Abstract
Cervical radiculopathic pain is a very common symptom that may occur with cervical spondylosis. Mechanical allodynia is often associated with cervical radiculopathic pain and is inadequately treated with current therapies. However, the precise mechanisms underlying cervical radiculopathic pain-associated mechanical allodynia have remained elusive. Compelling evidence from animal models suggests a role of large-diameter dorsal root ganglion neurons and plasticity of spinal circuitry attached with Aβ fibers in mediating neuropathic pain. Whether cervical radiculopathic pain condition induces plastic changes of large-diameter dorsal root ganglion neurons and what mechanisms underlie these changes are yet to be known. With combination of patch-clamp recording, immunohistochemical staining, as well as behavioral surveys, we demonstrated that upon chronic compression of C7/8 dorsal root ganglions, large-diameter cervical dorsal root ganglion neurons exhibited frequent spontaneous firing together with hyperexcitability. Quantitative analysis of hyperpolarization-activated cation current (Ih) revealed that Ih was greatly upregulated in large dorsal root ganglion neurons from cervical radiculopathic pain rats. This increased Ih was supported by the enhanced expression of hyperpolarization-activated, cyclic nucleotide-modulated channels subunit 3 in large dorsal root ganglion neurons. Blockade of Ih with selective antagonist, ZD7288 was able to eliminate the mechanical allodynia associated with cervical radiculopathic pain. This study sheds new light on the functional plasticity of a specific subset of large-diameter dorsal root ganglion neurons and reveals a novel mechanism that could underlie the mechanical allodynia associated with cervical radiculopathy.
Keywords: Cervical radiculopathic pain, mechanical allodynia, large-diameter dorsal root ganglion neurons, Ih current
Background
Chronic cervical radiculopathic pain (CRP) often results from neck trauma, cervical spondylosis, and intervertebral disc diseases. 1 CRP is characterized, in humans, by ongoing spontaneous pain and mechanical hypersensitivity to normally non-painful (allodynia) and painful (hyperalgesia).2,3 It has increasing prevalence and is inadequately controlled by currently available drugs, perhaps at least partially due to a lack of mechanism-based treatments.2,3 Currently, the mechanisms of CRP have remained unresolved. Several preclinical models have been developed to investigate the pathophysiology of CRP including our recently established chronic compression of C7/8 dorsal root ganglion (DRGs) model. 4 The advantage of this model is that it reflected actual pathophysiological processes of radiculopathic pain resulting from a chronic compression of cervical DRGs or its near nerve root seen in clinic. On this model, we recently demonstrated that among nociceptive DRG neurons, a specific subset of IB4− Aδ-fiber neurons but not IB4+ or IB4− C-fiber neurons exhibited hyperexcitability with persistent spontaneous discharges, and more importantly, displayed neuronal hypersensitivity to mechanical stimulation in CRP rats. This suggests a crucial role for this subset of IB4− Aδ-fiber nociceptive DRG neurons in the mechanical hyperalgesia associated with CRP. 4 However, mechanical allodynia under CRP conditions still remains elusive.
Apart from nociceptive DRG neurons, compelling evidence from animal models suggests a role of large Aβ-fiber, heavily myelinated neurons in mediating neuropathic pain. For example, in vitro recordings from dorsal roots and cell bodies of DRG showed spontaneous discharges and enhanced excitability of large Aβ-type neurons in various neuropathic pain models.5–9 More recently, plasticity of spinal circuitry involving primary afferent Aβ fibers has been shown in gating mechanical allodynia associated with tissue inflammation and nerve injury.10–14 We are therefore interested to know whether specific population of large-diameter DRG neurons is implicated in the development of CRP. If so, what mechanisms are involved in the large DRG neurons-mediated mechanical allodynia observed in CRP conditions?
