Significance
Bacterial surface polysaccharides form the interface between the bacterium and its environment. In Gram-positive bacteria, the intricate architecture of the peptidoglycan (PG) serves as the scaffolding to which other surface polysaccharides, including capsules, can be anchored. For pathogenic bacteria, proper localization of capsular polysaccharide (CPS) is essential to cause disease. In Streptococcus pneumoniae, CPS is also the primary target for vaccines. Here, we identify a structure for polysaccharide attachment to PG that involves a direct glycosidic linkage. Knowledge of this linkage lays the groundwork for identifying the enzyme(s) involved in its synthesis and for developing new targets for therapeutic interventions.
Keywords: capsule, Wzy, Gram positive, pneumococcus, glycobiology
Abstract
For many bacteria, including those important in pathogenesis, expression of a surface-localized capsular polysaccharide (CPS) can be critical for survival in host environments. In Gram-positive bacteria, CPS linkage is to either the cytoplasmic membrane or the cell wall. Despite the frequent occurrence and essentiality of these polymers, the exact nature of the cell wall linkage has not been described in any bacterial species. Using the Streptococcus pneumoniae serotype 2 CPS, which is synthesized by the widespread Wzy mechanism, we found that linkage occurs via the reducing end glucose of CPS and the β-D-N-acetylglucosamine (GlcNAc) residues of peptidoglycan (PG). Hydrofluoric acid resistance, 31P-NMR, and 32P labeling demonstrated the lack of phosphodiester bonds, which typically occur in PG–polysaccharide linkages. Component sugar analysis of purified CPS–PG identified only CPS and PG sugars in the appropriate ratios, suggesting the absence of an oligosaccharide linker. Time of flight mass spectrometry confirmed a direct glycosidic linkage between CPS and PG and showed that a single CPS repeat unit can be transferred to PG. The linkage was acetolysis susceptible, indicative of a 1,6 glycosidic bond between CPS and the GlcNAc C-6. The acetylation state of GlcNAc did not affect linkage. A direct glycosidic linkage to PG was also demonstrated for serotypes 8 and 31, whose reducing end sugars are glucose and galactose, respectively. These results provide the most detailed descriptions of CPS–PG linkages for any microorganism. Identification of the linkage is a first step toward identifying the linking enzyme and potential inhibitors of its activity.
Capsular polysaccharide (CPS) assembly and localization in bacteria is a complex, multienzyme process leading to anchoring of the CPS polymer on the outer surface of the cell. For pathogens, the protective layer of the CPS can be important for adhesion, biofilm formation, and resistance to complement-mediated opsonophagocytosis and lysis (1, 2). Although substantial information regarding the syntheses of these polymers has accumulated, less is known about the critical steps involved in their attachment to the bacterial surface.
A large number of diverse CPS structures can be elaborated, as exemplified in the Gram-positive pathogen Streptococcus pneumoniae, where 98 CPS serotypes differing in sugar composition and linkages are known (3, 4). S. pneumoniae infections, occurring primarily as pneumonia and sepsis in young children and the elderly, are a significant global disease burden (5). Elaboration of a CPS is essential for these bacteria to cause disease and to colonize the nasopharyngeal cavity (6–8). Despite their structural diversity, all but two of the 98 S. pneumoniae CPS serotypes are synthesized by the Wzy polymerase-dependent mechanism, which is the most common mechanism of CPS synthesis in Gram-positive and Gram-negative bacteria, and is also used in synthesis of many lipopolysaccharide O-antigens in Gram-negative bacteria (9, 10). Polymer synthesis in the Wzy pathway begins with transfer of a sugar-1-phosphate from a nucleotide diphosphosugar donor to the C55 lipid undecaprenyl-phosphate (Und-P). In S. pneumoniae serotype 2 CPS, Glcp-1-P is transferred from UDP-Glcp (11), and this is followed by addition of the remaining sugars (12, 13) to form the complete repeat unit (Fig. 1). Und-P-P-oligosaccharide repeat units are translocated to the outer face of the cytoplasmic membrane by Wzx and polymerized into high molecular weight (MW) polysaccharide by Wzy. Growth occurs at the reducing end, with single or multiple repeat units being transferred en bloc from Und-P-P to an acceptor Und-P-P-oligosaccharide repeat unit. Hydrolysis of the donor Und-P-P that remains after transfer yields Und-P, which is recycled back into the cell. In Gram-negative bacteria, CPS is ultimately transported across, and assembled on, the outer membrane (9, 14). For Gram-positive bacteria, CPS is covalently linked to the cell wall and cytoplasmic membrane (15–17); however, the mechanisms involved in ligation are unknown. Failure to transfer CPS from Und-P may result in lethality due to lipid intermediate accumulation and/or reduced turnover of Und-P for essential pathways such as peptidoglycan (PG) (12, 18).
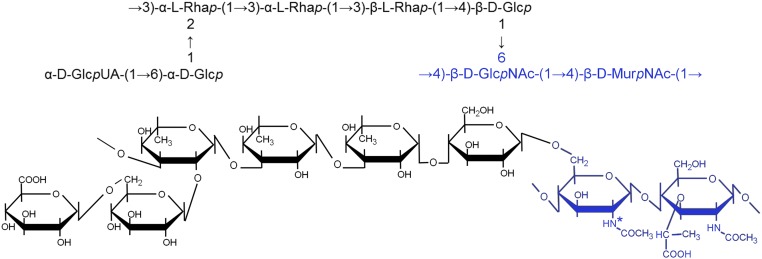
Fig. 1.
Proposed structure of serotype 2 CPS linked to PG, as determined in this study. The CPS structure is from refs. 11 and 25. PG residues are indicated in blue. Asterisk indicates 80% of the GlcNAc residues are deacetylated in S. pneumoniae (28). GlcUA, glucuronic acid; Rha, rhamnose.
A partial description of a CPS cell wall linkage in a Gram-positive bacterium has been reported for the Wzy-dependent Streptococcus agalactiae type III CPS, in which an oligosaccharide linker and phosphodiester bond are proposed to mediate linkage to the N-acetylglucosamine (GlcNAc) of PG (16). Exactly how these constituents are linked together or to GlcNAc has not been described. Other PG-linked polysaccharides include wall teichoic and teichuronic acids and the mycobacterial arabinogalactans. The linkage for each of these structures is to the N-acetylmuramic acid (MurNAc) C-6 via a phosphodiester bond. For teichoic acids and arabinogalactans, an oligosaccharide linker is also present (19–24). In this study, we provide a detailed description of a CPS linkage to PG, demonstrating that the S. pneumoniae type 2 CPS is linked via its reducing end Glc to the GlcNAc of PG by a direct 1,6 glycosidic bond.
Results
CPS Is Linked to PG.
