Abstract
The structure and chaperone function of DmHsp22WT, a small Hsp of Drosophila melanogaster localized within mitochondria were examined. Mutations of conserved arginine mutants within the alpha-crystallin domain (ACD) domain (R105G, R109G, and R110G) were introduced, and their effects on oligomerization and chaperone function were assessed. Arginine to glycine mutations do not induce significant changes in tryptophan fluorescence, and the mutated proteins form oligomers that are of equal or smaller size than the wild-type protein. They all form oligomer with one single peak as determined by size exclusion chromatography. While all mutants demonstrate the same efficiency as the DmHsp22WT in a DTT-induced insulin aggregation assay, all are more efficient chaperones to prevent aggregation of malate dehydrogenase. Arginine mutants of DmHsp22 are efficient chaperones to retard aggregation of CS and Luc. In summary, this study shows that mutations of arginine to glycine in DmHsp22 ACD induce a number of structural changes, some of which differ from those described in mammalian sHsps. Interestingly, only the R110G-DmHsp22 mutant, and not the expected R109G equivalent to human R140-HspB1, R116-HspB4, and R120-HspB5, showed different structural properties compared with the DmHsp22WT.
Keywords: Small heat shock protein (sHsp), Chaperone assays, Alpha-crystallin domain (ACD), DmHsp22, Mitochondria, Drosophila melanogaster
Introduction
Small heat shock proteins (sHsps) are low molecular weight chaperones involved in protein folding (Bakthisaran et al. 2015; Basha et al. 2012; Haslbeck and Vierling 2015; Jee 2016; McHaourab et al. 2009). They are found in all organisms including cyanobacterial viruses (Bourrelle-Langlois et al. 2016; Maaroufi and Tanguay 2013). Small Hsps are expressed under various stress conditions as well as during development in a cell-specific pattern (Arrigo and Tanguay 1991; Marin et al. 1993, 1996; Marin and Tanguay 1996; Michaud et al. 1997; Michaud and Tanguay 2003; Morrow and Tanguay 2003, 2012, 2015). Their molecular weight vary between 12 and 43 kDa (Sudnitsyna et al. 2012; Tyedmers et al. 2010), and their primary structure is subdivided in three domains: an alpha-crystallin domain (ACD), flanked by a highly variable N-terminal sequence (NTS) and a C-terminal extension (CTE) (Basha et al. 2012; Delbecq and Klevit 2013; Kappe et al. 2010). Small Hsps form homo- and hetero-oligomers with highly dynamic quaternary structure (Baldwin et al. 2011a, b; van Montfort et al. 2001), which can bind to misfolded proteins and prevent their aggregation. They are involved in maintaining protein homeostasis of the cell in an ATP-independent manner (Biswas and Das 2004; Boncoraglio et al. 2012; Ghosh et al. 2006; Giese and Vierling 2002; Hilton et al. 2013; Muchowski and Clark 1998; Stengel et al. 2010; Vos et al. 2008). The sHsp dimer is defined as the building block of higher-order sHsp structures. Dimerization in the ACD domain occurs in two different ways (Baldwin et al. 2011a; Hilton et al. 2013; Jehle et al. 2009; Laganowsky et al. 2010). Indeed, in mammalian sHsps, elongated ß6 + 7 strands of each monomer assemble together in an anti-parallel orientation to form dimers. However, in plant and lower organisms, dimerization occurs via reciprocal interaction between a ß6 strand of one monomer into the ß-sandwich of the neighboring monomer (Bagneris et al. 2009; Hilario et al. 2011; Kim et al. 1998; Laganowsky et al. 2010; Takeda et al. 2011; van Montfort et al. 2001). As sHsps are involved in many intracellular processes, it is not surprising that mutations within these proteins have been associated with different congenital diseases (Boncoraglio et al. 2012; Clark et al. 2012; Litt et al. 1998; Sun and MacRae 2005; Vicart et al. 1998). Among those, the mutation of a conserved arginine with a glycine have shown drastic effects: R120G in HspB5 is associated with myopathy, cardiomyopathy, and cataracts (Clark et al. 2012; Clark and Muchowski 2000) and R140G in HspB1 is associated with distal hereditary motor neuropathy and/or Charcot-Marie-Tooth disease type 2 (Houlden et al. 2008; Ikeda et al. 2009).
Drosophila melanogaster has 12 sHsps (Morrow and Tanguay 2015). DmHsp22 is a unique member of this family as it localizes to mitochondria where it is found in the matrix (Morrow et al. 2000). It shows a specific developmental expression pattern and has been associated with increased lifespan and resistance to oxidative stress (Michaud et al. 2002; Morrow et al. 2004).
Little is known about the quaternary structure of this protein. DmHsp22 containing three arginines (R) in the ACD at positions R105, R109, and R110 are found in almost all sHsps and are thought to play an important role in the higher structure of sHsps (Bova et al. 1999; Nefedova et al. 2013). Furthermore, the importance of conserved arginine, a charged and bulky amino acid involved in the formation of salt bridges through positive charge of guanidine group, needs to be characterized not only in humans.
Recently, Moutaoufik et al. (2016) described the structure and chaperone-like activity of three highly conserved arginine mutants within the ACD of Drosophila nuclear DmHsp27 (R122G, R131G, and R135G). Here, we examine the quaternary structure and chaperone-like activity of mitochondrial DmHsp22 and arginine mutants within the ACD domain.
Materials and methods
Amino acid sequence alignment
Sequence alignment of DmHsp22 with DmHsp27 and human sHsps including HspB1, HspB4, and HspB5 in the region of ACD was analyzed using multiple sequence alignment program (Clustal W).
Cloning and expression of recombinant proteins
DNA encoding D. melanogaster Hsp22WT was cloned in pETHSUK vector (generously provided by Dr. Stephen D. Weeks, KU Leuven, Belgium) at HindIII and KpnI sites by PCR (TransGen Biotech) (Weeks et al. 2007) and Gibson assembly (New England Biolabs). Arginine to glycine mutants in the ACD of the DmHsp22WT were constructed by using suitable oligomers (Invitrogen) (Table 1) and site-directed mutagenesis.
Table 1.
