ABSTRACT
The Hippo pathway is an important signaling pathway that controls cell proliferation and apoptosis. It is evolutionarily conserved in mammals and is stimulated by cell–cell contact, inhibiting cell proliferation in response to increased cell density. During early embryonic development, the Hippo signaling pathway regulates organ development and size, and its functions result in the coordinated balance between proliferation, apoptosis, and differentiation. Its principal effectors, YAP and TAZ, regulate signaling by the embryonic stem cells and determine cell fate and histogenesis. Dysfunction of this pathway contributes to cancer development in adults and children. Emerging studies have shed light on the upregulation of Hippo pathway members in several pediatric cancers and may offer prognostic information on rhabdomyosarcoma, osteosarcoma, Wilms tumor, neuroblastoma, medulloblastoma, and other brain gliomas. We review the results of such published studies and highlight the potential clinical application of this pathway in pediatric oncologic and pathologic studies. These studies support targeting this pathway as a novel treatment strategy.
KEYWORDS: Cancer, Hippo, pathways, pediatric, YAP
Introduction
The Hippo pathway is an important signaling pathway that controls cell proliferation and apoptosis. Dysfunction of this pathway often contributes to cancer development. It is stimulated in normal cells by cell–cell contact and inhibits cell proliferation in response to increased cell density. The pathway is evolutionarily conserved in mammals, and plays an important role in tissue homeostasis resulting in coordinated balance between proliferation, apoptosis, and differentiation.1,2 Hippo signaling pathway is activated with the phosphorylation of the large tumor suppressor kinases 1 and 2 (LATS1/2) by the STE-20 protein kinases (MST1/2) leading to activation of downstream members. Protein salvador homolog 1 (Sav1) and Mob kinase activator 1A (Mob1) are regulatory proteins that coordinate the phosphorylation of MST1/2 and LATS1/2 protein kinases, respectively. A principal member and downstream effector, Yes-associated protein (YAP), is a transcriptional coactivator that is phosphorylated and inactivated by the Hippo signaling cascade. Another member, the transcriptional coactivator with PDZ-binding motif (TAZ), shares similar functions to YAP in transcriptional activation and regulation.1-4 YAP and TAZ are phosphorylated by LATS1/2-Mob1 complex at specific amino acid residues. When Hippo signaling is attenuated, the phosphorylation of YAP and TAZ is reduced, leading to their nuclear localization. Inside the nucleus, they bind to one of the DNA-binding TEA domain (TEAD) family of transcription factors and activate target genes that are involved in cell proliferation, survival, and tissue growth (Fig. 1).
Figure 1.
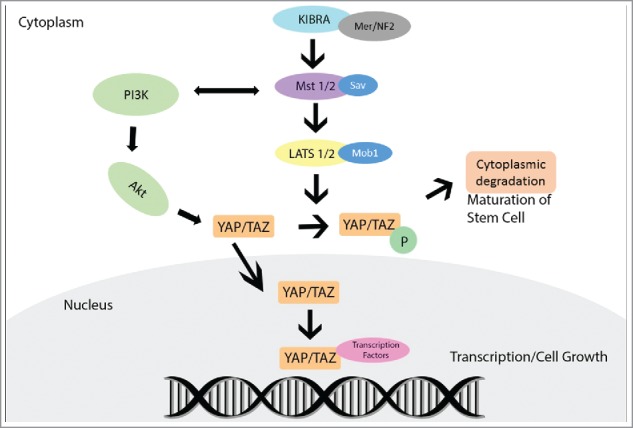
YAP protein interaction, activation, and inactivation within the canonical Hippo Pathway.
The Hippo pathway is regulated by several upstream pathways members. Merlin, a cytoskeletal protein and product of NF2 gene, activates MST, eventually leading to YAP phosphorylation. Another upstream Hippo member, the Kidney and Brain Protein (KIBRA), causes activation of LATS and YAP. Loss of KIBRA expression causes cells to display epithelial to mesenchymal transition features leading to tissue growth, which is concomitant with decreased LATS and YAP phosphorylation.5 The Hippo pathway is also regulated through cross-talk interaction with other intracellular signaling pathways. For example, MST1/2 activity is regulated by RAF-1, a product of the MAPK/ERK pathway, and by the RAS-association domain (RASSF) family proteins, which are implicated as tumor suppressors in many cancers. The phosphoinositide 3-kinase (PI3K) pathway regulates Hippo core components directly or through Protein kinase B (AKT) activation. Both YAP and TAZ interact with and are components of the Wnt signaling where they play a role as transcriptional mediators, similar to Beta-catenin.3,4
The Hippo signaling pathway regulates cell proliferation and apoptosis during organ development and is essential for the accurate formation and maintenance of tissues and organs.6 The nuclear/cytoplasmic distribution of YAP and TAZ is important in embryonic development through regulation of cell polarity and signaling by the embryonic stem cells.7,8 Nuclear localization of YAP and TAZ promotes progenitor cell renewal, facilitates tissue regeneration, and increases proliferation of undifferentiated progenitor cells in the liver, skin, intestines, and heart. Aberrant activation of nuclear TAZ and YAP transcriptional activity results in stem cell proliferation. Shifting of YAP to the cytoplasm results in cellular differentiation and maturation.8,9 Signals mediated by YAP and TAZ are also important in determining cell fate. Hippo pathway members influence mesenchymal stem cells and regulate their subsequent differentiation. Depletion of TAZ was found to promote adipogenesis, and increased nuclear TAZ activity resulted in osteogenesis.10,11 During further organ development and histogenesis, the Hippo pathway becomes essential in regulating final organ and cell size.9,10
The role of Hippo pathway in embryogenesis and organ development portends its importance in the development of many pediatric cancers. In this review, we discuss the role of Hippo pathway in pediatric malignancies with reference to the clinical applications of YAP as a diagnostic and prognostic biomarker in pathology and oncology.
