Abstract
To determine if mindfulness meditation (MM) in older adults improves cognition and, secondarily, if MM improves mental health and physiology, 134 at least mildly stressed 50–85 year olds were randomized to a six-week MM intervention or a waitlist control. Outcome measures were assessed at baseline and two months later at Visit 2. The primary outcome measure was an executive function/attentional measure (flanker task). Other outcome measures included additional cognitive assessments, salivary cortisol, respiratory rate, heart rate variability, Positive and Negative Affect Schedule (PANAS), Center for Epidemiologic Studies Depression (CESD), Perceived Stress Scale (PSS), Neuroticism-Extraversion-Openness (NEO) personality traits, and SF-36 health-related quality of life. 128 participants completed the study though Visit 2 assessments. There was no significant change in the primary or other cognitive outcome measures. Even after statistical adjustment for multiple outcomes, self-rated measures related to negative affect and stress were all significantly improved in the MM intervention compared to wait-list group (PANAS-negative, CESD, PSS, and SF-36 health-related quality of life Vitality and Mental Health Component). The SF-36 Mental Health Component score improved more than the minimum clinically important difference. There were also significant changes in personality traits such as Neuroticism. Changes in positive affect were not observed. There were no group differences in salivary cortisol, or heart rate variability. These moderate sized improvements in self-rated measures were not paralleled by improvements in cognitive function or physiological measures. Potential explanations for this discrepancy in stress-related outcomes are discussed to help improve future studies.
Keywords: meditation, stress, cognition, mental health, aging, adherence
INTRODUCTION
Currently, age-related cognitive decline stands as a major public health issue, with high societal costs and few preventative options. For example, within the United States, the prevalence of dementia is about 14% among persons over age 71 years old (Plassman et al., 2007) with a resultant high cost of $200 billion (Hurd, Martorell, Delavande, Mullen, & Langa, 2013). Cognitive impairment without dementia in those over 71 is even more prevalent, affecting about 22% of the population (Plassman et al., 2008). Despite the personal and societal costs and known modifiable risk factors such as hypertension and non-modifiable risk factors such as APOE genotype, there are very limited evidence-based recommendations for prevention of age-related cognitive decline (Daviglus et al., 2010; Institute of Medicine (U.S.). Committee on the Public Health Dimensions of Cognitive Aging, Blazer, Yaffe, & Liverman, 2015). Mildly improving cognition and delaying the onset of dementia by even 6 months with a widely available behavioral intervention would decrease the number of dementia cases by over 100,000 over 10 years (Brookmeyer, Gray, & Kawas, 1998).
Psychological stress is one important factor in the general population, and specifically with older adults that can be addressed by behavioral interventions. Psychological stress is very common and contributes to many physical and mental health problems. About 25% of surveyed American adults reported high stress, and 50% reported a major stressful event over the past year (NPR, Foundation, & Health, 2014). Chronic psychological stress has multiple effects on physiological systems (McEwen, 1998; Oken, Chamine, & Wakeland, 2015) and contributes to a broad range of diseases. Importantly, chronic psychological stress and excessive reactivity to stressors contributes to age-related cognitive decline, hippocampal injury, and neurodegenerative diseases, either directly or through stress mediators (Esch, Stefano, Fricchione, & Benson, 2002; Lupien et al., 1999; McEwen, 1998; Oken, Fonareva, & Wahbeh, 2011b; Stawski, Sliwinski, & Smyth, 2006). Neuroticism, which is elevated stress reactivity associated with negative emotions, has genetic, neurobiological, and environmental contributions (Barlow, Ellard, Sauer-Zavala, Bullis, & Carl, 2014; Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Oken et al., 2011b). High neuroticism contributes to many health disorders (Lahey, 2009) and is linked to increased age-related cognitive change and Alzheimer’s disease in longitudinal studies (although the neuropathology of this cognitive change is not related to amyloid deposition) (Wilson, Arnold, Schneider, Li, & Bennett, 2007; Wilson et al., 2003). Proneness to distress elicits deficits that are not specific but include frontal-executive function and perceptual speed (Wilson et al., 2007), similar to cognitive changes associated with affective disorders such as PTSD and depression (Davidson & McEwen, 2012; DeRubeis, Siegle, & Hollon, 2008). Additionally, depression may double the risk of later development of Alzheimer’s disease (Ownby, Crocco, Acevedo, John, & Loewenstein, 2006). Thus, reducing psychological distress or stress reactivity and their negative effects on cognition may be a modifiable risk factor with a potentially large impact on population health (Kremen, Lachman, Pruessner, Sliwinski, & Wilson, 2012).
Mindfulness meditation (MM) is a psychological intervention that reduces stress and stress reactivity in people with PTSD, depression, pain, and stress (Chiesa & Serretti, 2010; Grossman, Niemann, Schmidt, & Walach, 2004; Kabat-Zinn, 1982). The evidence for efficacy has not been definitive across the board, due in part to a lack of objective markers of improvement (Abbott et al., 2014; Goyal et al., 2014b; Ospina, Bond, Karkhaneh, & al, 2007). However, there is moderate evidence for the self-reported reduction of anxiety, depression and pain symptoms (Abbott et al., 2014; Goyal et al., 2014b; Khoury et al., 2013).
Meditation may produce cognitive benefits through two mechanisms: improving cognition by decreasing levels of stress or stress reactivity and/or improving attention training. There have been some studies evaluating the effect of MM on cognitive function in adults across the age range but the evidence from a systematic review is not strong because of many methodological limitations (Chiesa, Calati, & Serretti, 2011). Among older adults, MM has had an even more limited evaluation to improve cognitive function and reduce stress and stress reactivity. A systematic review on meditation effects on age-related cognitive decline found only six randomized controlled studies with variable methods, most with small sample sizes and high risk of bias (Gard, Holzel, & Lazar, 2014; Moynihan et al., 2013). There was some preliminary evidence of a meditation effect on cognitive function but nothing conclusive.
The primary goal of this randomized controlled trial (RCT) in stressed older adults was to determine if there were any beneficial cognitive effects of MM training. The secondary goals were to determine if there were beneficial effects of MM on self-rated mental health and on physiological measures related to stress.
METHOD
Participants
Participants consisted of generally healthy adults 50–85 years of age who reported at least mild levels of stress as evidenced by a Perceived Stress Scale (Cohen, Karmarck, & Mermelstein, 1983) score greater > 9 (Table 1). Participants with potential cognitive deficits were excluded to ensure all participants could fully engage in the standardized MM training and complete all assessments, including home adherence monitoring. The upper age cutoff helped to limit instances of multiple brain pathologies contributing to age-related cognitive alterations (White et al., 2005). Participants were recruited from the Portland, Oregon metropolitan area from June 2011 – January 2015 from paper and/or digital postings at Oregon Health & Sciences University (OHSU), Craigslist, neighborhood centers, public libraries, and local newspapers and newsletters.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
|
| Exclusion Criteria |
|
Following inquiries, participants were informed about the study and eligibility criteria. If interested, they underwent a 30-minute telephone eligibility screening using an OHSU Institutional Review Board (IRB)-approved Waiver of Authorization. The full study was approved by the IRB, and initial plan details were registered with ClinicalTrials.gov (NCT01386060) in expectation of specific grant funding that was never obtained. Participants provided informed consent during Visit 1. Exclusion criteria included significant untreated psychiatric disorders requiring medical care and underlying illnesses that might limit the benefit of the intervention, confound outcomes, or increase the likelihood of dropout (Table 1).