Primers to construct Drosophila melanogaster Hsp22 arginine mutants
| Gene Identity | Primer sequence | Length |
|---|---|---|
| Hsp22R105G | GCTATAGCTCCGGGCACTTCCTCCGCCG | 28 |
| GAGGAAGTGCCCGGAGCTATAGCCACC | 27 | |
| Hsp22R109G | CCAGGCACTTCCTCGGCCGATTCGTTCTGC | 30 |
| GCAGAACGAATCGGCCGAGGAAGTGCCTGG | 30 | |
| Hsp22R110G | GGCACTTCCTCCGCGGATTCGTTCTGCCG | 29 |
| CGGCAGAACGAATCCGCGGAGGAAGTGCC | 29 | |
| Hsp22R109GR110G | GGCACTTCCTCGGCGGATTCGTTCTGCCGG | 30 |
| CCGGCAGAACGAATCCGCGGAGGAAGTGCC | 30 |
Primers are listed in the 5′ → 3′ direction. The mutation sites are underlined
The DmHsp22WT and its arginine mutants were expressed in BL21 (DE3)-competent Escherichia coli (New England Biolabs). Transformed bacteria were grown in 10 ml LB broth Miller (EMD Chemicals Inc) containing 100 μg/ml ampicillin (Biobasic Canada) at 37 °C overnight. Growing bacteria was transferred into 490 ml broth Miller containing ampicillin at 37 °C until the optical density at 600 nm was equal to 0.4–0.6. Protein expression was done at 30 °C for 5 h with isopropyl-β-thiogalactoside (IPTG; Roche Applied Science) at a final concentration of 0.4 mM. Bacterial cells were harvested by centrifugation at 6000×g for 15 min and were frozen at −80 °C.
Purification of recombinant proteins
For protein purification, the bacterial pellet was resuspended in a binding buffer (20 mM Tris/HCl, pH 7.9, containing 0.5 M NaCl and 5 mM imidazole) supplemented with 0.01 mg/ml of DNAse and 4 mM of phenylmethyl sulfonyl fluoride (PMSF; Sigma-Aldrich) and lysed using French press (SLM Aminco). The bacterial lysate was centrifuged at 10,000×g for 30 min at 4 °C, and the supernatant obtained from centrifugation was loaded on 1.5 ml-charged nickel agarose beads (Ni-NTA; Qiagen) equilibrated with a 10 ml binding buffer. The column was washed with 40 ml of wash buffer (20 mM Tris/HCl, pH 7.9, containing 0.5 M NaCl and 60 mM imidazole). Proteins binding to the column were then eluted by applying 10 ml of elution buffer (20 Mm Tris/HCl, pH 7.9, containing 0.5 M NaCl and 1 M imidazole). Eluted proteins were then subjected to dialysis in TEN buffer (20 mM Tris/HCl, pH 7.9, containing 0.1 mM EDTA and 100 mM NaCl) three times for 2 h at 4 °C. Dialyzed samples were concentrated using Amicon Ultra-2 ml 10 kDa cutoff centrifugal filter units (Merck Millipore) to a 500 µl final volume. Protein concentration was measured using Bradford protein assay kit (Bio-Rad Protein Assay). The 6His-SUMO tag was then removed using a purified SUMO hydrolase enzyme for 1 h at 30 °C at a ratio of 1 μg SUMO hydrolase to 100 μg of each recombinant protein. The hydrolysate was then subjected to size-exclusion chromatography (SEC) on a Superose 6 10/300 GL column (GE Healthcare Life Sciences) to remove digested tag from the pure proteins. The purified proteins (5–10 mg/500 ml of growth media) were collected and kept at −80 °C in TEN buffer.
Fluorescence spectroscopy
Trp fluorescence intensity of DmHsp22WT and its arginine mutants was measured at 20 °C in buffer F (20 mM HEPES/NaOH, pH 7.9, containing 100 mM NaCl) as a function of wavelength (Bushueva et al. 1980). The protein concentration for this assay was 0.1 mg/ml. Fluorescence spectra was excited at 295 nm and recorded in the range of 300–400 nm (both slit width 5 nm) in thermostated cells (Varian Cary Eclipse spectrofluorometer).
Dynamic light scattering
Dynamic light scattering (DLS) experiments were performed using a DynaPro dynamic light scattering instrument (Wyatt Technology Corp, Santa Barbara, CA) (Michiel et al. 2009). The laser light source wavelength was 830 nm, and scatter light was collected at 90° using an optic fiber. Protein samples were first filtered through a 0.22 µm filter before loading. DLS buffer was 20 mM HEPES/NaOH (pH 7.9, 100 mM NaCl). The assay’s temperature was adjusted using a Peltier cell ranging from 8 to 55 °C and a protein concentration was between 3 and 5.6 mg/ml (136 to 255 μM). Each single-intensity measurement lasted for 10 s and totally up to 1 h for every condition. Dynamic regulation method version 6 (V6) software was used to obtain a hydrodynamic radius (Rh) and standard deviation (polydispersity (%P)) (Michiel et al. 2009, 2010).
Size-exclusion chromatography
Purification of the recombinant proteins and analysis of their quaternary structure were performed by SEC using a Superose 6 10/300 GL column (GE Healthcare Life Sciences) equilibrated with TEN buffer (20 mM Tris/HCl, pH 7.9, 0.1 mM EDTA and 100 mM NaCl). The proteins were centrifuged for 15 min at 10,000×g to remove aggregated proteins, and the supernatant was loaded onto the column. For analytical SEC, 100 μl of each sample containing 200 or 400 μg of proteins were injected into the column. Analytical SEC was run at room temperature at a flow rate of 0.5 ml/min in TEN buffer. Protein elution volume was monitored by absorbance at 280 nm. The column was calibrated with molecular weight standard immunoglobulin M (IGM) from bovine serum (970 kDa) and the High Molecular Weight Gel Filtration Calibration Kit (GE Healthcare) containing thyroglobulin (669 kDa), ferritin (440 kDa), aldolase (158 kDa), and conalbumin (75 kDa). Five hundred microliters of eluted fractions were collected to be analyzed later by means of SDS-PAGE and native gels.
SDS-PAGE and native gels
The purified proteins were analyzed on 12% SDS-PAGE under reducing condition and stained using Coomassie Brilliant Blue (Laemmli 1970). Native polyacrylamide gel electrophoresis was performed in Novex™ in 4–12% Tris-glycine gel (Life Technologies) under non-denaturing (native) conditions. In this system, proteins are separated based on their charge to mass ratios. Twenty-five micrograms of each newly purified protein was added to the native sample buffer and run at 4 °C with a constant voltage (125 V). Both upper buffer chamber and lower buffer chamber were filled with native electrophoresis buffer (100 mM Tris base and 10% glycine at pH 8.3). XCell SureLock Electrophoresis Cell was used to hold the gel and buffer. Protein weight standards (eight protein bands ranging in molecular weight from ~20 to 1200 kDa; Life Technologies) were used to determine molecular weight under native electrophoresis conditions.
Heat-induced aggregation assay
Chaperone-like activity of DmHsp22 and its arginine mutants was determined by their ability to prevent aggregation of several model substrates: luciferase (Luc; 0.1 μM; molecular weight (MW) 61 kDa; Promega), citrate synthase (CS) from pig (0.16 μM; MW 51 kDa; Sigma), and malate dehydrogenase (MDH) from pig heart (0.65 μM; MW 35 kDa; Roche).
