Abstract
The suppression of the immune system by overexposure to ultraviolet (UV) radiation has been implicated in the initiation and progression of photocarcinogenesis. Numerous changes occur in the skin on UVB exposure, including the generation of inflammatory mediators, DNA damage, epigenetic modifications, and migration and functional alterations in the antigen-presenting dendritic cells. Although each of these alterations can elicit a cascade of events that have the potential to modulate immune sensitivity alone, there is emerging evidence that there is considerable crosstalk between these cascades. The development of an understanding of UV-induced changes in the skin that culminate in UV-induced immunosuppression, which has been implicated in the risk of non-melanoma skin cancer, as a network of events has implications for the development of more effective chemopreventive strategies. In the current review article, we discuss the evidence of interactions between the various molecular targets and signaling mechanisms associated with UV-induced immunosuppression.
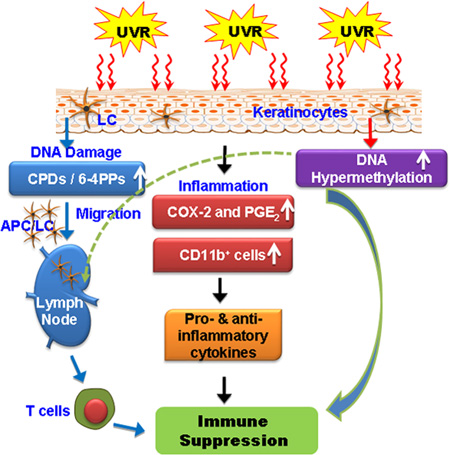
Graphical Abstract
Crosstalk among UV radiation-induced inflammatory mediators, DNA damage, and epigenetic regulators results in photo-immunosuppression. UVB radiation induced photodamage initiates migration of antigen presenting cells from skin to regional lymph nodes, where they present antigen to T-cells in an unusual way. UVB-induced inflammatory mediators and DNA damage affect epigenetic regulators and all together play crucial roles in suppression of immune system in UV radiation-exposed mouse skin. This suppression of immune system is implicated in skin cancer risk.
INTRODUCTION
MOLECULAR TARGETS CRUCIAL FOR UVB-INDUCED IMMUNOSUPPRESSION
Solar ultraviolet (UV) radiation is considered to be one of the most important environmental risk factors that affects skin physiology and has been associated with the pathogenesis of various skin disorders, including melanoma and non-melanoma skin cancers (1). The incidence of cutaneous malignancies is increasing as larger amounts of UV radiation reach the Earth's surface because of depletion of the ozone layer and as the population ages. Solar UV radiation is divided into three main categories based on wavelength: UVC (200–280 nm), UVB (280–320 nm), and UVA (320–400 nm). Although UV radiation represents only a small fraction of solar light, it is responsible for the majority of skin pathogenesis including carcinogenic activity (1). Almost the entire UVC fraction of solar light is absorbed by the ozone layer and may be very little fraction reaches to the Earth’s surface. UVB radiation induced damage is more severe than UVA damage as most of the UVB fraction is absorbed by the epidermal part of the skin (2).
It is known that acute or chronic exposure of the skin to UV radiation results in the development of inflammation, oxidative stress, DNA damage, epigenetic changes in skin cells, and suppression of immune sensitivity, which together underlie the development of cutaneous malignancies. The skin is a largest organ in the human body and provides internal organs with a barrier to physical and chemical injury, as well as acting as a primary immunological barrier. The immune system in the skin involves several different cell populations, including dendritic cells (DC), keratinocytes, macrophages, mast cells, B and T cells, plasma cells, and natural killer cells (3–5). DCs are the major antigen-presenting cells. In the skin, they are sub-classified into three subsets: Langerhans cells (LC), dermal dendritic cells and plasmacytoid dendritic cells (6). The epidermal keratinocytes can act as pro-inflammatory effector cells and respond to invading pathogens by coordinated production of pro-inflammatory cytokines and chemokines. Several studies have shown that epidermal keratinocytes incite cutaneous inflammation, which triggers systemic autoimmune responses by lymphocytes (7). Activated macrophages, known as mononuclear phagocytes, also are considered to be antigen-presenting cells, and migrate to sites of inflammation to encounter pathogens and degrade them through production of toxic intermediates (e.g., nitric oxide and reactive oxygen species). Mast cells are innate immune cells and contribute to allergic and inflammatory responses through release of cytokines, chemokines, lipid mediators, proteases and biogenic amines (5). Natural killer cells have the ability to kill virally infected and cancer cells, and are recruited to the skin under inflammatory conditions. In general, natural killer T cells also play an active role in the contact hypersensitivity (CHS) response (5).
The skin immune system encompasses both the innate immune system and the adaptive immune system. Innate immune responses are rapid and largely non-specific. They are mediated through macrophages, natural killer cells, and granulocytes and involve a series of enzymatically activated proteins. The innate immune system includes all defense mechanisms that are encoded in the germline genes of the host including the epithelial barriers, soluble proteins, and bioactive small molecules that are either constitutively present in biologic fluids or released from cells (4, 5). The innate immune system also includes cell-surface receptors that recognize specific molecular patterns expressed on the surfaces of invading microbes. The adaptive immune responses are highly specialized and create immunological memory after an initial response to a specific pathogen that leads to an enhanced response during subsequent encounters with that pathogen. These adaptive immune responses are slow to develop and are considered as a second line of defense following the innate immune responses. The cells of the adaptive immune system include the effectors of cellular immune responses, T and B lymphocytes. Lymphocytes, after development in the primary organs (thymus and bone marrow), migrate to secondary lymphoid organs, such as lymph nodes and spleen, and then migrate from the lymph nodes and spleen to other sites in the body to exert effector functions. The dynamic interactions of the components of the immune system play critical roles in maintaining homeostasis and protection from various risk factors. It has been recognized that the healthy immune system is necessary for restraining malignant disease, and that dysregulation of the immune system results in various pathological disorders including cancers and their progression (8, 9). In this review article, we discuss the effects of UV radiation-induced inflammatory mediators, DNA damage, and alterations in epigenetic regulators and the crosstalk among these effects, which results in suppression of immune reactivity/sensitivity in UV-exposed skin.