Procedure
Randomization and follow up
Following Visit 1, participants were randomized to a six-week one-on-one MM intervention or a waitlist control. All randomizations were performed by non-blinded research personnel using a computerized covariate adaptive randomization procedure (Pocock & Simon, 1975) aimed at balancing active and waitlist groups on age, gender, and baseline Perceived Stress Scale scores using a pre-determined projected median split for the continuous measures. The research assistant who led the MM training sessions performed the randomization, and the research assistants who conducted data-collection visits remained blinded. There were three assessment visits that were approximately three hours long and two months apart. Participants in the MM group received the intervention between Visits 1 and 2 but received no intervention between Visit 2 and Visit 3. In contrast, participants in the waitlist group received no intervention between Visits 1 and 2, but received the 6-week one-on-one intervention between Visits 2 and 3. This primary outcome paper analyzes only the Visit 1 and Visit 2 data. The outcome measures included: 1) cognitive measures; 2) self-rated measures of stress and affect; and 3) physiological measures of stress including salivary cortisol, blood pressure, respiration rate, heart rate and heart rate variability (HRV).
Mindfulness meditation
The MM in this RCT is a standardized and structured one-on-one program that has been fully described (Wahbeh, Lane, Goodrich, Miller, & Oken, 2014). It is based on Mindfulness-Based Cognitive Therapy (MBCT) (Segal, Williams, & Teasdale, 2002) and Mindfulness-Based Stress Reduction (MBSR) (Kabat-Zinn et al., 1992). The one-on-one intervention was chosen for several reasons, including ease of scheduling, ease of re-scheduling any missed appointments, and at least some participant preference compared to a group setting (Wahbeh, Svalina, & Oken, 2014). Participants attended 60–90 minute training sessions once a week for six weeks along with recommended daily home practice. The six MM trainings all followed a similar format, although the length of the sessions varied to some degree by weekly syllabus length and by participant characteristics. Most sessions began with a 30-minute guided meditation, followed by discussion about the participant’s meditation experience, presentation of new materials, and discussion of home practice. Formal meditation instruction included a 30-minute Body Scan, 30-minute Sitting Meditation, 30-minute Sitting with Difficulty Meditation, and 4-minute Breathing Space. The research assistant leading the MM intervention was educated in Buddhist meditation with previous experience teaching secular 1-on-1 MM to adults in RCTs. Participants were instructed to use the home practice audio recordings to practice at home 30–45 minutes a day as a goal but to practice at least some amount every day.
Waitlist
Participants randomized to the waitlist arm between Visit 1 and Visit 2 received the MM intervention after the waitlist period (i.e., between Visit 2 and Visit 3). This was done in part to facilitate recruitment and decrease disappointment following randomization.
Adherence and retention
Attendance at the weekly in-person sessions was tracked. Adherence to the MM home practice for the MM intervention was assessed by iMINDr, a software application on a study iPod Touch (Apple, Inc.) lent to the participants for the duration of the study (Wahbeh, Zwickey, & Oken, 2011) that tracked the guided meditation minutes played for home practice.
Measures
The outcomes were obtained at Visits 1, 2 and 3. The primary focus of the study was cognitive improvement. In addition to the cognitive outcome measures, there were other outcome measures related to mental health or stress-related physiology that might relate to or even mediate any cognitive improvements.
Cognitive assessments
Cognitive assessments were based on prior studies of cognitive changes from meditation interventions (Chiesa et al., 2011; Oken et al., 2010) and focused on attentionally demanding frontal/executive function tasks but also included assessments of episodic memory and reaction time. The cognitive outcome measures included the following: the Stroop Color and Word Test (Golden, 2002); a flanker attention test where participants decide whether a central arrow surrounded by flanker arrows is pointing left or right; the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) wordlist (episodic verbal memory test) (Morris, Heyman, Mohs, & Hughes, 1989); letter and category verbal fluency from the multiple form version of the Controlled Oral Word Associates test (Benton & Hamsher, 1989); WAIS Letter–Number Sequence that is a measure of working memory that entails sorting strings of verbally-presented letters and digits into alphabetic/numerical order (Wechsler, 2008); and simple and choice reaction time (Oken et al., 2008). To respond on the flanker task, participants tap on the left or right side of a touch-screen on a hand-held device. The test has 30 congruent (flankers in same direction) and 30 incongruent (flankers in opposite direction) trials and lasts 2.5 minutes.
Self-rated measures
Participants were assessed with measures that might be affected by the MM intervention or mediate the MM intervention effect on the objective measures. Forms were sent to participants prior to the in-lab assessment visits and were filled out at home, taking less than one hour to complete.
Stress
Perceived Stress Scale (Cohen, Karmarck, & Mermelstein, 1983).
Personality
While neuroticism has been considered a stable trait, changes in neuroticism are a possible outcome from meditation studies (Jacobs et al., 2011). Neuroticism was assessed with the 60-item NEO-FFI (Costa & McCrae, 2010) that evaluated other personality traits as well.
Affect
Positive and negative affect schedule (PANAS) (Watson, Clark, & Tellegen, 1988) and the Center for Epidemiologic Studies Depression Scale (CESD) (Radloff, 1977).
Fatigue and quality of life
The SF-36 health-related quality of life was administered to obtain: 1) the 4-question Vitality subscale (J.E Ware, 1993) and 2) the Physical and Mental Health Component summary scores (J. E. Ware, 2000).
Self-efficacy
The General Perceived Self-Efficacy (GPSE) Scale (Schwarzer & Jerusalem, 1995) was included because sense of control has been improved by meditation (Astin, 1997; Oken et al., 2010).
Mindfulness
Two factors highlighted in the Five Factor Mindfulness Questionnaire (Baer, Smith, Hopkins, Krietmeyer, & Toney, 2006) were used: the Mindful Attention Awareness Scale (Brown & Ryan, 2003) and the mindful non-judging subscale of the Kentucky Inventory of Mindfulness Skills (Baer, Smith, & Allen, 2004). These factors capture the primary goals of MM training—attention to the current moment and decreased reactivity to emotional stimuli. The full length of the assessment battery was felt to be too burdensome, so the Five Factor Questionnaire was not administered in its entirety.
Sleep
The Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) was administered because mind-body therapies improve sleep function (Neuendorf, Wahbeh, Chaime, et al., 2015) and may mediate stress effects on cognition (Oken, Fonareva, & Wahbeh, 2011a).
Expectancy and credibility
The Expectancy/Credibility (Devilly & Borkovec, 2000; Oken et al., 2008) and Teacher Credibility (Cherkin, Deyo, & Berg, 1991; Devilly & Borkovec, 2000) questionnaires were administered to determine if expectancy is associated with any improvements observed from the MM intervention.