To address the above questions, we set out to establish CRP models in rats and record ex vivo from intact large-diameter DRG neurons, comparing the excitability of neurons in CRP versus sham animals. The present study demonstrated that mechanical allodynia in a model of cervical radiculopathy in rats, is mediated at least in part by increased excitability in large-diameter DRG neurons, which is dependent on increased Ih and upregulation of hyperpolarization-activated, cyclic nucleotide-modulated (HCN) channels subunit 3 (HCN3). This sheds new light on the functional plasticity of a specific subset of large DRG neurons and reveals a novel mechanism that could underlie the mechanical allodynia associated with cervical radiculopathy.
Results
Chronic compression of cervical DRGs induces mechanical allodynia and thermal hyperalgesia as well as spontaneous pain in rats
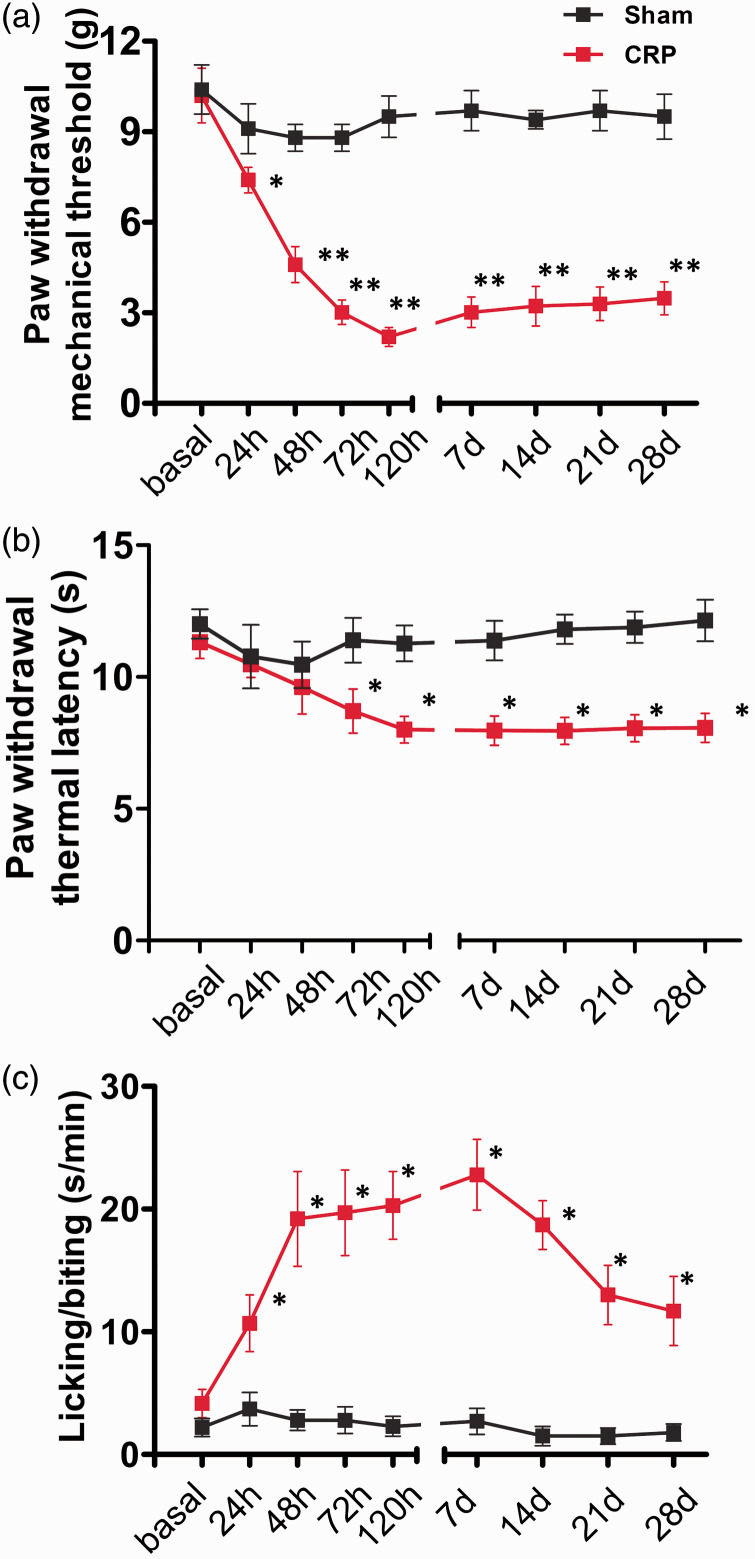
All the rats received either chronic compression of C7/C8 DRGs or sham operation. In general, all the animals subjected to compression appeared in good health and did not show any signs of autotomy. Development of chronic CRP was assessed at 1, 2, 3, 5, 7, 14, 21, 28 days following unilateral compression of C7/C8 DRGs. Following chronic compression of cervical DRGs, all the rats demonstrated dramatic mechanical allodynia in the ipsilateral forepaws, manifesting as a robust drop in the mechanical response threshold to Von Frey filaments, as compared to sham group (Figure 1(a), n = 10, P < 0.05). This mechanical allodynia starts to occur at one day postoperation, reaching to a steady sensitized state at three days post and persisting all the way up to the latest test period. In contrast to marked mechanical allodynia, compressed rats showed a minor degree of thermal hyperalgesia as well. Paw withdrawal latency to radiant heat stimuli was slightly but significantly reduced after chronic compression of C7/C8 DRGs, while this did not occur in sham group (Figure 1(b), n = 10, P < 0.05). Besides dramatic mechanical allodynia and minor thermal hyperalgesia, compressed rats also displayed pronounced spontaneous pain behaviors, as indicated by frequent lifting, licking, and biting of the injured forepaws (Figure 1(c), n = 10, P < 0.05). Analysis of the duration spent in licking/biting of the forepaws demonstrated a strong increase of licking/biting duration in rats subjected to chronic compression of the cervical DRGs, as compared to sham group. It can be inferred from the above that chronic compression of C7/C8 DRGs developed remarkable mechanical allodynia, slight thermal hyperalgesia, and obvious spontaneous pain behavior in rats. These pain behaviors observed in the present study is quite similar to what we have reported in a previous study. 4 More importantly, the pain behaviors manifested in rat CRP models mimic the symptoms of CRP patients observed in clinic.
Figure 1.
Chronic compression of C7/8 DRGs induces dramatic mechanical hypersensitivity, slight thermal hyperalgesia, and frequent spontaneous nociception in rats. (a) Magnitude and time course of mechanical hypersensitivity (allodynia and hyperalgesia) to plantar Von Frey filaments application observed in CRP rats as compared to sham rats. (b) Showing the development of slight thermal hyperalgesia to radiant heat stimuli in CRP rats. (c) Development of spontaneous nociception in CRP rats, manifesting as lifting, licking, biting of the injured forepaws. Duration of the time spent in all these behaviors was recorded. All data are expressed as mean ± SEM. *P < 0.05 as compared to their basal levels using repeated measures one-way analysis of variance.
CRP: cervical radiculopathic pain.
Changes of membrane properties and excitability in large-diameter DRG neurons after chronic compression of cervical DRGs
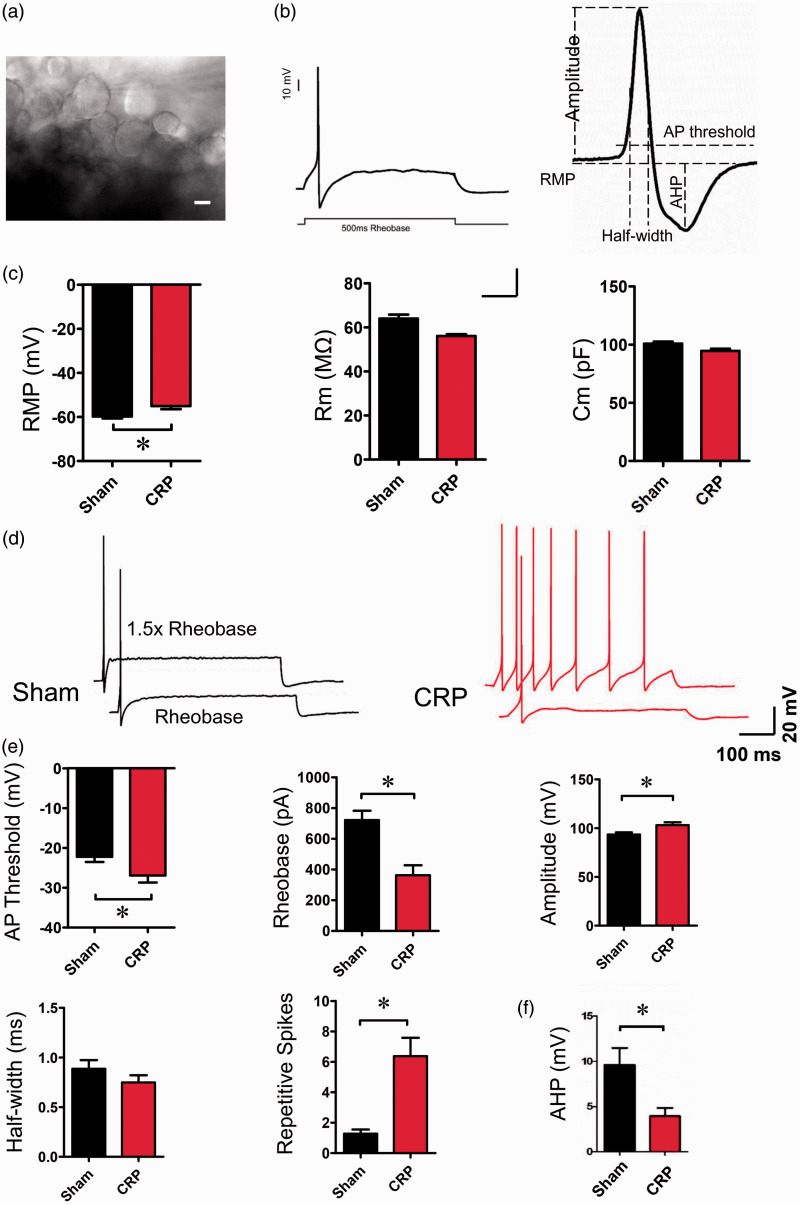
To explore whether large-diameter DRG neurons are involved in the development of CRP, we analyzed the membrane properties of large DRG neurons from ex vivo intact DRG preparations (Figure 2(a) and (b)). A total of 122 large-size (>40 µm) DRG neurons, containing 60 neurons from sham group and 62 from CRP group were recorded. Following chronic compression of C7/C8 DRGs, large DRG neurons exhibited dramatic changes in membrane properties and enhanced excitability. When examining passive membrane properties, we demonstrated that compressed large DRG neurons get more depolarized in resting membrane potential when compared to control neurons (Figure 2(c), P < 0.05, n = 29 vs. 26). The membrane capacitance and input resistance were not significantly different between two groups (Figure 2(c), P > 0.05, n = 19 vs. 19).
Figure 2.
