ABSTRACT
Enterochromaffin (EC) cells are the primary mechanosensors of the gastrointestinal (GI) epithelium. In response to mechanical stimuliEC cells release serotonin (5-hydroxytryptamine; 5-HT). The molecular details ofEC cell mechanosensitivity are poorly understood. Recently, our group found that human and mouseEC cells express the mechanosensitive ion channel Piezo2. The mechanosensitive currents in a humanEC cell model QGP-1 were blocked by the mechanosensitive channel blocker D-GsMTx4.
In the present study we aimed to characterize the effects of the mechanosensitive ion channel inhibitor spider peptide D-GsMTx4 on the mechanically stimulated currents from both QGP-1 and human Piezo2 transfected HEK-293 cells. We found co-localization of 5-HT and Piezo2 in QGP-1 cells by immunohistochemistry. QGP-1 mechanosensitive currents had biophysical properties similar to dose-dependently Piezo2 and were inhibited by D-GsMTx4. In response to direct displacement of cell membranes, human Piezo2 transiently expressed in HEK-293 cells produced robust rapidly activating and inactivating inward currents. D-GsMTx4 reversibly and dose-dependently inhibited both the potency and efficacy of Piezo2 currents in response to mechanical force. Our data demonstrate an effective inhibition of Piezo2 mechanosensitive currents by the spider peptide D-GsMTx4.
KEYWORDS: D-GsMTx4, enterochromaffin cell, mechanosensitive ion channel, mechanotransduction, Piezo2
Introduction
In the gastrointestinal (GI) epithelium enterochromaffin (EC) cells are known to be the primary mechanosensory cells, where their responses to mechanical forces define GI epithelium mechanosensitivity.1-3EC cells are responsible for synthetizing, storing and releasing about 95% of the total systemic serotonin (5-hydroxytryptamine, 5-HT).4 Application of mucosal pressure in small bowel leads to 5-HT release fromEC cells.3 Previous studies have implicated molecules such as G-protein coupled receptors5 and purine receptors P2Y and P2X,6-8 although none of these molecules are known to be primaryEC cell mechanosensors.
Our group is interested in understanding the molecular mechanisms ofEC cell mechanotransduction. We recently discovered that the mechano-gated ion channel Piezo2 is expressed in human and mouseEC cells from small bowel, where it is required for coupling of mechanical forces to 5-HT release.9 In this study, electrophysiological recordings using the tumor-derived humanEC cell model QGP-1, identified mechanosensitive currents that inactivate within milliseconds and that can be blocked by the spider peptide D-GsMTx4, a known mechano-gated channel inhibitor,10 and in particular Piezo1.11
Piezo proteins are mechanically activated cationic channels involved in several mechanotransduction processes and critical for survival in vertebrates.12-14 Piezo1 is expressed in multiple tissues, such as smooth muscle, red blood and vascular endothelial cells, and epithelial cells from kidney and bladder. Piezo1 is a mechanically gated cation channel that directly senses membrane tension15-17 in response to shear stress, cell volume changes18-23 and cell crowding.24 Multiple studies have associated Piezo1 mutations with diseases such as Xerocytosis.25,26
Unlike Piezo1, which appears to be important for general cellular mechanosensation, Piezo2 is important for specialized mechanosensation, such as in light touch perception, proprioception,13,18,27-29 and visceral sensation.30 Mutations in human Piezo2 lead to musculoskeletal diseases.31-33
Piezo channels are inhibited by some non-specific drugs, such as gadolinium (Gd3+) and the pore blocker ruthenium red (RR), which have been shown to inhibit mechano-gated channels.27 GsMTx4 is a peptide originally isolated from the venom of Grammostola spatulata spider that specifically targets mechano-gated channels.34 It acts as a gating modifier, meaning that it increases the membrane tension required for channel activation, which favors the closed state of the mechanosensitive ion channels.35 Piezo1 channels are known to be inhibited by GsMTx4.11,36 Because of their low stereospecificity, both enantiomers D-GsMTx4 and L-GsMTx4 have been shown to be equally effective in blocking Piezo1 mechanosensitive currents.35 However, to date no studies have examined whether GsMTx4 inhibits Piezo2 currents. Our previous work showed that D-GsMTx4 inhibited single cell mechanosensitive currents in theEC cell model QGP-1 and 5-HT release from Piezo2-expressingEC cells. In the present study, we expressed a human Piezo2 construct in HEK-293 cells and found that D-GsMTx4 dose-dependently and reversibly inhibits Piezo2 mechanosensitive currents, shifting the mid-point of sensitivity to membrane compression and decreasing peak response to force.
