Abstract
Ischemic stroke leads to cellular dysfunction, cell death, and devastating clinical outcomes. The cells of the brain react to such a cellular stress by a stress response with an upregulation of heat shock proteins resulting in activation of endogenous neuroprotective capacities. Several members of the family of small heat shock proteins (HspBs) have been shown to be neuroprotective. However, yet no systematic study examined all HspBs during cerebral ischemia. Here, we performed a comprehensive comparative study comprising all HspBs in an animal model of stroke, i.e., 1 h transient middle cerebral artery occlusion followed by 23 h of reperfusion. On the mRNA level out of the 11 HspBs investigated, HspB1/Hsp25, HspB3, HspB4/αA-crystallin, HspB5/αB-crystallin, HspB7/cvHsp, and HspB8/Hsp22 were significantly upregulated in the peri-infarct region of the cerebral cortex of infarcted hemispheres. HspB1 and HspB5 reached the highest mRNA levels and were also upregulated at the protein level, suggesting that these HspBs might be functionally most relevant. Interestingly, in the infarcted cortex, both HspB1 and HspB5 were mainly allocated to neurons and to a lesser extent to glial cells. Additionally, both proteins were found to be phosphorylated in response to ischemia. Our data suggest that among all HspBs, HspB1 and HspB5 might be most important in the neuronal stress response to ischemia/reperfusion injury in the brain and might be involved in neuroprotection.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-017-0794-9) contains supplementary material, which is available to authorized users.
Keywords: Stroke, Ischemia, Neuronal stress response, Heat shock proteins, HspB
Introduction
Stroke, an ischemic insult of the brain, is a leading cause of permanent disablement worldwide. Brain tissue is damaged on the one hand through ischemia caused by the artery occlusion. On the other hand, reperfusion after ischemia leads to additional tissue damage due to the generation of reactive oxygen species and subsequent inflammation after reoxygenation. The consequence is neuronal dysfunction and neuronal death. Neural cells try to cope with such stress events by endogenous cytoprotective mechanisms such as upregulation of heat shock proteins which prevent irreversible denaturation of proteins, counteract inflammation, or are able to inhibit apoptosis (Ritossa 1962; Stetler et al. 2010; Welch 1992). Heat shock proteins are classified into several protein families according to their molecular weight. Small heat shock proteins, called HspBs, are defined by their small molecular weight (12–43 kDa) and a characteristic, highly conserved α-crystallin domain (Kampinga et al. 2009; Kappe et al. 2003). This subgroup consists of ten family members, named HspB1 through HspB10, although many of them have alternative names related to their molecular weight or the tissue of their preferential expression. An 11th family member, HspB11, is still controversially discussed to belong to the HspB family or not (Bellyei et al. 2007; Kappe et al. 2010) but was nevertheless included in our study. The main function of HspBs is to prevent irreversible protein aggregation and denaturation by keeping partially unfolded proteins in a folding-competent state. For active refolding after the stress event, other ATP-dependent chaperones, such as Hsp70, are needed (Haslbeck et al. 2005; MacRae 2000). In addition to this chaperone function, several HspBs also exhibit anti-apoptotic (HspB1, HspB2, HspB4, HspB5, HspB6) or anti-inflammatory (HspB5) functions (Kamradt et al. 2002; Mehlen et al. 1996; Oshita et al. 2010; Ousman et al. 2007; Wagstaff et al. 1999).
HspBs are cytoprotective and vary in their expression pattern between tissues. Many of them are ubiquitously expressed with preferential expression in some tissues or organs whereas other HspBs show a specific expression in just one organ (Bhat and Nagineni 1989; Kirbach and Golenhofen 2011). For example, HspB4/αA-crystallin and HspB5/αB-crystallin are very abundant in the eye lens being important for maintaining lens transparency by their chaperone-like activities (Horwitz 2000). Other HspBs are preferentially expressed in muscle tissue, and their cardioprotective role is well documented (Golenhofen et al. 1998; Golenhofen et al. 2006; Ray et al. 2001; Sugiyama et al. 2000). In the brain, some HspBs are reported to be expressed but only at low levels (Kirbach and Golenhofen 2011; Quraishe et al. 2008). However, they can be upregulated by cellular stress events as well as in certain types of neurodegenerative diseases. Several reports suggest a neuroprotective role of HspBs (Akbar et al. 2003; Golenhofen and Bartelt-Kirbach 2016; Stetler et al. 2008).
During ischemia/reperfusion injury of the brain, the two best characterized HspBs, HspB1/Hsp25 and HspB5/αB-crystallin, have been studied. Both proteins were shown to be upregulated in ischemic and peri-infarct areas of the brain. Especially, HspB1 seems to have strong neuroprotective effects as HspB1 overexpression was shown to reduce infarct volume in animal models of transient or permanent brain ischemia (Badin et al. 2006; Shimada et al. 2014; van der Weerd et al. 2010). However, yet no systematic study exists investigating all HspB family members. We have previously shown that HspB1, HspB2, HspB3, HspB5, HspB6, HspB8, and HspB11 are expressed in rat brain under control conditions (Kirbach and Golenhofen 2011). Thus, we intended to do a comparative comprehensive study elucidating the expression and distribution of all HspBs after ischemia/reperfusion injury in cerebral cortex. For that purpose, we used an experimental model of 1 h transient middle cerebral artery occlusion (tMCAO) in rats followed by 23 h reperfusion. Cerebral cortex tissue was then analyzed for messenger RNA (mRNA) and protein levels of HspBs as well as their cellular distribution.
Methods
tMCAO
Animal care and experimental procedures were formally approved by the Review Board for the Care of Animal Subjects of the district government (North Rhine-Westphalia, Germany) and bred and maintained in a pathogen-free environment in a 12-h day and 12-h night cycle prior experiments. Food and water were available ad libitum. A total of 19 animals were used in the experiments described here.
The procedure of tMCAO operation was performed as previously described (Dang et al. 2011; Ulbrich et al. 2012). Briefly, 12-week-old male Wistar rats (300–350 g) were anesthetized with 2% isoflurane (Abbott, Germany), and the regional cerebral blood flow (CBF) was measured on the ipsi- and contralateral side during the whole procedure using Laser Doppler Flowmetry (Moor Instruments VMS-LDF2, UK). During operation, body temperature was kept constant at 37.0 ± 0.5 °C. After exposure of the common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA), the vagal nerve was preserved followed by ligation of the CCA and temporarily clipping of the ECA. Directly after measuring the baseline values of CBF, a commercially available catheter (Asahi PTCA Guide Wire Soft, Abbott Vascular) was subsequently introduced from the lumen of the CCA as far as a resistance was manually observed. After the catheter reached the origin of the middle cerebral artery, an immediate drop in baseline CBF occurred. Sham animals underwent the same procedure except the insertion of the catheter. Animals which only showed a reduction of CBF 50% or less after occlusion were excluded from the study. After 1 h of transient occlusion, the catheter was removed and the CBF was restored. After tMCAO, animals were kept under a heating lap until they finally woke up. Animals were randomly assigned to experimental groups and allowed to recover for 23 h after tMCAO.
