Zhao Y, Zhang XM, Zhu T, Fu WG. Polymorphisms in MMP-9 and TIMP-2 in Chinese patients with varicose veins. J Surg Res. 2011;168(1):e143–8. doi: 10.1016/j.jss.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 135.Andrade VL, Petruceli E, Belo VA, Andrade-Fernandes CM, Caetano Russi CV, Bosco AA, Tanus-Santos JE, Sandrim VC. Evaluation of plasmatic MMP-8, MMP-9, TIMP-1 and MPO levels in obese and lean women. Clin Biochem. 2012;45(6):412–5. doi: 10.1016/j.clinbiochem.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 136.Li Z, Guo S, Yao F, Zhang Y, Li T. Increased ratio of serum matrix metalloproteinase-9 against TIMP-1 predicts poor wound healing in diabetic foot ulcers. J Diabetes Complications. 2013;27(4):380–2. doi: 10.1016/j.jdiacomp.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 137.Lamfers ML, Grimbergen JM, Aalders MC, Havenga MJ, de Vries MR, Huisman LG, van Hinsbergh VW, Quax PH. Gene transfer of the urokinase-type plasminogen activator receptor-targeted matrix metalloproteinase inhibitor TIMP-1, ATF suppresses neointima formation more efficiently than tissue inhibitor of metalloproteinase-1. Circ Res. 2002;91(10):945–52. doi: 10.1161/01.RES.0000041418.51906.57. [DOI] [PubMed] [Google Scholar]
- 138.Eefting D, de Vries MR, Grimbergen JM, Karper JC, van Bockel JH, Quax PH. In vivo suppression of vein graft disease by nonviral, electroporation-mediated, gene transfer of tissue inhibitor of metalloproteinase-1 linked to the amino terminal fragment of urokinase (TIMP-1, ATF), a cell-surface directed matrix metalloproteinase inhibitor. J Vasc Surg. 2010;51(2):429–37. doi: 10.1016/j.jvs.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 139.Cadete VJJ, Arcand SA, Chaharyn BM, Doroszko A, Sawicka J, Mousseau DD, Sawicki G. Matrix Metalloproteinase-2 Is Activated During Ischemia/Reperfusion in a Model of Myocardial Infarction. Can J Cardiol. 2013;29(11):1495–1503. doi: 10.1016/j.cjca.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 140.Samadzadeh KMCK, Nguyen AT, Baker PM, Bains S, Lee ES. Monocyte activity is linked with abdominal aortic aneurysm diameter. J Surg Res. 2014 doi: 10.1016/j.jss.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 141.Gao LCL, Lu ZZ, Gao H, Wu L, Chen YX, Zhang CM, Jiang YK, Jing Q, Zhang Y, Yang HT. Activation of α1B-adrenoceptors contributes to intermittent hypobaric hypoxia-improved post-ischemic myocardial performance via inhibiting MMP-2 activation. Am J Physiol Heart Circ Physiol. 2014 doi: 10.1152/ajpheart.00772.2013. [DOI] [PubMed] [Google Scholar]
- 142.Foster CR, Daniel LL, Daniels CR, Dalal S, Singh M, Singh K. Deficiency of Ataxia Telangiectasia Mutated Kinase Modulates Cardiac Remodeling Following Myocardial Infarction: Involvement in Fibrosis and Apoptosis. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muradashvili N, Benton RL, Tyagi R, Tyagi SC, Lominadze D. Elevated level of fibrinogen increases caveolae formation; role of matrix metalloproteinase-9. Cell Biochem Biophys. 2014;69(2):283–94. doi: 10.1007/s12013-013-9797-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yao H, Hwang JW, Sundar IK, Friedman AE, McBurney MW, Guarente L, Gu W, Kinnula VL, Rahman I. SIRT1 redresses the imbalance of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-9 in the development of mouse emphysema and human COPD. Am J Physiol Lung Cell Mol Physiol. 2013;305(9):L615–24. doi: 10.1152/ajplung.00249.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.El Hajj EC, El Hajj MC, Voloshenyuk TG, Mouton AJ, Khoutorova E, Molina PE, Gilpin NW, Gardner JD. Alcohol modulation of cardiac matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs favors collagen accumulation. Alcohol Clin Exp Res. 2014;38(2):448–56. doi: 10.1111/acer.