The immunosuppressive effects of UVB radiation are well established. They have been demonstrated most clearly in terms of inhibition of the CHS response, which is considered to be a prototypic T-cell-mediated immune response (10, 11). The CHS response to contact allergens is the most used model to study UVB-induced immune suppression in mice and humans. In the skin, UVB-induced immune suppression is triggered by UVB-induced DNA damage in the form of generation of cyclobutane pyrimidine dimers (CPD) or thymine dimers. CPDs form instantaneously when skin cells are exposed to UV radiation. The formation of CPDs affects the function of antigen presenting cells, cytokine production, soluble mediators, epigenetic modifications, and ultimately their effect on the development of regulatory T cells (Treg) or suppressor T cells (12–15). UVB-induced Treg cells have important roles in UVB induced immune suppression and initiation of skin carcinogenesis. These cells are able to transfer antigen-specific immune suppression to normal animals that had not been exposed to UVB radiation (11, 12). In this review, we highlight the molecular targets/mechanisms result in UV-induced suppression of immune sensitivity and the potential crosstalk or combined effects of these mechanisms in UVB-exposed skin.
UVB-INDUCED DNA DAMAGE AND IMMUNE SUPPRESSION
The damage induced in skin cells by exposure to UV radiation is predominantly in the form of generation of CPDs. This generation of CPDs in UV-exposed skin has been recognized as a molecular trigger for both the initiation of suppression of immune sensitivity and initiation of non-melanoma skin cancer (16, 17). A comprehensive data analysis showed that repair or reduction of CPDs through application of DNA repair enzyme or agents responsible for CPDs repair reverses UVB-induced suppression of immune responses (13, 16–23). Of the various UV-induced DNA repair mechanisms, nucleotide excision repair (NER) is considered the most important and the repair of CPDs in UV-exposed skin normally is mediated through NER (17, 22, 23). Studies have been conducted in NER-deficient mice to investigate the role of DNA damage and CPDs in UVB-induced immune suppression. It was found that deficiency of NER increases susceptibility to UV-induced systemic immunosuppression (17, 24). UVB-induced CPDs formation has been shown to be involved in the enhanced expression of the immunosuppressive cytokines, interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α). Reduction in CPDs formation through the use of T4N5 endonuclease resulted in a significant inhibition of UV-induced production of these cytokines (25, 26). Some phytochemicals also have been shown to possess the capacity for repair of CPDs (16, 17, 20, 22). Oral administration of green tea polyphenols or topical application of silymarin (a plant flavonoid from milk thistle plant) stimulates rapid repair of damaged DNA in UVB-exposed skin and inhibits UVB-induced suppression of immune reactivity (16, 17, 27).
In addition to its role in promotion of production of immunosuppressive cytokines, UVB-induced DNA damage appears to play a key role in suppression of immune reactions in the skin through effects on antigen presenting cells. UVB-induced DNA damage is associated with the migration of antigen presenting cells (i.e., Langerhans cells in the epidermis) from the skin to the regional draining lymph nodes (27). Formation of CPDs in the antigen presenting cells of the skin also impairs the capacity of Langerhans cells to present antigen properly, which in turn results in an overall lack of immune sensitization (28). CPD-positive antigen presenting cells were detected in the lymph nodes of UV-exposed mice. These cells were determined to be of epidermal origin and show an impaired ability to present antigen (29, 30). Jans et al. (31) have shown differential role of basal keratinocytes in UV-induced immunosuppression and the development of skin tumors in mice. In this study, authors show that the majority of UV-induced adverse effects require the presence of CPDs in basal keratinocytes in the mouse skin. Using photolyase-transgenic mouse model, they have concluded that photolyase-mediated removal of CPDs, but not 6–4 photoproducts, from the genome of basal keratinocytes substantially decreases the risk of skin tumor development; however, removal of CPDs does not affect the UVB-induced suppression of immune sensitivity. These observations suggest a differential role of basal keratinocytes in UV- induced skin carcinogenesis.
UV-induced DNA damage contributes in the development of immune suppression. Kripke et al. (13) reported that application of liposomes containing the bacteriophage excision repair enzyme (T4N5) to the skin of UV-exposed mice, resulted in a decrease in the number of CPDs in epidermal DNA and, subsequently, decrease in the suppression of immune system. This abrogation of UVB-induced immune suppression by the T4N5-containing liposomes was associated with a reduction in the induction of suppressor T cells in the UV-exposed mice. Additional evidence comes from analysis of the roles of the immunoregulatory cytokine IL-12 in UVB-induced DNA damage-mediated immunosuppression in the skin (32). IL-12 has been shown to prevent UVB-induced immune suppression in laboratory animals (29, 30, 32). This prevention of UVB-induced immunosuppression by IL-12 was further shown to be due to its ability to reduce DNA damage or stimulate repair of damaged DNA via induction of DNA repair enzymes (30, 32). The preventive effect of IL-12 was not observed in DNA repair-deficient mice, thus providing the evidence that IL-12 is a key player in protecting the immune system from UV-induced damage (29, 30). The concept that the endogenous DNA repair mechanism requires the presence of IL-12 in mice was further indicated by the finding that subcutaneous injection of recombinant IL-12 into the UV-irradiated skin of IL-12-deficient mice enhanced the repair of UVB-induced CPDs (33, 34). Collectively, these studies indicate that a reduction in UV-induced DNA damage is associated with the inhibition of UV-induced immunosuppression and, therefore, DNA damage is regarded as the major molecular trigger of UV-induced immunosuppression.