Hippo pathway and cancer
Hippo signaling pathway in stem cells signifies an important role in cancer stem cells and tumor initiation. Dysregulation of Hippo pathway members evokes tumorigenesis in various human adult cancers, including breast, ovary, and liver. Hippo core kinases, MST1/2 and LATS1/2 are often described as tumor suppressors. Other Hippo pathway members, such as KIBRA, can also play a role in the development of cancers.5 However, YAP and TAZ are the main culprits in cancer pathogenesis, mainly through their interaction with the TEAD family of transcription factors.12 The exact mechanism of YAP in cancer development is still under investigation. Many previous studies have reported elevated YAP protein levels in various types of cancer, such as colorectal, gastric, and human hepatocellular carcinoma.12,13 In this context, YAP is often described as an oncogene and its increased expression in cancers correlates with poor prognosis. For example, increased YAP expression is associated with high clinical stage and short overall survival in colorectal and ovarian cancer.13,14 In other contexts, YAP functions as a tumor suppressor while inducing cell apoptosis. This alternate function of YAP has been noted in few morphologic studies of breast and prostate cancer, which have shown that YAP expression is lost in the tumor cells and retained in normal tissues.15,16 YAP has also been noted to induce apoptosis in several hematologic malignancies.17 This dual role as an oncogene as well as tumor suppressor gene makes it similar to FAT atypical cadherin 1, and may be related to its phosphorylation status and nuclear versus cytoplasmic localization as shown in a subset of head and neck squamous cell carcinomas.18,19
Because of its role in embryologic organ development, the Hippo pathway is thought to play a more significant role in the development of pediatric cancer. Many pediatric cancers represent arrest of cellular differentiation at the embryonal level and childhood cancers are frequently associated with congenital malformations, consistent with the notion that disruption of normal embryologic development is linked to oncogenesis.
Rhabdomyosarcoma
Major components of the mammalian Hippo pathway are expressed in fully differentiated skeletal muscles, progenitor cells, myoblasts, and myotubes. Both TAZ and YAP can bind to and function as coactivators of the paired box protein PAX3, an important transcription factor in early muscle development. Unlike canonical Hippo signaling, PAX3-TAZ and PAX3-YAP complex does not require TEAD factors to mediate activity, and PAX3 acts as the DNA-binding moiety.20 During normal muscle development and activation of Hippo kinases, YAP phosphorylation increases and translocates from the nucleus to the cytosol permitting myoblast differentiation.21,22 Nuclear expression of TAZ also increases the expression of myogenic genes and hastens myofiber formation and muscle differentiation through a MYOD1-dependent manner. In pathologic conditions, phosphorylation of TAZ leads to its cytoplasmic retention and delays myogenic differentiation.23 Similarly, YAP is retained in the nucleus, where it promotes satellite cells proliferation, and prevents their differentiation.
Rhabdomyosarcomas are tumors of skeletal muscle origin that exhibit overexpression of the myogenic regulatory proteins, MYOD1 and myogenin. Evidence of disruption of the Hippo pathway in rhabdomyosarcoma has emerged in few studies, principally through dysregulation of YAP. YAP is upregulated and both embryonal and alveolar rhabdomyosarcoma types show high expression of cytoplasmic and nuclear YAP protein. In alveolar rhabdomoyosarcoma (aRMS), PAX3-FOXO1 fusion transcript supports tumor initiation by upregulation of RASSF4. Enhanced RASSF4 expression in PAX3-FOXO1 fusion positive aRMS cell lines and tumors promotes tumorigenesis through inhibition of the Hippo pathway tumor suppressor MST1 and subsequent increased activity of YAP.24,25 YAP overexpression was found in RAS-driven rhabdomyosarcoma, where it supports cell growth, proliferation, and survival in vitro.24 YAP-deficient alveolar rhabdomyosarcoma cells were found to be significantly less proliferative than control cells.26 Additional data have also suggested that TAZ may play a role, albeit less prominent than that of YAP, in the pathogenesis of aRMS.23
YAP overexpression is also implicated as a key factor in the development of embryonal rhabdomyosarcoma (eRMS). A recent study using multiple genetically engineered mice has demonstrated that YAP protein overexpression transforms activated satellite cells leading to the development of embryonal rhabdomyosarcoma-like tumors.26 YAP combines with TEAD1 to upregulate pro-proliferative and oncogenic genes and maintain eRMS tumorigenesis by blocking MYOD1 activities. TAZ is also overexpressed in eRMS where it functions as an oncogene independent from YAP and activates the expression of other cancer-related genes. Knockdown of TAZ in human eRMS cell lines reduces their proliferation and anchorage-independent growth.23
Expression of YAP and TAZ in clinical cases of rhabdomyosarcoma signifies their potential applications as diagnostic and prognostic biomarkers. Both proteins show slightly more expression in eRMS than in aRMS. In immunohistochemical experiments of tissue microarray samples, YAP and TAZ are detected in 87% and 55% of eRMS and in 72% and 36% of aRMS respectively.23,26 Activity of both proteins is related to poor patient prognosis and adverse clinical outcome. In eRMS, YAP protein expression correlates with advanced clinical stage and its gene expression correlates with reduced survival.26 TAZ expression is also associated with short survival in eRMS.23 In this aspect, blocking or modulating YAP or TAZ activity may be considered as potential therapy for rhabdomyosarcoma.27
Ewing's sarcoma