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Udali S, Guarini P, Moruzzi S, Choi SW, Friso S. Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Aspects Med. 2013;34(4):883–901. doi: 10.1016/j.mam.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 147.Shiva Shankar LWTV. Epigenetic modulators mitigate angiogenesis through a complex transcriptomic network. Vasc Pharmacol. 2014;60:57–66. doi: 10.1016/j.vph.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 148.Hellebrekers DM, Jair KW, Vire E, Eguchi S, Hoebers NT, Fraga MF, Esteller M, Fuks F, Baylin SB, van Engeland M, Griffioen AW. Angiostatic activity of DNA methyltransferase inhibitors. Mol Cancer Ther. 2006;5(2):467–75. doi: 10.1158/1535-7163.MCT-05-0417. [DOI] [PubMed] [Google Scholar]
- 149.Ivanciu L, Gerard RD, Tang H, Lupu F, Lupu C. Adenovirus-mediated expression of tissue factor pathway inhibitor-2 inhibits endothelial cell migration and angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27(2):310–6. doi: 10.1161/01.ATV.0000254147.89321.cf. [DOI] [PubMed] [Google Scholar]
- 150.Miyazaki D, Nakamura A, Fukushima K, Yoshida K, Takeda S, Ikeda S. Matrix metalloproteinase-2 ablation in dystrophin-deficient mdx muscles reduces angiogenesis resulting in impaired growth of regenerated muscle fibers. Hum Mol Genet. 2011;20(9):1787–99. doi: 10.1093/hmg/ddr062. [DOI] [PubMed] [Google Scholar]
- 151.Gupta C, Kaur J, Tikoo K. Regulation of MDA-MB-231 cell proliferation by GSK-3beta involves epigenetic modifications under high glucose conditions. Exp Cell Res. 2014;324(1):75–83. doi: 10.1016/j.yexcr.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 152.Sato-Kusubata K, Jiang Y, Ueno Y, Chun TH. Adipogenic histone mark regulation by matrix metalloproteinase 14 in collagen-rich microenvironments. Mol Endocrinol. 2011;25(5):745–53. doi: 10.1210/me.2010-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Poplineau M, Dufer J, Antonicelli F, Trussardi-Regnier A. Epigenetic regulation of proMMP-1 expression in the HT1080 human fibrosarcoma cell line. Int J Oncol. 2011;38(6):1713–8. doi: 10.3892/ijo.2011.975. [DOI] [PubMed] [Google Scholar]
- 154.Kowluru RA, Santos JM, Mishra M. Epigenetic modifications and diabetic retinopathy. Biomed Res Int. 2013;2013:635284. doi: 10.1155/2013/635284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Osaki M, Inaba A, Nishikawa K, Sugimoto Y, Shomori K, Inoue T, Oshimura M, Ito H. Cysteine-rich protein 61 suppresses cell invasion via down-regulation of matrix metalloproteinase-7 expression in the human gastric carcinoma cell line MKN-45. Mol Med Rep. 2010;3(4):711–5. doi: 10.3892/mmr_00000322. [DOI] [PubMed] [Google Scholar]
- 156.Narayanan N, Tyagi N, Shah A, Pagni S, Tyagi SC. Hyperhomocysteinemia during aortic aneurysm, a plausible role of epigenetics. Int J Physiol Pathophysiol Pharmacol. 2013;5(1):32–42. [PMC free article] [PubMed] [Google Scholar]
- 157.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8(2):93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 158.Chen KC, Wang YS, Hu CY, Chang WC, Liao YC, Dai CY, Juo SH. OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: a novel mechanism for cardiovascular diseases. FASEB J. 2011;25(5):1718–28. doi: 10.1096/fj.10-174904. [DOI] [PubMed] [Google Scholar]
