Abstract
An abundance of evidence points to a pre-supplementary motor area (pre-SMA) role in human language. This study explores the pre-SMA resting state connectivity network and the nature of its connections to known language areas. We tested the hypothesis that by seeding the pre-SMA, one would be able to establish language laterality to known cortical and sub-cortical language areas. We analyzed data from 30 right-handed healthy controls and performed the resting state functional MRI (rfMRI). A seed based analysis using a manually drawn pre-SMA region of interest (ROI) template was applied. Time course signals in the pre-SMA ROI were averaged and cross-correlated to every voxel in the brain. Results show that the pre-SMA has significant left-lateralized functional connectivity to the pars opercularis within Broca's area. Among cortical regions, pre-SMA functional connectivity is strongest to the pars opercularis Additionally, pre-SMA connectivity was shown to exist to other cortical language-association regions, including Wernicke's Area, supramarginal gyri, angular gyri(, and middle frontal gyri. Among sub-cortical areas, considerable left-lateralized functional connectivity occurs to the caudate and thalamus while cerebellar sub-regions show right-lateralization. The current study reveals that the pre-SMA most strongly connects to the pars opercularis within Broca's area and that cortical connections to language areas are left-lateralizedamong a sample of right-handed patients. We provide rfMRI evidence that the functional connectivity of the pre-SMA is involved in semantic language processing and that this identification may be useful for establishing language laterality in pre-operative neurosurgical planning.
Keywords: Resting state functional MRI, resting state network, language network, supplementary motor area, pre-supplementary motor area, functional connectivity
Introduction
Clinical and neuroimaging task-fMRI studies have long shown preferential pre-SMA involvement within the supplemental motor area during language task performance. Whereas the rostral SMA proper has been shown to preferentially activate during the motor aspects of language function such as word articulation and motor output in task-fMRI studies, the pre-SMA exhibits more prominent activation in specific cognitive language tasks [1], including verb generation [2], semantic-based word generation [3], and word reading [4] among a myriad of others. Very recently, white matter connectivity between the pre-SMA and associated language network using diffusion tensor imaging (DTI) was described [5]. Based on these findings, we anticipate substantial resting state functional connectivity to language association areas discovered in previous task-based language studies.
Resting state affords paradigm-free functional imaging due to low-frequency blood oxygenation level-dependent (BOLD) fluctuations during rest. These signal fluctuations reflect connectivity between functionally related regions of the brain [6]. Resting state networks of motor, visual, auditory, memory, language, and attention systems have been studied. These networks are symmetric in signal activation and correspond to known functional areas from task-based fMRI studies [7]. Determining language lateralization in pre-operative neurosurgical planning is highly valuable for pre-operative planning [8]. Unlike task-based fMRI, which requires extensive cooperation by the often neurologically impaired pre-operative patient, resting state fMRI does not require task performance [8]. The ability to accurately localize lateralized language areas without the need for the neurosurgical patient to perform a complicated language paradigm would be a significant advantage. Recent studies attempting to characterize the language network in resting state have met with mixed results, with many unable to characterize lateralization of language networks [9; 10]. More recently, effective but highly involved data-driven approaches and global signal analyses have shown some success, but a simpler approach remains elusive [11].
The purpose of this study is to examine the resting state functional connectivity network of the pre-SMA and evaluate whether a simple seed analysis involving the pre-SMA can elicit lateralized connections to known language areas . With the convergence of aforementioned evidence from multiple imaging modalities suggesting a language role for the pre-SMA, we hypothesize that the pre-SMA should have significant resting state connectivity to primary and secondary brain language regions and can serve as a simple and reliable means of determining language laterality.
Materials and Methods
Subjects
The rfMRI data was obtained from the Cambridge resting state database, which was uploaded by Dr. Randy Buckner's group to the 1000 Functional Connectomes database (https://www.nitrc.org/projects/fcon_1000/). The first thirty right-handed subjects (mean age = 22, 11 male and 19 female) were included for data analysis.
