Abstract
In the present study, the complete sequence of the mitochondrial genome (mitogenome) of Daphnis nerii (Lepidoptera: Sphingidae) is described. The mitogenome (15,247 bp) of D.nerii encodes13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNAs), two ribosomal RNA genes (rRNAs) and an adenine (A) + thymine (T)-rich region. Its gene complement and order is similar to that of other sequenced lepidopterans. The 12 PCGs initiated by ATN codons except for cytochrome c oxidase subunit 1 (cox1) gene that is seemingly initiated by the CGA codon as documented in other insect mitogenomes. Four of the 13 PCGs have the incomplete termination codon T, while the remainder terminated with the canonical stop codon. This mitogenome has six major intergenic spacers, with the exception of A+T-rich region, spanning at least 10 bp. The A+T-rich region is 351 bp long, and contains some conserved regions, including ‘ATAGA’ motif followed by a 17 bp poly-T stretch, a microsatellite-like element (AT)9 and also a poly-A element. Phylogenetic analyses based on 13 PCGs using maximum likelihood (ML) and Bayesian inference (BI) revealed that D. nerii resides in the Sphingidae family.
1. Background
The oleander hawk moth, D.nerii (Lepidoptera: Sphingidae) is one of the most widely distributed species of Sphingidae. It occursin the tropical and subtropical regions ranging from Africa to south-east Asia. It was first reported on Guam in August, 2005 as a plant pest. It feeds on a variety of plant species ranging from shrubs to trees such as Catharanthus, Vinca, Adenium, Vitis, Tabernaemontana, Gardenia, Trachelospermum, Amsonia, Asclepias, Carissa, Rhazya, Thevetia, Jasminum and Ipomoea. While, Nerium oleander has been documented as the most preferred host of the D.nerii. The management of this species is extremely important and that require deep knowledge on its different biological aspects[1]. Although a few studies are available on its ecology, reproduction and development and so on but its genetic characteristics are rarely documented. To improve the management of the D.nerii, it is extremely important to know more knowledge about this pest, particularly its genetic characteristics and phylogentic position. Moreover, the study of mitogenome is an important subject to understand molecular evolution, comparative and evolutionary genomics, phylogenetics, and population genetics [2–4].
The metazoan mitogenome is a closed-circular DNA molecule, ranged in size from 14 to 19 kilobases (kb), including intergenic spacers being very short or absent[5]. It contains 13 protein-coding genes (PCGs), 2 ribosomal RNA genes (rRNAs), and 22 transfer RNA genes (tRNAs)[6]. In addition, there is one major non-coding region (control region) that in other Lepidopterans and in invertebrates is named as A+T-rich region because of its enormously high content in Adenines and Thymines. This control region is generally believed to control the initiation of transcription and replication of animal mitogenome[7].
The order Lepidoptera is one of the largest insect orders and includes greater than 160 000 described species that are classified into 45–48 superfamilies[8]. Sphingidae is one of the most diverse superfamilies, and contains 203 genera and 1348 species distributed worldwide. Despite this enormous species diversity, only two complete mitogenomes are available in GenBank (Table 1)[9]. Newly accessible Lepidoptera mitogenomes will provide further insight into our understanding of evolutionary relationships between these species. In this study, we described the complete sequence of the mitogenome of D. nerii and compared it with other Lepidoptera species sequenced to date to highlight evolution of Lepidopterans, particularly, phylogenetic relation-ships of Bombycoidea.
Table 1. Details of the lepidopteran mitogenomes used in this study.
| Superfamily | Family | Species | Size (bp) | GenBank accession no. | Reference |
|---|---|---|---|---|---|
| Bombycoidea | Bombycidae | Bombyx mandarina | 15,682 | NC_003395 | [33] |
| Bombyx mori | 15,643 | NC_002355 | Direct submission | ||
| Saturniidae | Actias selene | 15,236 | NC_018133 | [34] | |
| Eriogyna pyretorum | 15,327 | NC_012727.1 | [5] | ||
| Antheraea pernyi | 15,566 | AY242996 | [35] | ||
| Antheraea yamamai | 15,338 | NC_012739 | [36] | ||
| Sphingidae | Manduca sexta | 15,516 | NC_010266 | [9] | |
| Sphinx morio | 15,299 | NC_020780.1 | [37] | ||
| Notonagemia analis scribae | 15,303 | KU934302.1 | [38] | ||
| Daphnis nerii | 15,247 | This study | |||
| Noctuoidea | Lymantriidae | Lymantria dispar | 15,569 | NC_012893 | Unpublished |
| Amata formosae | 15,463 | KC513737 | [6] | ||
| Hyphantria cunea | 15,481 | NC_014058 | [28] | ||
| Noctuidae | Agrotis ipsilon | 15,377 | KF163965 | [39] | |
| Geometroidea | Geometridae | Apocheima cinerarium | 15,722 | KF836545 | [40] |
| Biston panterinaria | 15,517 | NC_020004 | [41] | ||
| Phthonandria atrilineata | 15,499 | NC_010522 | [27] | ||
| Biston thibetaria | 15,484 | KJ670146.1 | Unpublished | ||
| Biston suppressaria | 15,628 | KP278206 | [42] | ||
| Jankowskia athleta | 15,534 | KR822683 | [43] | ||
| Pyraloidea | Crambidae | Chilo suppressalis | 15,395 | NC_015612 | [32] |
| Elophila interruptalis | 15,351 | NC_021756 | [44] | ||
| Diatraea saccharalis | 15,490 | NC_013274 | [45] | ||
| Pyralidae | Corcyra cephalonica | 15,273 | NC_016866.1 | [46] | |
| Gelechioidea | Elachistidae | Promalactis suzukiella | 15,507 | NC_026697 | [47] |
| Tortricoidea | Tortricidae | Acleris fimbriana | 15,933 | NC_018754 | Unpublished |
| Adoxophyes orana | 15,343 | JX872403 | [48] | ||
| Papilionoidea | Papilionidae | Parnassius bremeri | 15,389 | NC_014053 | [49] |
| Papilio syfanius | 15,359 | NC_023978 | [50] | ||
| Papilio maraho | 16,094 | NC_014055 | [29] | ||
| Teinopalpus aureus | 15,242 | NC_014398 | Unpublished | ||
| Yponomeutoidea | Plutellidae | Plutella xylostella | 16,179 | JF911819 | [51] |
| Lyonetiidae | Leucoptera malifoliella | 15,646 | NC_018547 | [52] | |
| Hepialoidea | Hepialidae | Thitarodes renzhiensis | 16,173 | NC_018094 | [53] |
| Ahamus yunnanensis | 15,816 | NC_018095 | [53] | ||
| Thitarodes pui | 15,064 | NC_023530 | [54] |
2. Materials and methods
2.1 Experimental insects and DNA extraction
The D. nerii specimens were collected from Anhui Agricultural University, Anhui Province, China. The total DNA was extracted using the Genomic DNA Extraction Kit, according to the manufacturer's instructions (Aidlab Co., Beijing, China). The extracted DNA quality was examined by 1% agarose gel electrophoresis (w/v) and used to amplify the complete mitogenome of D. nerii.
2.2 PCR amplification and sequencing
We designed twelve pairs of primers from the conserved nucleotide sequences of known mitogenome of Lepidopteran species to determine the D. nerii mitogenome[10, 11]. The complete list of successful primer is given in Table 2 (Sangon Biotech Co., Shanghai, China). All amplifications were performed on an Eppendorf Mastercycler and Mastercycler gradient in 50μL reaction volumes, which contained 35μL sterilized distilled water, 5μL 10×Taq buffer (Mg2+ plus), 4 μL dNTP (25 mM), 1.5 μL extracted DNA as template, forward and reverse primers 2 μL each (10 μM) and 0.5 μL (1 unit) TaqDNA polymerase (Takara Co., Dalian, China). The PCR amplification conditions were as follows: an initial denaturation one cycle at 94°C for 4 min followed by 38 cycles, one cycle at 94°C for 30 s, one cycle at 48–59°C for 1–3 min (depending on the putative length of the fragments), and a final extension one cycle at 72°C for 10 min. The PCR products were detected by 1% agarose gel electrophoresis (w/v), and were purified using a DNA gel extraction kit (Transgen Co., Beijing, China), and directly sequenced with PCR primers.
