Abstract
Oral administration of antigen induces regulatory T cells that express latent membrane-bound TGF-beta (LAP) and that have been shown to play an important role in the induction of oral tolerance. We developed an in vitro model to study modulation of LAP+ on CD4+ T cells. The combination of anti-CD3 mAb, anti-CD28 mAb and recombinant IL-2 induced expression of LAP on naïve CD4+ T cells, independent of FoxP3 or exogenous TGF-β. In vitro generated CD4+LAP+FoxP3− T cells were suppressive in vitro, inhibiting proliferation of naïve CD4+ T cells and IL-17A secretion by Th17 cells. Assessing the impact of different cytokines and neutralizing antibodies against cytokines we found that LAP induction was decreased in the presence of IL-6 and IL-21, and to a lesser extent by IL-4 and TNFα. IL-6 abrogated the in vitro induction of CD4+LAP+ T cells by STAT3 dependent inhibition of Lrrc32 (GARP), the adapter protein that tethers TGF-beta to the membrane. Oral tolerance induction was enhanced in mice lacking expression of IL-6R by CD4+ T cells and by treatment of wild-type mice with neutralizing anti-IL-6 mAb. These results suggest that pro-inflammatory cytokines interfere with oral tolerance induction and that blocking the IL-6 pathway is a potential strategy for enhancing oral tolerance in the setting of autoimmune and inflammatory diseases.
INTRODUCTION
Oral tolerance has classically been defined as the specific suppression of cellular and/or humoral immune responses to an antigen by prior administration of the same antigen by the oral route (1, 2). Lower doses of antigen trigger generation of Tregs, whereas higher doses favor anergy/ depletion of antigen specific T cells (3). Low dose of antigen-induced oral tolerance has been shown to be dependent on the presence of Th3 type Tregs that are characterized by their surface expression of latency-associated peptide (LAP) (4, 5) maintaining TGF-β in a latent state, and the adapter protein GARP (glycoprotein A repetitions predominant) that tethers the LAP/TGF-β complex to the membrane (6). LAP can be found on both activated CD4+FoxP3+ T cells (7, 8), as well as on CD4+FoxP3− T cells (8). CD4+LAP+ T cells contribute to infectious tolerance by providing TGF-β that can be activated by acidification, protease, plasmin, matrix metalloproteases, thrombospondin-1, and certain αv integrins (9). Once active, TGF-β can induce FoxP3 expression in CD4+FoxP3− T cells and inhibit T cell proliferation, Th1 differentiation and maturation of dendritic cells (9). Many studies have shown the effectiveness of oral tolerance for preventing or treating autoimmune disease (10-12). Clinical trials, however, although proven to be safe have found only limited therapeutic effect in patients with autoimmune disorders (5, 13).
Given that LAP+ Th3 type cells are induced in vivo during oral tolerance, to further investigate factors related to the induction of oral tolerance, we developed an in vitro system for the induction of LAP+ cells. We reasoned that factors that affect the in vitro induction of LAP+ cells may in turn apply to the induction of oral tolerance in vivo. We found that blocking of certain cytokines inhibited the induction of LAP+ T cells in vitro, with the most prominent being IL-6. IL-6 is a major pro-inflammatory cytokine that blocks TGF-β-induced Treg differentiation (14, 15) and polarizes CD4+ T cells in the presence of TGF-β towards Th17 (16) that are pathogenic in many autoimmune diseases (17). Moreover, it has been shown that IL-6 renders effector T cells resistant to Tregs (18, 19). Given our findings related to IL-6 in the induction of LAP+ T cells in vitro and the multiple effects of IL-6 on T cell regulation, we were then able to test the effect of blocking IL-6 on the induction of oral tolerance.
MATERIALS AND METHODS
Mice
C57BL/6.FoxP3GFP, C57BL/6.FoxP3GFPIL-10 Thy1.1, OTII.FoxP3GFP and 2D2.FoxP3GFP mice were bred and housed in our animal facility. C57BL/6, C57BL/6.CD45.1, C57BL/6.IL-6−/−, C57BL/6, Tg(Cd4-cre)1Cwi (CD4Cre) and B6.129S1-Stat3tm1Xyfu/J (STAT3flox/flox) mice were bought from Jackson and the latter were interbred in our animal facility to obtain CD4CreSTAT3flox/flox mice. 129S6/SvEv-Stat1tm1Rds (STAT1−/−) and 129S6/SvEvTac (129S6) control mice were purchased from Taconics. All experiments were performed under specific pathogen-free conditions in our animal facility at the Harvard Institutes of Medicine and according to the animal protocol guidelines of the Committee on Animals of Harvard Medical School, which also approved the experiments.
Antibodies and FACS analysis
Cells were stained in Mg2+ and Ca2+ free HBSS with 2% FCS, 0.4% EDTA (0.5 M) and 2.5% HEPES (1M) and either directly acquired or fixed in PBS containing 2.5% formaldehyde (Sigma–Aldrich, Steinheim, Germany). FoxP3 (FJK-16s) was detected by intracellular staining according to the manufacturer's instructions (eBioscience, San Diego, CA). Cells were acquired on a FACS LSRII (BD) or FACS Fortessa (BD) and analysed using FlowJo software. Fixable viability dyes eFluor780 or eFluor506, Sytox Red and Fluorochrome conjugated antibodies to mouse CD3 (145-2C11), CD4 (RM4-5), CD8a (53-6.7), GARP (YGIC86), CD62L (MEL-14), CD44 (IM7), CD25 (PC61.5), Thy1.1 (HIS51), FoxP3 (FJK-16s) were purchased from eBioscience. Anti-mouse LAP (TW7-16B4) was from BioLegend. Results show FACS staining after gating on life (Sytox negative) CD4+ T cells, apart from Fig. 1B, D and F that were gated on total lymphocytes.
