Abstract
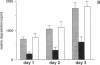
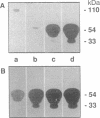
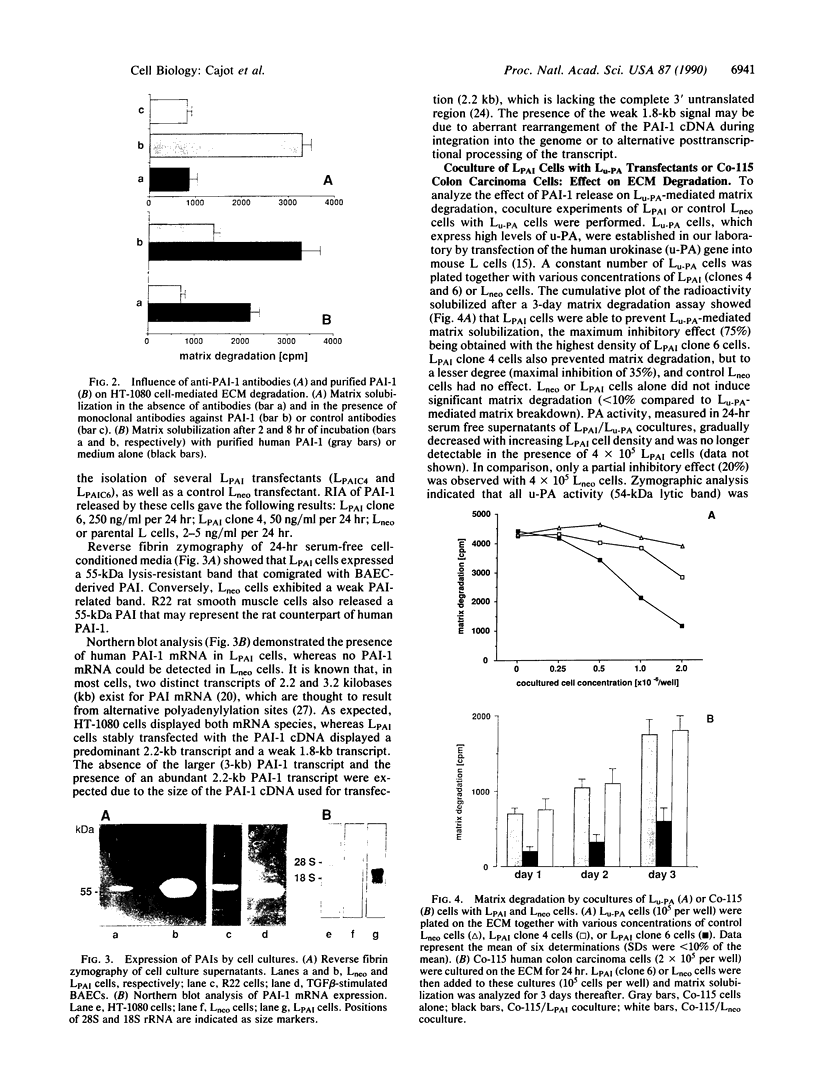
We have analyzed the role of plasminogen-activator inhibitor type 1 (PAI-1) in the regulation of tumor cell-mediated extracellular matrix degradation. Immunocytochemical analysis revealed PAI-1 associated with microgranular and fibrillar material of the extracellular matrix and demonstrated the presence of PAI-1 as a cell surface-associated antigen. Transforming growth factor beta significantly reduced matrix degradation mediated by HT-1080 human fibrosarcoma cells. This inhibition was correlated with an increase in PAI-1 antigen expression, whereas urinary-type plasminogen activator (u-PA) secretion was unaffected. In this experimental system, PAI-1 regulated extracellular matrix breakdown, as added PAI-1 inhibited matrix solubilization, whereas monoclonal antibodies to PAI-1 increased it. A cell line (LPAI) producing high levels of biologically active PAI-1 was established by transfection of a human PAI-1 cDNA clone into mouse L cells. Coculture experiments demonstrated that LPAI cells prevented matrix degradation by Lu-PA cells (L cells expressing high levels of u-PA) or Co-115 human colon carcinoma cells (expressing tissue-type plasminogen activator). These results indicate that PAI-1 may play a critical role in the regulation of extracellular matrix degradation during tumor cell invasion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aden D. P., Fogel A., Plotkin S., Damjanov I., Knowles B. B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979 Dec 6;282(5739):615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- Alessi M. C., Declerck P. J., De Mol M., Nelles L., Collen D. Purification and characterization of natural and recombinant human plasminogen activator inhibitor-1 (PAI-1). Eur J Biochem. 1988 Aug 15;175(3):531–540. doi: 10.1111/j.1432-1033.1988.tb14225.x. [DOI] [PubMed] [Google Scholar]
- Appella E., Robinson E. A., Ullrich S. J., Stoppelli M. P., Corti A., Cassani G., Blasi F. The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J Biol Chem. 1987 Apr 5;262(10):4437–4440. [PubMed] [Google Scholar]
- Bergman B. L., Scott R. W., Bajpai A., Watts S., Baker J. B. Inhibition of tumor-cell-mediated extracellular matrix destruction by a fibroblast proteinase inhibitor, protease nexin I. Proc Natl Acad Sci U S A. 1986 Feb;83(4):996–1000. doi: 10.1073/pnas.83.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajot J. F., Kruithof E. K., Schleuning W. D., Sordat B., Bachmann F. Plasminogen activators, plasminogen activator inhibitors and procoagulant analyzed in twenty human tumor cell lines. Int J Cancer. 1986 Nov 15;38(5):719–727. doi: 10.1002/ijc.2910380516. [DOI] [PubMed] [Google Scholar]
- Cajot J. F., Schleuning W. D., Medcalf R. L., Bamat J., Testuz J., Liebermann L., Sordat B. Mouse L cells expressing human prourokinase-type plasminogen activator: effects on extracellular matrix degradation and invasion. J Cell Biol. 1989 Aug;109(2):915–925. doi: 10.1083/jcb.109.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajot J. F., Sordat B., Kruithof E. K., Bachmann F. Human primary colon carcinomas xenografted into nude mice. I. Characterization of plasminogen activators expressed by primary tumors and their xenografts. J Natl Cancer Inst. 1986 Sep;77(3):703–712. doi: 10.1093/jnci/77.3.703. [DOI] [PubMed] [Google Scholar]
