Abstract
X-linked intellectual disability (ID) type Nascimento (MIM #300860), also known as ubiquitin-conjugating enzyme E2 A (UBE2A) deficiency syndrome, is a congenital malformation syndrome characterized by moderate to severe ID, speech impairment, dysmorphic facial features, genital anomalies and skin abnormalities. Here, we report a Japanese patient with severe ID and congenital cataract. We identified a novel hemizygous mutation (c.76G>A, p.Gly26Arg) in UBE2A by whole-exome sequencing.
Intellectual disability (ID) is a neurodevelopmental disorder that is characterized by significant limitations in intellectual functioning and adaptive behavior that are apparent before 18 years of age. An IQ score of <70 is characteristic. The prevalence of ID has been estimated at 1% of the worldwide population.1 To date, >700 causative genes have been reported for all forms of ID.1,2 Among these genes, >100 are implicated in X-linked ID.2
X-linked ID type Nascimento (MIM #300860), also known as ubiquitin-conjugating enzyme E2 A (UBE2A) deficiency syndrome, was first shown to be caused by a nonsense mutation in UBE2A in three affected males of a two-generation family.3 Their mothers were clinically normal. UBE2A, located on Xq24, encodes UBE2A. Ubiquitin-conjugating enzymes catalyze the covalent attachment of ubiquitin to substrate proteins. The protein ubiquitination pathway requires three ubiquitination enzymes, E1, E2 and E3. Ubiquitination of proteins is involved in the ubiquitin proteasome pathway required for the degradation of proteins.4 In subsequent studies, 19 mutations in UBE2A have been reported (seven missense or nonsense mutations, two small insertions or deletions, and 10 gross deletions).5–13 Common clinical findings of this syndrome are moderate to severe ID, speech impairment, dysmorphic facial features, genital anomalies and skin abnormalities.5,7,14 All patients carrying UBE2A mutations are males. Further, when the mothers of these patients were submitted to an X-chromosome inactivation assay, all of them showed skewed X-inactivation.
In this study, we identified a novel UBE2A mutation (c.76G>A, p.Gly26Arg) in a Japanese patient with severe ID and congenital cataract using whole-exome sequencing.
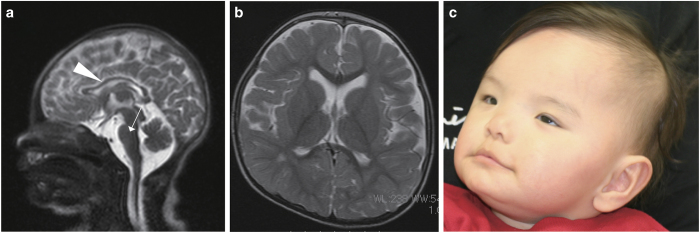
The patient was an 8-year-old Japanese male born at 39 weeks of gestation by normal vaginal delivery to a non-consanguineous 29-year-old gravida 1, para 1 mother and 33-year-old father. The mother was noted to have intrauterine growth restriction and oligohydramnios from the 36th week of gestation. APGAR scores were 8 at 1 min, and 9 at 5 min. His birth weight was 2,355 g (−1.8 s.d.), length 45.6 cm (−1.6 s.d.), and occipitofrontal circumference 32 cm (−0.9 s.d.). At birth, he required supplemental oxygen for tachypnea and retractive breathing, and was admitted to the neonatal intensive care unit. He had transient hypoglycemia due to a relative decrease in plasma volume due to polycythemia. Magnetic resonance imaging of his brain on the third day of life showed hypoplasia of the corpus callosum and the basilar part of the pons (Figure 1a). Brain magnetic resonance imaging at 1 year and 8 months of age showed some areas of hyperintensity in the deep white matter, mild delay of myelination and reduced white matter volume (Figure 1b). Echocardiography showed tetralogy of Fallot. At the age of 10 months, radical repair for the tetralogy of Fallot was performed. He was noted to have bilateral congenital cataract at 9 months of age, and he underwent cataract surgery at the age of 2 years. Physical therapy for lower limb hypertonicity was started at the age of 5 months. Bilateral lower limb triceps extension surgery for bilateral pes equinus was performed at the age of 3 years and 7 months. He had an episode of febrile convulsion at the age of 2 years and 2 months, but showed normal EEG patterns. His developmental milestones were severely delayed: rolling over at 7 months, sitting at 1 year and 6 months, crawling at 3 years and walking alone at 6 years of age. He could not speak meaningful words, and his developmental quotient score was <20 at the age of 8 years. He also had obsessive-compulsive behaviors.
