Abstract
Objectives
Medication adherence is a complex problem and can be evaluated using a variety of methods. There is no single or perfect strategy to assess adherence. The “best” measure depends on contextual factors. Our objective is to provide a practical, illustrative guide for selecting the most appropriate measure of medication adherence in common contexts.
Methods
We present three case studies – from the perspectives of an academic researcher, health care payer, and clinical care provider – to describe common problems and processes for measuring medication adherence, as well as proposing possible solutions.
Results
The most appropriate measure will depend on the context (tightly controlled clinical trial setting vs. clinical setting), intended purpose (research vs. clinical), available resources (data, personnel, materials, and funding), time (quick screening vs. comprehensive review), and phase of interest (initiation, implementation, persistence or discontinuation). Framing the problem of medication non-adherence and methods for measuring adherence are discussed using three representative case studies.
Conclusions
A simple tool is provided that may help stakeholders interested in medication adherence make decisions regarding the appropriate selection of measures.
Practice Implications
A medication adherence measure should be selected through the lens of each situation’s unique objectives, resources, and needs.
Keywords: medication adherence, measurement, case studies
1. The Problem of Medication Non-Adherence
Medication non-adherence is a significant, global public health concern. In fact, approximately 30–50% of patients with chronic diseases fail to take their medications as prescribed [1–3]. A myriad of factors contribute to non-adherence, spanning the levels of the individual patient (e.g., low health literacy), healthcare system (e.g., formulary restrictions), and often national health policy (e.g., insufficient prescription drug coverage) [4–6]. Because factors contributing to non-adherence are complex and occur on multiple levels, accordingly, solutions to improve adherence often require complex interventions (provider or system-related) and behavior change (patient-related) on multiple levels in order to ensure effectiveness [4, 7, 8]. Even then, the resulting improvement in adherence is often small [8].
Measuring non-adherence can also be challenging. Proper medication taking is not a single action, but instead a complex series of behavioral support including initiation, implementation and persistence, and discontinuation [9, 10]. Depending on which behavior(s) of medication adherence are of interest, it may be important to select a measure of medication adherence that adequately captures the relevant levels (individual, healthcare system, etc.) and phases (initiation, implementation, etc.). For example, consider patients diagnosed with Type II diabetes. They may face challenges in initiating new medications (e.g., metformin, insulin) along with implementation of a therapeutic lifestyle program and then must persist in continuing a comprehensive treatment plan over time. It is also critical that the patient understands the diagnosis and appreciates their individual role in self management, including the proper use of medical therapy. Central to successful diabetes control is not only persistence with prescribed regimens, but also discontinuing medications and perhaps replacing them with different therapies under a healthcare provider’s supervision. Depending on which phase of this trajectory is of interest, different measures might be better suited than others [11].
Predictors of medication non-adherence have been well-documented [1, 2] and there are systematic reviews describing various measures of adherence [12] and interventions to improve it [7, 8, 13–15]. However, guidance on selecting appropriate medication adherence measures is lacking. Navigating the myriad of measures requires thoughtful consideration of the advantages and disadvantages of medication adherence measurement methods. These advantages and disadvantages may be a matter of perspective; however, guidance is needed on how to choose the most appropriate medication adherence measure. The purpose of this article is to provide a practical, illustrative guide for selecting the most appropriate measure of medication adherence in common contexts. This guide complements existing medication adherence literature and expands the thought process regarding measurement selection through the presentation of case studies. This guide is intended for readers who are somewhat familiar with available measures of adherence. Readers seeking information on specific definitions pertaining to medication adherence and general issues relating to measurement may consult existing reviews on this topic [12, 16–19]. A medication adherence measure should be selected through the lens of each situation’s unique resources and needs.