Tissue processing
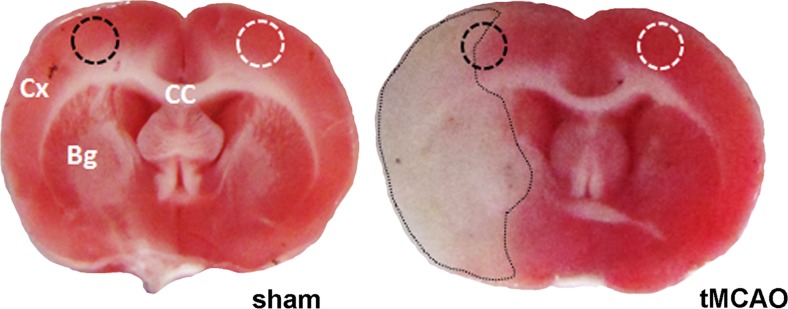
After ischemia, rats were transcardially perfused with 0.1% NaCl. Brains were rapidly removed, and six consecutive coronal sections (2 mm) were prepared and stained in a 2% 2,3,5-triphenyltetrazolium chloride (TTC, Fluka, Germany) solution (15 min, 37 °C). The unstained areas were defined as infarct area. TTC staining always delineates the transition zone from infarcted to non-infarcted area, thus representing the peri-infarct zone. Therefore, TTC-staining allowed to localizing the site of the transition zone which was dissected using a stereomicroscope for RNA and protein isolation. Equivalent regions were cut from the contralateral side or sham-operated animal brains.
For immunohistochemistry, animals were transcardially perfused with 2% (w/v) formaldehyde (Roth, Germany) containing 15% (v/v) saturated picric acid, pH 7.4 (AppliChem GmbH, Germany). Brains were dissected and post-fixed in the same solution overnight. Fixed brains were paraffin-embedded (Merck KGaA, Germany), and 5 μm coronal sections were made.
RNA isolation and cDNA synthesis
RNA from the peri-infarct area and control tissue was isolated with the NucleoSpin® RNA/Protein Kit (Machery-Nagel, Germany) as described previously (Dang et al. 2011). Purity of the RNA was controlled using 260:280 OD ratios (Nano-Drop 1000, Peqlab, Germany). Complementary DNA was synthesized using the M-MLV reverse transcription (RT)-kit and random hexanucleotide primers (Invitrogen, Germany) using 1 μg/ml of total RNA.
Real-time PCR
Measurement of HspB expression was performed as described previously (Kirbach and Golenhofen 2011). Briefly, 2 μl of 1:50 diluted complementary DNA (cDNA) was amplified in duplicates with the QuantiTect SYBR Green PCR Master Mix (Qiagen, Hilden, Germany) and the HspB primers used previously (Kirbach and Golenhofen 2011) in a LightCycler 1.0 (Roche Applied Science, Mannheim, Germany). cDNA from ipsilateral peri-infarct areas and corresponding contralateral areas from four animals with tMCAO and four sham-operated animals was analyzed. Expression was normalized to the geometric mean of the expression of the two reference genes cyclophilin A and Rpl13A. Threshold for expression was set at 0.1% of the reference gene expression. Afterwards, expression was normalized to the value of the respective contralateral side of the animal, and a mean value (±SEM) of all four animals was calculated. Statistical evaluation was performed with the non-parametric Mann-Whitney U test. Statistical significance was assumed for p < 0.05.
Protein isolation and Western blotting
Brain tissue was lysed with 1.5x Laemmli buffer, sonicated, and incubated at 95 °C, 5 min, prior to measurement of the protein concentration by amido black staining (Heinzel et al. 1965). Equal amount of protein was separated on 15% SDS polyacrylamide gels, followed by blotting onto Hybond nitrocellulose membranes (Amersham, GE Healthcare, Freiburg, Germany) or, in the case of HspB6, PVDF membrane (ThermoFisher Scientific, Waltham, MA, USA). Blotting was carried out in Towbin transfer buffer with SDS and 0.8 mA/cm2 in a Mini Trans Blot® chamber (Biorad, Munich, Germany). Membranes were blocked with 5% low fat milk (unless otherwise noted) in TBS/0.05% Tween 20 for 1 h followed by incubation with the respective primary antibody (Table 1) diluted in the respective blocking solution at 4 °C overnight. After incubation with secondary antibody (horseradish-peroxidase labeled goat anti-rabbit IgG (Jackson Immuno Research Laboratories, 1:3000) or rabbit anti-goat IgG (DAKO, #P0449, 1:2000)) for 1 h at room temperature, membranes were washed three times 5 min in TBS/0.05% Tween 20 and HRP was detected with the enhanced chemiluminescence system (Pierce ECL Western Blotting Substrate, Thermo Fisher Scientific, Waltham, MA, USA). Densitometric quantification of band intensity was carried out with ImageJ. Values obtained for HspBs were normalized to GAPDH and then calculated relative to contralateral side. Data are presented as arithmetic mean ± SEM. Statistical evaluation was performed with the non-parametric Mann-Whitney U test. Statistical significance was assumed for p < 0.05.
Table 1.