Table 2. Details of the primers used to amplify the mitogenome of D. nerii.
| Primer pair | Primer sequences (5’-3’) |
|---|---|
| F1 | TAAAAATAAGCTAAATTTAAGCTT |
| R1 | TATTAAAATTGCAAATTTTAAGGA |
| F2 | AAACTAATAATCTTCAAAATTAT |
| R2 | AAAATAATTTGTTCTATTAAAG |
| F3 | TGGAGCAGGAACAGGATGAAC |
| R3 | GAGACCADTACTTGCTTTCAG |
| F4 | ATTTGTGGAGCTAATCATAG |
| R4 | GGTCAGGGACTATAATCTAC |
| F5 | TCGACCTGGAACTTTAGC |
| R5 | GCAGCTATAGCCGCTCCTACT |
| F6 | TAAGCTGCTAACTTAATTTTTAGT |
| R6 | CCTGTTTCAGCTTTAGTTCATTC |
| F7 | CCTAATTGTCTTAAAGTAGATAA |
| R7 | TGCTTATTCTTCTGTAGCTCATAT |
| F8 | TAATGTATAATCTTCGTCTATGTAA |
| R8 | ATCAATAATCTCCAAAATTATTAT |
| F9 | ACTTTAAAAACTTCAAAGAAAAA |
| R9 | TCATAATAAATTCCTCGTCCAATAT |
| F10 | GGAGCTTCTACATGAGCTTTTGG |
| R10 | GTTTGCGACCTCGATGTTG |
| F11 | GGTCCCTTACGAATTTGAATATATCCT |
| R11 | AAACTAGGATTAGATACCCTATTAT |
| F12 | CTCTACTTTGTTACGACTTATT |
| R12 | TCTAGGCCAATTCAACAACC |
2.3 Sequence assembly and gene annotation
Sequence annotation was performed using blast tools available from the NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi), and SeqMan II program from the Lasergene software package (DNASTAR Inc.; Madison, USA). The protein-coding sequences were translated into putative proteins on the basis of the Invertebrate Mitochondrial Genetic Code. The skewness was measured by the method given by Junqueiraet al.[12], and the base composition of nucleotide sequences were described as: AT skew = [A−T]/[A+T], GC skew = [G−C]/[G+C]. The relative synonymous codon usage (RSCU) values were calculated using MEGA 5.1[13].
The tRNA genes were determined using the tRNAscan-SE software (http://lowelab.ucsc.edu/tRNAscan-SE/) [14], or predicted by sequence features of being capable of folding into the typical cloverleaf secondary structure with legitimate anticodon. The tandem repeats in the A+T-rich region were determined by the tandem repeats finder program (http://tandem.bu.edu/trf/trf.html)[15].
2.4 Phylogenetic analysis
To reconstruct the phylogenetic relationship among Lepidopterans, 36 complete or near-complete mitogenomes were downloaded from the GenBank database (Table 1). The mitogenomes of Drosophila melanogaster (U37541.1)[16] and Locusta migratoria (NC_001712)[17] were used as outgroup. The multiple alignments of the 13 PCGs concatenated nucleotide sequences were conducted using ClustalX version 2.0.[18]. Then concatenated set of nucleotide sequences from the 13 PCGs was used for phylogenetic analyses, which were performed using Maximum Likelihood (ML) method with the MEGA version 5.1 program[13] and Bayesian Inference (BI) with MrBayes 3.2 version program[19]. The ML analyses were used to infer phylogenetic trees with 1000 bootstrap replicates. BI analysis as the following conditions: the Markov chains were run for 100,000 generations with trees being sampled every 100 generations. The consensus trees were visualized by FigTree v1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/) program with adjustable settings.
3. Results and discussion
3.1 Genome structure, organization and composition
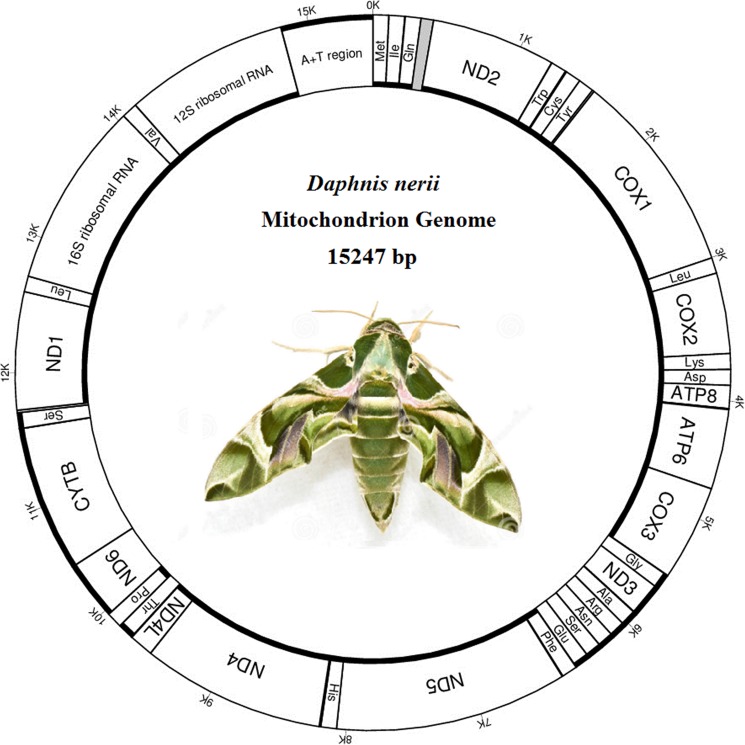
The complete sequence of the mitogenome of D.nerii is 15,247 bp in length (S1 File and Fig 1), which is well within the range observed in the whole sequenced Lepidoptera species with the size ranging from 15,682 bp in Bombyx mandarina (Bombycidae) to 15,064bp in Thitarodespui (Hepialidae) (Table 1). Alignment with previously sequenced lepidopteran mitogenomes revealed 38 mitogenome regions, including 13 protein-encoding regions (PCGs: atp6, atp8, cox1, cox2, cox3, cytb, nad1, nad2, nad3, nad4, nad5, nad6, and nad4L), two rRNA-encoding regions (large and small ribosomal RNA), 22 tRNA-encoding regions (transfer RNA) and a large non-coding-region with high A+T-rich composition that is usually found in most animal mtDNAs (Table 3). The gene arrangement and orientation of D.nerii mitogenome is trnM-trnI-trnQ that is different from the ancestral gene order trnI-trnQ-trnM[2].
Fig 1. Map of the mitogenome of D.nerii.
The tRNA genes are labeled according to the IUPAC-IUB single-letter amino acids: cox1, cox2 and cox3 refer to the cytochrome c oxidase subunits; cob refers to cytochrome b; nad1-nad6 refer to NADH dehydrogenase components; rrnL and rrnS refer to ribosomal RNAs.
Table 3. List of annotated mitochondrial genes of D. nerii.