Figure 1.
Brain magnetic resonance imaging (MRI) and photograph of the patient with a UBE2A mutation. (a) Brain MRI of the patient at birth. T2-weighted sagittal image shows hypoplasia of the corpus callosum (white arrowhead) and the basilar part of the pons (white arrow). (b) Brain MRI of the patient at 1 year and 8 months of age. T2-weighted axial image shows white matter abnormalities. (c) Patient at 1 year and 1 month of age. Photograph of the patient’s face showing strabismus, high forehead, hypertelorism, large ears, prominent philtrum and thin upper lip.
Physical examination at 1 year of age revealed mild growth delay with weight 8.36 kg (−1.0 s.d.), height 72.4 cm (−1.0 s.d.) and occipitofrontal circumference 42.5 cm (−2.6 s.d.). Dysmorphic facial appearance included strabismus, high forehead, hypertelorism, large ears, prominent philtrum and thin upper lip (Figure 1c and Supplementary Table S1). At 8 years 1 month, occipitofrontal circumference was 48.3 cm (−1.4 s.d.).
Standard karyotyping was normal, and cytogenetic microarray analysis using SurePrint G3 8×60 k (Agilent Technologies, Santa Clara, CA, USA) showed no pathogenic copy-number variations.
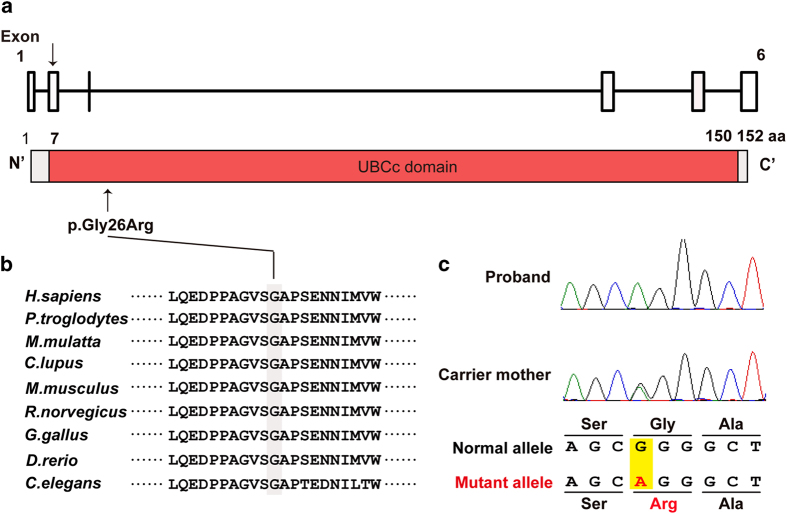
Genomic DNA was extracted from peripheral blood using a QIAcube (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Clinical information was obtained from the family members after written informed consent was secured, in accordance with the institutional review board of Kanagawa Children’s Medical Center. Genomic DNA from the patient was processed using a SureSelect Human All Exon V5+UTRs Kit (Agilent Technologies). Captured genomic DNA was sequenced on a HiSeq2500 (Illumina, San Diego, CA, USA) with 101 bp paired-end reads. Quality-controlled reads were aligned to the human reference genome (UCSC hg19, NCBI build 37.1) using the Burrows-Wheeler Aligner (BWA, version 0.7.10) (http://bio-bwa.sourceforge.net/index.shtml). PCR duplications were removed using Picard (version 1.118) (http://broadinstitute.github.io/picard/). Variants, including single-nucleotide variants and small insertions and deletions, were called using Genome Analysis Toolkit (GATK, version 3.2-2) (The UnifiedGenotyper and the HaplotypeCaller algorithms) (https://software.broadinstitute.org/gatk/) and were annotated using ANNOVAR (22 March 2015) (http://annovar.openbioinformatics.org/en/latest/). Out of all called variants within exons or ±10 bp from exon–intron boundaries, those registered in the NHLBI GO Exome Sequencing Project 6500, the 1000 Genomes Project, dbSNP138, the