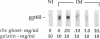Abstract
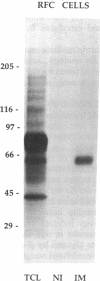
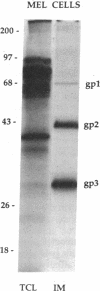
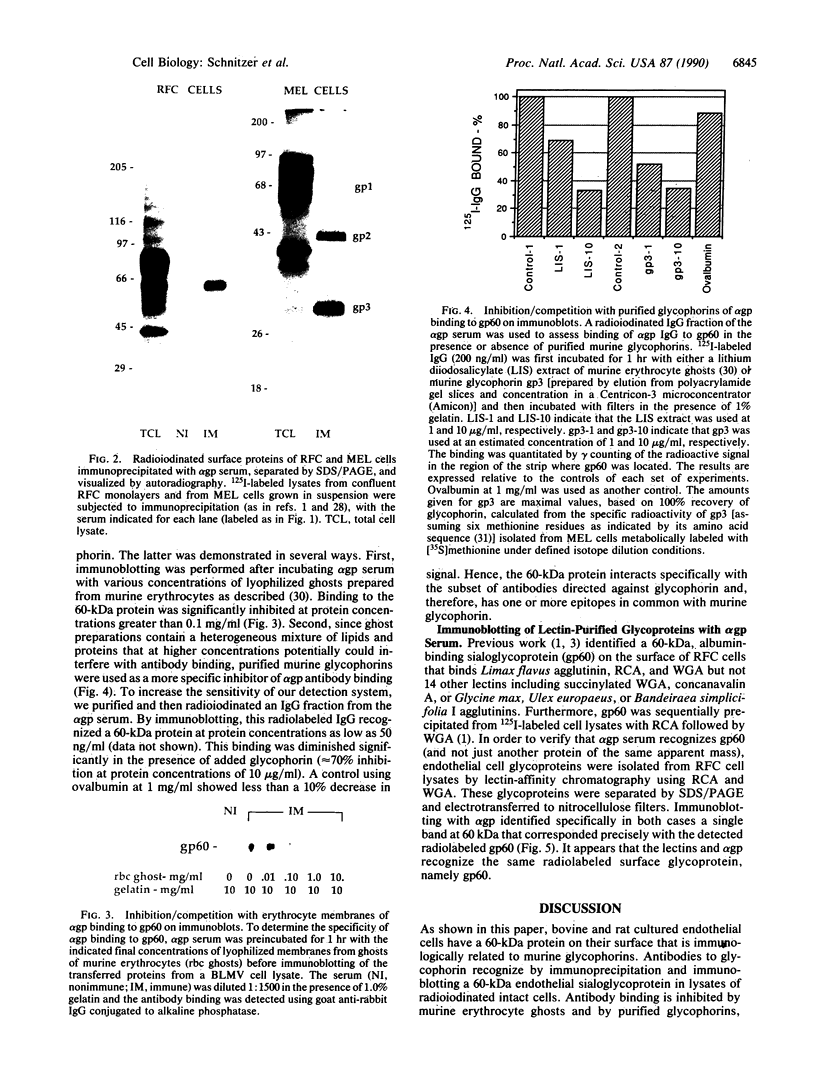
Glycophorins, the major sialoglycoproteins of red blood cells in many species, are generally considered to be specific to erythroid cells. Using polyclonal antibodies directed against mouse glycophorin (alpha gp), we have identified a glycoprotein antigenically related to glycophorin on the surface of bovine and rat cultured endothelial cells. Immunoblotting with alpha gp identified a single 60-kDa polypeptide on transfers of SDS/polyacrylamide gels of solubilized confluent endothelial monolayers. In addition, a 60-kDa polypeptide was immunoprecipitated by alpha gp from lysates of 125I-labeled intact endothelial cells. Controls with preimmune serum were negative. This antibody interaction was inhibited by murine erythrocyte ghosts and purified glycophorins. Our past work identified several endothelial surface sialoglycoproteins including a 60-kDa glycoprotein (gp60) that (i) interacts with albumin, (ii) binds Limax flavus, Ricinus communis, and Triticum vulgare agglutinins but not other lectins, (iii) is sequentially precipitated from 125I-labeled cell lysates by using R. communis agglutinin followed by T. vulgare agglutinin, and (iv) is sensitive to sialidase digestion. Immunoblotting of such precipitates with alpha gp demonstrates that lectins recognize the same glycoprotein, namely gp60. These results indicate that gp60, a major endothelial surface sialoglycoprotein, shares antigenic epitope(s) with glycophorin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. A., Marchesi V. T. Regulation of the association of membrane skeletal protein 4.1 with glycophorin by a polyphosphoinositide. Nature. 1985 Nov 21;318(6043):295–298. doi: 10.1038/318295a0. [DOI] [PubMed] [Google Scholar]
- Barsoum A. L., Bhavanandan V. P. Detection of glycophorin A-like glycoproteins on the surface of cultured human cells. Int J Biochem. 1989;21(6):635–643. doi: 10.1016/0020-711x(89)90383-2. [DOI] [PubMed] [Google Scholar]
- Barsoum A. L., Czuczman M. S., Bhavanandan V. P., Davidson E. A. Epitopes immunologically related to glycophorin A on human malignant and non-malignant cells in culture. Int J Cancer. 1984 Dec 15;34(6):789–795. doi: 10.1002/ijc.2910340609. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Barclay A. N., Sunderland C. A., Williams A. F. Identification of a glycophorin-like molecule at the cell surface of rat thymocytes. Nature. 1981 Feb 5;289(5797):456–460. doi: 10.1038/289456a0. [DOI] [PubMed] [Google Scholar]
- Chasis J. A., Reid M. E., Jensen R. H., Mohandas N. Signal transduction by glycophorin A: role of extracellular and cytoplasmic domains in a modulatable process. J Cell Biol. 1988 Oct;107(4):1351–1357. doi: 10.1083/jcb.107.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhermy D., Simeon J., Wautier M. P., Boivin P., Wautier J. L. Role of membrane sialic acid content in the adhesiveness of aged erythrocytes to human cultured endothelial cells. Biochim Biophys Acta. 1987 Nov 13;904(2):201–206. doi: 10.1016/0005-2736(87)90369-5. [DOI] [PubMed] [Google Scholar]