| Gene | Direction | Location | Size | Anti codon | Start codon | Stop codon | Intergenic Nucleotides |
|---|---|---|---|---|---|---|---|
| tRNAMet | F | 1–68 | 68 | CAT | — | — | 0 |
| tRNAIle | F | 69–136 | 68 | GAT | — | — | -3 |
| tRNAGln | R | 134–202 | 69 | TTG | — | — | 55 |
| nad2 | F | 256–1273 | 1018 | ATT | TAA | -2 | |
| tRNATrp | F | 1272–1341 | 70 | TCA | — | — | 2 |
| tRNACys | R | 1334–1397 | 64 | GCA | — | — | 0 |
| tRNATyr | R | 1398–1461 | 64 | GTA | — | — | 14 |
| cox1 | F | 1476–3004 | 1529 | CCA | T | 0 | |
| tRNALeu(UUR) | F | 3005–3071 | 67 | TAA | — | — | 0 |
| cox2 | F | 3072–3753 | 682 | ATG | T | 0 | |
| tRNALys | F | 3754–3824 | 71 | CTT | — | — | 1 |
| tRNAAsp | F | 3826–3892 | 67 | GTC | — | — | 0 |
| atp8 | F | 3893–4057 | 165 | ATC | TAA | -7 | |
| atp6 | F | 4051–4728 | 678 | ATG | TAA | -1 | |
| cox3 | F | 4728–5524 | 795 | ATG | TAA | 2 | |
| tRNAGly | F | 5527–5595 | 69 | TCC | — | — | 0 |
| nad3 | F | 5596–5948 | 353 | ATC | TAA | 3 | |
| tRNAAla | F | 5952–6017 | 66 | TGC | — | — | -1 |
| tRNAArg | F | 6017–6081 | 65 | TCG | — | — | 0 |
| tRNAAsn | F | 6082–6148 | 67 | GTT | — | — | -1 |
| tRNASer(AGN) | F | 6148–6214 | 67 | GCT | — | — | -1 |
| tRNAGlu | F | 6214–6279 | 66 | TTC | — | — | -2 |
| tRNAPhe | R | 6278–6344 | 67 | GAA | — | — | 0 |
| nad5 | R | 6345–8067 | 1723 | ATA | T | 0 | |
| tRNAHis | R | 8083–8139 | 57 | GTG | — | — | 24 |
| nad4 | R | 8150–9537 | 1388 | ATT | T | -1 | |
| nad4L | R | 9537–9821 | 285 | ATG | TAA | 4 | |
| tRNAThr | F | 9826–9890 | 65 | TGT | — | — | -1 |
| tRNAPro | R | 9890–9955 | 66 | TGG | — | — | 1 |
| nad6 | F | 9957–10488 | 532 | ATG | TAA | -1 | |
| cytb | F | 10488–11630 | 1143 | ATG | TAA | -6 | |
| tRNASer(UCN) | F | 11625–11689 | 65 | TGA | — | — | 18 |
| nad1 | R | 11708–12644 | 937 | ATG | TAA | 0 | |
| tRNALeu(CUN) | R | 12645–12711 | 67 | TAG | — | — | 0 |
| rrnL | R | 12712–14049 | 1338 | — | — | — | 0 |
| tRNAVal | R | 14050–14115 | 66 | TAC | — | — | 1 |
| rrnS | R | 14117–14895 | 778 | — | — | — | 1 |
| A+T-rich Region | 14897–15247 | 351 |
The comparison of D. nerii mitogenome composition and skewness level with other sequenced Lepidoptera species is represented in Table 4. The genome composition of the major strand is A: 40.81%, T: 39.48%, G: 7.58%, and C: 12.13%, with a total A+T content of 80.29%. Additionally, it exhibits positive AT skewness (0.017) and negative GC skewness (-0.231). The AT-skewness in other Lepidopteran mitogenomes sequenced to date, ranges from 0.057 (B. mandarina) to -0.027 (A. formosae), while the GC-skewness from -0.266 (A. formosae) to -0.174 (G. argentata). Moreover the positive AT skewness (0.017) indicates the occurrence of more As than Ts that has also been reported in several other lepidopteran species such as B. mandarina (0.057), H. cunea (0.010) and L. dispar (0.016).
Table 4. Composition and skewness in different Lepidopteran mitogenomes.
| Species | Size(bp) | A% | G% | T% | C% | A+T% | ATskewness | GCskewness |
|---|---|---|---|---|---|---|---|---|
| Whole genome | ||||||||
| D. nerii | 15,247 | 40.81 | 7.58 | 39.48 | 12.13 | 80.29 | 0.017 | -0.231 |
| M. sexta | 15,516 | 40.67 | 7.46 | 41.11 | 10.76 | 81.79 | -0.005 | -0.181 |
| S. morio | 15,299 | 40.64 | 7.58 | 40.53 | 11.23 | 81.17 | 0.001 | -0.194 |
| B. mandarina | 15,682 | 43.11 | 7.40 | 38.48 | 11.01 | 81.59 | 0.057 | -0.196 |
| A. pernyi | 15,566 | 39.22 | 7.77 | 40.94 | 12.07 | 80.16 | -0.021 | -0.216 |
| L. dispar | 15,569 | 40.58 | 7.57 | 39.30 | 12.55 | 79.88 | 0.016 | -0.248 |
| L. melli | 15,418 | 39.38 | 8.72 | 39.29 | 13.06 | 78.67 | 0.001 | -0.199 |
| H. cunea | 15,481 | 40.58 | 7.55 | 39.81 | 12.06 | 80.39 | 0.010 | -0.230 |
| A. formosae | 15,463 | 38.67 | 7.53 | 40.83 | 12.98 | 79.49 | -0.027 | -0.266 |
| G. argentata | 15,337 | 39.64 | 7.56 | 42.05 | 10.75 | 81.69 | 0.030 | -0.174 |
| C. pomonella | 15,253 | 39.92 | 7.88 | 40.21 | 11.99 | 80.13 | -0.004 | -0.207 |
| P.atrilineata | 15,499 | 40.78 | 7.67 | 40.24 | 11.31 | 81.02 | 0.007 | -0.192 |
| A. ilia | 15,242 | 39.77 | 7.75 | 40.68 | 11.80 | 80.45 | -0.011 | -0.207 |
| G. dimorpha | 15,831 | 39.99 | 7.77 | 40.85 | 11.39 | 80.84 | -0.011 | -0.189 |
| H. vitta | 15,282 | 39.58 | 7.81 | 40.34 | 12.27 | 79.92 | -0.010 | -0.222 |
| C. suppressalis | 15,395 | 40.64 | 7.39 | 40.03 | 11.94 | 80.67 | 0.007 | -0.235 |
| A. ipsilon | 15,377 | 40.38 | 7.71 | 40.87 | 11.04 | 81.25 | -0.006 | -0.178 |
| PCG | ||||||||
| D. nerii | 11,208 | 40.52 | 8.32 | 38.15 | 13.00 | 78.68 | 0.030 | -0.220 |
| M. sexta | 11,185 | 40.41 | 8.23 | 39.88 | 11.48 | 80.30 | 0.007 | -0.165 |
| S. morio | 11,179 | 40.28 | 8.27 | 39.56 | 11.89 | 79.84 | 0.009 | -0.180 |
| B. mandarina | 11,196 | 42.83 | 8.26 | 37.04 | 11.87 | 79.87 | 0.072 | -0.179 |
| A. pernyi | 11,204 | 39.22 | 7.77 | 40.94 | 12.07 | 80.16 | -0.021 | -0.216 |
| L. dispar | 11,227 | 39.67 | 8.44 | 38.16 | 13.73 | 77.83 | 0.019 | -0.239 |
| L. melli | 11,120 | 38.47 | 9.17 | 38.17 | 14.19 | 76.64 | 0.004 | -0.215 |
| H. cunea | 11,198 | 39.98 | 8.35 | 38.61 | 13.06 | 78.59 | 0.017 | -0.220 |
| A. formosae | 11,217 | 38.18 | 8.28 | 39.62 | 13.92 | 77.80 | -0.019 | -0.254 |
| G. argentata | 10,303 | 38.10 | 8.61 | 41.88 | 11.41 | 79.98 | -0.047 | -0.140 |
| C. pomonella | 11,199 | 39.55 | 8.69 | 39.00 | 12.76 | 78.55 | 0.007 | -0.190 |
| P.atrilineata | 11,203 | 40.23 | 8.59 | 38.87 | 12.31 | 79.10 | 0.017 | -0.178 |
