Abstract
Background
The mechanisms explaining the co-existence of asthma, eczema and rhinitis (allergic multimorbidity) are largely unknown. We investigated the mechanisms underlying multimorbidity between three main allergic diseases at a molecular level by identifying the proteins and cellular processes that are common to them.
Methods
An in silico study based on computational analysis of the topology of the protein interaction network was performed in order to characterize the molecular mechanisms of multimorbidity of asthma, eczema and rhinitis. As a first step, proteins associated to either disease were identified using data mining approaches, and their overlap was calculated. Secondly, a functional interaction network was built, allowing to identify cellular pathways involved in allergic multimorbidity. Finally, a network-based algorithm generated a ranked list of newly predicted multimorbidity-associated proteins.
Results
Asthma, eczema and rhinitis shared a larger number of associated proteins than expected by chance, and their associated proteins exhibited a significant degree of interconnectedness in the interaction network. There were 15 pathways involved in the multimorbidity of asthma, eczema and rhinitis, including IL4 signaling and GATA3-related pathways. A number of proteins potentially associated to these multimorbidity processes were also obtained.
Conclusions
These results strongly support the existence of an allergic multimorbidity cluster between asthma, eczema and rhinitis, and suggest that type 2 signaling pathways represent a relevant multimorbidity mechanism of allergic diseases. Furthermore, we identified new candidates contributing to multimorbidity that may assist in identifying new targets for multimorbid allergic diseases.
Introduction
During the last years, an increasing attention has been given to multimorbidity as the co-occurrence of two or more medical conditions within a person more often than would be expected by chance [1]. Asthma, eczema and rhinitis (so-called allergic diseases) are complex diseases which tend to co-occur in the same subjects. Children, in particular, frequently present concomitant or consecutive diseases [2,3], something that we refer to as allergic multimorbidity. The MeDALL (Mechanisms of the Development of Allergy) study [4], including both canonical epidemiological methods [5] and unsupervised cluster analysis [6], has shown that coexistence of asthma, eczema and rhinitis in the same child is more common than expected by chance alone, supporting the existence of a multimorbidity cluster. Although IgE sensitization is independently associated with excess multimorbidity of asthma, eczema and rhinitis, its presence accounted only for 38% of multimorbidity [5], suggesting that both IgE and non-IgE mechanisms are involved in the multimorbidity of these diseases [5,6].
Currently, the knowledge about the common mechanisms of allergic multimorbidity relies on few candidate mechanisms. Multiple genes have been identified through linkage and genome wide association studies (GWAS) that contribute to IgE sensitization as well as to asthma, eczema, rhinitis as individual entities. For example, of the ten genome-wide significant loci identified in a large meta-analysis of GWAS on allergic sensitization [7], five were significantly associated with asthma in the GABRIEL consortium asthma meta-analysis [8], but only two were associated with eczema [9]. One of these loci, the C11orf30-LRRC32 region may represent a common locus for allergic diseases through biological pathways involved in the regulation of IgE, polysensitization, eosinophilic inflammation and co-morbid allergic diseases [10]. Another common locus, STAT6, encodes a transcription factor expressed in B-cells, where it binds to DNA regions stimulating IgE production after B cell activation. In addition to IgE sensitization, many other mechanisms are likely to play a role in the allergic multimorbidity, including prevalent mutations (e.g. filaggrin) and rare genetic variants, structural variants associated to allergic diseases, and epigenetic mechanisms including DNA methylation and miRNA [11].
Despite the evidence of common mechanistic links between asthma, eczema and rhinitis, how these different mechanisms jointly contribute to the allergic multimorbidity is still unclear [10]. MeDALL has also made the hypothesis that allergic multimorbidity and polysensitization are associated with type 2 pathways [11, 12]. Diseases are seldom a consequence of a malfunction or expression change of one single protein, but rather the perturbation of intricate cellular networks, whose diversity and inter-dependence can also affect the activity of genes and proteins functionally linked to other disease-causing genes [13]. Thus, network-based analysis of biological networks, such as the metabolic network and the protein-protein interaction network, can help to identify the mechanisms of multimorbidities [14–16].
Following our findings in MeDALL showing that asthma, eczema and rhinitis in children constitute an allergic multimorbidity cluster suggesting common pathways [5, 6], we undertook an in silico study consisting in a computational network-based analysis to identify common proteins among these diseases and their corresponding cellular processes.
Materials and methods
Proteins associated to the multimorbidity of asthma, eczema and rhinitis
Data sources
We built a set of disease-associated genes from the following databases: Online Mendelian Inheritance in Man (OMIM) [17], ENSEMBL 77 Short Variations [18] databases (via BioMart [19]) and the Comparative Toxicogenomics Database (CTD) [20]. OMIM and CTD provide highly reliable gene-disease associations characterized through various experimental procedures and a process of expert curation of the literature. Since CTD contains a hierarchical collection of diseases, we considered genes associated to any of our diseases of interest and their descendants. Furthermore, only genes labeled as marker (i.e. experimentally associated with a disease) in the CTD database were used. Ensembl Variation contains curated information on disease-associated genetic variations from linkage data, genome-wide association studies and other genomic sources. Because the use of GWAS-derived data as a means for characterization of risk factors is debated [21–23], we wished to evaluate the influence that GWAS-derived data might have on our results. Thus, we repeated our analysis with a set of disease-associated gene set that excludes data derived solely from GWAS studies (S1 Text). Because the aim of our study was to explore the mechanisms of the diseases in the framework of the cellular network of protein interactions, the proteins encoded by the disease-associated genes were obtained in UniProt nomenclature [24]. Thus, only gene products known to exist at protein level (as defined by the UniProt database) were considered. Details on the databases and on the characterization of the gene-disease associations can be found in S1 Table and S2 Text.
Fraction of common proteins
The fraction of proteins associated to a pair of diseases was calculated as a classical Jaccard index [25]:
where |Ndis1 ∩ Ndis2| is the number of proteins common to disease 1 and disease 2, and is the number |Ndis1 U Ndis2| of distinct proteins in disease 1 and disease 2. Similarly, the Jaccard index for a triad of diseases was calculated as:
where |Ndis1 ∩ Ndis2 ∩ Ndis3| is the number of proteins common to disease 1, disease 2 and disease 3, and |Ndis1 U Ndis2 U Ndis3| is the number of distinct proteins in disease 1, disease 2 and disease 3.
Validation models
We devised two models to test the significance for the observed number of proteins common to any combination of asthma, eczema and rhinitis. In the first one, protein-disease associations were randomized from all proteins in the proteome (14,754) to generate a null distribution [26, 27]. This model tested whether the fraction of common proteins was higher than random expectation, and will be simply referred to as random model. We generated 103 instances of this model, and the statistical significance was assessed by means of a z-test. The second model tested if the observed fraction of common proteins was significantly higher than expected for any pair or trio of randomly chosen diseases belonging to the Immune System Diseases category of the CTD database. We generated 103 pairs and trios of these diseases avoiding the grouping of diseases that are subtypes (or descendants, in CTD terminology) of one another. The list of the selected immune system diseases and their associated proteins can be found in S2 Table. The list of all random pairs and trios of immune system diseases is available as S1 File. Because of the skewness in the distribution of the fraction of common proteins in the random models, the statistical significance was assessed by means of a comparison to the empirical distribution. All calculations in this study were performed with the R statistical software [28].
Network connectivity between asthma, eczema and rhinitis
Functional Interaction Network
In order to identify interactions between proteins associated to asthma, eczema and rhinitis, we built the functional interaction network (FIN). The FIN was obtained by combining data from: (1) the Reactome Functional Interaction Network (v. 2013), which contains pairwise protein interactions of different nature such as protein-protein interactions, gene expression interaction, metabolic interactions and signal transduction [29] (interactions annotated as predicted were discarded, as were those with score ≤ 0.5, as suggested by the authors); (2) the HIPPIE network, which integrates multiple experimental protein-protein interaction datasets [30] (only HIPPIE interactions scoring > 0.73 were considered, as they are accounted as high confidence by the authors); and (3) the innateDB database, which maintains a curated collection of protein-protein interaction data centered on innate immunity proteins [31]. We selected only those interactions from innatedDB with hpr < 20 and lpr < 20 [32, 33]. The FIN was graphically represented with the Cytoscape software [34].
Connectivity assessment
In order to assess if proteins common to any combination for asthma, eczema and rhinitis showed a larger connectivity in the FIN than expected by chance, we used the topological overlap, a generalized metric designed to measure the connectivity between two nodes in terms of the commonality of the nodes that they connect to [35, 36]. For any pair of proteins in the FIN, the topological overlap ranges from 0 (meaning absence of connectivity) to 1 (meaning maximal possible connectivity). The topological overlap (TO) between proteins a and b is defined as:
where |Na ∩ Nb| is the number of neighbors common to a and b (plus 1 if they are directly connected), and min(|Na|, |Nb|) is the smaller of the |Na| and |Nb| degrees. TOa,b = 0 if there is no connection between the a and b and if they do not share any neighbors. TOa,b = 1 if one set of neighbors is a subset of the other (although a and b may not be directly connected). We calculated the mean TO for proteins: a) common to the diseases under study, and b) unique to the diseases under study. An example of the calculation of the topological overlap can be found in S1 and S2 Figs.
Validation models
To obtain the random expectation for the topological overlap, we generated a null distribution based on 103 models where the disease-associated proteins were randomly exchanged by other proteins in the FIN, while keeping constant the number of proteins shared by the diseases and making sure that the exchanged proteins belonged in the same connectivity bin, so as to approximately keep the connectivity. The connectivity bin for a node of degree in the network d was calculated as round(ln(d)+1) [37]. This model tested whether the observed connectivity was higher than random expectation. The second model tested if the observed number of common proteins was significantly higher than expected for any pair or trio of randomly chosen immune system diseases. We selected immune system diseases as described in the previous section, adding the condition that at least one of their associated proteins should be present in the FIN. We generated 103 instances of each model. The statistical significance was tested as described in the previous section.
Cellular pathways shared between diseases
Information on the involvement of the proteins in cellular pathways was obtained from the BioCarta database [38] and mapped onto the FIN. The number of cellular pathways with more than 2 edges in the FIN was 273. We measured the fraction of edges rather than the fraction of nodes (i.e. proteins) because we considered that edges better represented the influence of the network connectivity. We calculated how similarly two diseases dis1 and dis2 perturbed a cellular pathway p by means of a Jaccard index, which we called Functional Similarity (FSim):
where epdis1 is the number of edges associated to the pathway p and disease 1, and epdis2 is the number of edges associated to the pathway p and disease 2. For three diseases, the Jaccard Index was defined as:
Where epdis3 is the number of edges associated to the pathway p and disease 3. An edge was considered associated to a disease if at least one of its nodes was associated to it. In order to avoid some pathways spanning throughout most of the FIN due to the presence of highly connected nodes, we considered an edge as associated to a cellular pathway only if both nodes were associated to it. The proteins associated to the cellular pathways used in this study, together with their interconnections in the FIN, are provided in S3 Table. For each pathway, this process gave us a distribution of observed Fsim values, which was tested against random expectation (using the model described in the previous section) and against the distribution obtained for random pairs/trios of randomly selected immune system diseases. Benjamini-Hochberg correction was used to account for multiple testing [39].