Physiological assessments
Physiological assessments were performed after participants had been seated for 30 mins and included: 1) systolic and diastolic blood pressure (average of two obtained in succession using an automatic digital inflation cuff); 2) respiration rate using light elastic piezoelectric strap around chest near the diaphragm (Ambu-Sleepmate, Maryland) recorded in three consecutive 5-minute blocks when participant was passively listening to emotionally neutral auditory recordings; and 3) electrocardiogram (ECG) for heart rate and conventional heart rate variability (HRV) frequency analysis measures (Task Force of the European Society of Cardiology, 1996; Thayer, Friedman, & Borkovec, 1996). Respiration rate was calculated in BrainVision. Analyzer (Brain Products, Germany). Breaths were labeled semi-automatically using a voltage trigger to label peak values. ECG was amplified using BioSemi amplifiers (BioSemi, Amsterdam), and ECG was processed using Kubios and BrainVision software. HRV measures from a 5-minute recording were low frequency (LF, 0.04–0.15 Hz) to high frequency (HF, 0.15 – 0.40 Hz) ratio and standard deviation of the RR interval (Mukherjee, Yadav, Yung, Zajdel, & Oken, 2011). We also measured resting heart rate since heart rate was reported to be more sensitive to mild mental stress than the typical HRV frequency analysis measures (Mukherjee et al., 2011). Saliva for cortisol was collected at home and analyzed as previously done (Oken et al., 2011b; Wahbeh & Oken, 2013) with saliva samples obtained on two days at three time points: immediately upon awakening, 30 minutes later and before bedtime. If participants followed directions and collected all samples, data was averaged across the two days. If for any reason a sample was missing, just a single day’s data were included in the analysis rather than the average, knowing the single day measure might produce significantly worse reliability (Kraemer et al., 2006). Thus, the cortisol outcome data for each visit consisted of a single salivary cortisol measure at three time points.
Data Analyses
All data analyses were done in Stata/IC 14 (StataCorp, College Station TX). Data were first inspected to ensure there were no outliers and extreme outliers (more than 4 standard deviations) were deleted. Data were assessed for normality using Shapiro-Wilk test. Data transformations were used in the event of non-normality (e.g., square root or Box-Cox). Fisher’s exact test was used to compare the rate of study completion.
The primary analysis for Visit 2 was analysis of covariance of outcome data by intervention group using the Visit 1 data as covariate. Given their relationship with cognition, age and years of education were entered as covariates for cognitive outcome measures and were kept in the model if their p values were less than 0.10. One goal of this study was to evaluate the MM effect sizes on all the outcome measures and these are reported as partial eta squared. For multiple comparisons, the type I rate was controlled by using the false discovery rate (FDR) (Benjamini & Hochberg, 1995) with an overall FDR rate of 0.05. Both the unadjusted p values and FDR corrected p values using R program p.adjust are provided in the outcomes table but p values mentioned in the results and discussion text are all corrected values. Pairwise Pearson’s correlation coefficients and unadjusted p values were calculated to better understand the relationships of the many self-rated measures.
The objective measure of mindfulness meditation home practice time was obtained from those who were randomized to receive the MM training immediately after Visit 1. The association between meditation home practice time and outcomes was assessed using a linear regression model with the dependent variable being the Visit 2 – Visit 1 difference. In this analysis, only outcomes that were significantly affected by MM in the above ANCOVA were evaluated.
As described, salivary cortisol was assessed at three time points following Visit 1 and Visit 2. While multilevel mixed model analysis of cortisol data has been done previously (Hruschka, Kohrt, & Worthman, 2005; Segerstrom, Boggero, Smith, & Sephton, 2014), we chose a simple ANCOVA analysis since there were only three data points. Outcomes were cortisol awakening response (CAR), the transient increase in cortisol for about 30 minutes after awakening, and the difference between the awakening cortisol minus the bedtime cortisol (slope). For the slope calculation, the 30 minute after awakening cortisol collection for the CAR was dropped as has been previously suggested (Hruschka et al., 2005; Kraemer et al., 2006).
RESULTS
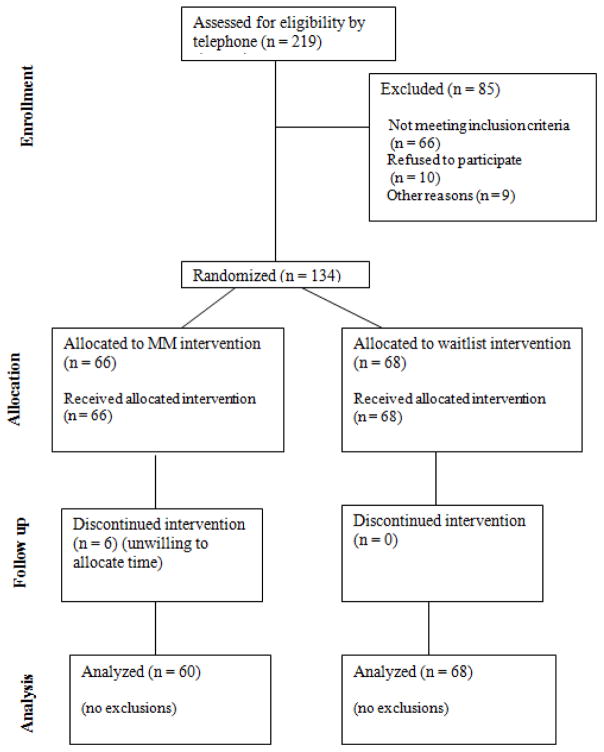
Following telephone screenings, 134 participants came to Visit 1 and were randomized to receive the MM beginning shortly after Visit 1 (n=66) or to a waitlist control (n=68) (Figure 1). The participant demographics (Table 2) were mostly women as is common for mind-body studies and primarily Caucasian non-Hispanic, with under-represented minority percentages comparable to the demographics of the Portland metropolitan area for this age range. Participants were also highly educated. Only one participant was over the age of 75 years old. The two groups were comparable in age, gender, years of education and baseline PSS between the two groups. There were 60 participants returning to Visit 2 in the MM group compared to all 68 participants in the waitlist group (p= 0.013).
Figure 1.
Flowchart of participants screened, enrolled, and completing the study (CONSORT figure).
Table 2.
Participant Demographics by Group
| Variable | Meditation | Waitlist |
|---|---|---|
| Number randomized (number female) | 66 (51) | 68 (56) |
| Age (mean ± std dev) | 60.2 ± 7.4 | 59.4 ± 6.3 |
| Years of Education (mean ± std dev) | 17.0 ± 2.5 | 16.4 ± 2.8 |
| Underrepresented groups (number) | ||
| Hispanic | 3 | 1 |
| African American | 1 | 1 |
| Asian | 2 | 4 |
| Perceived Stress Scale at Visit 1 (mean ± std dev) | 19.0 ± 6.1 | 18.5 ± 6.1 |
| Return at Visit 2 (number) | 60 | 68 |
The dropout rate by Visit 2 was only 9% in the MM group and 0% in the waitlist group (4.5% total out of all 134). The dropout rate was higher in the MM than waitlist group (Fisher’s Exact Test, p=0.013). The 6 dropouts had characteristics roughly comparable to the completers (age = 58.2 years, 4 women, education 14.5 years, and PSS 22.2). Participants who completed the MM intervention listened to the guided meditations an average of 30.3 + 11.8 mins/day (range 10.7 – 71.2).
There were no significant intervention effects on the flanker attention task or the rest of the cognitive outcomes, including working memory (Letter Number Sequencing), Stroop test, verbal fluency (letter or category), delayed verbal memory, simple reaction time or choice reaction time (Table 3). The outcomes related to negative affect and stress were almost all highly significantly improved from the MM intervention after adjusting for multiple comparisons (Table 3). This includes CESD, PANAS-negative (trait), and PSS with small to moderate effect sizes (e.g., partial eta squared for CESD was 0.11). Although the PANAS-positive did not significantly increase from MM training, the PANAS-negative was significantly decreased by MM. Some personality traits measured by the NEO-FFI were significantly affected by the intervention. Neuroticism was the a priori personality trait affected (p = 0.001), but Agreeableness (p=0.003) and Conscientiousness (p=0.002) also changed significantly. There was no significant change in Extraversion or Openness. Subjective sleep quality (PSQI) was not significantly improved.