Data Acquisition
Scanning was performed on a 3 Tesla TimTrio systems (Siemens) using a 12-channel phased-array head coil.. Structural images were acquired using a sagittal MP-RAGE three-dimensional T1-weighted sequence (TR = 2,530 ms, TE = 3.44 ms, FA = 7°, 1.0 mm isotropic voxels; FOV 256 × 256). For the rfMRI scan, patients were instructed to leave eyes open and not think about anything during the scan. T2*-weighted rfMRI data were obtained with a single-shot gradient echo echo-planar imaging (EPI) sequence in the axial orientation (TR = 3000ms, TE = 30 ms, 128×128 matrix, 3 mm isotropic voxels, flip angle = 90°, total number of volume = 119).
Data Pre-Processing
Preprocessing of the rfMRI data was performed with AFNI [12]. Initially each subject data was evaluated to detect if there is any severe head motion or artifactual signal. 3D volume registration using Fourier interpolation was performed to correct head motion and six motion parameters were recorded to regress out motion induced spurious variance. Linear drift was removed from the time series data. After spatial normalization [13], the data was spatially smoothed with a Gaussian filter (FWHM =4mm). Subsequently, time series were filtered using a bandpass of the interval 0.01-0.08 Hz region to remove physiological high-frequency noise, e.g., respiratory and cardiac [6] and to reflect spontaneous neuronal activity. During the procedure, non-brain tissues including skull were removed.
Connectivity Analysis with Pre-SMA ROI
A pre-SMA ROI was manually drawn utilizing an anatomical template in normalized space. Pre-SMA boundaries were defined posteriorly by the vertical anterior commissure line, anteriorly by the vertical line drawn from the genu of the corpus callosum, inferiorly by the cingulate gyrus, and superiorly by the brain surface [14; 15].
Masks of all anatomical brain regions available in the Talairach space were created. Individual masks were created for Broca's area and Wernicke's area; Broca's area was defined as Brodmann's area 44 and 45 [16], while Wernicke's area was defined as Brodmann's area 22 [17].
Seed-based correlation maps were generated for each subject by extracting the averaged BOLD fMRI time course from the pre-SMA ROI and then the Pearson correlation coefficient was computed between the ROI time courses and every voxel in the brain to generate correlation (r) map. All individual r-maps were converted to a normal distribution using Fisher's r-to-z transformation. For group analysis, all Fisher's z-score maps were entered into a paired t-test to detect the regions showing significant functional connectivity with the seed ROI. These t-maps were thresholded at p<0.05, corrected for multiple comparisons. Percent voxel activation for specific regions were calculated by dividing the number of threshold activated voxels by the total number of voxels within the region of interest.
Laterality Assessment in Both Hemispheres
Laterality was assessed by calculating the difference in voxel activation in the left versus right hemispheres for specific anatomic regions using a formula: laterality index (LI) =(L-R)/(L+R). Presence of hemispheric dominance is assessed by comparing the LI to a predefined threshold (LITH). We define LITH = 0.2, which means that left-sided voxel activation will be at least 50% more than right-sided voxel activation [18]: LI > LITH means left hemispheric dominance, LI < -LITH, means right hemispheric dominance, and |LI| ≤ LITH, bilateral dominance. For example, laterality of SMA functional connectivity with Broca's area was characterized by calculating the difference between voxel activation in the left Broca's area region versus right Broca's area region at a corresponding t-score (p < 0.01). Laterality is quantified by applying the equation after setting specific t-score thresholds (t>2, t>4, t>6, t>8, t>10). This approach stratifies voxel activation as a function of the relative connectivity strength of activated voxels within regions of interest.