Predicting multimorbidity-associated proteins
In the previous steps, we described the mechanisms of multimorbidity of asthma, eczema and rhinitis based on known protein-disease associations. In this section, we wished to use the information contained in the FIN to predict novel proteins potentially involved in the multimorbidity process. To do so, we employed the NetZcore algorithm of the GUILD package [40]. This algorithm requires an initial group of nodes, called seeds, to be weighted with a score, which will be iteratively assigned to those nodes in the network depending on their connectivity. We generated a scored list of potential disease-associated proteins for each disease independently, using a seed score = 1 for known disease-associated proteins, and 0 otherwise. In order to compute z-scores, 103 random networks were generated by randomly exchanging edges between pairs of nodes [41].
The resulting lists of candidate proteins were compared to proteins suggested to be related to multimorbidity in the literature. We used the text-mining tool Génie to extract gene names from PubMed abstracts related to multimorbidity between the diseases included in this study [42]. Génie relies on NCBIs curated associations between MedLine records and unambiguous gene identifiers, and employs a Bayesian classifier to associate them to the user’s query terms, outperforming similar text-mining tools (S2 Text for details). The complete set of keywords that we used to retrieve multimorbidity-related proteins (and the Génie parameters for the queries) can be found in S4 Table. The complete list of multimorbidity-associated proteins returned by Génie can be found in S5 Table. Because PubMed abstracts may contain predicted protein-disease associations, we excluded any abstract containing the words predicted or prediction. We also checked that we were not excluding abstracts labelling genes as predictors or mentioning genes with predictive value, which could have resulted in false negatives (S2 Text, S6 Table). A Fisher's Exact Test was used to test the statistical association between our predictions and multimorbidity-related proteins obtained by Génie. Since scientific articles about comorbid diseases may not actually employ the term “comorbidity” in the abstract, we carried out a supplementary analysis were the words “comorbidity” and “comorbid” were not present in the PubMed search (see S7 Table for the results).
Results
Proteins associated to the multimorbidity of asthma, eczema and rhinitis
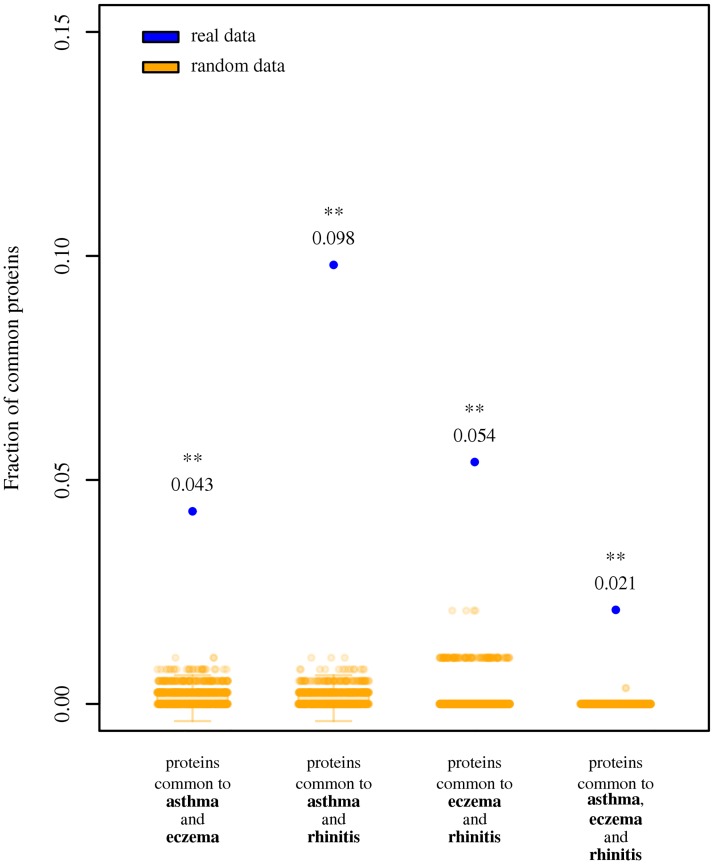
We observed 196 proteins associated to asthma, 49 to eczema, and 40 to rhinitis. S8 Table contains the list of all disease-associated proteins. S9 Table contains all disease-associated proteins for associations excluding GWAS-derived data. The number of proteins unique to one disease was 150 for asthma, 44 for eczema and 38 for rhinitis. The list of proteins common to any combination of diseases can be found in Table 1. The three pairs of diseases and the triad shared more proteins than could be expected by random chance (z-test; P < 0.01 in all cases; Fig 1; S3 Fig for absolute counts; S1 Text and S4 Fig for results excluding GWAS-derived data). Five proteins (IL4, IL13, IL1RL1, IL18R1 and TSLP) were common to all three diseases (z-test; P < 0.01 in all cases). Furthermore, they also shared a significantly larger fraction of proteins than observed for pairs and triads of randomly chosen immune system diseases (S5 Fig; empirical distribution test; P < 0.01 in all cases).
Table 1. List of proteins associated to at least two diseases.
The complete list of all disease-associated proteins can be found at S8 Table. Number of proteins associated to asthma and eczema: 16; to asthma and rhinitis: 35; to eczema and rhinitis: 5. To all three diseases: 5.
| Protein name (UniProt accession) | gene name (HGNC) | protein name | location | asthma | eczema | rhinitis |
|---|---|---|---|---|---|---|
| P05112 | IL4 | interleukin 4 | 5q31.1 | √ | √ | √ |
| P35225 | IL13 | interleukin 13 | 5q31.1 | √ | √ | √ |
| Q01638 | IL1RL1 | interleukin 1 receptor-like 1 | 2q12.1 | √ | √ | √ |
| Q13478 | IL18R1 | interleukin 18 receptor 1 | 2q12.1 | √ | √ | √ |
| Q969D9 | TSLP | thymic stromal lymphopoietin | 5q22.1 | √ | √ | √ |
| O95760 | IL33 | interleukin 33 | 9p24.1 | √ | √ | |
| P01584 | IL1B | interleukin 1 beta | 2q14.1 | √ | √ | |
| P05113 | IL5 | interleukin 5 | 5q31.1 | √ | √ | |
| P13501 | CCL5 | chemokine (C-C motif) ligand 5 | 17q12 | √ | √ | |
| P40425 | PBX2 | pre-B-cell leukemia homeobox 2 | 6p21.32 | √ | √ | |
| P51671 | CCL11 | chemokine (C-C motif) ligand 11 | 17q12 | √ | √ | |
| P51677 | CCR3 | chemokine (C-C motif) receptor 3 | 3p21.31 | √ | √ | |
| Q8IZI9 | IFNL3 | interferon, lambda 3 | 19q13.2 | √ | √ | |
| Q99466 | NOTCH4 | notch 4 | 6p21.32 | √ | √ | |
| Q9Y496 | KIF3A | kinesin family member 3A | 5q31.1 | √ | √ | |
| Q9Y4H4 | GPSM3 | G-protein signaling modulator 3 | 6p21.32 | √ | √ | |
| P01303 | NPY | neuropeptide Y | 7p15.3 | √ | √ | |
| P01906 | HLA-DQA2 | major histocompatibility complex, class II, DQ alpha 2 | 6p21.32 | √ | √ | |
| P01909 | HLA-DQA1 | major histocompatibility complex, class II, DQ alpha 1 | 6p21.32 | √ | √ | |
| P01912 | HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | 6p21.32 | √ | √ | |
| P01920 | HLA-DQB1 | major histocompatibility complex, class II, DQ beta 1 | 6p21.32 | √ | √ | |
| P13760 | HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | 6p21.32 | √ | √ | |
| P13761 | HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | 6p21.32 | √ | √ | |
| P16109 | SELP | selectin P | 1q24.2 | √ | √ | |
| P20039 | HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | 6p21.32 | √ | √ | |
| P21731 | TBXA2R | thromboxane A2 receptor | 19p13.3 | √ | √ | |
| P24394 | IL4R | interleukin 4 receptor | 16p12.1 | √ | √ | |
| P50135 | HNMT | histamine N-methyltransferase | 2q22.1 | √ | √ | |
| P84022 | SMAD3 | SMAD family member 3 | 15q22.33 | √ | √ | |
| Q13093 | PLA2G7 | phospholipase A2 group VII | 6p12.3 | √ | √ | |
| Q15399 | TLR1 | toll-like receptor 1 | 4p14 | √ | √ | |
| Q30134 | HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | 6p21.32 | √ | √ | |
| Q30167 | HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | 6p21.32 | √ | √ | |
| Q5Y7A7 | HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | 6p21.32 | √ | √ | |
| Q8NI36 | WDR36 | WD repeat domain 36 | 5q22.1 | √ | √ | |
| Q95IE3 | HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | 6p21.32 | √ | √ | |
| Q96D42 | HAVCR1 | hepatitis A virus cellular receptor 1 | 5q33.3 | √ | √ | |
| Q96QA5 | GSDMA | gasdermin A | 17q21.1 | √ | √ | |
| Q9GIY3 | HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | 6p21.32 | √ | √ | |
| Q9HBE5 | IL21R | interleukin 21 receptor | 16p12.1 | √ | √ | |
| Q9HBL0 | TNS1 | tensin 1 | 2q35 | √ | √ | |
| Q9NQ38 | SPINK5 | serine peptidase inhibitor, Kazal type 5 | 5q32 | √ | √ | |
| Q9TQE0 | HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | 6p21.32 | √ | √ | |
| Q9UIL8 | PHF11 | PHD finger protein 11 | 13q14.2 | √ | √ | |
| Q9UKT9 | IKZF3 | IKAROS family zinc finger 3 | 17q21.1 | √ | √ | |
| Q9UKW4 | VAV3 | vav guanine nucleotide exchange factor 3 | 1p13.3 | √ | √ |
Fig 1. Fraction of proteins associated to asthma, eczema and rhinitis.
Blue dots indicate the observed fraction of proteins. Orange scatter boxplots indicate random expectation. One asterisk: observed results are significantly larger than random expectation (z-test; P < 0.05). Two asterisks: observed results are significantly larger than random expectation (z-test; P < 0.01).
Network connectivity between asthma, eczema and rhinitis
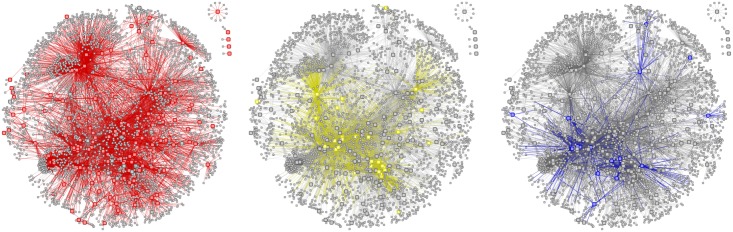
Functional Interaction Network (FIN) contained 9,847 proteins (nodes) involved in 154,164 functional interactions (edges), including 134 immune-related proteins. As not all the proteins associated to the three diseases had highly reliable experimentally-characterized interactions, the number of disease-associated proteins in the FIN was 163 for asthma, 43 for eczema and 32 for rhinitis. Fig 2 shows the portion of the FIN comprising proteins associated to the three diseases and their first-order neighbors (i.e. nodes directly connected to them). Fig 3A, 3B and 3C show the subnetworks for proteins associated to each disease. These figures also provide a visual representation of the pattern of interaction between disease-associate proteins in the FIN, and about what parts of the network are shared between diseases. The complete FIN is available in S2 File. The number of edges between proteins unique to each disease was lower than random chance for all pairs of diseases and for the triad (z-test; P < 0.01; S10 Table). We observed that almost all edges belonging to rhinitis-associated proteins are shared with asthma (only 5.4% of edges remain unique to rhinitis), a large overlap that is not seen in any other combination of diseases.