Membrane properties and cell excitability of large-diameter DRG neurons in sham and CRP rats. (a) Showing large-diameter DRG neurons from an ex vivo intact C7/8 DRG preparation are recorded. (b) A representative action potential (AP) of whole-cell configuration evoked by a depolarizing current injection (left panel) and analysis of various AP parameters are shown (right panel). (c) Analysis of the passive membrane properties including resting membrane potential (RMP), membrane resistance (Rm), and membrane capacitance (Cm) in sham and CRP group. (d) Typical traces showing the AP response to a depolarizing current injection at intensity of rheobase and 1.5X reheobase from sham and CRP DRG neurons, respectively. (e) Analysis of the active membrane properties including various AP parameters, i.e. AP threshold, rheobase, AP amplitude, AP half-width, and number of repetitive spikes to depolarizing current injection at 1.5X rheobase intensity. (f) Measurement of AHP from sham and CRP group. All data are expressed as mean ± SEM. Scale bars represent 25 µm in (a). *P < 0.05 indicates statistically significant differences between control and CRP rats (analysis of variance).
CRP: cervical radiculopathic pain.
The active membrane properties of large DRG neurons from CRP rats represented significant differences compared with controls. The augmented excitability in large DRG neurons from CRP rats is manifested as lowered action potential (AP) threshold, reduced rheobase, and increased AP amplitude (Figure 2(d) and (e), n = 20 vs. 22, P < 0.05). More importantly, the mean frequency of spikes induced by a depolarizing step current at 1.5X rheobase intensity was much higher in large DRG neurons tested from CRP rats than that from control rats (Figure 2(d) and (e), n = 18 vs. 18, P < 0.05). Furthermore, to determine the effect of afterhyperpolarization (AHP) on the repetitive firing of CRP DRG neurons, the properties of AHP in these large DRG neurons were analyzed. As shown in Figure 2(f), the amplitude of AHP from CRP DRG neurons was much lowered than that from control DRG neurons (Figure 2(f)). These results inferred us that hyperexcitability of large DRG neurons might be closely related to the development of CRP.
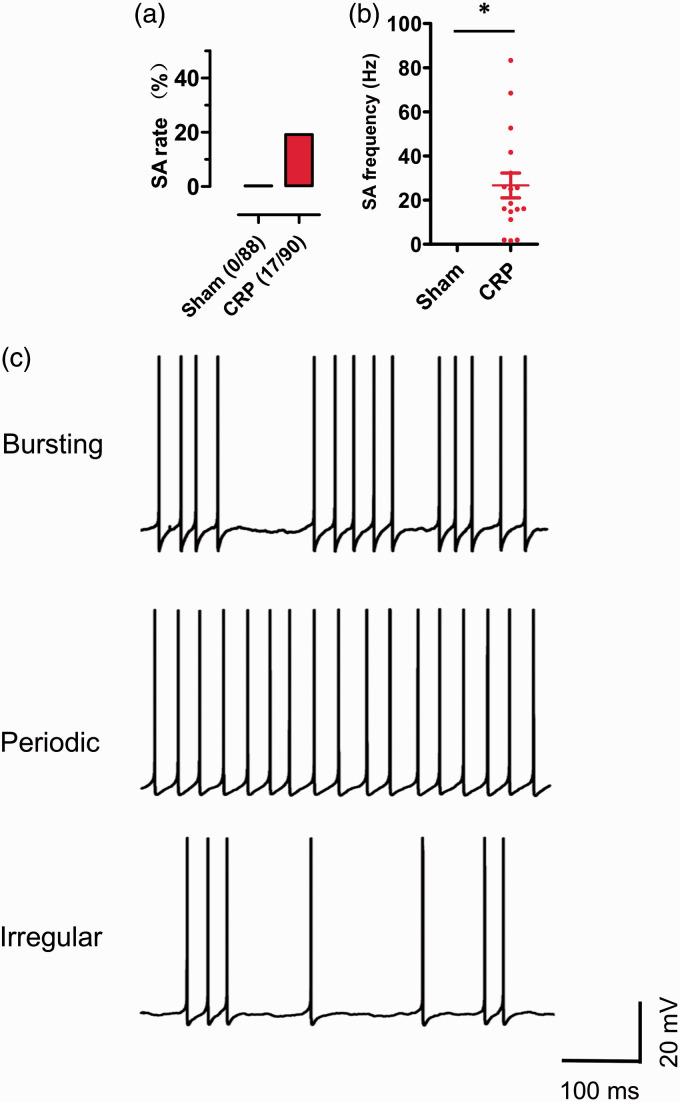
Chronic compression of cervical DRGs evokes high incidence of ongoing spontaneous firing in large DRG neurons in rats
Several lumbar radiculopathic pain models have reported that the injured lumbar DRG neurons displayed ectopic spontaneous discharges, most of the fibers were assumed to be A type.5,15 Most recently, we demonstrated that small IB4− Aδ-fiber DRG neurons exhibited frequent spontaneous firings in CRP models. 4 We further asked whether CRP induces spontaneous activity in large-diameter DRG neurons. As shown in Figure 3(a), upon testing at three days after chronic compression of cervical DRGs, 19% (17/90) of large DRG neurons showed frequent spontaneous activity (Figure 3(a), n = 17). The frequency of spontaneous firings average to be 26.66 ± 5.60 Hz (Figure 3(b), n = 17). This spontaneous activity can last for ∼2 h until whole-cell configuration was unstable and lost. In contrast, none of large DRG neurons from sham group showed spontaneous activity (Figure 3(a) and (b), n = 88). When examining the dynamic features of interspike interval series, three different spontaneous firing patterns were revealed, that is bursting, periodic, and irregular activity (Figure 3(c), n = 17). Among them, neurons with bursting activity accounts for about 47%, with intermittences during continuous spikes at mean firing frequency of 17.90 Hz (Figure 3(c), upper panel); 35.4% of neurons displayed periodic activity with high frequency of 50.73 Hz (Figure 3(c), middle panel). The other 17.6% belongs to irregular activity at low frequency of 1.86 Hz (Figure 3(c), lower panel).
Figure 3.
The occurrence of spontaneous activity (SA) in large-diameter DRG neurons in rats subjected to chronic compression. (a) Comparison of the percentage of cells displaying SA in large DRG neurons from sham and CRP group. (b) Summary of SA frequency in large DRG neurons in sham and CRP rats. (c) Three firing patterns of SA in large DRG neurons from CRP rats are shown: bursting activity (upper panel), periodic discharges (middle panel), and irregular firing (lower panel). *P < 0.05 indicates statistically significant differences between control and CRP rats (analysis of variance).
CRP: cervical radiculopathic pain.