Human Genetic Variation Database and our in-house Japanese exome data (57 individuals) were removed. Variants were confirmed as true positives by Sanger sequencing on an Applied Biosystems 3730×l DNA Analyzer (Life Technologies, Carlsbad, CA, USA). Sequence data were analyzed using Applied Biosystems Variant Reporter software (Gene Codes Corporation, Ann Arbor, MI, USA). The mean coverage of RefSeq protein-coding regions was 82.54 reads, with 94.6% being covered by 20 or more reads. Among the filtered variants, we focused on known ID-causing genes and found a novel hemizygous mutation in UBE2A (c.76G>A, p.Gly26Arg). The UBE2A protein contains a ubiquitin-conjugating enzyme catalytic (UBCc) domain. The amino acid substitution (p.Gly26Arg) localized in the UBCc domain (Figure 2a). This mutation was not registered in the NHLBI-Exome Sequencing Project 6500, the 1000 Genomes Project, dbSNP138, the Human Genetic Variation Database or in our in-house Japanese exome data. This missense mutation was predicted to be deleterious by SIFT (http://sift.jcvi.org/), Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/) and MutationTaster (http://www.mutationtaster.org/). The amino acid substitution (p.Gly26Arg) is evolutionarily conserved among different species (Figure 2b). Sanger sequencing of genomic DNA from the patient confirmed that the mutation was inherited from his healthy mother (Figure 2c).
Figure 2.
UBE2A mutation. (a) Novel hemizygous mutation (c.76G>A, p.Gly26Arg) identified in the patient. UBE2A comprises six exons, and the UBE2A protein contains an ubiquitin-conjugating enzyme catalytic (UBCc) domain predicted by SMART (http://smart.embl-heidelberg.de/). (b) The mutation occurred at an amino acid that is evolutionarily conserved in nine different species. The changed nucleotide is highlighted in the gray box. (c) Electropherogram of the patient and his mother.
An X-chromosome inactivation assay was performed using the human androgen receptor gene (HUMARA), as previously described.15 Genomic DNA was digested at 37 °C for 18 h with a methylation-sensitive enzyme (HpaII). PCR was performed using digested and undigested genomic DNA with the following primers: 6-FAM-labeled sense primer: 5′- GCTGTGAAGGTTGCTGTTCCTCAT-3′; antisense primer: 5′- TCCAGAATCTGTTCCAGAGCGTGC-3′. DNA fragment analysis was performed on an Applied Biosystems 3130×l DNA Analyzer (Life Technologies). Fragment data were analyzed using Peak Scanner Software 2 (Life Technologies). Skewed X-chromosome inactivation was observed in the patient’s mother (ratio 92:8).