Fig 2. Functional Interaction Networks of asthma, eczema and rhinitis.
Fraction of the Functional Interaction Networks comprising the proteins associated to asthma, eczema, rhinitis and all proteins connected to them (i.e. their direct neighbors in the network). A node represents a protein. A link between two nodes represents a functional connection between them. Isolated nodes represent proteins not directly connected neither to any other disease-associated protein nor to any of its direct neighbors. (A) Large red nodes represent asthma-associated proteins. Red links represent functional connections of these proteins. (B) Large yellow nodes represent eczema-associated proteins. Yellow links represent functional connections of these proteins. (C) Large blue nodes represent rhinitis-associated proteins. Blue links represent functional connections of these proteins.
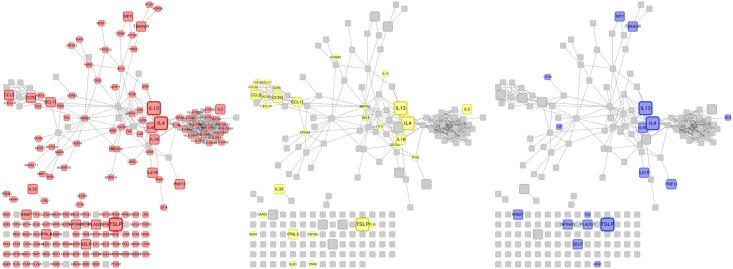
Fig 3. Functional Interaction Networks of asthma, eczema and rhinitis.
Fraction of the Functional Interaction Networks comprising the proteins associated to asthma, eczema and rhinitis. A node represents a protein. The size of the node represents the number of disease associations: the large nodes are associated to all diseases, the medium nodes are associated to two diseases, and the small nodes are associated to one disease. A link between two nodes represents a functional connection between them. These networks are a subset of the networks shown in Fig 2, where the direct neighbors have been removed. Isolated nodes at the bottom represent proteins not connected to any protein associated to asthma, eczema and rhinitis. (A) Red nodes represent asthma-associated proteins. (B) Yellow nodes represent eczema-associated proteins. (C) Blue nodes represent rhinitis-associated proteins.
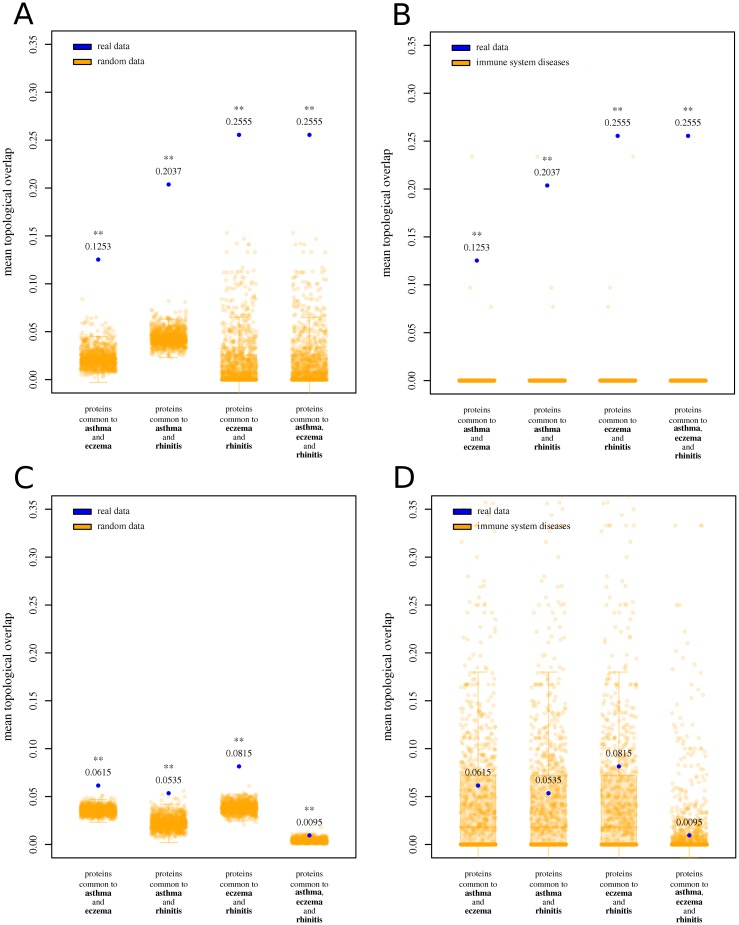
We captured the network-based relationship between the diseases under study with the topological overlap (TO). The TO between proteins common to any combination of diseases was significantly larger than random expectation, and also significantly larger than expected for random pairs/triads of immune-related diseases (z-test; P < 0.01 in both cases; Fig 4A and 4B). The same was true for proteins common to all three diseases (z-test; P < 0.01; Fig 4A and 4B). However, the TO between proteins unique to any combination of diseases, although significantly larger than random expectation (z-test; P < 0.01), was not significantly larger than that observed for random pairs/trios of immune-related diseases (Fig 4C and 4D). S6 Fig shows that proteins unique to each disease are more interconnected than random expectation for asthma, eczema and rhinitis, multimorbidity notwithstanding. For results excluding GWAS-derived data, see S1 Text and S7 Fig.
Fig 4. Mean topological overlap for proteins associated to asthma, eczema and rhinitis.
(A) Blue dots indicate the observed mean topological overlap (TO) for proteins common to asthma and eczema, asthma and rhinitis, eczema and rhinitis, and common to all three. Orange scatter boxplots indicate random expectation. (B) Blue dots indicate the observed mean TO for proteins common to the combinations of diseases shown in the previous figure. Orange scatter boxplots indicate observed TO values for pairs/trios of immune system diseases. (C) Blue dots indicate the observed mean TO between proteins unique to asthma and unique to eczema, unique to asthma and unique to rhinitis, unique to eczema and unique to rhinitis, and unique to each disease. Orange scatter boxplots indicate random expectation. (D) Blue dots indicate the observed mean TO for proteins unique to the combinations of diseases shown in the previous figure. Orange scatter boxplots indicate observed TO values for pairs/trios of immune system diseases. One asterisk: observed results are significantly larger than random expectation (P < 0.05). Two asterisks: observed results are significantly larger than random expectation (P < 0.01).
Cellular pathways shared between diseases
The origin of any disease can be traced to the perturbation of one or more cellular pathways. One common feature amongst comorbid diseases is that they share a common functionality, because the disruption of a multi-protein pathway can give rise to a number of diseases even if they do not have genes in common [43, 44]. We wished to identify individual pathways that could be linked to the multimorbidity between asthma, eczema and rhinitis, assuming that cellular pathways significantly perturbed by two or three of the diseases are more likely candidate mechanisms for multimorbidity. Fifteen cellular pathways revealed a significant functional similarity for distinct combinations of the three diseases (Table 2). The mechanisms of asthma and eczema were found to be identical for three pathways: Regulation of hematopoiesis by cytokines, GATA3 participate in activating the Th2 cytokine genes expression, and CCR3 signaling in eosinophils. The mechanisms of asthma and rhinitis were identical for the IL4 signaling pathway and the 4-1BB-dependent immune response pathways. Eczema and rhinitis did not show a complete functional similarity for any pathway, being GATA3 participate in activating the Th2 cytokine genes expression the pathway that both diseases share with the largest overlap (Fsim = 0.67). Other pathways were shared by pairs of diseases at different (but significant) degrees, of which two were exclusive of asthma and eczema: The role of eosinophils in the chemokine network of allergy, and Erythrocyte differentiation pathway. As for the triad of diseases, no pathway showed a perfected overlap (Fsim = 1). This indicates that there is no pathway (or part thereof) identically affected in all three diseases. However, two pathways show a significant three-way overlap: IL4 signaling pathway and GATA3 participate in activating the Th2 cytokine genes expression. S11 Table contains the functional similarity scores obtained excluding GWAS-derived data, which are discussed in S1 Text. Furthermore, all these observations were significantly larger than expected for pairs and trios of other immune-related diseases (empirical distribution test; P < 0.01).
Table 2. Functional similarity between asthma, eczema and rhinitis.
Numerical values show how similar is the use of a cellular pathway by pairs of trios of diseases. Similarity = 1 means that the diseases affect the pathway in exactly the same way. Similarity = 0 is represented by blank cells. Two asterisks: similarity is significantly larger than random expectation (z-test; P < 0.01). One asterisk: similarity is significantly larger than random expectation (z-test; P < 0.05). All significant similarities were also significantly larger than observed for pairs and trios of immune system diseases (empirical distribution test; P < 0.01).
| Cellular pathway | Overlap score for: | |||
|---|---|---|---|---|
| asthma and eczema | asthma and rhinitis | eczema and rhinitis | asthma, eczema and rhinitis | |
| Regulation of hematopoiesis by cytokines | 1.00 ** | |||
| CCR3 signaling in Eosinophils | 1.00 ** | |||
| The Role of Eosinophils in the Chemokine Network of Allergy | 0.57 ** | 0.14 | ||
| IL 4 signaling pathway | 0.53 ** | 1.00 ** | 0.53 ** | 0.53 ** |
| GATA3 participate in activating the Th2 cytokine genes expression | 1.00 ** | 0.67 * | 0.67 ** | 0.67 ** |
| Cytokine Network | 0.50 * | 0.33 | 0.50 ** | 0.30 ** |
| IL12 and Stat4 Dependent Signaling Pathway in Th1 Development | 0.28 | 0.40 | 0.33 ** | 0.23 ** |
| Th1/Th2 Differentiation | 0.25 | 0.48 | 0.42 ** | 0.23 * |
| Erythrocyte Differentiation Pathway | 0.50 * | |||
| The 4-1BB-dependent immune response | 0.22 | 1.00 * | 0.22 ** | 0.22 ** |
| Dendritic cells in regulating TH1 and TH2 Development | 0.56 ** | 0.25 | 0.33 ** | 0.22 * |
| Role of Tob in T-cell activation | 0.07 | 0.57 | 0.13 | 0.07 |
| Antigen Dependent B Cell Activation | 0.06 | 0.31 | 0.20 ** | 0.06 |
| Cytokines and Inflammatory Response | 0.23 | 0.24 | 0.25 * | 0.10 |
| IL2 Receptor Beta Chain in T cell Activation | 0.03 | 0.27 | 0.04 | 0.02 |
| Selective expression of chemokine receptors during T-cell polarization | 0.41 | 0.58 | 0.37 ** | 0.30 ** |
| IL 5 Signaling Pathway | 0.30 | 0.30 | 0.20 * | 0.10 |
| NFkB activation by Nontypeable Hemophilus influenzae | 0.25 | 0.25 | ||
| TGF beta signaling pathway | 0.58 | |||
| Adhesion and Diapedesis of Granulocytes | 0.11 | 0.33 | ||
| Cell Cycle: G1/S Check Point | 0.42 | |||
| Monocyte and its Surface Molecules | 0.40 | |||
| Antigen Processing and Presentation | 0.33 | |||
| Cells and Molecules involved in local acute inflammatory response | 0.29 | |||
| Bystander B Cell Activation | 0.27 | |||
| Activation of Csk by cAMP-dependent Protein Kinase Inhibits. . . | 0.26 | |||
| Lck and Fyn tyrosine kinases in initiation of TCR Activation | 0.26 | |||
| The Co-Stimulatory Signal During T-cell Activation | 0.26 | |||
| Signal transduction through IL1R | 0.25 | |||
| B Lymphocyte Cell Surface Molecules | 0.23 | |||
| IL10 Anti-inflammatory Signaling Pathway | 0.10 | |||
| Cystic Fibrosis Transmembrane Conductance Regulator And Beta 2. . . | 0.08 | |||
| Pertussis toxin-insensitive CCR5 Signaling in Macrophage | 0.07 | |||
| Role of ERBB2 in Signal Transduction and Oncology | 0.05 | |||
| IL 6 signaling pathway | 0.05 | |||
| Ceramide Signaling Pathway | 0.04 | |||
| NO2-dependent IL 12 Pathway in NK cells | 0.04 | |||
| NFAT and Hypertrophy of the heart (Transcription in the broken heart) | 0.04 | |||
| Signaling of Hepatocyte Growth Factor Receptor | 0.03 | |||
| TNFR1 Signaling Pathway | 0.03 | |||
| HIV-I Nef: negative effector of Fas and TNF | 0.03 | |||
| IL 2 signaling pathway | 0.03 | |||
| TNF/Stress Related Signaling | 0.03 | |||
| Keratinocyte Differentiation | 0.03 | |||
| MAPKinase Signaling Pathway | 0.02 | |||
Predicting multimorbidity-associated proteins
The 30 top-scoring protein candidates that are common to more than one disease are shown in Table 3. The complete list of candidates is shown in S12 Table (and in S13 Table for results excluding GWAS-derived data; see S1 Text for discussion). Although no gold-standard set for multimobidity-related proteins exists for the diseases under study, we observed a clear statistical association between our predictions and those extracted from biomedical literature by the computational tool Génie, with a significant p-value in all cases (Fisher's Exact test; P < 0.01; Table 4).
