Abstract
It has been postulated that one of the biggest impediments to a successful chemotherapy is the phenomena of multidrug resistance (MDR) in cancer cells. One of the main mechanisms of MDR is overexpression of the ATP-binding cassette (ABC) transporters in cancer cells which alters absorption, distribution, metabolism and excretion of various chemotherapeutic drugs. Efforts have been made to find effective inhibitors of ABC transporters. However, none has been approved clinically. This study shows that a novel compound 3-chloro-N-(2-hydroxyphenyl)-4-(3,3,3-trifluoro-2-hydroxy-2-methylpropanamido) benzamide (compound 7d), one of the 2-trifluoromethyl-2-hydroxypropionamide derivatives could reverse ABCG2 (BCRP)-mediated MDR. Cytotoxicity studies show that compound 7d sensitizes the ABCG2-overexpressing cells to chemotherapeutic drugs mitoxantrone and SN-38, which are well-established substrates of the ABCG2 transporter. Western blotting results indicate that compound 7d does not significantly alter the protein level of the ABCG2 transporter. Accumulation and efflux studies demonstrate that compound 7d increases intracellular accumulation of mitoxantrone by inhibiting the function of ABCG2. Overall, these findings indicate a potential use for compound 7d as an adjuvant agent for chemotherapy to inhibit the function of the clinically relevant ABC transporter and sensitize tumor cells to chemotheraputic drugs.
Keywords: 2-trifluoromethyl-2-hydroxypropionamide, ABCG2, multidrug resistance, ABC transporter
INTRODUCTION
Cancer chemotherapy becomes increasingly complicated because of the presence of multidrug resistance (MDR) [Gottesman, 2002]. MDR occurs due to the expression of ATP-binding cassette (ABC) transporters in cancer cells [Gottesman et al., 2002; Kathawala et al., 2015]. Current studies show that there are 48 human ABC transporter family members that are divided into 7 subfamilies from A to G according to the organization of their nucleotide-binding folds (NBFs) and 14 of them confers MDR [Dean et al., 2001; Tiwari et al., 2011]. Among those, ABC transporter subfamily B member 1 (ABCB1/Pgp), ABC transporter subfamily G member 2 (ABCG2/BCRP), and ABC transporter subfamily C member 1 (ABCC1/MRP1) have been reported as major factors in mediating resistance to certain anticancer drugs [Chen and Tiwari, 2011; Sodani et al., 2012]. ABCB1 was the first human ABC drug transporter to be discovered and it transported various chemotheraputic drugs such as Vinca alkaloids, anthracyclines, epipodophyllotoxins, and taxanes out of cells via an energy dependent process utilizing ATP [Gottesman et al., 2002; Juliano and Ling, 1976]. Similar to ABCB1, ABCC1 was discovered as another important transporter that conferred resistance to a range of chemotherapeutic substrates including anthracyclines, epipodophyllotoxins, Vinca alkaloids and taxanes [Cole et al., 1992; Sodani et al., 2012]. Subsequently, ABCG2 was identified that conferred resistance to diverse range of chemotherapeutics such as doxorubicin, mitoxantrone and topotecan [Doyle et al., 1998; Kathawala et al., 2015]. High ABCG2 expression has been found in a variety of solid tumors and in hematologic malignancies and has been correlated with poor clinical outcomes. Furthermore, ABCG2 is overexpressed in cancer stem cells that are able to self-renew and differentiate [Robey et al., 2007; Stacy et al., 2013]. Structurally, ABCG2 is a half-transporter composed of one nucleotide binding domain (NBD) and one transmembrane domain (TMD) that functions by homodimerization or heterodimerization [Mo and Zhang, 2012]. ABC transporters contribute to MDR by altering drug absorption, distribution, metabolism and excretion. For this reason, researchers have been looking for ways to inhibit ABC transporters and resensitize cancer cells to chemotherapeutic drugs [Goldberg et al., 1988; Polli et al., 2008; Shukla et al., 2011; Shukla et al., 2008; Tsuruo et al., 1981]. In recent years, tyrosine kinase inhibitors (TKI) have been in clinical use or used in clinical trials as targeted therapy for cancer treatment [Anreddy et al., 2014]. More recently, it has been reported that certain TKI may interact with ABC transporters by either being their substrates or inhibitors that compete with ATP on the catalytic site of the ATP-binding domain [Dai et al., 2008; Hegedus et al., 2002; Ozvegy-Laczka et al., 2005; Shi et al., 2009; Wang et al., 2014b]. Apart from the TKI, several studies have reported that ABC transporters can be inhibited by marine compounds, natural compounds and phosphodiesterase-5 (PDE-5) inhibitors that are mechanistically and structurally distinct [Kathawala et al., 2015]. One of the widely studied and an established ABCG2 reversal agent fumitremorgin C (FTC) is a fungal toxin of the diketopiperazines family of compounds. FTC is a specific, selective and potent inhibitor of ABCG2 [Rabindran et al., 2000]. However, no drug has been clinically approved as an inhibitor of ABC transporters due to their pharmacokinetic interactions and toxicity [Ahmed-Belkacem et al., 2006; Leonard et al., 2003; Wang et al., 2014a].
In this study, a variety of 2-trifluoromethyl-2-hydroxypropionamide derivatives were synthesized and their efficacy was evaluated. One compound, 3-chloro-N-(2-hydroxyphenyl)-4-(3,3,3-trifluoro-2-hydroxy-2-methylpropanamido)benzamide (compound 7d), was particularly active in ABCG2-overexpressing cells. Therfore, we conducted in vitro investigation of compound 7d such as, cytotoxicity, accumulation and alteration in protein expression in drug-selective and stably transfected ABCG2-overexpressing cells. Our results demonstrate that compound 7d could reverse ABCG2-mediated MDR by inhibiting the function of the transporter as opposed to alteration in protein levels.
MATERIALS AND METHODS
CHEMICALS
[3H]-mitoxantrone (4 Ci/mmol) was supplied by Moravek Biochemicals (Brea, CA). The monoclonal antibodies BXP-34 (used against ABCG2) were purchased from Signet Laboratories (Dedham, MA). The anti-actin monoclonal antibody (SC-8432) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The FTC was synthesized by the Thomas McCloud Developmental Therapeutics Program, Natural Products Extraction Laboratory, NCI, NIH (Bethesda, MD). Mitoxantrone, SN-38 and cisplatin were purchased from Tocris Bioscience (Ellisville, MO). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO) and other chemicals were purchased from Sigma Chemical (St. Louis, MO). Dulbecco Modified Eagle Medium (DMEM), fetal bovine serum (FBS), Penicillin/streptomycin and trypsin 0.25% were obtained from Hyclone Thermo Scientific (Logan, UT).
CELL LINES AND CELL CULTURE
HEK293 cells were transfected with either the empty pcDNA3.1 vector or the pcDNA3.1 vector containing the full length ABCG2 with amino acid 482 coding either arginine (R), glycine (G), or threonine (T) and cultured in medium with G418 at 2 mg/ml [Robey et al., 2003; Robey et al., 2008]. Stably transfected HEK293/pcDNA3.1, wild-type ABCG2-482-R2, mutant ABCG2-482-G2 and mutant ABCG2-482-T7 cells were obtained from Drs. Susan. E. Bates and Robert W. Robey (NIH, MD). H460 is a human non-small cell lung cancer cell line and the resistant subline H460/MX20 were established with an additional 20 nM of mitoxantrone [Robey et al., 2001]. DMEM culture medium supplemented with 10% FBS and 1% Penicillin/Streptomycin was used to culture all of the cell lines under 5% CO2 at 37°C as described previously [Yang et al., 2013; Zhang et al., 2014].