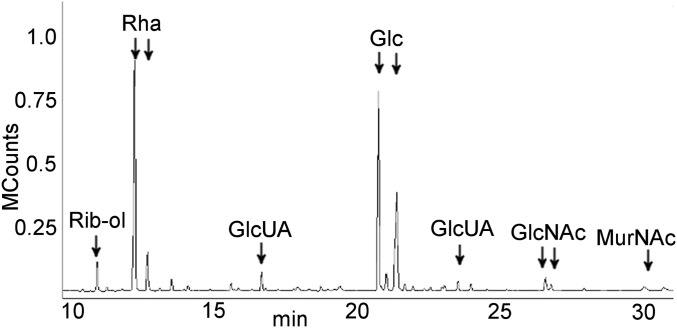
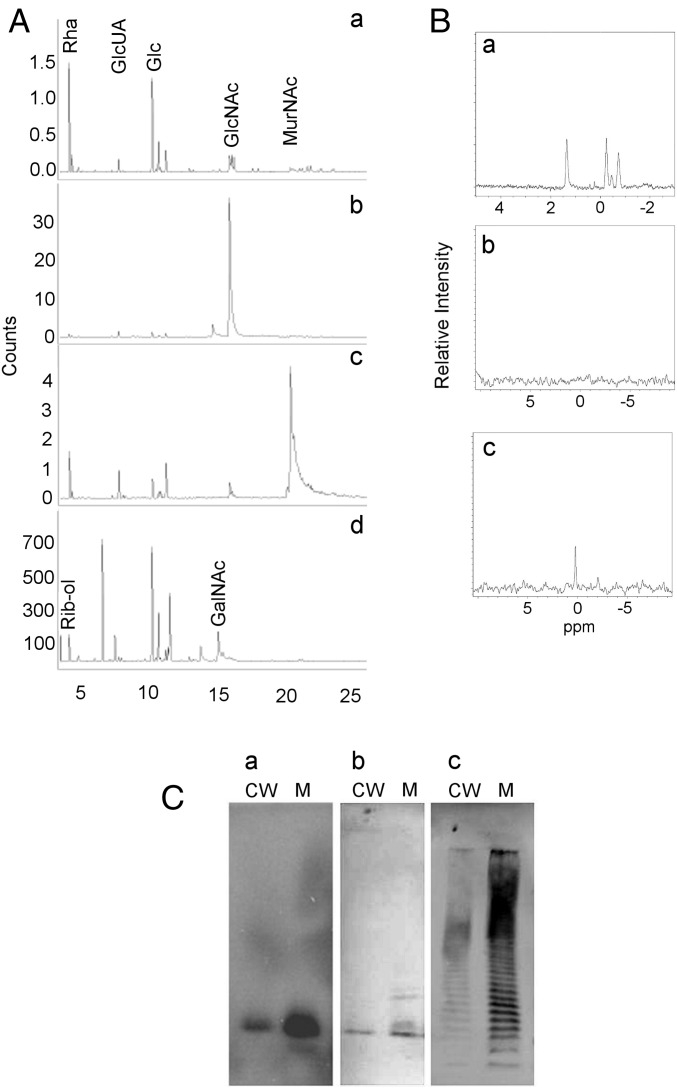
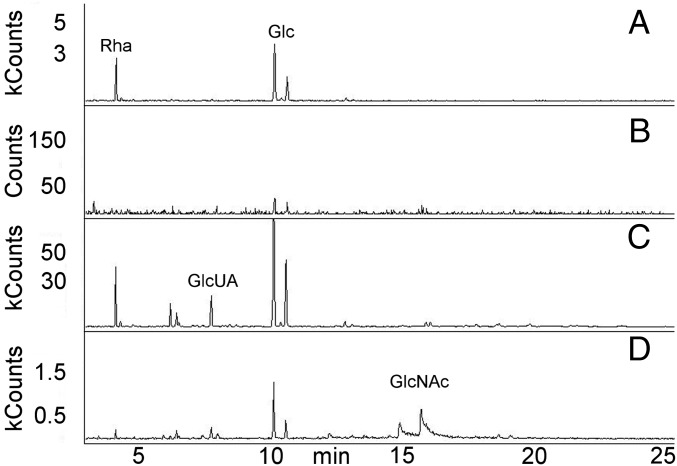
A cell wall fraction containing GlcNAc–MurNAc disaccharides and any linked components was generated by treatment of S. pneumoniae with mutanolysin, a β-1,4-N-acetylmuramidase that cleaves the PG backbone, and LytA, the pneumococcal N-acetylmuramoyl-L-alanine amidase that removes the peptide side chain from MurNAc. The negatively charged CPS was purified from the digest using anion exchange chromatography. CPS eluted in broad fractions, consistent with a range of polysaccharide sizes of varying numbers of repeat units. Gas chromatography and mass spectrometry (GC/MS) analysis identified the sugars present in CPS (Rha, Glc, and GlcUA) and PG (GlcNAc and MurNAc) (Fig. 2). Ribitol was detected in only small amounts, possibly indicative of teichoic acid and CPS on the same PG disaccharide or incomplete PG hydrolysis resulting in copurification of distantly linked CPS and teichoic acid. The molar ratios of Glc, Rha, and GlcUA were those expected from the published serotype 2 structure (11, 25). The GlcNAc and MurNAc ratio was consistent with that of PG. The results show no evidence for additional sugars, suggesting that no oligosaccharide linker is present in the CPS–PG structure.
Fig. 2.
CPS is linked to PG. GC/MS total ion chromatogram of cell wall-linked CPS. The molar ratios of Glc, Rha, and GlcUA are consistent with those of serotype 2 CPS. The ratios of the GlcNAc and MurNAc peaks reflect equimolar concentrations, as determined using purified sugar standards. Rib-ol, ribitol. MCounts, megacounts.
CPS Is Linked to GlcNAc.
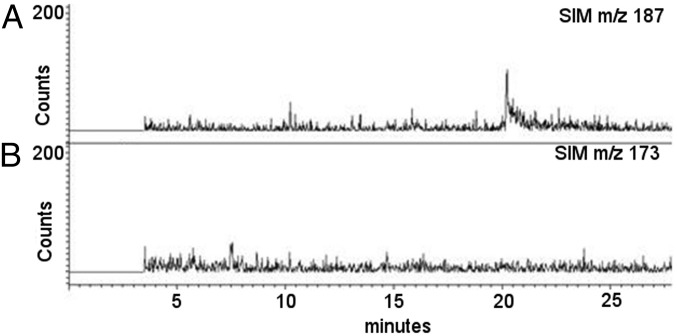
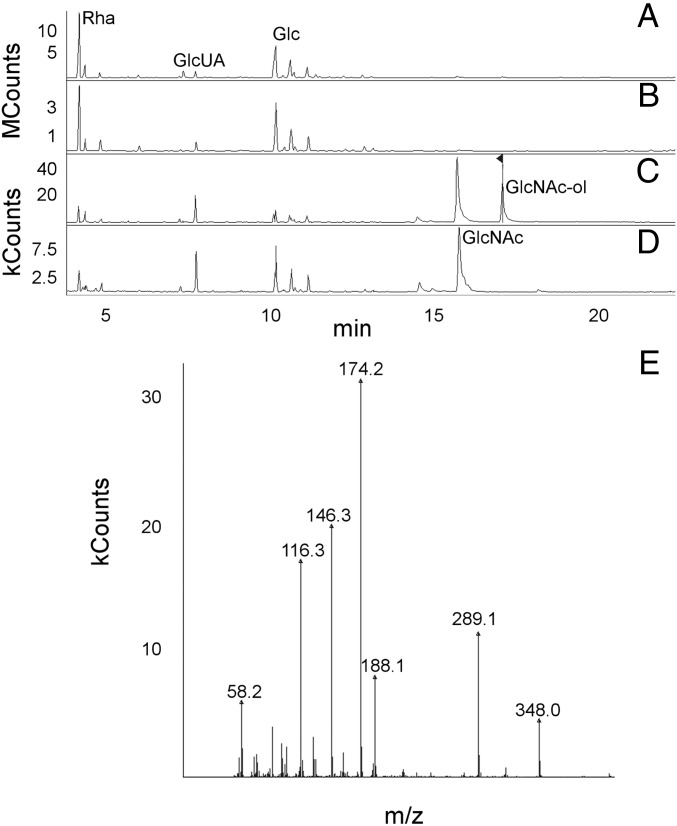
Purified CPS linked to PG disaccharides (which also contains low amounts of teichoic acid, as noted above, but henceforth referred to as CPS–PG) was subjected to β-N-acetylglucosaminidase to hydrolyze β-D-GlcNAc-1,4-β-D-MurNAc bonds. After ultrafiltration (3,000 Da cutoff), MurNAc but not GlcNAc was identified in the low MW fraction, indicating that the latter remained linked to high MW CPS (Fig. 3). Sodium borodeuteride (NaBD4) treatment of the high MW fraction resulted in reduction of GlcNAc to deuterated N-acetylglucosaminitol (GlcNAc-ol), indicating that MurNAc was released by the digestion and GlcNAc had remained at the reducing end of the high MW CPS (Fig. 4 A, C, and E). Similar treatment of a non–β-N-acetylglucosaminidase-digested control sample demonstrated no reduction of GlcNAc (Fig. 4 B and D). Sodium borodeuteride-reduced products were not detected for Glc, Rha, or GlcUA, consistent with linkage of their anomeric C-1 carbons to other sugars. Taken together, these data indicate that CPS and PG are covalently linked, and GlcNAc is located at the reducing end of CPS.