- Dolci E. D., Palade G. E. Ontogenetic expression of the murine erythrocyte glycophorins. J Cell Sci. 1989 May;93(Pt 1):191–197. doi: 10.1242/jcs.93.1.191. [DOI] [PubMed] [Google Scholar]
- Ghinea N., Eskenasy M., Simionescu M., Simionescu N. Endothelial albumin binding proteins are membrane-associated components exposed on the cell surface. J Biol Chem. 1989 Mar 25;264(9):4755–4758. [PubMed] [Google Scholar]
- Ghinea N., Fixman A., Alexandru D., Popov D., Hasu M., Ghitescu L., Eskenasy M., Simionescu M., Simionescu N. Identification of albumin-binding proteins in capillary endothelial cells. J Cell Biol. 1988 Jul;107(1):231–239. doi: 10.1083/jcb.107.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitescu L., Fixman A., Simionescu M., Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986 Apr;102(4):1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J., Parsons S. F., Anstee D. J., Bradley B. A. Evidence for the occurrence of human erythrocyte membrane sialoglycoproteins in human kidney endothelial cells. Vox Sang. 1988;55(2):104–108. doi: 10.1111/j.1423-0410.1988.tb05144.x. [DOI] [PubMed] [Google Scholar]
- Hawkins P., Anderson S. E., McKenzie J. L., McLoughlin K., Beard M. E., Hart D. N. Localization of MN blood group antigens in kidney. Transplant Proc. 1985 Apr;17(2):1697–1700. [PubMed] [Google Scholar]
- Huxley V. H., Curry F. E. Albumin modulation of capillary permeability: test of an adsorption mechanism. Am J Physiol. 1985 Feb;248(2 Pt 2):H264–H273. doi: 10.1152/ajpheart.1985.248.2.H264. [DOI] [PubMed] [Google Scholar]
- Jay A. W. Geometry of the human erythrocyte. I. Effect of albumin on cell geometry. Biophys J. 1975 Mar;15(3):205–222. doi: 10.1016/S0006-3495(75)85812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. I., Cook R. G., McEver R. P. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989 Mar 24;56(6):1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- Kasturi K., Harrison P. The cell specificity and biosynthesis of mouse glycophorins studied with monoclonal antibodies. Exp Cell Res. 1985 Mar;157(1):253–264. doi: 10.1016/0014-4827(85)90167-3. [DOI] [PubMed] [Google Scholar]
- Knupp C. L., Merrill R. H., Treadwell E. L. Coombs'-negative spherocytic hemolytic anemia and severe necrotizing renal vasculitis. South Med J. 1988 Feb;81(2):259–262. doi: 10.1097/00007611-198802000-00028. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Natori S., Obinata M. Isolation of the cDNA clone for mouse glycophorin, erythroid-specific membrane protein. Gene. 1989 Apr 30;77(2):325–332. doi: 10.1016/0378-1119(89)90080-2. [DOI] [PubMed] [Google Scholar]
- Mehta N. G. Role of membrane integral proteins in the modulation of red cell shape by albumin, dinitrophenol and the glass effect. Biochim Biophys Acta. 1983 Feb 16;762(1):9–18. doi: 10.1016/0167-4889(83)90110-6. [DOI] [PubMed] [Google Scholar]