THE SYNTHESIS OF 2-TRIFLUOROMETHYL-2-HYDROXYPROPIONAMIDE DERIVATIVES
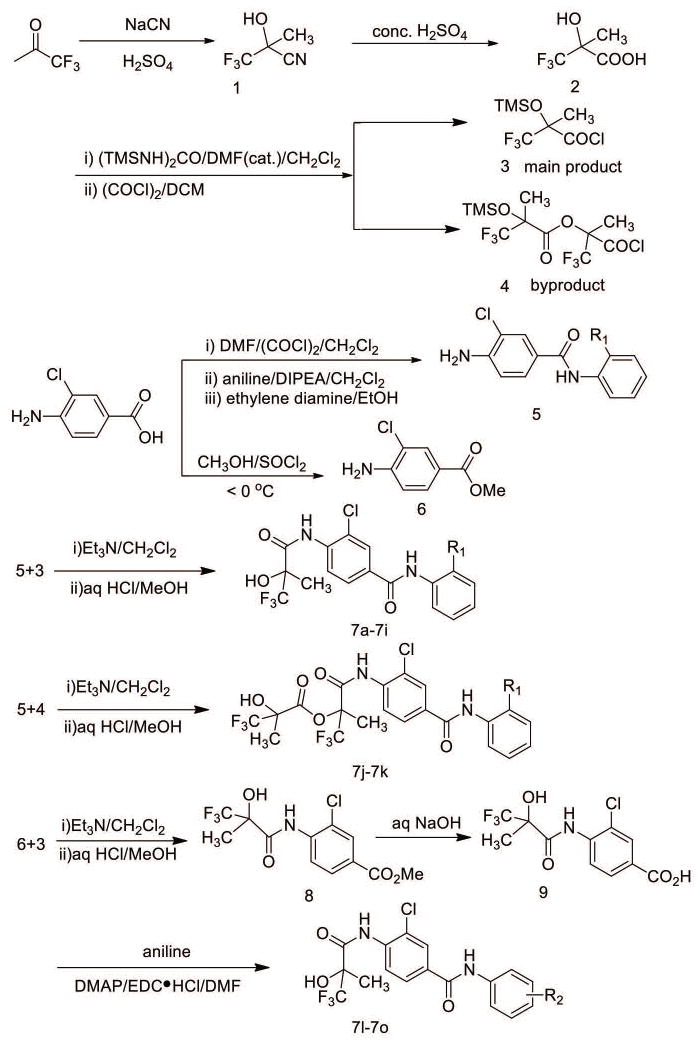
The synthesis of 3-chloro-N-phenyl-4-(3,3,3-trifluoro-2-hydroxy-2-methylpropan amido) benzamide derivatives 7a-7o is outlined in Figure 1. 3,3,3-trifluoro-2-methyl-2-((trimethylsilyl)oxy) propanoyl chloride (compound 3) was prepared according to the reported procedures. However, when 1,3-Bis(trimethylsilyl)urea was not enough, a byproduct 3-chloro-1,1,1-trifluoro-2-methyl-3-oxopropan-2-yl,3,3-trifluoro-2-methyl-2-((trimethylsilyl)oxy) propanoate (compound 4) was synthesized. Another important intermediate 4-amino-3-chloro-N-(2-hydroxyphenyl)benzamide was prepared according to the reported procedures with slight modification [Zhichkin et al., 2008]. Briefly, 4-amino-3-chlorobenzoic acid was simultaneously protected and activated with Vilsmeier reagent and then coupled with aniline to give 4-amino-3-chloro-N-(2-hydroxyphenyl)benzamide (compound 5) in good yield. Compound 5 reacted with compound 3 to give compound 7a-7i, while reacted with compound 4 to give compound 7j and 7k. 4-Amino-3-chlorobenzoic acid was easily transformed into 4-amino-3-chlorobenzoate (compound 6) in good yield. Then compounds 7l-7o were prepared according to the modified reported procedures using compound 6 and compound 3 [Bebernitz et al., 2000].
Figure 1. Synthesis of 3-chloro-N-phenyl-4-(3,3,3-trifluoro-2-hydroxy-2-methylpropanamido)benzamide derivatives 7a-7o.
Outline of scheme of 2-trifluoromethyl-2-hydroxypropionamide derivatives. Synthesis was carried out according to reported procedures as described in the “Materials and methods” section.
CELL PROLIFERATION ASSAY
Cells were harvested with trypsin and resuspended in a concentration of 6 × 103 cells/well. All cell lines were seeded evenly into 96 well plates with 180 μl/well. Reversal agents (20 μl/well) were added 1 h before adding different concentrations of chemotherapeutic drugs (20 μl/well). After 72 h of incubation, each well received 20 μl of MTT solution (4 mg/ml ). The plates were then incubated for another 4 h to permit viable cells to convert the yellow colored MTT into dark-blue formazan crystals. Subsequently, the medium was discarded. In order to dissolve the formazan crystals, 100 μl of DMSO was added into each well. An OPSYS Microplate reader from DYNEX Technologies, Inc. (Chantilly, VA) was used to measure absorbance at 570 nm. The degree of reversal of MDR was determined by dividing the IC50 (concentrations required to inhibit growth by 50%) for the drug resistant cells by the IC50 of the substrate in parental cells. The IC50 values were calculated from survival curves using the Bliss method [Shi et al., 2006].
PREPARATION OF CELL LYSATES AND WESTERN BLOT ANALYSIS
Cells were dissolved for 20 min on ice with a radioimmunoprecipitation assay (RIPA) buffer (PBS with 0.1% SDS, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 100 mg/ml p-aminophenylmethylsulfonyl fluoride) followed by centrifugation (12,000 rpm, 4°C for 20 min) to prepare the cell lysates. Equal amounts of total cell lysates (20 μg protein) were resolved by sodium dodecyl sulfate polycrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes. After incubation in 5% milk dissolved in TBST buffer (10 mM tris–HCl, pH 8.0, 150 mM NaCl, and 0.1% tween 20) for 1 h at room temperature, the PVDF membranes were immunoblotted overnight with monoclonal antibody BXP-34 against ABCG2 at 1:500 dilution or Actin at 1:1000 dilution as an internal loading control. The PVDF membrane was then incubated at room temperature with horseradish peroxide (HRP)-conjugated secondary antibody (1:1000 dilution) for another 2 h. The protein-antibody complex was detected by an enhanced chemiluminescence detection system (Amersham, NJ).
