Abstract
Cutaneous squamous cell carcinoma is the second most widespread cancer in humans and its incidence is rising. These tumours can evolve as poor-prognosis diseases, and therefore it is important to identify new markers to better predict its clinical evolution. Here, we identified the expression pattern of miRNAs at different stages of skin cancer progression in a panel of murine skin cancer cell lines. We determined that miR-203 and miR-205 are differentially expressed in this panel, and evaluated their potential use as biomarkers of prognosis in human tumours. MiR-205 was expressed in tumours with pathological features recognized as indicators of poor prognosis such as desmoplasia, perineural invasion and infiltrative growth pattern. MiR-205 was mainly expressed in undifferentiated areas and in the invasion front, and was associated with both local recurrence and the development of general clinical events of poor evolution. MiR-205 expression was an independent variable selected to predict events of poor clinical evolution using the multinomial logistic regression model described in this study. In contrast, miR-203 was mainly expressed in tumours exhibiting the characteristics associated with a good prognosis, was mainly present in well-differentiated zones, and rarely expressed in the invasion front. Therefore, the expression and associations of miR-205 and miR-203 were mostly mutually exclusive. Finally, using a logistic biplot we identified three clusters of patients with differential prognosis based on miR-203 and miR-205 expression, and pathological tumour features. This work highlights the utility of miR-205 and miR-203 as prognostic markers in cutaneous squamous cell carcinoma.
Keywords: Cutaneous Squamous Cell Carcinoma, miRNAs, miR-205, miR-203, P63, E-CADHERIN, Prognosis
INTRODUCTION
Cutaneous squamous cell carcinoma (CSCC) is the second most common cancer in humans after basal cell carcinoma 1, 2. The incidence of CSCC is rising dramatically. It is estimated that more than 700,000 new CSCC cases are diagnosed in the US per year 3, leading to high health care costs 4. The risk of developing CSCC along human life is between 7% and 11% 5. The incidence of CSCC varies in different geographical areas and countries 6. Owing to the high frequency of CSCC, it is the non-melanoma skin cancer that causes most metastases and deaths 3.
CSCC can evolve as a poor-prognosis disease. Although significant advances have been made in our understanding of the development of CSCC, there are many unknown aspects concerning its pathogenesis and prognosis. Thus, it is necessary to seek molecular markers that can help physicians to predict the biological behaviour and clinical evolution of CSCC. Recently, microRNAs (miRNAs or miRs) have emerged as a new class of molecules closely related to the pathogenesis of cancer 7. MiRNAs are small molecules of non-codifying RNA of 17–25 nucleotides that act as posttranscriptional regulators of mRNA expression 8. MiRNAs have been implicated in the pathogenesis of several forms of cancer 7, and their expression patterns may have prognostic value 9–11.
Despite the fact that important work has been done regarding the role of miRNAs in the Biology of CSCC 12, 13, and other skin cancers, such as basal cell carcinomas 14, the utility of miRNAs in defining the prognosis of the disease still requires further study. Although some authors have evaluated the expression patterns of miRNAs in skin cancer cell lines 15 and in CSCC 16, their potential prognostic value has not been explored in detail. In the present work, we identified differential miRNA expression patterns along different stages of CSCC progression in a well-established panel of murine skin cancer cell lines 17–20. Later, based on our findings and previous data in the literature, we selected miR-205 and miR-203 to evaluate their association with the clinical prognosis and evolution of human CSCC 21–27. Based on the expression of miR-203 and miR-205 and pathological tumour features, we predicted the prognosis of CSCC using multinomial logistic regression models. We also identified three clusters of CSCC with a logistic biplot, which highlights the utility of miR-203 and miR-205 expression to predict CSCC prognosis.
PATIENTS, MATERIAL AND METHODS
Patients and tumour variables
Seventy-nine human primary CSCCs were collected at the University Hospital of Salamanca in Spain. The collection and use of tumour samples were approved by the institutional Ethics Review Board of the University Hospital of Salamanca. Written informed consents for research using these tumour samples were obtained from all patients. We evaluated different pathological and clinical variables of evolution whose distribution is described on Supplementary Table S1.
Cell lines
We used a panel of murine cell lines that represent different stages of CSCC, a generous gift from Dr. Balmain (USCF). The panel includes (i) non-tumoural cell lines: C50, C5N; NK; (ii) papilloma cell lines: MSCP1 and P6; (iii) malignant, well-differentiated cell lines with squamous morphology: PDV, PDVC57, B9 and E4; and, (iv) poorly-differentiated cell lines, with the epithelial to mesenchymal transition (EMT) phenotype: H11, D3, A5, CarB and CarC 17–20. Cells were cultured in DMEM supplemented with 10% foetal bovine serum (FBS), 1% penicillin-streptomycin and 4 mM L-glutamine at 37°C and 5% of CO2.