- 159.Boon RAST, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, Vinciguerra M, Rosenthal N, Sciacca S, Pilato M, van Heijningen P, Essers J, Brandes RP, Zeiher AM, Dimmeler S. Micro-RNA-29 in aortic dilation: implications for aneurysm formation. Circ Res. 2011;109(10):1115–9. doi: 10.1161/CIRCRESAHA.111.255737. [DOI] [PubMed] [Google Scholar]
- 160.Chen KC, Liao YC, Hsieh IC, Wang YS, Hu CY, Juo SH. OxLDL causes both epigenetic modification and signaling regulation on the microRNA-29b gene: novel mechanisms for cardiovascular diseases. J Mol Cell Cardiol. 2012;52(3):587–95. doi: 10.1016/j.yjmcc.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 161.Turunen MP, Yla-Herttuala S. Epigenetic regulation of key vascular genes and growth factors. Cardiovasc Res. 2011;90(3):441–446. doi: 10.1093/cvr/cvr109. [DOI] [PubMed] [Google Scholar]
- 162.Wu D, Pan H, Zhou Y, Zhou J, Fan Y, Qu P. microRNA-133b downregulation and inhibition of cell proliferation, migration and invasion by targeting matrix metallopeptidase-9 in renal cell carcinoma. Mol Med Rep. 2014;9(6):2491–8. doi: 10.3892/mmr.2014.2116. [DOI] [PubMed] [Google Scholar]
- 163.Zhou X, Ren Y, Liu A, Han L, Zhang K, Li S, Li P, Kang C, Wang X, Zhang L. STAT3 inhibitor WP1066 attenuates miRNA-21 to suppress human oral squamous cell carcinoma growth in vitro and in vivo. Oncol Rep. 2014;31(5):2173–80. doi: 10.3892/or.2014.3114. [DOI] [PubMed] [Google Scholar]
- 164.Ye Z, Jingzhong L, Yangbo L, Lei C, Jiandong Y. Propofol inhibits proliferation and invasion of osteosarcoma cells by regulation of microRNA-143 expression. Oncol Res. 2014;21(4):201–7. doi: 10.3727/096504014X13890370410203. [DOI] [PubMed] [Google Scholar]
- 165.Ye P, Liu J, He F, Xu W, Yao K. Hypoxia-induced deregulation of miR-126 and its regulative effect on VEGF and MMP-9 expression. Int J Med Sci. 2014;11(1):17–23. doi: 10.7150/ijms.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Siddesha JM, Valente AJ, Yoshida T, Sakamuri SS, Delafontaine P, Iba H, Noda M, Chandrasekar B. Docosahexaenoic acid reverses angiotensin II-induced RECK suppression and cardiac fibroblast migration. Cell Signal. 2014;26(5):933–41. doi: 10.1016/j.cellsig.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Palmieri DCS, Geroldi A, Mura M, Mandich P, Palombo D. TNFα induces the expression of genes associated with endothelial dysfunction through p38MAPK-mediated down-regulation of miR-149. Biochem Biophys Res Commun. 2014;443(1):246–51. doi: 10.1016/j.bbrc.2013.11.092. [DOI] [PubMed] [Google Scholar]
- 168.Zhang HY, Qi M, Li SW, Qi T, Mei H, Huang K, Zheng LD, Tong QS. microRNA-9 Targets Matrix Metalloproteinase 14 to Inhibit Invasion, Metastasis, and Angiogenesis of Neuroblastoma Cells. Mol Canc Ther. 2012;11(7):1454–1466. doi: 10.1158/1535-7163.MCT-12-0001. [DOI] [PubMed] [Google Scholar]
- 169.Siragam V, Rutnam ZJ, Yang W, Fang L, Luo L, Yang X, Li M, Deng Z, Qian J, Peng C, Yang BB. MicroRNA miR-98 inhibits tumor angiogenesis and invasion by targeting activin receptor-like kinase-4 and matrix metalloproteinase-11. Oncotarget. 2012;3(11):1370–85. doi: 10.18632/oncotarget.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Morales R, Perrier S, Florent JM, Beltra J, Dufour S, De Mendez I, Manceau P, Tertre A, Moreau F, Compere D, Dublanchet AC, O’Gara M. Crystal structures of novel non-peptidic, non-zinc chelating inhibitors bound to MMP-12. J Mol Biol. 2004;341(4):1063–76. doi: 10.1016/j.jmb.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 171.Huang ST, Yang RC, Wu HT, Wang CN, Pang JHS. Zinc-Chelation Contributes to the Anti-Angiogenic Effect of Ellagic Acid on Inhibiting MMP-2 Activity, Cell Migration and Tube Formation. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rasmussen HS, McCann PP. Matrix metalloproteinase inhibition as a novel anticancer strategy: A review with special focus on batimastat and marimastat. Pharmacol Ther. 1997;75(1):69–75. doi: 10.1016/S0163-7258(97)00023-5. [DOI] [PubMed] [Google Scholar]
