Abstract
Angiostrongylus cantonensis, the rat lungworm, was the cause of neural larval migrans in two nine-banded armadillos (Dasypus novemcinctus) and one Virginia opossum (Didelphis virginiana) from the southeastern United States. Histologic findings in all three cases included eosinophilic meningoencephalitis with variable numbers of nematode larvae in the meninges or the neuroparenchyma. In two of the three cases, nematodes were extracted from brain tissue via a “squash prep” method. Identification of the nematodes was confirmed by amplification and sequence analysis of the partial cytochrome c oxidase subunit I gene from all three cases. Sequences (704bp) from the two cases from Louisiana were identical and 99.7% similar to nematodes detected in the armadillo from Florida. As A. cantonensis is now considered endemic in the southern United States, it should be considered as an important differential for any wild or domestic animal or human patient with neurological signs and eosinophilic meningitis. Many wildlife species frequently consume snails and slugs and could serve as sentinels for the detection of this parasite in regions where the presence of this parasite has not been confirmed. To the authors’ knowledge, this is the first report of neural larval migrans due to A. cantonensis in an armadillo and provides additional documentation that this nematode can cause disease in wildlife species in the southeastern United States.
Graphical abstract
Highlights
-
•
This is the first Angiostrongylus cantonensis report in the nine-banded armadillo.
-
•
This report supports established endemicity of rat lungworm in the southeastern US.
-
•
Wildlife species can serve as sentinels for A. cantonensis.
-
•
Angiostrongylus cantonensis is a differential for neurological disease in wildlife.
1. Introduction
Angiostrongylus cantonensis is a pulmonary nematode that was originally described in the Norway rat (Rattus norvegicus) and is an important zoonotic pathogen globally. The parasite is endemic in Asia, where it was first documented as a human pathogen in China in 1945 (Barratt et al., 2016). As of 2008, it was responsible for thousands of human cases of eosinophilic meningitis across 30 countries, with severe outbreaks reported in China, Taiwan, and Thailand (Wang et al., 2012). Angiostrongylus spp. are now recognized as the leading cause of eosinophilic meningitis in humans worldwide (Wang et al., 2008, Eamsobhana, 2014). This parasite is an important pathogen of domestic dogs and certain wildlife species and these hosts have been proposed as important biosentinels for angiostrongyliasis for humans (Lunn, 2007; Lv et al., 2008; Ma et al., 2013, Barratt et al., 2016).
In the definitive hosts (Norway rat or other rodents), L3 larvae develop for approximately two weeks in the central nervous system, after which they move to pulmonary arteries to mature and mate. In non-rodent hosts, the development of A. cantonensis to 4th and 5th larval stages in neural tissue can lead to neurologic signs, such as circling, ataxia, stupor, and paresis/paralysis of limbs. The parasite typically induces a granulomatous or eosinophilic histopathological response (Gardiner et al., 1990, Spratt, 2015). Neurological damage is due to both physical damage from the larvae and the host's immune reaction to the larvae, which is more intense to dead larvae than live larvae (Lunn, 2007, Cowie, 2013).
Angiostrongylus cantonensis was first reported in the United States in 1987 (Campbell and Little, 1988). Introduction of the nematode to North America is suspected to have been from infected rats aboard ships that docked in New Orleans (Campbell and Little, 1988). Since its introduction in the United States, A. cantonensis has been increasingly reported in humans and incidental mammalian hosts in Louisiana and the surrounding states. Early reports of infections in mammalian incidental hosts include a horse in Picayune, Mississippi, a captive howler monkey (Hylobates lar) in New Orleans, Louisiana, a wild wood rat (Neotoma floridanus) and four wild opossums (Didelphis virginiana) in New Orleans, Louisiana, a captive white-handed gibbon (Hylobates lar) in Miami, Florida, and a captive lemur (Varencia variegata rubra) in New Iberia, Louisiana (Gardiner et al., 1990, Costa et al., 2000, Kim et al., 2002, Duffy et al., 2004). Angiostrongylus cantonensis is believed to now be endemic in wildlife within the southeastern United States due to the presence of numerous gastropod species suspected to serve as intermediate hosts and a variety of rodent species that could potentially serve as definitive hosts (Kliks and Palumbo, 1992, Teem et al., 2013, York et al., 2015). Increased frequency of diagnosis and the expanding geographic distribution has been attributed to the spread of intermediate host gastropods, definitive rodent host species, and flexibility for potential host species of the lungworm (York et al., 2015). The presence of A. cantonensis in Oklahoma in a novel rat host (Sigmodon hispidus) indicates northern geographical expansion of the nematode (York et al., 2015). Cerebral A. cantonensis was confirmed in an African pygmy falcon (Polihierax semitorquatus) in California, although the source of infection was suspected to be from feeder geckos imported from Southeast Asia rather than due to ingestion of autochthonously infected gastropods (Burns et al., 2014).