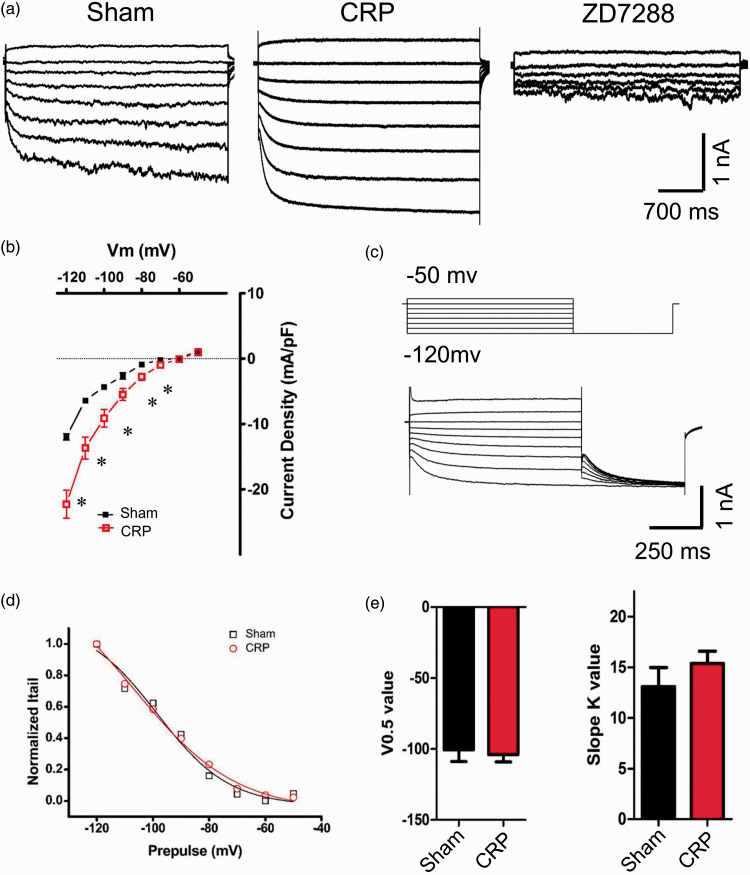
Chronic compression of cervical DRGs induces upregulation of Ih in large DRG neurons
Accumulating evidence has shown that hyperpolarization-activated cation current (Ih) plays a crucial role in affecting the excitability and driving repetitive firing in primary sensory neurons and pain pathophysiology.16–19 We are therefore interested to know whether Ih expression is altered following chronic compression of cervical DRGs and whether it is involved in the hyperexcitability of large DRG neurons from CRP rats. Consistent with previous reports, Ih displays as an inward current in response to hyperpolarizing potentials of −50 to −120 mV from a holding potential of −60 mV (Figure 4(a)). Following chronic compression of C7/C8 DRGs, Ih is greatly increased in magnitude, as compare to sham group (Figure 4(a), n = 7 vs. 7). This current can be blocked by bath application of ZD7288, a selective blocker of Ih (15 µM)20–22 (Figure 4(a)).Figure 4(b) shows I–V relations in large DRG neurons derived from sham and CRP rats. When normalized to cell capacitance, the median Ih current density in large DRG neurons from CRP rats was markedly enhanced compared to that from sham rats (Figure 4(b), n = 7 vs. 7, P < 0.05). However, the reversal potential for Ih between CRP (−33.64 ± 5.17 mV) and sham group (−30.94 ± 6.93 mV) are not significantly different.
Figure 4.
Upregulation of Ih currents in large DRG neurons from CRP rats. (a) Representative traces of Ih recorded in large DRG neurons from sham (left) and CRP group (middle) in response to hyperpolarizing voltage steps of −50 mV to −120 mV from a holding potential at −60 mV. Ih currents recorded were blocked by bath application of ZD7288 (15 μM). (b) I–V relations of Ih currents in large DRG neurons from sham and CRP group. Note that CRP induces upregulation of Ih currents greatly. (c) The activation curve of Ih from sham and CRP DRG neurons was constructed by measuring tail currents at −120 mV after application of prepulse potentials between −50 and −120 mV (middle inset) and fitted with a Boltzmann equation. (d) and (e) The midpoint (V0.5) for activation of Ih and slope K value in the CRP neurons (red) were not different from that recorded in sham control neurons (black). All data are expressed as mean ± SEM. *P < 0.05 as compared to sham control rats.
CRP: cervical radiculopathic pain.
Voltage-dependent activation and inactivation are physiologically important characteristics of ion channels that can directly influence the excitability of neurons. Consistent with our previous study, 4 the activation curve of Ih was constructed by measuring tail currents at −120 mV after application of prepulse potentials between −50 and −120 mV and fitted with a Boltzmann equation (Figure 4(c)). Analysis of the midpoint (V0.5) for activation of Ih in CRP large DRG neurons (−104.0 ± 5.1 mV) did not show significant variation from sham DRG neurons (−100.6 ± 8.2 mV) (Figure 4(d) and (e), n = 7 vs. 7, P > 0.05). Slope K value was not different between two groups as well (Figure 4(e), n = 7 vs. 7, P > 0.05). This is inconsistent with our previous report in IB4− Aδ neurons where CRP condition produces obvious shift of V0.5 of Ih in depolarizing direction. 4 This discrepancy might be due to different changes in kinetics of HCN channels responsible for Ih in different subgroups of DRG neurons under CRP states. Taken together, chronic compression of cervical DRGs produced obvious upregulation of Ih in large DRG neurons, this extends our previous report in nociceptive IB4− Aδ DRG neurons under CRP condition. 4
Chronic compression of cervical DRGs produces enhanced expression of HCN3 subunits in large DRG neurons
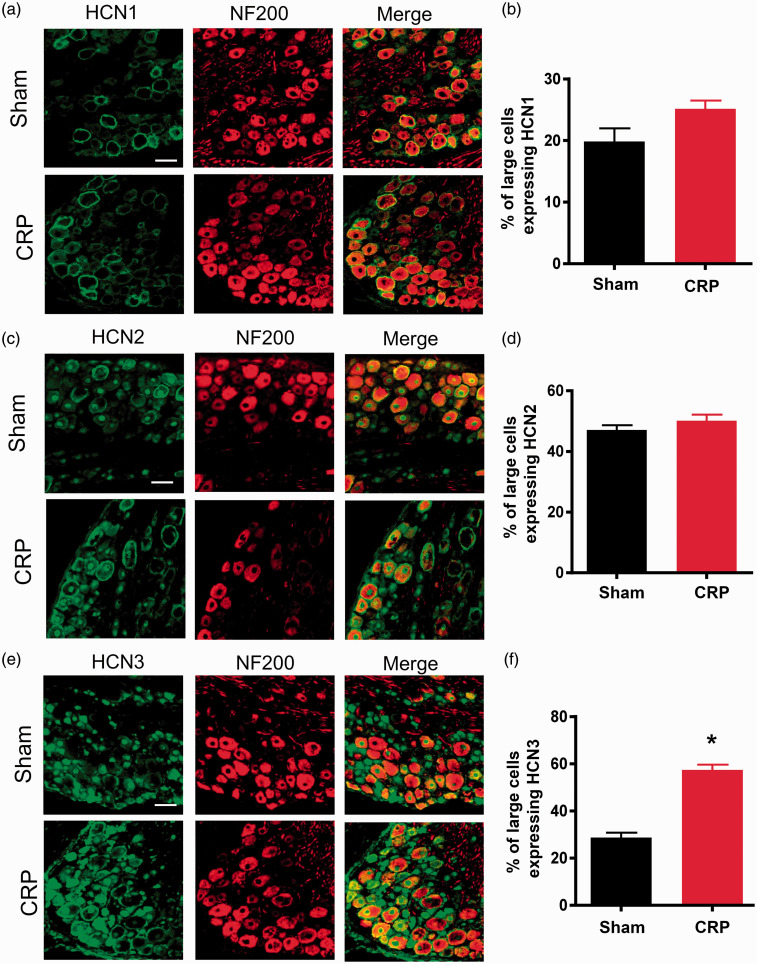
Having established that chronic C7/C8 DRGs compression-induced increase in excitability of large DRG neurons is associated with increased Ih amplitude and density in these neurons, we sought to determine whether chronic cervical DRGs compression alters HCN channel protein expression in DRG neurons, particularly in large DRG neurons. To address this question, we performed double immunofluorence staining with HCN channel protein subunits and neurofilament 200 (NF200), a marker for large-diameter DRG neurons. It has been identified that the family of HCN channels is responsible for Ih.23–25 The four presently known family members (HCN1–4) share substantial homology but differ significantly in their activation kinetics and responsiveness to cAMP.26–28 We focused on HCN1–3 because these isoforms are clearly expressed in rat DRG neurons, whereas the expression of HCN4 is uncertain. 29 Consistent with a previous report by Kouranova et al., 29 HCN1 channels are seen to be expressed in large cervical DRG neurons, manifesting as colocalization with NF200. Following chronic compression of cervical DRGs, HCN1 channels did not exhibit significant changes in NF200-positive large-diameter DRG neurons, as compared to sham group (see typical examples in Figure 5(a) and quantitative summary in Figure 5(b), P > 0.05, n = 23–25 sections/four rats). Similarly, neither HCN2 expression showed alteration in NF200-positive large DRG neurons upon chronic compression (see typical examples in Figure 5(c) and quantitative summary in Figure 5(d), P > 0.05, n = 23–25 sections/four rats). In striking contrast, HCN3 immunoreactivity is found to be enhanced dramatically in NF200-labeled large DRG neurons, as compared to sham group (see typical examples in Figure 5(e) and quantitative summary in Figure 5(f), P < 0.05, n = 23–25 sections/four rats). The above results suggest that enhanced expression of HCN3 in large-diameter DRG neurons might contribute to upregulation of Ih recorded from CRP rats.
