Abstract
Dihydroartemisinin (DHA) has been shown to inhibit the viability of various cancer cells. Previous studies have revealed that the mechanisms involved in the inhibitory effects of DHA are based on theactivation of p53 and the mitochondrial-related cell death pathway. However, the exact association between upstream signaling and the activation of cell death pathway remains unclear. In this study, we found that DHA treatment induced the upregulation of caveolin 1 (Cav1) and mitochondrial carrier homolog 2 (MTCH2) in HeLa cells, and this was associated with the DHA-induced inhibition of cell viability and DHA-induced apoptosis. Additionally, the overexpression of Cav1 and MTCH2 in HeLa cells enhanced the inhibitory effects of DHA on cell viability. Moreover, we also found that the upregulation of Cav1 contributed to the DHA-mediated p53 activation and the downregulation of the redox enzyme, NAD(P)H:quinone oxidoreductase 1 (NQO1), which have been reported to contribute to the activation of the cell death pathway. Of note, we also found that DHA induced the nuclear translocation and accumulation of both Cav1 and p53, indicating a novel potential mechanism, namely the regulation of p53 activation by Cav1. On the whole, our study identified Cav1 and MTCH2 as the molecular targets of DHA and revealed a new link between the upstream Cav1/MTCH2 upregulation and the downstream activation of the cell death pathway involved in the DHA-mediated inhibition of cell viability.
Keywords: dihydroartemisinin, anticancer, caveolin 1, mitochondrial carrier homolog 2, oxidative stress
Introduction
Dihydroartemisinin (DHA), recommended as an effective antimalarial herbal compound by the World Health Organization (WHO), is used worldwide to combat the parasite, Plasmodium falciparum, which causes malaria. Recent studies have demonstrated the inhibitory effects of DHA on the viability of various cancer cells (1–4). However, the mechanisms responsible for the anticancer effects of DHA have not yet been fully documented. It has been reported that DHA exerts inhibitory effects on cancer cells through the induction of apoptosis (3,4). DHA treatment has been shown to result in mitochondrial membrane depolarization, the release of cytochrome c and caspase activation (1,5). Bcl-2 family proteins, such as Bax, Bid and Noxa have also been shown to contribute to DHA-induced apoptosis (6,7). Moreover, p53 has been reported to facilitate apoptosis caused by DHA (5,8–10). These data suggest that the inhibitory effects of DHA on cancer cells are based on the activation of p53 and the mitochondrial-related cell apoptosis pathway. Despite these advances, however, the exact association between upstream signaling and the downstream activation of the cell death pathway following treatment with DHA remains unclear.
Caveolin 1 (Cav1) is an important component of caveolae, and is known to function as a scaffolding protein, regulating several signaling pathways (11–13). The loss of Cav1 has been demonstrated to be involved in tumorigenesis in several types of cancer, and the overexpression of Cav1 has been shown to inhibit cell and tumor growth (14–18). Thus, Cav1 is regarded as a potential tumor suppressor. In spite of the fact that a number of studies have been conducted to investigate the function of Cav1 in several types of cancer (14–18), studies reporting that Cav1 functions as a tumor suppressor by inhibiting the oxidative stress response pathway are limited (19). As important mediators of the apoptotic signaling pathway, reactive oxygen species (ROS) play important roles in the induction of cancer cell death. DHA has also reported to induce the generation of ROS as upstream signaling molecules that initiate the mitochondria-related apoptotic pathway (20,21). The increased generation of ROS suggests the inhibition of antioxidant gene expression in response to oxidative stress; thus, it is possible that proteins which inhibit the oxidative stress response pathways may function upstream of the activation of the cell death pathway following treatment with DHA. Of note, Cav1 has been shown to inhibit cellular antioxidant capacity through direct interaction with nuclear factor erythroid 2-related factor 2 (Nrf2) (22,23). Thus, it is reasonable that Cav1 may function upstream of the cell death pathway activated by DHA by inhibiting the Nrf2-related oxidative stress response pathway.
DHA has also been previously reported to trigger ROS-mediated Bid activation and mitochondrial translocation (7,21). Mitochondrial carrier homolog 2 (MTCH2) has been demonstrated to play an important role in facilitating the mitochondrial recruitment of truncated Bid (t-Bid) through direct interaction with t-Bid (24,25). In addition to facilitating apoptosis, the induction of MTCH2 also causes growth and motility arrest in vitro and the loss of tumorigenicity in vivo (26). These data suggest that MTCH2 may be considered as a novel therapeutic target.
In this study, we evaluated the anticancer effects of DHA and analyzed the expression of Cav1 and MTCH2 in a cervical cancer cell line treated with DHA, in an aim to elucidate the potential mechanisms involved in the anticancer effects of DHA.
Materials and methods
Cell culture
The HeLa cells were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). All cell lines were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (both from HyClone, Logan, UT, USA). All cell lines were incubated in a humidified atmosphere containing 5% CO2 at 37°C.
Reagents and antibodies
DHA was obtained from Sigma-Aldrich (St. Louis, MO, USA). Cav1 (polyclonal, rabbit anti-human; cat. no. 16447-1-AP; dilution 1:1,000), MTCH2 (polyclonal, rabbit anti-human; cat. no. 16888-1-AP; dilution 1:1,000), β-tubulin (monoclonal, mouse anti-human; cat. no. 66240-1, dilution 1:2,000), β-actin (polyclonal, rabbit anti-human; cat. no. 23660-1-AP; dilution 1:1,000), GAPDH (polyclonal, rabbit anti-human; cat. no. 10494-1-AP; dilution 1:1,000) and Myc (monoclonal, mouse anti-human; cat. no. 60003-2-Ig; dilution 1:1,000) antibodies were obtained from ProteinTech Group, Inc. (Chicago, IL, USA); p53 antibody (monoclonal, mouse anti-human; cat. no. P8999; dilution 1:1,000) was obtained from Sigma-Aldrich; NAD(P)H:quinone oxidoreductase 1 (NQO1) antibody (monoclonal, mouse anti-human; cat. no. #3187, dilution 1:1,000) was obtained from Cell Signaling Technology, Inc. (Beverly, MA, USA) and p84 antibody (monoclonal, mouse anti-human; cat. no. MA1-23261, dilution 1:1,000) was obtained from Invitrogen, Waltham, CA, USA. The secondary antibody (polyclonal, goat anti-rabbit/goat anti-mouse; cat. no. 042-06-15-06/042-06-18-06; dilution 1:10,000) was obtained from KPL, Inc. (Gaithersburg, MD, USA).