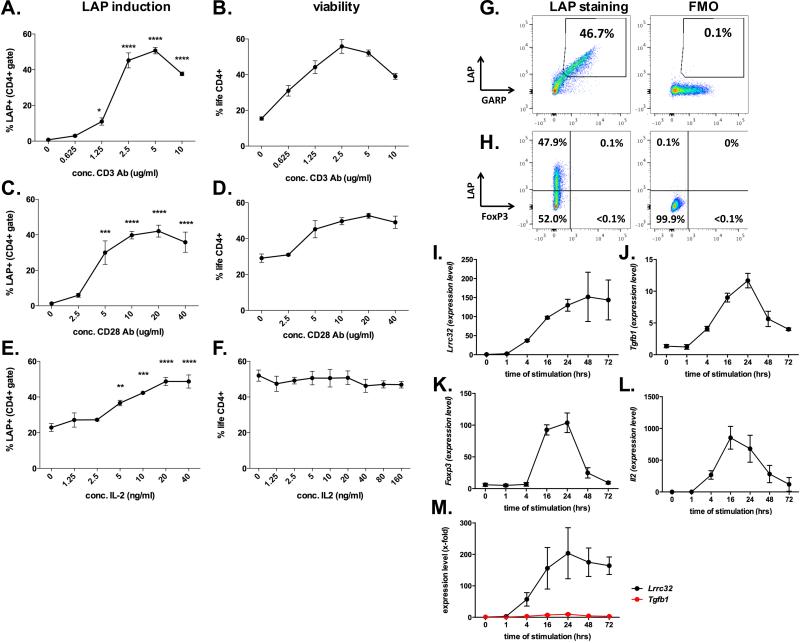
Fig. 1. In vitro induction of membrane bound TGF-β on naïve CD4+ T cells.
A-F. Percentage of LAP+ (A, C, E) and life CD4+ (B, D, F) T cells after culture of naïve CD4+ T cells with different titrations of plate bound anti-CD3 mAb (A, B), plate bound anti-CD28 mAb (C, D) and soluble IL-2 (E, F) keeping the other non-titrated variables constant (i.e. anti-CD3 mAb 1μg/ml, anti-CD28 mAb 10 μg/ml, IL-2 10 ng/ml). G-H. Representative dot blot showing LAP, GARP (G) and FoxP3 (H) staining as compared to FMO controls for LAP after three days of stimulation with anti-CD3 mAb (1μg/ml), anti-CD28 mAb (10 μg/ml) and IL-2 (10 ng/ml). I-L. Representative data from TaqMan PCR showing kinetics of gene expression of Lrrc32 (I; gene coding for GARP), Tgfb1 (J), Foxp3 (K) and Il2 (L) normalized to Gapdh under optimal conditions for LAP induction (anti-CD3 mAb 2.5μg/ml, anti-CD28 mAb 20 μg/ml, IL-2 20 ng/ml). M. X-fold induction of Llrc32 and Tgfb1 as compared to unstimulated naïve CD4+ T cells using data shown above (Fig.1I and K). Graphs show average values ± SEM for one representative experiment (out of three) with three independent samples. Statistical significant values are labeled: ***p<0.001, ****p<0.0001 (Ordinary one-way ANOVA with Tukey's multiple comparisons test)
Cell sorting
CD4+ T wells were enriched by magnetic activated cell sorting using the CD4+ isolation kit (Miltenyi) before surface staining and sorting on a FACS Aria in our cell sorting facility. The purity was superior to 98%.
T Cell Proliferation and Differentiation
Cells were cultured in IMDM supplemented with 10 % FCS, 5*105 M 2-mercaptoethanol and 100 units of penicillin and 100 μg of streptomycin per ml. In antigen-specific recall assays, 1 × 105/ml splenocytes or draining lymph node cells were cultured in 96 well round-bottom plates for 72 hrs with 100 μg/ml of MOG35-55 (University of California, Los Angeles) or endotoxin free OVA (InvivoGen), respectively. Supernatants were collected after 48 hours. During the last 16 hours, cells were pulsed with 1 μCi of 3H-thymidine (PerkinElmer) followed by harvesting on glass fiber filters and analysis of 3H-thymidine incorporation in a β-counter (1450 Microbeta, Trilux, PerkinElmer).
For in vitro T cell differentiation, naïve CD4+ cells from spleens and mesenteric lymph nodes were enriched by using anti-CD4 beads (Miltenyi) and further purified by flow cytometry (CD4+CD62L+CD44−Foxp3GFP− T cells or CD4+CD62L+CD44−CD25− T cells for the experiment using scurfy mice). T cells were stimulated for 3 days with plate-bound anti-CD3 mAb (145-2C11, 2.5 μg/ml) and anti-CD28 mAb (PV-1, 10 μg/mL). For antigen specific stimulation of CD4+ T cells from 2D2 mice, antigen presenting cells and MOG35-55 (10μg/ml) were added to the cell culture. The medium was supplemented with recombinant mouse cytokines and blocking mAb for differentiation of sorted naïve CD4+ T cells into Th0 (anti-IL-4 mAb (10 μg/ml, BVD4-1D11, BD), anti-IFNγ mAb (10 μg/ml, AN-18, BD)), Th1 (anti-IL-4 mAb (10 μg/ml), IL-12 (20 ng/ml, Biolegend)), Th2 (anti-IFNγ mAb (10 μg/ml), IL-4 (20 ng/ml, Biolegend)), Th17 (anti-IL-4 mAb (10 μg/ml), anti-IFNγ mAb (10 μg/ml), IL-6 (30 ng/ml, Biolegend), TGF-β1 (2 ng/ml, R&D systems)), iTreg (TGF-β1 (5 ng/ml), IL-2 (100 ng/ml, )) and iTh3 cells (IL-2 (20 ng/ml)). The mouse recombinant cytokines IL-1, IL-6, IL-7, IL-9, IL-10, IL-15, IL-21, IL-23, TNF-α and IFNγ were acquired from Biolegend
Neutralizing anti-mouse IL-6 mAb (MP5-20F3) and the isotype control rat IgG1 (HRPN) were from BioXCell (West Lebanon, NH, USA), anti-IL-4 mAb (11B11), anti-IL-10R mAb (1B1.3A), anti-IL-21 mAb (FFA21) and anti-IFNγ mAb (R4-6A2) were purchased from eBioscience.