Although there have been sporadic published reports, we have not previously diagnosed any cases in wildlife species at the Southeastern Cooperative Wildlife Disease Study despite encountering a caseload of regular submissions of neurological animals. Here we provide details, including molecular characterization, on three cases of A. cantonensis we diagnosed in the past two years, including recognition of a novel host.
2. Methods
The cases reported consist of wildlife carcasses and tissues that were submitted to the Southeastern Cooperative Wildlife Disease Study in Athens, GA for diagnostic evaluation (Table 1). Two carcasses were frozen prior to submission; tissues from the third case were received fresh. Standard procedures were followed for the gross examination of all organs, preservation for histopathologic examination, and ancillary testing appropriate for clinical history and postmortem findings. Specimens from major organs were collected, fixed in 10% neutral buffered formalin, routinely processed and embedded in paraffin wax, sectioned at 5 μm, and stained with hematoxylin and eosin. At the time of necropsy, small samples of brain, lung, liver, spleen, kidney, and small intestine were collected and frozen at −20 °C.
Table 1.
Summary of signalment, history, clinical signs, gross necropsy findings, and ancillary tests of angiostrongyliasis in free-ranging wildlife in the southeastern United States from 2015 to 2016.
| Case Identification | Date Found | Location Found | Age | Sex | Clinical signs | Gross findings | Ancillary tests | Ancillary test results |
|---|---|---|---|---|---|---|---|---|
| Armadillo A | October 2016 | Livingston Parish, LA | Adult | Male | Circling behavior in resident's driveway | Foot erosions, Hemoabdomen | FATa for rabies virus | FAT negative |
| Armadillo B | November 2015 | Tampa, FL | Adult | Male | Abnormal behavior, including circling | Meningeal vascular congestion, Foot erosions | FAT for rabies virus; viral isolation for arboviruses; PCRb for protozoa and nematode DNA | FAT negative; no viruses isolated; PCR negative for nematode DNA; PCR positive for Toxoplasma gondii |
| Opossum A | September 2016 | Ascension Parish, LA | Juvenile | Male | Circling behavior; Unable to stand upright | Emaciation | FAT for rabies virus; viral isolation for arboviruses | FAT negative; no viruses isolated |
FAT: Fluorescent antibody test.
PCR: polymerase chain reaction.
In armadillo A and opossum A, sections of fresh cerebrum were squashed between two glass plates and viewed with a trans-illumination light source (“squash prep” method) to identify nematodes present in the brain matter. Nematodes were extracted from the brain and viewed under dissecting and compound microscopes for morphological identification (Fig. 1a; Fig. 1b). DNA was extracted from the nematodes using a commercial DNA extraction kit (DNeasy, Qiagen, Valencia, California). The cytochrome-c oxidase I (COI) gene was amplified via polymerase chain reaction (PCR) using a cocktail of six M13-labeled primers for nematodes (Prosser et al., 2013). Amplicons were gel-purified using a gel-purification kit (Qiagen) and bi-directionally sequenced at the University of Georgia Genomics Facility (Athens, Georgia). Chromatograms were analyzed using Geneious R7 (Auckland, New Zealand) and the consensus sequence was compared to other sequences in the GenBank database. In the case of armadillo B, the “squash prep” method was attempted on lung and liver due to limited availability of brain tissue. No nematodes were detected. The PCR protocol described above was used to evaluate frozen brain tissue from Armadillo B.
