Abstract
Objective
Burn-related immunosuppression can promote human herpesviridae infections. However, the effect of these infections on morbidity and mortality after pediatric burn injuries is unclear.
Methods
We retrospectively analyzed pediatric patients with burns ≥10% of the total body surface area (TBSA) who were admitted between 2010 and 2015. On clinical suspicion of a viral infection, antiviral therapy was initiated. Viral infection was confirmed via Tzanck smear, viral culture, and/or PCR. Study endpoints were mortality, days of antiviral agent administration, type of viral test used, type of viral infection, and length of hospitalization.
Results
Of the 613 patients were analyzed, 28 presented with clinically diagnosed viral infections. The use of Tzanck smears decreased over the past 5 years, whereas PCR and viral cultures have become standard. Patients with viral infections had significantly larger burns (53±15% vs. 38±18%, p<0.001); however, length of stay per TBSA burn was comparable (0.5±0.4 vs. 0.6±0.2, p=0.211). The most commonly detected herpesviridae was herpes simplex virus 1. Two patients died due to sepsis, which was accompanied by HSV infection. The mortality rate among all patients (2.7%) was comparable to that in the infected group (7.1%, p=0.898). Acyclovir was given systemically for 9±8 days (N=76) and/or topically for 9±9 days for HSV (N=39, combination of both N=33). Ganciclovir was prescribed in three cases for CMV.
Conclusions
Viral infections occur more commonly in patients suffering from larger burns, and HSV infections can contribute to mortality.
Keywords: Viral cultures, cytomegalovirus, Tzanck smear, polymerase chain reaction, acyclovir
Introduction
The most common complications of burns are bacterial and viral infections causing burn wound and donor site infections, pneumonia, and sepsis [1]. Thermal injuries covering more than one-third of the total body surface area (TBSA) are associated with a severe inflammatory response [2–4]. Inflammation leading to immunosuppression of burn patients is related to phenotypic changes in T-cells [5] as well as defective natural killer cell activity against virus-infected cells [6]. Because of this, severely burned patients are prone to infections and sepsis [7]. Sepsis and infections enhance the hypermetabolic and catabolic response [2], which negatively affects the long-term outcomes of severely burned patients [4]. Along with bacterial infections, viral infections have been associated with increased morbidity and mortality in the severely burned patients [7–11]. Thus, prevention of infections and early treatment of sepsis are key to reducing morbidity and improving the long-term outcome of burn patients.
Primary infections and reactivation of viral infections with herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2), and cytomegalovirus (CMV) have been described during hospitalization after burns [12]. Moreover, severe viral infections can promote bacterial infections in burns and may increase morbidity as well as mortality in the temporarily immunocompromised patient [9,13,14]. Use of common antiviral drugs, including acyclovir and ganciclovir, for the treatment of viral infections in burns remains controversial [12,15,16].
Over the past 2 years, we have observed an increase in viral infections causing graft loss as well as donor site healing problems. This prompted us to retrospectively analyze our viral testing results, assess our antiviral treatment regimens, and investigate trends in use of different viral testing methods at our pediatric burn hospital.
Methods
Patients
We retrospectively analyzed records of pediatric burn patients who were admitted to our institution between January 2010 and August 2015 in this Institutional Review Board-approved study (IRB# 15-0101). To be included in the study, patients must have presented with deep partial- and/or full-thickness burns covering at least 10% of TBSA and must have been treated as inpatients in our pediatric burn center. The primary endpoint was mortality. Secondary endpoints included type of human herpes virus infection, type of viral screening, length of hospitalization, and days of antiviral agent administration. In addition, we collected demographic data including age, sex, type of burn, and burn size. Length of stay per TBSA burn (LOS/TBSA burn) was calculated by dividing the days of acute hospitalization by burn size (percent TBSA).