In vitro suppression assay
Congenic responder cells (naïve CD4+ T cells or in vitro differentiated Th0, Th1, Th2 or Th17 cells from C57BL/6.CD45.1 mice) were stained with proliferation dye efluor670 (eBioscience) and plated in 96-well round bottom plates (1×105 cells/well) in IMDM medium and stimulated with 1 μg/ml soluble anti-CD3 mAb in the presence of mitomycin C (50 μg/ml) treated antigen presenting cells (1×105 cells/well) before adding the same number of in vitro generated iTreg (CD4+FoxP3+) or iTh3 cells (CD4+LAP+FoxP3−) that had been sorted after 3 days of culture. Proliferation of responder cells was assessed after 72 hours of coculture by flow cytometry. Supernatants were kept for measuring cytokines.
Cytokine ELISA
Supernatants were harvested after 48 - 72 hours of culture and the concentrations of indicated cytokines were measured by quantitative capture ELISA according to the guidelines of the manufacturer (R&D Systems).
Real-time PCR
Indicated cell populations were harvested, RNA was extracted with mirVana kit (#AM1560; Applied Biosystems), reverse-transcribed with a high capacity cDNA reverse transcription kit (Applied Biosystems) and analyzed by quantitative RT-PCR using a Vii 7 Real-time PCR system (Applied Biosystems) with the following primers and probes (from Applied Biosystems; identifier in parentheses): Tgfb1 (Mm00441724_m1), Lrrc32 (Mm01273954_m1), FoxP3 (Mm00475156_m1) and Il-2 (Mm00434256_m1), Il6 (Mm00446190_m1), Il6ra (Mm00439653_m1), Il17a (Mm00439619_m1), Il21 (Mm00517640_m1), Il21r (Mm00600319_m1). The comparative threshold cycle method and the internal control Gapdh (Mm99999915-g1) was used for normalization of the target genes.
Oral tolerance induction
6 - 10 week old mice were fed, depending on the experiment, during 5 consecutive days with either OVA (8mg/ml) in the drinking water. Anti-IL-6 mAb or isotype control were administered by i.p. injection on days −1 (250 μg), +2 (125 μg) and +4 (125 μg) of OVA feeding. Tofacitinib (10 mg/kg; Selleckchem) was administered orally from day −1 until day 5 of OVA feeding
Delayed type hypersensitivity (DTH): Immunization protocol, recall and skin testing
Indicated mouse strains were injected s.c. with 50μg of OVA (Sigma) in CFA (Difco Laboratories) in the ventral flanks. In vitro recall response was measured at day 10 after immunization. Briefly, spleen cells were isolated and stimulated with indicated concentrations of LPS free OVA (Sigma) and proliferation was measured using 3H-thymidine incorporation. Skin testing was performed 3 weeks after immunization by s.c. injection 60 μg OVA into the left hind footpad and PBS in the right one. Footpad thickness was measured 24, 48 and 72 hours after challenge. The net increase of footpad thickness was expressed as the arithmetic mean ± SEM of each group.
Statistical Analysis
GraphPad Prism 6.0 was used for performing Student's t test for comparisons between two groups or where appropriate ordinary one-way ANOVA, followed by Tukey's or Dunnett's multiple comparisons test. A p-value < 0.05 was considered statistically significant.
RESULTS
In vitro induction of membrane TGF-β by anti-CD3 mAb, anti-CD28 mAb and recombinant IL-2
To better understand the impact of cytokines on expression of LAP on CD4+ T cells, we developed an in vitro system to generate LAP+GARP+CD4+ T cells. We FACS-sorted naïve CD4+ T cells and stimulated them with increasing concentrations of coated anti-CD3 mAb (Fig. 1A, B; 0 – 10 μg/ml), coated anti-CD28 mAb (Fig. 1C, D; 0 – 40μg/ml) and recombinant mouse IL-2 (IL-2) (Fig. 1E, F; 0 – 40 ng/ml) while keeping the other two variables constant, i.e., anti-CD3 mAb (1 μg/ml), anti-CD28 mAb (10 μg/ml) and IL-2 (10 ng/ml). LAP expression (Fig. 1A, C, E) and cell viability (Fig. 1B, D, F) were analyzed by flow cytometry after 3 days of culture. We found that the best condition to induce LAP while maximizing cell viability was by using 2.5 μg/ml or 5 μg/ml of anti-CD3 mAb (45.2% ± 4.2% and 50.7% ± 1.8% LAP+ T cells, 55.8% ± 3.8% and 52.1 ± 1.7% life CD4+ T cells, respectively; Fig. 1A, B), 10 – 20 μg/ml of anti-CD28 mAb (39.8% ± 2.1% and 42.1% ± 3.4% LAP+ T cells, 49.7% ± 2.0% and 52.8 ± 1.4% life CD4+ T cells respectively; Fig. 1C, D) and 20 ng/ml IL-2 (48.7% ± 2.3% LAP+ T cells and 50.8% ± 3.8% life CD4+ T cells; Fig. 1E, F). LAP-expressing CD4 T cells were also positive for GARP (Fig. 1G), but negative for FoxP3 (Fig. 1H). Analysis of gene expression by RT-PCR showed that both Lrrc32 (Fig. 1I) and Tgfb1 (Fig. 1J) were induced between 1 and 4 hours and increased until 24 hours of T cell stimulation. Lrrc32 mRNA was highly expressed up to at least 72 hours while Tgfb1 levels dropped after 24 hours, similarly to mRNA levels of Foxp3 (Fig. 1K) and Il2 (Fig. 1L). Even though the mRNA level of Tgfb1 surpassed the relative expression of Lrrc32, the x-fold induction as compared to unstimulated cells was superior for Lrrc32 (with a maximum of 204-fold at 24 hours as compared to 10-fold for Tgfb1, Fig. 1M). These observations indicate that the expression level of LAP is determined by the availability of GARP/LRRC32.