Here, we report a Japanese family with a patient diagnosed with severe ID and congenital cataract and a UBE2A missense mutation (c.76G>A, p.Gly26Arg) (Supplementary Table S1). To date, nine different missense or nonsense mutations, small insertions, or deletions in UBE2A have been reported to cause X-linked ID type Nascimento.3,6,7,10,11 All these amino acid substitutions are localized in the UBCc domain. Clinical features were compared between our patient and previously reported patients with identified missense or nonsense mutations, or small insertions in UBE2A (Supplementary Table S1). Brain magnetic resonance imaging of our case showed hypoplasia of the corpus callosum and the basilar part of the pons (Figure 1a). Previous studies have shown white matter abnormalities in two patients, and such abnormalities were also observed in our patient (Figure 1b and Supplementary Table S1). ID, speech impairment and foot abnormalities were observed in all patients with UBE2A mutations, including our patient. Skin abnormalities were observed in our patient. Synophrys was observed in previously reported patients but not in our patient, while congenital cataract was observed in our patient, but not in other patients with UBE2A mutations. However, congenital cataract was observed in two patients, one with a 350 kb deletion at Xq24 (including the genes SLC25A43, LOC100303728, SLC25A5, CXorf56, UBE2A, NKRF and SEPT6) and the other with a 240 kb deletion at Xq24 (including the genes SLC25A43, LOC100303728, SLC25A5, CXorf56, UBE2A and NKRF).9 To date, UBE2A has been reported to be associated with neurological diseases, such as Alzheimer’s disease, Parkinson’s disease, ID and other progressive, age-related neurodegenerative disorders, in addition to X-linked ID type Nascimento.16 UBE2A normally plays important roles in the ubiquitin proteasome system. Dysfunction of the ubiquitin proteasome system causes disruption of gap junctions, resulting in disturbance of calcium homeostasis, hyperactivation of calpain, dysregulated differentiation and cataract.17 Further analysis is required to elucidate the molecular mechanisms underlying these associations.
Ube2a −/y mice show impaired hippocampal learning,14 which is consistent with the ID of our patient and of previously described patients with UBE2A mutations. By contrast, the patients show motor delay, but Ube2a −/y mice do not show an overt motor deficit or abnormalities in social behavior.14 In addition, >50% of previous patients have suffered from seizures, but Ube2a −/y mice have no known epilepsy phenotype.14 Thus, the phenotype of patients with X-linked ID type Nascimento varies. Further study of more patients is necessary to validate phenotype–genotype correlations.
In this study, we used whole-exome sequencing to identify a novel mutation in UBE2A in a Japanese patient with severe ID and congenital cataract. Our findings strongly suggest that UBE2A mutations are causative for X-linked ID type Nascimento.
Acknowledgments
We thank the patient and his family for participating in this work. We also thank S. Tsuji (Department of Neurology, Graduate School of Medicine, The University of Tokyo), S Morishita, and J Yoshimura (Department of Computational Biology, Graduate School of Frontier Sciences, The University of Tokyo) for their advice, and K Ida and M Umegae for technical assistance. This work was supported by MEXT KAKENHI (no 221S0002) (KK), CREST, JST (KK), Grant-in-Aid for Young Scientists (B) (no 15K19660) (YT) and the Yokohama Foundation for Advancement of Medical Science (YT).
Footnotes
Supplementary Information for this article can be found on the Human Genome Variation website (http://www.nature.com/hgv)
The authors declare no conflict of interest.