- 173.Kocer SS, Walker SG, Zerler B, Golub LM, Simon SR. Metalloproteinase inhibitors, nonantimicrobial chemically modified tetracyclines, and ilomastat block Bacillus anthracis lethal factor activity in viable cells. Infect Immun. 2005;73(11):7548–57. doi: 10.1128/IAI.73.11.7548-7557.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, Kantor C, Gahmberg CG, Salo T, Konttinen YT, Sorsa T, Ruoslahti E, Pasqualini R. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol. 1999;17(8):768–74. doi: 10.1038/11703. [DOI] [PubMed] [Google Scholar]
- 175.Castro MM, Kandasamy AD, Youssef N, Schulz R. Matrix metalloproteinase inhibitor properties of tetracyclines:therapeutic potential in cardiovascular diseases. Pharmacol Res. 2011;64(6):551–60. doi: 10.1016/j.phrs.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 176.Luan ZX, Chase AJAC. New by.Statins inhibit secretion of metalloproteinases-1,-2,-3, and-9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003;23(5):769–775. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- 177.Mitani H, Egashira K, Kimura M. HMG-CoA reductase inhibitor, fluvastatin, has cholesterol-lowering independent “direct” effects on atherosclerotic vessels in high cholesterol diet-fed rabbits. Pharmacol Res. 2003;48(5):417–427. doi: 10.1016/S1043-6618(03)00184-1. [DOI] [PubMed] [Google Scholar]
- 178.Freskos JN, Asmelash B, Gaston KR, Karwa A, Marzan TA, Nickols MA, Rogers TE, Schoenstein T, Sympson CJ, Vu B. Design and synthesis of MMP inhibitors with appended fluorescent tags for imaging and visualization of matrix metalloproteinase enzymes. Bioorg Med Chem Lett. 2013;23(20):5566–5570. doi: 10.1016/j.bmcl.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 179.Santamaria S, Nuti E, Cercignani G, Marinelli L, La Pietra V, Novellino E, Rossello A. N-O-Isopropyl sulfonamido-based hydroxamates: Kinetic characterisation of a series of MMP-12/MMP-13 dual target inhibitors. Biochem Pharmacol. 2012;84(6):813–820. doi: 10.1016/j.bcp.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 180.La Pietra V, Marinelli L, Cosconati S, Di Leva FS, Nuti E, Santamaria S, Pugliesi I, Morelli M, Casalini F, Rossello A, La Motta C, Taliani S, Visse R, Nagase H, da Settimo F, Novellino E. Identification of novel molecular scaffolds for the design of MMP-13 inhibitors: a first round of lead optimization. Eur J of Med Chem. 2012;47(1):143–52. doi: 10.1016/j.ejmech.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 181.Jain P, Saravanan C, Singh SK. Sulphonamides: Deserving class as MMP inhibitors? Eur J Med Chem. 2013;60:89–100. doi: 10.1016/j.ejmech.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 182.Xu X, Mikhailova M, Chen Z, Pal S, Robichaud TK, Lafer EM, Baber S, Steffensen B. Peptide from the C-terminal domain of tissue inhibitor of matrix metalloproteinases-2 (TIMP-2) inhibits membrane activation of matrix metalloproteinase-2 (MMP-2) Matrix Biol. 2011;30(7–8):404–12. doi: 10.1016/j.matbio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nicolotti O, Catto M, Giangreco I, Barletta M, Leonetti F, Stefanachi A, Pisani L, Cellamare S, Tortorella P, Loiodice F, Carotti A. Design, synthesis and biological evaluation of 5-hydroxy, 5-substituted-pyrimidine-2,4,6-triones as potent inhibitors of gelatinases MMP-2 and MMP-9. Eur J Med Chem. 2012;58:368–376. doi: 10.1016/j.ejmech.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 184.Heim-Riether A, Taylor SJ, Liang S, Gao DA, Xiong Z, Michael August E, Collins BK, Farmer BT, 2nd, Haverty K, Hill-Drzewi M, Junker HD, Mariana Margarit S, Moss N, Neumann T, Proudfoot JR, Keenan LS, Sekul R, Zhang Q, Li J, Farrow NA. Improving potency and selectivity of a new class of non-Zn-chelating MMP-13 inhibitors. Bioorg Med Chem Lett. 2009;19(18):5321–4. doi: 10.1016/j.bmcl.2009.07.151. [DOI] [PubMed] [Google Scholar]