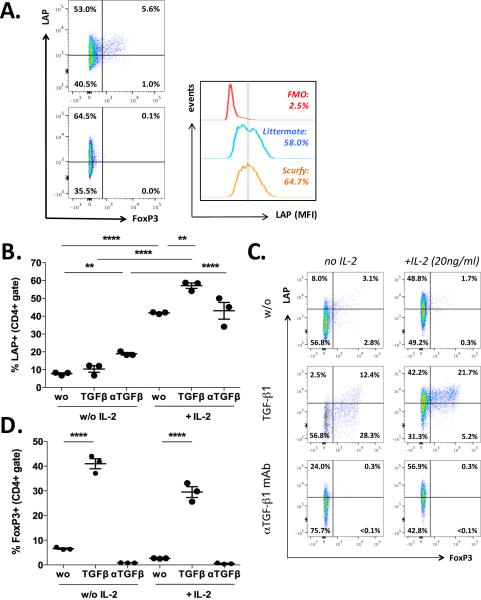
Because of the transient induction of Foxp3, we tested whether the expression of LAP was dependent on FoxP3. The observation that LAP induction was functional in naïve CD4+ T cells from scurfy mice, which are deficient for Foxp3, as compared to age matched littermates (Fig. 2A) demonstrated that LAP induction is independent of FoxP3. Consistent with this, we did not detect any major impact of extrinsic TGF-β1 on in vitro induction of LAP (Fig. 2B, C) as compared to FoxP3 induction (Fig. 2C, D). In the absence of IL-2, LAP expression was significantly increased when a neutralizing mAb against TGF-β (from 7.8 ± 0.5% to 18.9 ± 0.7%) was added, though adding recombinant TGF-β1 in the culture did not significantly change the induction of LAP expression (10.4 ± 1.8%). However, in the presence of IL-2, TGF-β1 increased the expression of LAP from 41.8 ± 0.3% to 57.1 ± 1.5%, whereas neutralization of TGF-β did not impair LAP induction (Fig. 2B, C).
Fig. 2. Induction of membrane bound TGF-β is independent on FoxP3 and exogenous TGF-β.
A. LAP induction on naïve CD4+ T cells from Scurfy mice as compared to heterozygous littermates after three day of culture with anti-CD3 mAb (2.5μg/ml), anti-CD28 mAb (20 μg/ml) and IL-2 (20 ng/ml). B-D. Percentage of LAP+ (B, C) or FoxP3+ T cells (C, D) after culturing naïve CD4+ T cells from wild-type mice with anti-CD3 mAb 2.5μg/ml and anti-CD28 mAb 20 μg/ml in the presence or absence of IL-2 (20 ng/ml), TGF-β1 (3 ng/ml) and/or neutralizing mAb against TGF-β (αTGF-β; 20 μg/ml). Graphs are representative for at least three independent experiments. Statistical significant values are labeled: **p<0.01, ****p<0.0001 (Ordinary one-way ANOVA with Tukey's multiple comparisons test).
To investigate the regulatory capacity of in vitro generated CD4+LAP+FoxP3− T cells we performed in vitro suppression assays. In vitro generated CD4+LAP+FoxP3− T cells significantly inhibited CD3 antibody induced proliferation of naïve CD4+ T cells (Supplementary Fig. 1A, B), increasing the percentage of non-proliferating cells from 9.0% ± 0.8% to 44.5% ± 0.6%), even though they were slightly less potent as compared to in vitro generated CD4+FoxP3+ Treg (Supplementary Fig. 1A, B; 55.2% ± 3.7%). The suppressive effect of CD4+LAP+FoxP3− T cells was partially dependent on TGF-β but independent of IL-10 as assessed by the use of blocking antibodies against TGF-β (27.6% ± 1.4% non-proliferating cells) or IL-10R (48.9% ± 1.1%), respectively (Supplementary Fig. 1C, D). We also analyzed the effect of CD4+LAP+FoxP3− T cells on cytokine secretion of in vitro polarized Th1 and Th17 cells. CD4+LAP+FoxP3− T cells inhibited secretion of IL-17A by in vitro generated Th17 cells (Supplementary Fig. 1E; 30.8 ± 15.4 pg/ml versus 365.9 ± 28.7 pg/ml) but not IFN-γ secretion by Th1 cells (Supplementary Fig. 1F; 25.6 ± 4.4 ng/ml versus 30.6 ± 3.8 pg/ml). In fact, CD4+LAP+FoxP3− T cells themselves secreted high levels of IFN-γ (22.1 ± 4.3 ng/ml).
Taken together, these data demonstrate that TCR signaling, combined with strong co-stimulation and IL-2, induces high expression of LAP on naïve CD4+ T cells in a FoxP3 and exogenous TGF-β independent fashion. These CD4+LAP+ T cells have regulatory activity in vitro.