Methods
Drugs
D-GsMTx4 was freshly made on the day of experiments by dissolving directly into the relevant extracellular solution.
Cell culture
QGP-1 cells: QGP-1 (passages 17 to 20) (kind gift of Dr. Valeria Giandomenico, Uppsala, Sweden) were cultured in modified RPMI 1640 with 10% FBS, 1% Penicillin-Streptomycin, and 1% L-Glutamine. Cells were grown to 50–60% confluence in T25 flasks. On the day of experiments, cells for electrophysiology experiments were lifted with trypsin-EDTA (0.5%) and plated onto the test chamber.
HEK-293 cells transfection: cDNA plasmid contained human Piezo2 and eGFP. Piezo2 was transiently expressed by transfection with Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA) into the human embryonic kidney cell line (HEK-293, American Type Culture Collection, Manassas, VA) and electrophysiological recordings were performed 24–48 hours after transfection.
Immunohistochemistry (IHC)
IHC protocol: Cells were grown to 50–60% confluence on poly-L-lysine (Sigma, St. Louis, MO) coated coverslips. Cells were fixed in 4% PFA-PB for 10 minutes separately, washed in PBS and permeabilized with 0.2% Triton-X in PBS and blocked with 200 µL/slide of 1% BSA/PBS/10% normal donkey serum in a humidity chamber. Primary antibodies (Table 1) were added in 200 µL/slide of 1% BSA/PBS/10% normal donkey serum and were incubated at 4°C overnight in humidity chamber. Slides were then rinsed 3 times for 5 minutes in PBS. Secondary antibodies (Table 1) were incubated for 30 minutes in the dark. Slides were mounted in slowfade gold with 4′,6-diamidino-2-phenylindole (DAPI, Life Technologies, Grand Island, NY) mounting buffer. Imaging was performed using Olympus BX51W1 (40×) (Olympus Corporation, Tokyo, Japan).
Table 1.
Antibodies used in this study.
| Source | Titer | Secondary | Source | Titer | |
|---|---|---|---|---|---|
| Piezo2 | Novus (NBP1-78624) | 1:250 (4ug/ml) | Cy3 | Jackson ImmunoReseach (111-165-003) | 1:500 (3ug/ml) |
| 5-HT | Abcam (ab66047) | 1:1000 (0.50ug/ml) | Cy5 | Jackson ImmunoResearch (705-175-147) | 1:500 (3ug/ml) |
Single cell electrophysiology
Solutions
The extracellular solution contained (in mM): 150 Na+, 5 K+, 2.5 Ca2+, 1 Mg2+,160 Cl−, 10 HEPES, and 5.5 glucose, pH 7.35, 300 mmol/kg; the intracellular solution contained (in mM): 140 Cs+, 150 Cl−, 4 Mg2+, 2 Ca2+, 10 HEPES, and 5 EGTA, pH 7.3, 300 mmol/kg.
Data acquisition
Standard whole cell voltage clamp were used as before.37-39 Electrodes (Kimble KG12 glass) were pulled by Sutter P97 puller (Sutter Instruments, Novato, CA), coated with R6101 (Dow Corning, Auburn, MI) and fire polished to 2–3 MΩ. Stimulation and data acquisition were done with an Axopatch 200B patch clamp amplifier, CyberAmp 320 signal conditioner, Digidata 1550A and pClamp 10.5 software (Molecular Devices, Sunnyvale, CA). Once the cells were voltage-clamped mechanical stimulation was applied via fire-polished glass microelectrodes (10–25% of cell size) driven by a piezo-transducer P-621.1CD attached to an E-625.CR controller (PI, Physik Instrumente, Germany).27,40 Cells were subjected to a series of mechanical steps of 0.3 µm increments or single steps of 3.5 or 5.0 µm depending on the experiment.
Data analysis
Whole cell patch clamp data were analyzed in pClamp 10.5 (Molecular Devices, Sunnyvale, CA). The peak currents within 10 msec of stimulus start were selected for analysis. pClamp (Molecular Devices, Sunnyvale, CA) and Origin 2016 (OriginLab Co., Northampton, MA, USA) were used for electrophysiology data analysis. Current-voltage relationships were fit with a linear function, V = A + B*I, where I is current, V is voltage, A is the y-intercept and B is the slope. Displacement-current curves were fit in using a Boltzmann function I = A2 + (A1 – A2)/(1 + exp[(x – x0)/dx]), where I is current, A1 is the y-intercept, A2 is peak, x is displacement, x0 is half-point displacement and dx is slope displacement. Error bars are standard errors (SE). Significance was assigned when P<0.05 by unpaired t-test with Welch's correction, using GraphPad Prism version 7 (GraphPad Software, San Diego California USA, www.graphpad.com).