Table 3. Potential disease-associated proteins predicted for asthma, eczema and rhinitis.
NetZcore prediction scores are shown as z-scores. Proteins are ranked according to their average z-score for all diseases. Empty cell: the protein was not predicted to be associated with the disease with z-score > 2.31 (corresponding to P < 0.01). Exp: the protein is experimentally known to be associated to the disease. This table only shows the 30 top-scoring proteins that were found to be associated to more than one disease. The complete list is available in S12 Table.
| Protein name (UniProt accession) | gene name (HGNC) | Protein name | z-score asthma | z-score eczema | z-score rhinitis |
|---|---|---|---|---|---|
| Q9Y496 | KIF3A | kinesin family member 3A | exp | exp | 10.26 |
| P24394 | IL4R | interleukin 4 receptor | exp | 4.21 | exp |
| Q9HBE5 | IL21R | interleukin 21 receptor | exp | 3.95 | exp |
| Q9UIL8 | PHF11 | PHD finger protein 11 | exp | 2.85 | exp |
| P16278 | GLB1 | galactosidase beta 1 | 5.56 | exp | 6.03 |
| O95715 | CXCL14 | chemokine (C-X-C motif) ligand 14 | exp | 10.85 | |
| P20036 | HLA-DPA1 | major histocompatibility complex, class II, DP alpha 1 | exp | 7.93 | |
| P04440 | HLA-DPB1 | major histocompatibility complex, class II, DP beta 1 | exp | 7.81 | |
| P13500 | CCL2 | chemokine (C-C motif) ligand 2 | exp | 7 | |
| P01903 | HLA-DRA | major histocompatibility complex, class II, DR alpha | exp | 6.96 | |
| Q29974 | HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | exp | 6.68 | |
| P01911 | HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | exp | 6.68 | |
| P14784 | IL2RB | interleukin 2 receptor subunit beta | exp | 2.79 | 2.35 |
| P29460 | IL12B | interleukin 12B | exp | 4.3 | |
| P08887 | IL6R | interleukin 6 receptor | exp | 4.29 | |
| Q8TE73 | DNAH5 | dynein, axonemal, heavy chain 5 | exp | 3.57 | |
| P35625 | TIMP3 | TIMP metallopeptidase inhibitor 3 | exp | 2.46 | |
| P17693 | HLA-G | major histocompatibility complex, class I, G | exp | 2.37 | |
| Q9P0G3 | KLK14 | kallikrein related peptidase 14 | 12.9 | 3.78 | 18.27 |
| Q9Y337 | KLK5 | kallikrein related peptidase 5 | 9.01 | 3.64 | 12.1 |
| P14091 | CTSE | cathepsin E | 11.16 | 13.22 | |
| P53634 | CTSC | cathepsin C | 11.16 | 13.22 | |
| Q9UBX1 | CTSF | cathepsin F | 11.16 | 13.22 | |
| Q99538 | LGMN | legumain | 10.55 | 12.39 | |
| O00287 | RFXAP | regulatory factor X associated protein | 10.46 | 12.36 | |
| P10619 | CTSA | cathepsin A | 10.24 | 12.06 | |
| P43235 | CTSK | cathepsin K | 9.86 | 11.47 | |
| P52732 | KIF11 | kinesin family member 11 | 9.64 | 11.55 | |
| O15066 | KIF3B | kinesin family member 3B | 9.61 | 11.51 | |
| O14782 | KIF3C | kinesin family member 3C | 9.4 | 11.28 |
Table 4. Statistical association between predicted multimorbidity-associated proteins and literature predictions.
Literature predictions were automatically extracted from PubMed abstracts using the Génie data mining tool. Statistical association was calculated by means of a Fisher's Exact Test. Confidence intervals are shown in parentheses.
| association | ||
|---|---|---|
| odds ratio | P | |
| Literature predictions for asthma and eczema | 14.09, 95% CI [6.69, 27.47] | 7.22·10−10 |
| Literature predictions for asthma and rhinitis | 10.22, 95% CI [3.78, 23.73] | 1.61·10−5 |
| Literature predictions for eczema and rhinitis | 3.78, 95% CI [1.93, 6.81] | 0.00011 |
| Literature predictions for asthma, eczema and rhinitis | 6.32, 95% CI [1.90, 16.58] | 0.0019 |
Discussion
In this paper, we presented a strategy to measure multimorbidity between asthma, eczema and rhinitis at cellular network level. Asthma, eczema and rhinitis share a larger number of associated proteins than expected by chance, and exhibit a significant degree of interconnectedness in the functional interaction network. Computational analysis of the network identified 13 cellular pathways as significantly overlapping between pairs of diseases, and thus potentially involved in allergic multimorbidity and polysensitization mechanisms. Three of these pathways were remarkable because they overlapped in all three pairs of diseases as well as in the three diseases simultaneously: IL2 activation, IL4 signaling pathway and GATA3 participate in activating the Th2 cytokine genes expression. Furthermore, the network analysis allowed predicting many additional proteins as new candidates contributing to multimorbidity. This study strongly supports the a priori MeDALL hypothesis proposing that in children asthma, eczema and rhinitis co-occur as an allergic multimorbidity cluster [5, 6] and that both IgE and non-IgE related pathways represent common mechanisms of the multimorbidity of allergic diseases [11, 12].
Strengths and weaknesses
Our study has some important strengths. This is, to our knowledge, the first time that the multimorbidity between asthma, eczema and rhinitis has been systematically explored with a computational approach. Although there are other network-based studies of asthma, underlying mechanisms of allergic multimorbidity were not considered [45, 46]. For our analysis, we integrated abundant curated evidence from different sources, and we relied on experimentally validated protein-disease associations. Because our aim was to provide a synthesis of current biological knowledge in a framework that can be readily interpreted by researchers, we selected a set of well-described cellular pathways. However, we acknowledge that drawback of our method may be that known tools to find biological plausible pathways may today under-represent relevant tissues that are less accessible (e.g. lung tissue with resident cells like epithelial cells) that have an important barrier and immunological function. For the construction of the FIN we used not only protein-protein interaction but also other kinds of protein-protein functional relationships (e.g. signaling, expression regulation, metabolic relationships) allowing the identification of a wide range of mechanisms. Our network-based analysis revealed information on protein interconnectivity and cellular pathways that could not have been uncovered otherwise. Finally, because our approach is not explicitly disease-centered, it can potentially be applied to any other groups of diseases.
On the other hand, our computational approach is subject to potential limitations, the main of which is the completeness and noisiness of the FIN [14, 23, 26]. We chose to consider only the most reliable interactions, which might have resulted in removing potentially true interactions (false negatives). Also, despite selecting only high-confidence interactions, some false interactions might have been included in this study (false positives). Furthermore, it is worth mentioning that, for simplicity, our network is undirected and independent of differential expression patterns or external factors (e.g. allergens). However, some studies have shown that the current interaction databases do allow the systematic investigation of disease mechanisms [23]. Although we applied the usual procedure of multiple testing correction in our statistical analysis to avoid false positives (type I errors), some authors have argued against its use on the grounds that these corrections inflate the number of false negatives (type II errors) [47]. Finally, it must be taken into account that our gene and protein selection is subject to some restrictions. First, we selected only curated disease-protein associations from several databases, so the quality of our dataset depends on their criteria as to establish what disease-protein associations are the most reliable. Consequently, we chose three manually-curated databases (CTD, OMIM and Ensembl Variation) that are widely employed in computational genotype-phenotype association studies. Part of our associations were GWAS-derived, and there is a debate on the use of this kind of data as a means for characterization of risk factors, based mainly on concerns about its statistical processing and the fact that, without additional functional information, it is often difficult in GWAS analysis to implicate a specific gene in a genotype-phenotype association [48]. However, when integrated with other gene-disease association methods, and particularly when applied to pathway and network analysis, it has been shown to increase statistical power and provide valid results. These results give us additional confidence on the soundness of our results. Second, we cannot exclude publication bias, and the fact that the associations between proteins and diseases may be the result of differences in the interest of researchers and funding sources. Third, as most of the studies captured in this computational study have been conducted in affluent countries where atopy is more common, we should be cautious when inferring our findings to populations where atopy plays a less relevant role in asthma, rhinitis and eczema. Finally, asthma in particular is considered an umbrella term encompassing multiple endotypes, which were not studied independently.
In the foreseeable future, improvements in the quality and coverage of protein interactions will allow for more accurate network-based studies. Although we selected proteins experimentally associated to the diseases under study, the shared proteins and mechanisms are in silico observations that will need experimental validation. However, some of the described mechanisms are consistent with the literature as described below.