IL-6 inhibits induction of LAP in vitro
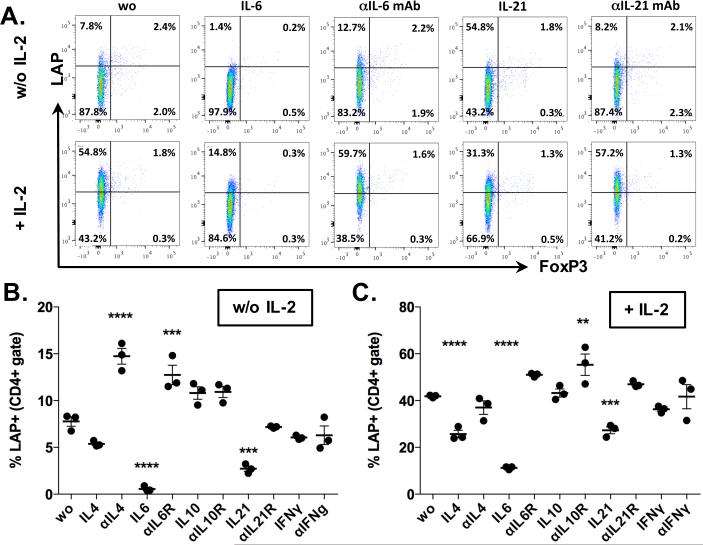
The above described cell culture system enabled us to screen for cytokines that modify the expression of LAP on CD4+ T cells. FACS-sorted naïve CD4+ T cells were stimulated with coated anti-CD3 mAb (2.5μg/ml) and anti-CD28 mAb (10 μg/ml) without (Fig. 3A, B Supplementary Fig. 2A, B) or with (Fig. 3A, C, Supplementary Fig. 2A, C) recombinant mouse IL-2 (20 ng/ml), in the presence or absence of cytokines or neutralizing antibodies against the indicated cytokines (Fig. 3A-C, Supplementary Fig. 2). We found that both IL-6 (20 ng/ml) and IL-21 (20 ng/ml) significantly inhibited LAP induction on CD4+ T cells in the presence or absence of IL-2 (Fig. 3A, B, C). Addition of either IL-6 or IL-21 to the IL-2 free cell culture decreased the percentage of LAP+ T cells from 7.8 ± 0.9% to 0.6 ± 0.3% or 2.7 ± 0.5%, respectively. In the presence of IL-2, IL-6 and IL-21 reduced the percentage of LAP+CD4+ T cells from 41.8 ± 0.6% to 11.2 ± 0.6% or 27.3 ± 2.6%, respectively. The inhibitory effect of IL-6 and IL-21 was reversed by addition of neutralizing mAb against these cytokines (Fig. 3A-C). While IL-4 also inhibited LAP (Fig. 3A, B, Supplementary Fig. 2), addition of IL-10, IFNγ, IL-1, IL-7, IL-9, IL-17A or IL-23 (all at 20 ng/ml) did not significantly affect LAP induction (Fig. 3A-C; Supplementary Fig. 2). However, we observed a small increase in LAP expression in the presence of anti-IL-10 mAb (Fig. 3B, C; Supplementary Fig. 2) and in the presence of TNFα (in absence of IL-2; Supplementary Fig. 2B), whereas IL-15 reduced the percentage of LAP+ T cells in the presence of IL-2 (Supplementary Fig. 2C). Because IL-6 was shown to have the highest inhibitory effect on LAP induction in both absence and presence of exogenous IL-2, and it is known to interfere with other pathways that are involved in tolerance induction, such as generation of regulatory T cells (14-16) and sensitivity of effector T cells to regulation (18, 19), we focused our next experiments on this cytokine. IL-6 also inhibited the induction of LAP on CD4+ T cells stimulated with their cognate antigen as shown by co-culture of naïve CD4+ T cells from 2D2 mice with antigen presenting cells and MOG35-55 (Supplementary Fig. 3).
Fig. 3. Influence of cytokines on in vitro induction of membrane bound TGF-β on naïve CD4+ T cells.
A-C. Percentage of CD4+LAP+ T cells after stimulation of naïve CD4+ T cells with coated anti-CD3 mAb (1μg/ml), anti-CD28 mAb (10 μg/ml) without (A, B) or with (A, C) IL-2 (20 ng/ml) and indicated cytokines (20 ng/ml) or neutralizing mAb (20 μg/ml). Graphs show average values ± SEM for one out of at least three independent experiments with three samples. Statistically significant values are labeled; **p<0.01, ***p<0.001, ****p<0.0001. (Ordinary one-way ANOVA with Dunnett's multiple comparisons test).
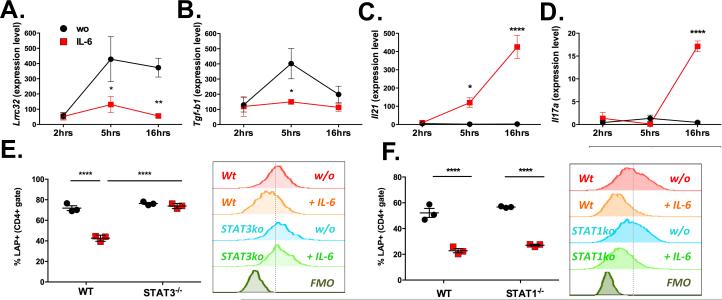
Analysis of mRNA levels of Lrc32 and Tgfb1 by RT-PCR showed that IL-6 significantly inhibited the transcription of both, Lrrc32 and Tgfb1 (Fig. 4A and B). While stimulation of naïve CD4+ T cells increased Lrrc32 mRNA levels from 156.8 ± 21.6 to 373.0 ± 61.6 at 16 hours after stimulation, addition of IL-6 maintained Lrrc32 mRNA at baseline levels (55.7 ± 10 .4 at 16 h; Fig. 4A). IL-6 also blocked the temporary increase of Tgfb1 mRNA that was observed 5 hours after stimulation Fig. 4B), and highly upregulated expression of Il21 mRNA at 16 hours (Fig. 4C). Induction of Il17 mRNA in response to IL-6 served as positive control for the biological function of IL-6 (Fig. 4D).
Fig. 4. IL-6R signaling inhibits llrc32 expression via STAT3.
A-D. Expression levels of Lrrc32 (A), Tgfb1 (B), Il21 (C) and Il17a (D) normalized to Gapdh after stimulation of naïve CD4+ T cells with coated anti-CD3 mAb, anti-CD28 mAb and IL-2 in the absence (black circles) presence of IL-6 (red squares) for 2, 5 or 16 hours. Graphs show average values ± SEM for three to five independent experiments. E-F. Percentage of CD4+LAP+ T cells after stimulation of naïve CD4+ T cells from CD4CreSTAT3flox/flox (STAT3−/−; E), STAT1−/− (F) mice as compared to wild-type (WT) mice with coated anti-CD3 mAb, anti-CD28 mAb and IL-2 in the absence (black circles) or presence of IL-6 (red squares) for 72 hours. Graphs show average values ± SEM for one representative experiment with three to four samples each and a representative histogram. Statistically significant values are labeled; ***p<0.001, ****p<0.0001 (Unpaired t-test for respective time-points in graphs A-G; Ordinary one-way ANOVA with Tukey's multiple comparisons test for graphs H-K).