Results
QGP-1 cells express Piezo2
QGP-1 is a widely usedEC cell model that produces and releases serotonin (5-HT) in response to chemical41-43 and mechanical stimulation.9 We first used immunohistochemistry (IHC) to determine whether QGP-1 cells contained 5-HT and expressed Piezo2. We found that QGP-1 expressed Piezo2 and contained 5-HT and that the labeling for Piezo2 and 5-HT overlapped (Fig. 1). Piezo2 labeling was mostly membrane bound, whereas 5-HT labeling was found homogenously distributed throughout the cytoplasm. Appropriately, negative controls lacking either 5-HT or Piezo2 primary antibodies were negative (Fig. 1, middle and bottom row, respectively).
Figure 1.
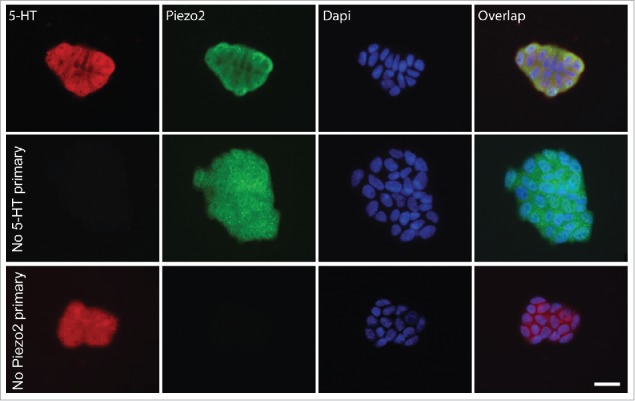
QGP-1 cells contain 5-HT and express Piezo2. Top row, epifluorescence images of Piezo2 and 5-HT immunohistochemistry with DAPI overlap. Middle row, omission of Piezo2 primary antibody eliminates Piezo2 labeling. Bottom row, omission of 5-HT antibody eliminates 5-HT labeling. Scale bar 50 µm.
D-GsMTx4 inhibition of QGP-1 cell mechanosensitive currents is dose-dependent
We previously showed that QGP-1 cells had mechanosensitive ionic currents.9 These QGP-1 mechanosensitive currents had properties that closely resembled Piezo2 currents, such as cationic permeability, linear current-voltage relationship and fast inactivation kinetics.9 The application of 5 µM D-GsMTx4 blocked about 90% of response to a maximum displacement stimulus of 5 µm.9 Further, Piezo2 siRNA knockdown almost completely removed the whole-cell current responses to mechanical stimulation. Our previous data, therefore, strongly suggested that QGP-1 mechanosensitive currents were carried by Piezo2.
Since D-GsMTx4 is known to specifically block mechanosensitive cation channels and in particular Piezo1,11 we wanted to examine the effects of this peptide on the current-distance sensitivity of mechanosensitive currents in QGP-1 cells. Whole-cell voltage clamped QGP-1 cells were stimulated with a piezo-driven blunt glass probe (Fig. 2). While other techniques such as tensile stress (stretch), shear stress and cell swelling (hypo-osmotic solutions) also allow the study of mechanosensation, compression of the cell membrane with a fine piezo-driven glass probe allows to record whole cell currents initiated by stimuli ranging from nanometers to millimeters within milliseconds. This makes the piezo driven glass probe an excellent tool for the study of fast activating and inactivating mechanosensitive channels. A displacement of 3.5 µm was applied to the cells and compared before and after application of 5 and 10 µM D-GsMTx4. We did not find mechanosensitive currents after treatment with 10 µM D-GsMTx4 (n = 4) (Fig. 2A). Application of 5 µM D-GsMTx4 significantly inhibited currents by 80.2 ± 18.4% (n = 4, *P < 0.05) (Fig. 2B).
Figure 2.