[3H]-MITOXANTRONE ACCUMULATION ASSAY
Equal amounts of HEK293/pcDNA3.1 or ABCG2-482-R2, ABCG2-482-G2 and, ABCG2-482-T7 cells (5 × 106) were preincubated at 37°C in DMEM (10% FBS) with and without compound 7d (5 μM and 10 μM) for 2 h. Subsequently, cells were incubated with 0.1 μM [3H]-mitoxantrone for another 2 h with or without compound 7d. The medium was removed after 2 h and the cells were rinsed three times with cold PBS. A 200 μl lysis buffer (pH 7.4, containing 1% Triton X-100 and 0.2% SDS) was added afterwards. A liquid scintillation analyzer from Packard Instrument Company, Inc. (Downers Grove, IL) was used to measure the radioactivity. FTC (2.5 μM) was used as a positive control for ABCG2 reversal.
[3H]-MITOXANTRONE EFFLUX ASSAY
Cells were exposed to the same procedure as stated in the drug accumulation experiment and then incubated in fresh medium at 37°C at various times (0, 30, 60, and 120 min) in the presence or absence of the inhibitor (compound 7d or FTC at 10 or 2.5 μM, respectively). After washing three times with ice-cold PBS, the cells were lysed using 200 μl lysis buffer and transferred to scintillation vials. Each sample was placed in scintillation fluid and radioactivity was measured in a liquid scintillation analyzer from Packard Instrument Company, Inc. (Downers Grove, IL).
STATISTICAL ANALYSIS
All experiments were repeated at least three times and the differences were determined using the two-tailed student’s t-test (Microsoft Excel 2010) and statistical significance was determined at p < 0.05.
RESULTS
CYTOTOXICITY OF COMPOUNDS 7a-o IN ABCG2-OVEREXPRESSING CELL LINES
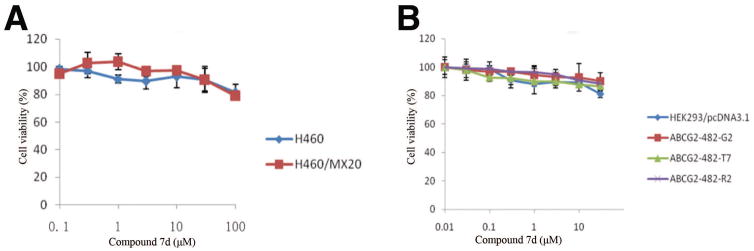
Cytotoxicity assay was performed after treating different cell lines with increasing concentrations of compounds 7a-7o to determine the effect of compounds 7a-7o on cell viability. It was shown that the non-toxic concentration of 7a-7o was more than 30 μM in HEK293/pcDNA3.1 and ABCG2 transfeced cell lines, as well as in the lung cancer cell line H460 and the ABCG2 resistant subline H460/MX20. The cell viability of compound 7d in H460 and H460/MX20 cells, and HEK293/pcDNA3.1 and ABCG2 transfected cell lines are shown in Figure 2A and 2B, respectively.
Figure 2. The cytotoxicity curves of compound 7d in different cell lines.
The cell viability curve of compound 7d in H460 and drug resistant subline H460/MX20 (A), the cell viability curve of compound 7d in HEK293/pcDNA3.1, ABCG2-482-R2, ABCG2-482-G2 and ABCG2-482-T7 cells (B). Cytoxicity was determined by MTT assay as described in the “Material and methods” section. Points with error bars represent the mean ± standard error. The above figures are representative of three independent experiments, each done in triplicates.
SCREENING OF CHEMO-RESISTANCE REVERSAL ACTIVITIES OF COMPOUNDS 7a-7o ON ABCG2-OVEREXPRESSING CELL LINES
A screening test was performed in H460 and its drug resistant subline H460/MX20, using mitoxantrone as the substrate for ABCG2 transporter. Compounds 7a-7o (10 μM) or positive control inhibitor of ABCG2 FTC (2.5 μM) was added. In the drug resistant subline H460/MX20, the resistance was 94.3 folds towards mitoxantrone compared to the parental H460 cell line. After adding 10 μM of compound 7a-7o, the resistance folds decreased significantly, especially with compunds 7d, 7e annd 7i. Compound 7d, 7e and 7i at 10 μM significantly decreased the resistance to mitoxantrone to 6.5, 5.2 and 8.3 resistance folds, respectively (Table 1). Based on these results, we chose compound 7d in our following experiments.
Table 1.
The effect of compounds 7a-7o at 10 μM on ABCG2-mediated drug resistance in H460 and H460/MX20 cell lines.
| Drugs | IC50 ± SDa (nM) (Resistance fold)
|
|
|---|---|---|
| H460 | H460/MX20 | |
| Mitoxantrone | 108.4 ± 27.0 (1.0) b | 10234.6 ± 391.2 (94.3) |
| +compound 7a 10 μM | 267.9 ± 65.2 (2.5) | 1683.1 ± 142.2 (15.5)* |
| +compound 7b 10 μM | 40.4 ± 12.8 (0.4) | 3998.6 ± 687.5 (36.9)* |
| +compound 7c 10 μM | 95.5 ± 10.4 (0.9) | 1032.6 ± 554.1 (9.5)* |
| +compound 7d 10 μM | 84.1 ± 15.0 (0.8) | 706.1 ± 183.0 (6.5)* |
| +compound 7e 10 μM | 172.8 ± 14.0 (1.6) | 562.8 ± 45.1 (5.2)* |
| +compound 7f 10 μM | 66.4 ± 10.9 (0.6) | 1461.8 ± 148.2 (13.5)* |
| +compound 7g 10 μM | 62.9 ± 4.2 (0.6) | 1179.2 ± 329.0 (10.9)* |
| +compound 7h 10 μM | 53.3 ± 2.1 (0.5) | 1224.3 ± 498.1 (11.3)* |
| +compound 7i 10 μM | 80.0 ± 16.3 (0.7) | 894.8 ± 55.4 (8.3)* |
| +compound 7j 10 μM | 165.2 ± 18.2 (1.5) | 1363.4 ± 268.3 (12.6)* |
| +compound 7k 10 μM | 98.1 ± 7.9 (0.9) | 1880.6 ± 107.0 (17.3)* |
| +compound 7l 10 μM | 93.1 ± 7.7 (0.9) | 3265.6 ± 423.1 (30.1)* |
| +compound 7m 10 μM | 110.6 ± 11.7 (1.0) | 5152.8 ± 645.8 (47.4)* |
| +compound 7n 10 μM | 101.6 ± 14.5 (0.9) | 5452.9 ± 630.7 (50.3)* |
| +compound 7o 10 μM | 120.1 ± 23.7(1.1) | 4261.4 ± 543.7 (39.3)* |
| +FTC 2.5 μM | 83.2 ±12.6 (0.8) | 583.6 ± 89.4 (5.4)* |
Values represent mean ± SD of at least three independent experiments, each performed in triplicate.
Resistance fold was calculated by dividing the IC50 values of mitoxantrone in H460/MX20 cells in the absence or presence of reversal agents by the IC50 values of substrates of H460 cell line.