Table 3.
Outcome Measures by Group
| Outcome Measures | MM | Waitlist | Group effect | |||||
|---|---|---|---|---|---|---|---|---|
| Visit 1 Mean (SD) | Visit 2 Mean (SD) | Visit 1 Mean (SD) | Visit 2 Mean (SD) | Unadj p | FDR p | partial eta squared | adjusted mean differ. | |
| Self-Rated | ||||||||
|
| ||||||||
| NEO- N | 24.8 (7.2) | 20.7 (7.7) | 24.4 (9.3) | 23.6 (9.7) | .0001 | .001** | .12 | 3.3 |
| PSS | 18.7 (5.9) | 15.2 (5.7) | 18.5 (6.1) | 18.5 (7.2) | .0001 | .001** | .11 | 3.5 |
| CESD1 | 17.6(8.5) | 12.4(7.6) | 19.4(10.5) | 18.5(10.9) | .0002 | .002** | .11 | 4.9 |
| NEO-A1 | 33.9(4.9) | 36.1(5.1) | 33.5(6.3) | 33.6(6.2) | .0006 | .003** | .09 | −2.2 |
| NEO-C | 28.6(7.5) | 31.0(6.3) | 31.1(7.1) | 30.7(7.3) | .0003 | .002** | .10 | −2.3 |
| NEO-E | 24.5(4.5) | 25.3(4.3) | 25.3(6.4) | 24.8(6.7) | .03 | .09 | .04 | −1.3 |
| NEO-O | 27.4(5.5) | 28.0(5.3) | 26.6(5.8) | 26.5(5.1) | .10 | .18 | .02 | −0.9 |
| PANAS-neg1 | 21.7(6.4) | 17.9(5.5) | 21.9(7.5) | 20.8(7.7) | .001 | .005** | .08 | 2.7 |
| PANAS-pos1 | 32.1(6.3) | 33.1(6.0) | 32.7(7.1) | 32.1(6.0) | .07 | .14 | .03 | −1.4 |
| GPSE1 | 29.6(3.5) | 30.3(3.7) | 30.1(4.2) | 29.2(4.0) | .003 | .009* | .07 | −1.5 |
| Sf-36 fatigue1 | 39.8(19.7) | 51.3(21.7) | 46.5(19.4) | 48.2(20.3) | .002 | .008* | .08 | 8.3 |
| SF-36 PCS1 | 50.4(6.5) | 49.1(6.8) | 50.2(6.7) | 50.6(7.2) | .07 | .14 | .03 | 1.6 |
| SF-36 MCS1 | 37.9(9.6) | 44.3(9.8) | 40.0(10.6) | 40.3(11.5) | .00003 | .001** | .13 | −5.7 |
| PSQI | 8.5(3.3) | 6.9(3.0) | 8.4(3.2) | 7.6(3.0) | .08 | .16 | .02 | 0.8 |
| MAAS1 | 51.8(10.2) | 57.6(10.1) | 51.5(12.1) | 54.8(11.2) | .05 | .14 | .03 | −2.6 |
| KIMS-NJ1 | 30.1(6.5) | 32.9(6.0) | 29.5(7.0) | 30.5(7.9) | .06 | .14 | .03 | −2.0 |
|
| ||||||||
| Cognitive | ||||||||
|
| ||||||||
| Letter Number Sequencing1 | 10.9(2.3) | 11.5(2.7) | 11.3(2.7) | 11.6(2.7) | .61 | .69 | .00 | −0.2 |
| Letter fluency1 | 48.0(14.3) | 46.9(13.4) | 47.0(14.2) | 49.5(14.2) | .13 | .23 | .03 | 3.2 |
| Category fluency | 21.4(5.9) | 16.5(4.7) | 21.4(5.6) | 17.0(4.1) | .88 | .94 | .00 | 0.6 |
| Stroop CW condition1 | 44.3(8.4) | 46.4(7.6) | 47.1(9.3) | 49.1(7.5) | .37 | .48 | .01 | 1.1 |
| Stroop CW interference | 49.0(8.3) | 48.8(7.0) | 46.6(9.8) | 49.5(6.8) | .38 | .48 | .01 | 1.3 |
| Word list- delayed | 6.8(2.0) | 7.5(1.8) | 6.8(2.0) | 7.3(1.9) | .40 | .49 | .01 | −0.2 |
| Choice RT (msec)1 | 470(67) | 462(70) | 463(70) | 465(59) | .21 | .33 | .01 | 7.3 |
| Simple RT (msec)1 | 270(46) | 267(43) | 272(43) | 279(40) | .07 | .14 | .03 | 10.0 |
| Flanker RT Incongruent (msec) | 753(107) | 703(119) | 792(165) | 726(148) | .37 | .4 | .01 | −3.6 |
|
| ||||||||
| Physiological | ||||||||
|
| ||||||||
| Respiration rate 1st five mins | 14.3(2.9) | 13.5(3.0) | 14.1(2.9) | 14.2(2.7) | .10 | .18 | .03 | 0.7 |
| Respiration rate 2nd five mins | 14.6(2.9) | 13.7(3.1) | 14.2(2.8) | 14.5(2.6) | .05 | .09 | .04 | 0.9 |
| Respiration rate 3rd five mins | 14.8(2.8) | 13.9(3.3) | 13.9(2.9) | 14.8(2.8) | .005 | .01* | .09 | 1.5 |
| CAR1 | .17(.21) | .13(.20) | .05(.46) | .12(.24) | .98 | .99 | .00 | −.01 |
| Cortisol slope1 | −.18(.27) | −.23(.14) | −.26(.20) | −.18(.83) | .07 | .14 | .03 | 0.1 |
| Systolic bp | 133(20) | 128(20) | 130(19) | 128(16) | .33 | .44 | .01 | 1.5 |
| Diastolic bp | 81(13) | 80(11) | 80(12) | 80(10) | .33 | .44 | .01 | 0.5 |
| Heart rate | 70(12) | 69(12) | 68(10) | 68(10) | .73 | .80 | .00 | 0.4 |
| HRV SDRR (msec) | 42(21) | 46(19) | 41(24) | 46(20) | .99 | .99 | .00 | 0.0 |
| HRV LF/HF ratio1 | 1.9(1.5) | 3.2(3.1) | 2.5(2.1) | 3.0(1.9) | .29 | .43 | .01 | −0.5 |
Note. Untransformed mean (standard deviation) of outcomes at the two visits by group. For ANCOVA group effect, there are unadjusted (unadj.) p values, FDR adjusted p values across all outcomes, partial eta squared effect size, and adjusted mean difference at Visit 2 (waitlist – MM). The number of participants for most analyses is 128. For FDR p values, * p<.05 and ** p<.005.