Laboratory diagnosis and antiviral drugs
When viral infections were suspected (blisters on donor sites or grafted areas, source for sepsis) by the attending physician, antiviral agents including acyclovir (for suspected HSV, EBV or VZV infections) and ganciclovir (for suspected CMV infections) were given until results of testing were available. If superficial lesions were visible, antiviral drugs were administered topical as well as systemically. If the test results were negative, antiviral therapy was stopped. Viral testing was performed using Tzanck smears, viral cultures, and/or PCR (Lyra Direct, Quidel Cooperation, San Diego, CA, USA). Tzanck smears were interpreted by a pathologist at the University of Texas Medical Branch.
Data analysis
Data were analyzed using SigmaStat 4.0 (Systat Software Inc., Chicago, IL, USA). For descriptive statistics, data were presented as mean ± standard deviation. Continuous outcomes were compared using Student’s t-test, and binary outcomes were compared using a Chi-Square test. A p value less than 0.05 was considered statistically significant.
Results
Patient characteristics
Six hundred and thirteen pediatric burn patients were analyzed, 99 (16%) had suspected and 28 (6%) patients had a confirmed viral infection (Table 1). The average time from burn to diagnosis of a viral infection was 14±13 days (median 11days , range 5–55 days). Age at burn, sex, LOS/TBSA burn, type of burn, year of admission, and mortality were comparable between all patients and those patients who had positive viral test results (Tables 1 and 2). Patients with clinically diagnosed viral infections had significantly larger burns (p<0.001), significantly larger full thickness burns full thickness burns (p=0.042), and their overall length of stay was significantly longer (p=0.003).
Table 1.
Patient Characteristics.
| All Admissions N=613 | Positive Viral Infections N=28 | p value* | |
|---|---|---|---|
| Age at burn, years | 7±6 | 8±6 | 0.225 |
| Sex, female:male | 247:366 | 11:17 | 1.000# |
| TBSA burn, % | 38±18 | 53±15 | <0.001 |
| TBSA full thickness burn, % | 25±22 | 32±24 | 0.042 |
| LOS cumulative, days | 22±22 | 29±13 | 0.003 |
| LOS non survivors, days | 31±52 | 35±3 | 0.345 |
| LOS/TBSA burn, days | 0.5±0.4 | 0.6±0.2 | 0.211 |
| Time to infection, days | NA | 14±13 | NA |
| Years of admission, year | 2012±1 | 2012±1 | 0.410 |
| Mortality, n (%) | 23 (2.7%) | 2 (7.1%) | 0.898# |
LOS: Length of stay; TBSA: Total Body Surface Area
Student’s t-tests were used to calculate p values, except as noted otherwise.
Chi-Square test.
Table 2.
Type of burns
| Burn Type | All Admissions N=613 | Positive Viral Infections N=28 | p value* |
|---|---|---|---|
| Flame | 303 | 17 | 0.781 |
| Scald | 254 | 10 | |
| Electrical | 45 | 1 | |
| Contact | 8 | 0 | |
| Chemical | 3 | 0 |
Chi-Square test
Viral testing
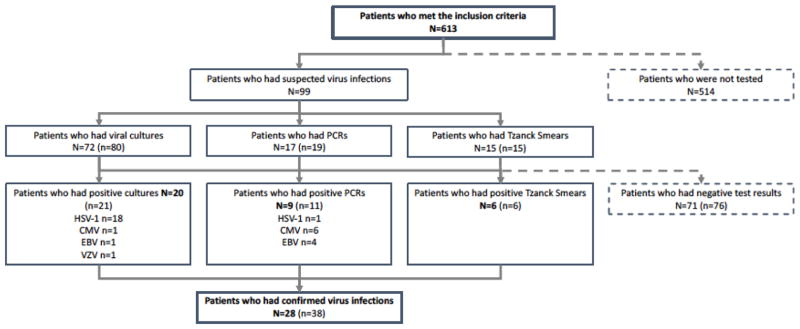
We performed 80 viral cultures on 72 (12%) patients and saw a total of 21 positive viral cultures in 20 patients (Figure 1). There were 18 positive viral cultures for HSV-1, 1 for varicella zoster virus (VZV), 1 for Epstein-Barr virus (EBV), and 1 for CMV. One patient had a positive culture for both CMV and HSV-1. A total of 19 PCRs were performed in 17 (3%) children, and there were 11 positive PCRs in 9 patients. One was positive for HSV-1, 4 for CMV, and 2 for EBV. Two patients had both a positive EBV and a CMV PCR result. Fifteen Tzanck smears were performed in 15 (2%) patients, and 6 were positive for herpes virus cytopathic effects. In one patient, PCR detected HSV-1 and the Tzanck smear showed cytopathic effects of herpes virus. Another case showed a positive culture for HSV-1 as well as a positive Tzanck smear test. In one patient, an active EBV infection was confirmed via PCR as well as viral culture. Two patients presented with a positive HSV (one HSV-1 and one HSV-2) infection and died due to sepsis, which was subsequently confirmed via autopsy.