2. Methods of Measuring Medication Adherence
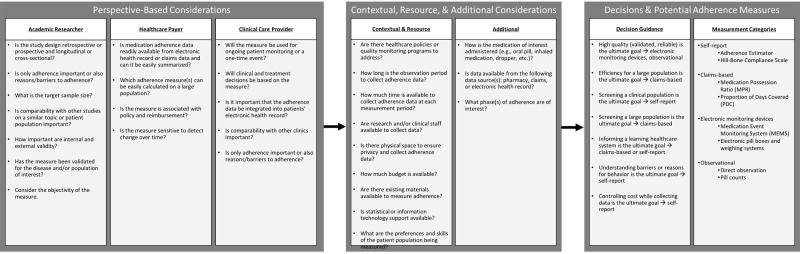
Measures of medication adherence are vast and include, but are not limited to, self-reported adherence, evaluation of biomarkers, electronic monitors and pillboxes, pharmacy-based measures, and assessment of a patient’s physiologic status among others [6, 12, 17–19]. There have been several systematic and other review articles detailing various measures of medication adherence [6, 16, 18, 20–24]. These reviews have classified adherence measures into direct or indirect measures. In other words, whether the medication-taking is directly observable (e.g., direct face-to-face observation) or indirect (e.g., pill counts, self-report, clinical response and biomarkers) [6, 16]. Beyond whether a measure is a direct or indirect assessment, measures of adherence differ in a number of additional ways. Measures may be better suited to measure a particular phase or phases of adherence (e.g., initiation, discontinuing). Additionally, measures rely on different data sources, vary with regard to validity and reliability, require differing amounts of time and expertise to collect, and have differing associated costs (Figure 1).
Figure 1.
Measure Selection Decision Making
While a combination of subjective and objective measurement is generally best [1], the most appropriate measure will depend on the context (tightly controlled clinical trial setting vs. clinical setting), intended purpose (research vs. clinical), available resources (data, personnel, materials, and funding), time (quick screening vs. comprehensive review), and phase of interest (initiation, implementation, or discontinuation). While many of these issues have previously been elucidated, we assert that an applied guide is needed. Thus, we present a practical consideration for selecting the “best” medication adherence measure from three perspectives: an academic researcher, health care payer, and clinical care provider.
3. Case Study 1: Academic Researcher
3.1. The Problem
Dr. Jones is a researcher at Acme University. She is designing an efficacy trial to demonstrate the therapeutic effect of an oral chemotherapy medication among patients with breast cancer. She knows that adherence is not often reported in cancer clinical trials, but is important in evaluating the effects of the medication [25]. The study population is comprised of adults receiving care in oncology clinics affiliated with academic medical centers. Dr. Jones will follow trial participants over a 10-month time period.
Because this is an efficacy trial, Dr. Jones has a tightly controlled research environment. She is looking for a measure of medication adherence that is objective, validated, and reliable. Dr. Jones requires a measure that has been used among patients with cancer. Her study results must be comparable with other published studies of oral chemotherapy to the best possible extent. Dr. Jones has research staff available to support the project, but is insufficiently staffed to personally observe participants taking their chemotherapy at home. She does, however, have a reasonable budget for adherence measurement.
Although the oncology clinics are affiliated with different academic medical centers that do not share pharmacy or electronic health record information with one another, Dr. Jones has access to participants’ individual electronic health record data. She could use this information to calculate adherence rates, but wants more granular information about participants’ daily behaviors. Specifically, because doses are designed to keep constant levels of drug in the body, taking the medication at the same time of day is important. Dr. Jones is concerned that patients might alter the time of day when they are taking their drugs to avoid experiencing side effects at an inconvenient time, so she decides against using a pharmacy or claims-based measure. In order to detect improvements during the trial, Dr. Jones wants to select a measure that is sensitive to longitudinal changes. She considers diaries or other measures of self-report, but has a low tolerance for recall bias.
3.2 The Process
Because Dr. Jones is interested in adherence to one specific medication, she considers using an electronic monitoring system. She searches the scientific literature and discovers several studies using electronic monitoring systems to assess adherence to oral chemotherapy regimens and hormonal therapy for breast cancer [26–28]. The monitors are expensive, but Dr. Jones determines that the cost is justified – there is a high correlation between electronic monitoring adherence estimates and clinical outcomes for many conditions. For example, a study among people with HIV found that the sensitivity of electronic monitoring of antiretroviral adherence to be 75% and the specificity was nearly 86% [29].
Dr. Jones is interested in a smart pill bottle, such as the Medication Event Monitoring System (MEMSCap), but is concerned that the bottle may be cumbersome for patients to open and/or that the using the smart pill bottle itself might be an intervention of sorts. She knows that patients sometimes remove medications and put them in their own weekly pillboxes. Because oral chemotherapy drugs have specific handling instructions, she is less concerned about the use of weekly pillboxes. Dr. Jones conducts a small pilot study and determines that participants can use the bottle.