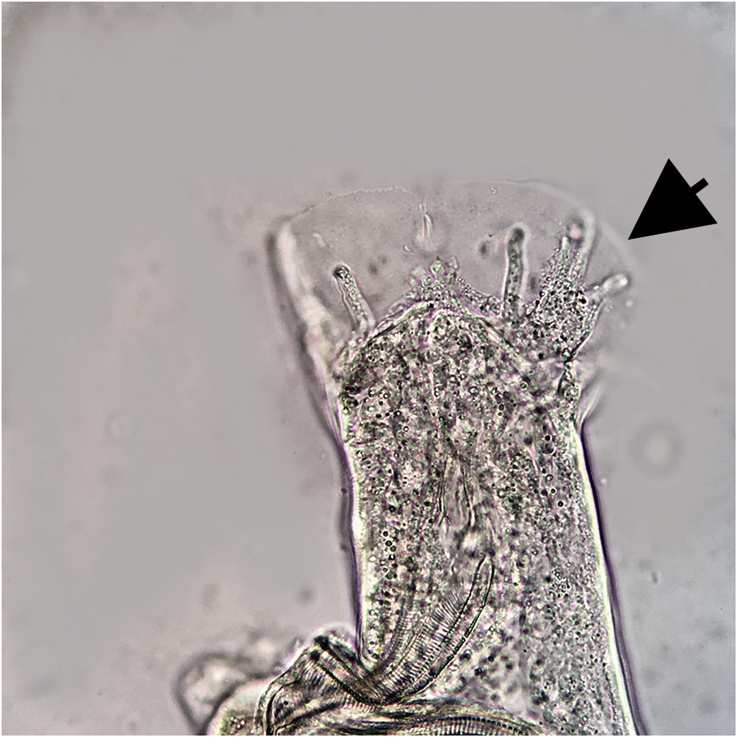
Fig. 1.
Caudal end of a male nematode extracted from the brain of Armadillo 1. Arrow indicates bursal rays.
Additional routine testing was conducted on these animals per protocols for neurological mammals (Table 1). Brain tissue was tested for rabies virus using a florescent antibody test (submitted to Athens Veterinary Diagnostic Laboratory) and Toxoplasma gondii and other apicomplexan parasites by PCR using primers Tg18s58F and Tg18s348R as described (Su et al., 2010). Amplicons were gel purified (Qiagen) and submitted to Georgia Genomics Facility (Athens, GA) for bi-directional sequencing. In addition, routine virus isolation for arboviruses was conducted in vero cells.
3. Results
3.1. Parasitology and histology findings
A squash prep of brain tissue from armadillo A yielded six nematode larvae and adults. These worms could easily be differentiated from other nematodes reported to migrate through the brain of mammals (e.g., Baylisascaris spp., Parelaphostrongylus spp.) by their large size and the presence of a metastrongyle bursa on male worms (Fig. 1). Adult parasites were identified as A. cantonensis based on several morphologic characteristics including size of worms (males were ∼23 mm and a female was 29 mm long), female with round tails and a vulva just anterior to a non-terminal anus, and males having a gubernaculum (50 μm) and two long (1.2 mm) similar spicules. One immature female nematode was detected in brain tissue of opossum A.
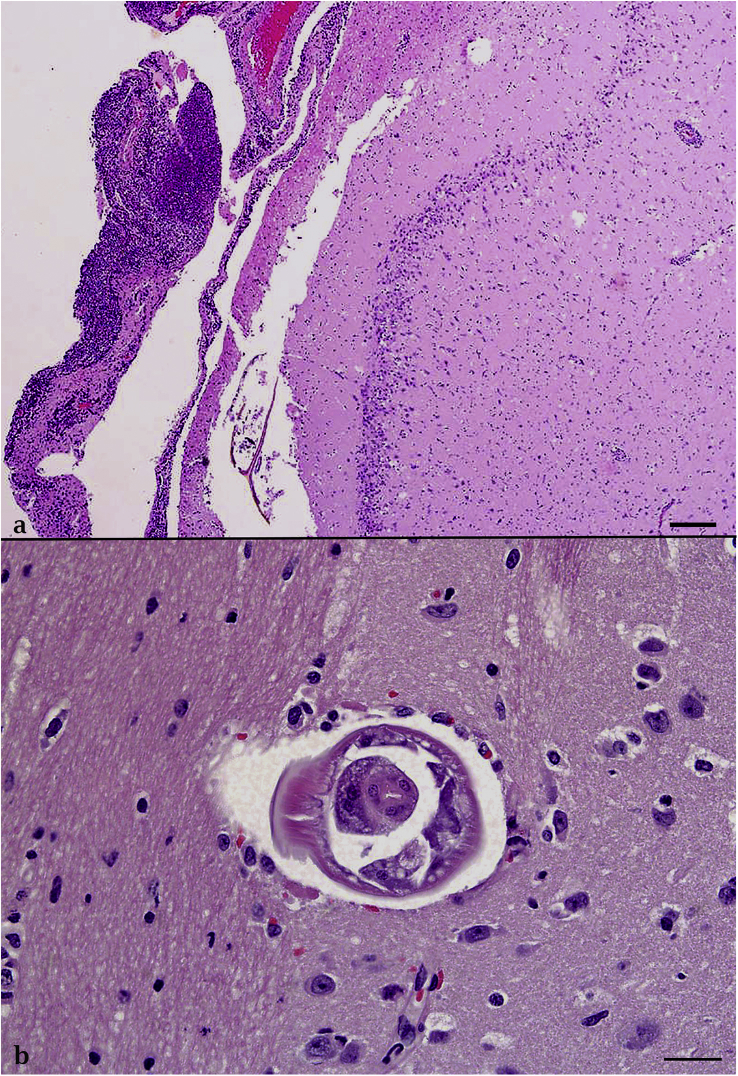
In all three cases, moderate to high numbers of eosinophils and lymphocytes and fewer neutrophils and macrophages multifocally expanded the meninges and perivascular regions in the cerebrum (Fig. 2a). Eosinophils infiltrated into the surrounding neuropil and randomly distributed eosinophilic granulomas and hemorrhages were present throughout the cerebrum (Fig. 2a). The cross section of one, 100–200 μm diameter nematode larva characterized by a smooth cuticle, coelomyarian musculature, lateral cords, and a distinct pharynx was present in the neuroparenchyma of armadillo A and B (Fig. 2b). In addition to the inflammation in the brain, armadillo B had multiple eosinophilic granulomas in the submucosal lymphoid tissue of the large intestines. One granuloma contained cross sections of occasionally mineralized or degenerate nematode larvae, but these larvae could not be definitively identified. Armadillo A had eosinophilic granulomas in the adventitia of the pulmonary artery.