| A. ilia | 11,148 | 39.41 | 8.41 | 39.49 | 12.69 | 78.89 | -0.001 | -0.203 |
| G. dimorpha | 11,232 | 39.51 | 8.81 | 39.18 | 12.49 | 78.69 | 0.004 | -0.173 |
| H. vitta | 11,202 | 38.76 | 8.61 | 39.43 | 13.20 | 78.19 | -0.009 | -0.210 |
| C. suppressalis | 11,230 | 40.42 | 8.16 | 38.48 | 12.95 | 78.90 | 0.025 | -0.227 |
| A. ipsilon | 11,226 | 39.69 | 8.44 | 40.14 | 11.72 | 79.83 | -0.006 | -0.163 |
| tRNA | ||||||||
| D. nerii | 1,586 | 41.74 | 7.38 | 40.79 | 10.09 | 82.53 | 0.012 | -0.155 |
| M. sexta | 1,554 | 40.99 | 7.92 | 41.06 | 10.04 | 82.05 | -0.001 | -0.118 |
| S. morio | 1,462 | 40.63 | 8.21 | 40.97 | 10.19 | 81.60 | -0.004 | -0.107 |
| B. mandarina | 1,472 | 41.78 | 7.81 | 39.95 | 10.46 | 81.73 | 0.022 | -0.145 |
| A. pernyi | 1,459 | 39.22 | 7.77 | 40.94 | 12.07 | 80.16 | -0.021 | -0.217 |
| L. dispar | 1,459 | 41.60 | 7.95 | 39.48 | 10.97 | 81.08 | 0.026 | -0.160 |
| L. melli | 1,486 | 40.58 | 8.55 | 40.24 | 10.63 | 80.82 | 0.004 | -0.109 |
| H. cunea | 1,463 | 41.83 | 7.86 | 39.99 | 10.32 | 81.82 | 0.022 | -0.135 |
| A. formosae | 1,457 | 40.43 | 7.96 | 40.36 | 11.26 | 80.78 | 0.001 | -0.172 |
| G. argentata | 1,468 | 41.35 | 8.24 | 40.19 | 10.22 | 81.54 | 0.014 | -0.107 |
| C. pomonella | 1,464 | 41.19 | 7.92 | 40.23 | 10.66 | 81.42 | 0.012 | -0.147 |
| P.atrilineata | 1,476 | 41.4 | 8.2 | 40.04 | 10.37 | 81.44 | 0.017 | -0.117 |
| A. ilia | 1,433 | 40.61 | 8.30 | 40.96 | 10.12 | 81.58 | -0.004 | -0.099 |
| G. dimorpha | 1,451 | 41.01 | 8.06 | 40.52 | 10.41 | 81.53 | 0.006 | -0.127 |
| H. vitta | 1,456 | 41.41 | 8.04 | 39.84 | 10.71 | 81.25 | 0.019 | -0.142 |
| C. suppressalis | 1,482 | 40.89 | 7.89 | 40.89 | 10.32 | 81.78 | 0.000 | -0.133 |
| A. ipsilon | 1,465 | 41.23 | 8.12 | 40.48 | 10.17 | 81.71 | 0.009 | -0.112 |
| rRNA | ||||||||
| D. nerii | 2,117 | 42.14 | 4.87 | 42.61 | 10.39 | 84.74 | -0.006 | -0.362 |
| M. sexta | 2,168 | 41.37 | 4.84 | 44.05 | 9.73 | 85.42 | -0.031 | -0.336 |
| S. morio | 2,152 | 41.73 | 4.83 | 43.08 | 10.36 | 84.8 | -0.016 | -0.364 |
| B. mandarina | 2,134 | 43.86 | 4.78 | 41.05 | 10.31 | 84.91 | 0.033 | -0.366 |
| A. pernyi | 2,144 | 39.22 | 7.77 | 40.94 | 12.07 | 80.16 | -0.021 | -0.217 |
| L. dispar | 2,150 | 42.79 | 4.79 | 41.81 | 10.60 | 84.60 | 0.012 | -0.377 |
| L. melli | 2,233 | 42.23 | 4.93 | 41.96 | 10.88 | 84.19 | 0.003 | -0.376 |
| H. cunea | 2,234 | 42.08 | 4.92 | 42.75 | 10.25 | 84.83 | -0.008 | -0.351 |
| A. formosae | 2,163 | 38.93 | 4.72 | 44.85 | 11.51 | 83.77 | -0.071 | -0.418 |
| G. argentata | 2,165 | 40.6 | 4.76 | 45.13 | 9.52 | 85.73 | -0.053 | -0.333 |
| C. pomonella | 2,147 | 40.48 | 5.03 | 43.92 | 10.57 | 84.4 | -0.041 | -0.355 |
| P.atrilineata | 2,203 | 42.85 | 4.58 | 43.08 | 9.49 | 85.93 | -0.003 | -0.349 |
| A. ilia | 2,109 | 40.11 | 4.98 | 44.86 | 10.05 | 84.97 | -0.056 | -0.337 |
| G. dimorpha | 2,181 | 41.13 | 4.95 | 43.83 | 10.09 | 84.96 | -0.032 | -0.342 |
| H. vitta | 2,194 | 41.43 | 4.88 | 43.25 | 10.44 | 84.69 | -0.021 | -0.363 |
| C. suppressalis | 2,171 | 41.27 | 4.97 | 43.67 | 10.09 | 84.94 | -0.028 | -0.340 |
| A. ipsilon | 2,162 | 41.58 | 5 | 43.57 | 9.85 | 85.15 | -0.023 | -0.327 |
| A+T-rich region | ||||||||
| D. nerii | 351 | 41.60 | 1.42 | 53.56 | 3.42 | 95.16 | -0.126 | -0.413 |
| M. sexta | 324 | 45.06 | 1.54 | 50.31 | 3.09 | 95.37 | -0.005 | -0.335 |
| S. morio | 316 | 44.3 | 2.53 | 48.42 | 4.75 | 92.72 | -0.044 | -0.305 |
| B. mandarina | 484 | 46.49 | 2.69 | 47.93 | 2.89 | 94.42 | -0.015 | -0.036 |
| A. pernyi | 552 | 39.22 | 7.77 | 40.94 | 12.07 | 80.16 | -0.021 | -0.216 |
| L. dispar | 435 | 40.58 | 7.57 | 39.30 | 12.55 | 79.88 | 0.016 | -0.248 |
| L. melli | 338 | 43.2 | 1.48 | 51.18 | 4.14 | 94.38 | -0.085 | -0.473 |
| H. cunea | 357 | 45.66 | 1.12 | 49.3 | 3.92 | 94.96 | -0.038 | -0.556 |
| A. formosae | 482 | 42.95 | 2.9 | 49.79 | 4.36 | 92.74 | -0.074 | -0.201 |
| G. argentata | 340 | 43.24 | 1.47 | 52.06 | 3.24 | 95.29 | -0.093 | -0.376 |
| C. pomonella | 351 | 43.3 | 1.14 | 52.42 | 3.13 | 95.73 | -0.095 | -0.466 |
| P.atrilineata | 457 | 40.7 | 0.66 | 57.55 | 1.09 | 98.25 | -0.172 | -0.246 |
| A. ilia | 403 | 42.93 | 3.23 | 49.63 | 4.22 | 92.56 | -0.072 | -0.133 |
| G. dimorpha | 848 | 41.63 | 1.30 | 54.83 | 2.24 | 96.46 | -0.137 | -0.266 |
| C. suppressalis | 348 | 42.24 | 0.29 | 53.16 | 4.31 | 95.4 | -0.114 | -0.874 |
| A. ipsilon | 332 | 46.08 | 1.51 | 48.8 | 3.61 | 94.88 | -0.029 | -0.41 |
3.2 Protein-coding genes and codon usage
The mitogenome of D.nerii contains 13 protein-coding genes. Most protein-coding genes (12 PCGs) begin with ATN (one with ATA, two with ATT, seven with ATG and two with ATC) codons, except for the cox1. The cox1 gene of D.nerii seems to be started with CCA codon as previously documented in Cerura menciana[20] and in Spoladea recurvalis[21]. Several authors have maintained the problematic translational start at the cox1 locus in many insect species, with TTAG, ACG, and TTG proposed as start codons for cox1[22–24]. A most common stop codon of the PCGs is TAA, but an incomplete termination stop codon T is present at cox1, cox2, nad5 and nad4. This has been well documented in other invertebrate mitogenomes and is a common evolutionary feature shared by mtDNA. The single T stop codon was recognized by endonucleases processing the polycistronic pre-mRNA transcription, and produced functional stop codons by polyadenylation from its contiguous PCGs[25].