Fig. 1.
Flow chart describing the number of tested patients and the amount of tests performed. N= number of patients, n= number of tests performed.
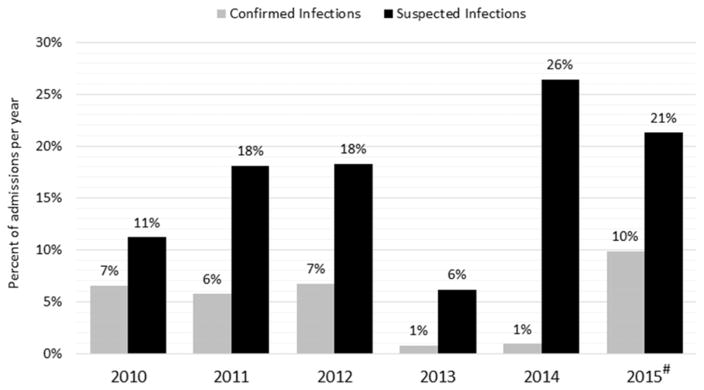
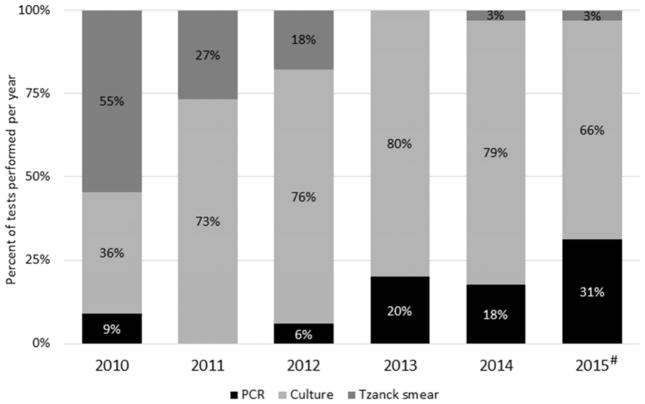
The number of patients who underwent viral testing peaked in 2014 and was the lowest in 2013 (Figure 2). However, the highest percentage of viral infections per admissions occurred in 2015. In 2010, the most common viral test used was the Tzanck smear test (55%); however, in 2014 and 2015 the Tzanck smear was used in 3% of the testing (Figure 3). The use of PCR for viral testing increased over the past years and was the second most used viral test (31%) in 2015.
Fig. 2.
Suspected and confirmed viral infections in pediatric burn admissions at our institution. #Represents data from 8 months of 2015.
Fig. 3.
Trends in viral testing over the last 5 years. #Represents data from 8 months of 2015.
Use of antiviral agents
Suspected HSV infections were treated systemically for 9±8 days (N=76, 12%, median 11 days, range 3–20 days).) and/or topically for 9±9 days (N=39, 6%, median 9 days, range 1–20 days; combination of both N=33, 5%) with acyclovir. Systemic administration of ganciclovir was used for a suspected CMV infection in three clinical cases.