MM = mindfulness meditation intervention group; Unadj = unadjusted; FDR = False Discovery Rate; NEO = Neuroticism-Extraversion-Openness Personality Inventory (-N = Neuroticism; -A = Agreeableness; C=Conscientiousness; -E = Extraversion; -O = Openness); PSS = Perceived Stress Scale; CESD = Center for Epidemiologic Studies Depression Scale; PANAS = Positive and Negative Affect Schedule (-neg = Negative Affect; -pos = Positive Affect); GPSE = General Perceived Self-Efficacy; SF-36 = Short Form 36-item health-related quality of life; PCS = Physical Component Summary Score; MCS = Mental Component Summary Score; PSQI = Pittsburgh Sleep Quality Inventory; MAAS = Mindful Attention Awareness Scale; KIMS-NJ = Kentucky Inventory of Mindfulness Skills—Non-Judgmental Factor; CW = Color Word; msec = milliseconds; RT = reaction time; mins = minutes; CAR = Cortisol Awakening Response; bp = blood pressure; HRV = Heart Rate Variability; SDRR = Standard Deviation of Inter-Beat Interval; LF = Low Frequency; HF = High Frequency.
Statistical transformation was used.
The SF-36 Vitality subscale demonstrated significant improvement (p = .008). The calculated Mental Health Component (MHC) also demonstrated significant improvement (p<.0.001), but the Physical Health Component (PHC) did not. Self-efficacy (GPSE) also improved with the intervention. Of note, the two mindfulness measures were not significantly improved. While there was no treatment effect on the mindfulness measures, there was a significant relationship between changes in the mindfulness measures and the mental health measures. For example, when entering the mindfulness change scores into the ANCOVA for Neuroticism, both the change in the KIMS-Non-judgmental and the MAAS significantly entered into the model (KIMS, p = 0.0001 and MAAS, p =0.01). There was no such relationship with the mindfulness change scores and the cognitive measures. Thus, even though there was not a clear treatment effect on the mindfulness measures, improvements in mental health correlated to improvements in mindfulness independent of group assignment. There was no such relationship between mindfulness changes and cognitive measures.
Of note, the pairwise correlations between all the self-rated measures were very high for most of the self-rated measures that were affected by the intervention (data not shown). This suggests there is some common underlying factor(s) being assessed by these measures.
Additionally, there were no significant intervention effects on salivary cortisol (awakening response or diurnal morning-bedtime difference), heart rate, or heart rate variability. There was, however, a significant decrease in respiration rate in the MM compared to waitlist group after 10 minutes of sitting and passively listening to an audio recording.
Participants in the MM intervention attended all one-on-one training sessions (these often required rescheduling) and as already mentioned practiced at home an average of 30.3 + 11.8 minutes per day. Using linear regression in the 60 participants who finished MM training before Visit 2, there was no significant relationship between minutes practiced and effects on outcome measures.
DISCUSSION
This randomized controlled trial of six-week MM training compared to waitlist control of 134 50–85 year olds was executed adequately, maintained blinding of the assessors, and had a dropout rate of only 4% through Visit 2. The six dropouts were all in the MM group, which may be related to the fact that those in the waitlist group were not burdened by coming to the lab on a weekly basis.
Demonstrating cognitive change from the MM intervention was the main goal of the study. There were no changes in the primary or secondary cognitive outcome measures. In contrast, there were significant improvements in most of the standardized, self-rated measures related to negative mood and stress. The finding that a meditation intervention produced benefits in psychological measures but not in cognitive or physiological measures is consistent with some meta-analyses of meditation interventions (Abbott et al., 2014; Goyal et al., 2014a) and another recent study (Wahbeh, Goodrich, Goy, & Oken, 2016). However, this trend in the literature contrasts with many individual MM intervention studies that demonstrate some effect on cognition or physiology with intervention lengths both longer and shorter than 6 weeks (Chiesa et al., 2011; Jha, Krompinger, & Baime, 2007; Jha, Stanley, Kiyonaga, Wong, & Gelfand, 2010; Kaul, Passafiume, Sargent, & O’Hara, 2010; Moore, Gruber, Derose, & Malinowski, 2012; Semple, 2010; Tang et al., 2007; Zeidan, Johnson, Diamond, David, & Goolkasian, 2010). Of note, adults with any significant cognitive deficits at screening were excluded from the study, so participants may have had less possibility of any improvement.
A systematic review of cognitive changes from meditation studies of all ages had limited conclusions because of quality of studies (Chiesa et al., 2011). Another systematic review of meditation focused on age-related cognitive decline and was composed of 6 RCTs (Gard et al., 2014). The authors also drew limited conclusions regarding the beneficial effect of any meditation type on cognitive function in part related to the studies mostly being small and having high risk of bias,.
There is a known relationship between cognition and chronic stress, especially with aging (Lupien, McEwen, Gunnar, & Heim, 2009; Stawski et al., 2006); thus, we must question why no cognitive changes accompanied the improved self-rated mental health and stress. One possible explanation could be that participants were performing at or near their maximal cognitive abilities at baseline. The likelihood of this is increased since participants did not have pathological depression, PTSD, or anxiety disorders, which all tend to produce impairments in cognition. It may also be the case that the selected cognitive measures were not optimal. The intention was to administer a fairly broad battery with a focus on frontal/executive measures, known to be more sensitive to negative affect and stress. Yet the cognitive benefits of MM could be related to decreased mind-wandering (Hasenkamp, Wilson-Mendenhall, Duncan, & Barsalou, 2012), which may not be apparent in conventional cognitive testing that requires high attentional focus for only relatively brief periods. Finally, cognitive changes may not have been detected because of the testing timeframe. One prior study of MBSR in adults whose mean age was greater than that in this study (73 vs. 60 years old) observed a significant immediate effect at 8 weeks of MBSR on the ratio of Trail Making Test part B/A before correcting for multiple outcomes (Moynihan et al., 2013). However, there was no effect when measured again at 11 or 32 weeks.
In contrast to the absence of cognitive changes, there were significant improvements in most of the self-rated measures related to negative mood and stress, with partial eta squared effect sizes ranging up to 0.12. Neuroticism, which is a risk factor for cognitive decline in aging (Wilson, Begeny, Boyle, Schneider, & Bennett, 2011; Wilson et al., 2003), was significantly decreased. While personality has traditionally been thought of more as a stable trait measure with genetic contributions, it can also be affected by environmental influences, as evidenced by a previous study that reported personality changes from a meditation intervention (Jacobs et al., 2011). Additionally, the SF-36 demonstrated significant MM-related changes in the Mental Health Component and Vitality subscale scores. The adjusted mean difference in the Mental Health Component (5.7) was greater than the minimum clinically important difference, which is 4 (Coteur, Feagan, Keininger, & Kosinski, 2009). Additionally, the adjusted mean difference in the Vitality subscore was 8.3, which is close to the minimum clinically important difference of about 9 (Coteur et al., 2009). The SF-36 Vitality subscore has also improved from a different mind-body intervention, yoga, in healthy older adults (Oken et al., 2006) and in people with multiple sclerosis (Oken et al., 2004). One self-report measure that did not improve was subjective sleep quality (PSQI), even though there is some evidence that mind-body medicine may improve sleep (Neuendorf, Wahbeh, Chamine, et al., 2015). While our study participants were not recruited for sleep problems, their average PSQI score at baseline was 8.4 (scores of 5 and above are considered “poor sleep quality”).