UVB-INDUCED INFLAMMATORY MEDIATORS AND IMMUNE SUPPRESSION
It is known that exposure of the skin to UVB radiation has many detrimental effects. Exposure to UVB induces inflammatory responses and generates inflammatory mediators in the skin that have immunosuppressive effects. UVB irradiation leads to increased blood flow and vascular permeability, resulting in edema, erythema, hyperplastic responses, as well as activation of cyclooxygenase-2 (COX-2) and higher production of prostaglandin (PG) metabolites (2). PGs are small molecule derivatives of arachidonic acid that are produced by the action of COX-2 on arachidonic acid. Almost immediately after UV exposure, keratinocytes secrete the lipid mediator of inflammation, platelet-activating factor (PAF) (35). This enhances COX-2 expression and production of PGE2, which is the most active PG metabolite generated from arachidonic acid. Treatment of keratinocytes with PAF upregulates the transcription of COX-2 and increases IL-10 levels and secretion of PGE2. It is well established that PGE2 regulates multiple aspects of inflammation and the function of various immune cells (36). It has been identified as a mediator of inflammation through its promotion of local vasodilatation and activation of neutrophils, macrophages, and mast cells during the early stages of inflammation (37–40). Immune cells that produce large amounts of PGE2 are considered to be the most powerful modulators of inflammatory processes and immune function (35). It has been demonstrated that COX-2 expression and PGE2 production by activated and non-activated human and murine DCs are involved in UVB-induced immune suppression and in the development of FOXP3+CD4+CD25+ adaptive regulatory T cells (41). PGE2 is predominantly produced in UVB-exposed skin by antigen presenting cells and keratinocytes and has marked autocrine and paracrine effects on their phenotype and function. The biological effects of PGE2 on immune and inflammatory cells are exerted by four G protein-coupled receptors on the plasma membrane, also known as EP receptors (EP1–EP4). The effects of PGE2 on dendritic cell biology depend on the nature of the maturation signals and tissue localization of the cells (42). In peripheral tissues, PGE2 seems to act as a potent activator of DCs and stimulates the surface expression of chemokine receptors that lead to promotion of DC migration to secondary lymphoid organs (43). When DCs migrate to secondary lymphoid organs, PGE2 has an inhibitory role and impedes the maturation of DCs, their expression of MHC class II molecules and their ability to activate T cells (44, 45). PGE2 exhibits both pro- and anti-inflammatory effects on DCs (46). The prostaglandin receptors, EP2 and EP4, have been shown to promote the development of IL-17-producing T cells in multiple models of infection and autoimmunity. The Th17-promoting activity of PGE2 is related to its ability to suppress the production of IL-12p70 while enhancing the levels of the Th17-supporting cytokine, IL-23 (47). Accumulating evidence demonstrates that the inflammatory axis represented by COX-2/PGE2 is involved in UVB-induced immune suppression and suggests that drugs that block COX-2 expression are capable of preventing UVB-induced immune suppression. Studies in animal models have shown that celecoxib and indomethacin (COX-2 inhibitors) as well as AH6809 (EP2 antagonist) reduce UVB-mediated suppression of the immune response (48, 49). The roles of inflammatory mediators, such as PGE2, also have been verified using COX-2-deficient mice, which are unable to synthesize PGs. COX-2-deficient mice were resistant to UVB-induced suppression of the CHS response, whereas the treatment of UVB-exposed COX-2-deficient mice with PGE2 resulted in suppression of immune reactivity.
To determine whether there is a link between PGE2 activity and DNA hypermethylation in UVB-exposed skin, COX-2-deficient mice that were administered PGE2 were treated with a DNA demethylating agent (5–Aza-dc) after UVB irradiation. The treatment with 5-Aza-dc resulted in a full sensitization reaction after DNFB challenge in the PGE2-treated COX-2-deficient mice, suggesting a role for PGE2-mediated DNA hypermethylation in UVB-induced immunosuppression (48). Moreover, a significant increase in DNA methylation and Dnmt activity was not observed when COX-2-deficient mice were subjected to multiple exposures to UVB radiation (48).
IL-12 also is involved in UVB-induced inflammation and has been shown to inhibit UVB-induced immunosuppression. IL-12-deficiency leads to enhancement of COX-2 expression and PGE2 production and contribute to greater immunosuppression. These effects of IL-12-deficiency in mice stimulate photocarcinogenesis in terms of tumor growth, progression and multiplicity (34). It has been shown that UVB exposure enhances the frequency of formation of CPDs, which subsequently leads to enhanced levels of inflammatory mediators. When UVB-induced CPDs are repaired or otherwise reduced in number, the levels of inflammation also are reduced (50).