Eighteen patients (3%) with a positive HSV-1 test received acyclovir systemically for 11±5 days and 10 patients with positive HSV-1 tests received topical acyclovir for 10±6 days. One patient who had a positive culture and PCR for EBV received 7 days of systemic and 6 days of topical acyclovir. Another patient with a positive PCR for CMV and EBV received acyclovir systemically for 4 days. In one case, ganciclovir was administered for 2 days, but a viral culture showed a positive HSV-1 infection, prompting discontinuation of ganciclovir and administration of topical and systemic acyclovir for another 13 days.
Discussion
Over the past 5 years, use of Tzanck smears for viral testing at our institution has been replaced with viral cultures and PCR. Although the largest number of patients were tested in 2014, only 1% were positive, and thus the percent of positive tests per admissions was comparable to that seen in 2013. Due to the fact that a screening for viruses was not done routinely on all admitted patients we are not able to determine the true incidence of viral infections in our pediatric burn population. Our results are representative of those patients who were suspected to have viral infections, according to the treating burn physician, and the recent trends of viral testing at our burn center. As one would expect, the most common viral infections were attributable to HSV-1, and the most common antiviral therapeutic agent was acyclovir, which was used both systemically and topically. Patients with more severe burns are more prone to developing viral infections, but there appears to be no correlation between viral infections and length of hospitalization. On average, viral infections were clinically detected around the 14th day post burn, and two cases were seen in which an HSV infection may have contributed to mortality, as confirmed by autopsy.
Tzanck smears have been used as a rapid, inexpensive, and minimally invasive tool to detect herpes infections by cytology over the last century, but diagnostic precision to discriminate between different types of herpesviridae or primary versus recurrent infection is limited [17]. This may account for their waning popularity at our institution. Instead, viral cultures and PCR have now become the standard for viral testing in pediatric burn patients. Antiviral therapeutic agents including acyclovir and ganciclovir are expensive and can even be toxic as some rare cases have shown [18]. For this reason, administration of these agents was initiated only when a viral infection was clinically suspected, for example when blisters occurred on the face or on donor sites or if blisters led to graft loss. Antiviral agents were administered until viral testing by PCR or viral culture was completed; in the case of a negative test results, the medication was discontinued. Because PCR allows for earlier detection of viral infections and is more sensitive than culture, it is becoming an important tool for determining the actual viral load. Viral cultures have been consistently used over the past 5 years. However, 76 patients received acyclovir systemically upon clinical suspicion, and only a quarter of those patients may have needed it.
Early treatment of herpes simplex infections in the respiratory tract can reduce mortality of critically ill patients [19]. In our studied cohort, one patient who contracted HSV-1 pneumonia following inhalation injury and one patient with HSV-2 infection of graft and donor sites expired. In both cases, sepsis was the primary cause of death, and the primary source of infection was herpesviridae. Our chart review showed that there was acyclovir administered systemically in the case where an active HSV-2 was seen on graft and donor sites. However, the patient who suffered from a HSV-1 pneumonia did not get any antiviral agent because the virus was detected at autopsy. HSV-1 infections in pediatric burn patients are rare; however, the overall HSV-2 seroprevalence rate in adults in the United States is 22% according to an analysis of more than 13,000 serum samples [20]. In this case, we suspect that the child was primarily infected during vaginal delivery because the mother had tested seropositive for HSV-2. Thus, reactivation of a HSV-2 infection due to burn was suspected. In addition to having a viral infection, this patient suffered from a multidrug resistant Pseudomonas aeruginosa infection. A combination of bacterial and viral infections can occur and may lead to worse outcomes and increase mortality in severely burned patients [7–11]. The second patient, who suffered from HSV-1 pneumonia following inhalation injury, died due to sepsis and disseminated intravascular coagulation. Although both patients were admitted at the second day post burn, the severity of the burn and resulting sepsis caused their death. The mortality rate in our studied cohort of 613 severely burned children was 2.7% and reflects the commonly low mortality rates seen in pediatric burns nowadays [21]. In virus-infected patients, the mortality rate was more than twice as high (7.1%). However, due the overall low mortality rate and low number of subjects, this finding was not statistically significant (p=0.898).