Total RNA isolation
Cells were collected and centrifuged at 1500 r.p.m. Total RNAs were isolated using the Qiagen™ kit (miRNA easy) following the manufacturer’s instructions. Briefly, RNAs were extracted with a mix of phenol-chloroform followed by precipitation in ethanol, and were purified through RNase-free columns. RNA concentrations were determined with a spectrophotometer (Nanodrop) and microfluidic chips (Agilent).
miRNA expression analysis
MiRNA expression was analysed with specific arrays miRCURY LNA microRNA Array, v.10.0 (Exiqon), following the manufacturer’s recommendations. Then, fluorescent reagents were reconstituted and cDNAs were synthesized and labelled. Following this, samples were heated at 95° C in darkness, hybridized, and washed with the robotic HS 4800 Pro system (Tecan®). Fluorescence was scanned with a GenePix 4000B (Axon Instruments™) microarray scanner. Image processing was accomplished with the Gene Prix Pro (v 6.0). The data for the analysis were generated from a single channel following manufacturer’s instructions. Raw data processing was performed with the ExiMiR package from R 28, 29. Quantile normalization was performed using the Robust Microarray Analysis (RMA) algorithm30 from the BioConductor (http://www.bioconductor.org/) tools suite. All expression data were deposited in the GEO database (GEO: GSE71923).
Tissue-array and in situ hybridization
Tissue samples embedded in paraffin were used to prepare tissue microarrays with a tissue arrayer device (Beecher Instruments, MD). Three 1-mm diameter tissue cylinders from each tumour were included. Different areas of each tumour were selected to analyse the heterogeneity of tumours. MiR-203 and miR-205 expression was detected in the tissue sections by in situ hybridization (ISH) using the miRCURY LNA™ microRNA ISH kit (Exiqon®) and following the manufacturer’s recommendations.
For the quantification of miRNA expression, we evaluated the percentage of positive cells, the intensity of the staining and the location as follows: the intensity of staining was considered negative when there were no stained cells or there were fewer than 5% of stained cells; weakly positive (+) when the percentage of stained cells was more than 5% and less than 25%; moderately positive (++) when more than 25% and less than 75%; intensely positive (+++) when more than 75% of the cells were stained. Analysis of miRNA expression by ISH was performed by three independent observers (J.C., C.R.C and E.C.A).
Immunohistochemistry
P63 and E-CADHERIN expressions were evaluated by immunohistochemistry with specific antibodies against P63 (Biocare, Clon BC4A4) and E-CADHERIN (Vitro, Clone EP700Y). We evaluated P63 and E-CADHERIN expression with the same semiquantitative method as that used to assess miRNA expression, described in the Supplementary Materials and Methods section.
Statistical analyses
To compare dichotomous variables we used the Chi-square or the Fisher exact tests, and to evaluate two independent samples, the Mann-Whitney U test. To assess more than two independent groups we used the Kruskal-Wallis test. To compare temporal intervals we used the Kaplan-Meier estimator, followed by the Mantel-Cox Log-Rank test. To build graphical representations of the statistical associations among variables, we used the Cytoscape (v.3.1.0) software, freely available at www.cytoscape.org (accessed December 11th 2014). To evaluate which variables predicted events of poor clinical evolution, we developed logistic regression models and used the Wald test. We considered P values < 0.05 as significant, and confidence intervals at 95%. To generate clusters of prognosis we used the logistic biplot 31.
RESULTS
Tumours with poor clinical evolution are associated with specific histopathological traits
We defined CSCCs with poor clinical evolution as those tumours that presented local recurrence, lymph nodal progression or metastases to distant organs. In the literature, a number of other types of histopathological tumour traits associated with poor clinical evolution of CSCC have been established, such as poor degree of differentiation, perineural infiltration, infiltrative growth pattern, desmoplasia, etc. 32–34. Therefore, we first evaluated to what extent the tumours in our cohort, known to have a poor clinical outcome, exhibited these histopathologic features, all associated with a poor prognosis (Table 1A). Undoubtedly, perineural infiltration was statistically more frequent in tumours with local recurrence (P = 0.002) and with lymph nodal progression (P = 0.048). Thicker tumours also showed more local recurrence (P = 0.009) and lymph nodal progression (P = 0.035). Nodal progression was also associated with a poor grade of differentiation (P = 0.006) (Table 1A). We did not find statistical association with dissemination to distant organs because of the low number of tumours with this clinical event (N = 1) in our series (Supplementary Table S1). As expected, we observed several associations among the different histopathological tumour traits, which in turn were also associated with poor or good prognosis based on the literature (Supplementary Table S2). In conclusion, in our cohort of CSCC we observed a number of associations among several pathological tumour traits with events of poor clinical evolution.