IL-6 inhibits in vitro induction of LAP in a STAT3 dependent manner
IL-6 binding to the IL-6R (a heterodimer consisting out of the IL-6Rα chain and gp130) triggers phosphorylation of the transcription factors STAT3 and STAT1. We investigated if IL-6 mediated inhibition of membrane TGF-beta was mediated by STAT3 or STAT1 by using mice deficient for STAT3 expression by CD4+ T cells (CD4CreSTAT3flox/flox) or knockout mice for STAT1 (STAT1−/−). IL-6 (20 ng/ml) inhibited the induction of LAP in response to anti-CD3 mAb, anti-CD28 mAb and IL-2 in wild-type mice (Fig. 4E-F), but this effect was completely abrogated in CD4+ T cells from CD4CreSTAT3flox/flox mice (73.9 ± 1.4% versus 42.6 ± 1.7%; Fig. 4E). However, STAT1 (Fig. 4F) was not required for the inhibitory effect of IL-6 on LAP/GARP induction. Thus, IL-6-induced LAP inhibition is mediated by the STAT3, but not STAT1, signaling pathway.
Analysis of CD4+LAP+ T cells in the absence of IL-6 under homeostatic conditions in vivo
To further investigate the effect of IL-6 on the generation of CD4+LAP+ T cells, we cultured naïve CD4+ T cells from either wild-type or IL-6−/− mice in the presence of anti-CD3 mAb, anti-CD28 mAb and IL-2, as described above, and found that CD4+ T cells from IL-6−/− mice expressed higher LAP levels as compared to wild-type mice (55.4 ± 3.3% versus 46.3 ± 0.8%; Supplementary Fig. 4A, B). To analyze the impact of IL-6 on LAP expression under homeostatic conditions in vivo, we performed FACS analysis of CD4+ T cells from several organs of IL-6−/− and wild-type mice. Both IL-6−/− and wild-type mice had comparable levels of CD4+LAP+ T cells in spleen (Supplementary Fig. 4C), mesenteric lymph nodes (mLN; Supplementary Fig. 4D) and Peyer's patches (PP; Supplementary Fig. 4E). However, we detected a significant increase in the frequency of CD4+LAP+ T cells in the lung (5.5 ± 0.5% versus 3.9 ± 0.5%; Supplementary Fig. 4F) and a slightly, but not statistically significant increase in the liver of IL-6−/− mice (3.9 ± 1.6% versus 2.2 ± 0.9%; Supplementary Fig. 4G). The frequency of CD4+FoxP3+ T cells was significantly increased in the spleen (10 .1 ± 0.9% versus 9.2 ± 0.5%; Supplementary Fig. 4H) and mLN (8.1 ± 0.2% versus 7.1 ± 0.4%; Supplementary Fig. 4I) and a positive trend in Peyer's patches (Supplementary Fig. 4J), lung (Supplementary Fig. 4K) and liver (Supplementary Fig. 4L) of IL-6−/− mice.
Blocking IL-6 signaling in CD4+ T cells enhances oral tolerance induction
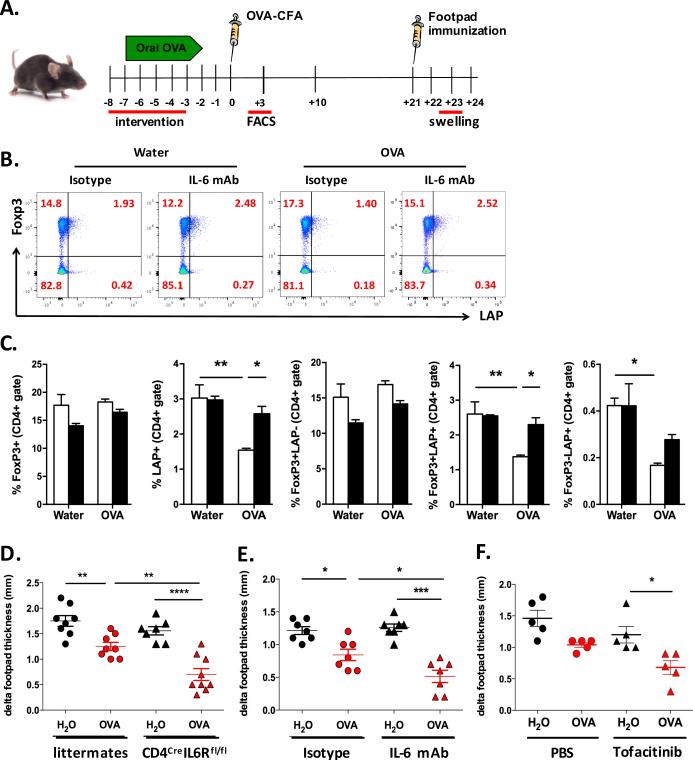
Based on our results above, we asked whether the absence of IL-6 would enhance oral tolerance induction in vivo. We used the classic delayed hypersensitive reaction (DTH) model, in which OVA feeding prior to immunization with OVA in CFA protects mice from footpad swelling after s.c. injection of OVA into the footpad (for experimental set-up see Fig. 5A). First we analyzed the frequency of CD4+FoxP3+ and CD4+LAP+ T cells three days after immunization. In contrast to what we observed under homeostatic conditions, neutralization of IL-6 during OVA feeding significantly increased the percentage of CD4+LAP+ T cells (2.6 ± 0.2% versus 1.5 ± 0.1%; Fig. 5B, C), in particular the percentage of CD4+FoxP3+LAP+ T cells (2.3 ± 0.1% versus 1.4 ± 0.1%; Fig. 5B, C) 3 days after CFA-OVA immunization, whereas there was no change in CD4+FoxP3+ T cell frequency (Fig. 5B, C). Of note, without previous neutralization of IL-6 the percentage of CD4+LAP+ (both FoxP3+ and FoxP3−) T cells from OVA fed mice was reduced as compared to controls (1.5 ± 0.1% as compared to 3.0 ± 0.4%; Fig. 5B, C). When assessing the footpad swelling in response to challenge with OVA 3 weeks after immunization we found that CD4CreIL-6Rflox/flox mice had significantly improved DTH response after OVA feeding as compared to wild-type mice (0.7 ± 0.1mm versus 0.9 ± 0.2mm footpad swelling; Fig. 5D). Similarly, blocking IL-6 during OVA feeding by i.p. injection of IL-6 mAb one day before OVA feeding, day +2 and day +4 significantly reduced footpad swelling as compared to mice that received isotype control (0.5 ± 0.1mm versus 0.8 ± 0.1mm; Fig. 5E). Importantly, the DTH response was not altered by injection of IL-6 mAb alone, without OVA feeding (1.3 ± 0.2mm versus 1.2 ± 0.2mm; Fig. 5E). We also observed a trend towards improved oral tolerance induction when mice were orally treated with the JAK inhibitor tofacitinib (10 mg/kg) during OVA feeding (1.0 ± 0.2mm versus 0.7 ± 0.1mm; Fig. 5F).