- 185.Gao DA, Xiong Z, Heim-Riether A, Amodeo L, August EM, Cao X, Ciccarelli L, Collins BK, Harrington K, Haverty K, Hill-Drzewi M, Li X, Liang S, Margarit SM, Moss N, Nagaraja N, Proudfoot J, Roman R, Schlyer S, Keenan LS, Taylor S, Wellenzohn B, Wiedenmayer D, Li J, Farrow NA. SAR studies of non-zinc-chelating MMP-13 inhibitors: improving selectivity and metabolic stability. Bioorg Med Chemi Lett. 2010;20(17):5039–43. doi: 10.1016/j.bmcl.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 186.Yuan H, Lu WQ, Wang LY, Shan L, Li HL, Huang J, Sun QY, Zhang WD. Synthesis of derivatives of methyl rosmarinate and their inhibitory activities against matrix metalloproteinase-1 (MMP-1) Eur J Med Chem. 2013;62:148–157. doi: 10.1016/j.ejmech.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 187.Farina AR, Cappabianca L, Di Ianni N, Ruggeri P, Ragone M, Merolle S, Gulino A, Mackay AR. Alendronate promotes plasmin-mediated MMP-9 inactivation by exposing cryptic plasmin degradation sites within the MMP-9 catalytic domain. FEBS Lett. 2012;586(16):2366–2374. doi: 10.1016/j.febslet.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 188.Chuang SY, Yang SH, Chen TY, Pang JHS. Cilostazol inhibits matrix invasion and modulates the gene expressions of MMP-9 and TIMP-1 in PMA-differentiated THP-1 cells. Eur J Pharmacol. 2011;670(2–3):419–426. doi: 10.1016/j.ejphar.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 189.Okada M, Kikuzuki R, Harada T, Hori Y, Yamawaki H, Hara Y. Captopril attenuates matrix metalloproteinase-2 and -9 in monocrotaline-induced right ventricular hypertrophy in rats. J Pharmacol Sci. 2008;108(4):487–94. doi: 10.1254/jphs.08174FP. [DOI] [PubMed] [Google Scholar]
- 190.Yamamoto D, Takai S, Miyazaki M. Inhibitory profiles of captopril on matrix metalloproteinase-9 activity. Eur J Pharmacol. 2008;588(2–3):277–9. doi: 10.1016/j.ejphar.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 191.Onal IK, Altun B, Onal ED, Kirkpantur A, Gul Oz S, Turgan C. Serum levels of MMP-9 and TIMP-1 in primary hypertension and effect of antihypertensive treatment. Eur J Intern Med. 2009;20(4):369–72. doi: 10.1016/j.ejim.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 192.Kuntze LB, Antonio RC, Izidoro-Toledo TC, Meschiari CA, Tanus-Santos JE, Gerlach RF. Captopril and Lisinopril Only Inhibit Matrix Metalloproteinase-2 (MMP-2)Activity at Millimolar Concentrations. Basic Clin Pharmacol Toxicol. 2014;114(3):233–239. doi: 10.1111/bcpt.12151. [DOI] [PubMed] [Google Scholar]
- 193.Wang JP, Zhang Q, Mei XH, Zhang XZ. Hydroxysafflor yellow A attenuates left ventricular remodeling after pressure overload-induced cardiac hypertrophy in rats. Pharmaceut Biol. 2014;52(1):31–35. doi: 10.3109/13880209.2013.805791. [DOI] [PubMed] [Google Scholar]