Figure 5.
Changes of expression of different HCN channels subunits in CRP rats compared to sham controls. (a), (c), and (e) Double immunofluorescence staining of DRG neurons with NF200 antibody together with HCN1 (a), HCN2 (c) and HCN3 (e) antibody, respectively, in CRP and sham control rats. (b) Quantitative summary showing that HCN1 immunoreactivity in NF200-positive large-diameter DRG neurons was not changed upon CRP operation. (d) HCN2 immunoreactivity in NF200-positive large DRG neurons was not significantly different between sham and CRP group. (f) HCN3 immunoreacitivity in NF200-positive large DRG neurons was largely increased in CRP rats compared to sham controls. All data are represented as mean ± SEM. Scale bars represent 50 µm. *P < 0.05 as compared to control rats.
CRP: cervical radiculopathic pain.
Blockade of Ih currents could eliminate mechanical allodynia and spontaneous pain observed in CRP rats
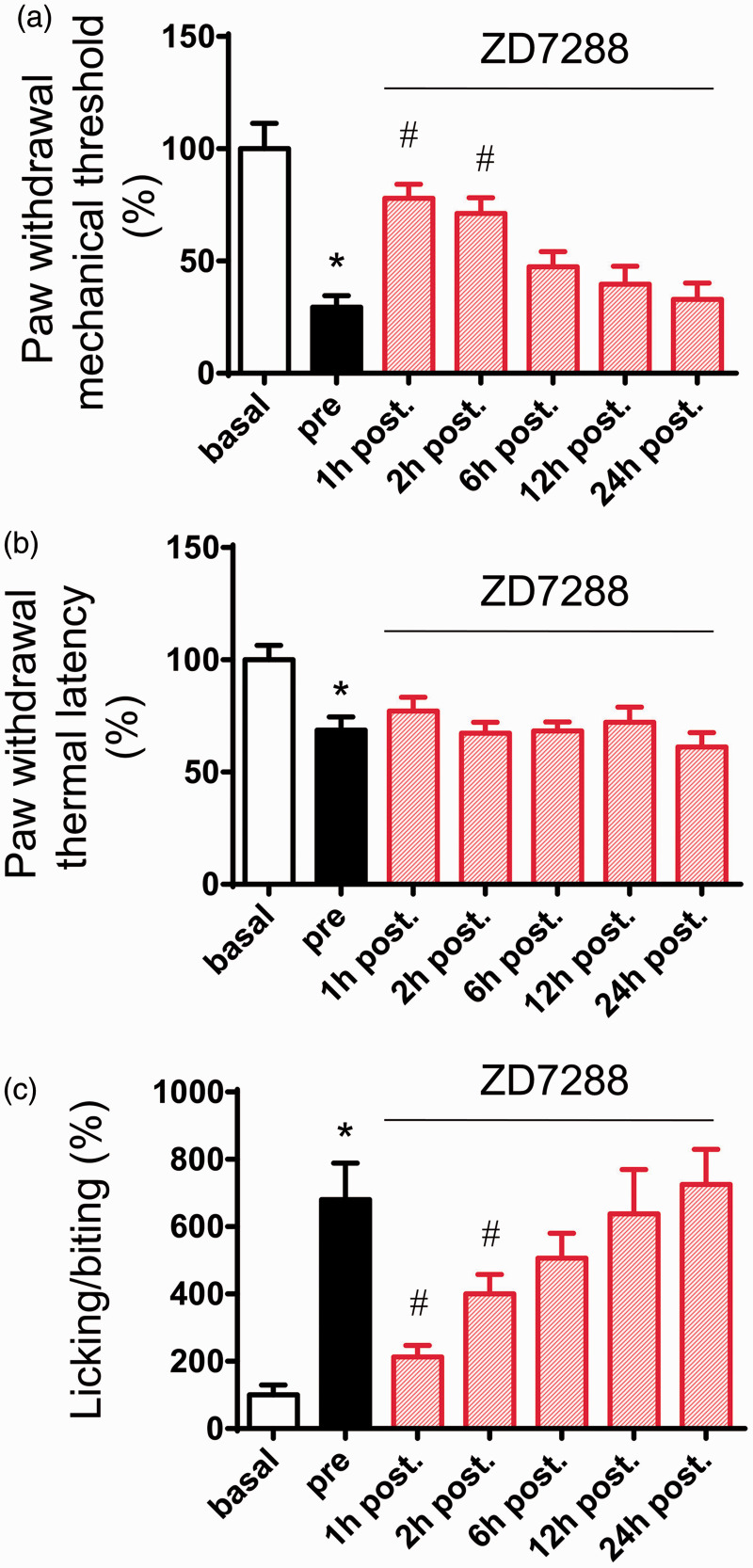
We next addressed whether these findings bear relevance to pain-related behavior in vivo. Intrathecal (i.t.) administration has been shown to be a feasible way to affect DRG sensory neurons.30,31 In the present study, ZD7288 (30 µg) was intrathecally delivered at three days after chronic compression of cervical DRGs when stable pain hypersensitivity has been established. As shown in Figure 6, i.t ZD7288 caused a dramatic inhibition of mechanical allodynia to von Frey hairs (Figure 6(a), n = 6, P < 0.05) but not thermal hyperalgesia to radiant heat stimuli (Figure 6(b), n = 6, P > 0.05). In parallel, spontaneous pain was greatly reduced by i.t. ZD7288 as well (Figure 6(c), n = 6, P < 0.05). Taken together, these results revealed a crucial role of upregulated Ih currents in spontaneous activity and hyperexcitability in large DRG neurons and behavioral hypersensitivity associated with CRP.
Figure 6.
Effects of i.t. injection of ZD7288 (30 µg) on behavioral mechanical allodynia (a), thermal hyperalgesia (b), and spontaneous pain (c) in CRP rats. Note that mechanical allodynia and spontaneous pain were largely alleviated by ZD7288, while thermal hyperalgesia was not (n = 6 for each group). All data are expressed as mean ± SEM. *P < 0.05 as compared to basal state, #P < 0.05 as compared to prestate (before ZD7288).
Discussion
Similar to symptoms of CRP in humans, chronic compression of cervical DRGs in rats produced dramatic mechanical allodynia and hyperalgesia as well as frequent spontaneous nociception. On CRP model in the present study, large DRG neurons exhibited augmented excitability and persistent spontaneous discharges. Ih current and HCN3 expression was dramatically upregulated in large DRG neurons from CRP rats. This study sheds new light on the functional plasticity of a specific subset of large DRG neurons and reveals a novel mechanism that could underlie the mechanical allodynia associated with cervical radiculopathy.