Fig. 3.
CPS is linked to the GlcNAc residue of PG. GC/MS analysis of low MW products following β-N-acetylglucosaminidase digestion of CPS–PG. (A) Selective ion monitoring for MurNAc at m/z 187. (B) Selective ion monitoring for GlcNAc at m/z 173.
Fig. 4.
GlcNAc is at the reducing end of CPS. CPS–PG was either treated with NaBD4 or first digested with β-N-acetylglucosaminidase followed by isolation of high MW product and subsequent NaBD4 treatment. (A) GC/MS total ion chromatogram of enzyme plus NaBD4-treated CPS–PG. (B) Total ion chromatogram of NaBD4-treated CPS–PG. (C) Selective ion monitoring at m/z 173 (GlcNAc) and 174 (deuterated GlcNAc-ol) of enzyme plus NaBD4-treated CPS–PG. The arrow is the reduced GlcNAc-ol. (D) Selective ion monitoring at m/z 173 and 174 of NaBD4-treated CPS–PG. (E) Electron impact mass spectrum of the GlcNAc-ol peak in C demonstrates the mass spectral fragmentation pattern of deuterated GlcNAc-ol, which resulted from reduction of GlcNAc after release of MurNAc by β-N-acetylglucosaminidase. kCounts, kilocounts; MCounts, megacounts.
CPS–PG Linkage Does Not Involve Phosphate Bonds.
To test for phosphodiester bonds, CPS–PG was subjected to digestion by 48% hydrofluoric acid (HF). After ultrafiltration (3,000 Da cutoff), all CPS sugars were identified in the high MW fraction in a ratio consistent with the serotype 2 CPS structure (Fig. 5A, a). GlcNAc and MurNAc were also present in this fraction (Fig. 5A, a–c). Consistent with the known phosphodiester bonds in S. pneumoniae teichoic acid repeat units (19, 22), HF treatment resulted in loss of the teichoic acid-specific ribitol and N-acetylgalactosamine from the high MW fraction (Fig. 5A, a) and their appearance in the low MW fraction, where neither CPS nor PG sugars were detected (Fig. 5A, d). The CPS–PG linkage is thus resistant to HF and likely not mediated by a phosphodiester bond.
Fig. 5.
The CPS–PG linkage does not contain phosphate bonds. (A) Resistance to HF. GC/MS analysis of HF-treated CPS–PG. (a) Total ion chromatogram of high MW fraction. (b) Selective ion monitoring of high MW fraction for GlcNAc at m/z 173. (c) Selective ion monitoring of high MW fraction for MurNAc at m/z 187. (d) Total ion chromatogram of low MW fraction demonstrating teichoic acid sugars ribitol (Rib-ol) and N-acetylgalactosamine (GalNAc). (B) Absence of phosphodiester bonds. 31P-NMR spectra of CPS–PG and HF-treated products. (a) CPS–PG before HF treatment. (b) High MW CPS–PG fraction obtained after HF treatment. (c) Low MW teichoic acid-containing fraction obtained after HF treatment. (C) Absence of phosphate. Cell wall (CW) and membrane (M) fractions from bacteria grown with 32P and separated by SDS-polyacrylamide gel electrophoresis. (a) Phosphorimage identifies molecules incorporating 32P. (b) Immunoblot using antiserum to teichoic acid. (c) Immunoblot using antiserum to CPS serotype 2.
To further test for phosphate-mediated linkages, CPS–PG was analyzed using 31P-NMR. Several distinct chemical shifts indicating phosphate and phosphate-mediated bonds were observed (Fig. 5B, a). After treatment with HF, phosphate was removed from the CPS-containing fraction (Fig. 5B, b) and identified in only the lower MW fraction (Fig. 5B, c), consistent with its association with teichoic acid. The complete removal of phosphate without hydrolyzing the CPS–PG bond demonstrates the lack of phosphodiester bond involvement.
To test for any phosphate in the CPS–PG structure, cells were grown in the presence of [32P]-phosphate and then separated into cell wall- and membrane-containing fractions. Following SDS/PAGE, 32P incorporation was observed in low MW products only, consistent with the small number of repeat units present in teichoic acid (26) and the detection of teichoic acid-specific bands by immunoblotting (Fig. 5C, a and b). The greater 32P incorporation observed in the membrane fraction reflects the higher amount of membrane-linked lipoteichoic acid, which also contains phosphodiester bonds (19). No 32P was detected in higher MW bands, indicative of the absence of phosphate in the CPS–PG linkage (Fig. 5C, a and c). The lack of 32P signal and CPS overlap also indicates that single repeat units of PG disaccharides rarely contain both CPS and teichoic acid (an outcome previously suggested by failure of antisera to teichoic acid to react with higher MW bands) (15, 27).
Linkage of CPS to PG Involves a Direct Glycosidic Bond.
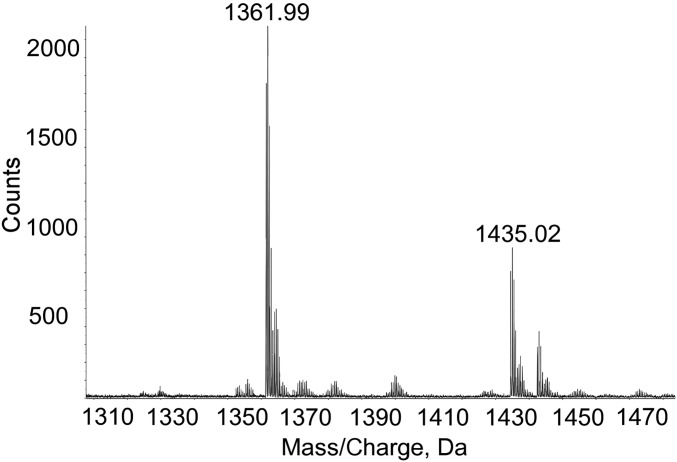
Component sugar analysis of CPS–PG with or without HF treatment identified the CPS and PG sugars in their expected ratios, showing no evidence of a linker (see above). To further test for a linkage unit, low MW CPS was purified by isolation and ultrafiltration of early elutions of CPS–PG from anion exchange chromatography. Triple Q-time-of-flight mass spectrometry (Q-TOF MS) demonstrated two major peaks (Fig. 6). The larger peak at m/z 1,361.99 corresponded to the mass expected for a single repeat unit of CPS linked by a glycosidic bond to a PG disaccharide with the loss of m/z 73, which is likely that of the lactate group on MurNAc. The smaller peak at 1,435.02 with a shift of m/z 73 represents the intact MurNAc on the PG. Taken together, these results show that CPS is directly linked to PG via a glycosidic bond, and a single repeat unit of CPS can be transferred to PG.
Fig. 6.
The CPS–PG linkage is a direct glycosidic bond. Mass spectrum of intact CPS–PG analyzed by triple Q-TOF MS. Peaks are as follows: 1,435.02 m/z, intact single repeat unit of CPS linked to a PG disaccharide; 1,361.99, intact single repeat unit of CPS linked to a PG disaccharide wherein the MurNAc has lost its lactate group.
Linkage of CPS to PG Does Not Require Deacetylation of GlcNAc.
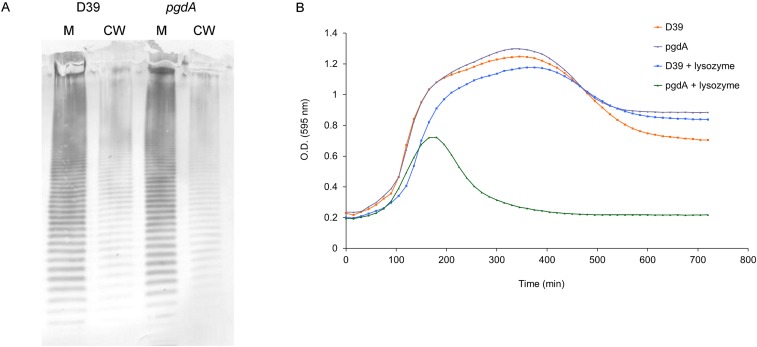
Approximately 80% of the S. pneumoniae PG GlcNAc is deacetylated at the 2-acetylamino position, resulting in resistance to lysozyme (28). Inactivation of the deacetylase-encoding gene (pgdA) did not alter CPS localization to the cell wall (Fig. S1). The acetylation state is thus not relevant, and there is likely no role for PgdA or the 2-acetylamino position of GlcNAc in the attachment of CPS to PG.