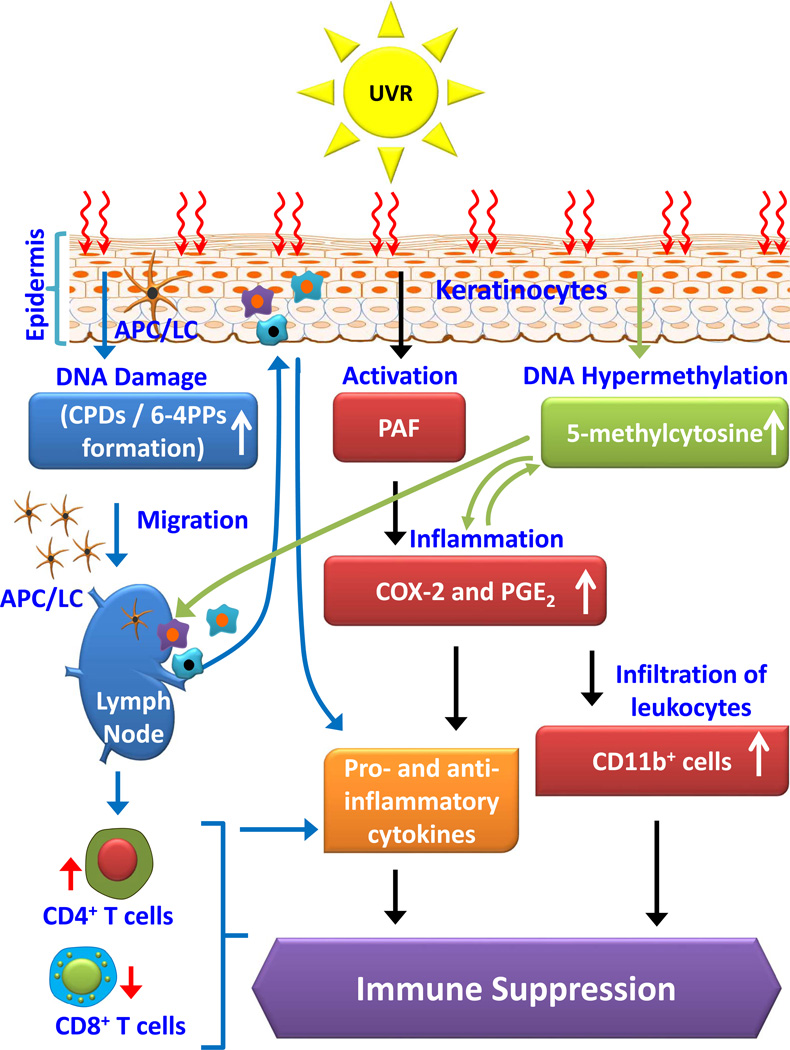
Furthermore, studies in humans and animals demonstrate that UV irradiation induces infiltration of the exposed skin with CD11b+ myeloid derived cells, which are able to produce the immune suppressive cytokines, including IL-10 and IL-4 (51–53). Depletion of CD11b+ cells inhibits the ability of UV-irradiated skin cells to induce regulatory T cells and suppression of CHS responses in mice (54–56). Hammerberg et al. (57) reported that in vivo treatment of mice with an anti-CD11b antibody reduced the number of infiltrating CD11b+ cells in the UV-exposed skin and inhibited UV-induced tolerance. UV irradiation of the skin also results in increased infiltration of Gr-1+ cells and higher levels of myeloperoxidase activity and increased levels of PGE2. The UV–induced infiltrating cells in the skin, dominated by activated macrophages and neutrophils, also are a potent source of inflammatory mediators and oxidative stress. These effects of infiltrating cells play a crucial role in suppression of immune sensitivity as well as increasing the risk of non-melanoma skin cancer. Although epidermal Langerhans cells were considered to be primary cells responsible for UV radiation-induced immune suppression (8, 58), some studies in mouse models suggest that the epidermal Langerhans cells may play a minor role in UV-induced immune suppression (59). Collectively, these studies suggest a strong association between UVB-induced inflammation and DNA damage and further suggest that both play a role in UVB-induced immunosuppression. The molecular mechanisms associated with inflammatory mediators and the DNA damage/repair pathways may thus engage in crosstalk with their intersection playing a pivotal role in suppression of immunity, as summarized in Figure 1.
Figure 1.
Schematic diagram depicting dynamic crosstalk among UV radiation-induced inflammatory mediators, DNA damage, and epigenetic regulators in photo-immunosuppression. UVB induced photodamage initiates migration of antigen presenting cells from skin to regional lymph nodes, where they present antigen to T-cells in a way that is not normal due to photodamage of antigen presenting cells. UVB-induced inflammatory mediators and DNA damage affect epigenetic modifications (DNA hypermethylation) and together with infiltrating myeloid derived cells play crucial roles in suppression of immune system in UV radiation-exposed mice. UVR, ultraviolet radiation; CPDs, cyclobutane pyrimidine dimers; 6–4 PPs, 6–4 photoproducts; APCs, antigen presenting cells; PAF, Platelet activation factor; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; CD11b+ cells, activated macrophages and neutrophil cell population.
UVB-INDUCED EPIGENETIC REGULATORS AND IMMUNE SUPPRESSION
The chronic inflammation, oxidative stress and DNA damage in the UV exposed skin have been shown to have marked effects on epigenetic regulators (60). For example, chronic inflammation accelerates DNA methylation changes in malignant and non-malignant skin lesions (61–63). Epigenetic regulators are known to play a central role in linking environmental cues and cellular responses.
Several investigators have shown the global effects of UV radiation on epigenetic check-points. Analysis of the promoter methylation status of genes in human non-melanoma skin cancer lesions revealed a high frequency of methylation of several tumor suppressor genes, including for example CDH1, CDH3, LAMA3, LAMC2, RASSF1A (62). Epigenetic changes also have been observed in human skin squamous cell carcinomas along with frequent inactivation of the RB1/p16 and p53 pathways. Nandakumar et al. (63) performed a systematic analysis of UV-exposed mouse skin and UV-induced skin tumor samples for DNA methylation and histone modifications. The results indicated a pattern of UV radiation-induced DNA hypermethylation in both the skin and the UV-induced skin tumors. Additionally, the levels of DNA methyltransferases (Dnmts) in the UV-exposed skin and UV-induced skin tumors were increased. The distribution pattern of Dnmt1, Dnmt3a and Dnmt3b in the UVB-exposed skin was altered and the distribution pattern of Dnmt3a-positive and Dnmt3b-positive cells in the basal layer of the UVB-exposed skin were distinct. DNA hypermethylation was observed throughout the UV-irradiated skin. This information suggests the possibility that de novo synthesis of Dnmt3a and Dnmt3b may act to induce a chromatin marker that is associated with proliferation on UVB-exposed skin cells and within the cells of UVB-induced skin tumors. Both the increase in the levels of global DNA methylation and the increase in the levels of Dnmt activity were observed in the UV-exposed skin prior to the appearance of skin tumors (62, 63).