Sarcomas in general are hypothesized to develop from mesenchymal stem/progenitor cells, which are able to differentiate into many cell types and give rise to several adult human tumors.28,29 Hippo pathways members are expressed in mesenchymal stem cells where they regulate their differentiation and fate.30 Thus, it is understandable that dysregulations of the Hippo pathway occur in many bone and soft tissue sarcomas.31,32 However, only a few oncogenic mutations are found in the Hippo pathway that result in its dysregulation. Instead, the most likely cause of perturbed Hippo signaling in sarcoma is the cross-talk with commonly mutated cancer genes such as KRAS, PIK3CA, CTNNB1, or FBXW7.33 In tissue microarray samples of human sarcomas, both YAP and TAZ are expressed in variety of tumors, including Ewing's sarcoma.31 YAP expression in Ewing's sarcoma is positively correlated with the expression of polycomb protein (BMI-1), which promotes the tumorigenicity of Ewing's sarcoma. YAP expressions levels are maintained and do not diminish in confluent Ewing's sarcoma tumor cells that express high levels of BMI-1. In contrast, YAP expression and nuclear localization are reduced in confluent BMI-1 knockdown cells, suggesting that silencing of BMI-1 restores contact inhibition by resuming normal activation of the Hippo-YAP growth-suppressor pathway.34 YAP nuclear expression is also documented in clinical human samples of Ewing's sarcoma, but this expression does not affect the patient's survival.35 These facts suggest that YAP expression may not have a major diagnostic role in Ewing's sarcoma but may be useful in selecting cases for YAP-targeted therapy. Other canonical Hippo pathway members have not yet been studied. However, the promoter regions of RASSF1A and RASSF2, which are closely related to the Hippo pathway, are found in a hypermethylated state in Ewing's sarcoma in correlation with worse clinical outcome.
Osteosarcoma
The Hippo pathway and its downstream targets help regulate proliferation of immature osteoblasts and their maturation into nonproliferating mature osteoblasts. It also regulates chondrocyte differentiation.36,37 Thus, aberrations in the Hippo signaling are proving to be important in the biology of osteosarcoma. These aberrations partly occur through the upregulation of Hedgehog (HH) signaling leading to increased expression of YAP and partly through upregulation of the sex-determining region Y-box 2 (Sox2) leading to inhibition of Mer/NF2 and KIBRA. Inhibition of HH signaling reduces YAP expression, and knockdown of YAP significantly inhibits osteosarcoma tumor progression and decreases cell proliferation and invasion.36,37 Sox2 is highly expressed in osteosarcoma where it maintains cancer stem cells.37,38 It disrupts the Hippo pathway in these cells through the inhibition of Mer/NF2 and KIBRA leading to increased YAP expression. Expression of Mer/NF2 in osteosarcoma cell lines leads to a more differentiated phenotype associated with the depletion of Sox2. Thus, in contrast to cancer stem cells, the more differentiated tumor cells are characterized by the expression of Mer/NF2 and decreased Sox2 and YAP expression. TAZ is also elevated in osteosarcoma cells, independent of Sox2, and stimulates osteogenic differentiation through Runx2 activation.38
Of the Hippo pathways members, only YAP and TAZ have been studied in human osteosarcoma tissues. A tissue microarray analysis has revealed high YAP protein expression in osteosarcoma when compared to the surrounding noncancerous tissue, and the expression correlates with advanced clinical stage.37 In another immunohistochemical study, both YAP and TAZ showed expression in up to 60% of osteosarcoma cases and that nuclear expression of these proteins served as independent prognostic factor.39 These experiments hint to the potential role of YAP and TAZ as prognostic indicators in osteosarcoma.
Neuroblastoma
YAP is expressed in early neural crests and regulates their phenotype and migration. YAP expression subsequently decreases upon maturation and differentiation of neural crest cells. Neuroblastoma is a common pediatric malignancy that arises from neural crests, and thus it is reasonable to extrapolate that Hippo pathway members are overexpressed in this tumor.40 YAP and TAZ activation has been described in neuroblastoma and shows a positive correlation with adverse prognostic features. Mutations of PTPN14, which encode a negative regulator of YAP, have been identified at neuroblastoma relapse.41 Migratory and invasive properties of human neuroblastoma cells have been found to be associated with high expression levels of TAZ. There is evidence that TAZ promotes epithelial to mesenchymal transition and metastasis of neuroblastoma.42 Although these experiments have suggested a potential role of YAP and TAZ as therapeutic targets, no experiments have been conducted so far on the immunohistochemical expression of these proteins in the different subtypes of clinical neuroblastoma cases.
Liver cancer
The mammalian Hippo pathway is thought to play an important role in the regulation of liver progenitor cells.43 Ablation of this pathway in mice has induced YAP expression and its localization to the nucleus, thus permitting hepatic tumorigenesis. Several studies have documented dysregulation of various Hippo members liver cancer, particularly in hepatocellular carcinoma (HCC). In a recent study on human HCC tissue, increased expression of YAP and downregulation of LATS were observed. Inhibition of LATS or MST1/2 has attenuated YAP phosphorylation and significantly increased YAP nuclear accumulation.43 In turn, the upregulation of the YAP inside the nucleus and its association with TEAD2 has led to transcriptional activation and cell invasion in HCC cells. Increase in YAP activity (nuclear localization and decreased phosphorylation) has also resulted in increased cell proliferation and HCC formation in mice.44 YAP expression by immunohistochemistry has been noted in adult and pediatric HCC clinical cases.45-48 Two recent studies have revealed YAP and TAZ are expressed in 60-70% of HCC cases and their expression has correlated with clinical stage and high serum alpha-fetoprotein levels.45,46 In a study of seven pediatric HCC cases, increase of YAP expression is identified at the protein and mRNA transcript levels.47 Increased YAP expression and activity is also noted in pediatric hepatoblastoma, where nuclear expression of the protein is noted in up to 73% of cases.45 However, no prognostic studies have yet shed light on the significance of YAP expression in pediatric liver tumors.