Fig. S1.
GlcNAc acetylation state does not affect CPS–PG linkage. (A) CPS immunoblot of S. pneumoniae D39 and its isogenic pgdA-negative derivative fractionated into cell wall (CW) and membrane (M) components. (B) Sensitivity of pgdA mutant to lysozyme, as demonstrated during growth in the presence or absence of the enzyme. O.D., optical density.
CPS Linkage Does Not Occur Through the GlcNAc C-2 or C-3.
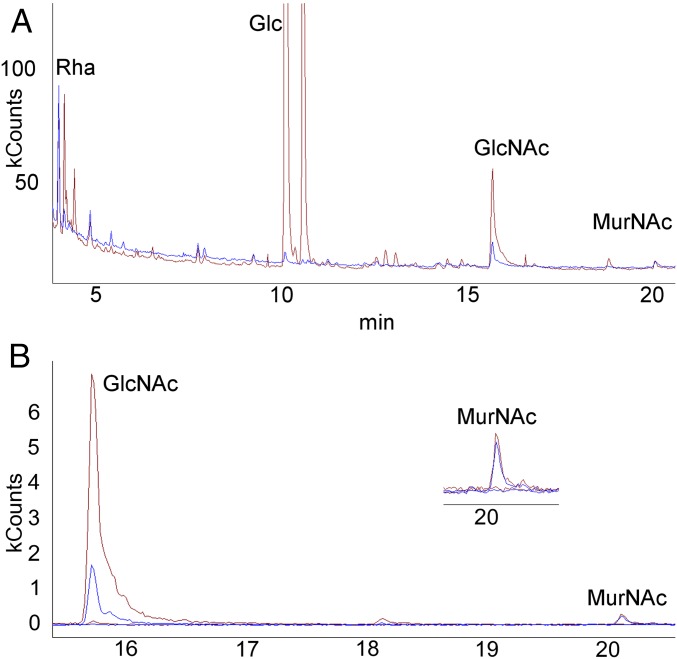
Smith degradation (29) was used to determine whether the GlcNAc C-2 and C-3 are protected from periodate oxidation, as would be expected if CPS linkage occurred at either of these sites. GC/MS of the reaction products demonstrated degradation of Glc but not Rha (Fig. 7A), consistent with the presence and absence, respectively, of vicinal hydroxyls in these sugars in CPS (Fig. 1). For the PG sugars, MurNAc was not degraded but GlcNAc was reduced to 16.7% of the amount contained in a nonperiodate-treated sample (Fig. 7 A and B). This value is consistent with the observation that more than 80% of S. pneumoniae PG GlcNAc is deacetylated and exists as glucosamine (28 and see above). In the absence of a linkage to the C-3, glucosamines are periodate susceptible. This result precludes the possibility of linkage occurring through the C-2 or C-3 of either the PG GlcNAc or the CPS backbone Glc, as well as the C-2, C-3, or C-4 of the side chain Glc.
Fig. 7.
The GlcNAc C-2 and C-3 are not involved in CPS linkage. GC/MS analysis following Smith degradation of CPS–PG. (A) Total ion chromatogram. The degradation product (blue) is overlaid with a nontreated control (red). Glc, but not Rha, is largely degraded. (B) Selective ion monitoring for PG sugars at m/z 173 and 187 following degradation (blue) overlaid with the untreated control (red). (Inset) MurNAc at higher magnification. kCounts, kilocounts.
Linkage of CPS to PG Occurs Through a Direct 1,6 Glycosidic Bond to GlcNAc.
Based on the known PG structure and the results described thus far, the glycosidic linkage between CPS and GlcNAc is unlikely to occur at the GlcNAc C-1 or C-4 (due to linkage to MurNAc), or C-2 or C-3 (due to susceptibility to Smith degradation and lack of effect of N-acetylation on CPS linkage). The GlcNAc C-6 is the least sterically hindered residue available for glycosidic linkages, and the analogous MurNAc C-6 is the attachment site for other PG-linked polysaccharides. Acetolysis preferentially cleaves 1,6 glycosidic bonds, with 1,4 linkages showing lesser susceptibility; linkages between 1, 2 and 1,3 residues are generally resistant (30, 31). The CPS backbone contains one 1,4 linkage and no 1,6 linkages; a single 1,6 linkage occurs between Glc and the terminal GlcUA in the side chain (Fig. 1). Following acetolysis of CPS–PG, the majority of the polymer was degraded but Glc and Rha were detected in the high MW fraction, indicative of incomplete acetolysis of 1,4 linkages (Fig. 8A). No GlcUA was identified in this fraction (Fig. 8A), demonstrating that all 1,6 bonds had been cleaved. Likewise, no GlcNAc was present in the high MW fraction, demonstrating that it was linked to CPS by a 1,6 bond (Fig. 8B). The lower MW fraction contained the majority of the Glc and Rha (Fig. 8C), and all of the GlcUA (Fig. 8C) and GlcNAc (Fig. 8D). The acetolysis labile nature of the CPS–PG linkage is indicative of a 1,6 glycosidic bond. As detailed in Discussion, the collective results provide strong evidence for linkage occurring between the C-6 of GlcNAc in the PG and C-1 of Glc in the CPS backbone.
Fig. 8.
The CPS–PG linkage is susceptible to acetolysis. GC/MS analysis of CPS–PG following acetolysis. (A) Total ion chromatogram of the high MW fraction. (B) Selective ion monitoring of the high MW fraction at m/z 173 revealed no GlcNAc. (C) Total ion chromatogram of the low MW fraction. (D) Selective ion monitoring of the low MW fraction at m/z 173. kCounts, kilocounts.
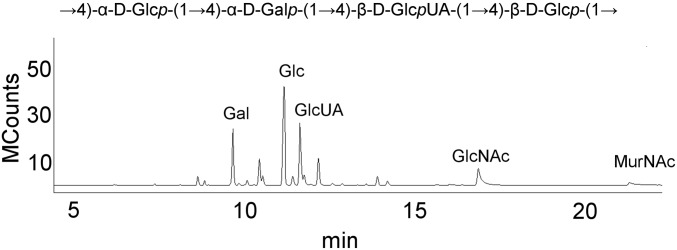
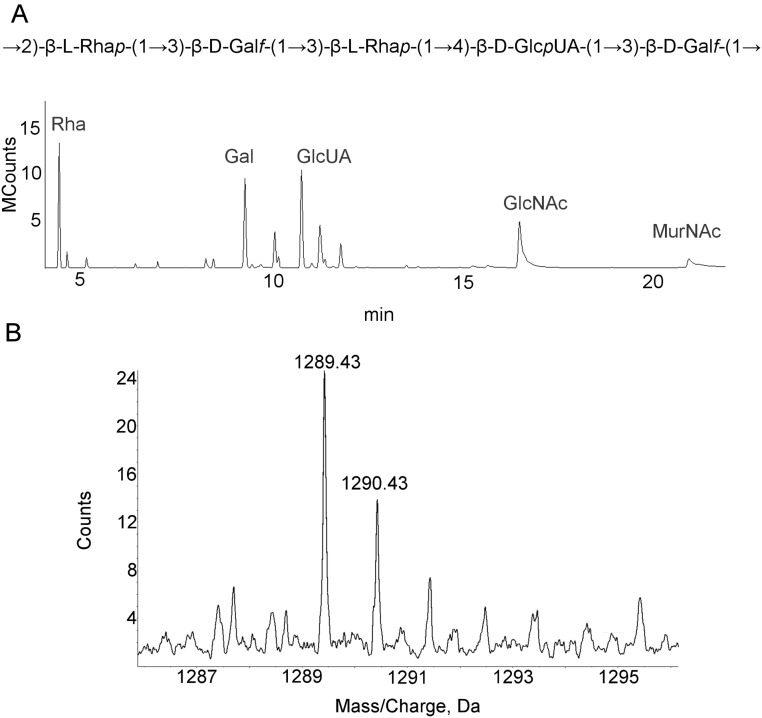
Direct Glycosidic Bonds Mediate CPS–PG Linkages of Other S. pneumoniae Serotypes.