: p<0.05
THE EFFECT OF COMPOUND 7d ON REVERSAL OF ABCG2-MEDIATED MDR IN DRUG-SELECTIVE AND STABLY TRANSFECTED ABCG2-OVEREXPRESSING CELL LINES
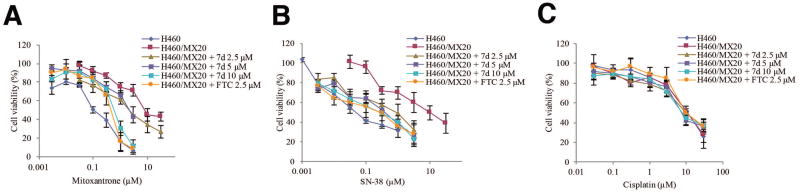
A concentration-dependent chemo-resistance reversal study was performed on the drug resistant subline H460/MX20 and its parental cell line H460 using different chemotheraputic drugs with various concentration of compound 7d (2.5 μM, 5 μM and 10 μM) or FTC (2.5 μM). The results are shown in Table 2. In parental cell line H460, the absence or presence of different concentrations of compound 7d did not alter the IC50 values of chemotheraputic drugs mitoxantrone and SN-38, probably because H460 did not express ABCG2 protein abunduntly (Figure 5). In the drug resistant subline H460/MX20, the resistance folds were about 86.2 folds towards mitoxantrone and 185.2 folds towards SN-38 compared to the H460 cells [Kuang et al., 2012]. After adding compound 7d at 2.5 μM, drug resistance decreased significantly to 22.0 folds towards mitoxantrone and 17.5 folds towards SN-38. Compound 7d at 5 μM further decreased the drug resistance to 20.8 folds towards mitoxantrone and 8.1 folds towards SN-38. After pretreating cells with 10 μM of compound 7d, the resistance to mitoxantrone significantly decreased to 6.3 folds towards mitoxantrone and 5.4 folds towards SN-38. FTC, the positive control inhibitor of ABCG2, at 2.5 μM decreased the drug resistance folds to 4.8 folds towards mitoxantrone and 5.0 folds towards SN-38 which was comparable with compound 7d. H460/MX20 cells does not confer resistance to cisplatin, which is not a substrate of ABCG2 (Table 2). From Figure 3A and 3B, it can be observed that curves shifted to the left after co-incubation of anticancer drug with compound 7d at 2.5 μM, 5 μM and 10 μM in H460/MX20 subline, while in Figure 3C compound 7d did not shift the cell viability curves for cisplatin.
Table 2.
Compound 7d effectively sensitizes drug selective ABCG2-overexpressing cancer cells to the substrate anticancer drugs
| Drugs | IC50 ± SDa (nM) (Resistance fold)
|
|
|---|---|---|
| H460 | H460/MX20 | |
| Mitoxantrone | 108.5 ± 18.9 (1.0) b | 9353.2 ± 876.8 (86.2) |
| +compound 7d 2.5 μM | 98.5 ± 9.5 (0.9) | 2390.0 ± 517.3 (22.0)* |
| +compound 7d 5 μM | 74.9 ± 16.0 (0.7) | 2253.3 ± 432.1 (20.8)* |
| +compound 7d 10 μM | 87.5 ± 6.6 (0.8) | 680.9 ± 256.1 (6.3)* |
| +FTC 2.5 μM | 86.8 ± 8.7 (0.8) | 525.4 ± 120.4 (4.8)* |
| SN-38 | 52.4 ± 5.6 (1.0) b | 9702.8 ± 1008.4 (185.2) |
| +compound 7d 2.5 μM | 55.4 ± 7.3 (1.1) | 914.6 ± 83.9 (17.5)* |
| +compound 7d 5 μM | 41.2 ± 13.2 (0.8) | 426.5 ± 52.6 (8.1)* |
| +compound 7d 10 μM | 46.2 ± 8.7(0.9) | 281.7 ± 49.3 (5.4)* |
| +FTC 2.5 μM | 40.6 ± 9.4(0.8) | 264.1 ± 28.0 (5.0)* |
| Cisplatin | 9608.9 ± 733.1 (1.0) b | 10356.6 ± 1065.2 (1.1) |
| +compound 7d 2.5 μM | 8917.6 ± 681.7 (0.9) | 9339.4 ± 729.4 (1.0) |
| +compound 7d 5 μM | 10323.4 ± 1087.8 (1.1) | 9162.6 ± 821.6 (1.0) |
| +compound 7d 10 μM | 8172.9 ± 765.5 (0.9) | 8251.4 ± 743.5 (0.9) |
| +FTC 2.5 μM | 11568.4 ± 980.1 (1.2) | 10767.2 ± 850.4 (1.1) |
Values represent mean ± SD of at least three independent experiments, each performed in triplicate.
Resistance fold was calculated by dividing the IC50 values of mitoxantrone, SN-38, or cisplatin of H460/MX20 cells in the absence or presence of reversal agents by the IC50 values of substrates of H460 cell line.
: p<0.05
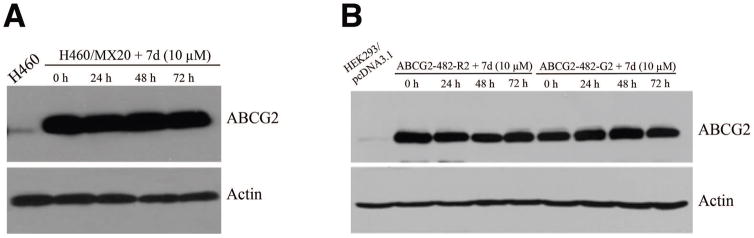
Figure 5. The effect of compound 7d on the expression of ABCG2 protein.
Cells were treated with compound 7d (10 μM) for 72 h. Western blot experiments for ABCG2 were performed on H460 and H460/MX20 cell lysates (A), and HEK293/pcDNA3.1, ABCG2-482-R2 and ABCG2-482-G2 transfected cell lysates (B) as described in “Materials and methods” section.
Figure 3. The effect of compound 7d on the sensitivity of drug resistant subline H460/MX20 to anticancer drugs.
H460 cells and H460/MX20 cells were seeded in 96-well plates 24 h before compound 7d was added. After 1 h preincubation of compound 7d, mitoxantrone (A), SN-38 (B), or cisplatin (C) was added and cells were further incubated for 72 h. Cell viability was measured by the MTT assay as described in the “Material and methods” section. Points with error bars represent the mean ± standard error. The above figures are representative of three independent experiments, each done in triplicates.