Primary antibodies used for Western blot and immunohistochemistry
| Antibody | Company | Species | Dilution (IHC) | Dilution (WB) |
|---|---|---|---|---|
| HspB1 | Enzo Life Sciences, #ADI-SPA-801 | Rabbit | 1:100 | 1:1000 |
| HspB1 PSer16 | Acris, #SP5104P | Rabbit | 1:100 | |
| HspB1 PSer86 | Acris, #SP5095P | Rabbit | 1:100 | |
| HspB5 | Enzo Life Sciences, #ADI-SPA-223 | Rabbit | 1:100 | |
| HspB5 | GeneTex #GTX62094 | Rabbit | 1:5000, 1% milk | |
| HspB5 PSer19 | Enzo Life Sciences, #ADI-SPA-225 | Rabbit | 1:100 | |
| HspB5 PSer45 | Enzo Life Sciences, #ADI-SPA-226 | Rabbit | 1:100 | |
| HspB5 PSer59 | Enzo Life Sciences, #ADI-SPA-227 | Rabbit | 1:100 | |
| HspB6 | R&D Systems, #AF4200 | Goat | 1:1000, PVDF | |
| HspB6 | Acris Antibodies GmbH, #BM2647 | Mouse | 1:100 | |
| HspB8 | Kind gift from Dr. Carra, Modena, Italy | Rabbit | 1:100 | |
| HspB8 | Cell Signaling, #3059 | Rabbit | 1:750 | |
| HspB11 | Proteintech #15732-1-AP | Rabbit | 1:50 | |
| HspB11 | Kind gift from Dr. Bellyei, Pecs, Hungary | Rabbit | 1:1000 | |
| GAPDH | Thermo Scientific, #MA5-15738 | 1:10,000 |
IHC immunohistochemistry, WB Western blotting
Immunohistochemistry
Paraffin-embedded coronal sections were rehydrated using a standard protocol (2 × 3 min xylol, 2 × 2 min 100% isopropyl alcohol, 2 min 95% isopropyl alcohol, 5 min 70% isopropyl alcohol, PBS). Afterwards, slices were immersed in hot 10 mM citrate buffer (pH 6.0) for 25 min. Endogenous peroxidase activity was blocked with 0.03% hydrogen peroxide in PBS/10% methanol for 10 min in the dark. Subsequently, unspecific binding sites were blocked with 0.5% Triton X-100 in PBS for 15 min followed by incubation in 3% BSA and 1% normal NGS for 1 h. Incubation with first antibody was carried out overnight at 4 °C in blocking solution. The antibodies and dilution used are listed in Table 1. After washing three times in PBS, slices were incubated with biotinylated secondary antibody (dilution 1:500) for 1 h at room temperature. Bound antibody was visualized by Vectastain Elite ABC Kit and ImmPACT Amec Red peroxidase substrate (Vector Labs, Burlingame, CA, USA) according to the manufacturer’s instructions. Slices were mounted in Mowiol 4-88 and scanned with Zeiss Mirax scanner (Zeiss, Oberkochen, Germany).
Results
mRNA expression of HspBs in the peri-infarct area of infarcted hemispheres
To investigate systematically and comprehensively all small heat shock proteins in response to cerebral infarction, we measured the mRNA levels of HspB1 to HspB11 and for comparison the inducible form of Hsp70 by real-time RT-PCR in the cerebral cortex in the peri-infarct area of infarcted hemispheres of rats subjected to 1 h of transient middle cerebral artery occlusion (tMCAO) followed by 23 h reperfusion. Figure 1 shows representative TTC-stained frontal brain sections of sham-operated and tMCAO-treated animals demonstrating the infarct spreading. Tissue samples from the peri-infarct zone and the equivalent region of the contralateral hemisphere were dissected and used for isolation of RNA. From the 11 HspBs investigated, eight HspBs were expressed under control conditions (contralateral hemispheres) in cerebral cortex (Table 2). HspB2, HspB4, and HspB10 were not expressed, defined by expression levels of less than 0.1% of reference gene expression. HspB1, HspB3, HspB7, HspB9, and HspB11 were expressed at low levels (below 1% of the reference gene expression) whereas HspB5, HspB6, and HspB8 showed high expression levels which are in concordance with our earlier findings in healthy rat brain tissue (Kirbach and Golenhofen 2011). In response to tMCAO, HspB2, HspB6, HspB9, HspB10, and HspB11 showed no significant increase. mRNA levels of HspB3, HspB4, and HspB7 increased significantly, but levels were still below 1% of the reference gene expression; thus, it can be assumed that they play only minor roles in the ischemic stress response (Table 2, electronic resource 1).
Fig. 1.
Representative TTC-stained frontal brain sections demonstrating the infarct spreading. TTC-stained brain sections of sham-operated (left) and tMCAO-treated (right) animals. The delineated white area in the tMCAO brain outlines the infarcted tissue. Black and white circles mark the micro-punched tissue pieces of the peri-infarct zone of the cerebral cortex (Cx) used for further biochemical and molecular analysis of the ipsi- and contralateral site. CC corpus callosum, Bg basal ganglia
Table 2.
HspB expression levels in tMCAO-treated rats
| Gene | Contralateral (% of reference genes) | Ipsilateral (% of reference genes) | Ipsilateral (in relation to contralateral) |
|---|---|---|---|
| HspB1 | 0.79 ± 0.17 | 62.68 ± 13.62 | 82.41 ± 10.41 |
| HspB2 | n.e. | n.e. | |
| HspB3 | 0.32 ± 0.09 | 0.77 ± 0.24 | 2.85 ± 0.56 |
| HspB4 | n.e. | 0.66 ± 0.48 | 41.29 ± 19.17 |
| HspB5 | 13.63 ± 2.87 | 65.15 ± 12.71 | 4.84 ± 0.21 |
| HspB6 | 9.03 ± 2.55 | 13.24 ± 0.96 | 1.94 ± 0.57 |
| HspB7 | 0.16 ± 0.04 | 0.50 ± 0.12 | 3.33 ± 0.54 |
| HspB8 | 1.78 ± 0.45 | 11.27 ± 2.15 | 6.98 ± 1.08 |
| HspB9 | 0.36 ± 0.14 | 0.56 ± 0.11 | 2.49 ± 0.46 |
| HspB10 | n.e. | n.e. | |
| HspB11 | 0.28 ± 0.04 | 0.36 ± 0.04 | 1.43 ± 0.31 |
| Hsp70i | 1.85 ± 0.33 | 360.88 ± 112.99 | 184.16 ± 55.94 |
mRNA amount of HspBs in the peri-infarct area 24 h after tMCAO measured by real-time RT-PCR, presented in percent of reference gene expression or relative to the respective values of the contralateral side. n = 4; data presented as arithmetic mean ± SEM; n.e. indicates not expressed (<0.1% of reference genes); italic printed values are statistically significant different compared to contralateral values (p < 0.05)
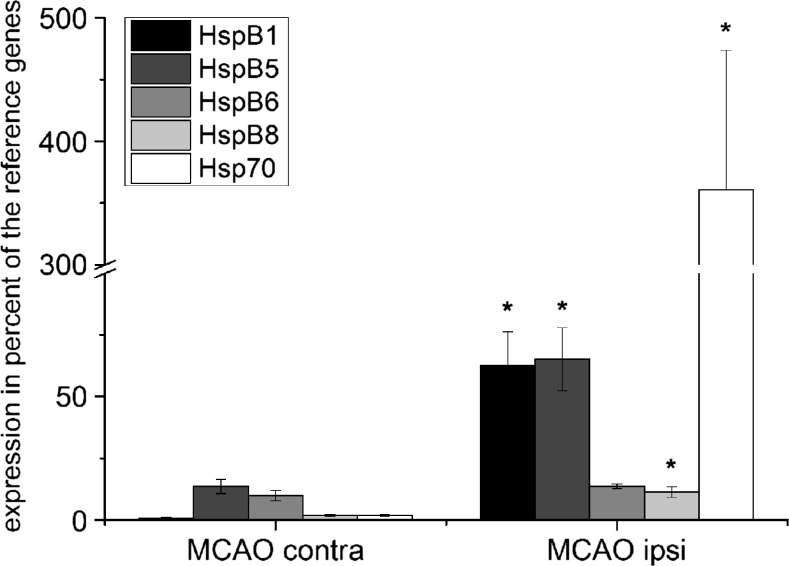
Only four out of the 11 HspBs were expressed at high levels (˃10% of reference genes) after tMCAO, namely HspB1, HspB5, HspB6, and HspB8 (Table 2, Fig. 2) with HspB1, HspB5, and HspB8 being significantly elevated compared to the contralateral hemisphere. The strongest relative increase after tMCAO (82.4 ± 10.4), reaching 62.68 ± 13.62% of the reference gene expression was achieved by HspB1. For comparison, the inducible Hsp70 was expressed at a similarly low level as HspB1 on the contralateral side and showed an even stronger relative increase (184.2 ± 55.9).