More than 75% of the 96 known S. pneumoniae Wzy serotypes, including serotype 2, initiate CPS synthesis with Glcp (3, 4). To examine the CPS–PG linkages in serotype 8, which also initiates with Glcp (Fig. S2), and serotype 31, which initiates with galactose (Galf) (Fig. S3A), isolated CPS–PG was treated with HF and purified by ultrafiltration. GC/MS analysis of the high MW fractions demonstrated only the CPS and PG sugars in the expected ratios (Figs. S2 and S3A). The absence of ribitol in this fraction was indicative of HF cleavage of the phosphodiester bonds of teichoic acid repeat units. Q-TOF MS of CPS–PG from serotype 31 revealed a peak corresponding to the mass expected for a single repeat unit of CPS linked by a direct glycosidic bond to a PG disaccharide (Fig. S3B). These results demonstrate the lack of phosphodiester bonds and oligosaccharide linkers in these CPS structures.
Fig. S2.
The serotype 8 CPS–PG linkage does not involve phosphodiester bonds or an oligosaccharide linker. GC/MS of high MW fraction from HF-treated CPS–PG. The serotype 8 structure is from ref. 48. MCounts, megacounts.
Fig. S3.
The serotype 31 CPS–PG linkage does not involve phosphodiester bonds or an oligosaccharide linker. (A) GC/MS of high MW fraction from HF-treated CPS–PG. The serotype 31 structure is from ref. 49. (B) Mass spectrum of intact CPS–PG analyzed by triple Q-TOF MS. The peaks are indicative of a single repeat unit of CPS linked to a PG disaccharide. MCounts, megacounts.
Discussion
Bacterial CPS synthesis occurs by three mechanisms: ABC transporter dependent (Gram negatives), synthase dependent (Gram positives), and Wzy dependent (Gram negatives and Gram positives) (9, 10). Cell surface attachment is the final step in each of these processes. In Gram-negative bacteria, linkage is to the outer membrane, either via a linker and a phosphatidylglycerol anchor (ABC transporter pathway) (32) or as a result of ionic interactions with an outer membrane protein that serves as a nucleation point (Wzy pathway) (14). In Gram-positive bacteria, cytoplasmic membrane linkage via phosphatidylglycerol occurs in the synthase pathway (33). In the Wzy pathway, CPS synthesized on Und-P is transferred to PG, and there is also evidence for transfer to an alternate membrane acceptor (18).
The most detailed descriptions of PG–polysaccharide linkages are for the teichoic acids, teichuronic acids, and mycobacterial arabinogalactans. The reducing ends of these polymers are linked to the MurNAc C-6 through a phosphodiester bond and, for teichoic acids and arabinogalactan, an oligosaccharide linker (polysaccharide–oligosaccharide–phosphodiester–MurNAc) (19–24). Similar components may comprise the S. agalactiae type III CPS–PG structure, except that this linkage is proposed to be CPS–phosphodiester–oligosaccharide–GlcNAc (16). However, complete analyses to confirm such a structure have not been reported. It was suggested that, like teichoic acid, the S. aureus CPS may be linked to the MurNAc C-6 (34); however, no experimental evidence has been presented for this possibility. Additional information regarding any aspect of CPS linkages to the cell wall is sparse.
The present study provides a detailed description of a CPS–PG linkage and demonstrates that, unlike other surface polysaccharide–PG linkages, it is mediated by a direct glycosidic bond. The results provide a clear demonstration that the phosphodiester bonds and oligosaccharide linkers common to other polysaccharide–PG linkages are missing here. Evidence for the lack of a phosphodiester bond came from resistance of the linkage to HF and absence of detectable phosphorous by either 31P NMR or [32P]-phosphate metabolic labeling. Likewise, there is no evidence for an oligosaccharide linker as: (i) GC/MS analyses demonstrated only CPS- and PG-specific sugars in the expected ratios, and (ii) the molecular mass of a single CPS repeat unit linked to a PG disaccharide was consistent with the presence of only these sugars.
Susceptibility of the CPS–GlcNAc linkage to acetolysis is indicative of a 1,6 glycosidic bond, and is consistent with observations making linkage to carbons other than the GlcNAc C-6 unlikely, as discussed above. During CPS synthesis, Glcp is the reducing sugar following addition of Glc-1-P to Und-P to form β-D-Glc-1-P-P-Und (11). As demonstrated here, the Glc anomeric C-1 remains bonded to another molecule in CPS–PG, as sodium borodeuteride reduction did not produce a deuterated glucitol. The observation of a single, complete CPS repeat unit linked to GlcNAc is consistent with a glycosyltransferase that recognizes the reducing end of CPS and forms a Glc-1,6-GlcNAc linkage; any other mechanism would likely require two enzymes to cleave and transfer an oligosaccharide internal to the polymer chain. Cleavage at the reducing end, between the Glc and terminal P of Und-P-P, would leave the latter intact. Similar to the polymerization reaction in reducing end polysaccharide synthesis (35), this would allow for recycling of Und-P following hydrolysis of the pyrophosphate bond.
Glcp is the most common initiating sugar in CPS synthesis in S. pneumoniae and other Gram-positive bacteria, but other sugars can be used (3). The observation that the CPS–PG linkages of serotypes 2, 8, and 31, which initiate with Glcp, Glcp, and Galf, respectively, are the same, suggests a common linking mechanism may occur across serotypes. The S. pneumoniae enzyme responsible for linking CPS to PG has not been identified, but proteins of the LytR–CpsA–Psr family are proposed to play a role in this and other bacteria (34, 36). The initial suggestion for a ligase activity of these proteins was based on copurification of a polyprenol phosphate, along with phosphotransferase activity associated with recombinant CpsA from S. pneumoniae (37). However, CPS attachment is not affected in S. pneumoniae mutants where cpsA alone is mutated (15, 36, 38) and, as shown here, linkage of CPS to PG does not involve phosphoryl transfer. Thus, proteins other than, or in addition to, CpsA must be involved.
The enzyme catalyzing the CPS–PG linkage is expected to be a glycosyltransferase that uses a polyprenol pyrophosphate sugar donor (i.e., a non-Leloir enzyme) and possesses an extracytoplasmic domain. Such domains are present in the S. pneumoniae Wzy polymerase, Wzx transporter, CpsA, CpsC, and the initiating glycosyltransferases of the WchA family (including CpsE for serotype 2) (3). Of these, only the Wzy and WchA enzymes are known to catalyze glycosidic linkages, and only Wzy is a non-Leloir enzyme. WchA enzymes use nucleotide sugar donors (Leloir enzymes) and their known active sites are in the cytoplasm. Similar to the proposed CPS–PG transfer mechanism, Wzy recognizes the reducing end of Und-P-P–linked polymers and generates a glycosidic linkage to a single Und-P-P–linked repeat unit. A bifunctional Wzy, capable of recognizing both the PG and CPS acceptor substrates, could potentially form the CPS–PG linkage. The 96 Wzy CPS serotypes comprise 44 serogroups, wherein the serogroups contain immunologically and chemically related serotypes (3, 4). Wzy proteins exhibit high diversity, with 40 homology groups that are largely serogroup specific (3). These enzymes could potentially provide the specificity necessary for the recognition of such diverse polymers, and the generation of a similar linkage to PG across serotypes.
CPS transfer to PG appears to occur essentially at random, as the polymer sizes on the membrane and cell wall are similar and range from very small to very large (15) (Fig. S1). Indeed, the results of the present study demonstrate that a single CPS repeat unit can be transferred to PG. Simultaneous modification of adjacent MurNAc and GlcNAc residues with teichoic acid and CPS, respectively, appears rare, as neither 32P nor immunoreactive teichoic acid colocalizes with CPS on PG disaccharides, and only low levels of teichoic acid sugars copurify with CPS–PG.