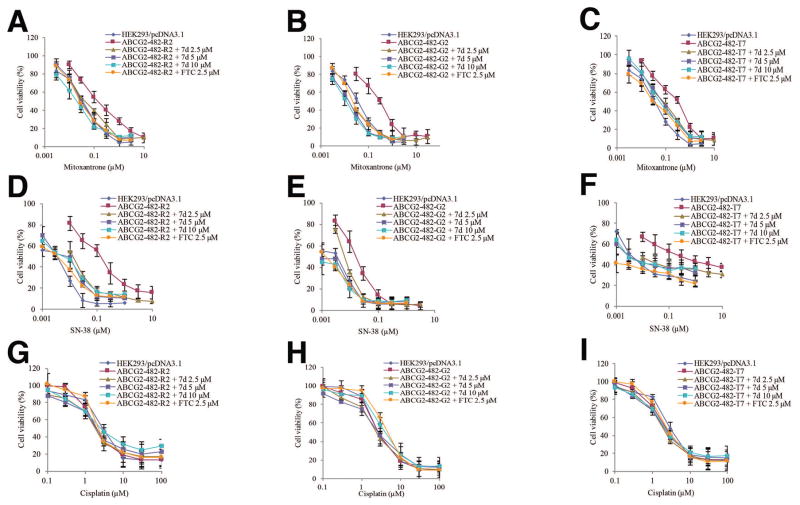
To minimize various factors that may contribute to drug resistance in cancer cell lines, a chemo-resistance reversal study in HEK293/pcDNA3.1 and ABCG2 stably transfected cell lines ABCG2-482-R2, ABCG2-482-G2, ABCG2-482-T7 was carried out. Table 3 indicates that ABCG2 transfected cell lines are resistant to ABCG2 substrates mitoxantrone and SN-38. Compound 7d at 2.5 μM, 5 μM and 10 μM enhanced the sensitivity of ABCG2 overexpressing cell lines to chemotherapeutic drugs mitoxantrone and SN-38 while it did not change the IC50 values of empty vector transfected cell line HEK293/pcDNA3.1. These results are consistent with the drug resistant cancer cell line H460/MX20 and its parental cell line H460. The curves in Figure 4 showed that after co-incubating anticancer drug with compound 7d, the curves shifted to the left in the presence of the substrates mitoxantrone and SN-38 in ABCG2 transfected cell lines ABCG2-482-R2, ABCG2-482-G2, ABCG2-482-T7. Cisplatin was used as a negative control substrate of ABCG2 (Table 3 and Figure 4).
Table 3.
Compound 7d effectively sensitizes stably transfected ABCG2-overexpressing cells to the substrate anticancer drugs
| Drugs | IC50±SDa (nM) (Resistance fold)
|
|||
|---|---|---|---|---|
| HEK293/pcDNA3.1 | ABCG2-482-R2 | ABCG2-482-G2 | ABCG2-482-T7 | |
| Mitoxantrone | 37.8 ± 2.3 (1.0) b | 158.9 ± 37.2 (4.2) | 309.3 ± 12.0 (8.2) | 299.8 ± 62.6 (7.9) |
| +compound 7d 2.5 μM | 37.6 ± 6.1 (1.0) | 39.4 ± 5.9 (1.0)* | 23.6 ± 2.0 (0.6)* | 60.1 ± 8.2 (1.6)* |
| +compound 7d 5 μM | 38.4 ± 4.7 (1.0) | 34.3 ± 6.2 (0.9)* | 18.9 ± 2.5 (0.5)* | 81.2 ± 13.8 (2.1)* |
| +compound 7d 10 μM | 38.0 ± 9.5 (1.0) | 20.8 ± 3.0 (0.6)* | 17.4 ± 3.9 (0.5)* | 73.7 ± 6.5 (1.9)* |
| +FTC 2.5 μM | 30.4 ± 2.2 (0.8) | 22.0 ± 8.9 (0.6)* | 21.5 ± 3.0 (0.6)* | 40.5 ± 4.9 (1.1)* |
| SN-38 | 3.3 ± 0.7 (1.0) b | 41.6 ± 4.3 (12.6) | 179.7 ± 17.2 (54.5) | 269.3 ± 51.8 (81.7) |
| +compound 7d 2.5 μM | 2.2 ± 0.5 (1.0) | 7.3 ± 1.5 (2.2)* | 21.4 ± 2.5 (6.5)* | 12.0 ± 1.1 (3.6)* |
| +compound 7d 5 μM | 2.0 ± 0.6 (0.6) | 0.8 ± 0.2 (0.25)* | 8.2 ± 1.3 (2.5)* | 6.5 ± 1.2 (2.0)* |
| +compound 7d 10 μM | 1.7 ± 0.6 (0.5) | 0.8 ± 0.3 (0.26)* | 11.0 ± 1.6 (3.3)* | 4.8 ± 0.8 (1.5)* |
| +FTC 2.5 μM | 2.8 ± 0.5 (0.8) | 1.0 ± 0.5 (0.3)* | 5.9 ± 0.7 (1.8)* | 5.4 ± 0.3 (1.7)* |
| Cisplatin | 3068.2 ± 253.4 (1.0) b | 2359.0 ± 247.5 (0.8) | 2931.2 ± 308.6 (1.0) | 2650.3 ± 324.1 (0.9) |
| +compound 7d 2.5 μM | 2614.7 ± 543.6 (0.9) | 2512.8 ± 485.1 (0.8) | 3051.6 ± 577.7 (1.0) | 2117.9 ± 643.7 (0.7) |
| +compound 7d 5 μM | 2823.6 ± 991.7 (0.9) | 2340.9 ± 342.9 (0.8) | 3107.4 ± 266.1 (1.0) | 2342.9 ± 203.5 (0.8) |
| +compound 7d 10 μM | 2635.6 ± 250.7 (0.9) | 2778.6 ± 159.3 (0.9) | 2606.6 ± 344.7 (0.8) | 2602.4 ± 250.0 (0.8) |
| +FTC 2.5 μM | 2211.3 ± 543.9 (0.7) | 2516.8 ± 163.9 (0.8) | 2911.2 ± 259.1 (0.9) | 2566.7 ± 253.0 (0.8) |
Values represent mean ± SD of at least three independent experiments, each performed in triplicate.
Resistance fold was calculated by dividing the IC50 values of mitoxantrone, SN-38, or cisplatin of ABCG2 overexpressing cell lines in the absence or presence of reversal agents by the IC50 values of substrates of HEK293/pcDNA3.1 cell line.
:P<0.05
Figure 4. The effect of compound 7d on the sensitivity of ABCG2 transfected cell lines to anticancer drugs.
HEK293/pcDNA3.1 cells and ABCG2 transfected cell linesABCG2-482-R2, ABCG2-482-G2 and ABCG2-482-T7 cells were seeded in 96-well plates for 24 h before compound 7d was added. After 1 h preincubation of 7d, mitoxantrone was added in ABCG2-482-R2 group (A), ABCG2-482-G2 group (B), ABCG2-482-T7 group (C); SN-38 was added in ABCG2-482-R2 group (C), ABCG2-482-G2 group (D), ABCG2-482-T7 group (E); cisplatin was added in ABCG2-482-R2 group (F), ABCG2-482-G2 group (G), ABCG2-482-T7 group (I) and cells were incubated for 72 h. Cell viability was measured by the MTT assay as described in the “Material and methods” section. Points with error bars represent the mean ± standard error. The above figures are representative of three independent experiments, each done in triplicates.
THE EFFECT OF COMPOUND 7d ON THE EXPRESSION LEVELS OF ABCG2 PROTEIN
To determine whether down-regulating the protein levels of ABCG2 or inhibiting the function of ABCG2 caused the MDR reversal, Western blotting was performed. After incubating ABCG2-overexpressing subline H460/MX20 with compound 7d (10 μM) at different time points (0, 24, 48, 72 h), the protein levels of ABCG2 were determined. Figure 5A clearly indicates that H460/MX20 expressed a much higher level of ABCG2 protein than that in the parental cell line H460, which did not change until 72 h. Similar results were obtained in the ABCG2-transfected cell lines as shown in Figure 5B.