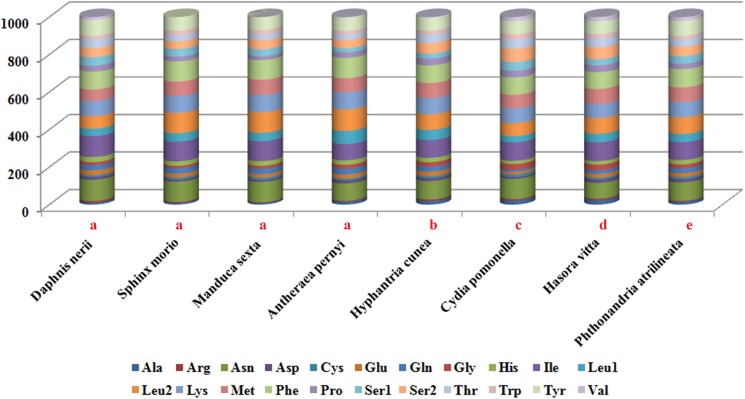
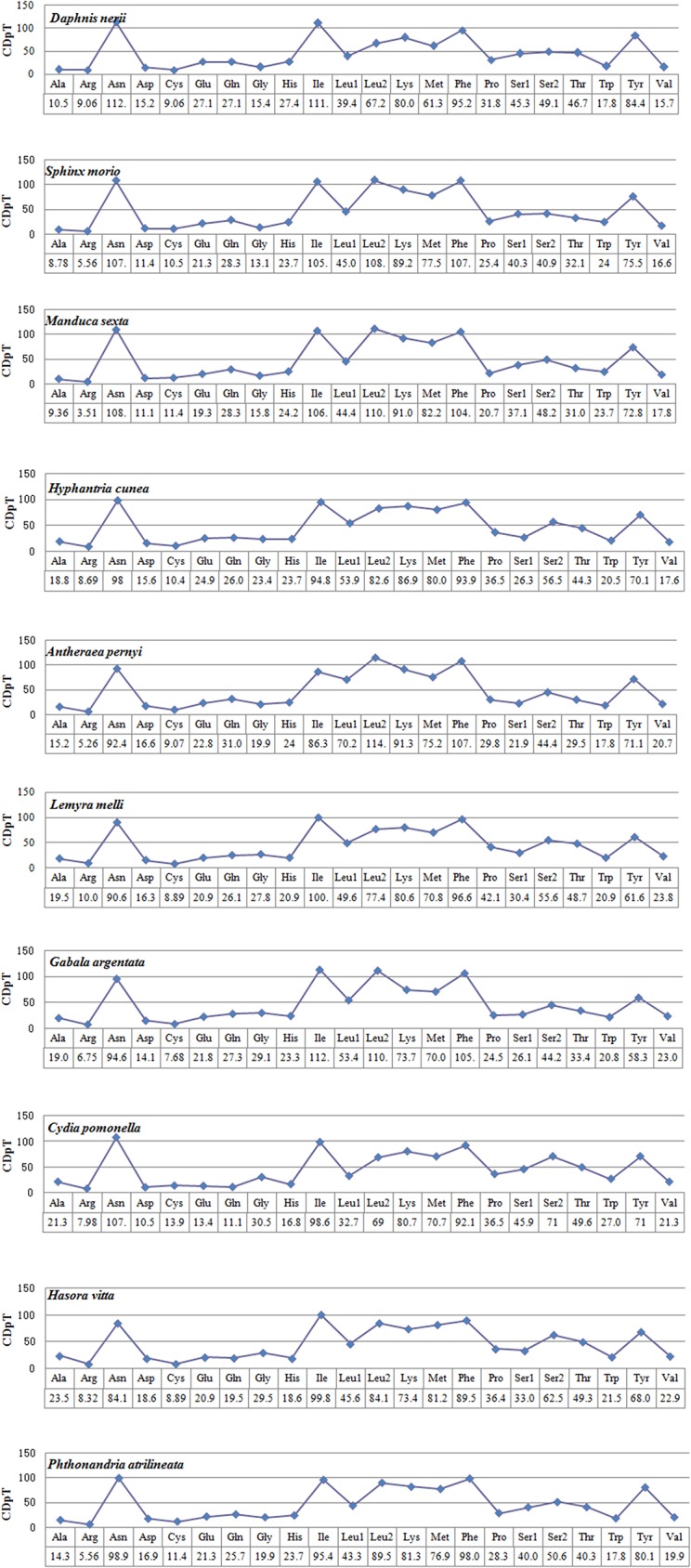
We analyzed the codon usage among eight Lepidopteran species, of which four belong to Bombycoidea and one each from Noctuoidea, Tortricoidea, Hesperioidea and Geometroidea (Fig 2). The results revealed that Asn, Ile, Leu2, Lys, Phe, Tyr and Met were the most frequently utilized amino acids. There were at least 4 codon families with no less than 60 codons per thousand codons (Leu2, Lys, Met, and Tyr), and 3 families with at least 80 codons per thousand codons (Asn, Ile and Phe) that were observed in the 8 insect species. The rarest used codon family was Arg. Codon distributions of four species in Bombycoidea are in consistency, and each amino acid has equal contents in different species (Fig 3).
Fig 2. Comparison of codon usage within the mitochondrial genome of members of the Lepidoptera.
Lowercase letters (a, b, c, d and e) above species name represent the superfamily to which the species belongs (a: Bombycoidea, b: Noctuoidea, c: Tortricoidea, d: Hesperioidea, e: Geometroidea).
Fig 3. Codon distribution in members of the Lepidoptera.CDspT = codons per thousand codons.
The Relative Synonymous Codon Usage (RSCU) was assessed in the PCGs for five available Lepidopteran superfamilies mitogenomes (Fig 4). All possible codon combinations are present in the PCGs of D.nerii except for the GCG. The absence of codons containing high GC content is also a characteristic feature of several Lepidopteran species such as M. sexta (CGG&CGC), H. cunea(GCG), G. argentata (GCG&CGC&CCG), P. atrilineata (CGG), C. pomonella (GCG), H. vitta (GCG), and so on. Further, these codons are likely to be less, and this featureis conserved in insect mitogenomes[20, 26].
Fig 4. The Relative Synonymous Codon Usage (RSCU) of the mitochondrial genome of six superfamilies in the Lepidoptera.
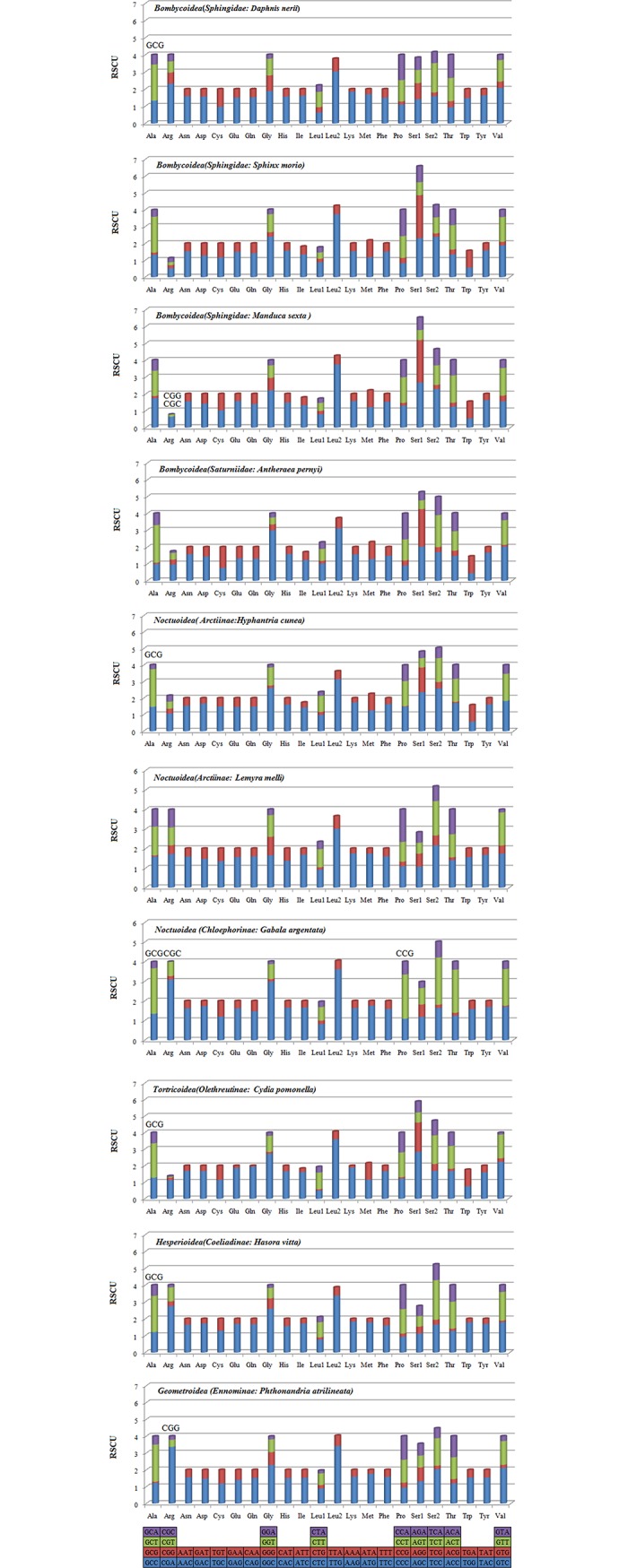
Codon families are plotted on the X axis. Codons indicated above the bar are not present in the mitogenome.
3.3 Ribosomal and transfer RNA genes
The mitogenome of D.nerii includes two rRNA genes usually present in other animals sequenced to date. The large ribosomal gene (rrnL) is 1338 bp long, and resided between tRNA Leu (CUN) and tRNA Val, whereas the small ribosomal gene (rrnS) is only 778 bp long, and located between tRNA Val and A+T-rich region (Table 3). The A+T content (84.74%) of two rRNAs fall within the range from 80.16% (A.pernyi) to 85.93% (P. atrilineata) of Lepidopterans. Both AT skewness (-0.006) and GC skewness (-0.362) are negative, that is similar to other previously sequenced Lepidopteran mitogenome[6, 27].