MTT and cell apoptosis assays
The cells were plated into 96-well plates and seeded at a density of 10,000 cells/well. The cells were treated with DHA or the vehicle (DMSO) for the indicated periods of time. The final volume of culture medium in each well was 100 µl. Ten microliters of MTT solution (concentration, 5 mg/ml) were added to the 100 µl of medium in each well. The plates were incubated at 37°C for 4 h, and the supernatant was then removed and 100 µl DMSO were added to each well. The absorbance signals were measured on a Thermo Scientific Multiskan FC spectrophotometer (cat. no. 51119000; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 490 nm. The cell apoptosis-inducing effects of drug treatment were measured using the CF488A-Annexin V and PI apoptosis assay kit (Biotium, Inc., Hayward, CA, USA). Samples and assays were prepared as described in the user manual and then mounted onto slides. Images were acquired using a Nikon fluorescence microscope [Nikon Instruments (Shanghai) Co., Ltd., Shanghai, China].
Western blot analysis
The cells were lysed using 1X SDS sample buffer and were separated by SDS-PAGE. Proteins were transferred onto PVDF membranes (Millipore Corp., Bedford, MA, USA). The membranes were first blocked with 5% milk for 1 h, then incubated overnight with the indicated antibodies. Following 3 washes with TBST (50 mM Tris-HCl, pH 7.5, 150 mM NaCl and 0.2% Tween-20), the blots were incubated with secondary antibodies [anti-rabbit secondary antibody (polyclonal, goat anti-rabbit; cat. no. 042-06-15-06; dilution 1:10,000) and anti-mouse secondary antibody (polyclonal, goat anti-mouse; cat. no. 042-06-18-06; dilution 1:10,000), KPL, Inc.] in the dark for 2 h. The blots were then washed again with TBST and images were acquired using the LI-COR Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA).
Establishment of stable cell lines
For stable Cav1/MTCH2 expression, the HeLa cells were transduced with Cav1-Myc or MTCH2-Myc lentiviral particles and stable cell lines were selected with blasticidin (Invitrogen). Lentiviral particles were our laboratory-made products using ViraPower Lentiviral Expression system following the manufacturer's protocol (Invitrogen, Thermo Fisher Scientific, Inc.).
Establishment of Cav1-knockout cell line
A Cav1-knockout cell line was generated using the CRISPER/Cas9 system from Sigma-Aldrich. The HeLa cells were first transfected with the expression vectors of Cas9 and two gRNAs, which targeted the AGCCACGGGCCAGCATGTC and TCGCTCAGCTCGTCTGCCA sequences in exon 1 of Cav1. Twenty-four hours following transfection, the cells were diluted and seeded in 96-well plates at 1 cell/well, and monoclonal cell lines without Cav1 expression were isolated as determined by western blot analysis.
Plasmid construction and cell transfection
The full-length Cav1 open reading frame (ORF) was amplified by PCR with a BamHI site-containing 5′ primer: 5′-gatcggatccgccaccatgtctgggggcaaatacgtag-3′, and an XbaI site-containing 3′ primer: 3′-gtagttgaacgtctttctttatagatctctag-5′, and cloned into the pcDNA3.1(+)-Myc vector (Invitrogen) to generate the Cav1-Myc expression construct. Full-length MTCH2 ORF was amplified by PCR with a HindIII site-containing 5′ primer: 5′-gatcaagctt-gccaccatggcggacgcggccagtcag-3′ and an XbaI site-containing 3′ primer: 3′-gatctctagaaattaacattttcaggtcac-5′, and cloned into the pcDNA3.1(+)-Myc vector to generate the MTCH2-Myc expression construct. For lentiviral particle generation, the cDNA fragment was transferred into the pLenti6 lentiviral vector, and the plasmid was then transfected with ViraPower Lentiviral Packaging mix into 293FT cells (cat. no. R70007; obtained from Thermo Fisher Scientific, Inc.) using Lipofectamine 2000 (all from Invitrogen). The virus-containing cell culture medium was harvested 40 h later and used to transduce the HeLa cells.
Colony formation assay
The HeLa cells were transfected with the Cav1-Myc or MTCH2 expression vector or empty vector [pcDNA3.1(+)-Myc vector (Invitrogen)] using Lipofec tamine 2000 (Invitrogen). At 48 h following transfection, the cells were selected with G418. The medium with G418 were changed every 3 days. Two weeks later, the cells were fixed and stained with crystal violet (cat. no. 548-62-9; obtained from Solarbio, Beijing, China).
Crude nuclear fraction isolation
The HeLa cells cultured in a 10-cm plate were treated with DHA at the indicated concentration for 36 h, and the cells were then lysed with 0.5% NP-40 lysis buffer (0.5% NP-40, 150 mM NaCl, 10 mM sodium phosphate and pH 7.4) for 20 min on ice, and cell lysates were collected in an EP tube and followed by centrifugation at >14,000 × g for 10 min at 4°C. The supernatant were collected as the cytoplasmic fraction, and the pellet were re-lysed in 1X SDS sample buffer and collected as the crude nuclear fraction.
Statistical analysis
Experimental data are expressed as the the means ± SEM. Data analysis was performed using ImageJ software and Origin 8.0 software. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
DHA inhibits the viability and induces the apoptosis of HeLa cells
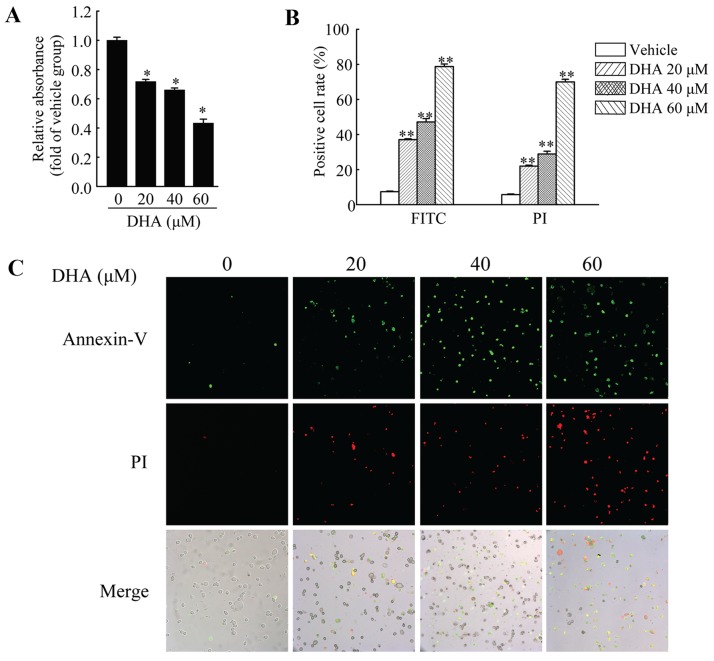
HeLa cells were used to investigate the anticancer effects of DHA in cervical carcinoma. First, we performed MTT assay to measure the viability of the HeLa cells. The cells were treated with DHA at the indicated concentrations for 36 h, and cell viability was then measured. The results revealed that DHA significantly inhibited the viability of the HeLa cells, and the inhibitory effects were dose-dependent (Fig. 1A). In addition, DHA also induced the apoptosis of the HeLa cells. Annexin V-FITC/PI staining assay was used to assess the apoptotic HeLa cells following treatment with DHA at the indicated concentrations. The results revealed that the number of Annexin V-FITC-positive and PI-positive cells was significantly increased in a dose-dependent manner following treatment with DHA (Fig. 1B and C).