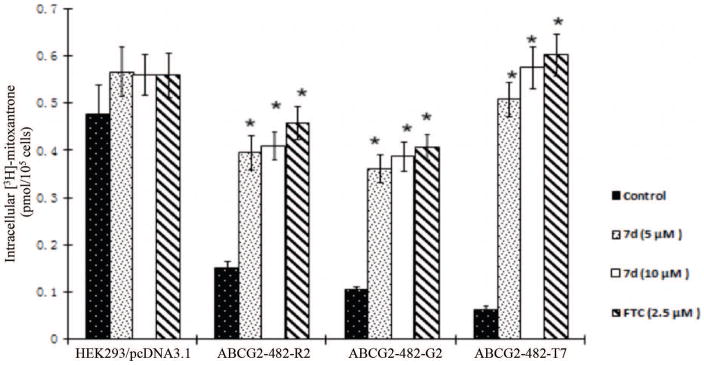
COMPOUND 7d INCREASES THE INTRACELLULAR ACCUMULATION OF [3H]-MITOXANTRONE IN CELLS OVEREXPRESSING ABCG2
In order to understand whether compound 7d sensitizes cells to chemotheraputic drugs by inhibiting the function of ABCG2, drug accumulation assay was performed. HEK293/pcDNA3.1 and ABCG2-transfected cells were incubated with [3H]-mitoxantrone with or without compound 7d or FTC at different concentrations for 2 h. Compound 7d (5 μM, 10 μM) significantly increased the intracellular concentrations of [3H]-mitoxantrone in ABCG2-transfected cells to the levels similar to those seen with FTC at 2.5 μM. However, compound 7d or FTC did not significantly influence the intracellular accumulation in HEK293/pcDNA3.1 cells (Fig. 6). These results suggest that the increased intracellular levels of [3H]-mitoxantrone in ABCG2 overexpressing cells might be due to the inhibitory effect of compound 7d on drug efflux function of ABCG2 transporter.
Figure 6. The effect of compound 7d on the accumulation of [3H]-mitoxantrone.
The accumulation [3H]-mitoxantrone in empty vector transfected HEK293/pcDNA3.1, ABCG2-482-R2, ABCG2-482-G2 and ABCG2-482-T7 cells with or without compound 7d treatment. Columns are the mean of triplicate determinations. *, p < 0.05 versus the control group.
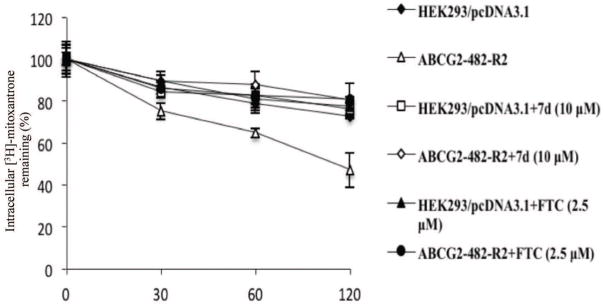
COMPOUND 7d DECREASES THE EFFLUX OF [3H]-MITOXANTRONE FROM CELLS OVEREXPRESSING ABCG2
To determine whether compound 7d inhibits the efflux of mitoxantrone, thus increasing the intracellular accumulation of [3H]-mitoxantrone, an efflux assay was performed. Figure 7 shows that the ABCG2 overexpressing cell line ABCG2-482-R2 extrudes a greater amount of [3H]-mitoxantrone at various time points (0, 30 min, 60 min, 120 min) than the parental cell line HEK293/pcDNA3.1. After preincubating with compound 7d (10 μM), the efflux of [3H]-mitoxantrone is significantly inhibited. Similar results were found with the group treated with FTC at 2.5μM. In the parental cell line HEK293/pcDNA3.1, compound 7d and FTC did not alter the rate of efflux of [3H]-mitoxantrone.
Figure 7. The effect of compound 7d on the efflux of [3H]-mitoxantrone.
The effect of compound 7d at 10 μM on retention of [3H]-mitoxantrone over a period of time in HEK293/pcDNA3.1 and ABCG2-482-R2. Data points represent the means ± SD.
DISCUSSION
The ABC transporters have been shown to cause efflux of the anti-cancer drugs from cancer cells, thus playing a major role in MDR [Breier et al., 2013; Sun et al., 2012]. Consequently, ABC tranporter inhibitors can be used in combination with traditional chemotherapeutic drugs to increase the effectiveness of treatment by increasing the intracellular concentration of anticancer drugs [Kathawala et al., 2013; Kwak et al., 2010; Shi et al., 2011]. Several preclinical and clinical studies have been carried out to evaluate the feasibility of this approach [Kathawala et al., 2014b; Kuppens et al., 2007; Morschhauser et al., 2007].
Anilide derivatives of 2-trifluoromethyl-2-hydroxypropionamide were originally found to possess anti-androgen activities [Morris et al., 1991]. It was reported that some members of the derivatives act as potassium channel openers, possessing undesirable hypotensive activity [Grant et al., 1994; Ohnmacht et al., 1996]. Moreover, these compounds can also act as potent inhibitors of pyruvate dehydrogenase kinase in vitro and in vivo [Bebernitz et al., 2000]. To our knowledge, this is the first report of the effect of 2-trifluoromethyl-2-hydroxypropionamide derivatives on ABCG2-mediated MDR. The results showed that 2-trifluoromethyl-2-hydroxypropionamide derivatives can be used at an acceptably safe concentrations up to 30 μM. These results suggested that compound 7d can specifically target ABCG2 which could possibly lower the side effects of 2-trifluoromethyl-2-hydroxypropionamide derivatives.
The wild-type ABCG2-482-R2, mutant ABCG2-482-G2 and mutant ABCG2-282-T7 cells shows different responses to ABCG2 inhibitors as well as different resistant folds to ABCG2 substrates. According to previous reports, mutant type ABCG2 showed strong resistance to anthracyclines, doxorubicin, rhodamine but little resistance to methotrexate; whereas wild-type ABCG2 showed weak resistance to anthracycline and rhodamine but high resistane to methotrexate [Allen et al., 2002; Volk et al., 2002]. Three-dimensional structure analysis showed that ABCG2 substrate recognition sites are located in transmembrane domain and position 482 is located in the central lumen of transmembrane domain, which explained why the change of arginine at 482 can affect the substrate spectrum of ABCG2 [Cai et al., 2010]. It is known that FTC can block both wild type and mutant type ABCG2, therefore it was used as a positive control of ABCG2 reversal agent. Our results showed compound 7d could senstitize both wild-type ABCG2-482-R2 and mutant ABCG2 transfected cell lines to chemotherapeutic drugs in a concentration-independent manner, which was comparable to FTC. Thus, we conclude that compound 7d could possibly be developed as a more useful inhibitor in the clinics.
Compound 7d could reverse ABCG2-mediated MDR by either downregulating the protein expression of ABCG2 or inhibiting the function of ABCG2. In order to determine the effect of compound 7d on the protein level of ABCG2, Western blotting was perfomed after incubating the cells with compound 7d for different lengths of time. Western blotting results indicated that compound 7d did not alter the expression levels of ABCG2 protein up to 72 h in both drug selected ABCG2-overexpressing subline H460/MX20 and ABCG2 stably transfected HEK293 cell lines.