Fig. 2.
mRNA amount of high-expressed HspBs in tMCAO-treated animals. mRNA amount in the peri-infarct areal 24 h after tMCAO measured by real-time RT-PCR. Expression was between 0.8 and 65% of reference gene expression. n = 4, data presented as mean ± SEM. Statistical significance was assumed for p < 0.05 (Mann-Whitney U test)
As an additional control, the expression levels of all HspBs were also determined in sham-operated animals. No significant differences of mRNA levels of all HspBs between cortex of both hemispheres was found (electronic resource 2), and mRNA levels were in the same range as the values of the healthy contralateral cortex of animals subjected to tMCAO.
Taken together, among all HspBs, HspB1 and HspB5 are most likely to play an important role in the response to cerebral infarction with gene expression rising to more than 60% of the reference gene expression. HspB6 and HspB8 may also be involved in this process reaching levels of 13.2 and 11.3%, respectively. The expression level of all other HspBs stayed below 1% of the reference gene expression, even after tMCAO.
Protein amount of HspBs in the peri-infarct area of the cortex
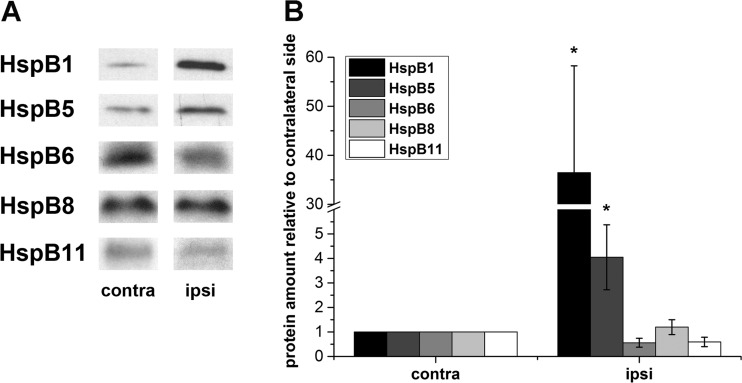
To investigate if mRNA levels of HspBs corresponded to the respective protein levels, the amount of the high-expressed HspBs (HspB1, HspB5, HspB6, HspB8) and HspB11 in the peri-infarct area of the infarcted hemispheres after tMCAO and the corresponding contralateral areas were measured by Western blotting (Fig. 3). HspB1 and HspB5 protein showed a high increase after tMCAO (36.5 ± 21.8- and 4.1 ± 1.3-fold, respectively) which fits to the elevated mRNA levels. In contrast, HspB6, HspB8, and HspB11 protein amounts did not change significantly. In sham-operated animals, no significant difference was measured between both hemispheres (electronic resource 3).
Fig. 3.
Protein amount of HspBs in the peri-infarct area of the cortex relative to contralateral side. Protein amount of HspBs was determined 24 h after tMCAO. a Representative Western blots and b densitometric analysis of the respective HspB normalized to GAPDH. Values are displayed in relation to the values of the contralateral side. n = 5, data presented as mean ± SEM. *p < 0.05 (Mann-Whitney U test)
Localization of HspBs in infarcted cortex and peri-infarct area