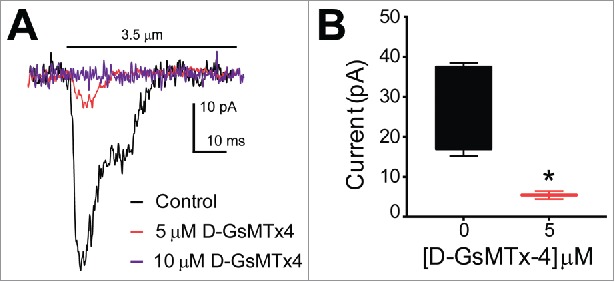
QGP-1 mechanosensitive currents are inhibited by the tarantula peptide D-GsMTx4 in a dose-dependent manner. QGP-1 cells were voltage-clamped and force was applied via graded membrane displacement by a piezo transducer driven glass probe. (A) Typical fast inward QGP-1 mechanosensitive currents in response to 3.5 µm membrane displacement in absence (control, black) and presence of 5 μM (red) and 10 μM (purple) D-GsMTx4. (B) Compared to the peak current in control, there was a significant decrease in peak current when Piezo2 was inhibited by 5 µM D-GsMTx4 (80.2 ± 18.4% inhibition, n = 4, *P < 0.05 by unpaired t-test with Welch's correction).
D-GsMTx4 blocks heterologously expressed Piezo2 currents in HEK-293 cells
Our data thus far suggested that theEC cell model QGP-1 possesses Piezo2 protein and mechanosensitive currents that closely resemble Piezo2 based on their biophysical properties. These currents were inhibited by D-GsMTx4. Therefore, we next examined whether D-GsMTx4 inhibited Piezo2 by applying it to HEK-293 cells transiently transfected with a human Piezo2 construct. Piezo2-transfected HEK-293 cells were voltage-clamped and mechanically stimulated by a piezo-electrically driven blunt glass probe as described above for QGP-1 cells. Increasing steps of 0.3 µm displacement generated increasing current responses (peak current −598.6 ± 76.7 pA, n = 3) and 5 µM D-GsMTx4 significantly decreased peak current by 55 ± 8.6% (−598.6 ± 76.7 to −269.6 ± 23.2 pA, n = 4, *P < 0.05) (Fig. 3A and B). Additionally, the current-distance relationships showed that 5 µM D-GsMTx4 significantly shifted the mid-point sensitivity from 3.4 ± 0.1 µm to 4.2 ± 0.1 µm (n = 4, *P < 0.05) (Fig. 3B). Therefore, our data show that D-GsMTx4 inhibits Piezo2. Consistent with other studies of GsMTx4 inhibition of mechanosensitive ionic currents44 we found that D-GsMTx4 induced a right shift in displacement-current sensitivity, suggesting a decrease in potency of mechanical stimuli on Piezo2 activation. We then assessed whether inhibition of Piezo2 currents by D-GsMTx4 is reversible (Fig. 3C) and currents elicited by a mid-point displacement of 3.5 µm were compared before and after application of the inhibitor. Indeed, we found that D-GsMTx4 (5 µM) reversibly inhibited 55.7 ± 21.4% of the peak mechanosensitive current. (Control −213.4 ± 57.5 pA, n = 4 versus 5 µM D-GsMTx4 −94.6 ± 20.2 pA, n = 5, *P < 0.05) and as shown in representative trace in green (Fig. 3C), after washout the mechanically-gated currents elicited by the same displacement were restored to control values. We then examined dose-dependence of D-GsMTx4 inhibition of Piezo2. The effects of 10 µM D-GsMTx4 could be examined when cells were challenged to a maximum stimulation (5 µm displacement), because we did not find currents at lower displacements. We analyzed individual cell responses to a single maximum stimulus and compared control responses (−634.9 ± 53.0 pA, n = 4) to application of 5 or 10 µM D-GsMTx4 (Fig. 3D and E). Control currents were significantly inhibited by both 5 and 10 µM D-GsMTx4 (−232.3 ± 37.2 and −67.6 ± 0.9 pA, respectively, n = 5, *P < 0.05). Non-transfected HEK-293 cells did not respond to mechanical stimulation (no changes in holding current before and after, n = 3) (Fig. 3D).
Figure 3.