Fig. 2.
Neuroparenchyma. Fig. 2a: Armadillo B. High numbers of eosinophils and lymphocytes expand the perivascular spaces. A focus of hemorrhage, eosinophils, and glial cells interrupts the neuroparenchyma. Photomicrographs are stained with hematoxylin and eosin (H&E). Bar = 200 μm. Fig. 2b: Armadillo A. Cross section of a nematode larva (200 μm width) within the thalamus is characterized by a smooth cuticle, coelomyarian musculature, lateral cords, and a distinct pharynx (consistent with a metastrongyle). No inflammatory cells surround the nematode. H&E, Bar = 50 μm.
Additional tests performed in each case are summarized in Table 1. Brain tissue from armadillo B was PCR positive for T. gondii (sequence confirmed), but protozoal organisms were not observed in histological sections of brain with routine staining and with immunohistochemical staining for T. gondii-specific antigens. This is considered an incidental finding in this case.
3.2. Molecular results
Sequences from the nematodes recovered from the brains of opossum A and armadillo A were identical (704 bp). These two sequences were 99.7% (702/704 base pairs) similar to the sequence recovered from brain tissue of armadillo B. The two Louisiana sequences were most similar to A. cantonensis AP017672 [from Taiwan] and LK950095 [from China] (99.6%, 701/704bp). The armadillo B sequence was most similar to A. cantonensis AP017672 [from Taiwan] and LK950095 [from China] (99.6%, 701/704bp)). The two sequences were submitted to GenBank (accession numbers MF000735 and MF000736).
4. Discussion
Angiostrongylus cantonensis has become established in the southeastern United States due to suitable climate, availability of intermediate gastropod hosts, and presence of numerous mammalian hosts in which the nematodes can achieve sexual maturity (Kim et al., 2002, York et al., 2015). The effect that this parasite will have on wildlife populations in the southeastern United States has yet to be determined.