The structure of the S. pneumoniae CPS linkage is distinct among PG-linked polysaccharides. Its identification opens the way for comparison with other CPS–PG linkages and provides the foundation for identifying the linking enzyme and potential inhibitors of its activity.
Materials and Methods
S. pneumoniae serotype 2 strain D39 was used for CPS isolation. Other strains, growth conditions, procedures for 32P incorporation, and immunoblotting are described in SI Materials and Methods. Monosaccharide analyses by GC/MS were performed essentially as described previously (39) and are detailed in SI Materials and Methods.
CPS–PG Preparation and Carbohydrate Treatments.
Protoplast and cell wall fractions were generated by incubation of bacteria with mutanolysin and autolysin (LytA). CPS–PG was purified from the cell wall fractions using DEAE cellulose anion exchange chromatography. Details for enzyme digestions, HF treatment, sodium borodeuteride reduction, Smith degradation (29), and acetolysis (as in ref. 40 with minor modifications) are provided in SI Materials and Methods. High and low MW products were separated using ultrafiltration (Amicon; 3,000 Da cutoff).
Structural Analyses.
Purified CPS–PG samples were analyzed using an AB Sciex 5600 Triple-TOF mass spectrometer. Data were collected in the high resolution TOF-MS positive ion mode over a mass range of 400–3,000 m/z. Postacquisition analysis was carried out using PeakView Software version 1.2 (AB Sciex). One-dimensional 31P-NMR was performed using a 500-MHz AVANCE III HD spectrometer with a triple-resonance broadband inverse (TBI) probe; 1,024 scans were collected. Additional details of both procedures are provided in SI Materials and Methods.
SI Materials and Methods
Bacterial Strains and Growth Conditions.
The S. pneumoniae serotype 2 parent D39 has been described (41). The serotype 8 isolate (DBL8) was kindly provided by David Briles, University of Alabama at Birmingham, Birmingham, AL. The serotype 31 isolate (3016-06) was obtained from the Centers for Disease Control and Prevention and kindly provided by Moon Nahm, University of Alabama at Birmingham. S. pneumoniae strains were grown statically at 37 °C in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY; components from Difco), or on blood agar plates [blood agar base (Becton Dickinson) containing 3% defibrinated sheep blood (Colorado Serum Company)], or tryptic soy agar with 5% sheep blood (Becton Dickinson). Blood agar plates were incubated at 37 °C in a candle jar. Escherichia coli DH5α and its LytA-expressing derivative TLlyt2 (see below) were grown at 37 °C in broth (1% tryptone, 0.5% yeast extract, 0.5% NaCl, and 0.1% glucose) with shaking or on agar plates (15 g agar/liter broth). Media were supplemented with erythromycin (30 µg/mL for E. coli and 0.3 µg/mL for S. pneumoniae) or ampicillin (100 µg/mL for E. coli), as appropriate.
Cloning and Expression of lytA for Purification of the N-Acetylmuramoyl-L-Alanine Amidase LytA (Autolysin).
The complete lytA gene with its native promoter was PCR amplified from D39 using primers PLytFw (5′-CTGGGTTGCCATGACAG-3′) and PLytRv (5′-CCTAATAATATGCGCTGTTCTG-3′). This product was ligated into pCR2.1–TOPO and transformed into E. coli DH5α to generate the lytA expression strain TLlyt2. For protein production, 500-mL cultures were grown in broth with ampicillin to midexponential phase and the bacteria were harvested by centrifugation at 20,000 × g. The cell pellet was washed in PBS (137 mM NaCl, 2.7 mM KCl, 5.4 mM disodium phosphate, 1.8 mM monopotassium phosphate, pH 7.4). After a further centrifugation, the pellet was suspended in 5 mL 10 mM EDTA. The cells were sonicated on ice using a Sonic Dismembrator 300 (Fisher) at 60% power, 10 times for 10 s each pulse, with a 60-s pause interval. The sonicate was centrifuged at 34,000 × g for 15 min to remove cellular debris, and the supernatant was filtered through a 0.22-µm filter to remove any remaining cells and cell debris. LytA was purified using affinity chromatography with DEAE, a choline analog, for interaction with the LytA choline binding domain (42). One milliliter of supernatant was added to a 1-mL packed column of DE53 DEAE-cellulose (Whatman) that had been equilibrated with 20 mM phosphate buffer, pH 7.0. The column was washed with 12-column volumes of 20 mM phosphate buffer, pH 7.0 containing 1.5 M NaCl. LytA was eluted with 1-column volume 20 mM phosphate buffer, pH 7.0 containing 1.5 M NaCl and 2% choline. This fraction was dialyzed against 50 mM Tris, pH 7.6 containing 30% glycerol, concentrated using Amicon Ultra 10 K filtration to 40 µL, and stored at 4 °C. Purity was assessed by Coomassie brilliant blue staining of proteins separated by SDS-10% polyacrylamide gel electrophoresis. Activity was examined by overnight incubation of purified LytA with the S. pneumoniae LytA-negative mutant AL2 (43) suspended in isotonic buffer [20% sucrose, 50 mM MgSO4, 50 mM Tris (pH 7.4)] at room temperature. Protoplast formation was confirmed by microscopic examination.
Generation of Peptidoglycan Deacetylase Mutant.
A pgdA insertion–duplication mutant was made using previously described methods (44). Primers P1F (5′-GTAGACTAGGACGTGGCAGACAC-3′) and P1R (5′-CCATGCAGACAAGTGTTGGTCAGAG-3′) were used to amplify an internal fragment of pgdA from S. pneumoniae D39 that was then ligated into pCR2.1-TOPO, subcloned into the S. pneumoniae suicide vector pJY4164 (44) and transformed into D39 with selection for erythromycin resistance (45). The PgdA mutant phenotype of lysozyme sensitivity (28) was confirmed by the addition of 100 µg/mL lysozyme to growing cultures and observation of a decrease in optical density over time (Fig. S1B).
Preparation of CPS Linked to PG.
Crude CPS covalently linked to PG was prepared from midexponential phase cultures (∼3 × 108 cfu/mL) grown in THY essentially as described previously (15), with the addition of LytA. Bacteria from 1,500-mL cultures were harvested by centrifugation, suspended in PBS, and centrifuged. The wash step was repeated. The cell pellet was concentrated 100-fold from the original culture volume in an isotonic buffer [20% sucrose, 50 mM MgSO4, 50 mM Tris (pH 7.4)]. Forty units per milliliter of mutanolysin (Sigma) and 2 µL/mL of purified recombinant LytA (see above) were added and the cells were incubated at room temperature overnight. Protoplast formation was confirmed microscopically. The protoplasts were removed by centrifugation at 16,000 × g for 20 min at 4 °C. For CPS–PG purification, the cell wall-containing supernatant was filtered (0.22 µm), dialyzed (3,000 Da cutoff) against water, and then passed over a DEAE-cellulose column. After a 10-column volume water wash, fractions were eluted with a 0–1 M gradient of ammonium acetate pH 7.6 with 60 mL of each eluent at a 1.0 mL/min flow rate. CPS-containing fractions identified by the Dische-Shettles methyl pentose assay to detect rhamnose (46) were dialyzed by ultrafiltration (Amicon Ultra 10 K). For hydrofluoric acid (HF) treatment, fractions containing CPS–PG were dried by vacuum centrifugation and dissolved in 48% HF for 36 h at 4 °C. Acid was removed under a stream of N2. The high molecular weight CPS and lower molecular weight products were separated by ultrafiltration (3,000 Da cutoff). Subsequent to the determination of HF resistance of the CPS–PG linkage, all CPS–PG preparations were treated with HF before further analysis to remove the phosphodiester-containing teichoic acid.
Carbohydrate Treatments.