Protein substrates were heat denatured at 42 °C for Luc and 45 °C for CS and MDH. Time duration for this assay was 30 min for Luc and 90 min for CS and MDH. All substrates were heat denatured in the absence or presence of either DmHsp22 or its arginine mutants equal to 0.05 μM for Luc assay, 0.33 μM for CS, and 0.65 μM for MDH. All assays were performed in 1 ml total volume in semi-micro-cuvettes (Sarstedt) by light scattering at 320 nm on a spectrophotometer with thermostated cells (Varian Cary 100, Montreal, Quebec, Canada) in HEPES buffer (50 mM HEPES-KOH, pH 7.5) (Fernando and Heikkila 2000; Morrow et al. 2006). Data are representative of three different assays and are expressed as the mean ± standard deviation.
DTT-induced insulin assay
Insulin solution from bovine pancreas (52 μM; Sigma) was used as protein substrate for dithiothreitol (DTT)-induced insulin aggregation assay at 37 °C. All the samples were centrifuged for 15 min at 10,000×g before starting the assay to prevent the presence of any aggregated protein. The assay mixture containing 52 μM insulin in the presence of 7.5 μM of DmHsp22 or its mutants was pre-incubated for 10 min at 37 °C in buffer I (50 mM potassium phosphate, pH 8, 100 mM NaCl). DTT-induced aggregation of insulin was followed by adding 60 μl of 1 M DTT (Bio-Rad) in order to reduce disulfide bonds in insulin (Muranova et al. 2015; Sluchanko et al. 2014) and was monitored in a spectrophotometer with thermostated cells (Varian Cary 100) at 340 nm.
Results
Sequence alignments and protein production
Sequence alignments of DmHsp22 with human HspB1, HspB4, HspB5, and D. melanogaster Hsp27 in the region of ACD are shown in Fig. 1a. In this sequence alignment, R105 and R109 in DmHsp22 are located at highly conserved places in the ACD primary structure. R109 is conserved with R135-DmHsp27, R140-HspB1, R116-HspB4, and R120-HspB5 while R105 from DmHsp22 is conserved with R131-DmHsp27, R136-HspB1, R112-HspB4, and R116-HspB5. Furthermore, the R110 residue in the primary structure of DmHsp22 is similar to the R117-HspB4 and is replaced with lysine residues in K136-DmHsp27, K141-HspB1, and K121-HspB5 according to the sequence alignment. Given the importance of ACD arginine for structure and function of other sHsps, each DmHsp22 arginine was mutated to glycine and a double mutant was also constructed (R109GR110G) (Fig. 1a). As can be seen in Fig. 1b, the purified proteins were soluble and pure in SDS-PAGE and were migrating around 20 kDa. The yield of each protein after purification was around 5–10 mg/500 ml of bacterial media.
Fig. 1.
Primary structure and sequence homology of DmHsp22. a Amino acid sequence alignment of ACDs of DmHsp22 (P02515) and DmHsp27 (P02518) and human HspB1 (P04792), HspB4 (P02489), and HspB5 (P02511) were performed using multiple sequence alignment program (Clustal W). Block columns represent completely conserved amino acid residues. b Twenty-five micrograms of each single arginine mutant in addition to the recombinant DmHsp22WT protein were loaded on 12% SDS-PAGE. M protein molecular weight marker. Proteins were visualized by using Coomassie Brilliant Blue staining G250
Fluorescence spectra of the DmHsp22WT and its ACD arginine mutants
Intrinsic fluorescence properties of tryptophan residues are sensitive indicators of proteins’ conformational behavior whose measurement depends on the microenvironment (Ghisaidoobe and Chung 2014). The N-terminal sequence of the DmHsp22 contains four tryptophan (W) residues (W8, W34, W42, and W48). The fluorescence spectra of DmHsp22 and the arginine mutants had a similar excitation maximum located at 330–350 nm (Fig. 2). The fluorescence intensities for arginine mutants R105G, R109G, and R110G were almost similar and slightly higher than that of DmHsp22WT, while the position and shape of maximum fluorescence was the same. Thus, tryptophan residues of these three arginine mutants did not show significant differences in tryptophan fluorescence exposure compared with the WT protein (Fig. 2). Therefore, tryptophan environment is stable in all the cases and the mutations do not seem to have a significant influence on tryptophan exposure within the N-terminal region.
Fig. 2.
Fluorescence spectra of the DmHsp22WT and its arginine mutants. The fluorescence spectra of proteins (0.1 mg/ml) at 20 °C were identified at the excitation wavelength of 295 nm and recorded in the range of 300–400 nm (both slits width 5 nm)
Thermal transition of Hsp22 and its ACD arginine mutants
sHsps are dynamic molecules under physiological conditions, and the rate of subunit dissociation and re-association is strongly dependent on temperature (Fu and Chang 2004), which is necessary for their chaperone-like activity (Fu et al. 2003). DLS was therefore performed to determine the size and the thermal stability of DmHsp22 and its ACD arginine mutants. DLS was performed at different temperatures ranging from 8 to 55 °C to investigate conformational changes in the oligomeric structure due to temperature. At 8 °C, DmHsp22 has a Rh of 7.9 nm and 31% of polydispersity (Fig. 3a). From 8 to 15 °C, the Rh shows a slight decrease and reaches 7.3 nm at 15 °C. From 15 to 45 °C, the Rh is stable in the case of WT protein, and from 45 to 55 °C, the Rh exhibits an increase to reach a Rh of 8.1 nm (Fig. 3d). The R105G mutant shows the same trend as DmHsp22WT (Data not shown). The behavior of R109G mutant is also very similar to that of DmHsp22WT protein with a Rh of about 7.9 nm and polydispersity of 42% at 8 °C (Fig. 3b). The Rh of R109G decreases slowly from 15 to 25 °C and reaches 7.3 nm at 25 °C (Fig. 3d). From 25 to 35 °C, the Rh increases lightly to reach 7.6 nm. From 35 to 45 °C, the Rh is stable and then goes up which subsequently rises as shown by a slight increase to 7.8 nm at 55 °C (Fig. 3d). The size of the particles formed by the R110G mutant is smaller than DmHsp22WT and R109G. In this mutant, the Rh is stable around 6 nm from 8 to 35 °C (Fig. 3c, d). At 45 °C, the Rh exhibits a continuous increase to reach 8.3 nm at 55 °C (Fig. 3d). Up to moderately high temperatures, the DLS analysis detected only one single population with a relatively large polydispersity (around 40%). It is worth noticing that for globular and monodisperse proteins, the polydispersity is expected to be below 20% (Roebben et al. 2015). For the DmHsp22WT, the percentage of polydispersity indicates a stable behavior of up to 55 °C ranging from 39 to 44%. In contrast, for the R109G and R110G mutants and at high temperature, the DLS started to detect two populations, each having a smaller polydispersity, 16 and 13% at 55 °C, respectively. The new population observed in DLS would be due to the appearance of a large oligomer corresponding to 24% of the mass for R109G and 48% of the mass for R110G at 55 °C.
Fig. 3.