Table 1. Associations of poor clinical evolution of CSCCs with.
A) histopathological tumor features, and B) miR-205 and mi-R203 expression. N.S. not significant. Red: significant P values. Blue: statistical trend.
| POOR PROGNOSIS CRITERIA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Local Recurrence | Nodal Progression | Events of Poor Clinical Evolution | |||||||||
|
| |||||||||||
| YES | NO | P-value | YES | NO | P-value | YES | NO | P-value | |||
|
A. Pathological Tumour Traits |
Growth Pattern | Expansive | 0 | 26 | N.S. | 1 | 15 | 0.064 | 1 | 25 | 0.066 |
| Mixed | 1 | 16 | 1 | 16 | 2 | 15 | |||||
| Infiltrative | 3 | 33 | 8 | 28 | 9 | 27 | |||||
|
| |||||||||||
|
Grade of Differentiation |
Good | 1 | 24 | N.S. | 1 | 24 | 0.006 | 2 | 23 | N.S. | |
| Moderate | 2 | 35 | 3 | 34 | 5 | 32 | |||||
| Poor | 1 | 16 | 6 | 11 | 5 | 12 | |||||
|
| |||||||||||
| YES | 3 | 11 | 4 | 10 | 5 | 9 | |||||
| Perineural Invasion | 0.002 | 0.048 | 0.018 | ||||||||
| NO | 1 | 64 | 6 | 59 | 7 | 58 | |||||
|
| |||||||||||
| YES | 2 | 19 | 3 | 18 | 3 | 18 | |||||
| Desmoplasia | N.S. | N.S. | N.S. | ||||||||
| NO | 2 | 56 | 7 | 51 | 9 | 49 | |||||
|
| |||||||||||
| Tumour Thickness | (Median in mm (SD)) | 11.5(14.5) | 5.28(4.5) | 0.009 | 6.25(5.25) | 5(4.50) | 0.035 | 8 (7.75 | 5 (4.58) | 0.003 | |
|
| |||||||||||
| Tumour Size | (Median in mm (SD)) | 21.5(44.5) | 18(9) | N.S. | 22(10.25) | 18(8) | N.S. | 22(10) | 18(9) | 0.074 | |
|
| |||||||||||
|
B. mIRNAs expression |
Expression | 0 | 29 | 2 | 27 | 2 | 27 | ||||
| miR-203 | N.S. | N.S. | N.S. | ||||||||
| No expression | 4 | 46 | 27 | 42 | 10 | 40 | |||||
|
| |||||||||||
| Expression | 4 | 35 | 7 | 32 | 10 | 29 | |||||
| miR-205 | 0.038 | N.S. | 0.011 | ||||||||
| No expression | 35 | 40 | 3 | 37 | 2 | 38 | |||||
Identification of miRNA differentially expressed in skin cancer cell lines with different grade of aggressiveness
Owing to the increasing importance of miRNAs in the pathogenesis of cancer 9–11, we considered the possibility that miRNAs could help to define the prognosis of CSCC. To identify miRNAs that could be associated with human CSCC with different grades of aggressiveness and prognosis, we identified miRNAs differentially expressed between groups of CSCC cell lines. Later, we chose some of these miRNAs to be evaluated in human CSCC prognosis, because of their importance in skin homeostasis.
As a result, we identified a number of miRNAs overexpressed in squamous non-malignant cell lines (C5N, NK, MSCP1, P6) when compared to a group of malignant cell lines with a squamous morphology (PCVC57, B9, E4) (Figure 1A and Supplementary Table S3A). Among the miRNAs most differentially expressed in the immortalized non-malignant group, we identified some miRNAs already known as tumour suppressors within different contexts, such as let-7, miR-34b, miR-200c, others with protumoural effects, such as miR-19a 35, 36, and others related to skin homeostasis, such as miR-203 and miR-205 14, 21, 23, 37, 38 (Figure 1A and Supplementary Table S3A). We also identified miRNAs downregulated in the EMT stage, within squamous CSCC cell lines (PCVC57, B9, E4) compared to spindle CSCC cell lines (D3, H11, CarB, CarC, A5). In our study, the miRNA most differentially expressed was miR-205, which is in agreement with the fact that it can inhibit EMT in CSCC 39 (Figure 1B and Supplementary Table S3B).