Wilms tumors
Genome-wide analysis has unraveled a potential role of the Hippo signaling system in kidney development through the regulation of the transcription factor WT-1.49,50 YAP expression in wild-type developing kidneys is found in the nephron progenitor cells, ureteric bud, and stroma. There is evidence to suggest that Fat4 from the stromal fibroblasts may drive phosphorylation of YAP in the progenitor cells. In the absence of this FAT4 signal, dephosphorylated YAP resides in the nucleus, which leads to increased proliferation and expansion of the progenitor cells.
Wilms tumor is an embryonic kidney tumor in young children that harbors WT-1 mutations in most cases. YAP is expressed in Wilms tumor cell lines and clinical tissue specimens in both the cytoplasm and the nucleus. Immunoblotting experiments have revealed that YAP and its phosphorylated counterpart (p-YAP) vary in content across 40 clinical Wilms tumor specimens. Tumors with unfavorable histology (i.e., anaplastic) have shown a 5.2-fold greater p-YAP content than those with favorable histology.50 Supportive immunohistochemical studies on Wilms tumor tissue still have to be published.
Brain tumors
The role of Hippo pathway in brain development is unclear. Deletion of TAZ and YAP in pre-migratory neural crest has resulted in craniofacial defects suggesting a role of this pathway in normal brain development.51 In normal brains, YAP was undetectable in neurons, but expressed in neural stem cells and astrocytes. At least in mice, there is evidence for YAP regulation of neocortical astrocytic differentiation and proliferation.51 These facts attests to the importance of this pathway in pediatric brain tumors, particularly gliomas where they are widely expressed.
Medulloblastoma
Sonic Hedgehog (HH) interacts with the Hippo pathway in a subset of medulloblastomas, leading to an upregulation and nuclear localization of YAP.52 Medulloblastoma falls into four distinct molecular subgroups, and YAP immunohistochemical nuclear staining is clinically validated and accepted as a surrogate marker for the classification of a medulloblastoma into the HH tumor subgroup. The HH subgroup tumors (YAP-positive) have a very good prognosis in infants and an intermediate prognosis in all other age groups.53,54 Though YAP nuclear expression has been noted in all histological subtypes of medulloblastomas, desmoplastic nodular medulloblastomas have a particularly high proportion of cases with YAP immunopositivity. The staining in desmoplastic nodular medulloblastomas is also peculiar in that it is highly expressed in the internodular areas and to a much lesser extent in the intranodular regions (Fig. 2).
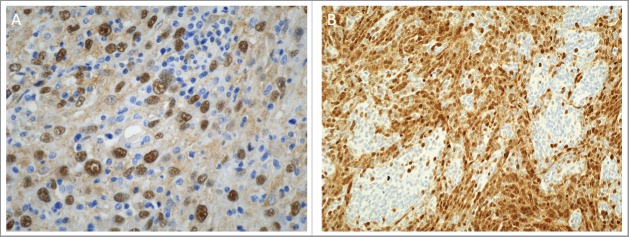
Figure 2.
Potential applications of YAP immunohistochemistry in clinical practice as a diagnostic or a prognostic marker. Immunohistochemistry for YAP was performed at The Children's Mercy Hospital, Kansas City, Missouri on the Leica Bond instrument using an antibody against the nonphosphorylated protein (Santa Cruz, Dallas, Texas). A. Nuclear staining for YAP is identified in embryonal rhabdomyosarcoma cells, while the infiltrating lymphocytes are negative (x400). B. YAP immunoreactivity in nodular desmoplastic medulloblastoma. Nuclear staining is present in the internodular region (x200).
Astrocytomas
A recent study has determined that YAP is expressed in astrocytomas that span the entire World Health Organization (WHO) grading range.53,54 It is frequently expressed in infiltrating astrocytoma and oligodendroglioma and less often in pilocytic astrocytoma. Pilocytic astrocytomas show variable staining in 30% of cases, and only 13% show a high level of nuclear staining. Other WHO II-WHO IV astrocytomas exhibit a higher degree of nuclear staining. As the WHO grade increases, from WHO II to WHO IV, YAP expression is increased. Additionally, pediatric glioblastoma cases are less likely to show a high degree of nuclear staining when compared to adult glioblastomas (40% vs. 66%). Furthermore, diffuse astrocytic tumors with high expression of YAP are associated with a reduction in overall survival when compared to similar tumors without high expression of YAP.55,56 In vitro experiments have shown that YAP signaling enhances glioblastoma growth and invasiveness implying that targeting this protein may offer a potential therapeutic option.57
Other brain tumors
Recently, YAP immunoreactivity was reported in a study to have strong nuclear staining in atypical teratoid rhabdoid tumors, medulloepitheliomas, and primitive neurectodermal tumors. This study included only a small number of these tumor subtypes. Further investigation is needed to determine the prevalence of YAP immunostaining in these cases.56
A recent study has highlighted the expression of TAZ in retinoblastoma in 65% of clinical cases. High TAZ expression in this tumor was found to be prognostically significant, associated with shorter overall survival and disease-free survival rates.58 Although no studies were done on clinical cases of germ cell tumors, there is a preliminary evidence suggesting that MST1/MST2 are required for teratoma formation, at least in mice.59
Hematologic malignancies
The role and significance of Hippo pathway in hematopoiesis are not well understood. A study using transgenic mouse model has shown that overexpression of YAP within the hematopoietic system has no effect on hematopoiesis or hematopoietic stem cell functions.60,61 MST1 and MST2, the core components of Hippo pathway, seem to act as a switch between self-renewal and differentiation in primitive hematopoiesis in one study using Xenopus model.62
The deregulation of Hippo pathway and its role in the pathogenesis of hematopoietic malignancies have been studied by several groups, but their results were not consistent. One study has shown that YAP can trigger DNA damage and induce apoptosis in myeloma and other leukemia/lymphoma cells and that low YAP levels prevent nuclear ABL1–induced apoptosis.17 Knockdown of MST1 results in increased YAP expression and triggers cell death.17 YAP has been found to be consistently downregulated in human hematologic cancers.63 One study has shown that loss of MST1 in mice enhances lymphoma development, and that MST1 is frequently decreased in clinical specimens of lymphoma and leukemia.64 In other studies, the expression levels of MST1/2 and YAP in hematologic malignancies are not significantly different from normal controls.65,66 The discrepancy of the results may reflect the etiologic and pathologic heterogeneity of the hematologic malignancies. Further studies with larger sample size and more components of this pathway should be performed in this area.