A number of preclinical models have been developed that attempt to mimic lumbar radiculopathic pain, including chronic compression of lumbar DRGs with a steel metal rod 5 or SURGIFLO, 32 application of nucleus pulposus to the lumbar DRGs and/or adjacent nerve roots with or without the inclusion of additional maneuvers such as root compression, pinch, or brief DRG displacement.33–35 However, given the different location and function, CRP cannot simply be assumed as the similar versions of the same cascades to the pain occurred in the lumbar spine. Hence, in the present study, we utilized a model involving chronic compression of cervical DRGs with a steel metal rod to mimic the symptoms of CRP. The advantage of this model over other current CRP models36–38 is that it reflected actual pathophysiological processes of radiculopathic pain resulting from a chronic compression of cervical DRGs or its near nerve root seen in clinic. On this model, the compressed rats display a dramatic mechanical allodynia and hyperalgesia together with spontaneous pain and slight thermal hyperalgesia, which is similar to the symptoms observed in patients suffering from CRP.
One of the most striking findings of the present study is that large-diameter cervical DRG neurons showed ongoing spontaneous activity and enhanced excitability following chronic compression of C7/8 DRGs. This extends one of our most recent studies showing that small nociceptive DRG neurons, especially IB4− Aδ DRG neurons displays hyperexcitability under CRP conditions. 4 These consecutive studies suggest that both small nociceptive Aδ DRG neurons and large nonnociceptive DRG neurons became hypersensitive under CRP conditions, which may mediate the mechanical hyperalgesia and allodynia associated with CRP. Similar to hyperexcitability of cervical large-diameter DRG neurons under CRP condition, ectopic spontaneous activity together with increased neuronal excitability in lumbar large DRG neurons have been demonstrated under various pathological states, e.g., lumbar DRG compression,8,15 chronic constriction injury of sciatic nerve, 39 peripheral, or central axotomy. 40 In clinic, mechanical allodynia is a typical symptom annoying the lives of patients with both cervical and lumbar radiculopathy. Thus, it indicates that hyperexcitability of large-diameter DRG neurons in cervical or lumbar segments might mediate mechanical allodynia observed under cervical or lumbar radiculopathic pain states. However, whether they share the similar underlying mechanisms of hyperexcitability remains to be further investigated.
Ionic mechanisms have been revealed in the generation of spontaneous activity and hyperexcitability in DRG neurons in various lumbar neuropathic pain states. This includes the involvement of A-type K+ currents, 41 TTX-resistant Na+ currents 42 , and Ca2+ currents. 43 In the present study, however, we demonstrated that CRP is associated with upregulated Ih density in large-diameter DRG neurons. This increased Ih might contribute to lower AHP amplitude in large DRG neurons derived from CRP rats as compared to sham rats, which in turn makes DRG neurons prone to repetitive firing and thus contributes to hyperexcitability of DRG neurons under CRP states. It has been reported in other types of neurons that Ca2+-activated K+ channels contributes to the AHP as well.44–46 Although we did not evaluate the changes of Ca2+-activated K+ channels in the present study, its contribution to the lowered AHP and DRG hyperexcitability observed in CRP rats cannot be excluded.
Furthermore, the exaggerated Ih is strongly supported by dramatic enhanced expression of HCN3 in large-diameter DRG neurons in CRP rats. It is assumed that HCN1, HCN3, and HCN2 channels are activated with different V0.5, being at −70 mV, −77 to −95 mV, and −95 mV, respectively.47–50 Increased expression of HCN3 in large-diameter DRG neurons is consistent with the V0.5 of activation of Ih in large DRG neurons observed in the present study. This extends our recent study showing that enhancement of Ih density has been found in small IB4- Aδ DRG neurons, which is supported by upregulation of HCN1 and HCN3 channels in small IB4- DRG neurons. 4 In striking contrast, chronic inflammation induced an upregulation of Ih in C but not Aδ nociceptors, which is dependent on increased expression of HCN2 channels. 51 These results inferred us that enhancement of Ih density in large DRG neurons contributes, at least in partial, to the spontaneous activity and hyperexcitability associated with CRP.
More recently, plasticity of spinal circuitry involving primary afferent Aβ fibers has been shown in gating mechanical allodynia associated with tissue inflammation and nerve injury.10–14 Previous studies have linked spontaneous afferent firing to the development of spontaneous pain, allodynia, and hyperalgesia.52,53 Thus, increased spontaneous activity and hyperexcitability in large-diameter DRG neurons may result in a substantial barrage of excitatory input to spinal dorsal horn; this exaggerated inputs together with plastic changes of spinal circuitry attached with Aβ fibers collectively contribute to mechanical allodynia associated with cervical radiculopathy.
In summary, our results revealed a novel hypothesis that mechanical allodynia in a model of cervical radiculopathy in rats, is mediated at least in part by increased excitability in large-diameter DRG neurons, which is dependent on increased Ih current and HCN3 expression. Therefore, targeting Ih current in large-diameter DRG neurons might represent a new potential therapeutic candidate for the treatment of chronic pain, including cervical radiculopathy.
Materials and methods
Animal and surgery
Experiments were performed on adult, male Sprague-Dawley rats (100–150 g). The rats were free access to food and water under a 12 h-day/12-h night cycle. All experimental protocols were approved by an institutional animal use and protection committee. The methods for CRP operation were described in detailed elsewhere. 4 In brief, the skin was incised on the left side along cervical vertebrae and the left paraspinal muscles separated from the mammillary process and the transverse process. The intervertebral foramen of vertebrae C6–7 and vertebrae C7T1 was clearly exposed. A fine, stainless L-shaped (at an angle of 60°) steel metal rod (3 mm in length, 0.63 mm in diameter) inserted into intervertebral foramen for steady compression of corresponding DRGs at a rostral direction at an angle of 90° to the dorsal middle line. After the rods were in place, the muscle and skin layers were sutured with administration of about 200 mg antibiotics.
Pain behavior tests
All behavioral testing was carried out in a double-blinded manner. Mechanical sensitivity was tested with manual application of Von Frey hairs to the plantar surface of forepaw. Each filament was applied 10 times, and the paw withdrawal response frequency (the percentage of positive responses to the stimulus) was recorded. The force of a particular filament required to elicit 50% frequency of paw withdrawal was expressed as the mechanical threshold. Thermal sensitivity was tested by application of infrared heat to the plantar surface of forepaw, and the response latency was measured from an automated device readout, as described previously in literature.4,54–57 To test spontaneous pain behavior, the rat was placed on the surface of a 2 mm-thick glass covered by a transparent Plexiglas box. The spontaneous pain was determined by counting the number of seconds the rat spent in tickling, lifting, sucking, and biting the injured forepaw during 1 min interval every 20 min. The mean duration of the spontaneous pain in an hour was recorded.
Intact DRG preparations and whole-cell patch-clamp recording
The procedure for an ex vivo preparation of intact whole mount DRG has been described elsewhere.4,58,59 In brief, C7 and C8 DRGs were carefully removed at postoperative three to five days from CRP rats or sham control rats. After removing the connective tissue, the ganglia were digested with a mixture of 0.4 mg/mL trypsin (Sigma) and 1.0 mg/ml type-A collagenase (Sigma) for 45 min at 37℃. The intact ganglia were then incubated in artificial cerebrospinal fluid (ACSF) oxygenated with 95% O2 and 5% CO2 at 28℃ for at least 1 h before transferring them to the recording chamber. DRG neurons were visualized with a 40X water-immersion objective using a microscope (BX51WI; Olympus, Tokyo, Japan) equipped with infrared differential interference contrast optics. Whole-cell current and voltage recordings were acquired with an Axon700B amplifier (Molecular Devices Corporation, Sunnyvale, CA, USA). Patch pipettes (4–7 MΩ) were pulled from borosilicate glass capillaries on P-97 puller (Sutter Instruments, USA). The series resistance was 10 to 20 MΩ. Neurons were selected for further study if they had a resting membrane potential negative than −50 mV and if they exhibited overshooting APs.