Results
The pre-SMA has statistically significant resting connectivity to the majority of the brain, but brain regional connectivity strength and laterality varies (Table 1). Connectivity is observed in cortical regions, basal ganglia, thalamic nuclei, and the cerebellum. The greatest degree of connectivity occurs from the pre-SMA to the pars opercularis of Broca's area, the caudate nuclei, and thalamic nuclei (Figure 1). Left-hemispheric dominance occurs among cortical language areas, whereas right-lateralization occurs in certain cerebellar sub-regions.
Table 1.
Coordinates, t-scores, and laterality indices of the most significantly activated regions (t>6.5, p≪0.0001).
| Brain RegionI | Talairach (Left) | t-Score Left | Talairach (Right) | t-Score Right | LI (t>6) | LI (t>8) | LI (t>10) |
|---|---|---|---|---|---|---|---|
| a) Cortical Regions | |||||||
| Broca's Area | (-50, 17, 1) | 8.09 | (49, 19, -1) | 7.21 | 0.00 | 0.33* | 0.66* |
| Wemicke's Area | (-60, -42, 10) | 6.53 | (60, -38, 7) | 5.93 | 0.12 | 0.36* | n/a |
| Superior Frontal Gyrus | (0, 16, 48) | 7.06 | (0, 16, 48) | 6.76 | 0.05 | 0.13 | 0.11 |
| Middle Frontal Gyrus | (-43, 3, 45) | 7.12 | (44, 3, 44) | 6.49 | 0.06 | 0.34* | 0.53* |
| Inferior Frontal Gyrus | (-50, 17, 1) | 6.73 | (49, 19, -1) | 6.32 | 0.01 | 0.22* | 0.39* |
| Supramarginal Gyrus | (-53, -53, 22) | 7.61 | (54, -46, 22) | 6.60 | 0.08 | 0.51* | n/a |
| Angular Gyrus | (-40, -59, 32) | 6.71 | (34, -55, 33) | 5.68 | 0.22* | 0.20* | n/a |
| Cingulate Gyrus | (0, -11, 39) | 6.53 | (0, -11, 39) | 6.20 | 0.02 | 0.08 | 0.11 |
| b) Basal Ganglia | |||||||
| Lentiform Nucleus | (-19, 1, 5) | 6.42 | (-14, -3, 5) | 5.87 | 0.10 | 0.49* | n/a |
| Claustrum | (-32, 3, 5) | 6.94 | (31, 10, 5) | 6.60 | 0.04 | 0.06 | n/a |
| Caudate | (-12, 9, 9) | 7.80 | (12, 8, 9) | 7.06 | 0.11 | 0.11 | 0.19 |
| Subthalamic nucleus | (-10, -13, -3) | 6.50 | (-11, 11, -3) | 7.08 | 0.07 | -0.76* | n/a |
| c) Thalamus | |||||||
| Thalamus | (-8, -12, 15) | 6.69 | (9, -4, 11) | 6.22 | 0.03 | 0.25* | n/a |
| Lateral Posterior Nucleus | (-15, -18, 14) | 7.77 | (17, -21, 14) | 7.40 | -0.07 | 0.20* | 0.85* |
| Lateral Dorsal Nucleus | (-12, -20, 16) | 8.62 | ((11, -17, 16) | 7.55 | 0.05 | 0.33* | -0.04 |
| Ventral Anterior Nucleus | (-12, -6, 6) | 7.49 | (8, -6, 6) | 7.12 | 0.11 | 0.02 | n/a |
| Ventral Lateral Nucleus | (-17, -16, 18) | 8.43 | (14, -16, 16) | 8.14 | -0.02 | 0.09 | 0.33* |
| Midline Nucleus | (-7, -18,16) | 8.57 | (-7, 19, 16) | 8.13 | 0.03 | 0.08 | -0.25* |
| Anterior Nucleus | (-8, -12, 15) | 9.12 | (9, -4, 11) | 8.60 | 0.03 | 0.05 | 0.31* |
| d) Cerebellum | |||||||
| Culmen Vermis | (0, -64, -3) | 6.53 | (0, -64, -3) | 6.02 | 0.10 | n/a | n/a |
| Uvula | (-15, -75, -32) | 7.25 | (24, -71, -25) | 7.71 | -0.02 | -0.14 | -0.32* |
| Culmen | (-34, -46, -27) | 6.58 | (35, -48, -27) | 6.37 | 0.05 | 0.01 | n/a |
| Declive | (-7, -71. -19) | 6.81 | (8, -72, -19) | 7.00 | 0.01 | -0.11 | n/a |
| Dentate | (-17, -58, -19) | 6.95 | (16, -57, -19) | 7.38 | 0.01 | -0.34* | n/a |
Fig. 1.