Conclusions
Hippo pathway members are dysregulated in various pediatric tumors, attesting to the importance of this pathway in the development of these embryonal cancers. Of all Hippo members, YAP is the most commonly studied protein on clinical cases of pediatric tumors. Evidence indicates that YAP expression affects patients' prognosis in rhabdomyosarcoma, osteosarcoma, HCC, Wilms tumor, and brain tumors. More useful clinical applications of YAP and other pathway members can be envisioned in the diagnosis and prognosis of pediatric tumors and await further research. Because of their wide expression, Hippo pathway members, particularly YAP, are potential novel treatment targets for those tumors that show overexpression.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to acknowledge the Department of Pathology of Children's Mercy Hospital for allowing us to use its facilities during preparation of this manuscript.
Author contributions
All authors have participated in the design, conceptualization, and write-up of the article. All authors have read the manuscript and approved its submission.
References
- 1.Bao Y, Hata Y, Ikeda M, Withanage K. Mammalian Hippo pathway: From development to cancer and beyond. J Biochem 2011; 149:361-79; PMID:21324984; https://doi.org/ 10.1093/jb/mvr021 [DOI] [PubMed] [Google Scholar]
- 2.Kodaka M, Hata Y. The mammalian Hippo pathway: Regulation and function of YAP1 and TAZ. Cell Mol Life Sci 2015; 72:285-306; PMID:25266986; https://doi.org/ 10.1007/s00018-014-1742-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae JS, Kim SM, Lee H. The Hippo signaling pathway provides novel anti-cancer drug targets. Oncotarget 2016; [Epub ahead of print] PMID:28035075; https://doi.org/ 10.18632/oncotarget.14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev 2014; 94:1287-312; PMID:25287865; https://doi.org/ 10.1152/physrev.00005.2014 [DOI] [PubMed] [Google Scholar]
- 5.Moleirinho S, Chang N, Sims AH, Tilston-Lünel AM, Angus L, Steele A, Boswell V, Barnett SC, Ormandy C, Faratian D, et al.. KIBRA exhibits MST-independent functional regulation of the Hippo signaling pathway in mammals. Oncogene 2013; 32:1821-30; PMID:22614006; https://doi.org/ 10.1038/onc.2012.196 [DOI] [PubMed] [Google Scholar]
- 6.Varelas X. The Hippo pathway effectors TAZ and YAP in development, hemostasis and disease. Development 2014; 141:1614-26; PMID:24715453; https://doi.org/ 10.1242/dev.102376 [DOI] [PubMed] [Google Scholar]
- 7.Hiemer SE, Varelas X. Stem cell regulation by the Hippo pathway. Biochim Biophys Acta 2013; 1830:2323-34; PMID:22824335; https://doi.org/ 10.1016/j.bbagen.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 8.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 2011; 13:877-83; PMID:21808241; https://doi.org/ 10.1038/ncb2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange AW, Sridharan A, Xu Y, Stripp BR, Perl AK, Whitsett JA. Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. J Mol Cell Biol 2015; 7:35-47; PMID:25480985; https://doi.org/ 10.1093/jmcb/mju046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: New connections between regulation of organ size and cancer. Curr Opin Cell Biol 2008; 20:638-46; PMID:18955139; https://doi.org/ 10.1016/j.ceb.2008.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, et al.. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 2005; 309:1074-8; PMID:16099986; https://doi.org/ 10.1126/science.1110955 [DOI] [PubMed] [Google Scholar]
- 12.Zhang K, Qi HX, Hu ZM, Chang YN, Shi ZM, Han XH, Han YW, Zhang RX, Zhang Z, Chen T, et al.. YAP and TAZ take center stage in cancer. Biochemistry 2015; 54:6555-66; PMID:26465056; https://doi.org/ 10.1021/acs.biochem.5b01014 [DOI] [PubMed] [Google Scholar]
- 13.Sun Z, Xu R, Li X, Ren W, Ou C, Wang Q, Zhang H, Zhang X, Ma J, Wang H, et al.. Prognostic value of yes-associated protein 1 (YAP1) in various cancers: A meta-analysis. PLoS One 2015; 10:e0135119; PMID:26263504; https://doi.org/ 10.1371/journal.pone.0135119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wierzbicki PM, Rybarczyk A. The Hippo pathway in colorectal cancer. Folia Histochem Cytobiol 2015; 53:105-19; PMID:26160682; https://doi.org/ 10.5603/FHC.a2015.0015 [DOI] [PubMed] [Google Scholar]
- 15.Hu X, Jia Y, Yu J, Chen J, Fu Q. Loss of YAP protein in prostate cancer is associated with Gleason score increase. Tumori 2015; 101:189-93; PMID:25908048; https://doi.org/ 10.5301/tj.5000238 [DOI] [PubMed] [Google Scholar]