DLS analysis of DmHsp22WT and arginine mutants of ACD shows their behavior by increasing the temperature. a–c Size estimation of the protein particles and polydispersity of homo-oligomers were reported as percentage of polydispersity of the oligomers of DmHsp22WT, R109G, and R110G at 8 °C. d Evolution of Rh as a function of temperature (8–55 °C). Rh hydrodynamic radius (nm), % poly D percentage of polydispersity, MW estimation of molecular weight (kDa)
Mutation of arginine residues in the ACD influences the oligomeric structure of DmHsp22
The results of DLS were next confirmed by SEC and native gel electrophoresis. These methods are complementary giving information on the molecular sizes of the oligomers as well as their distribution (Delbecq and Klevit 2013; Michiel et al. 2009, 2010; Moutaoufik et al. 2016). The oligomeric structure of DmHsp22 and its arginine mutants were investigated by size-exclusion chromatography. The DmHsp22WT protein showed one large single peak eluting at a volume of 12.8 ml on the SEC column and corresponding to an apparent molecular weight of ~790 kDa (Fig. 4a).
Fig. 4.
SEC of the DmHsp22WT and arginine mutants. a–e Analytical SEC of the WT and arginine mutants were performed on a Superose 6, 10/300 GL column at room temperature. Two different quantities of each protein after purification were injected on SEC column (400 and 200 μg of each sample as indicated in the curves). Elution volumes of proteins were measured at absorbance of 280 nm. In analytical SEC, DmHsp22WT protein was eluted as a single peak of ~790 kDa. For other injected samples, single peak of elute volume was at ~763, 680, Remove and 369 or 424 and 790 kDa corresponding to the R109G, R110G, R109GR110G at two different concentrations, and R105G, respectively. The molecular weight standards are indicated at the top of each curve. Each color in the curves show an identified sample as indicated in the curves (a–e). Solid lines representing higher quantity of each protein (400 μg), and dashed lines indicate lower quantity of each sample (200 μg)
The oligomers formed by DmHsp22WT at two concentrations are stable with respect to the position and shape of their peak, which means they are independent from the quantity of protein injected to the column (Fig. 4a). R109G was eluted as a single peak with a small shift towards an elution volume of ~13 ml compared with the DmHsp22WT protein, giving a molecular weight of ~763 kDa on SEC (Fig. 4b). R110G had a smaller apparent molecular weight of ~680 kDa compared with the DmHsp22WT (Fig. 4c). Elution profile of R109G and R110G were stable and independent from the protein quantity. The elution profile of the double R109GR110G mutant showed a wider elution profile at two different protein concentrations (Fig. 4d). Injecting 400 μg of this double mutant protein indicated an elution volume of ~15.9, corresponding to a single peak with an apparent molecular weight of 369 kDa, while decreasing the amount of the protein to 200 μg which resulted into an elution volume of ~15.5, corresponding to a single peak with an apparent molecular weight of ~424 kDa (Fig. 4d). Similar to the WT protein, R105G produces a curve with an elution volume of ~12.8, corresponding to an apparent molecular weight of ~790 kDa (Fig. 4e).
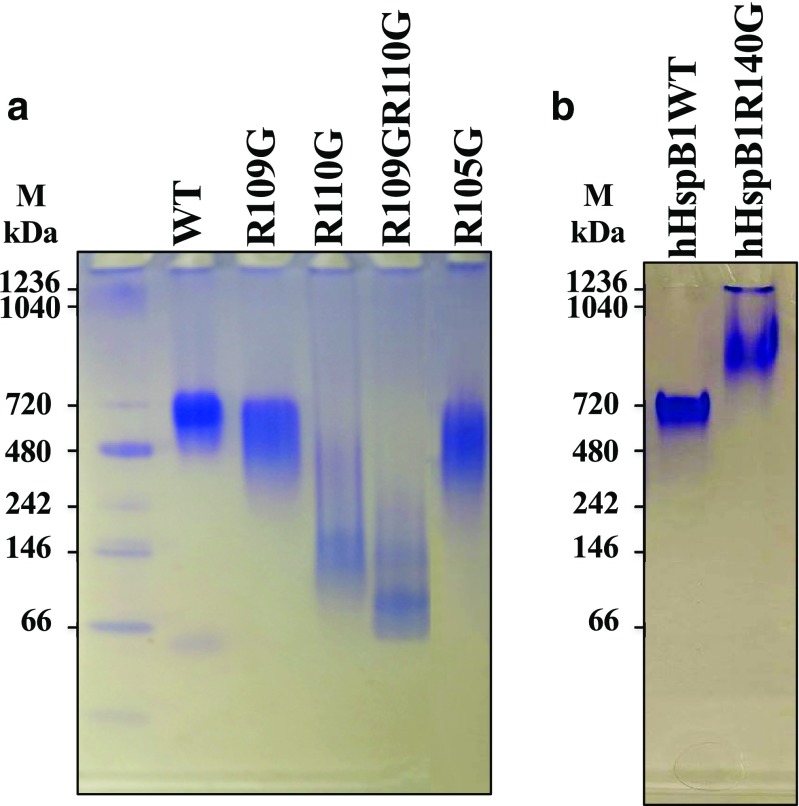
Native-PAGE electrophoresis was also used to confirm the result of SEC. For DmHsp22WT, R109G, and R105G, native electrophoresis supports the result of SEC and molecular weight of the proteins in native gel is comparable with the molecular weight observed by SEC. For example, R105G shows one band at lighter MW position than DmHsp22WT of ~720 kDa (Fig. 5a). In contrast, R110G and the double mutant R109GR110G do not form one single band in native gel, which makes interpretations difficult on their behavior on native gel. This might be due to the native gel method, which shows a different representation of dynamic subunit exchange behavior of proteins compared with SEC and DLS. Behavior of these arginine mutants of DmHsp22 differs from that of human HspB1 that form larger oligomers compared with the wild-type proteins (Fig. 5b) (Bova et al. 1999).
Fig. 5.
Novex™ 4–12% Tris-glycine native gel analysis. a Twenty-five micrograms of DmHsp22WT and its arginine mutants after Bradford protein assay were loaded on 4–12% Tris-glycine native gel at 4 °C to compare the oligomeric profiles of proteins. b Native gel analysis of the human R140G-HspB1 versus HspB1WT. Twenty-five micrograms of each purified protein were loaded on 4–12% Tris-glycine native gel and separated either at 4 °C. Proteins were visualized by using Coomassie brilliant blue staining G250
Chaperone-like activity
One of the common functions of many sHsps is their ability to prevent the aggregation of different protein substrates especially under stressful conditions. The chaperone-like activity of DmHsp22WT and its arginine mutants were tested in in vitro assays using different substrates namely Luc, insulin, CS, and MDH.