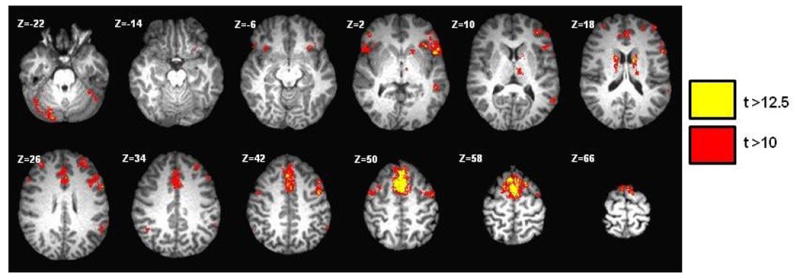
Resting state functional activity map generated using the seed ROI placed in the supplementary motor area (SMA). Broca's and Wernicke's area activations demonstrate a left sided dominance. The activation in the cerebellum is predominantly right-sided. (t=t score)
Connectivity between the Pre-SMA and Cortical Areas
The pre-SMA connects most significantly to language regions, with greatest connectivity occurring to the pars opercularis [19]. Similarly significant activation occurs in Wernicke's areas and known secondary language areas such as the supramarginal gyri, middle frontal gyri, and angular gyri (Table 1A). Among cortical language regions, Wernicke's area contains the weakest connectivity to the pre-SMA. Laterality indices for language regions are left-lateralized when threshold is set to high t-score ranges, with the pers opercularis possessing the greatest degree of left-lateralization (Figure 2). In addition, the pre-SMA demonstrates significant but relatively weaker connections to most other major cortical structures.
Fig. 2.
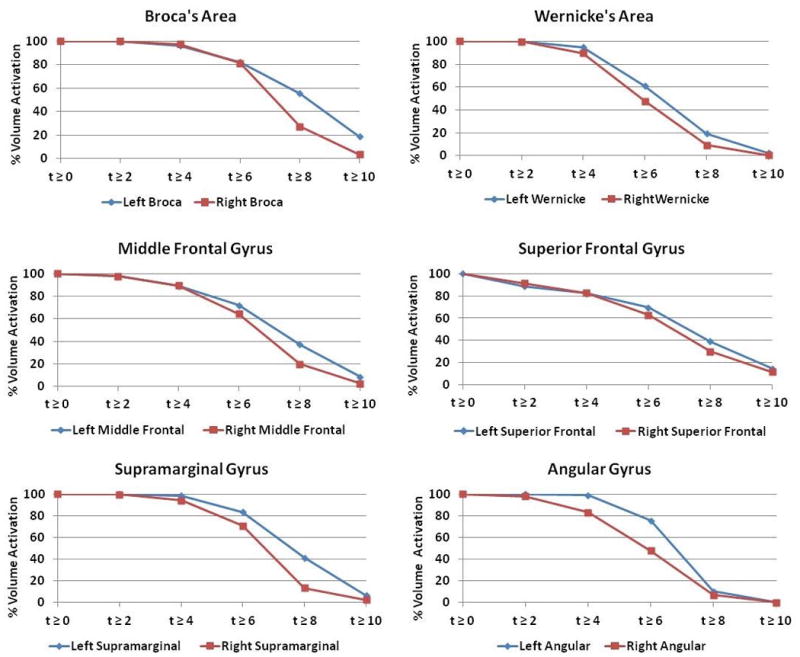
Percentage volume activation of most prominently activated cortical regions. Volume activation is graphed as a function of t-scores for both left (blue) and right (red) hemispheric regions.