With the exception of respiration rate, the physiological outcomes were not changed. The decline in respiration rate in the MM group was seen in the third 5-minute block of sitting quietly listening to auditory recordings. The reduction may represent a specific MM training-related change since mindful awareness of breathing was a central focus. There were no changes in other physiological measures related to stress, including HRV (LF/HF ratio and standard deviation of RR interval) or cortisol, which were strong theoretical candidates for sensitive measures. We sampled cortisol only three times a day for the two days following each of the visits. This limited sampling has been associated with worse intra-subject reliability for test-retest, high inter-subject coefficient of variation, and limited data to calculate area under the curve or diurnal cortisol slope. Thus, the study design and analysis may have been at least partially responsible for not detecting significant changes in cortisol.
Some of the mental health benefits of MM compared to waitlist may be related to simple attention and expectancy/placebo effects because there was only a passive waitlist control and no active control. The fact that objective measures demonstrated less change than the subjective self-rated measures lends credibility to this possibility since placebo is known to have a greater effect on subjective measures (Hrobjartsson & Gotzsche, 2001; Oken, 2008). However, the significant changes in the self-rated psychological measures were not correlated with the expectancy questionnaire scores. Also, some of the highly standardized assessments (e.g., Neuroticism) are not generally known to have significant improvements from any intervention, let alone demonstrate a placebo effect. Lastly, the effect size for mental health measures being above the minimum clinically important difference for some measures makes the expectancy effect explanation less likely.
As discussed, the absence of major changes in objective measures in contrast to the significant changes in self-rated measures is likely more than simple placebo effect, and there are many potential explanations. It is possible that the MM intervention used in this study was not sufficiently long in duration to affect cognitive or physiological changes. The widely used MBSR program typically includes 26 hours of class time, including eight 2.5-hour sessions and an all-day retreat (Kabat-Zinn et al., 1992). While shortened adaptations of the standardized MBSR and MBCT programs have demonstrated benefit, the ideal intervention duration is unknown. It may also be the case that outcomes need to be measured over a much longer time frame. It has been shown that physiological changes related to improvements in stress improvement or perseverative cognition (Ottaviani et al., 2014; Ottaviani et al., 2016) may take considerable time, and Visit 2 was only at two months.
There are many physiological measures related to stress (Oken et al., 2015) that may increase due to a short-term stressor; on the other hand, they may not decrease as much from a longer-term stress-reducing intervention. Other physiological changes not directly related to intracerebral processes, or what have been called allostatic load measures, have generally been more sensitive to longer-term cross-sectional differences (Evans & Schamberg, 2009; Juster, McEwen, & Lupien, 2009; Seeman, McEwen, Rowe, & Singer, 2001) and may have low sensitivity to a short-term intervention.
The outcomes may not be optimally chosen or well-performed. While we have previously demonstrated experimental and cross-sectional changes in cortisol and heart rate variability related to stress (Mukherjee et al., 2011; Wahbeh, Kishiyama, Zajdel, & Oken, 2008), cortisol is known to have high test-retest variability, and sampling at only three time points on only two days may be contributing to the absence of an intervention effect.
Ecological momentary assessment (EMA) (Shiffman, Stone, & Hufford, 2008) may produce more useful measures than single assessments in the laboratory environment. The laboratory induces an inherent change in peoples’ state, and they are not exposed to real-life stressors that would demonstrate their negative emotional reactivity and trigger coping mechanisms. The improvements related to meditation may primarily improve responsivity to a stressor (resilience). The objective measures may need to be direct measures of resilience to stress, either with experimental stressors or more sustained EMA.
The one-on-one class is likely not ideal for all participants although it has been quite acceptable and allows more flexibility for scheduling research participants. A recent survey comparing on-line, one-on-one, and group delivery of MM training suggested that one-on-one was at least as acceptable as group (Wahbeh, Lane, et al., 2014; Wahbeh, Svalina, et al., 2014). A group setting might produce improvements because of group cohesion and social support. However, the variability of group dynamics would add experimental noise to the intervention, and a group setting is less acceptable to some people with high introversion or PTSD.
It is likely that some people do not improve as a result of MM training from a mental health, cognitive or physiological perspective. It will be useful to better define those who are most likely to respond to MM training.
There is no way to assess the quality of meditation, so it is possible that the “dose,” as measured in number of hours practiced, was insufficient in this study to induce cognitive or physiological changes. The optimal dose of meditation needed to induce stress-relieving cognitive or physiological effects is not known. Those in the MM arm in this study practiced an average of 30 minutes per day during the 6-week intervention, as assessed by turning on the study iPod Touch to listen to the guided meditation audio for daily practice. Among the self-rated psychological measures that did demonstrate improvement with MM training compared to waitlist, there was no relationship of the degree of improvement with minutes practiced. There is little empirical data to justify how long one should practice meditation to achieve improvements in clinically relevant markers, and it would be helpful to have better knowledge of the dose response effect. It is also likely that some people do not improve as a result of MM training from a physiological, cognitive, or even mental health perspective. It will be useful to better define those who are most likely to respond to MM training.
While there was no relationship between mental health or cognitive improvement and minutes practiced, there was a relationship between changes in the mindfulness measures and mental health improvement. However, this relationship was even seen in the waitlist group, suggesting that the mindfulness change measures are not specific to MM interventions but do relate to mental health. Such relationships were not observed with the cognitive outcomes.
There are several additional limitations of this study. The age range of the study population was relatively narrow and participants were mostly women, Caucasian, and highly educated. While participants needed to report at least mild stress, they were not allowed to have very significant stress attributable to conditions such as generalized anxiety disorder, PTSD, or untreated depression. The latter populations may experience different effects of MM on cognitive or physiological outcomes. The MM was delivered in structured training sessions previously described, but the use of a single trainer limits the generalizability of this study’s results. Additionally, the effects of the intervention cannot be disentangled from the effects of the interventionist.
In summary, this study demonstrated significant benefits in many self-rated measures related to negative affect and stress, including clinically significant improvements. While other potential causes of the lack of observed changes in cognitive and physiological measures have been discussed, one possible explanation is expectancy effects given the use of only a wait-list control. In general, it is important to have an active control group that tries to match expectancy, but it is also important to have a passive control (usual care or wait-list) to see whether the non-specific expectancy effect is clinically significant even without having a specific treatment effect (Walach, 2001). Using psychological measures as outcomes, some have even suggested that the main effect of standard pharmacological treatments for depression is a non-specific placebo effect (Kirsch, 2010). Given the effect sizes for a MM intervention in this study along with its relatively low cost and risk, it is not unreasonable to consider MM useful for stressed older adults if the goal is to improve mental health, whatever the mechanism. In order to further understand the mechanism of improvement in mental health from MM, there remains a need for better experimental and analytical approaches. Since reactivity to stress is a biologically complicated system and different people have different physiological sequelae to stress, researchers may well benefit from methodologies that generate more relevant data and take better advantage of systems science methods and approaches (Mobus & Kalton, 2015; Oken et al., 2015).
Acknowledgments
This study was funded in part by Oregon Health & Science University and by grants from National Institutes of Health (AT005121 and UL1TR000128).
Roger Ellingson is acknowledged for engineering support. Jeff Proulx helped edit the paper. Preliminary results were presented at the International Conference on Integrative Medicine and Health 2016 annual meeting.
Footnotes
There were no conflicts of interest by any authors.