It appears that DNA hypermethylation and histone hypoacetylation may promote malignancies through decrease in the levels of tumor suppressor genes (62–64). UVB irradiation induces transcriptional silencing of the p16INK4a and RASSF1A and this has been correlated with the hypoacetylation of H3 and H4 histones in mouse skin (63, 64). An increase in the recruitment of MeCP2 and methyl-CpG binding domain 1 to p16INK4a and RASSF1A methylated heterochromatin also was evident on exposure of mouse skin to UVB. The authors of this study also suggested a link between the adverse effects of chronic exposure of UV radiation and DNA methylation and histone acetylation. These events are associated with reduced levels of tumor suppressor genes, most likely through the formation of a closed chromatin structure (62). The clinical relevance of the studies conducted in preclinical studies is evident by the analysis of human samples of squamous cell carcinoma, which also demonstrate the greater levels of DNA hypermethylation and increased DNMT activity compared to normal skin samples from humans. These information support the clinical significance of the role of epigenetic modifications in cutaneous malignancies associated with exposure of the skin to UVB radiation and suggest that these epigenetic alterations in skin contribute to the development of non-melanoma skin cancers (63, 64). As most of the epigenetic modifications are reversible; targeting of these events may lead to the development of preventative or therapeutic strategies for the non-melanoma skin cancers.
Another potential mechanism that may link epigenetic changes and immunosuppression is suggested by the detection of DNA hypermethylated cells in the regional lymph nodes of mice that had been subjected to UVB irradiation of the skin (Katiyar et al., unpublished data). This demonstrates that after exposure of the skin to UV radiation, DNA hypermethylated-antigen presenting cells migrate from the skin to the draining lymph nodes. Thus, cells that are photodamaged or have undergone UVB-induced functional alterations may affect the stimulation of T cells in lymph nodes. The presentation of antigens to T cells is a complex process in which defective antigen presentation can either abrogate stimulation of the T cell responses or skew the responses toward suppression. As the UVB irradiation modifies antigen processing in these antigen presenting cells, it would be anticipated that these cells would not present antigen to T cells in an appropriate manner. If this is the case, this epigenetic alteration in antigen presenting cells may result in suppression of the immune system. Further studies on this subject may lead to more detailed information concerning this previously unsuspected link between UVB-induced epigenetic alterations and the functioning of the immune system.
CROSSTALK AND THE INTER-RELATIONSHIP OF UVB-INDUCED PHOTODAMAGING EVENTS ON IMMUNE SUPPRESSION
Suppression of the immune system in the skin after UV radiation exposure is a crucial event that plays an essential role in initiation and progression of non-melanoma skin cancer. As summarized above, it has been demonstrated that UVB exposure can initiate several different photodamaging effects, including induction of inflammation and inflammatory mediators, DNA damage, oxidative stress, genetic and epigenetic changes and suppression of immune reactivity. A full understanding of the development of various skin diseases or skin disorders, including melanoma and non-melanoma skin cancers, requires elucidation of the inter-relationships between these photodamaging effects. A central question of critical importance to identification of effective strategic targeting of the mechanisms is which, if any, of the photodamaging events that have been identified is sufficient to drive the development of cutaneous malignancies or whether a network of events is required. For example, CPDs are generated immediately on exposure of skin to UVB radiation. This direct UVB-induced formation of CPDs is associated with the generation of inflammation. Furthermore, chronic and persistent inflammation, which can be induced by UVB exposure, also can damage DNA. UVB-induced CPDs formation has been recognized as a molecular trigger that plays a role in immune suppression. Reduction or repair of UVB-induced CPDs in the skin through the use of a DNA repair enzyme, or any agent which has the ability to reduce or repair CPDs, results in reversal of immune suppression (16, 27, 30).
UVB-induced infiltration of activated CD11b+ cells (myeloid derived cells/activated macrophages/ neutrophils) in the dermis and epidermis of the skin is a major source of inflammation as well as oxidative stress (56). This CD11b+ cell sub-type also plays a role in immune suppression in UV-exposed skin as verified by the use of anti-CD11b antibody. Intraperitoneal injection of an anti-CD11b antibody prevents the infiltration of CD11b+ cells in UVB-exposed skin and inhibits UVB-induced suppression of immune system as shown using the CHS model (54, 56, 57).
Epigenetic regulators play a crucial role in linking environmental cues and cellular responses. It has been recognized that various environmental factors, such as exposure of the skin to solar UV radiation, contribute to the development of skin tumors, which is promoted by the suppression of immunity. As described above, the authors’ laboratory has found that UVB irradiation induces DNA hypermethylation in skin cells, and DNA hypermethylation contributes to the downregulation of tumor suppressor genes (63, 64). The outcome of this study suggests a link between DNA methylation and histone acetylation in UV radiation-induced photodamaging effects. UV radiation exposure stimulates the activity of Dnmts, which may lead to the aberrant hypermethylation of DNA in skin cells. Furthermore, detection of DNA hypermethylation-positive antigen presenting cells in the lymph nodes of UVB-exposed mice suggests an association with improper presentation of antigen to T cells, which may give rise to the development of Treg or suppressor T cells that can play a role in immune suppression. To investigate the potential link between DNA methylation and UVB-induced immunosuppression, inbred C3H/HeN mice were exposed to UVB radiation with or without treatment with a DNA demethylating agent (5–Aza-dc) and the CHS response evaluated. The results demonstrated that treatment of mice with 5-Aza-dc reversed or inhibited UVB-induced suppression of immune sensitivity to a skin contact sensitizer (DNFB) and that this was accompanied by a lowering of the levels of global DNA methylation and Dnmt activity as compared to non-5Aza-dc-treated UVB exposed mice (48).