Connectivity between Pre-SMA and Sub-Cortical Structures (Thalamus, Basal Ganglia, and Cerebellum)
Notable connectivity exists to sub-cortical areas. The pre-SMA has statistically significant connectivity with multiple thalamic nuclei with varying extents of connectivity strength and lateralization. Connectivity is strongest in the lateral dorsal nuclei, lateral posterior nucleus, anterior nuclei, ventral lateral nuclei, ventral anterior nuclei, and midline nuclei (Table 1B). Among these regions, significant (LI≥0.2) left-lateralization of connectivity at high thresholds is seen in the posterior nucleus, lateral dorsal nuclei, ventral lateral nuclei, and anterior nuclei; significant right-lateralization is seen in the midline nucleus at high thresholds. The pre-SMA also has statistically significant connectivity with multiple basal nuclei with varying extents of connectivity strength and lateralization. Connectivity is strongest to the caudate, claustrum, subthalamic nucleus, and lentiform nucleus (Table 1C). Considerably stronger connectivity occurs in the head (t=6.90) and body (t=7.80) of the caudate compared to the tail (t=3.13). Finally, the pre-SMA has statistically significant connectivity to multiple cerebellar sub-regions (Table 1D). Strong activation occurs in the culmen vermis, uvula, culmen, declive, and dentate. Notably, cerebellar connections in the tonsil, inferior semi-lunar lobule, uvula, dentate, and tuber are right-lateralized at high thresholds.
Discussion
In the current study, the pre-SMA serves for the first time as a potential means to determine cortical language laterality. Our results reveal statistically significant pre-SMA resting state connectivity to both cortical and sub-cortical language regions
Though the pre-SMA has strong resting state functional connectivity to most cortical regions, it connects most strongly to the pars opercularis,region of Broca's area. These findings corroborate with DTI and paradigm-driven studies demonstrating structural and functional relationships between the pre-SMA and Broca's area during language tasks [5]. Revealingly, the next strongest areas of connections are all known language roles, such as Wernicke's area, middle frontal gyrus, supramarginal gyrus, and angular gyrus. These connections are also left-lateralized, which is consistent with known left-lateralization of language function in right-handed humans. These findings provide convincing resting state evidence of pre-SMA involvement in language. They additionally open the possibility for the application of the current method to the localization and lateralization of language areas in the pre-operative brain tumor patient using rfMRI, rather than task-based fMRI.
Notably, pre-SMA resting state connections are not limited to cortical language areas, as evidenced by findings in the thalamus, basal ganglia, and cerebellum. Definitive conclusions regarding the nature of these connections is limited based on the current study's methodology, particularly without the availability of concurrent task-based fMRI studies. However, it is possible to argue that these connections may be language-related in light of the current study's finding that the pre-SMA's strongest cortical relationship is a left-lateralized connection to Broca's area, specifically the pars opercularis. For example, thalamic connections shown in this study may be functionally equivalent to the thalamic activation observed in task-based fMRI during language performance. Since cortical evidence exists to support pre-SMA involvement in language, functional connectivity between the pre-SMA and thalamus may similarly reflect both regions' involvement in the language resting state network. For the thalamus, numerous task-based functional imaging studies have clearly shown thalamic participation in language [20] [21]. Similarly within the basal ganglia, substantial pre-SMA resting state connectivity occurs to the caudate, which has been shown to be involved in numerous linguistic tasks such as language syntactic operations [22]. Cerebellar activation has already been extensively documented in literature in the context of numerous language tasks [23]; activation that is right-hemispheric dominant in specific cerebellar sub-regions may reflect similarly right-hemispheric dominant cerebellar activation findings during language task performance in individuals with left-dominant frontal activation. Further work beyond the scope of the current study's methods is required to determine the specific nature of these pre-SMA to sub-cortical functional connections.