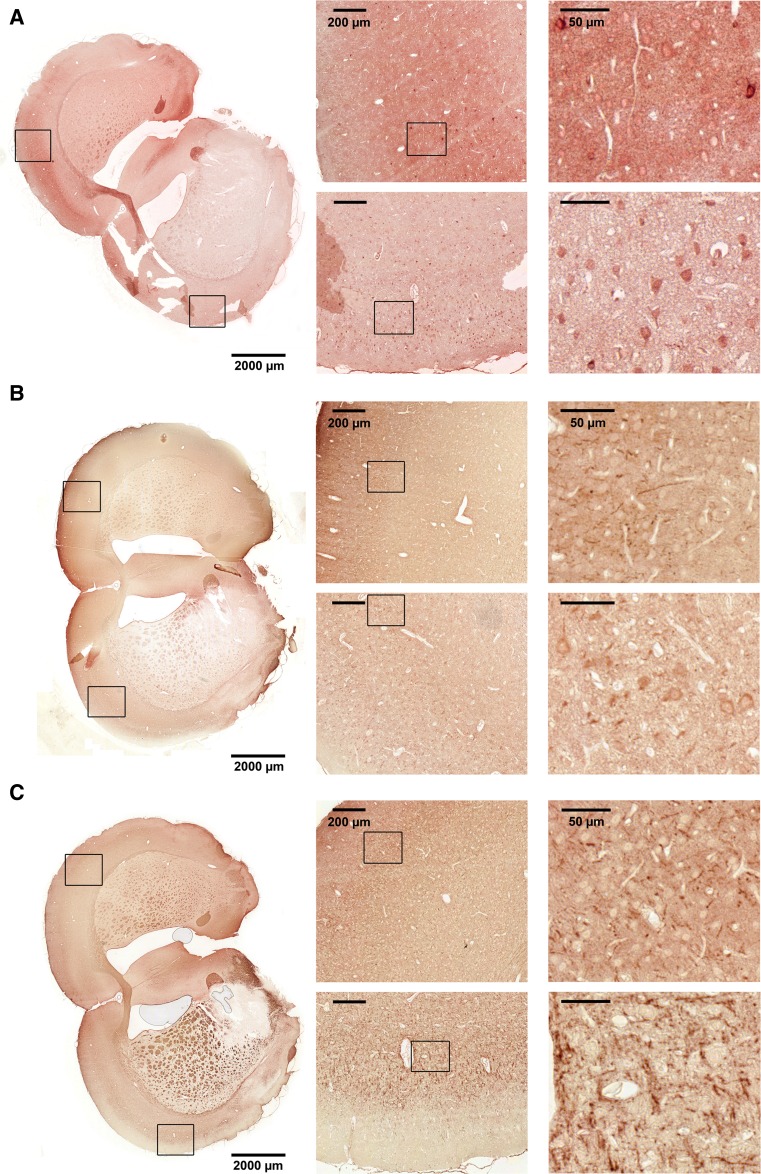
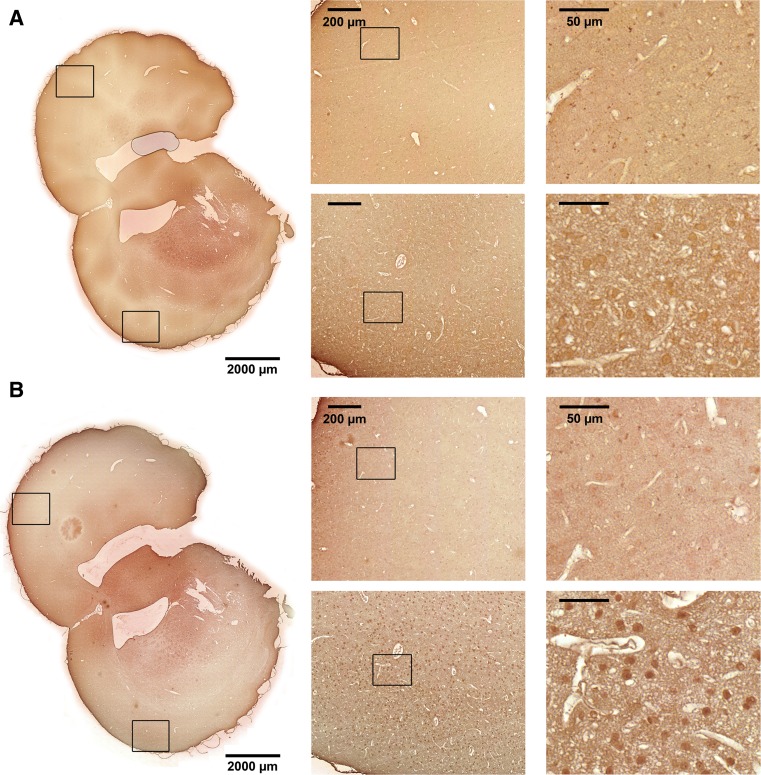
To investigate in which cell types HspBs were expressed and upregulated in response to tMCAO, immunostaining was performed. For that purpose, coronal sections of paraffin-embedded rat brains subjected to tMCAO were stained with specific HspB antibodies against HspB1, HspB5, HspB6, HspB8, and HspB11. For HspB6 no specific signals could be detected which is probably due to the low expression level or the low sensitivity of the antibody.
Figures 4, 5, and 6 show sections stained with the respective antibodies with enlargements of the core infarcted region as well as the corresponding region of the contralateral side (see also Fig. 1 for TTC-stained sections showing the area of infarction). If not noted otherwise, the staining pattern in the peri-infarct zone was similar as in the infarcted area and, thus, not shown in detail additionally.
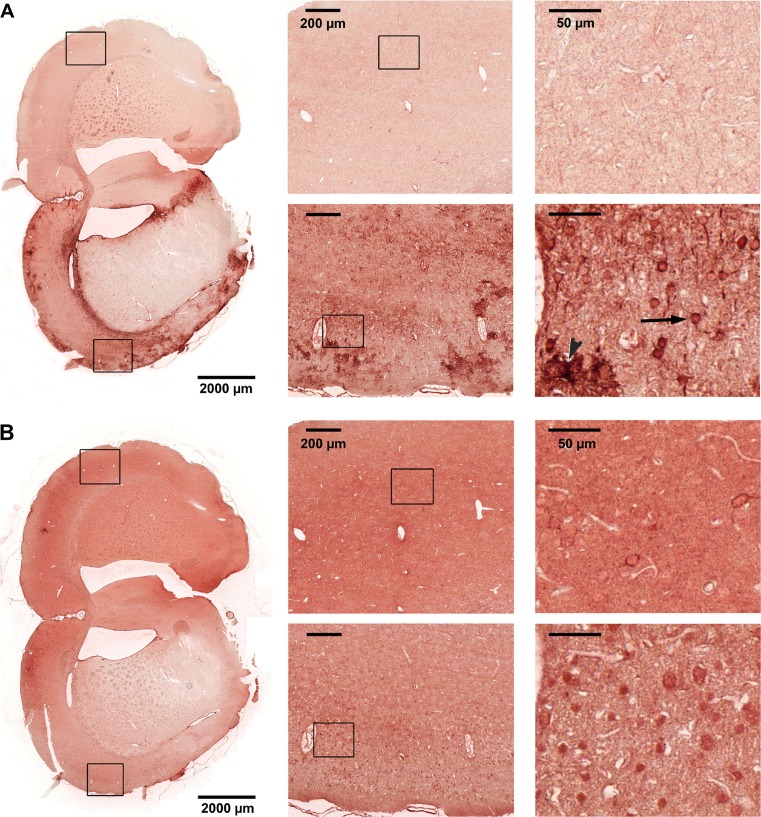
Fig. 4.
Immunohistochemical staining for HspB1 of coronal brain sections from tMCAO-treated animals. Representative images with two details from cortex of each side as indicated. Upper panels show the healthy hemisphere, lower panels the ischemic hemisphere. a HspB1, arrow points to a neuron, arrowhead to an astrocyte. b p HspB1-Ser86
Fig. 5.
Immunohistochemical staining for HspB5 of coronal brain sections from tMCAO-treated animals. Representative images with two details from cortex of each side as indicated. Upper panels show the healthy hemisphere, lower panels the ischemic hemisphere. a HspB5, b p HspB5-Ser19, c p HspB5-Ser59
Fig. 6.
Immunohistochemical staining for HspB8 and HspB11 of coronal brain sections from tMCAO-treated animals. Representative images with two details from cortex of each side as indicated. Upper panels show the healthy hemisphere, lower panels the ischemic hemisphere. a HspB8, b HspB11
HspB1 upregulation in the affected cortex could be clearly confirmed by this method (Fig. 4a). Strong immunoreactivity of HspB1 was observed in glia (astrocytes) and neurons in the infarcted but not in the contralateral cortex. Neuronal signals were mainly cytoplasmic with some neuronal processes being also positive. However, in the peri-infarct zone, no labeling of neurons could be observed (not shown).