CONCLUSION AND FUTURE PROSPECTS
This analysis and review of current literature and available information provides substantial insights into the links between UVB-induced inflammatory mediators, DNA damage and DNA methylation patterns in photo-immunosuppression, at least in laboratory animals. It suggests a network of intricate roles of inflammation in UVB-induced immunosuppression including regulation of DNA methylation patterns by inflammatory mediators and DNA damage in UVB-exposed skin, as summarized in Figure 1. In general, it can be speculated that changes in inflammatory mediators (e.g., PGs) biosynthesis may contribute to changes in DNA methylation patterns associated with a wide variety of skin diseases, including cutaneous malignancies, associated with chronic exposure to sunlight and other environmental factors (1, 65). Development of therapeutic strategies requires identification of interventions that are not only effective in mouse models but feasible and safe in humans. Importantly, the incidence of nonmelanoma skin cancer is reduced in subjects receiving a selective COX-2 inhibitor (celecoxib) compared to subjects receiving placebo (66). The development of the understanding of the network of events provides a rationale for considering the development of new strategies, such as using inhibitors of UVB-induced inflammation and demethylating agents alone or in combination for the prevention or treatment of nonmelanoma skin cancers. Importantly, new strategies for photo-chemoprevention may include the use of dietary agents or dietary supplements that have been demonstrated to neutralize or inhibit the photodamaging effects of solar UV light through multiple mechanisms. These agents may become a regular part of diet to maintain a healthy life style.
Acknowledgments
The work reported from Dr. Katiyar’s research laboratory was financially supported by the National Institutes of Health (NIH)/NCCAM/NCI (CA140197, AT002536, S.K.K.) and the Veterans Administration Merit Review Award (1I01BX001410-01, S.K.K.). The funding agencies had no role in data collection and data analysis. The content of this article also does not necessarily reflect the views or policies of the funding agencies as well as decision to publish this review article.
Footnotes
This article is part of the Special Issue honoring Dr. Hasan Mukhtar's 70th Birthday and his outstanding contributions to various aspects of photobiology research, including photocarcinogenesis and chemoprevention.
REFERENCES
- 1.Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J. Am. Acad. Dermatol. 2008;58:S129–S132. doi: 10.1016/j.jaad.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 2.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: Anti-inflammatory, anti-oxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CZ, Schaffert S, Fragoso R, Loh C. Regulation of immune responses and tolerance: the microRNA perspective. Immunol. Rev. 2013;253:112–128. doi: 10.1111/imr.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos JD, Zonneveld I, Das PK, Krieg SR, van der Loos CM, Kapsenberg ML. The skin immune system (SIS), distribution and immunophenotype of lymphocyte subpopulations in normal human skin. J. Invest. Dermatol. 1987;88:569–573. doi: 10.1111/1523-1747.ep12470172. [DOI] [PubMed] [Google Scholar]
- 5.Mann ER, Smith KM, Bernardo D, Al-Hassi HO, Knight SC, Hart AL. Review: Skin and the Immune System. J. Clin. Exp. Dermatol. Res. 2012;S2:003. [Google Scholar]
- 6.Zaba LC, Krueger JG, Lowes MA. Resident and "inflammatory" dendritic cells in human skin. J. Invest. Dermatol. 2009;129:302–308. doi: 10.1038/jid.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kupper TS. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J. Invest. Dermatol. 1990;94:146S–150S. doi: 10.1111/1523-1747.ep12876130. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz T. Mechanisms of UV-induced immunosuppression. Keio J. Med. 2005;54:165–171. doi: 10.2302/kjm.54.165. [DOI] [PubMed] [Google Scholar]
- 9.Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem. Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 10.Elmets CA, Bergstresser PR, Tigelaar RE, Wood PJ, Streilein JW. Analysis of the mechanism of unresponsiveness produced by haptens painted on skin exposed to low dose ultraviolet radiation. J. Exp. Med. 1983;158:781–794. doi: 10.1084/jem.158.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kripke ML. Photoimmunology. Photochem. Photobiol. 1990;52:919–924. doi: 10.1111/j.1751-1097.1990.tb08703.x. [DOI] [PubMed] [Google Scholar]
- 12.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat. Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 13.Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFabo EC, Kripke ML. Dose–response characteristics of immunologic unresponsiveness to UV-induced tumors produced by UV irradiation of mice. Photochem. Photobiol. 1979;30:385–390. doi: 10.1111/j.1751-1097.1979.tb07372.x. [DOI] [PubMed] [Google Scholar]
- 15.Cavanagh LL, Halliday GM. Dendritic epidermal T cells in ultraviolet irradiated skin enhance skin tumor growth by inhibiting CD4+T-cell-mediated immunity. Cancer Res. 1996;56:2607–2615. [PubMed] [Google Scholar]
- 16.Vaid M, Prasad R, Singh T, Elmets CA, Xu H, Katiyar SK. Silymarin inhibits ultraviolet radiation-induced immune suppression through DNA repair-dependent activation of dendritic cells and stimulation of effector T cells. Biochem. Pharmacol. 2013;85:1066–1076. doi: 10.1016/j.bcp.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katiyar SK, Vaid M, van Steeg H, Meeran SM. Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev. Res. (Phila) 2010;2:179–189. doi: 10.1158/1940-6207.CAPR-09-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of the molecular target for the suppression of contact hypersensitivity by ultraviolet radiation. J. Exp. Med. 1989;170:1117–1131. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stege H, Roza L, Vink AA, Grewe M, Ruzicka T, Grether-Beck S, Krutmann J. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc. Natl. Acad. Sci. USA. 2000;97:1790–1795. doi: 10.1073/pnas.030528897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katiyar SK, Perez A, Mukhtar H. Green tea polyphenol treatment to human skin prevents formation of ultraviolet light B-induced pyrimidine dimers in DNA. Clin. Cancer Res. 2000;10:3864–3869. [PubMed] [Google Scholar]