- 16.Jaramillo-Rodríguez Y, Cerda-Flores RM, Ruiz-Ramos R, López-Márquez FC, Calderón-Garcidueñas AL. YAP expression in normal and neoplastic breast tissue: An immunohistochemical study. Arch Med Res 2014; 45:223-8; PMID:24606817; https://doi.org/ 10.1016/j.arcmed.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 17.Cottini F, Hideshima T, Xu C, Sattler M, Dori M, Agnelli L, ten Hacken E, Bertilaccio MT, Antonini E, Neri A, et al.. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat Med 2014; 20:599-606; PMID:24813251; https://doi.org/ 10.1038/nm.3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Du YC, Zhou XJ, Liu H, Tang SC. The dual functions of YAP-1 to promote and inhibit cell growth in human malignancy. Cancer Metastasis Rev 2014; 33:173-81; PMID:24346160; https://doi.org/ 10.1007/s10555-013-9463-3 [DOI] [PubMed] [Google Scholar]
- 19.Ehsanian R, Brown M, Lu H, Yang XP, Pattatheyil A, Yan B, Duggal P, Chuang R, Doondeea J, Feller S, et al.. YAP dysregulation by phosphorylation or ΔNp63-mediated gene repression promotes proliferation, survival and migration in head and neck cancer subsets. Oncogene 2010; 29:6160-71; PMID:20729916; https://doi.org/ 10.1038/onc.2010.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami M, Tominaga J, Makita R, Uchijima Y, Kurihara Y, Nakagawa O, Asano T, Kurihara H. Transcriptional activity of Pax3 is co-activated by TAZ. Biochem Biophys Res Commun 2006; 339:533-9; PMID:16300735; https://doi.org/ 10.1016/j.bbrc.2005.10.214 [DOI] [PubMed] [Google Scholar]
- 21.Watt KI, Judson R, Medlow P, Reid K, Kurth TB, Burniston JG, Ratkevicius A, De Bari C, Wackerhage H. Yap is a novel regulator of C2C12 myogenesis. Biochem Biophys Res Commun 2010; 393:619-24; PMID:20153295; https://doi.org/ 10.1016/j.bbrc.2010.02.034 [DOI] [PubMed] [Google Scholar]
- 22.Judson RN, Tremblay AM, Knopp P, White RB, Urcia R, De Bari C, Zammit PS, Camargo FD, Wackerhage H. The Hippo pathway member Yap plays a key role in influencing fate decisions in muscle satellite cells. J Cell Sci 2012; 125:6009-19; PMID:23038772; https://doi.org/ 10.1242/jcs.109546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamed A, Sun C, De Mello V, Selfe J, Missiaglia E, Shipley J, Murray GI, Zammit PS, Wackerhage H. The Hippo effector TAZ (WWTR1) transforms myoblasts and its abundance is associated with reduced survival in embryonal rhabdomyosarcoma. J Pathol 2016; 240:3-14; PMID:27184927; https://doi.org/ 10.1002/path.4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slemmons KK, Crose LE, Rudzinski E, Bentley RC, Linardic CM. Role of the YAP oncoprotein in priming Ras-driven rhabdomyosarcoma. PLoS One 2015; 10:e0140781; PMID:26496700; https://doi.org/ 10.1371/journal.pone.0140781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crose LE, Galindo KA, Kephart JG, Chen C, Fitamant J, Bardeesy N, Bentley RC, Galindo RL, Chi JT, Linardic CM. Alveolar rhabdomyosarcoma-associated PAX3-FOXO1 promotes tumorigenesis via Hippo pathway suppression. J Clin Invest 2014; 124:285-96; PMID:24334454; https://doi.org/ 10.1172/JCI67087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tremblay AM, Missiaglia E, Galli GG, Hettmer S, Urcia R, Carrara M, Judson RN, Thway K, Nadal G, Selfe JL, et al.. The Hippo transducer YAP1 transforms activated satellite cells and is a potent effector of embryonal rhabdomyosarcoma formation. Cancer Cell 2014; 26:273-87; PMID:25087979; https://doi.org/ 10.1016/j.ccr.2014.05.029 [DOI] [PubMed] [Google Scholar]
- 27.Svalina MN, Keller C. YAPping about differentiation therapy in muscle cancer. Cancer Cell 2014; 26:154-5; PMID:25117705; https://doi.org/ 10.1016/j.ccr.2014.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lye KL, Nordin N, Vidyadaran S, Thilakavathy K. Mesenchymal stem cells: From stem cells to sarcomas. Cell Biol Int 2016; 40:610-8; PMID:26992453; https://doi.org/ 10.1002/cbin.10603 [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Ren Z, Du X, Hao M, Zhou W. The role of mesenchymal stem/progenitor cells in sarcoma: Update and dispute. Stem Cell Investig 2014; 1:18; PMID:27358864; https://doi.org/ 10.3978/j.issn.2306-9759.2014.10.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzeng HH, Hsu CH, Chung TH, Lee WC, Lin CH, Wang WC, Hsiao CY, Leu YW, Wang TH. Cell signaling and differential protein expression in neuronal differentiation of bone marrow mesenchymal stem cells with hypermethylated Salvador/Warts/Hippo (SWH) pathway genes. PLoS One 2015; 10:e0145542; PMID:26713735; https://doi.org/ 10.1371/journal.pone.0145542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fullenkamp CA, Hall SL, Jaber OI, Pakalniskis BL, Savage EC, Savage JM, Ofori-Amanfo GK, Lambertz AM, Ivins SD, Stipp CS, et al.. TAZ and YAP are frequently activated oncoproteins in sarcomas. Oncotarget 2016; 7:30094-108; PMID:27129148; https://doi.org/ 10.18632/oncotarget.8979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisinger-Mathason TS, Mucaj V, Biju KM, Nakazawa MS, Gohil M, Cash TP, Yoon SS, Skuli N, Park KM, Gerecht S, et al.. Deregulation of the Hippo pathway in soft-tissue sarcoma promotes FOXM1 expression and tumorigenesis. Proc Natl Acad Sci USA 2015; 112:E3402-11; PMID:26080399; https://doi.org/ 10.1073/pnas.1420005112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohamed AD, Tremblay AM, Murray GI, Wackerhage H. The Hippo signal transduction pathway in soft tissue sarcomas. Biochim Biophys Acta 2015; 1856:121-9; PMID:26050962; https://doi.org/ 10.1016/j.bbcan.