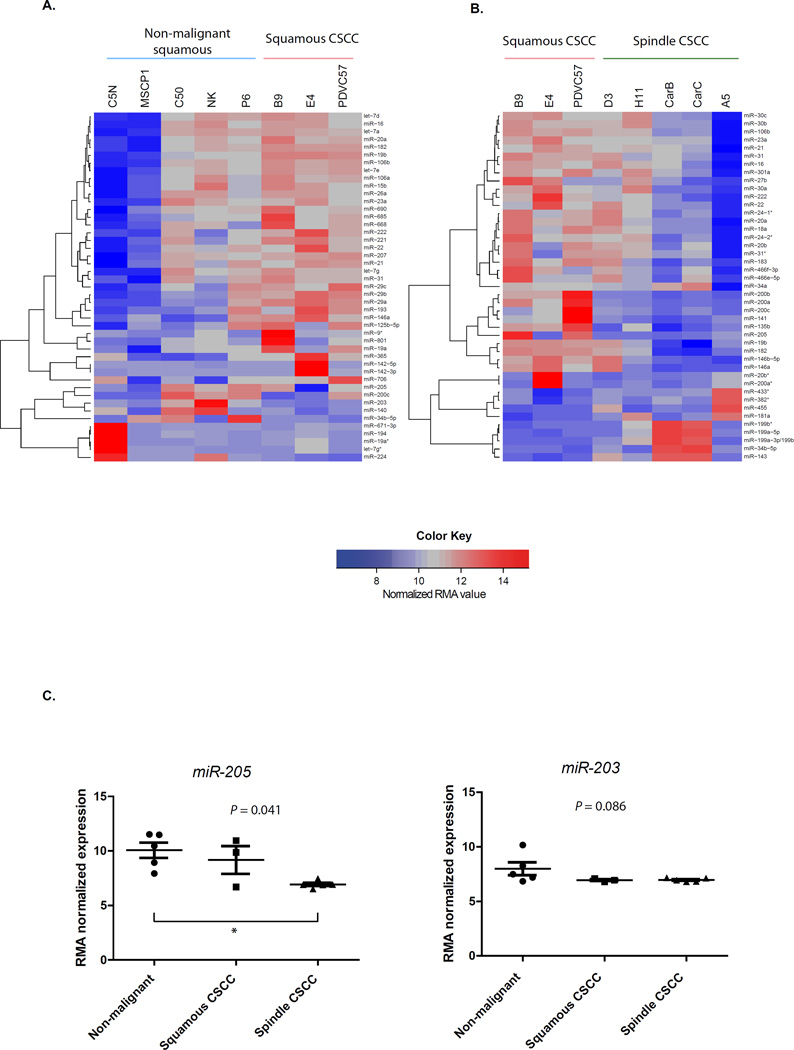
Figure 1. Expression patterns of miRNAs in murine skin cancer cell lines with different grade of aggressiveness.
A) The heatmap shows the 45 miRNAs most differentially expressed between CSCC cell lines (PCVC57, B9, E4) and non-malignant skin cell lines, which included immortalized keratinocytes (C50, C5N, NK) and cells from benign papillomas (MSCP1, P6) generated by the DMBA/TPA protocol of carcinogenesis. B) Heatmap showing the 43 miRNAs most differentially expressed between squamous CSCC cell lines (PCVC57, B9, E4) and the spindle group of CSCC cell lines (H11, A5, D3, CarB, CarC), that presented an EMT process. The lists of miRNAs from A and B heatmaps are shown in Supplementary Tables S3A and S3B, respectively. The A5 cell line was analysed from two different clones as an internal assay control and, as expected, both samples clustered together. In A and B, quantile normalization was performed using the Robust Microarray Analysis (RMA) algorithm, as indicated in the scale of the figure and in the material and methods section. C) Expression of the miR-205 (left) and the miR-203 (right) in the different groups of skin cell lines. The P-value indicated was obtained by the Kruskal-Wallis test. The asterisk indicates in between which groups there is statistical significance after applying the Dunn’s multiple comparison test.
Because of the importance of miR-203 and miR-205 in skin homeostasis, we initially focused on these two miRNAs 14, 21, 23, 37, 38. It was observed that miR-205 was more expressed in non-malignant and squamous CSCC cell lines than in spindle CSCC cells (P = 0.041). In contrast, miR-203 was more expressed in squamous non-malignant cell lines than in the malignant groups with a statistical trend (P = 0.086) (Figure 1C).
Mutually exclusive expression patterns of miR-203 and miR-205 in CSCC
Next, we evaluated the expression of miR-203 and miR-205 in human CSCCs. First, we confirmed the expression patterns of both miRNAs in the normal adjacent skin, as described within the literature. Thus, miR-203 was predominantly expressed in the upper layers of the skin 40, and miR-205 was mostly expressed in the basal and immediate suprabasal layers 41 (Figure 2A).