3.3 A Solution
As part of the trial design, Dr. Jones allows a 30-day observational run-in period when participants use the smart bottle, but their data is discarded. After this run-in period, the smart pill bottle data is collected for the research study. The smart pill bottle data provides Dr. Jones with specific information about when patients open their pill bottle each day, which affords her with detailed information about approximately when each dose is taken and/or when doses are missed.
The smart bottle allows Dr. Jones to report an individual’s specific medication taking behaviors, including time delays in taking the regimen on a particular day. This provides Dr. Jones with precise adherence information, which she can use to evaluate associations with clinical outcomes (disease progression, survival) and compare with other research studies. Given the high cost of newer oncology drugs, understanding adherence on a granular level may have importance beyond the trial setting. Dr. Jones also notes that the patients in her pilot study reported valuable information about their adherence beyond the information collected through the MEMSCap, such as the reason for delays in getting their medications refilled, often due to cost [30, 31]. Dr. Jones recalls that combining both objective and subjecting measures is best [1] and collects both smart pill bottle data and patient self-reported medication adherence.
4. Case Study 2: Health Care Payer
4.1. The Problem
Mr. Smith has read several articles suggesting that adherence to cardiovascular disease medications (e.g., regimens to lower cholesterol or blood pressure) may reduce downstream healthcare costs. He is interested in understanding adherence to multiple medications among enrollees in his insurance group. Mr. Smith wants to know not only about adherence, but also about associated healthcare use and spending. Understanding the characteristics of enrollees that are most likely to be non-adherent are important to allocate resources. For example, Mr. Smith’s company provides a case management program which incorporates a behavioral change component focused on improving the way enrollees take their medication. Obtaining adherence information would be useful for targeting which enrollees would benefit most from a case management program, thus reducing costs for the insurance group in the long run.
4.2. The Process
Mr. Smith has access to enrollees’ healthcare claims data beginning at the time of enrollment. He reasons that forgoing information about whether the medication was taken correctly (e.g., timing, with food, etc.) is reasonable in order to have information on the entire population of enrollees. Mr. Smith has access to statistical and programming support, but he is on a tight timeline to prepare a report for his management team. He decides that a pharmacy claims based measure is best, but struggles between medication possession ratio (MPR) and proportion of days covered (PDC) [32, 33].
Both measures rely on prescription refill information and both can be calculated with data available to Mr. Smith. MPR is a sum of the day’s support for fills and refills of the drug of interest within a particular time period divided by the number of days in that time period. MPR may overestimate adherence, for example, this likely happens when a patient refills a prescription early. PDC is more conservative estimate of refill. PDC adjusts for overlapping days of coverage (e.g., the early refill) and thus, it is impossible to have a PDC above 100%. PDC is also the measure endorsed by CMS [34].
4.3. A Solution
Mr. Smith presents both pharmacy refill measures (MPR and PDC) to his team. He acknowledges that having multiple measures to triangulate adherence would be best, but is limited in data availability. Mr. Smith selects PDC as the adherence measure because his team is interested in a medication regimen (e.g., multiple medications per patient as opposed to one drug), would prefer a more conservative estimate, and may make comparisons with reported CMS adherence metrics.
Based on the PDC approach to initiate an understanding of medication adherence, Mr. Smith is able to establish a focus on the importance of medication adherence in chronic disease management. Providers then are able to better understand which patients may benefit from education or other interventions to improve the appropriate use of medicines. Furthermore, the clinical team may pick up on patients’ cues or patient measures (e.g. ongoing control of blood pressure of reductions in LDL cholesterol) to link medication use to improved patient care.
5. Case Study 3: Clinical Care Provider
5.1. The Problem
Dr. Taylor oversees a primary care clinic serving a largely hypertensive population. She tasks her nurse manager, Mr. Schwartz, with integrating a medication adherence screen for patients diagnosed with hypertension into the clinic intake procedures. The screener information will be used to prompt a discussion between the intake nurse and patient about adherence and may trigger referral into an adherence improvement program before intensifying treatment.
5.2. The Process
Mr. Schwartz did not receive funding or additional staffing to support inclusion of the adherence screen. The clinic staff is already overburdened. Adherence information will need to be collected quickly at the point of care, preferably without requiring additional time from the nursing staff. Mr. Schwartz notes that there is existing infrastructure to collect information about patients’ pain and mental state (i.e., depression screen) while patients are in the waiting room. Patients report their level of pain and depression status on a paper form when they check in for their appointment. Mr. Schwartz thinks adding the adherence screener to the paper form makes sense. While patient-reported data is subject to recall bias, collecting it is relatively inexpensive, non-invasive, and quick. While not as precise or objective, self-report is at least moderately correlated with electronic monitoring [23] and has been validated against pharmacy claim-based adherence measures [35]. Even if the data are completely accurate, Mr. Schwartz reasons that patient reported information is appropriate to use as a screener and to trigger a referral to the adherence improvement program.