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 34.Hsu JH, Lawlor ER. BMI-1 suppresses contact inhibition and stabilizes YAP in Ewing sarcoma. Oncogene 2011; 30:2077-85; PMID:21170084; https://doi.org/ 10.1038/onc.2010.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed AA, Abedalthagafi M, Anwar AE, Bui MM. Akt and Hippo pathways in Ewing's sarcoma tumors and their prognostic significance. J Cancer 2015; 6:1005-10; PMID:26366214; https://doi.org/ 10.7150/jca.12703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mortus JR, Zhang Y, Hughes DP. Developmental pathways hijacked by osteosarcoma. Adv Exp Med Biol 2014; 804:93-118; PMID:24924170; https://doi.org/ 10.1007/978-3-319-04843-7_5 [DOI] [PubMed] [Google Scholar]
- 37.Chan LH, Wang W, Yeung W, Deng Y, Yuan P, Mak KK. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 over-expression. Oncogene 2014; 33:4857-66; PMID:24141783; https://doi.org/ 10.1038/onc.2013.433 [DOI] [PubMed] [Google Scholar]
- 38.Basu-Roy U, Bayin NS, Rattanakorn K, Han E, Placantonakis DG, Mansukhani A, Basilico C. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Commun 2015; 6:6411; PMID:25832504; https://doi.org/ 10.1038/ncomms7411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouvier C, Macagno N, Nguyen Q, Loundou A, Jiguet-Jiglaire C, Gentet JC, Jouve JL, Rochwerger A, Mattei JC, Bouvard D, et al.. Prognostic value of the Hippo pathway transcriptional coactivators YAP/TAZ and β1-integrin in conventional osteosarcoma. Oncotarget 2016; 7(40):64702-10; PMID:27608849; https://doi.org/ 10.18632/oncotarget.11876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hindley CJ, Condurat AL, Menon V, Thomas R, Azmitia LM, Davis JA, Pruszak J. The Hippo pathway member YAP enhances human neural crest cell fate and migration. Sci Rep 2016; 6:23208; PMID:26980066; https://doi.org/ 10.1038/srep23208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schramm A, Köster J, Assenov Y, Althoff K, Peifer M, Mahlow E, Odersky A, Beisser D, Ernst C, Henssen AG, et al.. Mutational dynamics between primary and relapse neuroblastomas. Nat Genet 2015; 47:872-7; PMID:26121086; https://doi.org/ 10.1038/ng.3349 [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, Xu Z, An Q, Jiang D, Wang L, Liang B, Li Z. TAZ promotes epithelial to mesenchymal transition via the upregulation of connective tissue growth factor expression in neuroblastoma cells. Mol Med Rep 2015; 11:982-8; PMID:25354978; https://doi.org/ 10.3892/mmr.2014.2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, et al.. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA 2010; 107:8248-53; PMID:20404163; https://doi.org/ 10.1073/pnas.0912203107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo C, Wang X, Liang L. LATS2-mediated YAP1 phosphorylation is involved in HCC tumorigenesis. Int J Clin Exp Pathol 2015; 8:1690-7; PMID:25973055 [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Wolfe A, Septer S, Edwards G, Zhong X, Abdulkarim AB, Ranganathan S, Apte U. Deregulation of Hippo kinase signaling in human hepatic malignancies. Liver Int 2012; 32:38-47; PMID:22098159; https://doi.org/ 10.1111/j.1478-3231.2011.02646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han SX, Bai E, Jin GH, He CC, Guo XJ, Wang LJ, Li M, Ying X, Zhu Q. Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J Immunol Res 2014; 2014:261365; PMID:24860833; https://doi.org/ 10.1155/2014/261365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaQuaglia MJ, Grijalva JL, Mueller KA, Perez-Atayde AR, Kim HB, Sadri-Vakili G, Vakili K. YAP subcellular localization and Hippo pathway transcriptome analysis in pediatric hepatocellular carcinoma. Sci Rep 2016; 6:30238; PMID:27605415; https://doi.org/ 10.1038/srep30238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer 2009; 115(19):4576-85; PMID:19551889; https://doi.org/ 10.1002/cncr.24495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kann M, Ettou S, Jung YL, Lenz MO, Taglienti ME, Park PJ, Schermer B, Benzing T, Kreidberg JA. Genome-wide analysis of Wilms' tumor 1-controlled gene expression in podocytes reveals key regulatory mechanisms. J Am Soc Nephrol 2015; 26:2097-104; PMID:25636411; https://doi.org/ 10.1681/ASN.2014090940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy AJ, Pierce