Proteins associated to the multimorbidity of asthma, eczema and rhinitis
We found that the number of disease-associated proteins shared by asthma, eczema and rhinitis could neither be explained by random chance nor by the fact that all diseases are related to the immune system. IL4, IL13, IL1RL1, IL18R1 and TSLP were the only proteins common to the three diseases, and thus are important candidates to explain allergic multimorbidity and polysensitization. IL4, IL13 and TSLP are cytokines that have been proposed to have a role in multimorbidity in non-atopic [49, 50] and atopic diseases [51]. It is known that both IL4 and IL13 are involved type 2 responses, in IgE production in asthma, rhinitis [52] and in eczema [53], as well as in the cellular inflammation of the three diseases [54] as well as in the regulation of the epithelial barrier function in the skin [55], the airways [56], and type 2 responses [57]. L1RL1 is part of the IL33 receptor complex, which drives TH2 inflammation. It has been associated to asthma [58], and also plays an important role in intermittent allergic rhinitis [59]. IL33 has been long associated with asthma but also with allergic rhinitis in murine models [60]. Furthermore, The associated region on chromosome 2q12 contains the family of IL1-receptor genes, IL1RL1 (IL1 receptor-like 1), IL18R1 (IL18 receptor 1), and IL18RAP (IL18 receptor accessory protein). Members of this family are abundantly expressed in the skin, and IL1RL1 is involved in Th2 responses and is important for the pathogenesis of eczema [61, 62]. TSLP is an epithelial IL7-like cytokine activating Th2 responses [63], and is responsible for a pattern of inflammation suggestive of multimorbidity between asthma and chronic obstructive pulmonary disease [64], and between different types of dermatitis [65]. IL4, IL13, IL1RL1, IL18R1 and TSLP are also the only proteins found to be common to eczema and rhinitis.
Proteins associated to asthma and eczema are all related to immunity (IL1ß, IL5, IL33, C-C motif chemokine 5, eotaxin). IL18 is also involved in the cell biology of the inflammasome [66]. IL33 is a crucial regulator of mast cell functions [67] and may be of great importance to understand multimorbidity and polysensitization as it as it modulates the expression of human β-defensin 2 in human primary keratinocytes, and may influence the susceptibility to bacterial superinfection in acute atopic dermatitis [68].
Amongst the proteins common to asthma and rhinitis,there is a number of members of the HLA-DRB and HLD-DQ families, which have major roles in T-cell activation. HLA plays a role in the development of the IgE response to the allergens, but genetic regulation appears to differ in mono- and polysensitized patients. Associations between HLA haplotypes or HLA-DQ/DR molecules and allergen sensitivity were confirmed only in patients either with low total serum IgE levels or monosensitized [69–71]. The presence of hepatitis A virus cellular receptor 1 (HAVCR1) is intriguing, although it may protect against atopy when hepatitis A virus infection rates were high [72]. We also found histamine N-methyltransferase (HNMT), a key protein in histidine metabolism, involved in the airways epithelium in asthma and rhinitis [73]. Finally, exclusion of disease-protein associations derived solely from GWAS studies did not noticeably alter our observations (S1 Text).
Network connectivity between asthma, eczema and rhinitis
Previous studies have concluded that biological network-level links between diseases contribute to the likelihood of individuals developing simultaneous conditions [74]. Visual inspection of Figs 2 and 3 suggests the existence of a region in the functional network is shared by all three diseases. We observed that the connectivity between proteins common to the diseases (measured as the average topological overlap) was significantly larger than random expectation. Although the type 2 response appears to be an important mechanism of multimorbidity and polysensitization, we also observed that the connectivity between proteins shared by asthma, eczema and rhinitis could not be explained solely by the fact that they all are immune-related diseases. Since network connectivity has been largely used as a measure of modularity (and, thus, of mutual functional influence) between proteins [75], these observations imply the existence of a core mechanism specific to the three diseases under study. On the other hand, proteins unique to each disease did not seem to contribute specifically to multimorbidity through their interactions with proteins unique to the other diseases (their modularity was not significantly larger than that of pairs/trios of other immune-related diseases). The analysis of the modularity between proteins unique to each disease suggested the existence of (at least) partial dissociated mechanisms unique to each disease, which would be responsible for their distinct patterns of occurrence and isolated symptomatology. This is supported by the fact that the number of edges between proteins unique to each disease was lower than random chance for all pairs of diseases and for the triad. Also, it has to be noted that, in terms of network functionality, rhinitis shares most of its mechanisms with asthma (a commonality that was not obvious when comparing nodes alone). This relationship has been observed elsewhere [11, 76].
Cellular pathways shared between diseases
The presence of type 2-related pathways amongst the top-scoring pathways affected by the three diseases supports the influence of the type 2 gene cluster in multimorbidity between asthma and eczema [77]. The pathway GATA3 participates in activating the Th2 cytokine genes expression show the highest overlap for the three diseases. Transcription factor GATA3 is highly expressed in peripheral blood ILC2s cells during inflammatory responses, and is essential for interleukin-4 expression [78]. Recently, therapeutic targeting GATA3 has proven beneficial in attenuating asthmatic responses [79]. ILC2, a type of innate lymphoid cells producing cytokines such as IL9, and IL13, plays an important role in eosinophilic asthma [80] in response to respiratory infections [81], and is over-expressed in eczema lesions and in allergic rhinitis subjects [82, 83]. The three diseases also show a significantly high similarity in the use of the pathway IL 4 signaling pathway suggesting the existence a core mechanism around ILC2s and IL4 that connects the mechanisms of the three diseases. It is also noteworthy the presence of two eosinophil-related pathways shared solely by asthma and eczema: CCR3 signaling in Eosinophils and The role of eosinophils in the chemokine network of allergy. Furthermore, the similarity that we observed in the use of cellular pathways by asthma, eczema and rhinitis could not be explained by their immune-related nature alone, despite the fact that some response mechanisms are shared among immune-related diseases [46]. This suggests that these mechanisms are specific for multimorbidity between the three diseases under study.
Predicting multimorbidity-associated proteins
We also used the network connectivity measures to predict proteins that could be associated with multimorbidity. Amongst the top-scoring proteins predicted to play a role in a three-way multimorbidity between asthma, eczema and rhinitis we identified interleukin receptors IL4R, IL21R and IL2RB. Both IL4R and IL21R are strongly related to the immune response, and are already known to be associated to asthma and rhinitis. IL2RB is currently associated to asthma and predicted to be associated also to eczema and rhinitis. All these receptors bind relevant cytokines regulating lymphocytic activity. Kallikrein-5 and kallikrein-14 (KLK5, KLK14) are two other proteins predicted to play a role in a three-way multimorbidity. Both proteins belong to a family of highly specific serine-proteinases whose deregulation is linked to several allergic diseases [84]. Recently, one kallikrein gene family member (KLKB1) was found to regulate soluble cleaved urokinase plasminogen activator receptor [85]. KLKB1 cleaves UPAR and negates soluble cleaved UPAR effects on primary human bronchial epithelial cells. Interestingly, scuPAR is encoded by UPAR, a gene found by positional cloning in asthma families [85]. Other proteins predicted to be related to multimorbidity are KIF3A, already known to be associated to asthma, eczema and the atopic march [86], and predicted to be implicated also in rhinitis. Interestingly, seven KIF3A SNPs were actually reported to be associated with rhinitis in one study that was not incorporated to the databases used in this study [87], thus supporting the reliability of the prediction method. Lastly, PHF11, a transcriptional activator of the Th1 effectors interleukin-2 (IL2) and interferon-γ (IFNG), is predicted to be associated to eczema. The highest-scoring candidate protein for comorbidity between asthma and eczema were C-X-C motif chemokine 14 (CXCL14), a known mediator in inflammatory processes, and myeloblastin (PRTN3), a matrix-degrading proteinase also related to asthma [88], and chemokine ligand CCL2, known to be upregulated by IL31, which in turn is one of the main inducers of skin pruritus in eczema [89]. As candidates for comorbidity between asthma and rhinitis, we identified in the top-scoring position several members of the class II MHC, owing probably to the number of proteins of the same family which are already known to be associated to both diseases. Also, we identified several members of the cathepsin family of proteases, known to be involved in many inflammatory processes, amongst them airway inflammation.
It has to be noted, though, that the identification of an association between a protein and a disease depends on the variable criteria used in different databases. It is thus possible that some of the predicted proteins in our study are reported as actually associated to the diseases according to some other databases or studies. Furthermore, the association test performed (Table 4) excluded predicted proteins from PubMed abstracts (see Methods). This minimized the number of false positives when comparing these literature-based protein sets to our predictions. However, because we used a keyword-based search, it is possible that some false positives may have been produced because of the diverse wording employed in abstracts. Also, excluding the words predicted and prediction (see Methods) might have introduced some false negatives by removing genes
Applying systems medicine to the understanding of allergic diseases
Asthma, eczema and rhinitis are salient examples of complex multimorbid allergic diseases. In the recent years, the importance of applying systems medicine approaches to unravel the complexity of chronic diseases has been highlighted [23, 46]. A central notion of this approach is that diseases are seldom a consequence of a malfunction or expression change of one single protein, but rather the perturbation of intricate cellular networks [13]. The MeDALL study was purposely designed to apply systems medicine approach to understanding the complexity of allergic diseases. With the present computational analysis of the mechanisms of asthma, eczema and rhinitis, we provided a valuable example of how systems medicine can contribute to the understanding of multimorbidity.
Conclusions
Asthma, eczema and rhinitis share a larger number of proteins than expected by chance, as well as a significantly higher degree of interconnectedness in the functional interaction network. The fact that these observations cannot be explained solely by their immune-related nature imply that other interrelated mechanisms are common to the three diseases.
Type 2 response appears to be an important mechanism of multimorbidity, consistent with our previous finding that multimorbidity and polysensitization are closely associated. However, the presence of non-type-2 cellular pathways and the connectivity between proteins shared by asthma, eczema and rhinitis could not be explained solely by the association of the three diseases to the immune system. This suggests that multimordity results from a wider range of interrelated cellular mechanisms.
Computational analysis of the network identified 15 cellular pathways potentially involved in allergic multimorbidity mechanisms. Two of these pathways were remarkable because they overlapped in all three diseases: IL4 signaling pathway and GATA3 participate in activating the Th2 cytokine genes expression.
Network analysis suggested additional proteins as new candidates contributing to multimorbidity. Those proteins were kallikreins, cathepsins and members of the class II MHC, among others. These results are of importance as they can lead to the assessment of new targets for multimorbid allergic diseases.
The study results strongly agrees with previous MeDALL findings showing that in children asthma, eczema and rhinitis co-occur as an allergic multimorbidity cluster likely due to common mechanisms.
Supporting information
(PDF)
(PDF)
(A) Disease A (orange ellipse) is associated to 6 proteins: p1, p2, p3, p4, p5, p6. Disease B (blue ellipse) is associated to 6 proteins: p4, p5, p6, p7, p8, p9. Three proteins are common to both diseases (p4, p5, p6; shown in the intersection of both diseases, in blue with orange border). (B) Mapping of the disease-associated proteins on to a dummy network. As in the previous figure, common proteins (p4, p5, p6) are shown in blue with orange border. (C) Calculation of the average topological overlap (TO) between the common proteins. (D) Calculation of the average topological overlap between the proteins unique to each disease.
(PNG)
(A) Disease A (orange ellipse) is associated to 7 proteins: p1, p2, p3, p4, p5, p6, p7. Disease B (blue ellipse) is associated to 7 proteins: p4, p5, p6, p7, p8, p9, p10. Disease C (purple ellipse) is associated to 5 proteins: p4, p5, p6, p7, p11. Proteins common to all diseases are p6 and p7 (shown as a blue circles with double purple-orange border). Proteins common solely to diseases A and B are p4 and p5 (shown as blue circles with orange border). There are no proteins common solely to diseases A and C, neither to diseases B and C. (B) Mapping of the disease-associated proteins on to a dummy network. (C) Calculation of the average topological overlap (TO) between the common proteins. (D) Calculation of the average topological overlap between the proteins unique to each disease.