5.3. A Solution
Mr. Schwartz is overwhelmed by the number of self-report medication adherence measures available [18, 24, 36, 37]. Mr. Schwartz wants to select a measure that has been successfully used to assess adherence among people with hypertension in a busy clinic setting; thus, he chooses the Hill-Bone Compliance Scale [38, 39]. Mr. Schwartz selects the Hill-Bone Compliance Scale for several reasons. While it is a 14-item self-administered scale, it only takes approximately 5 minutes to complete. It has good internal consistency, was developed to measure adherence to blood pressure medications and other issues of importance to a hypertensive population (e.g., sodium intake and appointment keeping), and has been associated with clinical outcomes like blood pressure control [18, 38, 39].
As a result of employing the Hill-Bone Compliance Scale, Mr. Schwartz can more easily reinforce with all of his patients that appropriate medication use is integral to their health and identify which patients may benefit from readily available resources. Reinforcement from educational websites, informational brochures, or community programs may then be availed to patients on a select basis to help further support proper medication utilization.
Discussion and Conclusion
5.1 Discussion
Medication adherence remains a major public health concern. While adherence is a problem for people of all ages, non-adherence is expected to continue as the population ages and becomes increasingly reliant on self-administered medications. There is increasing recognition of the importance of medication adherence in the health policy arena. Medication adherence is a critical element for chronic disease management – improving blood sugar, blood pressure and cholesterol control among people with diabetes. As a result, in the U.S. there have been changes to Medicare Part D benefits which may promote adherence and several of the Centers of Medicare and Medicare Services (CMS) star-rating measurements rely on medication adherence [40]. Additionally, part of the Europe 2020 Healthy Aging Initiative addresses adherence. Because of its increasing ties to reimbursement and improved patient outcomes, we anticipate increased emphasis on measuring adherence and subsequently improving it.
This shift in policy and focus comes at an opportune time. Technology is evolving to create new ways for measuring and improving adherence [41, 42]. While these methods may be innovative, it is important to remember that while technology may allow for many advances in the field, the “best” type of measure depends on the context, adherence phase, and purpose. Even once the category has been selected (e.g., claims based) there are numerous specific measures (e.g., PDC, MPR) from which to select. The case studies presented here provide three illustrative examples; however, there may not be a perfect solution. We suggest consulting available resources and thoughtfully evaluating the specific need when selecting a measure [6, 16, 18, 20–24, 36, 37].
5.2 Conclusion
Selecting an adherence measure requires careful consideration. There is no one best measure of medication adherence. Ideally, how one measures adherence should help inform how best to address the challenges of non-adherence.
6. Practice Implications
A medication adherence measure should be selected through the lens of each role’s unique resources and needs. Selection requires a balance between addressing the needs of stakeholders, time required for data collection, and time before being able to act on the information, as well as cost, and burden to patients and staff. In light of a lack of evidence- or consensus-based standard medication adherence measure for each stakeholder, it is likely necessary that individuals may benefit from use of multiple adherence measures. In general, understanding what information each medication adherence measure can and cannot provide will ensure better selection of measure(s) and subsequent use of these data.
HIGHLIGHTS.
Medication adherence is a complex problem that can be evaluated in numerous ways.
There is no single solution to improve adherence and no “best” measurement strategy.
We present case studies illustrating rationales for adherence measurement selection.
Selecting adherence measures requires consideration of stakeholders, purpose, cost, and time.
Acknowledgments
Dr. Zullig is supported by a VA Health Services Research and Development (HSR&D) Career Development Award (CDA 13-025). Dr. Bosworth is supported by a Research Career Scientist Award from VA Health Service Research and Development (VA HSR&D 08-27).
Footnotes
Conflicts of Interest
The authors have no conflicts to disclose. The views in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or of Duke University.