Fig. 5. Delayed type hypersensitivity (DTH) in the absence of IL-6R signaling.
A. Experimental set-up. Oral tolerance was induced by OVA feeding in the drinking water for five days before immunization with OVA-CFA. Interventions (i.e. anti-IL-6 mAb (IL-6 mAb i.p. day −8: 250μg, days −6 and −4: 125μg each; or tofacitinib (15mg/kg by gavage) took place during the time of tolerance induction, to wean off before immunization. Footpad challenge was performed 3 weeks after immunization. B-C. FACS analysis of spleen cells for expression of FoxP3GFP and LAP on CD4+ T cells three days after immunization with OVA-CFA. OVA fed mice or controls received anti-IL-6 mAb or isotype control as described in 5A. Graphs show representative dot blots (B) or average values ± SEM (C; n=4). D-F. DTH response of CD4CreIL6Rαflox/flox mice as compared to littermate controls (D; n=7-9/group), after treatment of wild-type mice with neutralizing anti-IL-6 mAb (E; n=7/group) or tofacitinib (F; n=5/group) as compared to isotype control (E) or PBS (F). Graphs show the difference of swelling between the OVA challenged footpad as compared to the PBS injected one. B and C show data from two independent experiments. Statistically significant values are labeled; *p<0.05, **p<0.01, ***p<0.001 (Ordinary one-way ANOVA with Tukey's multiple comparisons test).
DISCUSSION
We optimized an in vitro system that allowed us to investigate the function and regulation of membrane-bound TGF-β (LAP) on CD4+ T cells and to study the modulation of LAP and GARP expression by cytokines. To our knowledge this is the first study to optimize in vitro conditions for inducting membrane-bound TGF-β on naïve CD4+ T cells without co-expression of FoxP3. The expression of LAP and GARP on T cells has been mostly applied to FoxP3+CD4+ T cells. Edwards et al. found that LAP was enhanced on FoxP3+CD4+ T cells in vitro, though only a small subpopulation of FoxP3-CD4+ T cells expressed GARP after 72 h of stimulation (20), probably because of the strong TCR signaling and co-stimulation requirements we report in this study. We showed that membrane expression of TGF-β correlated with transcription of Lrrc32 but not Tgfb1, suggesting that LAP is regulated in CD4+ T cells by transcriptional control of Lrrc32. Consistent with this, it has been previously reported that GARP is essential for the surface expression of LAP on platelets and activated FoxP3+ regulatory T cells (6) and according to our data, this also applies to total CD4+ T cells. Indeed, it has been shown that LAP can be expressed on both FoxP3+ and FoxP3− CD4+ T cells (7, 8). We found that neither FoxP3 nor TGF-β1 were involved in LAP/GARP expression on CD4+ T cells in our in vitro system, indicating that only TCR activation and co-stimulatory factors are needed to LAP/GARP expression. In vitro induced CD4+LAP+GARP+ inhibited proliferation of naive T cells and secretion of IL-17 by in vitro induced Th17 cells. This effect was dependent on TGF-β and it has been recently shown that anti-GARP mAb can block the production of active TGF-β1 (21). However, these CD4+LAP+GARP+ T cells did not inhibit production of IFNγ by in vitro differentiated Th1 cells. We conclude that in vitro generated CD4+LAP+GARP+ are not equal to LAP+ cells that are induced during oral tolerance and that have been shown to suppress Th1 responses in vivo (22). However, this in vitro model is very interesting for identifying and studying compounds that modulate membrane bound TGF-β.
By testing the impact of cytokines on LAP expression in vitro, we found that IL-6 potently inhibited the expression of LAP on CD4+ T cells, an effect related to the blocking of Lrrc32 transcription. Furthermore, STAT3, but not STAT1, which is well known to mediate IL-6 functions (23), was involved in the blockage of LAP expression on CD4+ T cells by anti-CD3 mAb, anti-CD28 mAb and IL-2. Our finding that IL-6 blocks expression membrane-bound TGF-β1 on CD4+ T cells is consistent with the observation that IL-6 polarizes T cells towards Th17 in the presence of TGF-β (16) and inhibits the generation of FoxP3+ Treg (15, 18).
Although IL-21 also inhibited LAP expression on CD4+ T cells in a STAT3 dependent fashion in vitro we focused our studies on IL-6 for two main reasons: first, IL-6 had the strongest inhibitory effect on LAP induction; and second, the high translational potential for therapies targeting the IL-6R pathway, as anti-IL-6R mAb is an FDA approved drug for the treatment of rheumatoid arthritis. We found that oral tolerance was enhanced in the absence of IL-6R signaling. CD4CreIL-6Rflox/flox mice, in which CD4+ T cells are unable to response to IL-6, had significantly enhanced oral tolerance. Furthermore, blocking of IL-6 during the time of oral tolerance induction enhanced the therapeutic effect of OVA feeding, indicating that a short intervention with anti-IL6 was sufficient to enhance oral tolerance. The enhanced oral tolerance induction after neutralization of IL-6 correlated with an increase of CD4+LAP+, in particular of CD4+FoxP3+LAP+ T cells in the OVA fed group. Of note, the percentage of CD4+LAP+ T cells was decreased in spleens of OVA fed mice as compared to control mice when IL-6 was not neutralized even though oral tolerance was operational. Possible explanations might be that LAP+ T cells had migrated to the site of inflammation (immunization with CFA-OVA) and/or membrane LAP/TGF-b became activated and thus shed from the cell surface as a means of regulation. It seems unlikely that the low percentage of CD4+LAP+ in OVA fed mice reflects a decreased induction of these cells as our previous publications on other disease models reported an increase of tolerogenic CD4+LAP+ T cells in oral tolerance (22, 24, 25). Future studies will further address this observation.