(PNG)
Blue dots indicate the observed fraction of proteins. Orange scatter boxplots indicate random expectation. One asterisk: observed results are significantly larger than random expectation (z-test; P < 0.05). Two asterisks: observed results are significantly larger than random expectation (z-test; P < 0.01).
(PNG)
Blue dots indicate the observed fraction of proteins. (A) Orange scatter boxplots indicate random expectation. One asterisk: observed results are significantly larger than random expectation (z-test; P < 0.05). Two asterisks: observed results are significantly larger than random expectation (z-test; P < 0.01). (B) Orange scatter boxplots indicate fraction of associated proteins for pairs/trios of immune system diseases. One asterisk: observed results are significantly larger than random expectation (empirical distribution test; P < 0.05). Two asterisks: observed results are significantly larger than random expectation (empirical distribution test; P < 0.01).
(PNG)
Blue dots indicate the observed fraction of proteins. Orange scatter boxplots indicate fraction of associated proteins for pairs/trios of immune system diseases. One asterisk: observed results are significantly larger than random expectation (empirical distribution test; P < 0.05). Two asterisks: observed results are significantly larger than random expectation (empirical distribution test; P < 0.01).
(PNG)
Blue dots indicate the observed mean topological overlap (TO) between proteins uniquely associated to either asthma, eczema or rhinitis. Orange scatter boxplots indicate random expectation. One asterisk: observed results are significantly larger than random expectation (P < 0.05). Two asterisks: observed results are significantly larger than random expectation (P < 0.01).
(PNG)
(A) Blue dots indicate the observed mean topological overlap (TO) for proteins common to combinations of asthma, eczema and rhinitis. Orange scatter boxplots indicate random expectation. (B) Blue dots indicate the observed mean TO for proteins common to combinations of asthma, eczema and rhinitis. Orange scatter boxplots indicate observed TO values for pairs/trios of immune system diseases. (C) Blue dots indicate the observed mean TO for proteins unique to combinations of asthma, eczema and rhinitis. Orange scatter boxplots indicate random expectation. (D) Blue dots indicate the observed mean TO for proteins unique to combinations of asthma, eczema and rhinitis. Orange scatter boxplots indicate observed TO values for pairs/trios of immune system diseases. One asterisk: observed results are significantly larger than random expectation (P < 0.05). Two asterisks: observed results are significantly larger than random expectation (P < 0.01).
(PNG)
Tab-delimited text file. Diseases were extracted from the CTD database (see Methods in the main paper).
(GZ)
This network was generated by combining data from the HIPPIE, Reactome and InnateDB databases (see Methods in the main paper).
(GZ)
OMIM: On-line Mendelian Inheritance in Man; CTD: Comparative Toxicogenomics Database; EV84: Ensembl Variation 84.
(PDF)
Disease-associated proteins (provided as UniProt accessions), are separated by a semicolon. The network column indicates whether the disease has at least one associated protein present in then FIN (1) or not (0).
(XLS)
Pathway-associated proteins (provided as UniProt accessions) and interactions between pathway-associated proteins are separated by a semicolon.
(XLS)
The Génie tool (http://cbdm-01.zdv.uni-mainz.de/~jfontain/cms/) was used to extract gene names present in PubMed abstracts related to a topic of interest, as defined by a PubMed query.
(DOC)
Columns are as follows: Query: PubMed query used; Rank: position (rank) of the gene (ordered by ascending FDR); GeneID: gene name; n: number of PubMed abstracts manually associated to the gene; n_pos: number of PubMed abstracts that Génie has selected at P < 0.01; FDR: False Discovery Rate; Top 10 PMIDs: top 10 PMIDs for each gene, together with the associated p-value. More information at http://cbdm-01.zdv.uni-mainz.de/~jfontain/cms/.
(XLS)
The Génie tool was used to extract gene names present in PubMed abstracts related to a topic of interest, as defined by a PubMed query. Unlike S4 Table, this table does not exclude the terms predicted nor prediction, and includes the terms predictive or predictor.
(DOC)
Literature predictions were automatically extracted from PubMed abstracts using the Génie data mining tool. The terms used to query PubMed database were those shown in S4 Table minus the word “comorbidity”. Statistical association was calculated by means of a Fisher's Exact Test. Confidence intervals are shown in parentheses.
(DOC)
Gene names are provided as common (HGNC) gene names and protein names as UniProt accessions.
(XLS)
Gene names are provided as common (HGNC) gene names and protein names as UniProt accessions.
(XLS)
Mean random expectation in shown between parenthesis. The fraction of common edges was calculated using the Jaccard index (see Methods in the main paper). The fraction of common edges is statistically larger than random expectation in all four cases (p-value < 0.01). The fraction of unique edges is statistically lower than random expectation in all four cases (p-value < 0.01). The number of edges associated to asthma: 8560; number of edges associated to eczema: 2584; number of edges associated to rhinitis: 2898.
(DOC)
Numerical values show how similar is the use of a cellular pathway by pairs of trios of diseases. Similarity = 1 means that the diseases affect the pathway in exactly the same way. Similarity = 0 is represented by blank cells. Dark blue cells: similarity is significantly larger than random expectation (z-test; P < 0.01). Light blue cells: similarity is significantly larger than random expectation (z-test; P < 0.05). All significant similarities were also significantly larger than observed for pairs and trios of immune system diseases (empirical distribution test; P < 0.01).
(XLS)
Gene names are provided as common gene names and protein names as UniProt accessions. NetZcore prediction scores are shown as z-scores. Proteins are ranked according to their average z-score for all diseases. Empty cell: the protein was not predicted to be associated with the disease with z-score > 2.31 (corresponding to P < 0.01). Exp: the protein is experimentally known to be associated to the disease. Proteins predicted (or experimentally known) to be associated to more than one disease are candidates to multimorbidity.
(XLS)
Gene names are provided as common gene names and protein names as UniProt accessions. NetZcore prediction scores are shown as z-scores. Proteins are ranked according to their average z-score for all diseases. Empty cell: the protein was not predicted to be associated with the disease with z-score > 2.31 (corresponding to P < 0.01). Exp: the protein is experimentally known to be associated to the disease. Proteins predicted (or experimentally known) to be associated to more than one disease are candidates to multimorbidity.
(XLS)
Acknowledgments
We thank Ricard Solé, PhD and Dieter Maier, PhD for fruitful discussions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Spanish Ministry of Science and Innovation (MICINN) grant BIO2011-22568, and by Mechanisms of the Development of ALLergy (MeDALL), a collaborative project done within the EU under the Health Cooperation Work Programme of the Seventh Framework programme (grant agreement number 261357). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380: 37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 2.Ballardini N, Kull I, Lind T, Hallner E, Almqvist C, Ostblom E, et al. Development and comorbidity of eczema, asthma and rhinitis to age 12: data from the BAMSE birth cohort. Allergy 2012; 67: 537–44. 10.1111/j.1398-9995.2012.02786.x [DOI] [PubMed] [Google Scholar]
- 3.Hopper JL, Bui QM, Erbas B, Matheson MC, Gurrin LC, Burgess JA, et al. Does eczema in infancy cause hay fever, asthma, or both in childhood? Insights from a novel regression model of sibling data. J Allergy Clin Immunol 2012; 130: 1117–1122. 10.1016/j.jaci.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 4.Bousquet J, Anto J, Auffray C, Akdis M, Cambon-Thomsen A, Keil T, et al. MeDALL (Mechanisms of the Development of ALLergy): an integrated approach from phenotypes to systems medicine. Allergy 2011; 66: 596–604. 10.1111/j.1398-9995.2010.02534.x [DOI] [PubMed] [Google Scholar]
- 5.Pinart M, Benet M, Annesi-Maesano I, von Berg A, Berdel D, Carlsen KC, et al. Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: a population-based cohort study. Lancet Respir Med. 2014; 2: 131–40. 10.1016/S2213-2600(13)70277-7 [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Aymerich J, Benet M, Saeys Y, Pinart M, Basagaña X, Smit HA, et al. Phenotyping asthma, rhinitis, and eczema in MeDALL population-based birth cohorts: an allergic comorbidity cluster. Allergy 2015; 70: 973–84. 10.1111/all.12640 [DOI] [PubMed] [Google Scholar]
- 7.Bønnelykke K, Matheson MC, Pers TH, Granell R, Strachan DP, Alves AC, et al. Meta-analysis of genome-wide association studies identifies ten loci influencing allergic sensitization. Nat Genet. 2013; 45: 902–6. 10.1038/ng.2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010; 363: 1211–21. 10.1056/NEJMoa0906312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paternoster L, Standl M, Chen CM, Ramasamy A, Bønnelykke K, Duijts L, et al. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat Genet. 2011; 44: 187–92. 10.1038/ng.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koppelman GH, Nawijn MC. Recent advances in the epigenetics and genomics of asthma. Curr Opin Allergy Clin Immunol. 2011; 11: 414–9. 10.1097/ACI.0b013e32834a9573 [DOI] [PubMed] [Google Scholar]
- 11.Bousquet J, Anto JM, Wickman M, Keil T, Valenta R, Haahtela T, et al. Are allergic multimorbidities and IgE polysensitization associated with the persistence or re-occurrence of foetal Type 2 signalling? The MeDALL hypothesis. Allergy 2015; 70: 1062–78. 10.1111/all.12637 [DOI] [PubMed] [Google Scholar]
- 12.Bousquet J, Anto JM, Akdis M, Auffray C, Keil T, Momas I, et al. Paving the way of systems biology and precision medicine in allergic diseases: The MeDALL success story. Allergy. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidal M, Cusick ME, Barabási AL. Interactome networks and human disease. Cell 2011; 144: 986–98. 10.1016/j.cell.2011.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barabási AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. 2011; 12: 56–68. 10.1038/nrg2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piro RM. Network medicine: linking disorders. Hum Genet. 2012. December;131(12): 1811–20. 10.1007/s00439-012-1206-y [DOI] [PubMed] [Google Scholar]
- 16.Kontou PI, Pavlopoulou A, Dimou NL, Pavlopoulos GA, Bagos PG. Network analysis of genes and their association with diseases. Gene. 2016. June 2;590(1): 68–78. 10.1016/j.gene.2016.05.044 [DOI] [PubMed] [Google Scholar]