HspB1 can be phosphorylated at two positions in rat, namely serine 16 and serine 86. Since it is known from several tissues that phosphorylation often determines, its subcellular localization immunohistochemistry was additionally performed using phosphospecific HspB1 antibodies. No signals in either hemisphere were observed with the pHspB1-Ser16-specific antibody suggesting no relevant phosphorylation at this site (data not shown). For pHspB1-Ser86, an overall intense staining in the unaffected (contralateral) hemisphere with strong labeling of single glial cells could be observed. Interestingly, in the infarcted hemisphere, pHspB1-Ser86 was specifically localized in neurons (Fig. 4b). This staining was seen in the neuronal somata as and in the nuclei.
For HspB5, a diffuse staining of the healthy contralateral hemisphere could be observed with single glial cells exhibiting a stronger signal. Neurons, however, were not stained (Fig. 5a). In contrast, in the ischemic cortex, neurons were stained prominently in the soma and in the nucleus. HspB5 is known to be phosphorylated in response to cellular stress and has three potential phosphorylation sites, serine 19, serine 45, and serine 59. Using phosphospecific antibodies, we sought to investigate cellular localization of the phosphoforms as well. No specific signal was detected with the pHspB5-Ser45 specific antibody (not shown). Localizations of pHspB5-Ser19 and pHspB5-Ser59 are shown in Fig. 5b, c. Both antibodies stained elongated structures most likely representing neuronal processes. pHspB5-Ser19 was additionally localized in neuronal somata in the infarcted regions.
Similar to HspB1 and HspB5, HspB8 and HspB11 also showed a neuronal localization in the ischemic cortex (Fig. 6).
Thus, our data show that several HspBs are upregulated not only in glia but also in neurons after tMCAO.
Discussion
Small heat shock proteins (HspBs) are known to be neuroprotective after various cellular stress conditions such as heat, hypoxia, oxidative stress, or ischemia. In general, HspBs are upregulated in response to stress conditions and some of them are additionally regulated by their phosphorylation. The ten members of the HspB family not only share similar characteristics but also display very distinct functions. Unfortunately, most studies focus only on single family members, making it difficult to compare HspBs with each other from different studies which used different experimental models. Here, we systematically analyzed all ten HspBs and HspB11 in a rat model of 1 h tMCAO followed by 23 h reperfusion. HspB1 and HspB5 were strongly upregulated in the peri-infarct area on mRNA and protein level as reported already previously (see below). Interestingly, all the other HspBs were not regulated or reached only low amounts even after upregulation.
HspB1 (Hsp27)
HspB1 is well known to be of protective value in cerebral infarction. HspB1 transgenic mice showed significantly reduced cortical damage after permanent MCAO (van der Weerd et al. 2010). In addition, it was shown that intravenous injection of HspB1 in mice reduced infarct volume after tMCAO (Teramoto et al. 2013). Our data show that HspB1 is upregulated after tMCAO in astrocytes and in neurons indicating that it protects glial cells as well as neurons. Localization of HspB1 in reactive astrocytes during ischemic conditions has been shown also by Imura et al. (1999) whereas Popp et al. (2009) described additionally neuronal expression. Interestingly, in hippocampus after ischemia, Kato et al. (1994) observed time-dependent expression of HspB1 in different cell types. Whereas 1 day after ischemia, HspB1 was found in CA1 pyramidal neurons, and after 3 and 7 days, it was localized in reactive glia. In a model of kainic acid-induced seizure activity, HspB1 was found to be expressed in control conditions in hippocampal neurons whereas it was upregulated after kainic-acid treatment in glial cells (Kato et al. 1999). Interestingly, here we could show that HspB1 is phosphorylated at serine 86 in neurons in response to ischemia suggesting that phosphorylation might be important for the neuroprotective effect of HspB1. Indeed, injection of phospho-HspB1 was more effective than non-phosphorylated HspB1 in an in vivo model of tMCAO (Shimada et al. 2014). The relevance of HspB1 phosphorylation for neuroprotection after ischemia was also ascertained by phosphorylation mutants and inhibition of protein kinase D (Stetler et al. 2012). Protein kinase D is known to phosphorylate serine 82 of human HspB1 which is equivalent to rat serine 86 (Kostenko and Moens 2009). Thus, our data support previous studies, emphasizing the important role of phospho-HspB1-Ser86 in neuroprotection after ischemia/reperfusion injury. Regulation of HspBs by phosphorylation is a very fast event (within minutes or hours) as known from studies of ischemic myocardium (Golenhofen et al. 1998, 1999). Thus, one might suggest that phosphorylation of HspBs might be involved in the early response whereas upregulation of the protein levels occurs with some delays and may become important after days. However, expression levels may not be solely crucial for neuroprotection. Other factors may modify and influence the stress response as it has been exemplarily shown for the process of aging (Gong et al. 2004).
HspB5 (αB-crystallin)
Similar to HspB1, the neuroprotective capacity of HspB5 has been reported by the use of genetically modified animal models. After experimental stroke, HspB5-/- mice are characterized by an increased lesion size compared to wild-type mice (Arac et al. 2011). Whereas Kato et al. (1994) did not observe significant alterations in HspB5 content after cerebral ischemia, other groups reported upregulation. In peri-lesional areas after 2 h tMCAO, HspB5 was found to be expressed in astrocytes (Imura et al. 1999). Another group observed upregulation of HspB5 in reactive astrocytes to a later time point, namely after 2 days of reperfusion which was sustained for several days (Piao et al. 2005). In addition, they found HspB5 also upregulated in somata and processes of pyramidal neurons of the peri-infarct zone after 1 h tMCAO and 4 h reperfusion. However, this upregulation returned to basal levels after 1 day of reperfusion. We analyzed HspB5 expression 24 h after tMCAO, which might be the reason that we did not see an upregulation of HspB5 in astrocytes reported to occur later. Interestingly, Piao et al. (2005) also found that the levels of kinases upstream of HspB5 (p38MAPK, MAPKAPK-2 (Kato et al. 1998)) rose gradually with a peak at 4 days after MCAO. They suggested that the respective phosphorylated HspB5 might play a major role in the ischemic stress response. This fits to our finding of phosphorylation of HspB5 at serine19 and serine 59 in neurons within the infarct area. In cultured hippocampal neurons at control conditions, phosphorylation of HspB5 has been shown to occur with preferential localization of the phosphoforms within the neuronal processes (Schmidt et al. 2012). Furthermore, we reported recently that HspB5 regulates dendritic complexity and stabilizes the dendritic tree from heat-shock induced rarefaction in a phosphorylation-dependent manner (Bartelt-Kirbach et al. 2016). Thus, one might speculate that phospho-HspB5 protects neuronal cytoarchitecture also during ischemia/reperfusion injury in the brain.