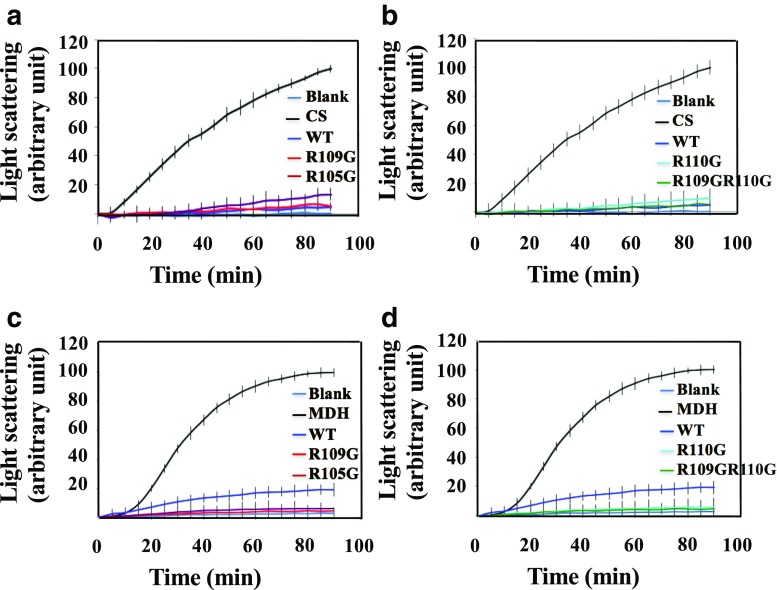
DmHsp22WT prevented heat-induced aggregation of Luc with 93% efficiency at a molar ratio of 0.5:1 μM of DmHsp22 to Luc. Mutants R110G and R109GR110G were similarly effective with a very light decrease compared with the WT (87 and 89% efficiency, respectively) (Fig. 6a, b). DmHsp22WT provided a 95% protection against aggregation of CS at a molar ratio of 2:1. Mutants R105G and R110G also retarded aggregation of CS (88 and 90%, respectively) (Fig. 7a, b). Interestingly, DmHsp22WT is less efficient than the mutants to prevent heat-induced aggregation of MDH at a molar ratio of 1:1 of WT to MDH (0.65 μM). The percentage of efficiency observed by WT was about 81%, while all the mutants showed a 94–96% efficiency to protect MDH as a substrate (Fig. 7c, d).
Fig. 6.
Chaperone-like activity of DmHsp22WT and its arginine mutants by using luciferase (Luc) (a, b) and insulin (c, d) as protein substrates. The heat and DTT-induced aggregation of Luc and insulin were assayed using absorbance at 320 nm for Luc assay and 340 nm for DTT-induced insulin aggregation assay at different temperatures (42 °C for Luc and 37 °C for DTT-induced aggregation of insulin). a, b Heat-induced aggregation of Luc (0.1 μM) was measured for 30 min in HEPES-KOH 50 mM (pH, 7.5) to make the molar ratio of 0.5:1 for sHsp to substrate. c, d DTT-induced aggregation of insulin (52 μM) in 10 mM potassium phosphate buffer (pH, 7.9) containing 100 mM NaCl was measured in absence or presence of DmHsp22WT or arginine mutants (7.57 μM). The final molar ratio of sHsps to insulin is 1:7. These results are representative of three independent experiments
Fig. 7.
Chaperone-like activity of DmHsp22WT and arginine mutants using citrate synthase (CS) (a, b) and malate dehydrogenase (MDH) (c, d) as protein substrates. The heat-induced aggregation of CS and MDH was measured using absorbance at 320 nm at 45 °C. a, b Heat-induced aggregation of CS (0.1665 μM) was assayed either alone or in presence of DmHsp22WT and four arginine mutants (0.33 μM) to give molar ratio of 2:1 for sHsps to substrate with the same buffer as Luc assay. c, d Aggregation of MDH (0.65 μM) was determined by light scattering using sHsps with concentration equal to 0.65 μM to give the ratio of 1:1 with the HEPES buffer. Data are representative of three independent experiments
In the reduction-induced aggregation of insulin assay, all the samples including DmHsp22WT and all the arginine mutants possessed efficiency to protect aggregation of substrate with the ratio of 1:7 (Hsp22/insulin). In this case, the percentage of protection was between 92 and 95% for all the proteins (Fig. 6c, d). We obtained the same results at 24 °C for insulin assay (data not shown).
In summary, mutating the arginine residues to glycine in the ACD at positions R105, R109, and R110 of DmHsp22 did not change their chaperone-like properties in these common assays.
Discussion
The highly conserved ACD has been suggested to play a role in assembly of the oligomeric structure of sHsps (Bagneris et al. 2009; Baranova et al. 2011; Clark et al. 2011; Jehle et al. 2009; Laganowsky et al. 2010). Results from gel filtration showed that mitochondrial DmHsp22WT forms oligomer in its native state with molecular weight in the range of HspB1 and HspB5 (Morrow et al. 2000). It was therefore of interest to see how this mitochondrial small Hsp and its arginine mutants are organized. In SEC, DmHsp22WT forms one single symmetric peak with an apparent molecular weight around 790 kDa, which is larger than oligomeric peaks formed in the case of human HspB1 and HspB5 whose average molecular masses are around 450 and 620 kDa at 20 °C, respectively (Bova et al. 1999; Kumar et al. 1999; Skouri-Panet et al. 2012; Sudnitsyna et al. 2012).
Arginine to glycine missense mutation in the ACD of human sHsps has been reported to be associated with human congenital diseases and was found to modify protein structure and chaperone-like activity (Bova et al. 1999). Thus, we examined the influence of such mutations on the structure and function of DmHsp22. Our study showed that a mutation in R109G equivalent with R140-HspB1, R116-HspB4, and R120-HspB5 in humans located in the highly conserved ACD did not result in the structural disruption of the oligomeric structure. This contrasts with the mutations in human R140G-HspB1, R116C-HspB4, and R120G-HspB5, which give rise to higher molecular weight oligomers compared with the WT protein (Bova et al. 1999; Cobb and Petrash 2000; Kumar et al. 1999; Michiel et al. 2009; Nefedova et al. 2013). According to our results, R109 is not involved in oligomer formation which is in agreement with the fact that in some variants of DmHsp22 in D. melanogaster, arginine 109 residue is replaced with glycine (Southgate et al. 1983).
In contrast, we found that the neighboring R110 residue, which is conserved in R117-HspB4 and is replaced with lysine amino acid in human HspB1 and HspB5 and in DmHsp27 could induce an effect on the oligomeric structure of DmHsp22 observed as formation of smaller oligomer by three different methods, DLS, size-exclusion chromatography, and native gel electrophoresis. The conformational changes due to R110G are similar to the mutant R148G-Hsp27 from Chinese hamster (Chavez Zobel et al. 2005). Similarly, the R110G double mutant (R109GR110G) disrupted the oligomeric structure to smaller oligomers. The influence of two mutants R110G and R109GR110G on the structure of DmHsp22 showed that R110 (and not R109) residue was involved in the formation of oligomeric structure of DmHsp22 and its dimeric structure. Mutant R105G in the ACD of DmHsp22, which is equivalent with R136-HspB4 in human and R131-DmHsp27 gives oligomer with the same molecular weight as the WT protein as shown in SEC analysis. Interestingly, such a mutation in DmHsp27 forms larger oligomers equal to 1100 kDa according to the SEC result (Moutaoufik et al. 2016).