In severely burned children, we advocate viral culture for children with clinical suspicion of viral infections. Although evidence that support the choice of specific antiviral agents is still lacking and the effect of these agents in the pediatric burn population has to be studied in more detail, we believe that upon clinical suspicion, an antiviral agent should be administered topical and systemically. It is important to initiate the treatment early on to provide the best benefit of the agents. We do not give antiviral drugs to each admitted patient and we do not recommend to perform routinely a viral screening test. The benefit of antiviral agents on survival and other clinical outcomes has yet to be elucidated in prospective randomized controlled trials.
Because of its retrospective design, our study has several limitations. We were not able to collect detailed information about the anatomical locations where the cultures, PCR`s as well as Tzanck smears were collected. Furthermore, we were not able to describe the prevalence of viral infections because patients underwent testing only if requested by the attending physician. Thus, a general screening would be needed to answer this question. Further, Tzanck smears are very sensitive but unspecific and thus cannot be compared to viral cultures or PCR. A prospective study would need to be designed to focus on the pros and cons of various viral testing methods. To distinguish between a virus reactivation and a primary infection, we would have needed to perform antibody testing. This testing was not performed, as the aim of our current study was to analyze PCR, Tzanck smear, and viral culture results.
Conclusions
Viral cultures have become the most commonly performed viral test at our pediatric burn center. Patients with a larger burn size and deeper burns are more likely to have positive viral test results; however, there seems to be no correlation between a prolonged length of stay and viral infections in our studied cohort. Herpes simplex virus infections may contribute to mortality and thus an antiviral agent should be administered topical and systemically upon suspicion. However, prospective trials to determine the overall seroprevalence as well as the efficacy of antiviral agents in burns are warranted.
Highlights.
Viruses can cause burn wound and donor site infections, pneumonia, and sepsis.
Viral cultures are the most commonly performed viral test at our burn center, followed by PCRs and Tzanck smears.
Patients with large full-thickness burns are more likely to have positive viral test results.
Herpes simplex virus infections may contribute to mortality.
Acknowledgments
Sources of Support: This study was supported by the National Institutes of Health (P50GM060338, UL1TR000071, T32GM008256, R01GM056687, R01112936, R01HD049471) and Shriners of North America (71006, 71008, 71009, 80100, 84080, 84291).
Footnotes
Parts of this research were presented in abstract form at the Surgical Infection Society 36th Annual Meeting in 2016 in Palm Beach, Florida, USA.
Conflicts of interest: None
Authors’ contributions
All authors made substantial contributions to the development or design of the work (DNH, JOL, MC, ONL, PW, RPC) or to the acquisition, analysis, or interpretation of data for the work (GH, HKH, JOL, MC, PW) and the drafting of the work or revising the intellectual content (all authors).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ABA-National Burn Repository 2013 Report Dataset Version 9.0. n.d http://www.ameriburn.org/2013NBRAnnualReport.pdf.