- Milici A. J., Watrous N. E., Stukenbrok H., Palade G. E. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987 Dec;105(6 Pt 1):2603–2612. doi: 10.1083/jcb.105.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Doyle M. J., Jaffe E. A. Synthesis and secretion of thrombospondin by cultured human endothelial cells. J Cell Biol. 1982 May;93(2):343–348. doi: 10.1083/jcb.93.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzikowski C. Z., Steuden I., Wiedlocha A., Kieler J., Tromholt V. The Thomsen-Friedenreich antigen on human urothelial cell lines detectable by peanut lectin and monoclonal antibody raised against human glycophorin A. Anticancer Res. 1989 Jan-Feb;9(1):103–107. [PubMed] [Google Scholar]
- Rehfeld S. J. The interaction of albumin and concanavalin A with normal and sickle human erythrocytes. Biochem Biophys Res Commun. 1975 Sep 16;66(2):586–591. doi: 10.1016/0006-291x(75)90550-1. [DOI] [PubMed] [Google Scholar]
- Sage H., Vernon R. B., Funk S. E., Everitt E. A., Angello J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J Cell Biol. 1989 Jul;109(1):341–356. doi: 10.1083/jcb.109.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarris A. H., Palade G. E. Isolation and partial characterization of the sialoglycoprotein fraction of murine erythrocyte ghosts. J Cell Biol. 1982 Jun;93(3):583–590. doi: 10.1083/jcb.93.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger E. E., Hamelin M. Interaction of serum proteins with lung endothelial glycocalyx: its effect on endothelial permeability. Am J Physiol. 1984 Aug;247(2 Pt 2):H206–H217. doi: 10.1152/ajpheart.1984.247.2.H206. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E. Analysis of steric partition behavior of molecules in membranes using statistical physics. Application to gel chromatography and electrophoresis. Biophys J. 1988 Dec;54(6):1065–1076. doi: 10.1016/S0006-3495(88)83043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., Carley W. W., Palade G. E. Albumin interacts specifically with a 60-kDa microvascular endothelial glycoprotein. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6773–6777. doi: 10.1073/pnas.85.18.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., Carley W. W., Palade G. E. Specific albumin binding to microvascular endothelium in culture. Am J Physiol. 1988 Mar;254(3 Pt 2):H425–H437. doi: 10.1152/ajpheart.1988.254.3.H425. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E. Glycocalyx electrostatic potential profile analysis: ion, pH, steric, and charge effects. Yale J Biol Med. 1988 Sep-Oct;61(5):427–446. [PMC free article] [PubMed] [Google Scholar]
- Ulmer J. B., Dolci E. D., Palade G. E. Glycophorin expression in murine erythroleukaemia cells. J Cell Sci. 1989 Feb;92(Pt 2):163–171. doi: 10.1242/jcs.92.2.163. [DOI] [PubMed] [Google Scholar]
- Villeval J. L., Le Van Kim C., Bettaieb A., Debili N., Colin Y., el Maliki B., Blanchard D., Vainchenker W., Cartron J. P. Early expression of glycophorin C during normal and leukemic human erythroid differentiation. Cancer Res. 1989 May 15;49(10):2626–2632. [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells. Identification of a large precursor polypeptide chain. J Biol Chem. 1983 Feb 25;258(4):2065–2067. [PubMed] [Google Scholar]
- Williams A. R. The effect of bovine and human serum albumins on the mechanical properties on human erythrocyte membranes. Biochim Biophys Acta. 1973 Apr 25;307(1):58–64. doi: 10.1016/0005-2736(73)90024-2. [DOI] [PubMed] [Google Scholar]