Carbohydrates were reduced using 10 mg/mL NaBD4 for 2 h in 100 mM ammonium hydroxide. Reactions were stopped with acetic acid and drying under N2. Samples were washed multiple times with 5% methanolic acetic acid, dried, and then washed with methanol and dried. For β-N-acetylglucosaminidase treatment of purified CPS linked to PG, samples were incubated with the enzyme [50 mM (Sigma) in sodium acetate buffer, pH 5.5, and 10 mM MgCl2] at 37 °C for 7 d. Released monosaccharides were separated from the intact PG-linked CPS by ultrafiltration (3,000 Da cutoff). Smith degradation was performed as previously described (29).
Acetolysis.
Acetolysis was performed as previously described (40) with some modifications. Dried CPS–PG was dissolved in 100-μL anhydrous formamide in a Teflon-lined screw cap test tube. One milliliter of a 1:1 (vol/vol) mixture of acetic anhydride and pyridine was added and the cap was sealed. This solution was incubated at 40 °C for 24 h, dried under a stream of N2, suspended in 1 mL methanol, and dried under N2. The peracetylated polysaccharide was dissolved in 1 mL of a 10:10:1 (vol/vol/vol) solution of acetic anhydride, acetic acid, and sulfuric acid, incubated at 40 °C for 7 d, and evaporated under a stream of N2 following addition of 100 μL pyridine. The resulting viscous residue was dissolved in 1 mL methanol and dried under N2. This wash step was repeated twice more. The acetolyzed product was extracted with 1 mL chloroform and washed five times with 2 mL H2O. The organic layer was dried under a stream of N2 and the residue was dissolved in 2.2 mL methanol. To this solution, 136 μL 0.5 M methanolic sodium methoxide was added dropwise (47). After 1 h at room temperature, the reaction was terminated by the addition of 136 μL ethyl acetate. The samples were dried in vacuo and dissolved in 1 mL H2O. Higher molecular weight polysaccharide was separated from low molecular weight acetolyzed product by ultrafiltration (Amicon Ultra 3,000 Da), and each fraction was analyzed for component sugars by GC/MS.
GC/MS Analyses.
Samples to be analyzed by GC/MS were dried in vacuo, dissolved in 3 N methanolic HCl at 80 °C for 16 h in sealed 2-mL glass ampoules, and dried under vacuum centrifugation. The residue was washed several times in anhydrous methanol and dried. Amino sugars were N-acetylated by dissolving the sample in 200 μL methanol and adding 20 μL pyridine and 20 μL acetic anhydride in a sealed vial. After 20 min at room temperature, the samples were dried under vacuum, dissolved in methanol, and transferred to glass conical inserts in sample vials. After evaporation to dryness, samples were trimethylsilylated by dissolving in 100 μL Tri-Sil HTP reagent (Pierce) under argon for 30 min at 80 °C.
GC/MS was performed on a Varian 4000 GC-MS/MS system (Agilent Technologies) fitted with a VF-5 ms 30-m (0.25-mm internal diameter) capillary column. The flow rate was kept constant at 1 mL/min. Column temperature was maintained at 160 °C for 3 min and then increased to 260 °C at 3 °C/min and held at 260 °C for 2 min. The effluent was analyzed by MS, using the electron impact ionization mode.
32P Incorporation and Immunoblotting Analyses.
For 32P experiments, S. pneumoniae D39 was cultured in 8 mL THY supplemented with 200 μL of 1 mCi/mL [32P] phosphate salt. Cell wall and protoplast fractions were generated using bacteria from midexponential phase cultures as described above. Three pairs of 20-µL aliquots of each fraction were separated on a single SDS-10% polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane, which was then cut into thirds. One membrane was exposed to a phosphor screen (General Electric Healthcare) overnight, and bands were visualized in a Storm 820 phosphorimager (General Electric Healthcare). The other two membranes were immunoblotted using antisera specific for either serotype 2 CPS or teichoic acid. Analyses of CPS and teichoic acid by immunoblot were performed as previously described (15, 18).
Q-TOF MS Analysis.
Purified CPS–PG samples were analyzed at the UAB Targeted Metabolomics and Proteomics Laboratory using an AB Sciex 5600 Triple-Q-TOF mass spectrometer with direct infusion at a flow rate of 5 µL/min. The data were collected in the high-resolution Q-TOF-MS positive ion mode over a mass range of 400–3,000 m/z. The ion spray voltage was set at 2,300 V, the declustering potential was 30 V, and the interface temperature was set at 180 °C. Postacquisition analysis was carried out using PeakView Software version 1.2 (AB Sciex) for spectrum creation.
NMR Spectroscopy Analyses.
Samples were dried and suspended in 100% D2O (D, 99.96%). One-dimensional 31P-NMR was performed at the UAB Cancer Center High-Field NMR Facility using a 500 MHz AVANCE III HD spectrometer with a TBI probe. A total of 1,024 scans were collected.
Acknowledgments
We thank Yuriy Knirel for advice on acetolysis and Landon Wilson for expertise with Q-TOF MS instrumentation. This work was supported by NIH Awards R01 AI28457 (to J.Y.), T32 AI07041 (to T.R.L.), P30 CA13148 [University of Alabama at Birmingham (UAB) Cancer Center High-Field NMR Facility], and the Mizutani Foundation for Glycoscience (J.Y.). The UAB Targeted Metabolomics and Proteomics Laboratory is funded in part by NIH Awards S10 RR027822, P30 DK079337 (UAB O’Brien Acute Kidney Injury Center), P30 AR50948 (UAB Skin Disease Research Center), and the UAB Lung Health Center and UAB Center for Free Radical Biology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620431114/-/DCSupplemental.
References
- 1.Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun. 2010;78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Limoli DH, Jones CJ, Wozniak DJ. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr. 2015;3 doi: 10.1128/microbiolspec.MB-0011-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentley SD, et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2006;2:e31. doi: 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geno KA, Saad JS, Nahm MH. Discovery of novel pneumococcal serotype, 35D: A natural WciG-deficient variant of serotype 35B. J Clin Microbiol. 2017;55:1416–1425. doi: 10.1128/JCM.00054-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilishvili T, Noggle B, Moore MR. 2012 Pneumococcal Disease. VPD Surveillance Manual (Centers for Disease Control, Atlanta), 5th Ed, Chap 11. Available at https://www.cdc.gov/vaccines/pubs/surv-manual/chpt11-pneumo.pdf. Accessed May 2, 2017.