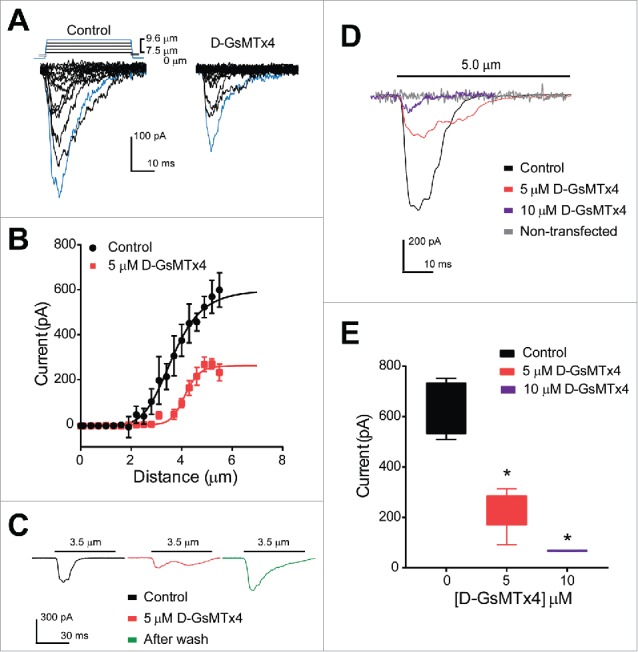
Human Piezo2 transiently transfected in HEK-293 cells produce mechanosensitive currents that are inhibited by D-GsMTx4. (A) A stepwise (0.3 µm step) increase in cell membrane deformation resulted in a set of fast activating and inactivating mechanically-induced inward currents, with blue traces highlighting current in response to a maximum stimulus, in both absence (Control) and presence (D-GsMTx4) of 5 µM D-GsMTx4. (B) Peak current-displacement relationship (n = 4) fitted by a two-state Boltzmann function in Control solution (Black, midpoint of 3.5 ± 0.1 µm) and perfused with 5 µM D-GsMTx4 (Red, midpoint of 4.2 ± 0.1 µm). (C) Inward currents elicited by 3.5 µm displacement in control solution (black) are inhibited by 55.7 ± 21.4% after application of 5 µM D-GsMTx4 (red) and washout (Green) restores peak current after exposure with 5 µM D-GsMTx4. (D) Typical inward currents evoked by a 5.0 µm displacement (Imax) in control solution (Black) and application of 5 µM D-GsMTx4 (Red) or 10 µM D-GsMTx4 (purple). A typical non-transfected HEK-293 cell response to mechanical stimulus is shown in gray. (E) Current-concentration whisker plot in presence of 0 (Black, −634.9 ± 53.0 pA), 5 µM (Red, −232.3 ± 37.2 pA) and 10 µM (Blue, −67.6 ± 0.9 pA) D-GsMTx4 (*P < 0.05 by unpaired t-test with Welch's correction).
Discussion
EC cells are the primary mechanosensory cells in the GI epithelium, where they are known to release 5-HT in response to mechanical stimulation. In a recent study, our group showed that Piezo2 contributes to the mechanosensitive currents and 5-HT release by a humanEC cell model QGP-1.45
In the present study, we examined the inhibitory properties of the stereoisomer of the tarantula peptide GsMTx4 (D-GsMTx4) in both QGP-1 and human Piezo2 transiently transfected in HEK-293 cells.
We first immunolabelled QGP-1 cells using both Piezo2 and 5-HT antibodies and found strong expression of Piezo2 in 5-HT positive QGP-1 cells (Fig. 1). Controls using no 5-HT primary (second row) and no Piezo2 primary (third row) antibodies were adequately negative. After confirming Piezo2 expression, we tested the effects of 5 and 10 µM D-GsMTx4 on currents elicited by 3.5 µm displacement (mid-point sensitivity)9 in QGP-1 cells. Our previous work showed that currents elicited by a maximum stimulus of 5.0 µm displacement were significantly inhibited by application of 5 µM D-GsMTx4 and here we decided to test the effects of the blocker to a smaller mechanical stimulus. As previously described, cells were voltage-clamped and compressed using a piezo-driven glass poker. QGP-1 mechanosensory currents were inhibited by 5 µM D-GsMTx4 and fully blocked by 10 µM D-GsMTx4 (Fig. 2A and B), suggesting a dose-dependency of the QGP-1 mechanosensitive ion channel block by D-GsMTx4. Our data presented here support the use of QGP-1 cells as an adequate model to study the role of Piezo2 in the mechanosensitivity ofEC cells.