There has been increasing public attention and concern worldwide regarding human and animal angiostrongyliasis due to an increase in reported cases in endemic countries, nonendemic countries, and among international travelers (Slom et al., 2002, Lv et al., 2008). Angiostrongylus cantonensis has been cited as a “neglected pathogen” due to lack of awareness in the general public and potential underreporting within the medical community (Eamsobhana, 2014). There is a lack of standardization in diagnosis of angiostrongyliasis and although there are some serological tests available for humans, diagnostic tests for veterinary species are lacking. Early enzyme-linked immunosorbent assays (ELISAs) developed for humans were not highly specific (Wilkins et al., 2013). Molecular detection holds potential for the future diagnosis of angiostrongyliasis, although this method is not globally available (Wilkins et al., 2013, Qvarnstrom et al., 2016). Interruption of the life cycle of A. cantonensis is likely the most effective way to reduce the risk of infection to humans, which is mostly attempted by public education programs focused on the dangers of consumption of raw mollusks, implementing proper washing protocols for vegetables, and increasing control of rat populations (Hollyer, 2013).
Angiostrongylus cantonensis is an important differential diagnosis for neurological disease in free-ranging wildlife species. The rat lungworm has likely been underdiagnosed in neurological wildlife due to the necessity for histopathologic identification of eosinophilic meningoencephalitis and the infrequent use of “squash-preps” to detect nematodes. This method is a convenient and cost-effective tool that can be utilized in suspected cases of nematode migration. Increasing awareness of the parasite among biologists, veterinarians, state wildlife agencies, and pathologists will hopefully lead to more documentation of the parasite.
Conflicts of interest
None.
Acknowledgments
We wish to thank the Florida Fish and Wildlife Conservation Commission and Louisiana Department of Wildlife and Fisheries for submission of these cases. Funding was provided by the sponsorship of the Southeastern Cooperative Wildlife Disease Study by the fish and wildlife agencies of Alabama, Arkansas, Florida, Georgia, Kentucky, Kansas, Louisiana, Maryland, Mississippi, Missouri, Nebraska, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Virginia, and West Virginia, USA. Support from the states to SCWDS was provided in part by the Federal Aid to Wildlife Restoration Act (50 Stat. 917).
References
- Barratt J., Chan D., Sandaradura I., Malik R., Spielman D., Lee R., Marriott D., Harkness J., Ellis J., Stark D. Angiostrongylus cantonensis: a review of its distribution, molecular biology, and clinical significance as a human pathogen. Parasitology. 2016;143:1087–1118. doi: 10.1017/S0031182016000652. [DOI] [PubMed] [Google Scholar]
- Burns R., Bicknese E., Qvarnstrom Y., DeLeon-Carnes M., Drew C., Gardiner C., Rideout B. Cerebral Angiostrongylus cantonensis infection in a captive African pygmy falcon (Polihierax semitorquatus) in southern California. J. Vet. Diagn Invest. 2014;26(5):695–698. doi: 10.1177/1040638714544499. [DOI] [PubMed] [Google Scholar]
- Campbell B., Little M. The finding of Angiostrongylus cantonensis in rats in New Orleans. Am. J. Trop. Med. Hyg. 1988;38(3):568–573. doi: 10.4269/ajtmh.1988.38.568. [DOI] [PubMed] [Google Scholar]
- Costa L., McClure J., Snider T., III, Stewart T. Case report: verminous meningoencephalitis by Angiostrongylus (= Parastrongylus) cantonensis in an American miniature horse. Equine Vet. Educ. 2000;12(1):2–6. [Google Scholar]
- Cowie R. Biology, systematics, life cycle, and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawaii J. Med. Public Health. 2013;72(6 Suppl. 2):6–10. [PMC free article] [PubMed] [Google Scholar]
- Duffy M., Miller C., Kinsella J., de Lahunta A. Parastrongylus cantonensis in a nonhuman primate, Florida. Emerg. Infect. Dis. 2004;10(12):2207–2210. doi: 10.3201/eid1012.040319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eamsobhana P. Eosinophilic meningitis caused by Angiostrongylus cantonensis – a neglected disease with escalating importance. Trop. Biomed. 2014;31(4):569–578. [PubMed] [Google Scholar]
- Gardiner C., Wells S., Gutter A., Fitzgerald L., Anderson D., Harris R., Nichols D. Eosinophilic meningoencephalitis due to Angiostrongylus cantonensis as the cause of death in captive non-human primates. Am. J. Trop. Med. Hyg. 1990;42(1):70–74. doi: 10.4269/ajtmh.1990.42.70. [DOI] [PubMed] [Google Scholar]
- Hollyer J. Telling consumers, gardeners, and farmers about the possible risk of rat lungworm in the local food supply in Hawai‘i. Hawaii J. Med. Public Health. 2013;72(6 Suppl. 2):82. [Google Scholar]
- Kim D., Stewart T., Bauer R., Mitchell M. Parastrongylus (=Angiostrongylus) cantonensis now endemic in Louisiana wildlife. J. Parasitol. 2002;88(5):1024–1026. doi: 10.1645/0022-3395(2002)088[1024:PACNEI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kliks M., Palumbo N. Eosinophilic meningitis beyond the Pacific Basin: the global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Soc. Sci. Med. 1992;34(2):199–212. doi: 10.1016/0277-9536(92)90097-a. [DOI] [PubMed] [Google Scholar]
- Lunn, J., 2007. Canine neural Angiostrongyliasis. World Small Animal Veterinary Association World Congress Proceedings. 12–175.