- 6.Hardy GG, Magee AD, Ventura CL, Caimano MJ, Yother J. Essential role for cellular phosphoglucomutase in virulence of type 3 Streptococcus pneumoniae. Infect Immun. 2001;69:2309–2317. doi: 10.1128/IAI.69.4.2309-2317.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magee AD, Yother J. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun. 2001;69:3755–3761. doi: 10.1128/IAI.69.6.3755-3761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson AL, et al. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun. 2007;75:83–90. doi: 10.1128/IAI.01475-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 10.Yother J. Capsules of Streptococcus pneumoniae and other bacteria: Paradigms for polysaccharide biosynthesis and regulation. Annu Rev Microbiol. 2011;65:563–581. doi: 10.1146/annurev.micro.62.081307.162944. [DOI] [PubMed] [Google Scholar]
- 11.Cartee RT, Forsee WT, Bender MH, Ambrose KD, Yother J. CpsE from type 2 Streptococcus pneumoniae catalyzes the reversible addition of glucose-1-phosphate to a polyprenyl phosphate acceptor, initiating type 2 capsule repeat unit formation. J Bacteriol. 2005;187:7425–7433. doi: 10.1128/JB.187.21.7425-7433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James DB, Gupta K, Hauser JR, Yother J. Biochemical activities of Streptococcus pneumoniae serotype 2 capsular glycosyltransferases and significance of suppressor mutations affecting the initiating glycosyltransferase Cps2E. J Bacteriol. 2013;195:5469–5478. doi: 10.1128/JB.00715-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James DB, Yother J. Genetic and biochemical characterizations of enzymes involved in Streptococcus pneumoniae serotype 2 capsule synthesis demonstrate that Cps2T (WchF) catalyzes the committed step by addition of β1-4 rhamnose, the second sugar residue in the repeat unit. J Bacteriol. 2012;194:6479–6489. doi: 10.1128/JB.01135-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bushell SR, et al. Wzi is an outer membrane lectin that underpins group 1 capsule assembly in Escherichia coli. Structure. 2013;21:844–853. doi: 10.1016/j.str.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bender MH, Cartee RT, Yother J. Positive correlation between tyrosine phosphorylation of CpsD and capsular polysaccharide production in Streptococcus pneumoniae. J Bacteriol. 2003;185:6057–6066. doi: 10.1128/JB.185.20.6057-6066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng L, Kasper DL, Krick TP, Wessels MR. Characterization of the linkage between the type III capsular polysaccharide and the bacterial cell wall of group B Streptococcus. J Biol Chem. 2000;275:7497–7504. doi: 10.1074/jbc.275.11.7497. [DOI] [PubMed] [Google Scholar]
- 17.Sørensen UB, Henrichsen J, Chen HC, Szu SC. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb Pathog. 1990;8:325–334. doi: 10.1016/0882-4010(90)90091-4. [DOI] [PubMed] [Google Scholar]
- 18.Xayarath B, Yother J. Mutations blocking side chain assembly, polymerization, or transport of a Wzy-dependent Streptococcus pneumoniae capsule are lethal in the absence of suppressor mutations and can affect polymer transfer to the cell wall. J Bacteriol. 2007;189:3369–3381. doi: 10.1128/JB.01938-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer W, Behr T, Hartmann R, Peter-Katalinić J, Egge H. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoid acid (C polysaccharide) Eur J Biochem. 1993;215:851–857. doi: 10.1111/j.1432-1033.1993.tb18102.x. [DOI] [PubMed] [Google Scholar]
- 20.Kojima N, Araki Y, Ito E. Structure of the linkage units between ribitol teichoic acids and peptidoglycan. J Bacteriol. 1985;161:299–306. doi: 10.1128/jb.161.1.299-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeil M, Daffe M, Brennan PJ. Evidence for the nature of the link between the arabinogalactan and peptidoglycan of mycobacterial cell walls. J Biol Chem. 1990;265:18200–18206. [PubMed] [Google Scholar]
- 22.Mosser JL, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970;245:287–298. [PubMed] [Google Scholar]
- 23.Neuhaus FC, Baddiley J. A continuum of anionic charge: Structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev. 2003;67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward JB. Teichoic and teichuronic acids: Biosynthesis, assembly, and location. Microbiol Rev. 1981;45:211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansson PE, Lindberg B, Anderson M, Lindquist U, Henrichsen J. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 2, a reinvestigation. Carbohydr Res. 1988;182:111–117. doi: 10.1016/0008-6215(88)84095-3. [DOI] [PubMed] [Google Scholar]
- 26.Yother J, Leopold K, White J, Fischer W. Generation and properties of a Streptococcus pneumoniae mutant which does not require choline or analogs for growth. J Bacteriol. 1998;180:2093–2101. doi: 10.1128/jb.180.8.2093-2101.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yother J. Integration of capsular polysaccharide biosynthesis with metabolic and virulence pathways in. In: Brogden KA, et al., editors. Streptococcus pneumoniae. Virulence Mechanisms of Bacterial Pathogens. 4th Ed. ASM Press; Washington, DC: 2006. pp. 51–65. [Google Scholar]
- 28.Vollmer W, Tomasz A. The pgdA gene encodes for a peptidoglycan N-acetylglucosamine deacetylase in Streptococcus pneumoniae. J Biol Chem. 2000;275:20496–20501. doi: 10.1074/jbc.M910189199. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein IJ, Hay GW, Lewis BA, Smith F. Controlled degradation of polysaccharides by periodate oxidation, reduction, and hydrolysis. In: Whistler RL, editor. Methods in Carbohydrate Chemistry. Vol 5. Academic Press; New York: 1965. pp. 361–370. [Google Scholar]
- 30.Aspinall GO. The selective degradation of carbohydrate polymers. Pure Appl Chem. 1977;49:1105–1134. [Google Scholar]
- 31.Rosenfeld L, Ballou CE. Acetolysis of disaccharides: Comparative kinetics and mechanism. Carbohydr Res. 1974;32:287–298. [Google Scholar]
- 32.Willis LM, et al. Conserved glycolipid termini in capsular polysaccharides synthesized by ATP-binding cassette transporter-dependent pathways in Gram-negative pathogens. Proc Natl Acad Sci USA. 2013;110:7868–7873. doi: 10.1073/pnas.1222317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cartee RT, Forsee WT, Yother J. Initiation and synthesis of the Streptococcus pneumoniae type 3 capsule on a phosphatidylglycerol membrane anchor. J Bacteriol. 2005;187:4470–4479. doi: 10.1128/JB.187.13.4470-4479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan YG, Kim HK, Schneewind O, Missiakas D. The capsular polysaccharide of Staphylococcus aureus is attached to peptidoglycan by the LytR-CpsA-Psr (LCP) family of enzymes. J Biol Chem. 2014;289:15680–15690. doi: 10.1074/jbc.M114.567669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robbins PW, Bray D, Dankert BM, Wright A. Direction of chain growth in polysaccharide synthesis. Science. 1967;158:1536–1542. doi: 10.1126/science.158.3808.1536. [DOI] [PubMed] [Google Scholar]
- 36.Eberhardt A, et al. Attachment of capsular polysaccharide to the cell wall in Streptococcus pneumoniae. Microb Drug Resist. 2012;18:240–255. doi: 10.1089/mdr.2011.0232. [DOI] [PubMed] [Google Scholar]
- 37.Kawai Y, et al. A widespread family of bacterial cell wall assembly proteins. EMBO J. 2011;30:4931–4941. doi: 10.1038/emboj.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morona JK, Morona R, Paton JC. Attachment of capsular polysaccharide to the cell wall of Streptococcus pneumoniae type 2 is required for invasive disease. Proc Natl Acad Sci USA. 2006;103:8505–8510. doi: 10.1073/pnas.0602148103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calix JJ, et al. Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae Serotype 20 strains: Discovery of a new pneumococcal serotype. J Biol Chem. 2012;287:27885–27894. doi: 10.1074/jbc.M112.380451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oyamada H, et al. Structural analysis of cell wall mannan of Candida sojae, a new yeast species isolated from defatted soybean flakes. Arch Microbiol. 2008;189:483–490. doi: 10.1007/s00203-007-0339-1. [DOI] [PubMed] [Google Scholar]
- 41.Avery OT, Macleod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanz JM, Lopez R, Garcia JL. Structural requirements of choline derivatives for ‘conversion’ of pneumococcal amidase. A new single-step procedure for purification of this autolysin. FEBS Lett. 1988;232:308–312. doi: 10.1016/0014-5793(88)80759-2. [DOI] [PubMed] [Google Scholar]
- 43.Berry AM, Lock RA, Hansman D, Paton JC. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect Immun. 1989;57:2324–2330. doi: 10.1128/iai.57.8.2324-2330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yother J, Handsome GL, Briles DE. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J Bacteriol. 1992;174:610–618. doi: 10.1128/jb.174.2.610-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardy GG, Caimano MJ, Yother J. Capsule biosynthesis and basic metabolism in Streptococcus pneumoniae are linked through the cellular phosphoglucomutase. J Bacteriol. 2000;182:1854–1863. doi: 10.1128/jb.182.7.1854-1863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dische Z, Shettles LB. A new spectrophotometric test for the detection of methylpentose. J Biol Chem. 1951;192:579–582. [PubMed] [Google Scholar]
- 47.Slomiany BL, Meyer K. Isolation and structural studies of sulfated glycoproteins of hog gastric mucosa. J Biol Chem. 1972;247:5062–5070. [PubMed] [Google Scholar]
- 48.Jones JKN, Perry MB. The structure of the type VIII pneumococcus specific polysaccharide. J Am Chem Soc. 1957;79:2787–2793. [Google Scholar]
- 49.Batavyal L, Roy N. Structure of the capsular polysaccharide of Diplococcus pneumoniae type 31. Carbohydr Res. 1983;119:300–302. [Google Scholar]