According to the results of DLS, increasing temperature from 8 to 55 °C resulted in very few modifications in Rh and polydispersity for the DmHsp22 and the R109 mutant (Rh of 7–8 nm). In the case of the R110 mutant, the Rh is smaller (6 nm at 8 °C) and increases to approximately 9–10 nm at high temperature. Human HspB5 possesses the lowest polydispersity at 37 °C (Michiel et al. 2009). Different mutants of HspB5 showed their own behavior in DLS. For example, mutant R120G-HspB5 remains stable between 37 and 45 °C (Rh, 10.9 nm) then increases the Rh as a function of temperature and due to the formation of large MW aggregates (Michiel et al. 2009).
The chaperone-like activity of DmHsp22WT in vitro was previously shown using citrate synthase and luciferase (Morrow et al. 2006), and its characteristic as being a good chaperone in vitro may be an advantage for mitochondria, which are particularly sensitive to stress (Li et al. 2002; Marcillat et al. 1989; Zhang et al. 1990). The R110G and R109GR110G mutants caused a very light likely non-significant decrease in chaperone-like activity compared with DmHsp22WT using Luc as a model substrate. The effect observed are similar with the mutant K141Q of HspB1, which shows a chaperone-like activity slightly smaller than that of the WT protein in a lysozyme assay (Nefedova et al. 2013).
DmHsp22 and the ACD arginine mutants possess the same efficiency to protect insulin from aggregation with the molar ratio presented here equal to the 1:7 for sHsp/insulin. DmHsp22 protein and mutants R109G and R109GR110G are able to protect CS aggregation with the molar ratio of 2:1 for sHsp/CS to the same extent. However, a very slight decrease in efficiency was observed in the case of R105G and R110G. Interestingly, all the mutants are a bit more efficient than DmHsp22WT in protecting aggregated MDH. This result is similar to a number of investigations, which indicate that mutations in certain residues of HspB1 (S135F, for example) increase the chaperone-like activity of corresponding mutants compared with the WT protein (Almeida-Souza et al. 2010). While our manuscript was finalized, Mymrikov et al. 2016 published a detailed study on the chaperone assays and reported that the efficiency of each human sHsp to suppress aggregation of different substrates was dependent on the substrate used and each human sHsps have its own chaperone activity (Mymrikov et al. 2016).
In summary, mutations of three neighboring arginine residues located in the arginine-enriched region of the ACD have little influence on the DmHsp22 structure and chaperone-like activity. In contrast to the R140-HspB1, R116-HspB4, and R120-HspB5, R109-DmHsp22 does not seem to be involved neither in the formation of oligomers nor in dimeric interaction. The interactions between E93 and R110 of DmHsp22 in ionic bridge formation will be the subject of our future investigation plan. Finally, mutating the conserved R residues in the ACD did not significantly affect their activity as chaperone in a number of substrates in vitro assays.
Acknowledgements
We would like to thank Jérémie Hamel from the Institute for Integrative Systems Biology (IBIS) for help with SEC. The authors also thank Céline Férard and Fériel Skouri-Panet from IMPMC. SF is supported by CNRS. This work has been supported by grants from the Canadian Institutes of Health Research (CIHR) to RMT. MTM was supported by studentships from PROTEO.
References
- Almeida-Souza L, et al. Increased monomerization of mutant HSPB1 leads to protein hyperactivity in Charcot-Marie-Tooth neuropathy. J Biol Chem. 2010;285:12778–12786. doi: 10.1074/jbc.M109.082644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP, Tanguay RM. Expression of heat shock proteins during development in Drosophila. Results Probl Cell Differ. 1991;17:106–119. doi: 10.1007/978-3-540-46712-0_8. [DOI] [PubMed] [Google Scholar]
- Bagneris C, Bateman OA, Naylor CE, Cronin N, Boelens WC, Keep NH, Slingsby C. Crystal structures of alpha-crystallin domain dimers of alphaB-crystallin and Hsp20. J Mol Biol. 2009;392:1242–1252. doi: 10.1016/j.jmb.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Bakthisaran R, Tangirala R, Rao CM. Small heat shock proteins: role in cellular functions and pathology. Biochim Biophys Acta. 2015;1854:291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Baldwin AJ, Hilton GR, Lioe H, Bagneris C, Benesch JL, Kay LE. Quaternary dynamics of alphaB-crystallin as a direct consequence of localised tertiary fluctuations in the C-terminus. J Mol Biol. 2011;413:310–320. doi: 10.1016/j.jmb.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Baldwin AJ, Lioe H, Hilton GR, Baker LA, Rubinstein JL, Kay LE, Benesch JL. The polydispersity of alphaB-crystallin is rationalized by an interconverting polyhedral architecture. Structure. 2011;19:1855–1863. doi: 10.1016/j.str.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Baranova EV, Weeks SD, Beelen S, Bukach OV, Gusev NB, Strelkov SV. Three-dimensional structure of alpha-crystallin domain dimers of human small heat shock proteins HSPB1 and HSPB6. J Mol Biol. 2011;411:110–122. doi: 10.1016/j.jmb.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Basha E, O'Neill H, Vierling E. Small heat shock proteins and alpha-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci. 2012;37:106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas A, Das KP. Role of ATP on the interaction of alpha-crystallin with its substrates and its implications for the molecular chaperone function. J Biol Chem. 2004;279:42648–42657. doi: 10.1074/jbc.M404444200. [DOI] [PubMed] [Google Scholar]
- Boncoraglio A, Minoia M, Carra S. The family of mammalian small heat shock proteins (HSPBs): implications in protein deposit diseases and motor neuropathies. Int J Biochem Cell Biol. 2012;44:1657–1669. doi: 10.1016/j.biocel.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Bourrelle-Langlois M, Morrow G, Finet S, Tanguay RM. In vitro structural and functional characterization of the small heat shock proteins (sHSP) of the Cyanophage S-ShM2 and its host, Synechococcus sp. WH7803. PLoS One. 2016;11:e0162233. doi: 10.1371/journal.pone.0162233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, Horwitz J. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci U S A. 1999;96:6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushueva TL, Busel EP, Burstein EA. Some regularities of dynamic accessibility of buried fluorescent residues to external quenchers in proteins. Arch Biochem Biophys. 1980;204:161–166. doi: 10.1016/0003-9861(80)90019-3. [DOI] [PubMed] [Google Scholar]
- Chavez Zobel AT, Lambert H, Theriault JR, Landry J. Structural instability caused by a mutation at a conserved arginine in the alpha-crystallin domain of Chinese hamster heat shock protein 27. Cell Stress Chaperones. 2005;10:157–166. doi: 10.1379/CSC-102.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JI, Muchowski PJ. Small heat-shock proteins and their potential role in human disease. Curr Opin Struct Biol. 2000;10:52–59. doi: 10.1016/S0959-440X(99)00048-2. [DOI] [PubMed] [Google Scholar]