In order to look into whether compound 7d could inhibit the function of ABCG2, accumulation assay was performed on HEK293/pcDNA3.1 and ABCG2-overexpressing cell lines. The accumulation results indicated that in ABCG2 overexpressing cell lines, the intracellular concentration of [3H]-mitoxantrone increased significantly in presence of compound 7d with minimal increase in HEK293/pcDNA3.1 cells. Futhermore, the efflux assay showed that compound 7d significantly reduced the efflux of [3H]-mitoxantrone at different time points, thus indicating that increasing accumulation of [3H]-mitoxantrone inside the cells is due to reduced efflux activity. Overall, these results suggested that compound 7d can reverse ABCG2-mediated resistance to chemotheraputic drugs by reducing the efflux and increasing the intracellular concentration of cancer chemotheraputic drugs as opposed to decreasing the protein level of ABCG2.
Collectively, our study reported that compound 7d at safe concentrations could reverse ABCG2-mediated MDR to the ABCG2 chemotherapeutic substrate drugs in a concentration-dependent manner. These results warrant a preclinical investigation of compound 7d and its development as a potential ABCG2 inhibitor to surmount ABCG2-mediated MDR in clinics. Given that ABCG2 is an established cancer stem cell marker and clinically important transporter, we believe these results will provide a novel direction for the development of ABCG2 reversal agents.
Acknowledgments
CONTRACT GRANT SPONSOR: 1) National Institutes of Health; 2) St. John’s University Research Seed Grant.
CONTRACT GRANT NUMBER: 1) 1R15CA143701; 2) 579-1110-7002.
We thank Drs. Susan E. Bates and Robert W. Robey (NIH, MD) for providing FTC, H460, H460/MX20, HEK293/pcDNA3.1 and ABCG2-transfected cell lines.
Footnotes
CONFLICTS OF INTEREST: No potential conflicts of interest were disclosed.
References
- Ahmed-Belkacem A, Pozza A, Macalou S, Perez-Victoria JM, Boumendjel A, Di Pietro A. Inhibitors of cancer cell multidrug resistance mediated by breast cancer resistance protein (BCRP/ABCG2) Anticancer Drugs. 2006;17:239–43. doi: 10.1097/00001813-200603000-00001. [DOI] [PubMed] [Google Scholar]
- Allen JD, Jackson SC, Schinkel AH. A mutation hot spot in the Bcrp1 (Abcg2) multidrug transporter in mouse cell lines selected for Doxorubicin resistance. Cancer Res. 2002;62:2294–9. [PubMed] [Google Scholar]
- Anreddy N, Gupta P, Kathawala RJ, Patel A, Wurpel JN, Chen ZS. Tyrosine kinase inhibitors as reversal agents for ABC transporter mediated drug resistance. Molecules. 2014;19:13848–77. doi: 10.3390/molecules190913848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebernitz GR, Aicher TD, Stanton JL, Gao J, Shetty SS, Knorr DC, Strohschein RJ, Tan J, Brand LJ, Liu C, Wang WH, Vinluan CC, Kaplan EL, Dragland CJ, DelGrande D, Islam A, Lozito RJ, Liu X, Maniara WM, Mann WR. Anilides of (R)-trifluoro-2-hydroxy-2-methylpropionic acid as inhibitors of pyruvate dehydrogenase kinase. J Med Chem. 2000;43:2248–57. doi: 10.1021/jm0000923. [DOI] [PubMed] [Google Scholar]
- Breier A, Gibalova L, Seres M, Barancik M, Sulova Z. New insight into p-glycoprotein as a drug target. Anticancer Agents Med Chem. 2013;13:159–70. [PubMed] [Google Scholar]
- Cai X, Bikadi Z, Ni Z, Lee EW, Wang H, Rosenberg MF, Mao Q. Role of basic residues within or near the predicted transmembrane helix 2 of the human breast cancer resistance protein in drug transport. J Pharmacol Exp Ther. 2010;333:670–81. doi: 10.1124/jpet.109.163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZS, Tiwari AK. Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases. FEBS J. 2011;278:3226–45. doi: 10.1111/j.1742-4658.2011.08235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–4. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR, Jr, Huang Y, Robey RW, Liang YJ, Chen LM, Shi CJ, Ambudkar SV, Chen ZS, Fu LW. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–14. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–17. [PubMed] [Google Scholar]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–70. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg H, Ling V, Wong PY, Skorecki K. Reduced cyclosporin accumulation in multidrug-resistant cells. Biochem Biophys Res Commun. 1988;152:552–8. doi: 10.1016/s0006-291x(88)80073-1. [DOI] [PubMed] [Google Scholar]
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Grant TL, Ohnmacht CJ, Howe BB. Anilide tertiary carbinols: a novel series of K+ channel openers. Trends Pharmacol Sci. 1994;15:402–4. doi: 10.1016/0165-6147(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Hegedus T, Orfi L, Seprodi A, Varadi A, Sarkadi B, Keri G. Interaction of tyrosine kinase inhibitors with the human multidrug transporter proteins, MDR1 and MRP1. Biochim Biophys Acta. 2002;1587:318–25. doi: 10.1016/s0925-4439(02)00095-9. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–62. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kathawala RJ, Chen JJ, Zhang YK, Wang YJ, Patel A, Wang DS, Talele TT, Ashby CR, Jr, Chen ZS. Masitinib antagonizes ATP-binding cassette subfamily G member 2-mediated multidrug resistance. Int J Oncol. 2014a;44:1634–42. doi: 10.3892/ijo.2014.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathawala RJ, Gupta P, Ashby CR, Jr, Chen ZS. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Updat. 2015;18:1–17. doi: 10.1016/j.drup.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Kathawala RJ, Sodani K, Chen K, Patel A, Abuznait AH, Anreddy N, Sun YL, Kaddoumi A, Ashby CR, Jr, Chen ZS. Masitinib Antagonizes ATP-Binding Cassette Subfamily C Member 10-Mediated Paclitaxel Resistance: A Preclinical Study. Mol Cancer Ther. 2014b doi: 10.1158/1535-7163.MCT-13-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathawala RJ, Wang YJ, Ashby CR, Jr, Chen ZS. Recent advances regarding the role of ABC subfamily C member 10 (ABCC10) in the efflux of antitumor drugs. Chin J Cancer. 2013 doi: 10.5732/cjc.013.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang YH, Patel JP, Sodani K, Wu CP, Liao LQ, Patel A, Tiwari AK, Dai CL, Chen X, Fu LW, Ambudkar SV, Korlipara VL, Chen ZS. OSI-930 analogues as novel reversal agents for ABCG2-mediated multidrug resistance. Biochem Pharmacol. 2012;84:766–74. doi: 10.1016/j.bcp.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens IE, Witteveen EO, Jewell RC, Radema SA, Paul EM, Mangum SG, Beijnen JH, Voest EE, Schellens JH. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res. 2007;13:3276–85. doi: 10.1158/1078-0432.CCR-06-2414. [DOI] [PubMed] [Google Scholar]
