Abstract
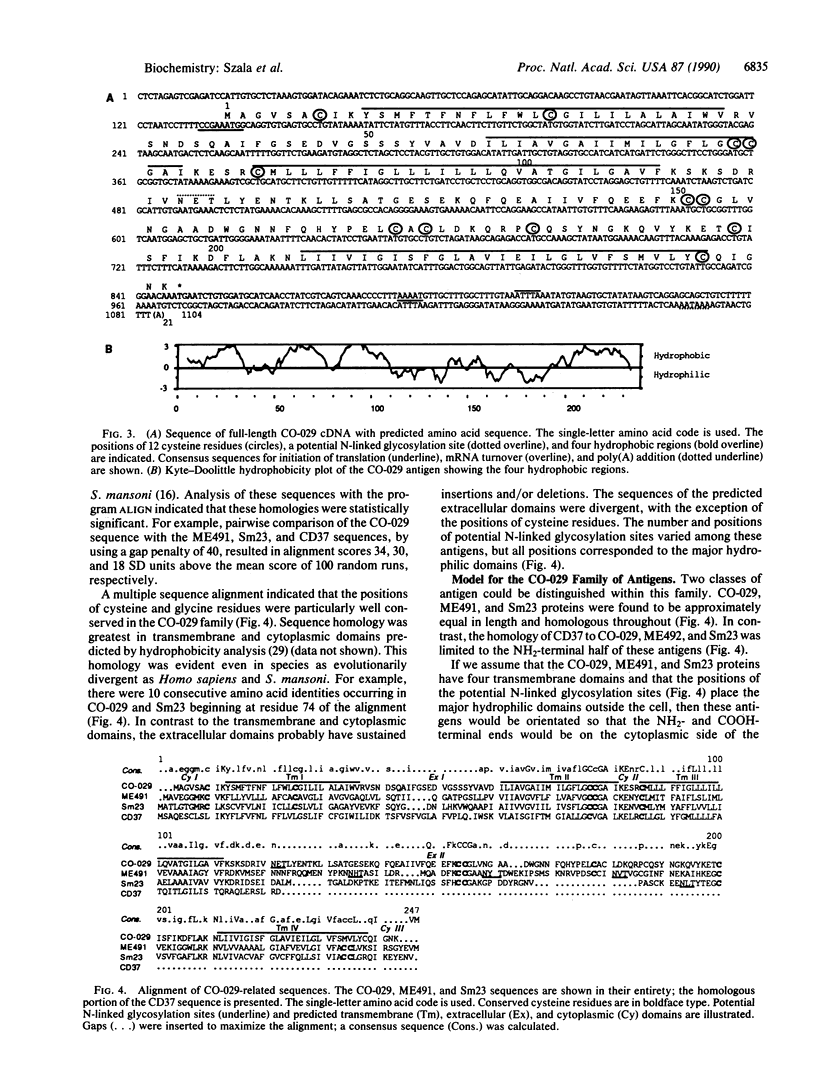
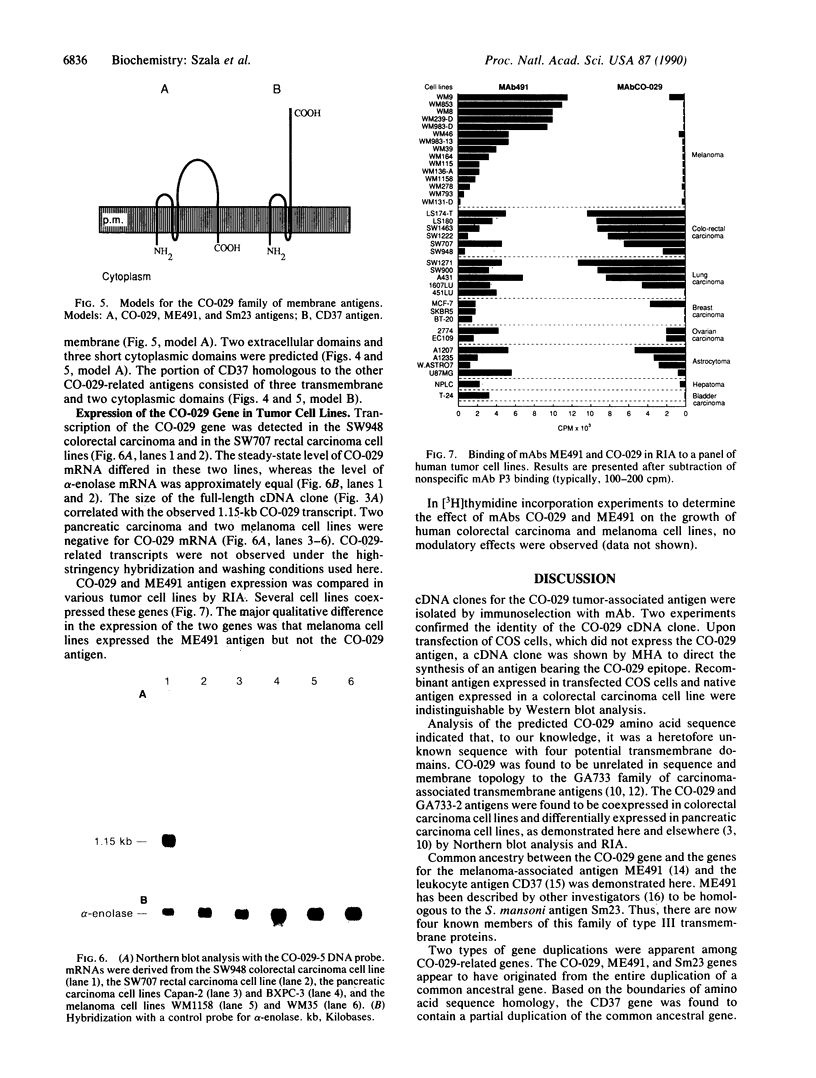
The human tumor-associated antigen CO-029 is a monoclonal antibody-defined cell surface glycoprotein of 27-34 kDa. By using the high-efficiency COS cell expression system, a full-length cDNA clone for CO-029 was isolated. When transiently expressed in COS cells, the cDNA clone directed the synthesis of an antigen reactive to monoclonal antibody CO-029 in mixed hemadsorption and immunoblot assays. Sequence analysis revealed that CO-029 belongs to a family of cell surface antigens that includes the melanoma-associated antigen ME491, the leukocyte cell surface antigen CD37, and the Sm23 antigen of the parasitic helminth Schistosoma mansoni. CO-029 and ME491 antigen expression and the effect of their corresponding monoclonal antibodies on cell growth were compared in human tumor cell lines of various histologic origins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Classon B. J., Williams A. F., Willis A. C., Seed B., Stamenkovic I. The primary structure of the human leukocyte antigen CD37, a species homologue of the rat MRC OX-44 antigen. J Exp Med. 1989 Apr 1;169(4):1497–1502. doi: 10.1084/jem.169.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giallongo A., Feo S., Moore R., Croce C. M., Showe L. C. Molecular cloning and nucleotide sequence of a full-length cDNA for human alpha enolase. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6741–6745. doi: 10.1073/pnas.83.18.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardet C., Vacca A., Schmidt-Kessen A., Schreyer M., Carrel S., Mach J. P. Immunochemical characterization of two antigens recognized by new monoclonal antibodies against human colon carcinoma. J Immunol. 1986 Feb 15;136(4):1497–1503. [PubMed] [Google Scholar]
- Göttlinger H. G., Funke I., Johnson J. P., Gokel J. M., Riethmüller G. The epithelial cell surface antigen 17-1A, a target for antibody-mediated tumor therapy: its biochemical nature, tissue distribution and recognition by different monoclonal antibodies. Int J Cancer. 1986 Jul 15;38(1):47–53. doi: 10.1002/ijc.2910380109. [DOI] [PubMed] [Google Scholar]