- Clark AR, Naylor CE, Bagneris C, Keep NH, Slingsby C. Crystal structure of R120G disease mutant of human alphaB-crystallin domain dimer shows closure of a groove. J Mol Biol. 2011;408:118–134. doi: 10.1016/j.jmb.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AR, Lubsen NH, Slingsby C. sHSP in the eye lens: crystallin mutations, cataract and proteostasis. Int J Biochem Cell Biol. 2012;44:1687–1697. doi: 10.1016/j.biocel.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Cobb BA, Petrash JM. Structural and functional changes in the alpha A-crystallin R116C mutant in hereditary cataracts. Biochemistry. 2000;39:15791–15798. doi: 10.1021/bi001453j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbecq SP, Klevit RE. One size does not fit all: the oligomeric states of alphaB crystallin. FEBS Lett. 2013;587:1073–1080. doi: 10.1016/j.febslet.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando P, Heikkila JJ. Functional characterization of Xenopus small heat shock protein, Hsp30C: the carboxyl end is required for stability and chaperone activity. Cell Stress Chaperones. 2000;5:148–159. doi: 10.1379/1466-1268(2000)005<0148:FCOXSH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Chang Z. Temperature-dependent subunit exchange and chaperone-like activities of Hsp16.3, a small heat shock protein from mycobacterium tuberculosis. Biochem Biophys Res Commun. 2004;316:291–299. doi: 10.1016/j.bbrc.2004.02.053. [DOI] [PubMed] [Google Scholar]
- Fu X, Liu C, Liu Y, Feng X, Gu L, Chen X, Chang Z. Small heat shock protein Hsp16.3 modulates its chaperone activity by adjusting the rate of oligomeric dissociation. Biochem Biophys Res Commun. 2003;310:412–420. doi: 10.1016/j.bbrc.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Ghisaidoobe AB, Chung SJ. Intrinsic tryptophan fluorescence in the detection and analysis of proteins: a focus on Forster resonance energy transfer techniques. Int J Mol Sci. 2014;15:22518–22538. doi: 10.3390/ijms151222518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JG, Houck SA, Doneanu CE, Clark JI. The beta4-beta8 groove is an ATP-interactive site in the alpha crystallin core domain of the small heat shock protein, human alphaB crystallin. J Mol Biol. 2006;364:364–375. doi: 10.1016/j.jmb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Giese KC, Vierling E. Changes in oligomerization are essential for the chaperone activity of a small heat shock protein in vivo and in vitro. J Biol Chem. 2002;277:46310–46318. doi: 10.1074/jbc.M208926200. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Vierling E. A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J Mol Biol. 2015;427:1537–1548. doi: 10.1016/j.jmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario E, Martin FJ, Bertolini MC, Fan L. Crystal structures of Xanthomonas small heat shock protein provide a structural basis for an active molecular chaperone oligomer. J Mol Biol. 2011;408:74–86. doi: 10.1016/j.jmb.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Hilton GR, Lioe H, Stengel F, Baldwin AJ, Benesch JL. Small heat-shock proteins: paramedics of the cell. Top Curr Chem. 2013;328:69–98. doi: 10.1007/128_2012_324. [DOI] [PubMed] [Google Scholar]
- Houlden H, Laura M, Wavrant-De Vrieze F, Blake J, Wood N, Reilly MM. Mutations in the HSP27 (HSPB1) gene cause dominant, recessive, and sporadic distal HMN/CMT type 2. Neurology. 2008;71:1660–1668. doi: 10.1212/01.wnl.0000319696.14225.67. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Abe A, Ishida C, Takahashi K, Hayasaka K, Yamada M. A clinical phenotype of distal hereditary motor neuronopathy type II with a novel HSPB1 mutation. J Neurol Sci. 2009;277:9–12. doi: 10.1016/j.jns.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Jee H. Size dependent classification of heat shock proteins: a mini-review. Journal of exercise rehabilitation. 2016;12:255–259. doi: 10.12965/jer.1632642.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle S, et al. AlphaB-crystallin: a hybrid solid-state/solution-state NMR investigation reveals structural aspects of the heterogeneous oligomer. J Mol Biol. 2009;385:1481–1497. doi: 10.1016/j.jmb.2008.10.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe G, Boelens WC, de Jong WW. Why proteins without an alpha-crystallin domain should not be included in the human small heat shock protein family HSPB. Cell Stress Chaperones. 2010;15:457–461. doi: 10.1007/s12192-009-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Kumar LV, Ramakrishna T, Rao CM. Structural and functional consequences of the mutation of a conserved arginine residue in alphaA and alphaB crystallins. J Biol Chem. 1999;274:24137–24141. doi: 10.1074/jbc.274.34.24137. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laganowsky A, et al. Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Science: a Publication of the Protein Society. 2010;19:1031–1043. doi: 10.1002/pro.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Mao HP, Ruchalski KL, Wang YH, Choy W, Schwartz JH, Borkan SC. Heat stress prevents mitochondrial injury in ATP-depleted renal epithelial cells. American Journal of Physiology Cell Physiology. 2002;283:C917–C926. doi: 10.1152/ajpcell.00517.2001. [DOI] [PubMed] [Google Scholar]
- Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet. 1998;7:471–474. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- Maaroufi H, Tanguay RM. Analysis and phylogeny of small heat shock proteins from marine viruses and their cyanobacteria host. PLoS One. 2013;8:e81207. doi: 10.1371/journal.pone.0081207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcillat O, Zhang Y, Davies KJ. Oxidative and non-oxidative mechanisms in the inactivation of cardiac mitochondrial electron transport chain components by doxorubicin. The Biochemical Journal. 1989;259:181–189. doi: 10.1042/bj2590181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin R, Tanguay RM. Stage-specific localization of the small heat shock protein Hsp27 during oogenesis in Drosophila melanogaster. Chromosoma. 1996;105:142–149. doi: 10.1007/BF02509495. [DOI] [PubMed] [Google Scholar]
- Marin R, Valet JP, Tanguay RM. hsp23 and hsp26 exhibit distinct spatial and temporal patterns of constitutive expression in Ddrosophila adults. Dev Genet. 1993;14:69–77. doi: 10.1002/dvg.1020140109. [DOI] [PubMed] [Google Scholar]
- Marin R, Landry J, Tanguay RM. Tissue-specific posttranslational modification of the small heat shock protein HSP27 in Drosophila. Exp Cell Res. 1996;223:1–8. doi: 10.1006/excr.1996.0052. [DOI] [PubMed] [Google Scholar]