- 194.Lin CW, Chen PN, Chen MK, Yang WE, Tang CH, Yang SF, Hsieh YS. Kaempferol Reduces Matrix Metalloproteinase-2 Expression by Down-Regulating ERK1/2 and the Activator Protein-1 Signaling Pathways in Oral Cancer Cells. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0080883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kamat PK, Kalani A, Givvimani S, Sathnur PB, Tyagi SC, Tyagi N. Hydrogen Sulfide Attenuates Neurodegeneration and Neurovascular Dysfunction Induced by Intracerebral-Administered Homocysteine in Mice. Neuroscience. 2013;252:302–319. doi: 10.1016/j.neuroscience.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sen U, Munjal C, Qipshidze N, Abe O, Gargoum R, Tyagi SC. Hydrogen sulfide regulates homocysteine-mediated glomerulosclerosis. Am J Nephrol. 2010;31(5):442–55. doi: 10.1159/000296717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, Shah KS, Passmore JC, Tyagi SC. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol. 2009;297(2):F410–9. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lavin B, Gomez M, Pello OM, Castejon B, Piedras MJ, Saura M, Zaragoza C. Nitric Oxide Prevents Aortic Neointimal Hyperplasia by Controlling Macrophage Polarization. Arterioscler Thromb Vasc Biol. 2014;34(8):1739. doi: 10.1161/ATVBAHA.114.303866. [DOI] [PubMed] [Google Scholar]
- 199.Zucker S, Drews M, Conner C, Foda HD, DeClerck YA, Langley KE, Bahou WF, Docherty AJ, Cao J. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP) J Biol Chem. 1998;273(2):1216–22. doi: 10.1074/jbc.273.2.1216. [DOI] [PubMed] [Google Scholar]
- 200.Overall CM, Tam E, McQuibban GA, Morrison C, Wallon UM, Bigg HF, King AE, Roberts CR. Domain interactions in the gelatinase ATIMP-2MT1-MMP activation complex, The ectodomain of the 44-kDa form of membrane type-1 matrix metalloproteinase does not modulate gelatinase A activation. J Biol Chem. 2000;275(50):39497–506. doi: 10.1074/jbc.M005932200. [DOI] [PubMed] [Google Scholar]
- 201.Kai HS, Butler GS, Morrison CJ, King AE, Pelman GR, Overall CM. Utilization of a novel recombinant myoglobin fusion protein expression system to characterize the tissue inhibitor of metalloproteinase (TIMP)-4, TIMP-2 C-terminal domain, tails by mutagenesis, The importance of acidic residues in binding the MMP-2 hemopexin C-domain. J Biol Chem. 2002;277(50):48696–707. doi: 10.1074/jbc.M209177200. [DOI] [PubMed] [Google Scholar]
- 202.Sawicki G, Menon V, Jugdutt BI. Improved balance between TIMP-3 and MMP-9 after regional myocardial ischemia-reperfusion during AT1 receptor blockade. J Card Fail. 2004;10(5):442–9. doi: 10.1016/j.cardfail.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 203.Sakamoto T, Seiki M. Cytoplasmic tail of MT1-MMP regulates macrophage motility independently from its protease activity. Genes Cells. 2009;14(5):617–26. doi: 10.1111/j.1365-2443.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- 204.Kazes I, Elalamy I, Sraer JD, Hatmi M, Nguyen G. Platelet release of trimolecular complex components MT1-MMP/TIMP2/ MMP2: involvement in MMP2 activation and platelet aggregation. Blood. 2000;96(9):3064–9. [PubMed] [Google Scholar]
- 205.Yin Z, Sada AA, Reslan OM, Narula N, Khalil RA. Increased MMPs expression and decreased contraction in the rat myometrium during pregnancy and in response to prolonged stretch and sex hormones. Am J Physiol Endocrinol Metab. 2012;303(1):E55–70. doi: 10.1152/ajpendo.00553.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.de Meijer VE, Sverdlov DY, Popov Y, Le HD, Meisel JA, Nose V, Schuppan D, Puder M. Broad-spectrum matrix metalloproteinase inhibition curbs inflammation and liver injury but aggravates experimental liver fibrosis in mice. PLoS One. 2010;5(6):e11256. doi: 10.1371/journal.pone.0011256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bencsik P, Paloczi J, Kocsis GF, Pipis J, Belecz I, Varga ZV, Csonka C, Gorbe A, Csont T, Ferdinandy P. Moderate inhibition of myocardial matrix metalloproteinase-2 by ilomastat is cardioprotective. Pharmacol Res: the official journal of the Italian Pharmacological Society. 