One of the reasons that oral antigen alone may not be sufficient to induce clinical relevant tolerance in humans could relate to ongoing inflammation in the host and our results suggest that it could be related in part to the inhibitory effect of IL-6 on inducing Tregs.
In summary, our data demonstrate that IL-6 inhibits regulatory T cells induction not only by blocking the de novo generation of Foxp3+ Tregs (14, 15) and inducing Th17 cells (16), but also by inhibiting expression of membrane-bound TGF-β. Thus, neutralization of IL-6 during tolerance-promoting therapies, particularly those relying on the induction of regulatory T cells, could improve such tolerance inducing strategies. Since anti-IL-6R mAb (tocilizumab) is an FDA approved for the treatment of subjects with rheumatoid arthritis it could readily be tested clinically in this context.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Deneen Kozoriz for her excellent technical support in cell sorting.
Funding
This work was supported by the National Institutes of Health R01 grant AI43458 to H.L.W. and by the Nutricia Research Foundation Grant 2013-T1 to C.K.
REFERENCES
- 1.Vaz NM, Maia LC, Hanson DG, Lynch JM. Inhibition of homocytotropic antibody responses in adult inbred mice by previous feeding of the specific antigen. Journal of Allergy and Clinical Immunology. 1977;60:110–115. doi: 10.1016/0091-6749(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 2.Hanson DG, Vaz NM, Maia LC, Hornbrook MM, Lynch JM, Roy CA. Inhibition of specific immune responses by feeding protein antigens. Int. Arch. Allergy Appl. Immunol. 1977;55:526–532. doi: 10.1159/000231966. [DOI] [PubMed] [Google Scholar]
- 3.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 5.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol. Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran DQ, Tran DQ, Andersson J, Andersson J, Wang R, Wang R, Ramsey H, Ramsey H, Unutmaz D, Unutmaz D, Shevach EM, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF- on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci USA. 2009;106:13445–13450. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O'Shea JJ, Shevach EM. CD4+FoxP3+ regulatory T cells confer infectious tolerance in a TGF--dependent manner. J. Exp. Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M-L, Yan B-S, Bando Y, Kuchroo VK, Weiner HL. Latency- associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta- mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J. Immunol. 2008;180:7327–7337. doi: 10.4049/jimmunol.180.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran DQ. TGF- : the sword, the wand, and the shield of FOXP3+ regulatory T cells. Journal of Molecular Cell Biology. 2012;4:29–37. doi: 10.1093/jmcb/mjr033. [DOI] [PubMed] [Google Scholar]
- 10.Homann D, Dyrberg T, Petersen J, Oldstone MB, von Herrath MG. Insulin in oral immune “tolerance”: a one-amino acid change in the B chain makes the difference. J. Immunol. 1999;163:1833–1838. [PubMed] [Google Scholar]
- 11.Bergerot I, Ploix C, Petersen J, Moulin V, Rask C, Fabien N, Lindblad M, Mayer A, Czerkinsky C, Holmgren J, Thivolet C. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4610–4614. doi: 10.1073/pnas.94.9.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maron R, Guerau-de-Arellano M, Zhang X, Weiner HL. Oral administration of insulin to neonates suppresses spontaneous and cyclophosphamide induced diabetes in the NOD mouse. J. Autoimmun. 2001;16:21–28. doi: 10.1006/jaut.2000.0471. [DOI] [PubMed] [Google Scholar]
- 13.Faria AMC, Weiner HL. Oral Tolerance: Therapeutic Implications for Autoimmune Diseases. Clinical and Developmental Immunology. 2006;13:143–157. doi: 10.1080/17402520600876804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominitzki S, Fantini MC, Neufert C, Nikolaev A, Galle PR, Scheller J, Monteleone G, Rose-John S, Neurath MF, Becker C. Cutting edge: trans- signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25 T cells. J. Immunol. 2007;179:2041–2045. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 15.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. U.S.A. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 17.Singh RP, Hasan S, Sharma S, Nagra S, Yamaguchi DT, Wong DTW, Hahn BH, Hossain A. Th17 cells in inflammation and autoimmunity. Autoimmunity Reviews. 2014;13:1174–1181. doi: 10.1016/j.autrev.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 19.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Bäckström BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature Medicine. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards JP, Fujii H, Zhou AX, Creemers J, Unutmaz D, Shevach EM. Regulation of the Expression of GARP/Latent TGF- 1 Complexes on Mouse T Cells and Their Role in Regulatory T Cell and Th17 Differentiation. J. Immunol. 2013;190:5506–5515. doi: 10.4049/jimmunol.1300199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuende J, Liénart S, Dedobbeleer O, van der Woning B, De Boeck G, Stockis J, Huygens C, Colau D, Somja J, Delvenne P, Hannon M, Baron F, Dumoutier L, Renauld J-C, De Haard H, Saunders M, Coulie PG, Lucas S. Monoclonal antibodies against GARP/TGF-β1 complexes inhibit the immunosuppressive activity of human regulatory T cells in vivo. Science Translational Medicine. 2015;7:284ra56–284ra56. doi: 10.1126/scitranslmed.aaa1983. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa H, Ochi H, Chen ML, Frenkel D, Maron R, Weiner HL. Inhibition of Autoimmune Diabetes by Oral Administration of Anti-CD3 Monoclonal Antibody. Diabetes. 2007;56:2103–2109. doi: 10.2337/db06-1632. [DOI] [PubMed] [Google Scholar]
- 23.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, Wu H, Chen M-L, Gandhi R, Miller A, Maron R, Weiner HL. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+CD25−LAP+ T cells. Nature Medicine. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, Center E, Tsokos G, Weiner H. Suppression of murine SLE by oral anti-CD3: inducible CD4+CD25-LAP+ regulatory T cells control the expansion of IL-17+ follicular helper T cells. Lupus. 2009;18:586–596. doi: 10.1177/0961203308100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.