- McHaourab HS, Godar JA, Stewart PL. Structure and mechanism of protein stability sensors: chaperone activity of small heat shock proteins. Biochemistry. 2009;48:3828–3837. doi: 10.1021/bi900212j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud S, Tanguay RM. Expression of the Hsp23 chaperone during Drosophila embryogenesis: association to distinct neural and glial lineages. BMC Dev Biol. 2003;3:9. doi: 10.1186/1471-213X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud S, Marin R, Westwood JT, Tanguay RM. Cell-specific expression and heat-shock induction of Hsps during spermatogenesis in Drosophila melanogaster. J Cell Sci. 1997;110(Pt 17):1989–1997. doi: 10.1242/jcs.110.17.1989. [DOI] [PubMed] [Google Scholar]
- Michaud S, Morrow G, Marchand J, Tanguay RM. Drosophila small heat shock proteins: cell and organelle-specific chaperones? Prog Mol Subcell Biol. 2002;28:79–101. doi: 10.1007/978-3-642-56348-5_5. [DOI] [PubMed] [Google Scholar]
- Michiel M, Skouri-Panet F, Duprat E, Simon S, Ferard C, Tardieu A, Finet S. Abnormal assemblies and subunit exchange of alphaB-crystallin R120 mutants could be associated with destabilization of the dimeric substructure. Biochemistry. 2009;48:442–453. doi: 10.1021/bi8014967. [DOI] [PubMed] [Google Scholar]
- Michiel M, Duprat E, Skouri-Panet F, Lampi JA, Tardieu A, Lampi KJ, Finet S. Aggregation of deamidated human betaB2-crystallin and incomplete rescue by alpha-crystallin chaperone. Exp Eye Res. 2010;90:688–698. doi: 10.1016/j.exer.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Montfort RL, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- Morrow G, Tanguay RM. Heat shock proteins and aging in Drosophila melanogaster. Semin Cell Dev Biol. 2003;14:291–299. doi: 10.1016/j.semcdb.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Morrow G, Tanguay RM. Small heat shock protein expression and functions during development. Int J Biochem Cell Biol. 2012;44:1613–1621. doi: 10.1016/j.biocel.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Morrow G, Tanguay RM (2015) Drosophila small heat shock proteins: an update on their features and functions. In: Tanguay RM, Hightower LE (eds) The big book on small heat shock proteins. Springer International Publishing, Cham, pp 579–606. doi:10.1007/978-3-319-16077-1_25
- Morrow G, Inaguma Y, Kato K, Tanguay RM. The small heat shock protein Hsp22 of Drosophila melanogaster is a mitochondrial protein displaying oligomeric organization. J Biol Chem. 2000;275:31204–31210. doi: 10.1074/jbc.M002960200. [DOI] [PubMed] [Google Scholar]
- Morrow G, Samson M, Michaud S, Tanguay RM. Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2004;18:598–599. doi: 10.1096/fj.03-0860fje. [DOI] [PubMed] [Google Scholar]
- Morrow G, Heikkila JJ, Tanguay RM. Differences in the chaperone-like activities of the four main small heat shock proteins of Drosophila melanogaster. Cell Stress Chaperones. 2006;11:51–60. doi: 10.1379/CSC-166.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutaoufik MT, Morrow G, Maaroufi H, Ferard C, Finet S, Tanguay RM. Oligomerization and chaperone-like activity of Drosophila melanogaster small heat shock protein DmHsp27 and three arginine mutants in the alpha-crystallin domain. Cell Stress Chaperones. 2016 doi: 10.1007/s12192-016-0748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski PJ, Clark JI. ATP-enhanced molecular chaperone functions of the small heat shock protein human alphaB crystallin. Proc Natl Acad Sci U S A. 1998;95:1004–1009. doi: 10.1073/pnas.95.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranova LK, Weeks SD, Strelkov SV, Gusev NB. Characterization of mutants of human small heat shock protein HspB1 carrying replacements in the N-terminal domain and associated with hereditary motor neuron diseases. PLoS One. 2015;10:e0126248. doi: 10.1371/journal.pone.0126248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mymrikov EV, Daake M, Richter B, Haslbeck M, Buchner J. The chaperone activity and substrate spectrum of human small heat shock proteins. J Biol Chem. 2016 doi: 10.1074/jbc.M116.760413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefedova VV, Datskevich PN, Sudnitsyna MV, Strelkov SV, Gusev NB. Physico-chemical properties of R140G and K141Q mutants of human small heat shock protein HspB1 associated with hereditary peripheral neuropathies. Biochimie. 2013;95:1582–1592. doi: 10.1016/j.biochi.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Roebben G, et al. Reference materials and representative test materials to develop nanoparticle characterization methods: the NanoChOp project case. Frontiers in Chemistry. 2015;3:56. doi: 10.3389/fchem.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouri-Panet F, Michiel M, Ferard C, Duprat E, Finet S. Structural and functional specificity of small heat shock protein HspB1 and HspB4, two cellular partners of HspB5: role of the in vitro hetero-complex formation in chaperone activity. Biochimie. 2012;94:975–984. doi: 10.1016/j.biochi.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Sluchanko NN, Roman SG, Chebotareva NA, Gusev NB. Chaperone-like activity of monomeric human 14-3-3zeta on different protein substrates. Arch Biochem Biophys. 2014;549:32–39. doi: 10.1016/j.abb.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Southgate R, Ayme A, Voellmy R. Nucleotide sequence analysis of the drosophila small heat shock gene cluster at locus 67B. J Mol Biol. 1983;165:35–57. doi: 10.1016/S0022-2836(83)80241-1. [DOI] [PubMed] [Google Scholar]
- Stengel F, et al. Quaternary dynamics and plasticity underlie small heat shock protein chaperone function. Proc Natl Acad Sci U S A. 2010;107:2007–2012. doi: 10.1073/pnas.0910126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudnitsyna MV, Mymrikov EV, Seit-Nebi AS, Gusev NB. The role of intrinsically disordered regions in the structure and functioning of small heat shock proteins. Curr Protein Pept Sci. 2012;13:76–85. doi: 10.2174/138920312799277875. [DOI] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. Small heat shock proteins: molecular structure and chaperone function. Cellular and Molecular Life Sciences: CMLS. 2005;62:2460–2476. doi: 10.1007/s00018-005-5190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Hayashi T, Abe T, Hirano Y, Hanazono Y, Yohda M, Miki K. Dimer structure and conformational variability in the N-terminal region of an archaeal small heat shock protein, StHsp14.0. J Struct Biol. 2011;174:92–99. doi: 10.1016/j.jsb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 2010;11:777–788. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- Vicart P, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47:7001–7011. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- Weeks SD, Drinker M, Loll PJ. Ligation independent cloning vectors for expression of SUMO fusions. Protein Expr Purif. 2007;53:40–50. doi: 10.1016/j.pep.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJ. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem. 1990;265:16330–16336. [PubMed] [Google Scholar]