Other HspBs
Up to now, there exist only few reports dealing with the HspBs other than HspB1 and HspB5 during cerebral ischemia. This might be due to (1) their low expression in the brain which makes it difficult to detect them by the use of antibodies and (2) the fact that they have been identified later than HspB1 and HspB5. Our real-time RT-PCR data show that HspB2 and HspB10 were not expressed in the cortex at control conditions or after tMCAO. HspB3, HspB7, HspB9, and HspB11 were constitutively expressed at very low levels with a significant induction of HspB3 and HspB7 after tMCAO. HspB4 was found to be expressed only after tMCAO. However, mRNA levels of all these HspBs even after induction were still relatively low compared to HspB1 and HspB5, suggesting that they only play a minor role in the ischemic stress response. HspB8 was expressed at similar levels as HspB1 and HspB5 but increased only on mRNA but not on protein level in the peri-infarct zone. However, it localized to neurons similar to HspB1 and HspB5. HspB8 was previously reported to be expressed in rat cortex and to be upregulated 72 h after ischemia/reperfusion injury (Tao et al. 2015). It was also neuroprotective in a model of ischemia/reperfusion injury in cultured neuroblastoma cells by inhibiting apoptosis (Yang et al. 2015).
mRNA and protein levels of HspB11, which is controversially discussed to belong to the HspB family (Bellyei et al. 2007; Kappe et al. 2010), were not significantly altered in response to tMCAO in our study. This fits to previous data that HspB11 is not stress-responsive after several kinds of cellular stresses (Bartelt-Kirbach and Golenhofen 2014; Bellyei et al. 2007; Kirbach and Golenhofen 2011). However, our immunohistochemical data show that HspB11 is localized in neurons in the peri-infarct area. Since HspB11 has been first described as an inhibitor of apoptotic cell death in various cell lines (Bellyei et al. 2007), it might play a role in neuronal cell survival even without stress-induced induction.
In conclusion, our data suggest that among all HspBs, HspB1 and HspB5 might be most important in the neuronal stress response to ischemia/reperfusion injury in the brain since their protein levels were upregulated to the highest extent. In addition, ischemia-induced phosphorylation of HspB1 and HspB5 in neurons was observed which is thought to be important for their activation. Other HspBs seem to play a minor role in cerebral ischemia as judged by their low expression level and low degree of induction. However, some of them were allocated to neurons and, thus, may act in concert with HspB1 or HspB5 and contribute to neuroprotection.
Electronic supplementary material
(PDF 133 kb)
(PDF 77 kb)
(PDF 139 kb)
Acknowledgements
We thank Bianca Mekle, Diana Reinhard, and Stephanie Sues for their excellent technical assistance.
Compliance with ethical standards
Animal care and experimental procedures were formally approved by the Review Board for the Care of Animal Subjects of the district government (North Rhine-Westphalia, Germany) and bred and maintained in a pathogen-free environment in a 12-h day and 12-h night cycle prior experiments.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-017-0794-9) contains supplementary material, which is available to authorized users.
References
- Akbar MT, Lundberg AM, Liu K, Vidyadaran S, Wells KE, Dolatshad H, Wynn S, Wells DJ, Latchman DS, de Belleroche J. The neuroprotective effects of heat shock protein 27 overexpression in transgenic animals against kainate-induced seizures and hippocampal cell death. J Biol Chem. 2003;278:19956–19965. doi: 10.1074/jbc.M207073200. [DOI] [PubMed] [Google Scholar]
- Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci U S A. 2011;108:13287–13292. doi: 10.1073/pnas.1107368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badin RA, Lythgoe MF, van der Weerd L, Thomas DL, Gadian DG, Latchman DS. Neuroprotective effects of virally delivered HSPs in experimental stroke. J Cereb Blood Flow Metab. 2006;26:371–381. doi: 10.1038/sj.jcbfm.9600190. [DOI] [PubMed] [Google Scholar]
- Bartelt-Kirbach B, Golenhofen N. Reaction of small heat-shock proteins to different kinds of cellular stress in cultured rat hippocampal neurons. Cell Stress Chaperones. 2014;19:145–153. doi: 10.1007/s12192-013-0452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt-Kirbach B, Moron M, Glomb M, Beck CM, Weller MP, Golenhofen N (2016) HspB5/alphaB-crystallin increases dendritic complexity and protects the dendritic arbor during heat shock in cultured rat hippocampal neurons. Cell Mol Life Sci 73:3761–3775 [DOI] [PMC free article] [PubMed]
- Bellyei S, Szigeti A, Pozsgai E, Boronkai A, Gomori E, Hocsak E, Farkas R, Sumegi B, Gallyas F., Jr Preventing apoptotic cell death by a novel small heat shock protein. Eur J Cell Biol. 2007;86:161–171. doi: 10.1016/j.ejcb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Bhat SP, Nagineni CN. Alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989;158:319–325. doi: 10.1016/S0006-291X(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Dang J, Mitkari B, Kipp M, Beyer C. Gonadal steroids prevent cell damage and stimulate behavioral recovery after transient middle cerebral artery occlusion in male and female rats. Brain Behav Immun. 2011;25:715–726. doi: 10.1016/j.bbi.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Bartelt-Kirbach B (2016) The impact of small heat shock proteins (HspBs) in Alzheimer’s and other neurological diseases. Curr Pharm Des 22:4050–4062 [DOI] [PubMed]
- Golenhofen N, Ness W, Koob R, Htun P, Schaper W, Drenckhahn D. Ischemia-induced phosphorylation and translocation of stress protein alpha B-crystallin to Z lines of myocardium. Am J Phys. 1998;274:H1457–H1464. doi: 10.1152/ajpheart.1998.274.5.H1457. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Htun P, Ness W, Koob R, Schaper W, Drenckhahn D. Binding of the stress protein alpha B-crystallin to cardiac myofibrils correlates with the degree of myocardial damage during ischemia/reperfusion in vivo. J Mol Cell Cardiol. 1999;31:569–580. doi: 10.1006/jmcc.1998.0892. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Redel A, Wawrousek EF, Drenckhahn D. Ischemia-induced increase of stiffness of alphaB-crystallin/HSPB2-deficient myocardium. Pflugers Arch. 2006;451:518–525. doi: 10.1007/s00424-005-1488-1. [DOI] [PubMed] [Google Scholar]
- Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Heinzel W, Vogt A, Kallee E, Faller W. A new method for the quantitative determination of antibody and antigen protein, with a sensitivity to five micrograms. J Lab Clin Med. 1965;66:334–340. [PubMed] [Google Scholar]
- Horwitz J. The function of alpha-crystallin in vision. Semin Cell Dev Biol. 2000;11:53–60. doi: 10.1006/scdb.1999.0351. [DOI] [PubMed] [Google Scholar]