2014;80:36–42. doi: 10.1016/j.phrs.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 208.Zhang X, Bresee J, Fields GB, Edwards WB. Near-infrared triple-helical peptide with quenched fluorophores for optical imaging of MMP-2 and MMP-9 proteolytic activity in vivo. Bioorg Med Chem Lett. 2014;24(16):3786–90. doi: 10.1016/j.bmcl.2014.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003;23(5):769–75. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- 210.Nagashima H, Aoka Y, Sakomura Y, Sakuta A, Aomi S, Ishizuka N, Hagiwara N, Kawana M, Kasanuki H. A 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, cerivastatin, suppresses production of matrix metalloproteinase-9 in human abdominal aortic aneurysm wall. J Vasc Surg. 2002;36(1):158–63. doi: 10.1067/mva.2002.123680. [DOI] [PubMed] [Google Scholar]
- 211.Vincent L, Chen W, Hong L, Mirshahi F, Mishal Z, Mirshahi-Khorassani T, Vannier JP, Soria J, Soria C. Inhibition of endothelial cell migration by cerivastatin, an HMG-CoA reductase inhibitor: contribution to its anti-angiogenic effect. FEBS Lett. 2001;495(3):159–66. doi: 10.1016/S0014-5793(01)02337-7. [DOI] [PubMed] [Google Scholar]
- 212.Chen YJ, Chang LS. Simvastatin induces NFkappaB/p65 down-regulation and JNK1/c-Jun/ATF-2 activation, leading to matrix metalloproteinase-9 (MMP-9) but not MMP-2 down-regulation in human leukemia cells. Biochem Pharmacol. 2014;92(4):530–43. doi: 10.1016/j.bcp.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 213.Manu KA, Shanmugam MK, Li F, Chen L, Siveen KS, Ahn KS, Kumar AP, Sethi G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J Mol Med (Berl) 2014;92(3):267–76. doi: 10.1007/s00109-013-1095-0. [DOI] [PubMed] [Google Scholar]
- 214.Andrade VL, do Valle IB, Sandrim VC. Simvastatin therapy decreases MMP-9 levels in obese women. J Clin Pharmacol. 2013;53(10):1072–7. doi: 10.1002/jcph.146. [DOI] [PubMed] [Google Scholar]
- 215.Janardhanan R, Yang B, Vohra P, Roy B, Withers S, Bhattacharya S, Mandrekar J, Kong H, Leof EB, Mukhopadhyay D, Misra S. Simvastatin reduces venous stenosis formation in a murine hemodialysis vascular access model. Kidney Int. 2013;84(2):338–52. doi: 10.1038/ki.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kang S, Kim ES, Moon A. Simvastatin and lovastatin inhibit breast cell invasion induced by H-Ras. Oncol Rep. 2009;21(5):1317–22. doi: 10.3892/or_00000357. [DOI] [PubMed] [Google Scholar]
- 217.Slawinska-Brych A, Zdzisinska B, Kandefer-Szerszen M. Fluvastatin inhibits growth and alters the malignant phenotype of the C6 glioma cell line. Pharmacol Rep. 2014;66(1):121–9. doi: 10.1016/j.pharep.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 218.Ozaki H, Miyashita Y, Watanabe H, Shirai K. Enhancement of MMP-9 activity in THP-1 cells by 7-ketocholesterol and its suppression by the HMG-CoA reductase inhibitor fluvastatin. J Atheroscler Thromb. 2005;12(6):308–14. doi: 10.5551/jat.12.308. [DOI] [PubMed] [Google Scholar]
- 219.Leu HB, Chen JW, Wu TC, Ding YA, Lin SJ, Charng MJ. Effects of fluvastatin, an HMG-CoA reductase inhibitor, on serum levels ofinterleukin-18 and matrixmetalloproteinase-9 in patients with hypercholesterolemia. Clin Cardiol. 2005;28(9):423–8. doi: 10.1002/clc.4960280907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Pochetti G, Gavuzzo E, Campestre C, Agamennone M, Tortorella P, Consalvi V, Gallina C, Hiller O, Tschesche H, Tucker PA, Mazza F. Structural insight into the stereoselective inhibition of MMP-8 by enantiomeric sulfonamide phosphonates. J Med Chem. 2006;49(3):923–31. doi: 10.1021/jm050787+. [DOI] [PubMed] [Google Scholar]