We next tested whether Piezo2 channels are inhibited by D-GsMTx4 by testing inhibition in transiently over-expressing human Piezo2 in HEK-293 cell line. HEK-293 cells did not have mechanosensitive currents prior to transfection, consistent with previous studies of mock transfections used as negative controls for Piezo1 experiments in HEK-293T cells.27 When Piezo2 was transfected into HEK-293 cells, a ladder of displacement (0.3 µm steps) generated mechano-gated currents that activated and inactivated within milliseconds (Fig. 3A). Application of 5 µM D-GsMTx4 significantly inhibited peak current responses and it significantly shifted the current-distance relationship. A right shift (decrease) in sensitivity is found in presence of the D-GsMTx4 with a change in mid-point sensitivity from 3.4 ± 0.1 µm (Control) to 4.2 ± 0.1 µm (5 µM D-GsMTx4) (Fig. 3B). Our findings are consistent with previous studies that showed that GsMTx4 is a gating modifier of mechanosensitive ion channels.[refs] Next, we tested the reversibility of the inhibition by D-GsMTx4 and found that washout can restore Piezo2 currents after exposure to 5 µM D-GsMTx4 (Fig. 3C). These findings support previous reports on Piezo-dependent Ca2+ influx inhibition by the peptide in chondrocytes46 and Piezo1 currents in HEK-293 cells.44
As previously shown in QGP-1 cells, currents elicited by a maximum displacement (5 µm) were inhibited by the blocker. In Piezo2-expressing HEK-293 cells, application of both 5 and 10 µM D-GsMTx4 significantly Piezo2 mechanosensitive currents in response to maximum displacements, showing the peptide not only affects sensitivity but also the potency of the responses to mechanical stimulation (Fig. 3D and E). Previous studies have shown that GsMTx4 shifts Piezo1 sensitivity to mechanical stimulation and reduces the efficacy of the responses. Other less potent blockers, such as RR and Gd3+ (30 µM) have been shown to reversibly block about 80% of Piezo2-dependent currents in HEK-293T cells, when subjected to a maximum stimulus of 5 µm displacement.
In summary, we found expression of Piezo2 in 5-HT positive humanEC cell model QGP-1 and characterized the biophysical effects of D-GsMTx4 in HEK-293 cells transfected with human Piezo2. Mechanically stimulated Piezo2 currents are dose-dependently and reversibly inhibited by D-GsMTx4, which decreases both the potency and efficacy of mechanosensitive Piezo2 currents.
Abbreviations
- 5-HT
5-hydroxytryptamine
- DAPI
4′,6-diamino-2-phenylindole
- EC
enterochromaffin
- Gd3+
gadolinium
- GI
gastrointestinal
- IHC
immunohistochemistry
- PB
phosphate buffer
- PFA
paraformaldehyde
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Mrs. Jennifer Rud for administrative assistance.
Funding
Financial support to AB from NIH K08 (DK106456) and 2015 American Gastroenterological Association Research Scholar Award (AGA RSA), Pilot and Feasibility Grant from Mayo Clinic Center for Cell Signaling in Gastroenterology (NIH P30DK084567), and to GF from NIH R01 (DK52766).
References
- [1].Bulbring E, Lin RC. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol. 1958; 140(3):381-407; PMID: 13514713 [PMC free article] [PubMed] [Google Scholar]
- [2].Bulbring E, Crema A. Observations concerning the action of 5-hydroxytryptamine on the peristaltic reflex. Br J Pharmacol. 1958; 13(4):444-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bulbring E, Crema A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J Physiol. 1959; 146(1):18-28; PMID: 13655213; https://doi.org/ 10.1113/jphysiol.1959.sp006175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cote F, Thevenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, Hanoun N, Saurini F, Lechat P, et al.. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003; 100(23):13525-30; PMID: 14597720; https://doi.org/ 10.1073/pnas.2233056100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim M, Javed NH, Yu JG, Christofi F, Cooke HJ. Mechanical stimulation activates Galphaq signaling pathways and 5-hydroxytryptamine release from human carcinoid BON cells. J Clin Invest. 2001; 108(7):1051-9; PMID: 11581306; https://doi.org/ 10.1172/JCI12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Christofi FL. Purinergic receptors and gastrointestinal secretomotor function. Purinergic signalling. 2008; 4(3):213-36; PMID: 18604596; https://doi.org/ 10.1007/s11302-008-9104-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chin A, Svejda B, Gustafsson BI, Granlund AB, Sandvik AK, Timberlake A, Sumpio B, Pfragner R, Modlin IM, Kidd M. The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. American journal of physiology Gastrointestinal and liver physiology. 2012; 302(3):G397-405; PMID: 22038827; https://doi.org/ 10.1152/ajpgi.00087.