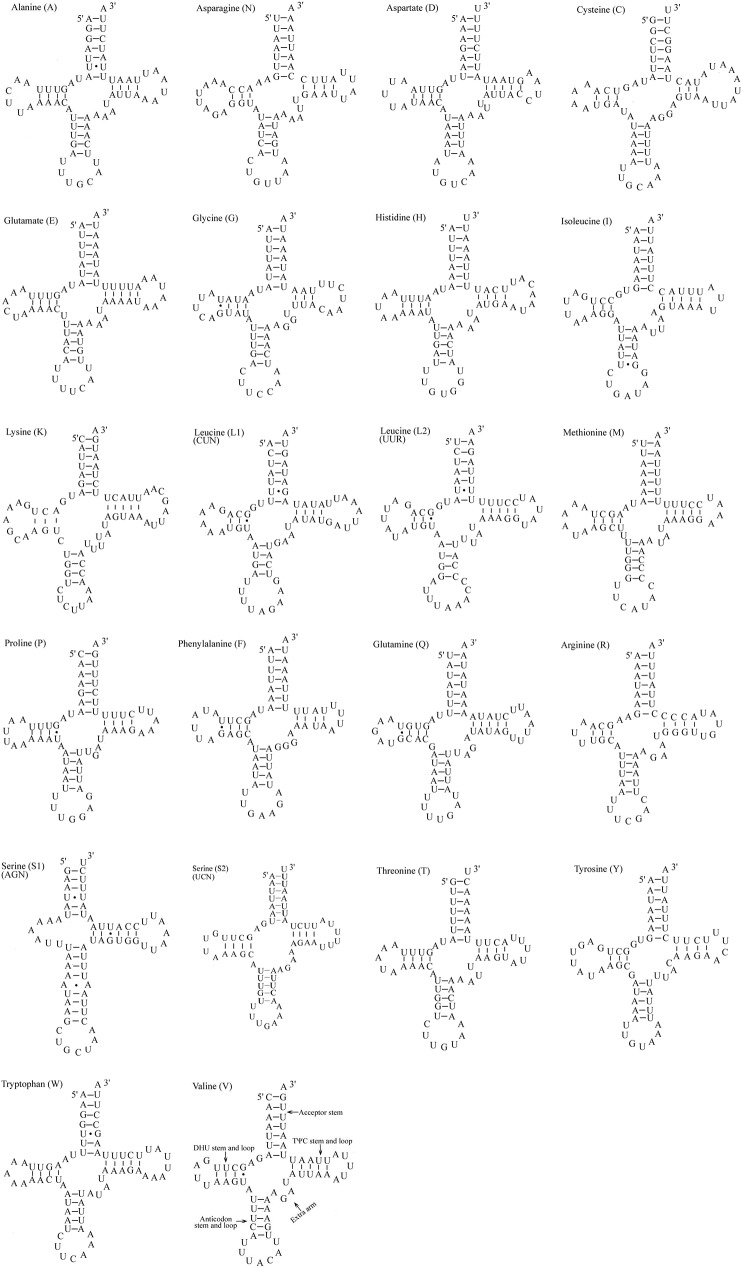
The D.nerii harbors an entire set of 22 tRNA (ranging from 64 to 71 nucleotides in length) commonly present in most of Lepidopteran mitogenomes. This region is highly A+T biased, accounting for 82.53%, and exhibit positive AT-skewness (0.012), while negative GC skewness (-0.155) (Table 4). All tRNA spresented the typical cloverleaf secondary structure but trnS1 lacked the DHU stem (Fig 5) similar to several other previously sequenced Lepidopterans[10, 28]. Moreover 14 of the 22 tRNA genes were coded by the H-strand and remainder eight by the L-strand.
Fig 5. Putative secondary structures of the 22 tRNA genes of the D.nerii mitogenome.
A total of 15 mismatched bps in the D.nerii tRNAs were identified. Most of them are G-U wobble pairs scatter throughout ten tRNAs (two in acceptor stem, seven in DHU, one in anticodon stem, and one in TψC), a A-A mismatch in the anticodon stem of the trnS1 and three U-U mismatches in acceptor stem of the trnA, trnL2 and trnS1 (Fig 5).
3.4 Overlapping and intergenic spacer regions
The mitogenome of D.nerii contains 12 overlapping regions with a total length of 26 bp. The six overlapping regions are resided between tRNA and tRNA (trnW and trnC, trnA and trnR, trnD and trnS1, trnS1 and trnE, trnE and trnF, trnT and trnP), two between tRNA and protein (nad2 and trnW, trnS2 and cytb), and four between protein and protein (atp6 and atp8, atp6 and cox3, nad4 and nad4L, nad6 and cytb). The length of these sequences varies from 1 bp to 7 bp with the longest overlapping region present between atp6 and atp8 (Table 3), which is usually found in Lepidopteran mitogenomes[29, 30]. Further, we observed the longest region in ten Lepidopteran species (Fig 6), Which indicates the seven nucleotides sequence ATGATAA is a strikingly, common feature across Lepidopteran mitogenomes[6]. The mitogenome of D.nerii has 12 intergenic spacers in a total of 126 bp with a length varying in 1 to 55 bp. Of which there are four major intergenic spacers at least 10 bp in length (Table 3). The longest intergenic spacer (55bp) is located between the trnQ and nad2, with an extremely high A+T nucleotides content, this characteristic feature has been frequently described in Lepidopteran mitogenomes[21]. The 19 bp spacer between trnS2 (UCN) and nad1 contains the motif ATACTAA (Fig 7A) that is highly conserved region and found in most insect mtDNAs, and it seems to be a possible mitochondrial transcription termination peptide-binding site (mtTERM protein)[31].
Fig 6. Alignment of overlapping region between atp8 and atp6 across Lepidoptera and other insects.
The numbers on the right refer to intergenic nucleotides.
Fig 7.
(A) Alignment of the intergenic spacer region between trnS2 (UCN) and nad1 of several Lepidopteran insects. The shaded ‘ATACTAA’ motif is conserved across the Lepidoptera order. (B) Features present in the A+T-rich region of D.nerii. The sequence is shown in the reverse strand. The ATATG motif is shaded. The poly-T stretch is underlined while the poly-A stretch is double underlined. The single microsatellite T/A repeats sequence are indicated by dotted underlining.
3.5 The A+T-rich region
The A + T-rich region of D.nerii mitogenome is located between the rrnS and trnM with a length of 351 bp that is remarkably shorter than G. dimorpha (848 bp) and longer than S. morio (316 bp), but average when compared with that of other Lepidopteran mitogenomes and (Table 4). This region harbors the highest A+T content (95.16%) in the mtDNA, and most negative AT skewness (-0.126) and GC skewness (-0.413) (Table 4). We identified several short repeating sequences scattered throughout the entire region, including the motif ATAGA followed by a 17 bp poly-T stretch, a microsate-like (AT)9 element and a poly-A element upstream of trnM gene similar to other Lepidopteran mitogenomes (Fig 7B). The length of poly-T stretch varies from species to species[6, 20], while ATAGA region is conserved in Lepidoptera species[9].
3.6. Phylogenetic analyses
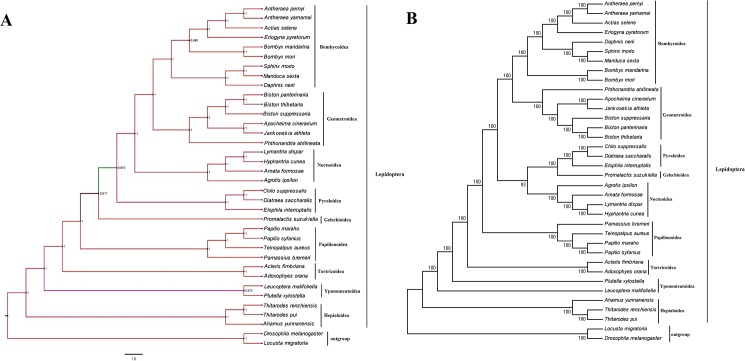
To reconstruct the phylogenetic relationship among Lepidopteran insects, the nucleotide sequences of the 13 PCGs were firstly aligned and then concatenated. The phylogenetic analyses showed that D.nerii has a close relationship to M. sexta and S. morio that was well supported from both BI and ML analyses (Fig 8A and 8B). The D. nerii is within the family Sphingidae (Bombycoidea) and clustered with other superfamilies, including the Geometroidea, Noctuoidea, Pyraloidea, Gelechioidea, Papilionoidea, Tortricoidea, Yponomeutoidea and Hepialoidea. Further the analyses revealed that Sphingidae is more closely related to Bombycidae than Saturniidae. Interestingly, Bombycoidea was more closely related to Noctuoideain ML methods, while in BI method Bombycoidea closely related to Geometroidea. These phylogenetic relationships are consistent with previously reportedstudies of Lepidopterans[11, 32]. We concluded from the present study that more research on the diverse Lepidoptera species is needed, to be able to understand better the relationships among them.