Figure 1.
Inhibition of cell viability and induction of cell apoptosis by dihydroartemisinin (DHA) in HeLa cells. (A) The viability of HeLa cells was measured by MTT assay following treatment with the vehicle or DHA for 36 h. Values were normalized against the vehicle control group. (B and C) Cell apoptosis assay of HeLa cells treated with the vehicle or DHA for 36 h. (B) Quantification of the ratio of positive cells to total cells. The number of positive cells was counted using ImageJ software. Data are presented as the means ± SEM of 3 independent experiments. *P<0.05, **P<0.01 compared with the vehicle control group.
DHA induces the increased expression of endogenous Cav1 and MTCH2
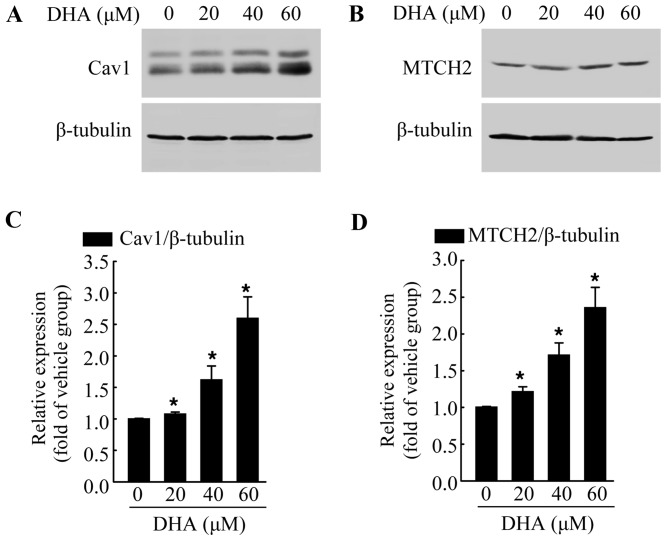
The exact mechanisms involved in the anticancer effects of DHA are not yet fully understood. Thus, in this study, we examined the expression of various cancer-related proteins, in an aim to identify the potential target of DHA. The cells were exposed to DHA at the indicated concentrations for 36 h, and then subjected to western blot analysis. We found that DHA significantly increased the protein levels of Cav1 (Fig. 2A and C) and MTCH2 (Fig. 2B and D) in a dose-dependent manner when compared with the untreated control group. Cav1 and MTCH2 have been reported to act as tumor suppressors (27–29). We also detected the increased expression of Cav1 and MTCH2 following treatment with DHA; thus it is possible that Cav1 and MTCH2 may mediate the inhibitory effects of DHA on HeLa cells.
Figure 2.
Dihydroartemisinin (DHA) increases the protein level of caveolin 1 (Cav1) and mitochondrial carrier homolog 2 (MTCH2) in HeLa cells. (A) HeLa cells were treated with the vehicle or DHA for 36 h and then harvested for western blot analysis to detect the protein level of Cav1. β-tubulin was used as a loading control. (B) HeLa cells were treated with te vehicle or DHA for 36 h and then harvested for western blot analysis to detect the protein level of MTCH2. β-tubulin was used as a loading control. (C and D) Quantification of the protein levels of Cav1 and MTCH2 in A and B, respectively. Values were normalized against β-tubulin. Data are presented as the means ± SEM of 3 independent experiments. *P<0.05 compared with the vehicle control group.
Overexpression of Cav1 and MTCH2 inhibits colony formation of HeLa cells
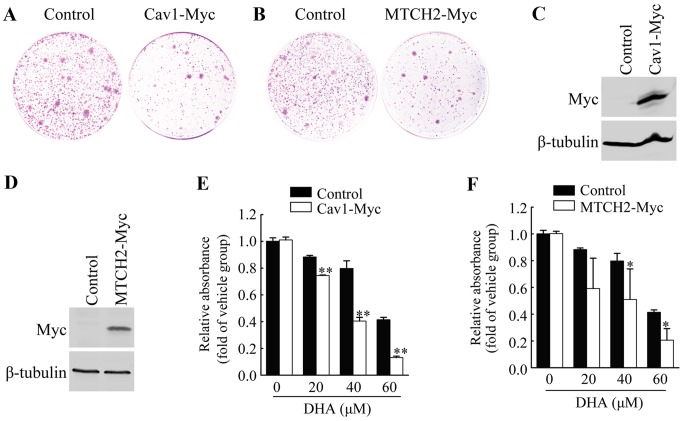
To further examine the functional involvement of Cav1 and MTCH2 in the inhibitory effects of DHA on HeLa cell cell viability, we first performed colony formation assays by introducing the Cav1-Myc expression vector or MTCH2-Myc expression vector or control vector into the HeLa cells, and measuring the growth of G418-resistant colonies. Cav1 overexpression resulted in the significant inhibition of colony formation (Fig. 3A), and similar results were observed in the cells overexpressing MTCH2 (Fig. 3B).
Figure 3.
Overexpression of caveolin 1 (Cav1) or mitochondrial carrier homolog 2 (MTCH2) enhances the sensitivity to dihydroartemisinin (DHA). (A) G418-resistant colonies of HeLa cells transfected with empty vector (Control) or Cav1-Myc expression vector (Cav1-Myc). (B) G418-resistant colonies of HeLa cells transfected with empty vector (Control) or MTCH2-Myc expression vector (MTCH2-Myc). (C) Western blot analysis of HeLa cells stably transduced with Cav1-Myc virus (HeLa/Cav1-Myc) and HeLa cells stably transduced with empty viral vector (HeLa/Control). β-tubulin was used as loading control. (D) Western blot analysis of HeLa cells stably transduced with MTCH2-Myc virus (HeLa/MTCH2-Myc) and HeLa cells stably transduced with empty vector virus (HeLa/Control). β-tubulin was used as loading control. (E) The viability of HeLa/Cav1-Myc cells and HeLa/Control cells was measured by MTT assay after being treated with the vehicle or DHA at the indicated concentrations for 36 h. (F) The viability of HeLa/MTCH2-Myc cells and HeLa/Control cells were measured by MTT assay after being treated with the vehicle or DHA at the indicated concentrations for 36 h. Values were normalized against the vehicle control group. Data are presented as the means ± SEM of 3 independent experiments. *P<0.05, **P<0.01 compared with the vehicle control group.