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Linan-Rico A, Wunderlich JE, Grants IS, Frankel WL, Xue J, Williams KC, Harzman AE, Enneking JT, Cooke HJ, Christofi FL. Purinergic Autocrine Regulation of Mechanosensitivity and Serotonin Release in a HumanEC Model: ATP-gated P2×3 Channels inEC are Downregulated in Ulcerative Colitis. Inflamm Bowel Dis. 2013; 19(11):2366-79; PMID: 23917247; https://doi.org/ 10.1097/MIB.0b013e31829ecf4d [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, Kashyap PK, Grover M, Oeckler R, Gottlieb PA, Li HJ, et al.. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol. 2016; 595(1):79-91; Epub ahead of print. https://doi.org/ 10.1113/JP272718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon. 2007; 49(2):249-70; https://doi.org/ 10.1016/j.toxicon.2006.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bae C, Sachs F, Gottlieb PA. The Mechanosensitive Ion Channel Piezo1 Is Inhibited by the Peptide GsMTx4. Biochemistry (Mosc). 2011; 50(29):6295-300; https://doi.org/ 10.1021/bi200770q [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, et al.. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014; 516(7529):121-5; PMID: 25471886; https://doi.org/ 10.1038/nature13980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, et al.. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014; 509(7502):622-6; PMID: 24717433; https://doi.org/ 10.1038/nature13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, et al.. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A. 2014; 111(28):10347-52; PMID: 24958852; https://doi.org/ 10.1073/pnas.1409233111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, Ng CA, Sachs F, Gottlieb PA, Martinac B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun. 2016; 7:10366; PMID: 26785635; https://doi.org/ 10.1038/ncomms10366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. Elife. 2015; 4:e12088; https://doi.org/ 10.7554/eLife.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, Martinac B, Patapoutian A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016; 17(7):1739-46; https://doi.org/ 10.1016/j.celrep.2016.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y, et al.. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell reports. 2015; 13(6):1161-71; https://doi.org/ 10.1016/j.celrep.2015.09.072 [DOI] [PubMed] [Google Scholar]
- [19].Faucherre A, Kissa K, Nargeot J, Mangoni ME, Jopling C. Piezo1 plays a role in erythrocyte volume homeostasis. Haematologica. 2014; 99(1):70-5; PMID: 23872304; https://doi.org/ 10.3324/haematol.2013.086090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Miyamoto T, Mochizuki T, Nakagomi H, Kira S, Watanabe M, Takayama Y, Suzuki Y, Koizumi S, Takeda M, Tominaga M. Functional Role for Piezo1 in Stretch-evoked Ca2+ Influx and ATP Release in Urothelial Cell Cultures. J Biol Chem. 2014; 289(23):16565-75; PMID: 24759099; https://doi.org/ 10.1074/jbc.M113.528638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cinar E, Zhou S, DeCourcey J, Wang Y, Waugh RE, Wan J. Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc Natl Acad Sci U S A. 2015; 112(38):11783-8; PMID: 26351678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Martins P, Fakhry J, de Oliveira EC, Hunne B, Fothergill LJ, Ringuet M, Reis DD, Rehfeld JF, Callaghan B, Furness JB. Analysis of enteroendocrine cell populations in the human colon. Cell Tissue Res. 2016; [Epub ahead of print] PMID: 27844204; https://doi.org/ 10.1007/s00441-016-2530-7 [DOI] [PubMed] [Google Scholar]
- [23].Peyronnet R, Martins JR, Duprat F, Demolombe S, Arhatte M, Jodar M, Tauc M, Duranton C, Paulais M, Teulon J, et al.. Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO reports. 2013; 14(12):1143-8; PMID: 24157948; https://doi.org/ 10.1038/embor.2013.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Slattum GM, Rosenblatt J. Tumour cell invasion: an emerging role for basal epithelial cell extrusion. Nat Rev Cancer. 2014; 14(7):495-501; PMID: 24943812; https://doi.org/ 10.1038/nrc3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci U S A. 2013;110(12):E1162-8; PMID: 23487776; https://doi.org/ 10.1073/pnas.1219777110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zarychanski R, Schulz VP, Houston BL, Maksimova Y, Houston DS, Smith B, Rinehart J, Gallagher PG. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120(9):1908-15; PMID: 22529292; https://doi.org/ 10.1182/blood-2012-04-422253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55-60; PMID: 20813920; https://doi.org/ 10.1126/science.1193270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ikeda R, Gu JG. Piezo2 channel conductance and localization domains in Merkel cells of rat whisker hair follicles. Neurosci Lett. 2014;583:210-5; PMID: 24911969; https://doi.org/ 10.1016/j.neulet.2014.05.055 [DOI] [PubMed] [Google Scholar]