- 17.Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. OMIM.org: Online Mendelian Inheritance in Man, an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015; 43: D789–98. 10.1093/nar/gku1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S, et al. Ensembl 2014. Nucleic Acids Res. 2014; 42: D749–55. 10.1093/nar/gkt1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasprzyk A. BioMart: driving a paradigm change in biological data management. Database 2011; 2011: bar049 10.1093/database/bar049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis AP, Murphy CG, Johnson R, Lay JM, Lennon-Hopkins K, Saraceni-Richards C, et al. The Comparative Toxicogenomics Database: update 2013. Nucleic Acids Res 2013; 41: D1104–14. 10.1093/nar/gks994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraft P, Zeggini E, Ioannidis JP. Replication in genome-wide association studies. Stat Sci. 2009. November 1;24(4):561–573 10.1214/09-STS290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, Bucan M, Grant SF, Schellenberg G, Hakonarson H. Strategies for genetic studies of complex diseases. Cell. 2010. August 6;142(3):351–3 10.1016/j.cell.2010.07.025 [DOI] [PubMed] [Google Scholar]
- 23.Schork AJ, Thompson WK, Pham P, Torkamani A, Roddey JC, Sullivan PF, et al. All SNPs are not created equal: genome-wide association studies reveal a consistent pattern of enrichment among functionally annotated SNPs. PLoS Genet. 2013. April;9(4):e1003449 10.1371/journal.pgen.1003449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UniProt Consortium. UniProt: a hub for protein information. Nucleic Acids Res. 2015; 43: D204–12. 10.1093/nar/gku989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deza MM, Deza E. Encyclopedia of Distances. Springer, 1 edition, August 2009. [Google Scholar]
- 26.Menche J, Sharma A, Kitsak M, Ghiassian SD, Vidal M, Loscalzo J, et al. Disease networks. Uncovering disease-disease relationships through the incomplete interactome. Science 2015; 347: 1257601 10.1126/science.1257601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Zhu C, Jacomy A, Lu LJ, Jegga AG. The orphan disease networks. Am J Hum Genet. 2011. June 10;88(6): 755–66. 10.1016/j.ajhg.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2013. [Google Scholar]
- 29.Croft D, Mundo AF, Haw R, Milacic M, Weiser J, Wu G, et al. The Reactome pathway knowledgebase. Nucleic Acids Res 2014; 42: D472–7. 10.1093/nar/gkt1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer MH, Fontaine JF, Vinayagam A, Porras P, Wanker EE, Andrade-Navarro MA. HIPPIE: Integrating protein interaction networks with experiment based quality scores. PLoS One 2012; 7: e31826 10.1371/journal.pone.0031826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breuer K, Foroushani AK, Laird MR, Chen C, Sribnaia A, Lo R, et al. InnateDB: systems biology of innate immunity and beyond—recent updates and continuing curation. Nucleic Acids Res 2013; 41: D1228–33. 10.1093/nar/gks1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turinsky AL, Razick S, Turner B, Donaldson IM, Wodak SJ. Navigating the global protein-protein interaction landscape using iRefWeb. Methods Mol Biol. 2014;1091: 315–31. 10.1007/978-1-62703-691-7_22 [DOI] [PubMed] [Google Scholar]
- 33.Turinsky AL, Razick S, Turner B, Donaldson IM, Wodak SJ. Literature curation of protein interactions: measuring agreement across major public databases. Database (Oxford). 2010. December 22;2010:baq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cline MS, Smoot M, Cerami E, Kuchinsky A, Landys N, Workman C, et al. Integration of biological networks and gene expression data using Cytoscape. Nat Protoc 2007; 2: 2366–82. 10.1038/nprot.2007.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravasz E, Somera AL, Mongru DA, Oltvai ZN, Barabási AL. Hierarchical organization of modularity in metabolic networks. Science 2002; 297: 1551–5. 10.1126/science.1073374 [DOI] [PubMed] [Google Scholar]
- 36.Yip AM, Horvath S. Gene network interconnectedness and the generalized topological overlap measure. BMC Bioinformatics 2007; 8: 22 10.1186/1471-2105-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCormack T, Frings O, Alexeyenko A, Sonnhammer EL. Statistical assessment of crosstalk enrichment between gene groups in biological networks. PLoS One. 2013;8(1):e54945 10.1371/journal.pone.0054945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura D. BioCarta. Biotech Software & Internet Report. 2001; 2: 117–120. [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc Ser B 1995; 57: 289–300. [Google Scholar]
- 40.Guney E, Oliva B. Exploiting protein-protein interaction networks for genome-wide disease-gene prioritization. PLoS One 2012; 7: e43557 10.1371/journal.pone.0043557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maslov S, Sneppen K. Specificity and stability in topology of protein networks. Science 2002; 296: 910–3. 10.1126/science.1065103 [DOI] [PubMed] [Google Scholar]
- 42.Fontaine JF, Priller F, Barbosa-Silva A, Andrade-Navarro MA. Génie: literature-based gene prioritization at multi genomic scale. Nucleic Acids Research 2011; 39: W455–61. 10.1093/nar/gkr246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhernakova A, van Diemen CC, Wijmenga C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet. 2009. January;10(1): 43–55. 10.1038/nrg2489 [DOI] [PubMed] [Google Scholar]
- 44.Lee DS, Park J, Kay KA, Christakis NA, Oltvai ZN, Barabási AL. The implications of human metabolic network topology for disease comorbidity. Proc Natl Acad Sci U S A. 2008. July 22;105(29): 9880–5. 10.1073/pnas.0802208105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randhawa V, Bagler G. Identification of SRC as a potent drug target for asthma, using an integrative approach of protein interactome analysis and in silico drug discovery. OMICS 2012; 16: 513–26. 10.1089/omi.2011.0160 [DOI] [PubMed] [Google Scholar]
- 46.Sharma A, Menche J, Huang C, Ort T, Zhou X, Kitsak M, et al. A disease module in the interactome explains disease heterogeneity, drug response and captures novel pathways and genes. Hum Mol Genet 2015; pii: ddv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1: 43–6. [PubMed] [Google Scholar]
- 48.Wang K, Bucan M, Grant SF, Schellenberg G, Hakonarson H. Strategies for genetic studies of complex diseases. Cell. 2010. August 6;142(3):351–3 10.1016/j.cell.2010.07.025 [DOI] [PubMed] [Google Scholar]
- 49.Brietzke E, Mansur RB, Grassi-Oliveira R, Soczynska JK, McIntyre RS. Inflammatory cytokines as an underlying mechanism of the comorbidity between bipolar disorder and migraine. Med Hypotheses 2012; 78: 601–5. 10.1016/j.mehy.2012.01.036 [DOI] [PubMed] [Google Scholar]
- 50.Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of 'overspill' of inflammatory mediators from the lungs? Review of the evidence. Thorax 2010; 65: 930–6. 10.1136/thx.2009.130260 [DOI] [PubMed] [Google Scholar]
- 51.Nabe T. Interleukin -33: New Therapeutic Target for Atopic Diseases. J Pharmacol Sci 2014; 126: 85–91. [DOI] [PubMed] [Google Scholar]
- 52.Bottema RW, Nolte IM, Howard TD, Koppelman GH, Dubois AE, de Meer G, et al. Interleukin 13 and interleukin 4 receptor-α polymorphisms in rhinitis and asthma. Int Arch Allergy Immunol 2010; 153: 259–67. 10.1159/000314366 [DOI] [PubMed] [Google Scholar]
- 53.Oiso N, Fukai K, Ishii M. Interleukin 4 receptor alpha chain polymorphism Gln551Arg is associated with adult atopic dermatitis in Japan. Br J Dermatol 2000; 142: 1003–6. [DOI] [PubMed] [Google Scholar]
- 54.Chanez P, Vignola AM, Vic P, Guddo F, Bonsignore G, Godard P, et al. Comparison between nasal and bronchial inflammation in asthmatic and control subjects. Am J Respir Crit Care Med. 1999;159(2): 588–95. 10.1164/ajrccm.159.2.9801022 [DOI] [PubMed] [Google Scholar]
- 55.Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132(3 Pt 2): 751–62. 10.1038/jid.2011.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saatian B, Rezaee F, Desando S, Emo J, Chapman T, Knowlden S, et al. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013;1(2):e24333 10.4161/tisb.24333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity. 2015;43(1): 29–40. 10.1016/j.immuni.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 58.Grotenboer NS, Ketelaar ME, Koppelman GH, Nawijn MC. Decoding asthma: translating genetic variation in IL33 and IL1RL1 into disease pathophysiology. J Allergy Clin Immunol. 2013. March;131(3): 856–65. 10.1016/j.jaci.2012.11.028 [DOI] [PubMed] [Google Scholar]
- 59.Glück J, Rymarczyk B, Rogala B. Serum IL-33 but not ST2 level is elevated in intermittent allergic rhinitis and is a marker of the disease severity. Inflamm Res. 2012. June;61(6): 547–50. 10.1007/s00011-012-0443-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akasaki S, Matsushita K, Kato Y, Fukuoka A, Iwasaki N, Nakahira M, Fujieda S, Yasuda K, Yoshimoto T. Murine allergic rhinitis and nasal Th2 activation are mediated via TSLP- and IL-33-signaling pathways. Int Immunol. 2016. February;28(2): 65–76. 10.1093/intimm/dxv055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vladich FD, Brazille SM, Stern D, et al. IL-13 R130Q, a common variant associated with allergy and asthma, enhances effector mechanisms essential for human allergic inflammation. J Clin Invest 2005; 115: 747–754 10.1172/JCI22818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 2010; 10: 103–110. 10.1038/nri2692 [DOI] [PubMed] [Google Scholar]
- 63.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med 2006; 203: 269–73. 10.1084/jem.20051745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2009; 41: 631–8. 10.1165/rcmb.2009-0220TR [DOI] [PubMed] [Google Scholar]
- 65.Simpson EL. Comorbidity in Atopic Dermatitis. Curr Dermatol Rep 2012; 1: 29–38. 10.1007/s13671-011-0003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma D, Kanneganti TD. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol. 2016;213(6): 617–29. 10.1083/jcb.201602089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saluja R, Khan M, Church MK, Maurer M. The role of IL-33 and mast cells in allergy and inflammation. Clin Transl Allergy. 2015;5: 33 10.1186/s13601-015-0076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alase A, Seltmann J, Werfel T, Wittmann M. Interleukin-33 modulates the expression of human beta-defensin 2 in human primary keratinocytes and may influence the susceptibility to bacterial superinfection in acute atopic dermatitis. Br J Dermatol. 2012;167(6): 1386–9. 10.1111/j.1365-2133.2012.11140.x [DOI] [PubMed] [Google Scholar]
- 69.Marsh DG, Chase GA, Freidhoff LR, Meyers DA, Bias WB. Association of HLA antigens and total serum immunoglobulin E level with allergic response and failure to respond to ragweed allergen Ra3. Proc Natl Acad Sci U S A. 1979;76(6): 2903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fischer GF, Pickl WF, Fae I, Ebner C, Ferreira F, Breiteneder H, et al. Association between IgE response against Bet v I, the major allergen of birch pollen, and HLA-DRB alleles. Hum Immunol. 1992;33(4): 259–65. [DOI] [PubMed] [Google Scholar]
- 71.Soriano JB, Ercilla G, Sunyer J, Real FX, Lazaro C, Rodrigo MJ, et al. HLA class II genes in soybean epidemic asthma patients. Am J Respir Crit Care Med. 1997;156(5): 1394–8. 10.1164/ajrccm.156.5.9701064 [DOI] [PubMed] [Google Scholar]
- 72.McIntire JJ, Umetsu SE, Macaubas C, Hoyte EG, Cinnioglu C, Cavalli-Sforza LL, et al. Immunology: hepatitis A virus link to atopic disease. Nature 2003; 425: 576 10.1038/425576a [DOI] [PubMed] [Google Scholar]
- 73.Yan L, Galinsky RE, Bernstein JA, Liggett SB, Weinshilboum RM. Histamine N-methyltransferase pharmacogenetics: association of a common functional polymorphism with asthma. Pharmacogenetics 2000; 10: 261–6. [DOI] [PubMed] [Google Scholar]
- 74.Park J, Lee DS, Christakis NA, Barabási AL. The impact of cellular networks on disease comorbidity. Mol Syst Biol. 2009;5: 262 10.1038/msb.2009.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han JD, Bertin N, Hao T, Goldberg DS, Berriz GF, Zhang LV, et al. Evidence for dynamically organized modularity in the yeast protein-protein interaction network. Nature 2004; 430: 88–93. 10.1038/nature02555 [DOI] [PubMed] [Google Scholar]