The large-diameter C7 and C8 DRG neurons (>40 µm) were recorded. The ACSF contained (in mM): 124 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.0 MgCl2, 2.0 CaCl2, 25 NaHCO3, and 10 Glucose. The pipette solution contained (in mM): 140 KCl, 2 MgCl2, 10 Hepes, 2 Mg-ATP, pH 7.4. Osmolarity was adjusted to 290 to 300 mOsm. All chemicals were obtained from Sigma, St. Louis, MO, USA. Data were acquired with a digidata 1322A acquisition system (molecular devices) using pCLAMP 9.0 software. Signals were low pass filtered at 5 kHz, sampled at 10 kHz, and analyzed offline.
Ih current analysis
For recording of Ih, ACSF and normal intrapipette solution mentioned above were used. To activate Ih, hyperpolarizing potentials of −50 to −120 mV were delivered in increments of 10 mV from a holding potential of −60 mV for duration of 3.5 s. The current was obtained by the steady state current minus current at the beginning of the step. Tail current analysis was taken to analyze the steady state inactivation of Ih. The current amplitude was converted into conductance using Ohm’s law. Conductance was normalized and fitted with a Boltzmann function: G = Gmax/(1 + exp((V0.5 − Vm)/K)), where V0.5 is the half-activation voltage, and K is the slope factor.
Immunofluorescence labeling
Rats were perfused with 4% paraformaldehyde, and DRG was extracted and postfixed overnight in 4% paraformaldehyde. Immunofluorescence staining was performed on cryosections (16 µm) using standard reagents and protocols. Briefly, the sections were incubated with a solution containing 0.3% Triton X-100 and 1% bovine serum albumin for 30 min at room temperature. The sections then were incubated with NF200 antibody (1:200, Sigma) and anti-HCN1, anti-HCN2, anti-HCN3 antibody, respectively (1:200, Alomone, Israel) for 24 h. After three rinses, the sections were further incubated with the secondary antibodies Alexa Fluor 594 (goat anti-mouse IgG, 1:500, Invitrogen) and Alexa Fluor 488 (donkey anti-rabbit IgG, 1:500, Invitrogen) for 4 h at room temperature. The sections were then mounted with anti-fade medium and stored at 4℃. All images were captured with an Olympus confocal microscope and processed with Adobe Photoshop software.
I.t. administration
The rats were anaesthetized by 1% pentobarbital sodium. A midline incision was made along T8 to L2, and the muscle attached to spinous process removed. With the tip of the sharp scissor, a 1-mm hole on the right vertebra was made until dura, and clean cerebrospinal fluid (CSF) was exposed. The catheter was send through the hole slowly until it reached C7/C8. After a flush with 10 μl artificial CSF, the exterior end of catheter was sealed by heat. The rats were allowed to recover for three days. Any rat showing motor deficits would be excluded. ZD7288 was dissolved in distilled water and administrated in a volume of 5 μl for i.t. injection. After the administration, another 5 μl artificial CSF was injected to flush the catheter before the heat seal.
Statistical analysis
All data are expressed as mean ± SEM. Student’s t-test or an analysis of variance for random measures was carried out, followed by either a post hoc Fisher’s test or Dunnett’s test. P < 0.05 was considered significant.
Author contributions
Da-Lu Liu carried out electrophysiological and behavioral experiments. Xu Wang, Wen-Guang Chu, and Na Lu performed immunostaining experiments and analysis. Wen-Juan Han and Yi-Kang Du participated in behavioral tests. San-Jue Hu and Sheng-Xi Wu provided technical advice and instruction on the experiment. Rou-Gang Xie and Ceng Luo designed, drafted, and finished the final manuscript. Da-Lu Liu, Xu Wang, and Wen-Guang Chu contributed equally to this work. All authors approved the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Natural Science Foundation of China (NSFC) grants (No. 31471059, 31671088) to Ceng Luo; grants from State Key Laboratory of Neuroscience, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (No. SKLN-2015B02) to Ceng Luo, National Science and Technology Support Program of China (No. 31400949) to Rou-Gang Xie.
References
- 1.Carette S, Fehlings MG. Clinical practice. Cervical radiculopathy. N Engl J Med 2005; 353: 392–399. [DOI] [PubMed] [Google Scholar]
- 2.Radhakrishnan K, Litchy WJ, O’Fallon WM, et al. Epidemiology of cervical radiculopathy. A population-based study from Rochester, Minnesota, 1976 through 1990. Brain 1994; 117: 325–335. [DOI] [PubMed] [Google Scholar]
- 3.Schoenfeld AJ, George AA, Bader JO, et al. Incidence and epidemiology of cervical radiculopathy in the United States military: 2000 to 2009. J Spinal Disord Tech 2012; 25: 17–22. [DOI] [PubMed] [Google Scholar]
- 4.Liu DL, Lu N, Han WJ, et al. Upregulation of Ih expressed in IB4-negative Aδ nociceptive DRG neurons contributes to mechanical hypersensitivity associated with cervical radiculopathic pain. Sci Rep 2015; 5: 16713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain 1998; 77: 15–23. [DOI] [PubMed] [Google Scholar]
- 6.Kim YI, Na HS, Kim SH, et al. Cell type-specific changes of the membrane properties of peripherally-axotomized dorsal root ganglion neurons in a rat model of neuropathic pain. Neuroscience 1998; 86: 301–309. [DOI] [PubMed] [Google Scholar]
- 7.Liu CN, Wall PD, Ben-Dor E, et al. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain 2000; 85: 503–521. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol 1999; 82: 3359–3366. [DOI] [PubMed] [Google Scholar]
- 9.Zhu YF, Henry JL. Excitability of Aβ sensory neurons is altered in an animal model of peripheral neuropathy. BMC Neuroscience 2012; 13: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Y, Dong H, Gao Y, et al. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest 2013; 123: 4050–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan B, Cheng L, Bourane S, et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell 2014; 159: 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster E, Wildner H, Tudeau L, et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 2015; 85: 1289–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peirs C, Williams SP, Zhao X, et al. Dorsal horn circuits for persistent mechanical pain. Neuron 2015; 87: 797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gangadharan V, Kuner R. Unravelling spinal circuits of pain and mechanical allodynia. Neuron 2015; 87: 673–675. [DOI] [PubMed] [Google Scholar]
- 15.Song XJ, Hu SJ, Greenquist KW, et al. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol 1999; 82: 3347–3358. [DOI] [PubMed] [Google Scholar]
- 16.Chaplan SR, Guo HQ, Lee DH, et al. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci 2003; 23: 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo L, Chang L, Brown SM, et al. Role of peripheral hyperpolarization-activated cyclic nucleotide-modulated channel pacemaker channels in acute and chronic pain models in the rat. Neuroscience 2007; 144: 1477–1485. [DOI] [PubMed] [Google Scholar]
- 18.Emery EC, Young GT, Berrocoso EM, et al. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 2011; 333: 1462–1466. [DOI] [PubMed] [Google Scholar]
- 19.Ingram SL, Williams JT. Modulation of the hyperpolarization-activated current (Ih) by cyclic nucleotides in guinea-pig primary afferent neurons. J Physiol 1996; 492: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.BoSmith RE, Briggs I, Sturgess NC. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol 1993; 110: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasparini S, DiFrancesco D. Action of the hyperpolarization-activated current (Ih) blocker ZD 7288 in hippocampal CA1 neurons. Pflugers Arch 1997; 435: 99–106. [DOI] [PubMed] [Google Scholar]