Overexpression of Cav1 and MTCH2 enhances the sensitivity of HeLa cells to DHA
We established HeLa cell lines stably expressing Cav1 or MTCH2 (Fig. 3C and D). HeLa cells stably transduced with Cav1-Myc virus (HeLa/Cav1-Myc) and HeLa cells stably transduced with the empty viral vector (HeLa/Control) were treated with the vehicle or DHA at the indicated concentrations for 36 h, and cell viability was then measured by MTT assay. We found that the stable expression of Cav1 enhanced the inhibitory effects of DHA on cell viability (Fig. 3E). The stable expression of MTCH2 also significantly enhanced the inhibitory effects of DHA on cell viability (Fig. 3F). These results suggested that the upregulation of Cav1 and MTCH2 was responsible for the enhanced inhibitory effects of DHA on cell viability.
Cav1 regulates p53 activation in HeLa cells
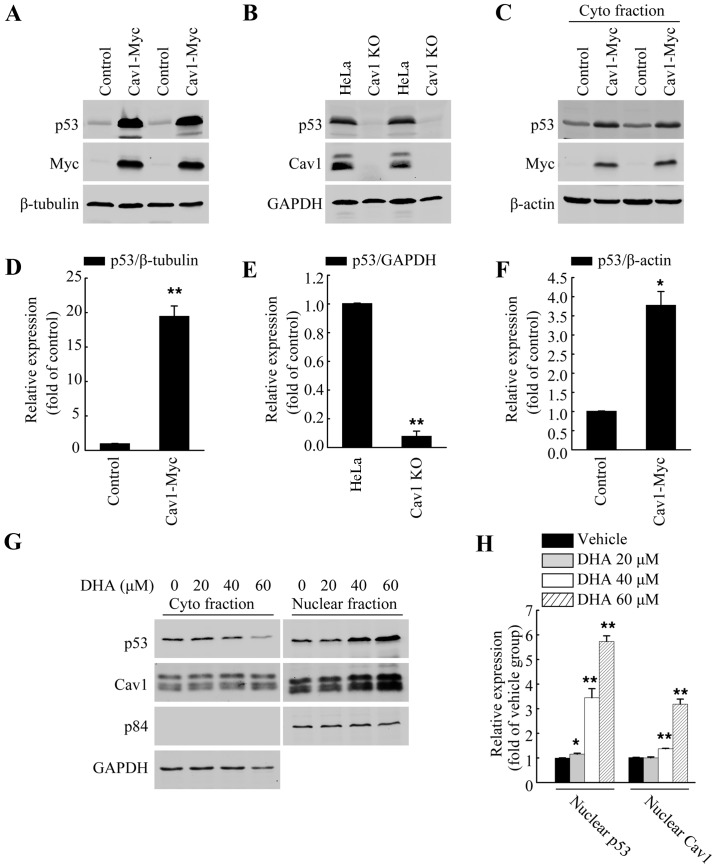
p53 has been reported to facilitate apoptosis caused by DHA (5,8–10). Previous studies have also demonstrated a direct association between Cav1 and p53 in regulating stress-induced premature senescence (SIPS) in mouse embryonic fibroblasts (30,31). Thus, in this study, we wished to evaluate the role of Cav1 in p53 activation in HeLa cells. Direct functional analysis in the form of gene knockout and overexpression analysis were used. After establishing a stable cell line expressing Cav1 (HeLa/Cav1-Myc) with a stably transduced Cav1-Myc vector and generated a Cav1-knockout cell line (HeLa/Cav1-KO) using the CRISPER/cas9 system, we prepared whole cell lysates using SDS sample buffer, and then performed western blot analysis. Our results demonstrated that the overexpression of Cav1 in the HeLa cells was associated with a significant increase (>20-fold) in the protein level of p53 (Fig. 4A and D), while the silencing of Cav1 caused a decrease in the protein level of p53 when compared with the control group (Fig. 4B and E). Of note, when we harvested the cells with NP40 lysis buffer, we found that the protein level of p53 in the soluble fraction of HeLa/Cav1-Myc was significantly increased (Fig. 4C and F) and the increase in p53 expression was much lower than that in whole cell lysate fraction. These data suggested that the majority of the increased p53 protein may exist in the insoluble nuclear fraction.
Figure 4.
Caveolin 1 (Cav1) regulates p53 activation following treatment with dihydroartemisinin (DHA). (A) Western blot analysis of HeLa/Cav1-Myc cells and HeLa/Control cells to detect the protein level of p53. β-tubulin was used as a loading control. (B) Western blot analysis of HeLa cells (HeLa) and Cav1-knockout cell line (Cav1-KO) to detect the protein level of p53. GAPDH was used as a loading control. (C) Western blot analysis of HeLa/Cav1-Myc cells and HeLa/Control cells to detect the protein level of p53. β-actin was used as a loading control. Cells were harvested using 0.5% NP 40 lysis buffer. (D-F) Quantification of the protein levels of p53 in panels A, B and C, respectively. Values were normalized against β-tubulin, GAPDH and β-actin, respectively. (G) The cytoplasmic fraction and nuclear fraction of HeLa cells treated with the indicated concentrations of DHA for 36 h were isolated separately and then subjected to western blot analysis to detect the protein level of p53 and Cav1. GAPDH and p84 were used as loading controls. (H) Quantification of the protein levels of nuclear p53 and nuclear Cav1 in panel G. Values were normalized against nuclear marker p84. Data are presented as the means ± SEM of 3 independent experiments. *P<0.05, **P<0.01 compared with the control group.
Upregulation of Cav1 is associated with the nuclear localization and stabilization of p53 following treatment with DHA
Apart from being localized in the plasma membrane, Cav1 has also been reported to be localized in the nucleus (23), and the increased nuclear localization of Cav1 has also observed under H2O2-induced oxidative stress (32). In this study, to further elucidate the potential association between Cav1 upregulation and p53 stabilization following treatment with DHA, the HeLa cells were cultured in a 10-cm plate and treated with DHA at the indicated concentrations for 36 h, and the crude nuclear fractions and cytosolic fraction were then collected and subjected to western blot analysis. We found DHA treatment increased the nuclear localization of both Cav1 and p53 (Fig. 4G and H). Since we aldready demonstrated that Cav1 expression was increased following treatment with DHA (Fig. 2A and C), and that it directly regulates the stabilization of p53 in HeLa cells (Fig. 4A–F), these data indicated that the upregulation and nuclear localization of Cav1 may contribute to the activation of p53 in HeLa cells treated with DHA.