Compliance with Ethical Standards:
There were no conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Abbott RA, Whear R, Rodgers LR, Bethel A, Thompson Coon J, Kuyken W, … Dickens C. Effectiveness of mindfulness-based stress reduction and mindfulness based cognitive therapy in vascular disease: A systematic review and meta-analysis of randomised controlled trials. Journal of Psychosomatic Research. 2014;76(5):341–351. doi: 10.1016/j.jpsychores.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Astin JA. Stress reduction through mindfulness meditation. Effects on psychological symptomatology, sense of control, and spiritual experiences. Psychotherapy and Psychosomatics. 1997;66(2):97–106. doi: 10.1159/000289116. [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Allen KB. Assessment of mindfulness by self-report: the Kentucky Inventory of mindfulness skills. Assessment. 2004;11(3):191–206. doi: 10.1177/1073191104268029. [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietmeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Ellard KK, Sauer-Zavala S, Bullis JR, Carl JR. The origins of neuroticism. Perspectives on Psychological Science. 2014;9(5):481–196. doi: 10.1177/1745691614544528. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Benton AL, Hamsher KDS. Multilingual Aphasia Examination. Iowa City, Iowa: AJA Associates; 1989. [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s Disease in the United States and the Public Health Impact of Delaying Disease Onset. American Journal of Public Health. 1998;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology. 2003;84:822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatric Research. 1989;28(2):192–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. The American Journal of Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkin D, Deyo RA, Berg AO. Evaluation of a physician intervention to improve primary care for low-back pain: II. Impact on patients. Spine. 1991;16:1173–1178. doi: 10.1097/00007632-199110000-00008. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Calati R, Serretti A. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clinical Psychology Review. 2011;31(3):449–464. doi: 10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. A systematic review of neurobiological and clinical features of mindfulness meditations. Psychological Medicine. 2010;40:1239–1252. doi: 10.1017/S0033291709991747. [DOI] [PubMed] [Google Scholar]
- Cohen S, Karmarck T, Mermelstein R. A Global Measure of Perceived Stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Costa PT, McCrae RR. NEO Inventories: Professional manual. Lutz, FL: Psychological Assessment Resources, Inc; 2010. [Google Scholar]
- Coteur G, Feagan B, Keininger DL, Kosinski M. Evaluation of the meaningfulness of health-related quality of life improvements as assessed by the SF-36 and the EQ-5D VAS in patients with active Crohn’s disease. Alimentary Pharmacology and Therapeutics. 2009;29(9):1032–1041. doi: 10.1111/j.1365-2036.2009.03966.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nature Neuroscience. 2012;15(5):689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Cox NJ, … Trevisan M. NIH state-of-the-science conference statement: Preventing Alzheimer’s disease and cognitive decline. NIH Consensus State-of-the-science Statements. 2010;27(4):1–30. [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nature Reviews Neuroscience. 2008;9(10):788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. Journal of Behavioral Therapy and Experimental Psychiatry. 2000;31:73–86. doi: 10.1016/S0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Esch T, Stefano GB, Fricchione GL, Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuroendocrinology Letters. 2002;23(3):199–208. [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences. 2009;106:6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard T, Holzel BK, Lazar SW. The potential effects of meditation on age-related cognitive decline: a systematic review. Ann N Y Acad Sci. 2014;1307:89–103. doi: 10.1111/nyas.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test Manual. Wood Dale, IL: Stoelting Co; 2002. [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, … Haythornthwaite JA. Meditation Programs for Psychological Stress and Well-being: A Systematic Review and Meta-analysis. JAMA Internal Medicine. 2014a doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, … Haythornthwaite JA. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Internal Medicine. 2014b;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits: A meta-analysis. Journal of Psychosomatic Research. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage. 2012;59(1):750–760. doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hrobjartsson A, Gotzsche PC. Is the Placebo Powerless? An analysis of Clinical Trials Comparing Placebo with No Treatment. The New England Journal of Medicine. 2001;344(21):1594–1620. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, Worthman CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30(7):698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary Costs of Dementia in the United States. The New England Journal of Medicine. 2013;368(14):1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer Dan G, Yaffe Kristine, Liverman Catharyn T. Institute of Medicine (U.S.). Committee on the Public Health Dimensions of Cognitive Aging. Cognitive aging : progress in understanding and opportunities for action. National Academies Press; 2015. [PubMed] [Google Scholar]
- Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell DA, … Saron CD. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664–681. doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(2):109–119. doi: 10.3758/CABN.7.2.109. [DOI] [PubMed] [Google Scholar]
- Jha AP, Stanley EA, Kiyonaga A, Wong L, Gelfand L. Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion. 2010;10(1):54–64. doi: 10.1037/a0018438. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic Load Biomarkers of Chronic Stress and Impact on Health and Cognition. Neuroscience & Biobehavioral Reviews. 2009 doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. General Hospital Psychiatry. 1982;4(1):33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, Fletcher KE, Pbert L, … Santorelli SF. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149(7):936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- Kaul P, Passafiume J, Sargent CR, O’Hara BF. Meditation acutely improves psychomotor vigilance, and may decrease sleep need. Behavioral and Brain Functions. 2010;6:47. doi: 10.1186/1744-9081-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, … Hofmann SG. Mindfulness-based therapy: a comprehensive meta-analysis. Clinical Psychology Review. 2013;33(6):763–771. doi: 10.1016/j.cpr.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Kirsch Irving. The emperor’s new drugs : exploding the antidepressant myth. New York, NY: Basic Books; 2010. [Google Scholar]
- Knopman DS, Roberts RO, Geda YE, Pankratz VS, Christianson TJ, Petersen RC, Rocca WA. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34(1):34–42. doi: 10.1159/000255464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Giese-Davis J, Yutsis M, O’Hara R, Neri E, Gallagher-Thompson D, … Spiegel D. Design decisions to optimize reliability of daytime cortisol slopes in an older population. The American Journal of Geriatric Psychiatry. 2006;14(4):325–333. doi: 10.1097/01.JGP.0000201816.26786.5b. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Lachman ME, Pruessner JC, Sliwinski M, Wilson RS. Mechanisms of age-related cognitive change and targets for intervention: social interactions and stress. J Gerontol A Biol Sci Med Sci. 2012;67(7):760–765. doi: 10.1093/gerona/gls125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB. Public health significance of neuroticism. The American Psychologist. 2009;64(4):241–256. doi: 10.1037/a0015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Nair NPV, Briere S, Maheu F, Tu MT, Lemay M, … Meaney MJ. Increased cortisol levels and impaired cognition in human aging: Implication for depression and dementia later in life. Reviews in the Neurosciences. 1999;10(2):117–139. doi: 10.1515/REVNEURO.1999.10.2.117. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. The New England Journal of Medicine. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Mobus GE, Kalton MC. Principles of Systems Science. Springer; 2015. [Google Scholar]
- Moore A, Gruber T, Derose J, Malinowski P. Regular, brief mindfulness meditation practice improves electrophysiological markers of attentional control. Frontiers in Human Neuroscience. 2012;6:18. doi: 10.3389/fnhum.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J, Heyman A, Mohs R, Hughes M. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part 1. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/WNL.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Moynihan JA, Chapman BP, Klorman R, Krasner MS, Duberstein PR, Brown KW, Talbot NL. Mindfulness-based stress reduction for older adults: effects on executive function, frontal alpha asymmetry and immune function. Neuropsychobiology. 2013;68(1):34–43. doi: 10.1159/000350949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Yadav R, Yung I, Zajdel D, Oken BS. Sensitivity to mental effort and test-retest reliability of heart rate variability measures in healthy seniors. Clinical Neurophysiology. 2011;122:2059–2066. doi: 10.1016/j.clinph.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuendorf R, Wahbeh H, Chaime I, Yu J, Hutshison K, Oken BS. The Effects of Mind-Body Interventions on Sleep Quality: A Systematic Review. Evidence-Based Complementary and Alternative Medicine. 2015 doi: 10.1155/2015/902708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuendorf R, Wahbeh H, Chamine I, Yu J, Hutchison K, Oken BS. The Effects of Mind-Body Interventions on Sleep Quality: A Systematic Review. Evid Based Complement Alternat Med. 2015;2015:902708. doi: 10.1155/2015/902708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NPR, Foundation, Robert Wood Johnson, & Health, Harvard School of Public. The Burden of Stress in America. 2014 http://www.rwjf.org/content/dam/farm/reports/surveys_and_polls/2014/rwjf414295.