- Kwak JO, Lee SH, Lee GS, Kim MS, Ahn YG, Lee JH, Kim SW, Kim KH, Lee MG. Selective inhibition of MDR1 (ABCB1) by HM30181 increases oral bioavailability and therapeutic efficacy of paclitaxel. Eur J Pharmacol. 2010;627:92–8. doi: 10.1016/j.ejphar.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8:411–24. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- Mo W, Zhang JT. Human ABCG2: structure, function, and its role in multidrug resistance. Int J Biochem Mol Biol. 2012;3:1–27. [PMC free article] [PubMed] [Google Scholar]
- Morris JJ, Hughes LR, Glen AT, Taylor PJ. Non-steroidal antiandrogens. Design of novel compounds based on an infrared study of the dominant conformation and hydrogen-bonding properties of a series of anilide antiandrogens. J Med Chem. 1991;34:447–55. doi: 10.1021/jm00105a067. [DOI] [PubMed] [Google Scholar]
- Morschhauser F, Zinzani PL, Burgess M, Sloots L, Bouafia F, Dumontet C. Phase I/II trial of a P-glycoprotein inhibitor, Zosuquidar.3HCl trihydrochloride (LY335979), given orally in combination with the CHOP regimen in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2007;48:708–15. doi: 10.1080/10428190701190169. [DOI] [PubMed] [Google Scholar]
- Ohnmacht CJ, Russell K, Empfield JR, Frank CA, Gibson KH, Mayhugh DR, McLaren FM, Shapiro HS, Brown FJ, Trainor DA, Ceccarelli C, Lin MM, Masek BB, Forst JM, Harris RJ, Hulsizer JM, Lewis JJ, Silverman SM, Smith RW, Warwick PJ, Kau ST, Chun AL, Grant TL, Howe BB, Neilson KL, et al. N-aryl-3,3,3-trifluoro-2-hydroxy-2-methylpropanamides: KATP potassium channel openers. Modifications on the western region. J Med Chem. 1996;39:4592–601. doi: 10.1021/jm960365n. [DOI] [PubMed] [Google Scholar]
- Ozvegy-Laczka C, Cserepes J, Elkind NB, Sarkadi B. Tyrosine kinase inhibitor resistance in cancer: role of ABC multidrug transporters. Drug Resist Updat. 2005;8:15–26. doi: 10.1016/j.drup.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Polli JW, Humphreys JE, Harmon KA, Castellino S, O’Mara MJ, Olson KL, John-Williams LS, Koch KM, Serabjit-Singh CJ. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino }methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:695–701. doi: 10.1124/dmd.107.018374. [DOI] [PubMed] [Google Scholar]
- Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, Poruchynsky MS, Bates SE. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–8. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RW, Honjo Y, van de Laar A, Miyake K, Regis JT, Litman T, Bates SE. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2) Biochim Biophys Acta. 2001;1512:171–82. doi: 10.1016/s0005-2736(01)00308-x. [DOI] [PubMed] [Google Scholar]
- Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- Robey RW, Shukla S, Finley EM, Oldham RK, Barnett D, Ambudkar SV, Fojo T, Bates SE. Inhibition of P-glycoprotein (ABCB1)- and multidrug resistance-associated protein 1 (ABCC1)-mediated transport by the orally administered inhibitor, CBT-1((R)) Biochem Pharmacol. 2008;75:1302–12. doi: 10.1016/j.bcp.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Liang YJ, Chen ZS, Wang XW, Wang XH, Ding Y, Chen LM, Yang XP, Fu LW. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol Ther. 2006;5:39–47. doi: 10.4161/cbt.5.1.2236. [DOI] [PubMed] [Google Scholar]
- Shi Z, Tiwari AK, Patel AS, Fu LW, Chen ZS. Roles of sildenafil in enhancing drug sensitivity in cancer. Cancer Res. 2011;71:3735–8. doi: 10.1158/0008-5472.CAN-11-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Tiwari AK, Shukla S, Robey RW, Kim IW, Parmar S, Bates SE, Si QS, Goldblatt CS, Abraham I, Fu LW, Ambudkar SV, Chen ZS. Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine kinase inhibitor AG1478. Biochem Pharmacol. 2009;77:781–93. doi: 10.1016/j.bcp.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Ohnuma S, Ambudkar SV. Improving cancer chemotherapy with modulators of ABC drug transporters. Curr Drug Targets. 2011;12:621–30. doi: 10.2174/138945011795378540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Sauna ZE, Ambudkar SV. Evidence for the interaction of imatinib at the transport-substrate site(s) of the multidrug-resistance-linked ABC drug transporters ABCB1 (P-glycoprotein) and ABCG2. Leukemia. 2008;22:445–7. doi: 10.1038/sj.leu.2404897. [DOI] [PubMed] [Google Scholar]
- Sodani K, Patel A, Kathawala RJ, Chen ZS. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer. 2012;31:58–72. doi: 10.5732/cjc.011.10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy AE, Jansson PJ, Richardson DR. Molecular pharmacology of ABCG2 and its role in chemoresistance. Mol Pharmacol. 2013;84:655–69. doi: 10.1124/mol.113.088609. [DOI] [PubMed] [Google Scholar]
- Sun YL, Patel A, Kumar P, Chen ZS. Role of ABC transporters in cancer chemotherapy. Chin J Cancer. 2012;31:51–7. doi: 10.5732/cjc.011.10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari AK, Sodani K, Dai CL, Ashby CR, Jr, Chen ZS. Revisiting the ABCs of multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12:570–94. doi: 10.2174/138920111795164048. [DOI] [PubMed] [Google Scholar]
- Tiwari AK, Sodani K, Wang SR, Kuang YH, Ashby CR, Jr, Chen X, Chen ZS. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol. 2009;78:153–61. doi: 10.1016/j.bcp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41:1967–72. [PubMed] [Google Scholar]
- Volk EL, Farley KM, Wu Y, Li F, Robey RW, Schneider E. Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Res. 2002;62:5035–40. [PubMed] [Google Scholar]
- Wang YJ, Kathawala RJ, Zhang YK, Patel A, Kumar P, Shukla S, Fung KL, Ambudkar SV, Talele TT, Chen ZS. Motesanib (AMG706), a potent multikinase inhibitor, antagonizes multidrug resistance by inhibiting the efflux activity of the ABCB1. Biochem Pharmacol. 2014a;90:367–78. doi: 10.1016/j.bcp.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Zhang YK, Kathawala RJ, Chen ZS. Repositioning of Tyrosine Kinase Inhibitors as Antagonists of ATP-Binding Cassette Transporters in Anticancer Drug Resistance. Cancers (Basel) 2014b;6:1925–52. doi: 10.3390/cancers6041925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Kathawala RJ, Chufan EE, Patel A, Ambudkar SV, Chen ZS, Chen X. Tivozanib reverses multidrug resistance mediated by ABCB1 (P-glycoprotein) and ABCG2 (BCRP) Future Oncol. 2013 doi: 10.2217/fon.13.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kathawala RJ, Wang YJ, Zhang YK, Patel A, Shukla S, Robey RW, Talele TT, Ashby CR, Jr, Ambudkar SV, Bates SE, Fu LW, Chen ZS. Linsitinib (OSI-906) antagonizes ATP-binding cassette subfamily G member 2 and subfamily C member 10-mediated drug resistance. Int J Biochem Cell Biol. 2014;51:111–9. doi: 10.1016/j.biocel.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhichkin PE, Peterson LH, Beer CM, Rennells WM. The use of formamidine protection for the derivatization of aminobenzoic acids. J Org Chem. 2008;73:8954–9. doi: 10.1021/jo8017186. [DOI] [PubMed] [Google Scholar]