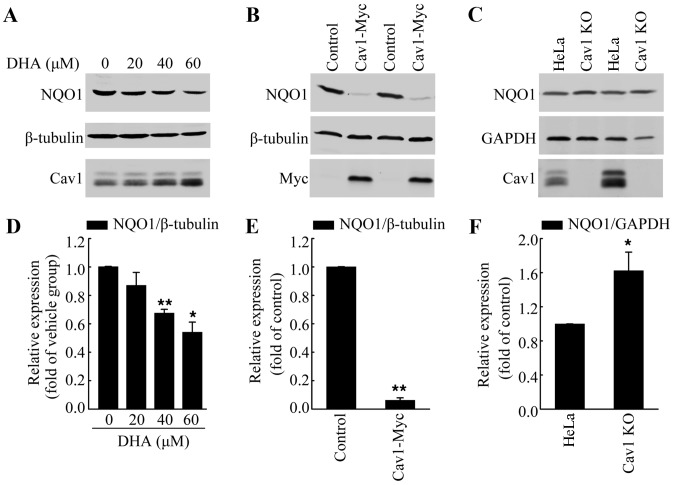
Upregulation of cav1 contributes to the decreased expression of NQO1 following treatment with DHA
Increased ROS formation has been observed in response to DHA treatment, which was the main course of DHA-induced apoptosis (33,34). Notably, Cav1 has been reported to regulate cellular antioxidant capacity by inhibiting the transcriptional activity of Nrf2 (22,23). Thus, in this study, we analyzed the protein level of NQO1, a direct target of Nrf2, in HeLa cells treated with DHA to determine whether Cav1 is involved in DHA-induced oxidative stress. We treated the HeLa cells with DHA at the indicated concentrations for 36 h, and then performed western blot analysis. Consist with our hypothesis, we found that DHA treatment led to the downregulation of NQO1, which was associated with the upregulation of Cav1 (Fig. 5A and D). Moreover, the NQO1 protein level in the HeLa/Cav1-Myc cells was significantly lower than that in the HeLa/Control cells (Fig. 5B and E), while depletion of Cav1 resulted in an increase of NQO1 protein level (Fig. 5C and F). These results suggested that the increased Cav1 expression was the possible upstream regulator of NQO1.
Figure 5.
Caveolin 1 (Cav1) contributes to the downregulation of NAD(P)H:quinone oxidoreductase 1 (NQO1) following treatment with dihydroartemisinin (DHA). (A) HeLa cells were treated with the vehicle or DHA for 36 h and then harvested for western blot analysis to detect the protein level of NQO1 and Cav1. β-tubulin was used as a loading control. (B) Western blot analysis of HeLa/Cav1-Myc cells and HeLa/Control cells to detect the protein level of NQO1. β-tubulin was used as a loading control. (C) Western blot analysis of HeLa cells (HeLa) and Cav1 knockout cell line (Cav1-KO) to detect the protein level of NQO1. GAPDH was used as a loading control. (D-F) Quantification of the protein levels of NQO1 in panels A, B and C, respectively. Values were normalized against the vehicle group or loading control, respectively. Data are presented as the means ± SEM of 3 independent experiments. *P<0.05, **P<0.01 compared with the control group.
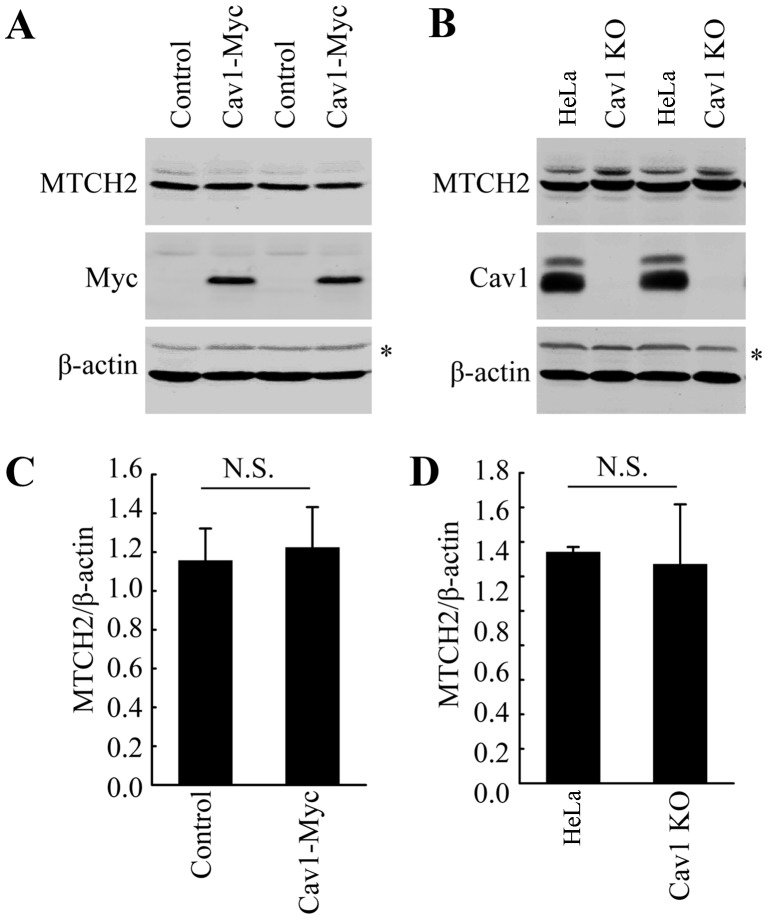
Cav1 does not directly influence the expression of MTCH2
As indicated above, DHA treatment increased the expression of both Cav1 and MTCH2 (Fig. 2). Thus, we wished to examine the potential association between Cav1 and MTCH2. We already demonstrated the nuclear translocation of Cav1 in the HeLa cells treated with DHA (Fig. 4). Thus, we hypothesized that Cav1 may regulate the expression of MTCH2 via the activation of MTCH2. However, direct functional analysis, such as gene knockout and overexpression analysis did not support our hypothesis. Neither Cav1 overexpression nor Cav1 depletion affected the expression of MTCH2 (Fig. 6A–D). These data suggested that the induction of Cav1 and MTCH2 in HeLa cells treated with DHA may not be involved in the same pathway and further studies are required to elucidate the mechanisms involved in the upregulation of MTCH2 induced by DHA.
Figure 6.