- Herlyn D., Herlyn M., Ross A. H., Ernst C., Atkinson B., Koprowski H. Efficient selection of human tumor growth-inhibiting monoclonal antibodies. J Immunol Methods. 1984 Oct 12;73(1):157–167. doi: 10.1016/0022-1759(84)90041-3. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Clark W. H., Jr, Mastrangelo M. J., Guerry D. P., 4th, Elder D. E., LaRossa D., Hamilton R., Bondi E., Tuthill R., Steplewski Z. Specific immunoreactivity of hybridoma-secreted monoclonal anti-melanoma antibodies to cultured cells and freshly derived human cells. Cancer Res. 1980 Oct;40(10):3602–3609. [PubMed] [Google Scholar]
- Herlyn M., Steplewski Z., Herlyn D., Koprowski H. CO 17-1A and related monoclonal antibodies: their production and characterization. Hybridoma. 1986 Jul;5 (Suppl 1):S3–10. [PubMed] [Google Scholar]
- Hoover H. C., Jr, Hanna M. G., Jr Active immunotherapy in colorectal cancer. Semin Surg Oncol. 1989;5(6):436–440. doi: 10.1002/ssu.2980050610. [DOI] [PubMed] [Google Scholar]
- Hotta H., Ross A. H., Huebner K., Isobe M., Wendeborn S., Chao M. V., Ricciardi R. P., Tsujimoto Y., Croce C. M., Koprowski H. Molecular cloning and characterization of an antigen associated with early stages of melanoma tumor progression. Cancer Res. 1988 Jun 1;48(11):2955–2962. [PubMed] [Google Scholar]
- Hotta H., Takahashi N., Homma M. Transcriptional enhancement of the human gene encoding for a melanoma-associated antigen (ME491) in association with malignant transformation. Jpn J Cancer Res. 1989 Dec;80(12):1186–1191. doi: 10.1111/j.1349-7006.1989.tb01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Mitchell K., Herlyn M., Herlyn D., Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979 Nov;5(6):957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Linnenbach A. J., Wojcierowski J., Wu S. A., Pyrc J. J., Ross A. H., Dietzschold B., Speicher D., Koprowski H. Sequence investigation of the major gastrointestinal tumor-associated antigen gene family, GA733. Proc Natl Acad Sci U S A. 1989 Jan;86(1):27–31. doi: 10.1073/pnas.86.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowicz-Szulczynska E. M., Koprowski H. Nuclear uptake of monoclonal antibody to a surface glycoprotein and its effect on transcription. Arch Biochem Biophys. 1989 Jun;271(2):366–379. doi: 10.1016/0003-9861(89)90286-5. [DOI] [PubMed] [Google Scholar]