J, de Caestecker C, Libes J, Neblett D, de Caestecker M, Perantoni AO, Tanigawa S, Anderson JR, Dome JS, et al.. Aberrant activation, nuclear localization, and phosphorylation of Yes-associated protein-1 in the embryonic kidney and wilms tumor. Pediatr Blood Cancer 2014; 61:198-205; PMID:24115727; https://doi.org/ 10.1002/pbc.24788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Xiao Y, Hsu CW, Martinez-Traverso IM, Zhang M, Bai Y, Ishii M, Maxson RE, Olson EN, Dickinson ME, et al.. Yap and Taz play a crucial role in neural crest-derived craniofacial development. Development 2016; 143:504-15; PMID:26718006; https://doi.org/ 10.1242/dev.126920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Z, Hu J, Pan J, Wang Y, Hu G, Zhou J, Mei L, Xiong WC. YAP stabilizes SMAD1 and promotes BMP2-induced neocortical astrocytic differentiation. Development 2016; 143:2398-409; PMID:27381227; https://doi.org/ 10.1242/dev.130658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandez-L A, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, Taylor MD, Kenney AM. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates sonic hedgehog-driven neural precursor proliferation. Genes Dev 2009; 23:2729-41; PMID:19952108; https://doi.org/ 10.1101/gad.1824509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, et al.. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol 2012; 123:465-72; PMID:22134537; https://doi.org/ 10.1007/s00401-011-0922-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, et al.. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 2011; 29:1408-14; PMID:20823417; https://doi.org/ 10.1200/JCO.2009.27.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu YC, Wang YZ. Role of yes-associated protein 1 in gliomas: Pathologic and therapeutic aspects. Tumour Biol 2015; 36:2223-7; PMID:25750037; https://doi.org/ 10.1007/s13277-015-3297-2 [DOI] [PubMed] [Google Scholar]
- 57.Orr BA, Bai H, Odia Y, Jain D, Anders RA, Eberhart CG. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J Neuropathol Exp Neurol 2011; 70:568-77; PMID:21666501; https://doi.org/ 10.1097/NEN.0b013e31821ff8d8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Artinian N, Cloninger C, Holmes B, Benavides-Serrato A, Bashir T, Gera J. Phosphorylation of the Hippo pathway component AMOTL2 by the mTORC2 kinase promotes YAP signaling, resulting in enhanced glioblastoma growth and invasiveness. J Biol Chem 2015; 290(32):19387-401; PMID:25998128; https://doi.org/ 10.1074/jbc.M115.656587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Xue C, Cui H, Huang Z. High expression of TAZ indicates a poor prognosis in retinoblastoma. Diagn Pathol 2015; 10:187; PMID:26464030; https://doi.org/ 10.1186/s13000-015-0415-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li P, Chen Y, Mak KK, Wong CK, Wang CC, Yuan P. Functional role of Mst1/Mst2 in embryonic stem cell differentiation. PLoS One 2013; 8(11):e79867; PMID:24224013; https://doi.org/ 10.1371/journal.pone.0079867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jansson L, Larsson J. Normal hematopoietic stem cell function in mice with enforced expression of the Hippo signaling effector YAP1. PLoS One 2012; 7(2):e32013; PMID:22363786; https://doi.org/ 10.1371/journal.pone.0032013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nejigane S, Takahashi S, Haramoto Y, Michiue T, Asashima M. Hippo signaling components, Mst1 and Mst2, act as a switch between self-renewal and differentiation in Xenopus hematopoietic and endothelial progenitors. Int J Dev Biol 2013; 57(5):407-14; PMID:23873372; https://doi.org/ 10.1387/ijdb.130010st [DOI] [PubMed] [Google Scholar]
- 63.Cottini F, Anderson KC, Tonon G. Awakening the Hippo co-activator YAP1, a mercurial cancer gene, in hematologic cancers. Mol Cell Oncol 2014; 1(3):e970055; PMID:27308358; https://doi.org/ 10.4161/23723548.2014.970055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim TS, Lee DH, Kim SK, Shin SY, Seo EJ, Lim DS. Mammalian sterile 20–like kinase 1 suppresses lymphoma development by promoting faithful chromosome segregation. Cancer Res 2012; 72(20):5386-95; PMID:22926556; https://doi.org/ 10.1158/0008-5472.CAN-11-3956 [DOI] [PubMed] [Google Scholar]
- 65.Safari S, Movafagh A, Zare-Adollahi D, Ghadiani M, Riazi-Isfahani S, Safavi-Naini N, Omrani MD. MST1/2 and YAP1 gene expression in acute myeloid leukemia. Leuk Lymphoma 2014; 55(9):2189-91; PMID:24303784; https://doi.org/ 10.3109/10428194.2013.867493 [DOI] [PubMed] [Google Scholar]
- 66.Machado-Neto JA, de Melo Campos P, Olalla Saad ST, Traina F. YAP1 expression in myelodysplastic syndromes and acute leukemias. Leuk Lymphoma 2014; 55(10):2413-5; PMID:24605912; https://doi.org/ 10.3109/10428194.2014.891028 [DOI] [PubMed] [Google Scholar]