Caveolin 1 (Cav1) does not directly influence the expression of mitochondrial carrier homolog 2 (MTCH2). (A) Western blot analysis of HeLa/Cav1-Myc cells and HeLa/Control cells to detect the protein level of MTCH2. β-actin was used as a loading control. Non-specific bands are indicated by an asterisk (*). (B) Western blot analysis of HeLa cells (HeLa) and Cav1-knockout cell line (Cav1-KO) to detect the protein level of MTCH2. β-actin was used as a loading control. Non-specific bands are inciated by an asterisk (*). (C and D) Quantification of the protein levels of MTCH2 in panels A and B, respectively. Values were normalized against β-actin. Data are presented as the means ± SEM of 3 independent experiments. N.S., not significant (P>0.05 compared with the control group).
Discussion
Previous studies have revealed that the anti-proliferative effects of DHA are based on the mitochondrial-related activation of cell death pathway induced by high levels of ROS (33,34), However, the detailed associatoin between the upstream signaling and downstream activation of cell death pathway following treatment with DHA remains unclear. Cav1, a reported cancer suppressor, is an important component of caveolae and functions as a scaffolding protein in regulating several signaling pathways (11–13). Recently, Cav1 was also reported to function as a modulator of oxidative stress by inhibiting the expression of Nrf2 (22,23). These data suggest a possible role of Cav1 in mediating elevated ROS generation, which inhibits cell viability in DHA-treated cells. However, the exact role of Cav1 in this progression remains unclear. Of note, we found that DHA treatment increased the expression of Cav1 in HeLa cells and the overexpression of Cav1 enhanced the sensitivity to DHA. These data suggested a potential link between the activation of the cell death pathway triggered by the upregulation of Cav1 and the inhibition of the Nrf2-mediated signaling pathway following treatment with DHA.
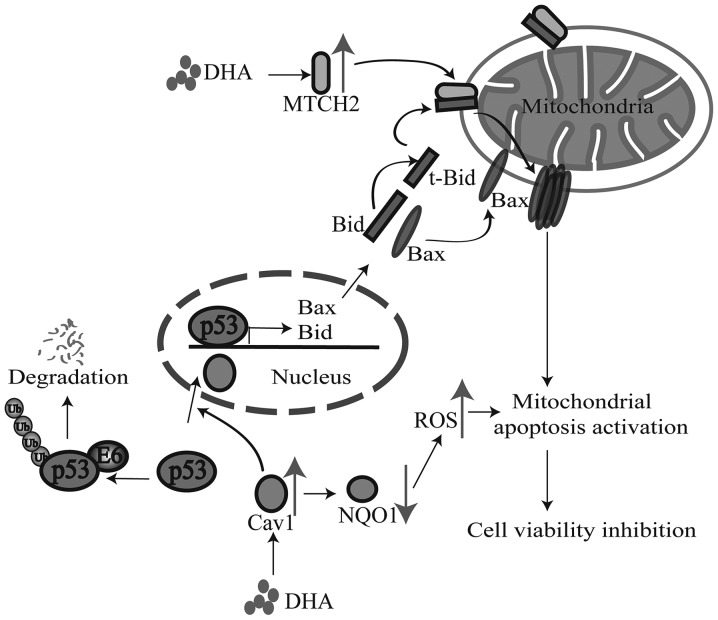
The Nrf2-mediated signaling pathway plays a critical role in protecting cells from oxidative stress induced cell death by transcriptionally activating antioxidant genes, including NQO1. NQO1 expression is increased in response to oxidative stress and an elevated NQO1 expression is essential for reducing ROS levels and maintaining the function of the mitochondria under oxidative stress (35). However, to the best of our knowledge, the involvement of NQO1 in the generation of high levels of ROS induced by DHA treatment has not been reported to date. Notably, the current study indicated that a potential association may exist between NQO1 and the DHA-induced inhibition of cell viability. DHA has been reported to induce high levels of ROS (33,34), which indicates the inhibition of the ability to remove free radicals following treatment with DHA. In other words, the elevated generation of ROS may indicate decreased antioxidant gene expression. In support of this assumption, we found that DHA treatment inhibited the expression of NQO1 which was associated with decreased cell viability. These data suggested that the downregulation of NQO1 following treatment with DHA may contribute to the elevated ROS generation and to the eventual inhibition of cell viability. Moreover, we also demonstrated that Cav1 may be responsible for the decreased expression of NQO1 following treatment wih DHA. The decreased expression of NQO1 was associated with the elevated expression of Cav1 following treatment with DHA. Functional analysis, namely Cav1 overexpression and Cav1 depletion also indicated that Cav1 regulated the protein level of NQO1 in HeLa cells. Since previous researchers have demonstrated the direct association between Cav1 upregulation and the inhibition of Nrf2 activation (22,23), our results suggested that Cav1 may play an important role in triggering the activation of the cell death pathway by inhibiting the Nrf2-mediated signaling pathway following treatment with DHA (Fig. 7).
Figure 7.
The possible mechanism involved in the anticancer effect of dihydroartemisinin (DHA).
DHA has been reported to induce the activation of the cell death pathway, including Bid activation and mitochondria translocation (7,21). The tumor suppressor, p53, plays an important role in mediating the cell death pathway. It has also been reported that p53 regulates the expression of Bid (36) and facilitates the apoptosis caused by DHA (5,8–10). However, the exact role of p53 in the DHA-mediated inhibition of cell viability remains unclear. Previous studies have shown a direct association between Cav1 and p53 in regulating SIPS in mouse embryonic fibroblasts (30,31). Thus, it is possible that Cav1 may also regulate p53 activation following treatment with DHA. In line with our hypothesis, we found that although the basal level of p53 in HeLa cells was low, the overexpression of Cav1 significantly increased the protein level of p53, whereas the depletion of Cav1 induced the downregulation of p53 in HeLa cells. Of note, previous studies have indicated that Cav1 regulates the stabilization of p53 by inhibiting the interaction of p53 and its ubiquitin ligase, mouse double minute 2 homolog (Mdm2); however, in HeLa cells, the proteasome-dependent degradation of p53 is mainly promoted by HPV E6 protein and inhibiting the interaction of p53 and Mdm2 cannot lead to the stabilization of p53 (37,38). These data suggested that Cav1 may regulate the stabilization of p53 in a different manner, rather than disassociating p53 from Mdm2 in HeLa cells. The mechanisms through which Cav1 induces the stabilization of p53 in HeLa cells are not yet clear. In the current study, we found that DHA treatment induced the accumulated nuclear localization of both Cav1 and p53. It has been reported that inhibiting the nuclear export of p53 with small molecule nuclear export inhibitors, such as leptomycin B and actinomycin D leads to the activation of p53 in HeLa cells (37,38). Combined with our findings, these data suggest that Cav1 may facilitate the nuclear translocation or inhibit the nuclear export of p53 to regulate the stability of p53 (Fig. 7); further studies are required to elucidate the complete mechanisms involved.