- Erickson L. A., Lawrence D. A., Loskutoff D. J. Reverse fibrin autography: a method to detect and partially characterize protease inhibitors after sodium dodecyl sulfate--polyacrylamide gel electrophoresis. Anal Biochem. 1984 Mar;137(2):454–463. doi: 10.1016/0003-2697(84)90113-1. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing V. J., Law L. W., Corti A., Appella E., Blasi F. Modulation of metastatic potential by cell surface urokinase of murine melanoma cells. Cancer Res. 1988 Mar 1;48(5):1270–1278. [PubMed] [Google Scholar]
- Jones P. A., De Clerck Y. A. Extracellular matrix destruction by invasive tumor cells. Cancer Metastasis Rev. 1982;1(4):289–317. doi: 10.1007/BF00124214. [DOI] [PubMed] [Google Scholar]
- Khokha R., Waterhouse P., Yagel S., Lala P. K., Overall C. M., Norton G., Denhardt D. T. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989 Feb 17;243(4893):947–950. doi: 10.1126/science.2465572. [DOI] [PubMed] [Google Scholar]
- Kruithof E. K., Nicolosa G., Bachmann F. Plasminogen activator inhibitor 1: development of a radioimmunoassay and observations on its plasma concentration during venous occlusion and after platelet aggregation. Blood. 1987 Nov;70(5):1645–1653. [PubMed] [Google Scholar]
- Laiho M., Saksela O., Keski-Oja J. Transforming growth factor-beta induction of type-1 plasminogen activator inhibitor. Pericellular deposition and sensitivity to exogenous urokinase. J Biol Chem. 1987 Dec 25;262(36):17467–17474. [PubMed] [Google Scholar]
- Laug W. E. Inhibition of human tumor cell-associated fibrinolysis by vascular bovine smooth muscle cells. J Natl Cancer Inst. 1985 Aug;75(2):345–352. [PubMed] [Google Scholar]
- Loskutoff D. J., Linders M., Keijer J., Veerman H., van Heerikhuizen H., Pannekoek H. Structure of the human plasminogen activator inhibitor 1 gene: nonrandom distribution of introns. Biochemistry. 1987 Jun 30;26(13):3763–3768. doi: 10.1021/bi00387a004. [DOI] [PubMed] [Google Scholar]
- Loskutoff D. J., van Mourik J. A., Erickson L. A., Lawrence D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2956–2960. doi: 10.1073/pnas.80.10.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf R. L., Richards R. I., Crawford R. J., Hamilton J. A. Suppression of urokinase-type plasminogen activator mRNA levels in human fibrosarcoma cells and synovial fibroblasts by anti-inflammatory glucocorticoids. EMBO J. 1986 Sep;5(9):2217–2222. doi: 10.1002/j.1460-2075.1986.tb04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf R. L., Van den Berg E., Schleuning W. D. Glucocorticoid-modulated gene expression of tissue- and urinary-type plasminogen activator and plasminogen activator inhibitor 1 and 2. J Cell Biol. 1988 Mar;106(3):971–978. doi: 10.1083/jcb.106.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Tsuboi R., Robbins E., Rifkin D. B. In vitro angiogenesis on the human amniotic membrane: requirement for basic fibroblast growth factor-induced proteinases. J Cell Biol. 1989 Feb;108(2):671–682. doi: 10.1083/jcb.108.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimuro J., Loskutoff D. J. Purification of a protein from bovine plasma that binds to type 1 plasminogen activator inhibitor and prevents its interaction with extracellular matrix. Evidence that the protein is vitronectin. J Biol Chem. 1989 Jan 15;264(2):936–939. [PubMed] [Google Scholar]