- Oken BS. Placebo effects: clinical aspects and neurobiology. Brain. 2008;131:2812–2823. doi: 10.1093/brain/awn116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Chamine I, Wakeland W. A systems approach to stress, stressors, and resilience in humans. Behavioural Neuroscience. 2015;282:144–154. doi: 10.1155/2015/902708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Flegal K, Zajdel D, Kishiyama S, Haas M, Peters D. Expectancy effect: impact of pill administration on cognitive performance in healthy seniors. Journal of Clinical and Experimental Neuropsychology. 2008;30:7–17. doi: 10.1080/13803390701775428. [DOI] [PubMed] [Google Scholar]
- Oken BS, Fonareva I, Haas M, Wahbeh H, Lane JB, Zajdel DP, Amen AM. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. Journal of Alternative and Complementary Medicine. 2010;16:1031–1038. doi: 10.1089/acm.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Fonareva I, Wahbeh H. Stress-related cognitive dysfunction in dementia caregivers. Journal of Geriatric Psychiatry and Neurology. 2011a;24:192–199. doi: 10.1177/0891988711422524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Fonareva I, Wahbeh H. Stress-related cognitive dysfunction in dementia caregivers. Journal of Geriatric Psychiatry and Neurology. 2011b;24:192–199. doi: 10.1177/0891988711422524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Kishiyama S, Zajdel D, Bourdette D, Carlsen J, Haas M, … Mass M. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology. 2004;62(11):2058–2064. doi: 10.1212/01.WNL.0000129534.88602.5C. [DOI] [PubMed] [Google Scholar]
- Oken BS, Zajdel D, Kishiyama S, Flegal K, Dehen C, Haas M, … Leyva J. Randomized controlled 6-month trial of yoga in healthy seniors. Alternative Therapies in Health and Medicine. 2006;12(1):40–47. [PMC free article] [PubMed] [Google Scholar]
- Ospina MB, Bond K, Karkhaneh M, et al. Meditation practices for health: state of the research (AHRQ) Evidence Report/Technology Assessment (Full Report) 2007;155:1–263. Publication No. 07-E010. [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C, Shahabi L, Tarvainen M, Cook I, Abrams M, Shapiro D. Cognitive, behavioral, and autonomic correlates of mind wandering and perseverative cognition in major depression. Front Neurosci. 2014;8:433. doi: 10.3389/fnins.2014.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, Brosschot JF. Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychol Bull. 2016;142(3):231–259. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, … Wallace RB. Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, … Wallace RB. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. doi: 10.2307/2529712. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Schwarzer R, Jerusalem M. Generalized self-efficacy scale. In: Weinman J, Wright S, Johnston M, editors. Measures in health psychology: a user’s portfolio. Causal and control beliefs. Windsor, UK: Nfer-Nelson; 1995. pp. 35–37. [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based Cognitive Therapy for Depression: A new approach to preventing relapse. New York: Guilford; 2002. [Google Scholar]
- Segerstrom SC, Boggero IA, Smith GT, Sephton SE. Variability and reliability of diurnal cortisol in younger and older adults: implications for design decisions. Psychoneuroendocrinology. 2014;49:299–309. doi: 10.1016/j.psyneuen.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple RJ. Does mindfulness meditation enhance attention? a randomized contrlled trial. Mindfulness. 2010;1:121–130. doi: 10.1007/s12671-010-0017-2. [DOI] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Stawski RS, Sliwinski MJ, Smyth JM. Stress-related cognitive interference predicts cognitive function in old age. Psychology and Aging. 2006;21:535–544. doi: 10.1037/0882-7974.21.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, … Posner MI. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology NASPE. Heart rate variability - standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39(4):255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Wahbeh H, Goodrich E, Goy E, Oken BS. Mechanistic pathways of mindfulness meditation in combat veterans with posttraumatic stress disorder. Journal of Clinical Psychology. 2016;72:365–383. doi: 10.1002/jclp.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Kishiyama S, Zajdel D, Oken B. Salivary cortisol awakening response in mild Alzheimer’s disease, caregivers, and non-caregivers. Alzheimer’s Disease & Related Disorders. 2008;22:181–183. doi: 10.1097/WAD.0b013e31815a9dff. [DOI] [PubMed] [Google Scholar]
- Wahbeh H, Lane JB, Goodrich E, Miller M, Oken BS. One-on-one mindfulness meditation trainings in a research setting. Mindfulness. 2014;5:88–99. doi: 10.1007/s12671-012-0155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Oken B. Salivary cortisol lower in posttraumatic stress disorder. Journal of Traumatic Stress. 2013;26:1–8. doi: 10.1002/jts.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Svalina MN, Oken BS. Group, One-on-One, or Internet?: Preferences for mindfulness meditation delivery format and their predictors. Open Medicine Journal. 2014;1:66–74. doi: 10.2174/1874220301401010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Zwickey H, Oken B. One method for objective adherence measurement in mind-body medicine. The Journal of Alternative and Complementary Medicine. 2011;17:1–3. doi: 10.1089/acm.2010.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walach H. The efficacy paradox in randomized controlled trials of CAM and elsewhere: beware of the placebo trap. Journal of Alternative and Complementary Medicine. 2001;7:213–218. doi: 10.1089/107555301300328070. [DOI] [PubMed] [Google Scholar]
- Ware JE. SF-36 health survey update. Spine. 2000;25(24):3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- Ware JE. SF-36 Health Survey: Manual interpretation Guide. Boston: The Health Institute; 1993. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Fourth Edition. San Antonio, TX: Pearson; 2008. [Google Scholar]
- White L, Small BJ, Petrovitch H, Ross GW, Masaki K, Abbott RD, … Markesbery W. Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu-Asia Aging Study. Journal of Geriatric Psychiatry and Neurology. 2005;18(4):224–227. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and late-life dementia. Psychosomatic Medicine. 2007;69(1):47–53. doi: 10.1097/01.psy.0000250264.25017.21. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Begeny CT, Boyle PA, Schneider JA, Bennett DA. Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatr Psychiatry. 2011;19(4):327–334. doi: 10.1097/JGP.0b013e31820119da00019442-201104000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Evans DA, Bienias JL, Mendes De Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61(11):1479–1485. doi: 10.1212/01.WNL.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Johnson SK, Diamond BJ, David Z, Goolkasian P. Mindfulness meditation improves cognition: evidence of brief mental training. Consciousness and Cognition. 2010;19(2):597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]