Recent studies have indicated MTCH2 as a potential cancer suppressor (26) and apoptosis facilitator via its interaction with t-Bid (24,25). We also found that the protein level of MTCH2 was increased in HeLa cells treated with DHA, indicating that MTCH2 may also contribute to the DHA-induced inhibition of cell viability in cancer cells. MTCH2 facilitates the mitochondrial translocation of t-Bid (24,25). We also demonstrated the nuclear translocation of p53 following treatment with DHA; thus, we assumed that DHA may induce the nuclear translocation and activation of p53 by upregulating Cav1. The activation of p53 then leads to upregulation of Bid and MTCH2 facilitates the translocation of cleaved Bid to the mitochondria, and eventually leads to the activation of the mitochondrial cell death pathway (Fig. 7). Despite the positive correlation between Cav1 and MTCH2 expression following treatment with DHA, it is worth noting that Cav1 cannot directly regulate the expression of MTCH2, as demonstrated by the functional overexpression of Cav1 and the depletion of Cav1. Further studies are required in order to elucidate the detailed mechanisms involved in the upregulation of MTCH2 in cells treated with DHA.
In conclusion, according to our findings, we made a hypothesis that Cav1 and MTCH2 may function coordinately as upstream signaling sensors and the upregulation of Cav1 and MTCH2 enhances the inhibitory effects of DHA on cell viability by inducing the nuclear activation of p53, the down-regulation of NQO1 and the mitochondrial translocation of tBid [although we did not examine the exact role of Bid in this study, we hypothesize that it may play a role according to previous studies (24,25) and ours], which eventually leads to the activation of the downstream cell death pathway (Fig. 7). Our study not only elucidated the potential mechanisms responsible for the anticancer effects of DHA, but also provide promising targets for cancer therapy.
Acknowledgments
The present study was supported by research grants from the Natural Science Foundation of Chengdu University (no. 2011XJZ14) and the Natural Science Foundation of China (no. 51402027).
References
- 1.Cao L, Duanmu W, Yin Y, Zhou Z, Ge H, Chen T, Tan L, Yu A, Hu R, Fei L, et al. Dihydroartemisinin exhibits anti-glioma stem cell activity through inhibiting p-AKT and activating caspase-3. Pharmazie. 2014;69:752–758. [PubMed] [Google Scholar]
- 2.Lucibello M, Adanti S, Antelmi E, Dezi D, Ciafrè S, Carcangiu ML, Zonfrillo M, Nicotera G, Sica L, De Braud F, et al. Phospho-TCTP as a therapeutic target of Dihydroartemisinin for aggressive breast cancer cells. Oncotarget. 2015;6:5275–5291. doi: 10.18632/oncotarget.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu M, Sun L, Zhou J, Zhao Y, Deng X. Dihydroartemisinin-induced apoptosis is associated with inhibition of sarco/endoplasmic reticulum calcium atpase activity in colorectal cancer. Cell Biochem Biophys. 2015;73:137–145. doi: 10.1007/s12013-015-0643-3. [DOI] [PubMed] [Google Scholar]
- 4.Liao K, Li J, Wang Z. Dihydroartemisinin inhibits cell proliferation via AKT/GSK3β/cyclinD1 pathway and induces apoptosis in A549 lung cancer cells. Int J Clin Exp Pathol. 2014;7:8684–8691. [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang CZ, Zhang H, Yun J, Chen GG, Lai PBS. Dihydroartemisinin exhibits antitumor activity toward hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol. 2012;83:1278–1289. doi: 10.1016/j.bcp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Zhao X, Zhong H, Wang R, Liu D, Waxman S, Zhao L, Jing Y. Dihydroartemisinin and its derivative induce apoptosis in acute myeloid leukemia through Noxa-mediated pathway requiring iron and endoperoxide moiety. Oncotarget. 2015;6:5582–5596. doi: 10.18632/oncotarget.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu YY, Chen TS, Wang XP, Li L. Single-cell analysis of dihydroartemisinin-induced apoptosis through reactive oxygen species-mediated caspase-8 activation and mitochondrial pathway in ASTC-a-1 cells using fluorescence imaging techniques. J Biomed Opt. 2010;15:046028. doi: 10.1117/1.3481141. [DOI] [PubMed] [Google Scholar]
- 8.Cabello CM, Lamore SD, Bair WB, III, Qiao S, Azimian S, Lesson JL, Wondrak GT. The redox antimalarial dihydroartemisinin targets human metastatic melanoma cells but not primary melanocytes with induction of NOXA-dependent apoptosis. Invest New Drugs. 2012;30:1289–1301. doi: 10.1007/s10637-011-9676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji Y, Zhang YC, Pei LB, Shi LL, Yan JL, Ma XH. Anti-tumor effects of dihydroartemisinin on human osteosarcoma. Mol Cell Biochem. 2011;351:99–108. doi: 10.1007/s11010-011-0716-6. [DOI] [PubMed] [Google Scholar]
- 10.Zhang CZ, Pan Y, Cao Y, Lai PB, Liu L, Chen GG, Yun J. Histone deacetylase inhibitors facilitate dihydroartemisinin-induced apoptosis in liver cancer in vitro and in vivo. PLoS One. 2012;7:e39870. doi: 10.1371/journal.pone.0039870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmons GE, Jr, Taylor HE, Hildreth JE. Caveolin-1 suppresses human immunodeficiency virus-1 replication by inhibiting acetylation of NF-κB. Virology. 2012;432:110–119. doi: 10.1016/j.virol.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 13.Wang XM, Kim HP, Song R, Choi AM. Caveolin-1 confers anti-inflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am J Respir Cell Mol Biol. 2006;34:434–442. doi: 10.1165/rcmb.2005-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huertas-Martínez J, Rello-Varona S, Herrero-Martín D, Barrau I, García-Monclús S, Sáinz-Jaspeado M, Lagares-Tena L, Núñez-Álvarez Y, Mateo-Lozano S, Mora J, et al. Caveolin-1 is down-regulated in alveolar rhabdomyosarcomas and negatively regulates tumor growth. Oncotarget. 2014;5:9744–9755. doi: 10.18632/oncotarget.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bender FC, Reymond MA, Bron C, Quest AF. Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. Cancer Res. 2000;60:5870–5878. [PubMed] [Google Scholar]