- Lv S., Zhang Y., Steinmann P., Zhou X. Emerging angiostrongyliasis in mainland China. Emerg. Infect. Dis. 2008;14(1):161–164. doi: 10.3201/eid1401.061529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G., Dennis M., Rose K., Spratt D., Spielman D. Tawny frogmouths and brushtail possums as sentinels for Angiostrongylus cantonensis, the rat lungworm. Vet. Parasitol. 2013;192:158–165. doi: 10.1016/j.vetpar.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Prosser, S., Velarde-Aguilar, M., León-Régagnon, V., Herbert, P., 2013. Advancing nematode barcoding: A primer cocktail for the cytochrome c oxidase subunit I gene from vertebrate parasitic nematodes. Mol Ecol Resour. Blackwell Publishing Ltd. 1–8. http://dx.doi.org/10.1111/1755-0998.12082 (Accessed 3 March 2017). [DOI] [PubMed]
- Qvarnstrom Y., Xayavong M., da Silva A., Park S., Whelen A., Calimlim P., Sciulli R., Honda S., Higa K., Kitsutani P., Chea N., Heng S., Johnson S., Graeff-Teixeira C., Fox L., da Silva A. Real-time polymerase chain reaction detection of Angiostrongylus cantonensis DNA in cerebrospinal fluid from patients with eosinophilic meningitis. Am. J. Trop. Med. Hyg. 2016;94:176–181. doi: 10.4269/ajtmh.15-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slom T., Cortese M., Gerber S., Jones R., Holtz T., Lopez A., Zambrano Z., Sufit R., Sakolvaree Y., Chaicumpa W., Herwaldt B., Johnson S. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. New Eng. J. Med. 2002;346:668–675. doi: 10.1056/NEJMoa012462. [DOI] [PubMed] [Google Scholar]
- Spratt D. Species of Angiostrongylus (Nematoda: Metastrongyloidea) in wildlife: a review. Int. J. Parasitol. 2015;4:178–189. doi: 10.1016/j.ijppaw.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C., Shwab E.K., Zhou P., Zhu X.Q., Dubey J.P. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology. 2010;137:1–11. doi: 10.1017/S0031182009991065. [DOI] [PubMed] [Google Scholar]
- Teem J., Gvarnstrom Y., Bishop H., da Silva A., Carter J., White-Mclean J., Smith T. The occurrence of the rat lungworm, Angiostrongylus cantonensis, in nonindigenous snails in the Gulf of Mexico region of the United States. Hawaii J. Med. Public Health. 2013;72(6 Suppl. 2):11–14. [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Lai D., Zhu X., Chen X., Lun Z. Human angiostrongyliasis. Lancet Infect. Dis. 2008;8:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- Wang Q., Wu Z., Wei J., Owen R., Lun Z. Human Angiostrongylus cantonensis: an update. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31(4):389–395. doi: 10.1007/s10096-011-1328-5. [DOI] [PubMed] [Google Scholar]
- Wilkins P., Qvarnstrom Y., Whelen A., Saucier C., da Silva A., Eamsobhana P. The current status of laboratory diagnosis of Angiostrongylus cantonensis infections in humans using serologic and molecular methods. Hawaii J. Med. Public Health. 2013;72:55–57. [PMC free article] [PubMed] [Google Scholar]
- York E., Creecy J., Lord W., Caire W. Geographic range expansion for the rat lungworm in North America. Emerg. Infect. Dis. 2015;21(7):1234–1236. doi: 10.3201/eid2107.141980. [DOI] [PMC free article] [PubMed] [Google Scholar]