- 2.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 3.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PloS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zedler S, Bone RC, Baue AE, von Donnersmarck GH, Faist E. T-cell reactivity and its predictive role in immunosuppression after burns. Crit Care Med. 1999;27:66–72. doi: 10.1097/00003246-199901000-00028. [DOI] [PubMed] [Google Scholar]
- 6.Klimpel GR, Herndon DN, Fons M, Albrecht T, Asuncion MT, Chin R, et al. Defective NK cell activity following thermal injury. Clin Exp Immunol. 1986;66:384–92. [PMC free article] [PubMed] [Google Scholar]
- 7.D’Avignon LC, Hogan BK, Murray CK, Loo FL, Hospenthal DR, Cancio LC, et al. Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: an autopsy series. Burns J Int Soc Burn Inj. 2010;36:773–9. doi: 10.1016/j.burns.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Nash G, Asch MJ, Foley FD, Pruitt BA. Disseminated cytomegalic inclusion disease in a burned adult. JAMA. 1970;214:587–8. [PubMed] [Google Scholar]
- 9.Byers RJ, Hasleton PS, Quigley A, Dennett C, Klapper PE, Cleator GM, et al. Pulmonary herpes simplex in burns patients. Eur Respir J. 1996;9:2313–7. doi: 10.1183/09031936.96.09112313. [DOI] [PubMed] [Google Scholar]
- 10.Hamprecht K, Pfau M, Schaller H-E, Jahn G, Middeldorp JM, Rennekampff H-O. Human Cytomegalovirus Infection of a Severe-Burn Patient: Evidence for Productive Self-Limited Viral Replication in Blood and Lung. J Clin Microbiol. 2005;43:2534–6. doi: 10.1128/JCM.43.5.2534-2536.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peppercorn A, Veit L, Sigel C, Weber DJ, Jones S, Cairns BA. Overwhelming disseminated herpes simplex virus type 2 infection in a patient with severe burn injury: case report and literature review. J Burn Care Res Off Publ Am Burn Assoc. 2010;31:492–8. doi: 10.1097/BCR.0b013e3181db51cb. [DOI] [PubMed] [Google Scholar]
- 12.Wurzer P, Guillory A, Parvizi D, Clayton RP, Branski LK, Kamolz L-P, et al. Human herpes viruses in burn patients: A systematic review. Burns J Int Soc Burn Inj. 2016 doi: 10.1016/j.burns.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt SJ, Tribble CG, Lakeman AD, Hayden FG. Herpes simplex burn wound infections: epidemiology of a case cluster and responses to acyclovir therapy. Surgery. 1985;98:338–43. [PubMed] [Google Scholar]
- 14.Sen S, Szoka N, Phan H, Palmieri T, Greenhalgh D. Herpes simplex activation prolongs recovery from severe burn injury and increases bacterial infection risk. J Burn Care Res Off Publ Am Burn Assoc. 2012;33:393–7. doi: 10.1097/BCR.0b013e3182331e28. [DOI] [PubMed] [Google Scholar]
- 15.Munster AM, Moran KT, Thupari J, Allo M, Winchurch RA. Prophylactic intravenous immunoglobulin replacement in high-risk burn patients. J Burn Care Rehabil. 1987;8:376–80. doi: 10.1097/00004630-198709000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Moran KT, Thupari JN, O’Reilly TJ, Munster AM. Effect of immunoglobulin G therapy on serum antibody titers to cytomegalovirus in burn patients. Am J Surg. 1988;155:294–7. doi: 10.1016/s0002-9610(88)80718-9. [DOI] [PubMed] [Google Scholar]
- 17.Kelly B, Shimoni T. Reintroducing the Tzanck smear. Am J Clin Dermatol. 2009;10:141–52. doi: 10.2165/00128071-200910030-00001. [DOI] [PubMed] [Google Scholar]
- 18.Storch GA. Diagnostic Virology. Clin Infect Dis. 2000;31:739–51. doi: 10.1086/314015. [DOI] [PubMed] [Google Scholar]
- 19.Traen S, Bochanen N, Ieven M, Schepens T, Bruynseels P, Verbrugghe W, et al. Is acyclovir effective among critically ill patients with herpes simplex in the respiratory tract? J Clin Virol Off Publ Pan Am Soc Clin Virol. 2014;60:215–21. doi: 10.1016/j.jcv.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Fleming DT, McQuillan GM, Johnson RE, Nahmias AJ, Aral SO, Lee FK, et al. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337:1105–11. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 21.Kraft R, Herndon DN, Al-Mousawi AM, Williams FN, Finnerty CC, Jeschke MG. Burn size and survival probability in paediatric patients in modern burn care: a prospective observational cohort study. Lancet. 2012;379:1013–21. doi: 10.1016/S0140-6736(11)61345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]