References
- 1.Sabaté E. Adherence to long-term therapies: evidence for action: World Health Organization. 2003 [PubMed]
- 2.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Medical care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Wertheimer AI, Santella TM. Medication Compliance Research: Still So Far To Go. Journal of Applied Research. 2003;3(3) [Google Scholar]
- 4.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clinic proceedings. 2011;86(4):304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal B, Mosca L. Lifestyle and psychosocial risk factors predict non-adherence to medication. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2010;40(2):228–233. doi: 10.1007/s12160-010-9212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osterberg L, Blaschke T. Adherence to medication. The New England journal of medicine. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 7.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. The Cochrane database of systematic reviews. 2008;(2):Cd000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Haynes RB, McDonald H, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications. The Cochrane database of systematic reviews. 2002;(2):Cd000011. doi: 10.1002/14651858.CD000011. [DOI] [PubMed] [Google Scholar]
- 9.Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, Lewek P, et al. A new taxonomy for describing and defining adherence to medications. British journal of clinical pharmacology. 2012;73(5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchins DS, Zeber JE, Roberts CS, Williams AF, Manias E, Peterson AM. Initial Medication Adherence-Review and Recommendations for Good Practices in Outcomes Research: An ISPOR Medication Adherence and Persistence Special Interest Group Report. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2015;18(5):690–699. doi: 10.1016/j.jval.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Iglay K, Cartier SE, Rosen VM, Zarotsky V, Rajpathak SN, Radican L, Tunceli K. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Current medical research and opinion. 2015;31(7):1283–1296. doi: 10.1185/03007995.2015.1053048. [DOI] [PubMed] [Google Scholar]
- 12.Lam WY, Fresco P. Medication Adherence Measures: An Overview. BioMed research international. 2015;2015:217047. doi: 10.1155/2015/217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conn VS, Ruppar TM, Chase JA, Enriquez M, Cooper PS. Interventions to Improve Medication Adherence in Hypertensive Patients: Systematic Review and Meta-analysis. Current hypertension reports. 2015;17(12):94. doi: 10.1007/s11906-015-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demonceau J, Ruppar T, Kristanto P, Hughes DA, Fargher E, Kardas P, De Geest S, Dobbels F, Lewek P, Urquhart J, et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs. 2013;73(6):545–562. doi: 10.1007/s40265-013-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2003;60(7):657–665. doi: 10.1093/ajhp/60.7.657. [DOI] [PubMed] [Google Scholar]
- 16.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clinical therapeutics. 1999;21(6):1074–1090. doi: 10.1016/S0149-2918(99)80026-5. discussion 1073. [DOI] [PubMed] [Google Scholar]
- 17.Krousel-Wood M, Holt E, Joyce C, Ruiz R, Dornelles A, Webber LS, Morisky DE, Frohlich ED, Re RN, He J, et al. Differences in cardiovascular disease risk when antihypertensive medication adherence is assessed by pharmacy fill versus self-report: the Cohort Study of Medication Adherence among Older Adults (CoSMO) Journal of hypertension. 2015;33(2):412–420. doi: 10.1097/HJH.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen TM, La Caze A, Cottrell N. What are validated self-report adherence scales really measuring?: a systematic review. British journal of clinical pharmacology. 2014;77(3):427–445. doi: 10.1111/bcp.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. Journal of clinical epidemiology. 1997;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 20.Culig J, Leppee M. From Morisky to Hill-bone; self-reports scales for measuring adherence to medication. Collegium antropologicum. 2014;38(1):55–62. [PubMed] [Google Scholar]
- 21.Jimmy B, Jose J. Patient medication adherence: measures in daily practice. Oman medical journal. 2011;26(3):155–159. doi: 10.5001/omj.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann A, Aslani P, Ahmed R, Celio J, Gauchet A, Bedouch P, Bugnon O, Allenet B, Schneider MP. Assessing medication adherence: options to consider. International journal of clinical pharmacy. 2014;36(1):55–69. doi: 10.1007/s11096-013-9865-x. [DOI] [PubMed] [Google Scholar]