Fig 8.
Tree showing the phylogenetic relationships among Lepidopteran insects, constructed using (A) Bayesian inference (BI). (B) Maximum Likelihood method (ML). Bootstrap values (1000 repetitions) of the branches are indicated. Drosophila melanogaster (U37541.1) and Locustamigratoria (NC_001712) were used as outgroups.
Supporting information
(SEQ)
Acknowledgments
We would like to thank the native English speaking scientists Muhammad Nadeem Abbas for editing our manuscript. This work was supported by the earmarked fund for modern Argoindustry Technology Research System (CARS-22 SYZ10), the National Natural Science Foundation of China (31301715), the Sericulture Biotechnology Innovation Team (2013xkdt-05), the National Natural Science Foundation of China (31472147), the Ph.D. Programs in Biochemistry and Molecular Biology (xk2013042), the National Natural Science Foundation of China (31402018), and the Graduate Student Innovation Fund of Anhui Agricultural University (2015–34).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The modern Argoindustry Technology Research System (CARS-22 SYZ10), the Biology Key Subjects of Anhui Province, the National Natural Science Foundation of China (31301715), the Sericulture Biotechnology Innovation Team (2013xkdt-05), the National Natural Science Foundation of China (31472147), the Ph.D. Programs in Biochemistry and Molecular Biology (xk2013042), the National Natural Science Foundation of China (31402018), and the Graduate Student Innovation Fund of Anhui Agricultural University (2015-34).
References
- 1.Moore A, Miller RH. Daphnis nerii (Lepidoptera: Sphingidae), a New Pest of Oleander on Guam, Including Notes on Plant Hosts and Egg Parasitism. Proc Hawaiian Entomol Soc. 2008;40:67–70. [Google Scholar]
- 2.Boore JL. Animal mitochondrial genomes. Nucleic acids research. 1999;27(8):1767–80. PubMed Central PMCID: PMC148383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babbucci M, Basso A, Scupola A, Patarnello T, Negrisolo E. Is it an ant or a butterfly? Convergent evolution in the mitochondrial gene order of Hymenoptera and Lepidoptera. Genome biology and evolution. 2014;6(12):3326–43. PubMed Central PMCID: PMC4466343. doi: 10.1093/gbe/evu265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron SL. Insect mitochondrial genomics: implications for evolution and phylogeny. Annual review of entomology. 2014;59:95–117. doi: 10.1146/annurev-ento-011613-162007 [DOI] [PubMed] [Google Scholar]
- 5.Jiang ST, Hong GY, Yu M, Li N, Yang Y, Liu YQ, et al. Characterization of the complete mitochondrial genome of the giant silkworm moth, Eriogyna pyretorum (Lepidoptera: Saturniidae). International journal of biological sciences. 2009;5(4):351–65. PubMed Central PMCID: PMC2686093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu HF, Su TJ, Luo AR, Zhu CD, Wu CS. Characterization of the Complete Mitochondrion Genome of Diurnal Moth Amata emma (Butler) (Lepidoptera: Erebidae) and Its Phylogenetic Implications. PloS one. 2013;8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolstenholme DR. Animal mitochondrial DNA: structure and evolution. International review of cytology. 1992;141:173–216. [DOI] [PubMed] [Google Scholar]
- 8.Hao J, Sun Q, Zhao H, Sun X, Gai Y, Yang Q. The Complete Mitochondrial Genome of Ctenoptilum vasava (Lepidoptera: Hesperiidae: Pyrginae) and Its Phylogenetic Implication. Comparative and functional genomics. 2012;2012:328049 PubMed Central PMCID: PMC3335176. doi: 10.1155/2012/328049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron SL, Whiting MF. The complete mitochondrial genome of the tobacco hornworm, Manduca sexta, (Insecta: Lepidoptera: Sphingidae), and an examination of mitochondrial gene variability within butterflies and moths. Gene. 2008;408(1–2):112–23. doi: 10.1016/j.gene.2007.10.023 [DOI] [PubMed] [Google Scholar]
- 10.Dai LS, Zhu BJ, Qian C, Zhang CF, Li J, Wang L, et al. The complete mitochondrial genome of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Mitochondrial DNA. 2016;27(2):1512–3. doi: 10.3109/19401736.2014.953116 [DOI] [PubMed] [Google Scholar]
- 11.Liu QN, Zhu BJ, Dai LS, Liu CL. The complete mitogenome of Bombyx mori strain Dazao (Lepidoptera: Bombycidae) and comparison with other lepidopteran insects. Genomics. 2013;101(1):64–73. doi: 10.1016/j.ygeno.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 12.Junqueira AC, Lessinger AC, Torres TT, da Silva FR, Vettore AL, Arruda P, et al. The mitochondrial genome of the blowfly Chrysomya chloropyga (Diptera: Calliphoridae). Gene. 2004;339:7–15. doi: 10.1016/j.gene.2004.06.031 [DOI] [PubMed] [Google Scholar]
- 13.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular biology and evolution. 2011;28(10):2731–9. doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic acids research. 1997;25(5):955–64. PubMed Central PMCID: PMC146525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic acids research. 1999;27(2):573–80. PubMed Central PMCID: PMC148217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis DL, Farr CL, Kaguni LS. Drosophila melanogaster mitochondrial DNA: completion of the nucleotide sequence and evolutionary comparisons. Insect molecular biology. 1995;4(4):263–78. [DOI] [PubMed] [Google Scholar]
- 17.Flook PK, Rowell CH, Gellissen G. The sequence, organization, and evolution of the Locusta migratoria mitochondrial genome. Journal of molecular evolution. 1995;41(6):928–41. [DOI] [PubMed] [Google Scholar]
- 18.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic acids research. 1997;25(24):4876–82. PubMed Central PMCID: PMC147148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic biology. 2012;61(3):539–42. PubMed Central PMCID: PMC3329765. doi: 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai L, Qian C, Zhang C, Wang L, Wei G, Li J, et al. Characterization of the Complete Mitochondrial Genome of Cerura menciana and Comparison with Other Lepidopteran Insects. PloS one. 2015;10(8):e0132951 PubMed Central PMCID: PMC4550444. doi: 10.1371/journal.pone.0132951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He SL, Zou Y, Zhang LF, Ma WQ, Zhang XY, Yue BS. The Complete Mitochondrial Genome of the Beet Webworm, Spoladea recurvalis (Lepidoptera: Crambidae) and Its Phylogenetic Implications. PloS one. 2015;10(6):e0129355 PubMed Central PMCID: PMC4474886. doi: 10.1371/journal.pone.0129355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee ES, Shin KS, Kim MS, Park H, Cho S, Kim CB. The mitochondrial genome of the smaller tea tortrix Adoxophyes honmai (Lepidoptera: Tortricidae). Gene. 2006;373:52–7. doi: 10.1016/j.gene.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 23.Ogoh K, Ohmiya Y. Complete mitochondrial DNA sequence of the sea-firefly, Vargula hilgendorfii (Crustacea, Ostracoda) with duplicate control regions. Gene. 2004;327(1):131–9. doi: 10.1016/j.gene.2003.11.011 [DOI] [PubMed] [Google Scholar]
- 24.Lutz-Bonengel S, Sanger T, Pollak S, Szibor R. Different methods to determine length heteroplasmy within the mitochondrial control region. International journal of legal medicine. 2004;118(5):274–81. doi: 10.1007/s00414-004-0457-0 [DOI] [PubMed] [Google Scholar]
- 25.Lu C, Liu YQ, Liao SY, Li B, Xiang ZH, Han H, et al. Complete Sequence Determination and Analysis of Bombyx mori Mitochondrial Genome. Journal of Agricultural Biotechnology. 2002;10:163–70. [Google Scholar]