- 16.Bélanger MM, Roussel E, Couet J. Caveolin-1 is down-regulated in human lung carcinoma and acts as a candidate tumor suppressor gene. Chest. 2004;125(Suppl):106S. doi: 10.1378/chest.125.5_suppl.106S. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Pan L, Pu H, Wang Y, Zhang X, Li C, Yang Z. Loss of caveolin-1 promotes endothelial-mesenchymal transition during sepsis: a membrane proteomic study. Int J Mol Med. 2013;32:585–592. doi: 10.3892/ijmm.2013.1432. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, He W, Li Z, Chang W, Xin Y, Huang T. Caveolin-1 functions as a key regulator of 17β-estradiol-mediated autophagy and apoptosis in BT474 breast cancer cells. Int J Mol Med. 2014;34:822–827. doi: 10.3892/ijmm.2014.1836. [DOI] [PubMed] [Google Scholar]
- 19.Trimmer C, Sotgia F, Whitaker-Menezes D, Balliet RM, Eaton G, Martinez-Outschoorn UE, Pavlides S, Howell A, Iozzo RV, Pestell RG, et al. Caveolin-1 and mitochondrial SOD2 (MnSOD) function as tumor suppressors in the stromal microenvironment: a new genetically tractable model for human cancer associated fibroblasts. Cancer Biol Ther. 2011;11:383–394. doi: 10.4161/cbt.11.4.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao H, Gu H, Qu X, Sun J, Song B, Gao W, Liu J, Shao Q. Involvement of the mitochondrial pathway and Bim/Bcl-2 balance in dihydroartemisinin-induced apoptosis in human breast cancer in vitro. Int J Mol Med. 2013;31:213–218. doi: 10.3892/ijmm.2012.1176. [DOI] [PubMed] [Google Scholar]
- 22.Volonte D, Liu Z, Musille PM, Stoppani E, Wakabayashi N, Di YP, Lisanti MP, Kensler TW, Galbiati F. Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Mol Biol Cell. 2013;24:1852–1862. doi: 10.1091/mbc.E12-09-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Liu H, Zhou JS, Cao JF, Zhou XB, Choi AM, Chen ZH, Shen HH. Caveolin-1 inhibits expression of antioxidant enzymes through direct interaction with nuclear erythroid 2 p45-related factor-2 (Nrf2) J Biol Chem. 2012;287:20922–20930. doi: 10.1074/jbc.M112.352336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz C, Zaltsman-Amir Y, Mostizky Y, Kollet N, Gross A, Friedler A. Molecular basis of the interaction between proapoptotic truncated BID (tBID) protein and mitochondrial carrier homologue 2 (MTCH2) protein: key players in mitochondrial death pathway. J Biol Chem. 2012;287:15016–15023. doi: 10.1074/jbc.M111.328377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaltsman Y, Shachnai L, Yivgi-Ohana N, Schwarz M, Maryanovich M, Houtkooper RH, Vaz FM, De Leonardis F, Fiermonte G, Palmieri F, et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat Cell Biol. 2010;12:553–562. doi: 10.1038/ncb2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leibowitz-Amit R, Tsarfaty G, Abargil Y, Yerushalmi GM, Horev J, Tsarfaty I. Mimp, a mitochondrial carrier homologue, inhibits Met-HGF/SF-induced scattering and tumorigenicity by altering Met-HGF/SF signaling pathways. Cancer Res. 2006;66:8687–8697. doi: 10.1158/0008-5472.CAN-05-2294. [DOI] [PubMed] [Google Scholar]
- 27.Yu K, Ganesan K, Tan LK, Laban M, Wu J, Zhao XD, Li H, Leung CH, Zhu Y, Wei CL, et al. A precisely regulated gene expression cassette potently modulates metastasis and survival in multiple solid cancers. PLoS Genet. 2008;4:e1000129. doi: 10.1371/journal.pgen.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arigoni M, Barutello G, Riccardo F, Ercole E, Cantarella D, Orso F, Conti L, Lanzardo S, Taverna D, Merighi I, et al. miR-135b coordinates progression of ErbB2-driven mammary carcinomas through suppression of MID1 and MTCH2. Am J Pathol. 2013;182:2058–2070. doi: 10.1016/j.ajpath.2013.02.046. [DOI] [PubMed] [Google Scholar]
- 29.Han F, Gu D, Chen Q, Zhu H. Caveolin-1 acts as a tumor suppressor by down-regulating epidermal growth factor receptor-mitogen-activated protein kinase signaling pathway in pancreatic carcinoma cell lines. Pancreas. 2009;38:766–774. doi: 10.1097/MPA.0b013e3181b2bd11. [DOI] [PubMed] [Google Scholar]
- 30.Bartholomew JN, Volonte D, Galbiati F. Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res. 2009;69:2878–2886. doi: 10.1158/0008-5472.CAN-08-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volonte D, Zou H, Bartholomew JN, Liu Z, Morel PA, Galbiati F. Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6) J Biol Chem. 2015;290:4202–4214. doi: 10.1074/jbc.M114.598268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chrétien A, Piront N, Delaive E, Demazy C, Ninane N, Toussaint O. Increased abundance of cytoplasmic and nuclear caveolin 1 in human diploid fibroblasts in H(2)O(2)-induced premature senescence and interplay with p38alpha(MAPK) FEBS Lett. 2008;582:1685–1692. doi: 10.1016/j.febslet.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Hosoya K, Murahari S, Laio A, London CA, Couto CG, Kisseberth WC. Biological activity of dihydroartemisinin in canine osteosarcoma cell lines. Am J Vet Res. 2008;69:519–526. doi: 10.2460/ajvr.69.4.519. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Hu W, Zhang JL, Wu XH, Zhou HJ. Dihydroartemisinin induces autophagy and inhibits the growth of iron-loaded human myeloid leukemia K562 cells via ROS toxicity. FEBS Open Bio. 2012;2:103–112. doi: 10.1016/j.fob.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Kim SK, Kim HK, Mattson MP, Hyun DH. Mitochondrial function in human neuroblastoma cells is up-regulated and protected by NQO1, a plasma membrane redox enzyme. PLoS One. 2013;8:e69030. doi: 10.1371/journal.pone.0069030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS. BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol. 2002;4:842–849. doi: 10.1038/ncb866. [DOI] [PubMed] [Google Scholar]
- 37.Koivusalo R, Mialon A, Pitkänen H, Westermarck J, Hietanen S. Activation of p53 in cervical cancer cells by human papillomavirus E6 RNA interference is transient, but can be sustained by inhibiting endogenous nuclear export-dependent p53 antagonists. Cancer Res. 2006;66:11817–11824. doi: 10.1158/0008-5472.CAN-06-2185. [DOI] [PubMed] [Google Scholar]
- 38.Hietanen S, Lain S, Krausz E, Blattner C, Lane DP. Activation of p53 in cervical carcinoma cells by small molecules. Proc Natl Acad Sci USA. 2000;97:8501–8506. doi: 10.1073/pnas.97.15.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]