- 23.Shi L, Liu J, Fonseca V, Walker P, Kalsekar A, Pawaskar M. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health and quality of life outcomes. 2010;8:99. doi: 10.1186/1477-7525-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stirratt MJ, Dunbar-Jacob J, Crane HM, Simoni JM, Czajkowski S, Hilliard ME, Aikens JE, Hunter CM, Velligan DI, Huntley K, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Translational behavioral medicine. 2015;5(4):470–482. doi: 10.1007/s13142-015-0315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergsbaken JJ, Eickhoff JC, Buss BA, Mably MS, Kolesar JM. Assessment of adherence with oral anticancer agents in oncology clinical trials: A systematic review. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2016;22(1):105–113. doi: 10.1177/1078155214567163. [DOI] [PubMed] [Google Scholar]
- 26.Bourmaud A, Henin E, Tinquaut F, Regnier V, Hamant C, Colomban O, You B, Ranchon F, Guitton J, Girard P, et al. Adherence to oral anticancer chemotherapy: What influences patients' over or non-adherence? Analysis of the OCTO study through quantitative-qualitative methods. BMC research notes. 2015;8:291. doi: 10.1186/s13104-015-1231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simons S, Ringsdorf S, Braun M, Mey UJ, Schwindt PF, Ko YD, Schmidt-Wolf I, Kuhn W, Jaehde U. Enhancing adherence to capecitabine chemotherapy by means of multidisciplinary pharmaceutical care. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19(7):1009–1018. doi: 10.1007/s00520-010-0927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993;11(6):1189–1197. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]
- 29.Deschamps AE, De Geest S, Vandamme AM, Bobbaers H, Peetermans WE, Van Wijngaerden E. Diagnostic value of different adherence measures using electronic monitoring and virologic failure as reference standards. AIDS patient care and STDs. 2008;22(9):735–743. doi: 10.1089/apc.2007.0229. [DOI] [PubMed] [Google Scholar]
- 30.Zullig LL, Peppercorn JM, Schrag D, Taylor DH, Jr, Lu Y, Samsa G, Abernethy AP, Zafar SY. Financial distress, use of cost-coping strategies, and adherence to prescription medication among patients with cancer. Journal of Oncology Practice. 2013;9(6S):60s–63s. doi: 10.1200/JOP.2013.000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bestvina CM, Zullig LL, Rushing C, Chino F, Samsa GP, Altomare I, Tulsky J, Ubel P, Schrag D, Nicolla J. Patient-oncologist cost communication, financial distress, and medication adherence. Journal of Oncology Practice. 2014;10(3):162–167. doi: 10.1200/JOP.2014.001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nau DP. Proportion of days covered (PDC) as a preferred method of measuring medication adherence. Springfield, VA: Pharmacy Quality Alliance; 2012. [Google Scholar]
- 33.Sattler EL, Lee JS, Perri M., 3rd Medication (re)fill adherence measures derived from pharmacy claims data in older Americans: a review of the literature. Drugs & aging. 2013;30(6):383–399. doi: 10.1007/s40266-013-0074-z. [DOI] [PubMed] [Google Scholar]
- 34.Enhancements to Medicare Part D Patient Safety Reports and Website. [ https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/downloads/2010PtSafetyReportEnhan_memo_093010.pdf]
- 35.Krousel-Wood M, Islam T, Webber LS, Re R, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in hypertensive seniors. The American journal of managed care. 2009;15(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- 36.Clifford S, Perez-Nieves M, Skalicky AM, Reaney M, Coyne KS. A systematic literature review of methodologies used to assess medication adherence in patients with diabetes. Current medical research and opinion. 2014;30(6):1071–1085. doi: 10.1185/03007995.2014.884491. [DOI] [PubMed] [Google Scholar]
- 37.Garfield S, Clifford S, Eliasson L, Barber N, Willson A. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC medical research methodology. 2011;11:149. doi: 10.1186/1471-2288-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim MT, Hill MN, Bone LR, Levine DM. Development and testing of the Hill-Bone Compliance to High Blood Pressure Therapy Scale. Progress in cardiovascular nursing. 2000;15(3):90–96. doi: 10.1111/j.1751-7117.2000.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 39.Song Y, Han HR, Song HJ, Nam S, Nguyen T, Kim MT. Psychometric evaluation of hill-bone medication adherence subscale. Asian nursing research. 2011;5(3):183–188. doi: 10.1016/j.anr.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 40.McKethan A, Benner J, Brookhart A. Seizing The Opportunity To Improve Medication Adherence. HealthAffairs Blog. 2012;2016 [Google Scholar]
- 41.Granger BB, Bosworth HB. Medication adherence: emerging use of technology. Current opinion in cardiology. 2011;26(4):279–287. doi: 10.1097/HCO.0b013e328347c150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zullig L, Shaw R, Bosworth H. Behavioral health care and technology: using science-based innovations to transform practice. Oxford: Oxford University Press; 2014. Applying technology to medication management and adherence . [Google Scholar]