- 26.Lu HF, Su TJ, Luo AR, Zhu CD, Wu CS. Characterization of the complete mitochondrion genome of diurnal Moth Amata emma (Butler) (Lepidoptera: Erebidae) and its phylogenetic implications. PloS one. 2013;8(9):e72410 PubMed Central PMCID: PMC3771990. doi: 10.1371/journal.pone.0072410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Wei ZJ, Hong GY, Jiang ST, Wen LP. The complete nucleotide sequence of the mitochondrial genome of Phthonandria atrilineata (Lepidoptera: Geometridae). Molecular biology reports. 2009;36(6):1441–9. doi: 10.1007/s11033-008-9334-0 [DOI] [PubMed] [Google Scholar]
- 28.Liao F, Wang L, Wu S, Li YP, Zhao L, Huang GM, et al. The complete mitochondrial genome of the fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae). International journal of biological sciences. 2010;6(2):172–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu LW, Lees DC, Yen SH, Lu CC, Hsu YF. The Complete Mitochondrial Genome Of the near-Threatened Swallowtail, Agehana Maraho (Lepidoptera: Papilionidae): Evaluating Sequence Variability And Suitable Markers for Conservation Genetic Studies. Entomol News. 2010;121(3):267–80. [Google Scholar]
- 30.Zhu BJ, Liu QN, Dai LS, Wang L, Sun Y, Lin KZ, et al. Characterization of the complete mitochondrial genome of Diaphania pyloalis (Lepidoptera: Pyralididae). Gene. 2013;527(1):283–91. doi: 10.1016/j.gene.2013.06.035 [DOI] [PubMed] [Google Scholar]
- 31.Taanman JW. The mitochondrial genome: structure, transcription, translation and replication. Biochimica et biophysica acta. 1999;1410(2):103–23. [DOI] [PubMed] [Google Scholar]
- 32.Chai HN, Du YZ, Zhai BP. Characterization of the complete mitochondrial genomes of Cnaphalocrocis medinalis and Chilo suppressalis (Lepidoptera: Pyralidae). International journal of biological sciences. 2012;8(4):561–79. PubMed Central PMCID: PMC3334671. doi: 10.7150/ijbs.3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yukuhiro K, Sezutsu H, Itoh M, Shimizu K, Banno Y. Significant levels of sequence divergence and gene Rearrangements have occurred between the mitochondrial Genomes of the wild mulberry silkmoth, Bombyx mandarina, and its close relative, the domesticated silkmoth, Bombyx mori. Molecular biology and evolution. 2002;19(8):1385–9. [DOI] [PubMed] [Google Scholar]
- 34.Liu QN, Zhu BJ, Dai LS, Wei GQ, Liu CL. The complete mitochondrial genome of the wild silkworm moth, Actias selene. Gene. 2012;505(2):291–9. doi: 10.1016/j.gene.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 35.Liu YQ, Li YP, Pan MH, Dai FY, Zhu XW, Lu C, et al. The complete mitochondrial genome of the Chinese oak silkmoth, Antheraea pernyi (Lepidoptera: Saturniidae). Acta Bioch Bioph Sin. 2008;40(8):693–703. [PubMed] [Google Scholar]
- 36.Kim SR, Kim MI, Hong MY, Kim KY, Kang PD, Hwang JS, et al. The complete mitogenome sequence of the Japanese oak silkmoth, Antheraea yamamai (Lepidoptera: Saturniidae). Molecular biology reports. 2009;36(7):1871–80. doi: 10.1007/s11033-008-9393-2 [DOI] [PubMed] [Google Scholar]
- 37.Kim MJ, Choi SW, Kim I. Complete mitochondrial genome of the larch hawk moth, Sphinx morio (Lepidoptera: Sphingidae). Mitochondrial DNA. 2013;24(6):622–4. doi: 10.3109/19401736.2013.772155 [DOI] [PubMed] [Google Scholar]
- 38.Min JK, Kim JS, Kim I. Complete mitochondrial genome of the hawkmoth Notonagemia analis scribae (Lepidoptera: Sphingidae). 2016;1(1):416–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu QL, Cui WX, Wei SJ. Characterization of the complete mitochondrial genome of the black cutworm Agrotis ipsilon (Lepidoptera: Noctuidae). Mitochondrial DNA. 2015;26(1):139–40. doi: 10.3109/19401736.2013.815175 [DOI] [PubMed] [Google Scholar]
- 40.Liu SX, Xue DY, Cheng R, Han HX. The complete mitogenome of Apocheima cinerarius (Lepidoptera: Geometridae: Ennominae) and comparison with that of other lepidopteran insects. Gene. 2014;547(1):136–44. doi: 10.1016/j.gene.2014.06.044 [DOI] [PubMed] [Google Scholar]
- 41.Yang XS, Xue DY, Han HX. The complete mitochondrial genome of Biston panterinaria (Lepidoptera: Geometridae), with phylogenetic utility of mitochondrial genome in the Lepidoptera. Gene. 2013;515(2):349–58. doi: 10.1016/j.gene.2012.11.031 [DOI] [PubMed] [Google Scholar]
- 42.Chen SC, Wang XQ, Wang JJ, Hu X, Peng P. The complete mitochondrial genome of a tea pest looper, Buzura suppressaria (Lepidoptera: Geometridae). Mitochondrial DNA. 2015:1–2. [DOI] [PubMed] [Google Scholar]
- 43.Xu YM, Chen SC, Wang XQ, Peng P, Li PW. The complete mitogenome of Jankowskia athleta (Lepidoptera: Geometridae). Mitochondrial DNA. 2015:1–2. [DOI] [PubMed] [Google Scholar]
- 44.Park JS, Kim MJ, Kim SS, Kim I. Complete mitochondrial genome of an aquatic moth, Elophila interruptalis (Lepidoptera: Crambidae). Mitochondrial DNA. 2014;25(4):275–7. doi: 10.3109/19401736.2013.800504 [DOI] [PubMed] [Google Scholar]
- 45.Li W, Zhang X, Fan Z, Yue B, Huang F, King E, et al. Structural characteristics and phylogenetic analysis of the mitochondrial genome of the sugarcane borer, Diatraea saccharalis (Lepidoptera: Crambidae). DNA and cell biology. 2011;30(1):3–8. doi: 10.1089/dna.2010.1058 [DOI] [PubMed] [Google Scholar]
- 46.Wu YP, Li J, Zhao JL, Su TJ, Luo AR, Fan RJ, et al. The complete mitochondrial genome of the rice moth, Corcyra cephalonica. Journal of insect science. 2012;12:72 PubMed Central PMCID: PMC3593705. doi: 10.1673/031.012.7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park JS, Kim SS, Kim KY, Kim I. Complete mitochondrial genome of Suzuki's Promolactis moth Promalactis suzukiella (Lepidoptera: Oecophoridae). Mitochondrial DNA Part A DNA mapping, sequencing, and analysis. 2016;27(3):2093–4. [DOI] [PubMed] [Google Scholar]
- 48.Wu QL, Liu W, Shi BC, Gu Y, Wei SJ. The complete mitochondrial genome of the summer fruit tortrix moth Adoxophyes orana (Lepidoptera: Tortricidae). Mitochondrial DNA. 2013;24(3):214–6. doi: 10.3109/19401736.2012.748044 [DOI] [PubMed] [Google Scholar]
- 49.Kim MI, Baek JY, Kim MJ, Jeong HC, Kim KG, Bae CH, et al. Complete nucleotide sequence and organization of the mitogenome of the red-spotted apollo butterfly, Parnassius bremeri (Lepidoptera: Papilionidae) and comparison with other lepidopteran insects. Molecules and cells. 2009;28(4):347–63. doi: 10.1007/s10059-009-0129-5 [DOI] [PubMed] [Google Scholar]
- 50.Dong Y, Zhu LX, Ding MJ, Wang JJ, Luo LG, Liu Y, et al. Complete mitochondrial genome of Papilio syfanius (Lepidoptera: Papilionidae). Mitochondrial DNA. 2016;27(1):403–4. doi: 10.3109/19401736.2014.898278 [DOI] [PubMed] [Google Scholar]
- 51.Wei SJ, Shi BC, Gong YJ, Li Q, Chen XX. Characterization of the Mitochondrial Genome of the Diamondback Moth Plutella xylostella (Lepidoptera: Plutellidae) and Phylogenetic Analysis of Advanced Moths and Butterflies. DNA and cell biology. 2013;32(4):173–87. doi: 10.1089/dna.2012.1942 [DOI] [PubMed] [Google Scholar]
- 52.Wu YP, Zhao JL, Su TJ, Li J, Yu F, Chesters D, et al. The Complete Mitochondrial Genome of Leucoptera malifoliella Costa (Lepidoptera: Lyonetiidae). DNA and cell biology. 2012;31(10):1508–22. doi: 10.1089/dna.2012.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao YQ, Ma CA, Chen JY, Yang DR. The complete mitochondrial genomes of two ghost moths, Thitarodes renzhiensis and Thitarodes yunnanensis: the ancestral gene arrangement in Lepidoptera. BMC genomics. 2012;13 doi: 10.1186/1471-2164-13-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yi J, Que S, Xin T, Xia B, Zou Z. Complete mitochondrial genome of Thitarodes pui (Lepidoptera: Hepialidae). Mitochondrial DNA. 2016;27(1):109–10. doi: 10.3109/19401736.2013.873926 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SEQ)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.