- 221.Becker DP, Villamil CI, Barta TE, Bedell LJ, Boehm TL, Decrescenzo GA, Freskos JN, Getman DP, Hockerman S, Heintz R, Howard SC, Li MH, McDonald JJ, Carron CP, Funckes-Shippy CL, Mehta PP, Munie GE, Swearingen CA. Synthesis and structure-activity relationships of beta- and alpha-piperidine sulfone hydroxamic acid matrix metalloproteinase inhibitors with oral antitumor efficacy. J Med Chem. 2005;48(21):6713–30. doi: 10.1021/jm0500875. [DOI] [PubMed] [Google Scholar]
- 222.Mori M, Massaro A, Calderone V, Fragai M, Luchinat C, Mordini A. Discovery of a New Class of Potent MMP Inhibitors by Structure-Based Optimization of the Arylsulfonamide Scaffold. ACS Med Chem Lett. 2013;4(6):565–9. doi: 10.1021/ml300446a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yuan H, Lu W, Wang L, Shan L, Li H, Huang J, Sun Q, Zhang W. Synthesis of derivatives of methyl rosmarinate and their inhibitory activities against matrix metalloproteinase-1 (MMP-1) Eur J Med Chem. 2013;62:148–57. doi: 10.1016/j.ejmech.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 224.Luo Y, Zhang L, Wang WY, Hu QF, Song HP, Su YL, Zhang YZ. Alendronate retards the progression of lumbar intervertebral disc degeneration in ovariectomized rats. Bone. 2013;55(2):439–48. doi: 10.1016/j.bone.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 225.Omote Y, Deguchi K, Kono S, Liu N, Liu W, Kurata T, Yamashita T, Ikeda Y, Abe K. Neurovascular protection of cilostazol in stroke-prone spontaneous hypertensive rats associated with angiogenesis and pericyte proliferation. J Neurosci Res. 2014;92(3):369–74. doi: 10.1002/jnr.23327. [DOI] [PubMed] [Google Scholar]
- 226.Yu BC, Lee DS, Bae SM, Jung WK, Chun JH, Urm SH, Lee DY, Heo SJ, Park SG, Seo SK, Yang JW, Choi JS, Park WS, Choi IW. The effect of cilostazol on the expression of matrix metalloproteinase-1 and type I procollagen in ultraviolet-irradiated human dermal fibroblasts. Life Sci. 2013;92(4–5):282–8. doi: 10.1016/j.lfs.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 227.Ishiguro M, Mishiro K, Fujiwara Y, Chen H, Izuta H, Tsuruma K, Shimazawa M, Yoshimura S, Satoh M, Iwama T, Hara H. Phosphodiesterase-III inhibitor prevents hemorrhagic transformation induced by focal cerebral ischemia in mice treated with tPA. PLoS One. 2010;5(12):e15178. doi: 10.1371/journal.pone.0015178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Devel L, Beau F, Amoura M, Vera L, Cassar-Lajeunesse E, Garcia S, Czarny B, Stura EA, Dive V. Simple pseudo-dipeptides with a P2′ glutamate: a novel inhibitor family of matrix metalloproteases and other metzincins. J Biol Chem. 2012;287(32):26647–56. doi: 10.1074/jbc.M112.380782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Devel L, Garcia S, Czarny B, Beau F, LaJeunesse E, Vera L, Georgiadis D, Stura E, Dive V. Insights from selective non-phosphinic inhibitors of MMP-12 tailored to fit with an S1′ loop canonical conformation. J Biol Chem. 2010;285(46):35900–9. doi: 10.1074/jbc.M110.139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Johnson J, George S, Newby A, Jackson C. Divergent effects of matrix metalloproteinases 3,7,9,12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. PNAS. 2005;103(42):15575–80. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kevorkian L, Young D, Darrah C, Donell S, Shepstone L, Porter S, Brockbank S, Edwards D, Parker A, Clark I. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50(1):131–41. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]