- 21.Katiyar SK, Bergamo BM, Vyalil PK, Elmets CA. Green tea polyphenols: DNA photodamage and photoimmunology. J. Photochem. Photobiol. B. 2001;65:109–114. doi: 10.1016/s1011-1344(01)00248-2. [DOI] [PubMed] [Google Scholar]
- 22.Katiyar SK, Mantena SK, Meeran SM. Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism. PLoS One. 2011;6:e21410. doi: 10.1371/journal.pone.0021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaid M, Sharma SD, Katiyar SK. Proanthocyanidins inhibit photocarcinogenesis through enhancement of DNA repair and xeroderma pigmentosum group A-dependent mechanism. Cancer Prev. Res. (Phila) 2010;12:1621–1629. doi: 10.1158/1940-6207.CAPR-10-0137. [DOI] [PubMed] [Google Scholar]
- 24.Miyauchi-Hashimoto H, Tanaka K, Horio T. Enhanced inflammation and immunosuppression by ultraviolet radiation in xeroderma pigmentosum group A (XPA) model mice. J. Invest. Dermatol. 1996;107:343–348. doi: 10.1111/1523-1747.ep12363295. [DOI] [PubMed] [Google Scholar]
- 25.Nishigori C, Yarosh DB, Ullrich SE, Vink AA, Bucana CD, Roza L, Kripke ML. Evidence that DNA damage triggers interleukin 10 cytokine production in UV irradiated murine keratinocytes. Proc. Natl. Acad. Sci. USA. 1996;93:10354–10359. doi: 10.1073/pnas.93.19.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kibitel J, Hejmadi V, Alas L, O'Connor A, Sutherland BM, Yarosh D. UV-DNA damage in mouse and human cells induces the expression of tumor necrosis factor alpha. Photochem. Photobiol. 1998;67:541–546. [PubMed] [Google Scholar]
- 27.Katiyar SK. UV-induced immune suppression and photocarcinogenesis: Chemoprevention by dietary botanical agents. Cancer Lett. 2007;255:1–11. doi: 10.1016/j.canlet.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vink AA, Moodycliffe AM, Shreedhar V, Ullrich SE, Roza L, Yarosh DB, Kripke ML. The inhibition of antigen-presenting activity of dendritic cells resulting from UV irradiation of murine skin is restored by in vitro photo-repair of cyclobutane pyrimidine dimers. Proc. Natl. Acad. Sci. USA. 1997;94:5255–5260. doi: 10.1073/pnas.94.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz A, Maeda A, Kernebeck K, van Steeg H, Beissert S, Schwartz T. Prevention of UV radiation induced immunosuppression by IL-12 is dependent on DNA repair. J. Exp. Med. 2005;201:173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meeran SM, Mantena SK, Katiyar SK. Prevention of ultraviolet radiation-induced immunosuppression by (−)-epigallocatechin-3-gallate in mice is mediated through interleukin 12- dependent DNA repair. Clin. Cancer Res. 2006;12:2272–2280. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 31.Jans J, Garinis GA, Schul W, van Oudenaren A, Moorhouse M, Smid M, Sert YG, van der Velde A, Rijksen Y, de Gruijl FR, van der Spek PJ, Yasui A, Hoeijmakers JH, Leenen PJ, van der Horst GT. Differential role of basal keratinocytes in UV-induced immunosuppression and skin cancer. Mol. Cell Biol. 2006;26:8515–8526. doi: 10.1128/MCB.00807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarz A, Stander S, Berneburg M, Bohm M, Kulms D, van Steeg H, Grosse-Heitmeyer K, Krutmann J, Schwarz T. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat. Cell Biol. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- 33.Meeran SM, Mantena SK, Meleth S, Elmets CA, Katiyar SK. Interleukin-12-deficient mice are at greater risk of ultraviolet Radiation-induced skin tumors and malignant transformation of papillomas to carcinomas. Mol. Cancer Ther. 2006;5:825–832. doi: 10.1158/1535-7163.MCT-06-0003. [DOI] [PubMed] [Google Scholar]
- 34.Katiyar SK. Interleukin-12 and photocarcinogenesis. Toxicol. Appl. Pharmacol. 2007;224:220–227. doi: 10.1016/j.taap.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pei Y, Barber LA, Murphy RC, Johnson CA, Kelley SW, Dy LC, Fertel RH, Nguyen TM, Williams DA, Travers JB. Activation of the epidermal platelet- activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J. Immunol. 1998;161:1954–1961. [PubMed] [Google Scholar]
- 36.Phipps RP, Stein SH, Roper RL. A new view of prostaglandin E regulation of the immune response. Immunol. Today. 1991;12:349–352. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- 37.Yu Y, Chadee K. Prostaglandin E2 stimulates IL-8 gene expression in human colonic epithelial cells by a posttranscriptional mechanism. J. Immunol. 1998;161:3746–3752. [PubMed] [Google Scholar]
- 38.Nakayama T, Mutsuga N, Yao L, Tosato G. Prostaglandin E2 promotes degranulation-independent release of MCP-1 from mast cells. J. Leukoc. Biol. 2006;79:95–104. doi: 10.1189/jlb.0405226. [DOI] [PubMed] [Google Scholar]
- 39.Wang XS, Lau HY. Prostaglandin E potentiates the immunologically stimulated histamine release from human peripheral blood-derived mast cells through EP1/EP3 receptors. Allergy. 2006;61:503–506. doi: 10.1111/j.1398-9995.2006.01043.x. [DOI] [PubMed] [Google Scholar]
- 40.Weller CL, Collington SJ, Hartnell A, Conroy DM, Kaise T, Barker JE, Wilson MS, Taylor GW, Jose PJ, Williams TJ. Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proc. Natl. Acad. Sci. USA. 2007;104:11712–11717. doi: 10.1073/pnas.0701700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gately S. The contributions of cyclooxygenase-2 to tumor angiogenesis. Cancer Metastasis Rev. 2000;19:19–27. doi: 10.1023/a:1026575610124. [DOI] [PubMed] [Google Scholar]