References
- Vissers LE, Gilissen C, Veltman JA. Genetic studies in intellectual disability and related disorders. Nat Rev Genet 2016; 17: 9–18. [DOI] [PubMed] [Google Scholar]
- Thevenon J, Michot C, Bole C, Nitschke P, Nizon M, Faivre L et al. RPL10 mutation segregating in a family with X-linked syndromic intellectual disability. Am J Med Genet A 2015; 167A: 1908–1912. [DOI] [PubMed] [Google Scholar]
- Nascimento RM, Otto PA, de Brouwer AP, Vianna-Morgante AM. UBE2A, which encodes a ubiquitin-conjugating enzyme, is mutated in a novel X-linked mental retardation syndrome. Am J Hum Genet 2006; 79: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiquitin-proteasome system. J Biosci 2006; 31: 137–155. [DOI] [PubMed] [Google Scholar]
- Thunstrom S, Sodermark L, Ivarsson L, Samuelsson L, Stefanova M. UBE2A deficiency syndrome: a report of two unrelated cases with large Xq24 deletions encompassing UBE2A gene. Am J Med Genet A 2015; 167A: 204–210. [DOI] [PubMed] [Google Scholar]
- Haddad DM, Vilain S, Vos M, Esposito G, Matta S, Kalscheuer VM et al. Mutations in the intellectual disability gene Ube2a cause neuronal dysfunction and impair parkin-dependent mitophagy. Mol Cell 2013; 50: 831–843. [DOI] [PubMed] [Google Scholar]
- Czeschik JC, Bauer P, Buiting K, Dufke C, Guillen-Navarro E, Johnson DS et al. X-linked intellectual disability type Nascimento is a clinically distinct, probably underdiagnosed entity. Orphanet J Rare Dis 2013; 8: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Orii KO, Kobayashi J, Hayashi S, Imamura A, Imoto I et al. Novel deletion at Xq24 including the UBE2A gene in a patient with X-linked mental retardation. J Hum Genet 2010; 55: 244–247. [DOI] [PubMed] [Google Scholar]
- de Leeuw N, Bulk S, Green A, Jaeckle-Santos L, Baker LA, Zinn AR et al. UBE2A deficiency syndrome: Mild to severe intellectual disability accompanied by seizures, absent speech, urogenital, and skin anomalies in male patients. Am J Med Genet A 2010; 152A: 3084–3090. [DOI] [PubMed] [Google Scholar]
- Budny B, Badura-Stronka M, Materna-Kiryluk A, Tzschach A, Raynaud M, Latos-Bielenska A et al. Novel missense mutations in the ubiquitination-related gene UBE2A cause a recognizable X-linked mental retardation syndrome. Clin Genet 2010; 77: 541–551. [DOI] [PubMed] [Google Scholar]
- Niranjan TS, Skinner C, May M, Turner T, Rose R, Stevenson R et al. Affected kindred analysis of human X chromosome exomes to identify novel X-linked intellectual disability genes. PLoS ONE 2015; 10: e0116454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker T, Zahir FR, Griffith M, Delaney A, Chai D, Tsang E et al. Single exon-resolution targeted chromosomal microarray analysis of known and candidate intellectual disability genes. Eur J Hum Genet 2014; 22: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen MH, de Leeuw N, de Brouwer AP, Pfundt R, Hehir-Kwa JY, Yntema HG et al. Interpretation of clinical relevance of X-chromosome copy number variations identified in a large cohort of individuals with cognitive disorders and/or congenital anomalies. Eur J Med Genet 2012; 55: 586–598. [DOI] [PubMed] [Google Scholar]
- Bruinsma CF, Savelberg SM, Kool MJ, Jolfaei MA, Van Woerden GM, Baarends WM et al. An essential role for UBE2A/HR6A in learning and memory and mGLUR-dependent long-term depression. Hum Mol Genet 2016; 25: 1–8. [DOI] [PubMed] [Google Scholar]
- Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 1992; 51: 1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Alexandrov PN, Jaber V, Lukiw WJ. Deficiency in the ubiquitin conjugating enzyme UBE2A in Alzheimer's disease (AD) is linked to deficits in a natural circular miRNA-7 sponge (circRNA; ciRS-7). Genes 2016; 7: e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Lyu L, Chin D, Gao J, Sun X, Shang F et al. Altered ubiquitin causes perturbed calcium homeostasis, hyperactivation of calpain, dysregulated differentiation, and cataract. Proc Natl Acad Sci USA 2015; 112: 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Data Citations
- Tsurusaki Yoshinori.HGV Database. 2017. 10.6084/m9.figshare.hgv.1361. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Tsurusaki Yoshinori.HGV Database. 2017. 10.6084/m9.figshare.hgv.1361. [DOI]