This study shows for the first time that the pre-SMA is an effective region of interest for eliciting lateralized cortical language regions. For neurosurgical patients, identification of language lateralization provides critical information to deciding the appropriateness of neurosurgical intervention and improving post-operative morbidity [8]. Task-based fMRI is already regularly employed in cortical tumor and epilepsy resections, but its use is limited by the patient's ability to perform the task, minimize movement in the scanner, or simply be conscious. Resting state fMRI addresses these limitations by examining changes in spontaneous BOLD signal rather than signal changes induced by tasks. To elicit the language network, various groups have employed a seed analysis involving Broca's and Wernicke's ROIs and complicated ICA, data-driven or global signal approaches among others, showing mixed results with regards to language lateralization [11; 24; 25]. Our resting state analysis is a novel, effective, and relatively simple approach to determining the critical information of language lateralization, especially in patient groups that are unable to produce task-based activation maps. We believe this study is a convincing step towards understanding the clinical utility of resting state in neurosurgical pre-operative planning. Additionally, future studies now have a method of correlating pre-SMA functional connectivity changes with clinical language performance in the setting of disease. For example, the effects of stroke on language function can be assessed by comparing the pre-SMA resting state network in stroke patients to those in healthy controls. Similar work in stroke patients has already been performed to assess motor outcomes [26].
Our current study has limitations. First, our sample size (N=30), while sufficient to extract a meaningful result with healthy subjects, requires a larger population to make firm judgments. Second, the band pass frequency range (0.01Hz – 0.1Hz) applied in this study can directly exclude relatively high frequency oscillations from white matter (0.073 – 0.25Hz), respiratory (0.1 – 0.5Hz), and cardiac (0.6 – 1.2Hz) sources. However, to account for non-neuronal noise, data-driven independent components analysis (ICA) may be a more powerful tool for regressing out signals from regions such as white matter or ventricles that are likely to have a high degree of physiological artifact relative to neuronal activity [27], than seed based analysis alone. Third, the pre-SMA in the current study was based on anatomical landmarks from key older studies. To further validate this study's approach for determining pre-SMA functional connectivity, results from functionally defined seed regions from task-based fMRI should be compared to the results of this study.
Conclusion
This study demonstrates the ability of a pre-SMA seed analysis to elicit the lateralized language network. The pre-SMA resting state network significantly involves the cortical and sub-cortical language areas. Cortical language connections exhibit left-hemispheric dominance at high connectivity thresholds in right-handed healthy subjects. Additionally, sub-cortical involvement in the basal ganglia, thalamus, and cerebellum correlate well to findings in task-based fMRI literature. We provide solidifying evidence that the pre-SMA plays a major role in language function and also propose that the pre-SMA can serve as an effective seed analysis ROI for determining language lateralization and localizing brain language areas.