- Mimuro J., Schleef R. R., Loskutoff D. J. Extracellular matrix of cultured bovine aortic endothelial cells contains functionally active type 1 plasminogen activator inhibitor. Blood. 1987 Sep;70(3):721–728. [PubMed] [Google Scholar]
- Nielsen L. S., Andreasen P. A., Grøndahl-Hansen J., Huang J. Y., Kristensen P., Danø K. Monoclonal antibodies to human 54,000 molecular weight plasminogen activator inhibitor from fibrosarcoma cells--inhibitor neutralization and one-step affinity purification. Thromb Haemost. 1986 Apr 30;55(2):206–212. [PubMed] [Google Scholar]
- Ny T., Sawdey M., Lawrence D., Millan J. L., Loskutoff D. J. Cloning and sequence of a cDNA coding for the human beta-migrating endothelial-cell-type plasminogen activator inhibitor. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6776–6780. doi: 10.1073/pnas.83.18.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L. In vivo invasion of modified chorioallantoic membrane by tumor cells: the role of cell surface-bound urokinase. J Cell Biol. 1988 Dec;107(6 Pt 1):2437–2445. doi: 10.1083/jcb.107.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek H., Veerman H., Lambers H., Diergaarde P., Verweij C. L., van Zonneveld A. J., van Mourik J. A. Endothelial plasminogen activator inhibitor (PAI): a new member of the Serpin gene family. EMBO J. 1986 Oct;5(10):2539–2544. doi: 10.1002/j.1460-2075.1986.tb04532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J., Hedman K., Nielsen L. S., Danø K., Vaheri A. Ultrastructural localization of plasma membrane-associated urokinase-type plasminogen activator at focal contacts. J Cell Biol. 1988 Jan;106(1):87–95. doi: 10.1083/jcb.106.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J., Saksela O., Salonen E. M., Andreasen P., Nielsen L., Danø K., Vaheri A. Distinct localizations of urokinase-type plasminogen activator and its type 1 inhibitor under cultured human fibroblasts and sarcoma cells. J Cell Biol. 1987 Apr;104(4):1085–1096. doi: 10.1083/jcb.104.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y., Okada M., Noro A., Matsuda M. Interaction of tissue-type plasminogen activator and plasminogen activator inhibitor 1 on the surface of endothelial cells. J Biol Chem. 1988 Feb 5;263(4):1960–1969. [PubMed] [Google Scholar]
- Saksela O. Plasminogen activation and regulation of pericellular proteolysis. Biochim Biophys Acta. 1985 Nov 12;823(1):35–65. doi: 10.1016/0304-419x(85)90014-9. [DOI] [PubMed] [Google Scholar]
- Schleef R. R., Podor T. J., Dunne E., Mimuro J., Loskutoff D. J. The majority of type 1 plasminogen activator inhibitor associated with cultured human endothelial cells is located under the cells and is accessible to solution-phase tissue-type plasminogen activator. J Cell Biol. 1990 Jan;110(1):155–163. doi: 10.1083/jcb.110.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. W., Pöllänen J., Tapiovaara H., Leung K. C., Sim P. S., Salonen E. M., Rønne E., Behrendt N., Danø K., Vaheri A. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol. 1989 May;108(5):1987–1995. doi: 10.1083/jcb.108.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]