- Imura T, Shimohama S, Sato M, Nishikawa H, Madono K, Akaike A, Kimura J. Differential expression of small heat shock proteins in reactive astrocytes after focal ischemia: possible role of beta-adrenergic receptor. J Neurosci. 1999;19:9768–9779. doi: 10.1523/JNEUROSCI.19-22-09768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Sam S, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277:38731–38736. doi: 10.1074/jbc.M201770200. [DOI] [PubMed] [Google Scholar]
- Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones. 2003;8:53–61. doi: 10.1379/1466-1268(2003)8<53:THGECS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe G, Boelens WC, de Jong WW. Why proteins without an alpha-crystallin domain should not be included in the human small heat shock protein family HSPB. Cell Stress Chaperones. 2010;15:457–461. doi: 10.1007/s12192-009-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Liu Y, Kogure K, Kato K. Induction of 27-kDa heat shock protein following cerebral ischemia in a rat model of ischemic tolerance. Brain Res. 1994;634:235–244. doi: 10.1016/0006-8993(94)91926-7. [DOI] [PubMed] [Google Scholar]
- Kato K, Ito H, Kamei K, Inaguma Y, Iwamoto I, Saga S. Phosphorylation of alphaB-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J Biol Chem. 1998;273:28346–28354. doi: 10.1074/jbc.273.43.28346. [DOI] [PubMed] [Google Scholar]
- Kato K, Katoh-Semba R, Takeuchi IK, Ito H, Kamei K. Responses of heat shock proteins hsp27, alphaB-crystallin, and hsp70 in rat brain after kainic acid-induced seizure activity. J Neurochem. 1999;73:229–236. doi: 10.1046/j.1471-4159.1999.0730229.x. [DOI] [PubMed] [Google Scholar]
- Kirbach BB, Golenhofen N. Differential expression and induction of small heat shock proteins in rat brain and cultured hippocampal neurons. J Neurosci Res. 2011;89:162–175. doi: 10.1002/jnr.22536. [DOI] [PubMed] [Google Scholar]
- Kostenko S, Moens U. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol Life Sci. 2009;66:3289–3307. doi: 10.1007/s00018-009-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae TH. Structure and function of small heat shock/alpha-crystallin proteins: established concepts and emerging ideas. Cell Mol Life Sci. 2000;57:899–913. doi: 10.1007/PL00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Schulze-Osthoff K, Arrigo AP. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- Oshita SE, Chen F, Kwan T, Yehiely F, Cryns VL. The small heat shock protein HspB2 is a novel anti-apoptotic protein that inhibits apical caspase activation in the extrinsic apoptotic pathway. Breast Cancer Res Treat. 2010;124:307–315. doi: 10.1007/s10549-010-0735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O’Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- Piao CS, Kim SW, Kim JB, Lee JK (2005) Co-induction of αB-crystallin and MAPKAPK-2 in astrocytes in the penumbra after transient focal cerebral ischemia. Exp Brain Res 163(4):421–429 [DOI] [PubMed]
- Popp A, Jaenisch N, Witte OW, Frahm C. Identification of ischemic regions in a rat model of stroke. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quraishe S, Asuni A, Boelens WC, O’Connor V, Wyttenbach A. Expression of the small heat shock protein family in the mouse CNS: Differential anatomical and biochemical compartmentalization. Neuroscience. 2008;153:483–491. doi: 10.1016/j.neuroscience.2008.01.058. [DOI] [PubMed] [Google Scholar]
- Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK. Transgene overexpression of αB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. FASEB J. 2001;15:393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- Ritossa FM. A new puffing pattern induced by a temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- Schmidt T, Bartelt-Kirbach B, Golenhofen N. Phosphorylation-dependent subcellular localization of the small heat shock proteins HspB1/Hsp25 and HspB5/alphaB-crystallin in cultured hippocampal neurons. Histochem Cell Biol. 2012;138:407–418. doi: 10.1007/s00418-012-0964-x. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Tanaka R, Shimura H, Yamashiro K, Urabe T, Hattori N. Phosphorylation enhances recombinant HSP27 neuroprotection against focal cerebral ischemia in mice. Neuroscience. 2014;278:113–121. doi: 10.1016/j.neuroscience.2014.07.073. [DOI] [PubMed] [Google Scholar]
- Stetler RA, Cao G, Gao Y, et al. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008;28:13038–13055. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler RA, Gan Y, Zhang W, Liou AK, Gao Y, Cao G, Chen J. Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Prog Neurobiol. 2010;92:184–211. doi: 10.1016/j.pneurobio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler RA, Gao Y, Zhang L, et al. Phosphorylation of HSP27 by protein kinase D is essential for mediating neuroprotection against ischemic neuronal injury. J Neurosci. 2012;32:2667–2682. doi: 10.1523/JNEUROSCI.5169-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Suzuki A, Kishikawa M, Akutsu R, Hirose T, Waye MM, Tsui SK, Yoshida S, Ohno S. Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J Biol Chem. 2000;275:1095–1104. doi: 10.1074/jbc.275.2.1095. [DOI] [PubMed] [Google Scholar]
- Tao X, Lu W, Deng J, Hu Z, Lei Q, Zhang J, Song T, Liu J, Zheng L, He J. HspB8 expression in brain tissue after cerebral ischemic reperfusion and atorvastatin intervention in Sprague-Dawley rats. Neurol Res. 2015;37:229–237. doi: 10.1179/1743132814Y.0000000427. [DOI] [PubMed] [Google Scholar]
- Teramoto S, Shimura H, Tanaka R, Shimada Y, Miyamoto N, Arai H, Urabe T, Hattori N. Human-derived physiological heat shock protein 27 complex protects brain after focal cerebral ischemia in mice. PLoS One. 2013;8:e66001. doi: 10.1371/journal.pone.0066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich C, Zendedel A, Habib P, Kipp M, Beyer C, Dang J. Long-term cerebral cortex protection and behavioral stabilization by gonadal steroid hormones after transient focal hypoxia. J Steroid Biochem Mol Biol. 2012;131:10–16. doi: 10.1016/j.jsbmb.2012.01.007. [DOI] [PubMed] [Google Scholar]
- van der Weerd L, Tariq Akbar M, Aron Badin R, Valentim LM, Thomas DL, Wells DJ, Latchman DS, Gadian DG, Lythgoe MF, de Belleroche JS. Overexpression of heat shock protein 27 reduces cortical damage after cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:849–856. doi: 10.1038/jcbfm.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff MJ, Collaco-Moraes Y, Smith J, de Belleroche JS, Coffin RS, Latchman DS. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J Biol Chem. 1999;274:5061–5069. doi: 10.1074/jbc.274.8.5061. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Yang B, Zhang H, Mo X, Xiao H, Hu Z. HspB8 is neuroprotective during oxygen glucose deprivation and reperfusion. Curr Neurovasc Res. 2015;12:63–72. doi: 10.2174/1567202612666150102152350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 133 kb)
(PDF 77 kb)
(PDF 139 kb)