Acknowledgments
Sources of Funding: Memorial Sloan Kettering Medical Student Research Fellowship
Abbreviations
- fMRI
functional MRI
- rfMRI
Resting state functional MRI
- BOLD
Blood oxygen level-dependent
- pre-SMA
pre-supplementary motor area
- ROI
Region of interest
- SMA
Supplementary motor area
- DTI
diffusion tensor imaging
- LI
laterality index
Footnotes
Sources of Conflict: None Declared
References
- 1.Peck KK, Bradbury M, Psaty EL, Brennan NP, Holodny AI. Joint activation of the supplementary motor area and presupplementary motor area during simultaneous motor and language functional MRI. Neuroreport. 2009;20(5):487–491. doi: 10.1097/WNR.0b013e3283297d71. [DOI] [PubMed] [Google Scholar]
- 2.Etard O, Mellet E, Papathanassiou D, Benali K, Houde O, Mazoyer B, et al. Picture naming without Broca's and Wernicke's area. 2000;11(3):617–622. doi: 10.1097/00001756-200002280-00036. [DOI] [PubMed] [Google Scholar]
- 3.Tremblay P, Gracco VL. Contribution of the frontal lobe to externally and internally specified verbal responses: fMRI evidence. Neuroimage. 2006;33(3):947–957. doi: 10.1016/j.neuroimage.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Fiez Ja, Balota Da, Raichle ME, Petersent SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;241:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- 5.Jenabi M, Peck KK, Young RJ, Brennan N, Holodny AI. Probabilistic fiber tracking of the language and motor white matter pathways of the supplementary motor area (SMA) in patients with brain tumors. J Neuroradiol. 2013;41(5):342–349. doi: 10.1016/j.neurad.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 7.Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamran M, Hacker CD, Allen MG, Mitchell TJ, Leuthardt EC, Snyder AZ, et al. Resting-State Blood Oxygen Level Dependent Functional Magnetic Resonance Imaging for Presurgical Planning. Neuroimaging Clin N Am. 2014;24(4):557–571. doi: 10.1016/j.nic.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller AM, Meyer M. Language in the brain at rest: new insights from resting state data and graph theoretical analysis. Front Hum Neurosci. 2014;8(April):228. doi: 10.3389/fnhum.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tie Y, Rigolo L, Norton IH, Huang RY, Wu W, Orringer D, et al. Defining language networks from resting-state fMRI for surgical planning- A feasibility study. Hum Brain Mapp. 2014;35(3):1018–1030. doi: 10.1002/hbm.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAvoy M, Mitra a, Coalson RS, Petersen SE, Raichle ME. Unmasking Language Lateralization in Human Brain Intrinsic Activity. Cereb Cortex. 2015:1–14. doi: 10.1093/cercor/bhv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 13.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain 3-Dimensional proportional system: an approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- 14.Picard N, Strick PL. Motor areas of the median wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 15.Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11(6):663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 16.Hagoort P. On Broca, brain, and binding: A new framework. Trends Cogn Sci. 2005;9(9):416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs Ma, Barker PB, et al. Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Ann Neurol. 2001;50(5):561–566. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- 18.Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging. 2008;26(5):594–601. doi: 10.1016/j.mri.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, et al. Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: An in vivo MRI analysis. Eur J Neurosci. 1999;11(9):3033–3046. doi: 10.1046/j.1460-9568.1999.00718.x. [DOI] [PubMed] [Google Scholar]
- 20.Alain C, Reinke K, McDonald KL, Chau W, Tam F, Pacurar A, et al. Left thalamo-cortical network implicated in successful speech separation and identification. Neuroimage. 2005;26(2):592–599. doi: 10.1016/j.neuroimage.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 21.von Kriegstein K, Patterson RD, Griffiths TD. Task-Dependent Modulation of Medial Geniculate Body Is Behaviorally Relevant for Speech Recognition. Curr Biol. 2008;18(23):1855–1859. doi: 10.1016/j.cub.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotz Sa, Schwartze M, Schmidt-Kassow M. Non-motor basal ganglia functions: A review and proposal for a model of sensory predictability in auditory language perception. Cortex. 2009;45(8):982–990. doi: 10.1016/j.cortex.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Price CJ. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomasi D, Volkow ND. Resting functional connectivity of language networks: characterization and reproducibility. Mol Psychiatry. 2012;17(8):841–854. doi: 10.1038/mp.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ter Minassian A, Ricalens E, Nguyen The Tich S, Dinomais M, Aubé C, Beydon L. The presupplementary area within the language network: a resting state functional magnetic resonance imaging functional connectivity analysis. Brain Connect. 2014;4(6):440–53. doi: 10.1089/brain.2014.0263. [DOI] [PubMed] [Google Scholar]
- 26.Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67(3):365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]