- 42.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 43.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 44.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin E2 enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J. Immunol. 2002;168:2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 45.Harizi H, Juzan M, Grosset G, Rashedi M, Gualde N. Dendritic cells issued in vitro from bone marrow produce PGE (2) that contributes to the immunomodulation induced by antigen-presenting cells. Cell Immunol. 2001;209:19–28. doi: 10.1006/cimm.2001.1785. [DOI] [PubMed] [Google Scholar]
- 46.Goodwin JS. Are prostaglandins pro-inflammatory, anti-inflammatory, both or neither? J. Rheumatol. Suppl. 1991;28:26–29. [PubMed] [Google Scholar]
- 47.Kalinski P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prasad R, Katiyar SK. Prostaglandin E2 Promotes UV radiation induced immune suppression through DNA hypermethylation. Neoplasia. 2013;15:795–804. doi: 10.1593/neo.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katiyar SK. Dietary proanthocyanidins inhibit UV radiation-induced skin tumor development through functional activation of the immune system. Mol. Nutr. Food Res. 2016;60:1374–1382. doi: 10.1002/mnfr.201501026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meeran SM, Akhtar S, Katiyar SK. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J. Invest. Dermatol. 2009;129:1258–1270. doi: 10.1038/jid.2008.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang K, Hammerberg C, Meunier L, Cooper KD. CD11b+ macrophages that infiltrate human epidermis after in vivo ultraviolet exposure potently produce IL-10 and represent the major secretory source of epidermal IL-10 protein. J. Immunol. 1994;153:5256–5264. [PubMed] [Google Scholar]
- 52.Teunissen MB, Piskin MG, Nuzzo SD, Sylva-Steenland RMR, deRie MA, Bos JD. Ultraviolet B radiation induces a transient appearance of IL-4+ neutrophils, which support the development of Th2 responses. J. Immunol. 2002;168:3732–3739. doi: 10.4049/jimmunol.168.8.3732. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida Y, Kang L, Berger M, Chen G, Gilliam AC, Moser A, Wu L, Hammerberg C, Cooper KD. Monocyte induction of IL-10 and down-regulation of IL-12 by iC3b deposited in ultraviolet-exposed human skin. J. Immunol. 1998;161:5873–5879. [PubMed] [Google Scholar]
- 54.Hammerberg C, Duraiswamy N, Cooper KD. Active induction of unresponsiveness (tolerance) to DNFB by in vivo ultraviolet-exposed epidermal cells is dependent upon infiltrating class II MHC+ CD11b bright monocytic/macrophagic cells. J. Immunol. 1994;153:4915–4924. [PubMed] [Google Scholar]
- 55.Hammerberg C, Katiyar SK, Carroll MC, Cooper KD. Activated complement component 3 (C3) is required for ultraviolet induction of immunosuppression and antigenic tolerance. J. Exp. Med. 1998;187:1133–1138. doi: 10.1084/jem.187.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mittal A, Elmets CA, Katiyar SK SK. CD11b+ cells are the major source of oxidative stress in UV radiation-irradiated skin: Possible role in photoaging and photocarcinogenesis. Photochem. Photobiol. 2003;77:259–264. doi: 10.1562/0031-8655(2003)077<0259:ccatms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 57.Hammerberg C, Duraiswamy N, Cooper KD. Reversal of immunosuppression inducible through ultraviolet-exposed skin by in vivo anti-CD11b treatment. J. Immunol. 1996;157:5254–5261. [PubMed] [Google Scholar]
- 58.Schwarz A, Noordegraaf M, Maeda A, Torii K, Clausen BE, Schwarz T. Langerhans cells are required for UVR-induced immunosuppression. J. Invest. Dermatol. 2010;130:1419–1427. doi: 10.1038/jid.2009.429. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Jameson SC, Hogquist KA. Epidermal langerhans cells are not required for UV-induced immunosuppression. J. Immunol. 2009;183:5548–5553. doi: 10.4049/jimmunol.0900235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katiyar SK, Singh T, Prasad R, Sun Q, Vaid M. Epigenetic alterations in ultraviolet radiation-induced skin carcinogenesis: Interaction of bioactive dietary components on epigenetic targets. Photochem. Photobiol. 2012;88:1066–1074. doi: 10.1111/j.1751-1097.2011.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sathyanarayana UG, Moore AY, Li L, Padar A, Majmudar K, Stastny V, Makarla P, Suzuki M, Minna JD, Feng Z, Gazdar AF. Sun exposure related methylation in malignant and non-malignant skin lesions. Cancer Lett. 2007;245:112–120. doi: 10.1016/j.canlet.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 62.Murao K, Kubo Y, Ohtani N, Hara E, Arase S. Epigenetic abnormalities in cutaneous squamous cell carcinomas: frequent inactivation of the RB1/p16 and p53 pathways. Br. J. Dermatol. 2006;155:999–1005. doi: 10.1111/j.1365-2133.2006.07487.x. [DOI] [PubMed] [Google Scholar]
- 63.Nandakumar V, Vaid M, Tollefsbol TO, Katiyar SK. Aberrant DNA hypermethylation patterns lead to transcriptional silencing of tumor suppressor genes in UVB-exposed skin and UVB-induced skin tumors of mice. Carcinogenesis. 2011;32:597–604. doi: 10.1093/carcin/bgq282. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Katiyar SK. Epigenetic regulation by dietary phytochemicals in photocarcinogenesis. Curr. Pharmacol. Rep. 2015;1:52–59. [Google Scholar]
- 65.Katiyar SK. Proanthocyanidins from grape seeds inhibit UV radiation-induced immune suppression in mice: Detection and analysis of molecular and cellular targets. Photochem. Photobiol. 2015;91:156–162. doi: 10.1111/php.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elmets CA, Viner JL, Pentland AP, Cantrell W, Lin HY, Bailey H, Kang S, Linden KG, Heffernan M, Duvic M, Richmond E, Elewski BE, Umar A, Bell W, Gordon GB. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial. J. Natl. Cancer Inst. 2010;102:1835–1844. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]