- Ross A. H., Herlyn D., Iliopoulos D., Koprowski H. Isolation and characterization of a carcinoma-associated antigen. Biochem Biophys Res Commun. 1986 Feb 26;135(1):297–303. doi: 10.1016/0006-291x(86)90976-9. [DOI] [PubMed] [Google Scholar]
- Ross A. H., Lubeck M., Steplewski Z., Koprowski H. Identification and characterization of the CO17-1A carcinoma-associated antigen. Hybridoma. 1986 Jul;5 (Suppl 1):S21–S28. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela B. A., Steplewski Z., Koprowski H. Colon carcinoma-associated glycoproteins recognized by monoclonal antibodies CO-029 and GA22-2. Hybridoma. 1989 Aug;8(4):481–491. doi: 10.1089/hyb.1989.8.481. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Maher P. A., Yaffe M. P. On the transfer of integral proteins into membranes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1960–1964. doi: 10.1073/pnas.84.7.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad J., Hamilton A. E., Beavers L. S., Gamboa G. C., Apelgren L. D., Taber L. D., Sportsman J. R., Bumol T. F., Sharp J. D., Gadski R. A. Molecular cloning and characterization of a human adenocarcinoma/epithelial cell surface antigen complementary DNA. Cancer Res. 1989 Jan 15;49(2):314–317. [PubMed] [Google Scholar]
- Szala S., Froehlich M., Scollon M., Kasai Y., Steplewski Z., Koprowski H., Linnenbach A. J. Molecular cloning of cDNA for the carcinoma-associated antigen GA733-2. Proc Natl Acad Sci U S A. 1990 May;87(9):3542–3546. doi: 10.1073/pnas.87.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettendorff M., Iliopoulos D., Tempero M., Kay D., DeFreitas E., Koprowski H., Herlyn D. Idiotypic cascades in cancer patients treated with monoclonal antibody CO17-1A. Proc Natl Acad Sci U S A. 1989 May;86(10):3787–3791. doi: 10.1073/pnas.86.10.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M. D., Henkle K. J., Mitchell G. F. An immunogenic Mr 23,000 integral membrane protein of Schistosoma mansoni worms that closely resembles a human tumor-associated antigen. J Immunol. 1990 Apr 15;144(8):3195–3200. [PubMed] [Google Scholar]