- [29].Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, Patapoutian A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci. 2015;18(12):1756-62; PMID: 26551544; https://doi.org/ 10.1038/nn.4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang J, Zhang J, Yang H, Li K, Lei X, Xu C. The potential role of Piezo2 in the mediation of visceral sensation. Neurosci Lett. 2016;630:158-63; https://doi.org/ 10.1016/j.neulet.2016.07.058 [DOI] [PubMed] [Google Scholar]
- [31].Coste B, Houge G, Murray MF, Stitziel N, Bandell M, Giovanni MA, Philippakis A, Hoischen A, Riemer G, Steen U, et al.. Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proc Natl Acad Sci U S A. 2013;110(12):4667-72; PMID: 23487782; https://doi.org/ 10.1073/pnas.1221400110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Okubo M, Fujita A, Saito Y, Komaki H, Ishiyama A, Takeshita E, Kojima E, Koichihara R, Saito T, Nakagawa E, et al.. A family of distal arthrogryposis type 5 due to a novel PIEZO2 mutation. Am J Med Genet A. 2015; 167A(5):1100-6. PMID: 25712306; https://doi.org/ 10.1002/ajmg.a.36881 [DOI] [PubMed] [Google Scholar]
- [33].Chesler AT, Szczot M, Bharucha-Goebel D, Ceko M, Donkervoort S, Laubacher C, Hayes LH, Alter K, Zampieri C, Stanley C, et al.. The Role of PIEZO2 in Human Mechanosensation. The New England journal of medicine. 2016; 375(14):1355-1364; PMID: 27653382; https://doi.org/ 10.1056/NEJMoa1602812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol. 2000;115(5):583-98; PMID: 10779316; https://doi.org/ 10.1085/jgp.115.5.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Suchyna TM, Tape SE, Koeppe RE, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004; 430(6996):235-40; PMID: 15241420; https://doi.org/ 10.1038/nature02743 [DOI] [PubMed] [Google Scholar]
- [36].Gottlieb PA, Sachs F. Piezo1: properties of a cation selective mechanical channel. Channels. 2012; 6(4):214-9; PMID: 22790400; https://doi.org/ 10.4161/chan.21050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Strege P, Beyder A, Bernard C, Crespo-Diaz R, Behfar A, Terzic A, Ackerman M, Farrugia G. Ranolazine inhibits shear sensitivity of endogenous Na (+) current and spontaneous action potentials in HL-1 cells. Channels (Austin). 2012; 6(6):457-62; PMID: 23018927; https://doi.org/ 10.4161/chan.22017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Saito YA, Strege PR, Tester DJ, Locke GR 3rd, Talley NJ, Bernard CE, Rae JL, Makielski JC, Ackerman MJ, Farrugia G. Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am J Physiol Gastrointest Liver Physiol. 2009; 296(2):G211-8; PMID: 19056759; https://doi.org/ 10.1152/ajpgi.90571.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Sarr MG, Farrugia G. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2003; 285(6):G1111-21; PMID: 12893628; https://doi.org/ 10.1152/ajpgi.00152.2003 [DOI] [PubMed] [Google Scholar]
- [40].Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, et al.. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014; 509(7502):617-21; PMID: 24717432; https://doi.org/ 10.1038/nature13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Doihara H, Nozawa K, Kojima R, Kawabata-Shoda E, Yokoyama T, Ito H. QGP-1 cells release 5-HT via TRPA1 activation; a model of human enterochromaffin cells. Mol Cell Biochem. 2009; 331(1-2):239-45; PMID: 19507004; https://doi.org/ 10.1007/s11010-009-0165-7 [DOI] [PubMed] [Google Scholar]
- [42].Kojima R, Nozawa K, Doihara H, Keto Y, Kaku H, Yokoyama T, Itou H. Effects of novel TRPA1 receptor agonist ASP7663 in models of drug-induced constipation and visceral pain. Eur J Pharmacol. 2014; 723:288-93; https://doi.org/ 10.1016/j.ejphar.2013.11.020 [DOI] [PubMed] [Google Scholar]
- [43].Schulze A, Hartung P, Schaefer M, Hill K. Transient receptor potential ankyrin 1 (TRPA1) channel activation by the thienopyridine-type drugs ticlopidine, clopidogrel, and prasugrel. Cell Calcium. 2014; 55(4):200-7; https://doi.org/ 10.1016/j.ceca.2014.02.014 [DOI] [PubMed] [Google Scholar]
- [44].Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50(29):6295-300; PMID: 21696149; https://doi.org/ 10.1021/bi200770q [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, Kashyap P, Grover M, Oeckler R, Gottlieb PA, Li HJ, et al.. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol. 2016; 595(1):79-91. https://doi.org/ 10.1113/JP272718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, Wu J, Beicker KN, Coles J, Zauscher S, et al.. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci U S A. 2014; 111(47):E5114-22. https://doi.org 10.1073/pnas.1414298111 [DOI] [PMC free article] [PubMed] [Google Scholar]