- 76.Liu AH, Babineau DC, Krouse RZ, Zoratti EM, Pongracic JA, O'Connor GT, et al. Pathways through which asthma risk factors contribute to asthma severity in inner-city children. J Allergy Clin Immunol. 2016. October;138(4):1042–1050. 10.1016/j.jaci.2016.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marenholz I, Esparza-Gordillo J, Lee YA. Shared genetic determinants between eczema and other immune-related diseases. Curr Opin Allergy Clin Immunol 2013; 13: 478–86 10.1097/ACI.0b013e328364e8f7 [DOI] [PubMed] [Google Scholar]
- 78.Barnes PJ. Role of GATA-3 in allergic diseases. Curr Mol Med 2008; 8: 330–334. [DOI] [PubMed] [Google Scholar]
- 79.Krug N, Hohlfeld JM, Kirsten AM, Kornmann O, Beeh KM, Kappeler D, et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N Engl J Med 2015; 372: 1987–95. 10.1056/NEJMoa1411776 [DOI] [PubMed] [Google Scholar]
- 80.Apter AJ. Advances in adult asthma diagnosis and treatment in 2014. J Allergy Clin Immunol 2015; 135: 46–53. 10.1016/j.jaci.2014.10.050 [DOI] [PubMed] [Google Scholar]
- 81.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol 2015; 16: 45–56. 10.1038/ni.3049 [DOI] [PubMed] [Google Scholar]
- 82.Salimi M, Ogg G. Innate lymphoid cells and the skin. BMC Dermatol 2014; 14: 18 10.1186/1471-5945-14-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Doherty TA, Scott D, Walford HH, Khorram N, Lund S, Baum R, et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol 2014; 133: 1203–5. 10.1016/j.jaci.2013.12.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sotiropoulou G, Pampalakis G. Targeting the kallikrein-related peptidases for drug development. Trends Pharmacol Sci 2012; 33: 623–34. 10.1016/j.tips.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 85.Portelli MA, Siedlinski M, Stewart CE, Postma DS, Nieuwenhuis MA, Vonk JM, et al. Genome-wide protein QTL mapping identifies human plasma kallikrein as a post-translational regulator of serum uPAR levels. FASEB J 2014; 28: 923–34. 10.1096/fj.13-240879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marenholz I, Esparza-Gordillo J, Ruschendorf F, Bauerfeind A, Strachan DP, Spycher BD, et al. Meta-analysis identifies seven susceptibility loci involved in the atopic march. Nat Commun. 2015;6: 8804 10.1038/ncomms9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kovacic MB, Myers JM, Wang N, Martin LJ, Lindsey M, Ericksen MB, et al. Identification of KIF3A as a novel candidate gene for childhood asthma using RNA expression and population allelic frequencies differences. PLoS One. 2011;6(8):e23714 10.1371/journal.pone.0023714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guay C, Laviolette M, Tremblay GM. Targeting serine proteases in asthma. Curr Top Med Chem 2006; 6: 393–402. [DOI] [PubMed] [Google Scholar]
- 89.Wollenberg A, Feichtner K. Atopic dermatitis and skin allergies—update and outlook. Allergy. 2013. December;68(12): 1509–19. 10.1111/all.12324 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(A) Disease A (orange ellipse) is associated to 6 proteins: p1, p2, p3, p4, p5, p6. Disease B (blue ellipse) is associated to 6 proteins: p4, p5, p6, p7, p8, p9. Three proteins are common to both diseases (p4, p5, p6; shown in the intersection of both diseases, in blue with orange border). (B) Mapping of the disease-associated proteins on to a dummy network. As in the previous figure, common proteins (p4, p5, p6) are shown in blue with orange border. (C) Calculation of the average topological overlap (TO) between the common proteins. (D) Calculation of the average topological overlap between the proteins unique to each disease.
(PNG)
(A) Disease A (orange ellipse) is associated to 7 proteins: p1, p2, p3, p4, p5, p6, p7. Disease B (blue ellipse) is associated to 7 proteins: p4, p5, p6, p7, p8, p9, p10. Disease C (purple ellipse) is associated to 5 proteins: p4, p5, p6, p7, p11. Proteins common to all diseases are p6 and p7 (shown as a blue circles with double purple-orange border). Proteins common solely to diseases A and B are p4 and p5 (shown as blue circles with orange border). There are no proteins common solely to diseases A and C, neither to diseases B and C. (B) Mapping of the disease-associated proteins on to a dummy network. (C) Calculation of the average topological overlap (TO) between the common proteins. (D) Calculation of the average topological overlap between the proteins unique to each disease.
(PNG)
Blue dots indicate the observed fraction of proteins. Orange scatter boxplots indicate random expectation. One asterisk: observed results are significantly larger than random expectation (z-test; P < 0.05). Two asterisks: observed results are significantly larger than random expectation (z-test; P < 0.01).
(PNG)
Blue dots indicate the observed fraction of proteins. (A) Orange scatter boxplots indicate random expectation. One asterisk: observed results are significantly larger than random expectation (z-test; P < 0.05). Two asterisks: observed results are significantly larger than random expectation (z-test; P < 0.01). (B) Orange scatter boxplots indicate fraction of associated proteins for pairs/trios of immune system diseases. One asterisk: observed results are significantly larger than random expectation (empirical distribution test; P < 0.05). Two asterisks: observed results are significantly larger than random expectation (empirical distribution test; P < 0.01).
(PNG)
Blue dots indicate the observed fraction of proteins. Orange scatter boxplots indicate fraction of associated proteins for pairs/trios of immune system diseases. One asterisk: observed results are significantly larger than random expectation (empirical distribution test; P < 0.05). Two asterisks: observed results are significantly larger than random expectation (empirical distribution test; P < 0.01).
(PNG)
Blue dots indicate the observed mean topological overlap (TO) between proteins uniquely associated to either asthma, eczema or rhinitis. Orange scatter boxplots indicate random expectation. One asterisk: observed results are significantly larger than random expectation (P < 0.05). Two asterisks: observed results are significantly larger than random expectation (P < 0.01).
(PNG)
(A) Blue dots indicate the observed mean topological overlap (TO) for proteins common to combinations of asthma, eczema and rhinitis. Orange scatter boxplots indicate random expectation. (B) Blue dots indicate the observed mean TO for proteins common to combinations of asthma, eczema and rhinitis. Orange scatter boxplots indicate observed TO values for pairs/trios of immune system diseases. (C) Blue dots indicate the observed mean TO for proteins unique to combinations of asthma, eczema and rhinitis. Orange scatter boxplots indicate random expectation. (D) Blue dots indicate the observed mean TO for proteins unique to combinations of asthma, eczema and rhinitis. Orange scatter boxplots indicate observed TO values for pairs/trios of immune system diseases. One asterisk: observed results are significantly larger than random expectation (P < 0.05). Two asterisks: observed results are significantly larger than random expectation (P < 0.01).
(PNG)
Tab-delimited text file. Diseases were extracted from the CTD database (see Methods in the main paper).
(GZ)
This network was generated by combining data from the HIPPIE, Reactome and InnateDB databases (see Methods in the main paper).
(GZ)
OMIM: On-line Mendelian Inheritance in Man; CTD: Comparative Toxicogenomics Database; EV84: Ensembl Variation 84.
(PDF)
Disease-associated proteins (provided as UniProt accessions), are separated by a semicolon. The network column indicates whether the disease has at least one associated protein present in then FIN (1) or not (0).
(XLS)
Pathway-associated proteins (provided as UniProt accessions) and interactions between pathway-associated proteins are separated by a semicolon.
(XLS)
The Génie tool (http://cbdm-01.zdv.uni-mainz.de/~jfontain/cms/) was used to extract gene names present in PubMed abstracts related to a topic of interest, as defined by a PubMed query.
(DOC)
Columns are as follows: Query: PubMed query used; Rank: position (rank) of the gene (ordered by ascending FDR); GeneID: gene name; n: number of PubMed abstracts manually associated to the gene; n_pos: number of PubMed abstracts that Génie has selected at P < 0.01; FDR: False Discovery Rate; Top 10 PMIDs: top 10 PMIDs for each gene, together with the associated p-value. More information at http://cbdm-01.zdv.uni-mainz.de/~jfontain/cms/.
(XLS)
The Génie tool was used to extract gene names present in PubMed abstracts related to a topic of interest, as defined by a PubMed query. Unlike S4 Table, this table does not exclude the terms predicted nor prediction, and includes the terms predictive or predictor.
(DOC)
Literature predictions were automatically extracted from PubMed abstracts using the Génie data mining tool. The terms used to query PubMed database were those shown in S4 Table minus the word “comorbidity”. Statistical association was calculated by means of a Fisher's Exact Test. Confidence intervals are shown in parentheses.
(DOC)
Gene names are provided as common (HGNC) gene names and protein names as UniProt accessions.
(XLS)
Gene names are provided as common (HGNC) gene names and protein names as UniProt accessions.
(XLS)
Mean random expectation in shown between parenthesis. The fraction of common edges was calculated using the Jaccard index (see Methods in the main paper). The fraction of common edges is statistically larger than random expectation in all four cases (p-value < 0.01). The fraction of unique edges is statistically lower than random expectation in all four cases (p-value < 0.01). The number of edges associated to asthma: 8560; number of edges associated to eczema: 2584; number of edges associated to rhinitis: 2898.
(DOC)
Numerical values show how similar is the use of a cellular pathway by pairs of trios of diseases. Similarity = 1 means that the diseases affect the pathway in exactly the same way. Similarity = 0 is represented by blank cells. Dark blue cells: similarity is significantly larger than random expectation (z-test; P < 0.01). Light blue cells: similarity is significantly larger than random expectation (z-test; P < 0.05). All significant similarities were also significantly larger than observed for pairs and trios of immune system diseases (empirical distribution test; P < 0.01).
(XLS)
Gene names are provided as common gene names and protein names as UniProt accessions. NetZcore prediction scores are shown as z-scores. Proteins are ranked according to their average z-score for all diseases. Empty cell: the protein was not predicted to be associated with the disease with z-score > 2.31 (corresponding to P < 0.01). Exp: the protein is experimentally known to be associated to the disease. Proteins predicted (or experimentally known) to be associated to more than one disease are candidates to multimorbidity.
(XLS)
Gene names are provided as common gene names and protein names as UniProt accessions. NetZcore prediction scores are shown as z-scores. Proteins are ranked according to their average z-score for all diseases. Empty cell: the protein was not predicted to be associated with the disease with z-score > 2.31 (corresponding to P < 0.01). Exp: the protein is experimentally known to be associated to the disease. Proteins predicted (or experimentally known) to be associated to more than one disease are candidates to multimorbidity.
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.