- 22.Yao H, Donnelly DF, Ma C, et al. Upregulation of the hyperpolarization-activated cation current after chronic compression of the dorsal root ganglion. J Neurosci 2003; 23: 2069–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santoro B, Grant SG, Bartsch D, et al. Interactive cloning with the SH3 domain of N-src identifies a new brain specific ion channel protein, with homology to eag and cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 1997; 94: 14815–14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santoro B, Liu DT, Yao H, et al. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 1998; 93: 717–729. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig A, Zong X, Jeglitsch M, et al. A family of hyperpolarization-activated mammalian cation channels. Nature 1998; 393: 587–591. [DOI] [PubMed] [Google Scholar]
- 26.Santoro B, Tibbs GR. The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann NY Acad Sci 1999; 868: 741–764. [DOI] [PubMed] [Google Scholar]
- 27.Ishii TM, Takano M, Ohmori H. Determinants of activation kinetics in mammalian hyperpolarization-activated cation channels. J Physiol 2001; 537: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaupp UB, Seifert R. Molecular diversity of pacemaker ion channels. Annu Rev Physiol 2001; 63: 235–257. [DOI] [PubMed] [Google Scholar]
- 29.Kouranova EV, Strassle BW, Ring RH, et al. Hyperpolarization-activated cyclic nucleotide-gated channel mRNA and protein expression in large versus small diameter dorsal root ganglion neurons: correlation with hyperpolarization-activated current gating. Neuroscience 2008; 153: 1008–1019. [DOI] [PubMed] [Google Scholar]
- 30.Li G, Ma F, Gu Y, et al. Analgesic tolerance of opioid agonists in mutant mu-opioid receptors expressed in sensory neurons following intrathecal plasmid gene delivery. Mol Pain 2013; 9: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Kang L, Li G, et al. Intrathecal leptin inhibits expression of the P2X2/3 receptors and alleviates neuropathic pain induced by chronic constriction sciatic nerve injury. Mol Pain 2013; 9: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu X, Yang L, Wang S, et al. A rat model of radicular pain induced by chronic compression of lumbar dorsal root ganglion with SURGIFLO. Anesthesiology 2008; 108: 113–121. [DOI] [PubMed] [Google Scholar]
- 33.Strong JA, Xie W, Bataille FJ, et al. Preclinical studies of low back pain. Mol Pain 2013; 9: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces europhysiologic and histologic changes in porcine cauda equina nerve roots. Spine (Phila Pa 1976) 1993; 18: 1425–1432. [PubMed] [Google Scholar]
- 35.Kawakami M, Tamaki T, Weinstein JN, et al. Pathomechanism of pain-related behavior produced by allografts of intervertebral disc in the rat. Spine 1996; 21: 2101–2107. [DOI] [PubMed] [Google Scholar]
- 36.Hubbard RD, Winkelstein BA. Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: mechanical factors in painful neck injuries. Spine (Phila Pa 1976) 2005; 30: 1924–1932. [DOI] [PubMed] [Google Scholar]
- 37.Wu A, Lauschke JL, Morris R, et al. Characterization of rat forepaw function in two models of cervical dorsal root injury. J Neurotrauma 2009; 26: 17–29. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues-Filho R, Santos AR, Bertelli JA, et al. Avulsion injury of the rat brachial plexus triggers hyperalgesia and allodynia in the hindpaws: a new model for the study of neuropathic pain. Brain Res 2003; 982: 186–194. [DOI] [PubMed] [Google Scholar]
- 39.Zhao FY, Spanswick D, Martindale JC, et al. GW406381, a novel COX-2 inhibitor, attenuates spontaneous ectopic discharge in sural nerves of rats following chronic constriction injury. Pain 2007; 128: 78–87. [DOI] [PubMed] [Google Scholar]
- 40.Ma C, Shu Y, Zheng Z, et al. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol 2003; 89: 1588–1602. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 1999; 19: 4644–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amir R, Argoff CE, Bennett GJ, et al. The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain 2006; 7: S1–S29. [DOI] [PubMed] [Google Scholar]
- 43.Lu SG, Zhang XL, Luo ZD, et al. Persistent inflammation alters the density and distribution of voltage-activated calcium channels in subpopulations of rat cutaneous DRG neurons. Pain 2010; 151: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corotto FS, Michel WC. Mechanisms of afterhyperpolarization in lobster olfactory receptor neurons. J Neurophysiol 1998; 80: 1268–1276. [DOI] [PubMed] [Google Scholar]
- 45.Otmakhova NA, Lisman JE. Contribution of Ih and GABAB to synaptically induced afterhyperpolarisations in CA1: a brake on the NMDA response. J Neurophysiol 2004; 92: 2027–2039. [DOI] [PubMed] [Google Scholar]
- 46.Takigawa T, Alzheimer C. Interplay between activation of GIRK current and deactivation of Ih modifies temporal integration of excitatory input in CA1 pyramidal cells. J Neurophysiol 2003; 89: 2238–2244. [DOI] [PubMed] [Google Scholar]
- 47.Wahl-Schott C, Biel M. HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci 2009; 66: 470–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altomare C, Terragni B, Brioschi C, et al. Heteromeric HCN1-HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J Physiol 2003; 549: 347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stieber J, Stockl G, Herrmann S, et al. Functional expression of the human HCN3 channel. J Biol Chem 2005; 280: 34635–34643. [DOI] [PubMed] [Google Scholar]
- 50.Baruscotti M, Bucchi A, Difrancesco D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol Ther 2005; 107: 59–79. [DOI] [PubMed] [Google Scholar]
- 51.Weng X, Smith T, Sathish J, et al. Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not Adelta-nociceptors. Pain 2012; 153: 900–914. [DOI] [PubMed] [Google Scholar]
- 52.Devor M, Seltzer Z. Pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD, Melzack R. (eds). Textbook of pain, Edinburgh, UK: Churchill Livingstone, 1999, pp. 129–164. [Google Scholar]
- 53.Djouhri L, Koutsikou S, Fang X, et al. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci 2006; 26: 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen RG, Kong WW, Ge DL, et al. Bilateral mechanical and thermal hyperalgesia and tactile allodynia after chronic compression of dorsal root ganglion in mice. Neurosci Bull 2011; 27: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J, Guan SM, Sun W, et al. Melittin, the major pain-producing substance of Bee Venom. Neurosci Bull 2016; 32: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang F, Hua LM, Jiao YL, et al. Activation of mammalian target of rapamycin contributes to pain nociception induced in rats by BmK I, a sodium channel-specific modulator. Neurosci Bull 2014; 30: 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo C, Gangadharan V, Bali KK, et al. Presynaptically localized cyclic GMP-dependent protein kinase 1 is a key determinant of spinal synaptic potentiation and pain hypersensitivity. PLoS Biol 2012; 10: e1001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun W, Miao B, Wang XC, et al. Reduced conduction failure of the main axon of polymodal nociceptive C-fibres contributes to painful diabetic neuropathy in rats. Brain 2012; 135: 359–375. [DOI] [PubMed] [Google Scholar]
- 59.Sun W, Miao B, Wang XC, et al. Gastrodin inhibits allodynia and hyperalgesia in painful diabetic neuropathy rats by decreasing excitability of nociceptive primary sensory neurons. PLoS One 2012; 7: e39647. [DOI] [PMC free article] [PubMed] [Google Scholar]