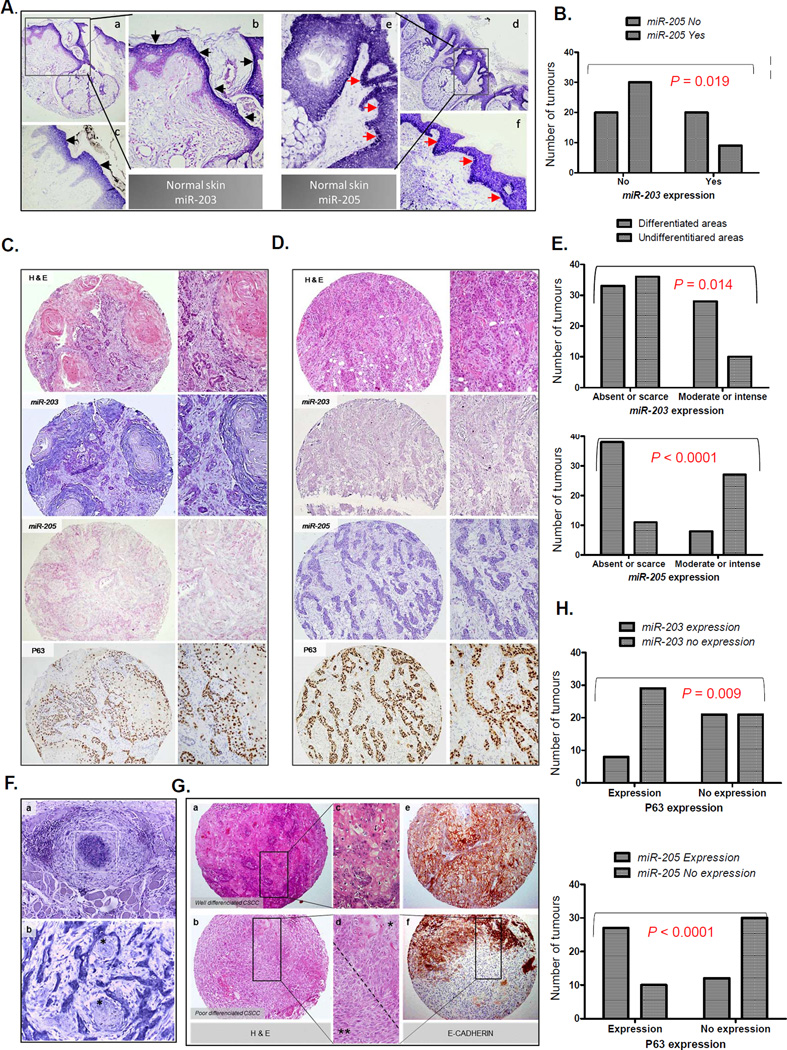
Figure 2. Expression of miR-203 and miR-205 in human CSCC.
A) Expression of miR-203 and miR-205 in normal skin as determined by in situ hybridization (ISH). Markedly, miR-203 is expressed in the epidermal upper layers of the epidermis (a, b (100x) and c (400x), black arrowheads), whereas miR-205 is expressed in the basal and immediately suprabasal layers of the epithelia (d, e (100x) and f (400x), red arrowheads). B) Mutually exclusive expression of miR-205 and miR-203 in CSCC. Pearson’s Chi-square test. C) Example of a well-differentiated CSCC. The haematoxylin and eosin (H&E) section shows intense keratinization in the central zones of the tumoural lobules (100x and 400x). The pictures show miR-203 and miR205 expression by ISH and P63 expression by immunohistochemistry (IQ) from the same tumour, as indicated in the pictures. MiR-203 is highly expressed, whereas miR-205 and P63 were poorly expressed. D) Example of a poorly-differentiated CSCC. The H&E section shows strands of epithelial cells without keratinization (100x). MiR-205 and P63 were highly expressed, whereas miR-203 is poorly expressed. In C and D, pictures on the right correspond to more augmented details (400x) from the ones on the left (100x). E) Quantification of miR-203 and miR-205 expression in differentiated and undifferentiated areas of CSCC. Pearson’s Chi-square test. F) Upper figure: Strong miR-205 expression in CSCC cells that are forming an intravascular tumoural thrombus (a, and white square) (400x). Lower figure: High miR-205 expression present in tumoral cells in the front of invasion with perineural infiltration (b) (400x). Nerves are indicated by an asterisk (ӿ). G) H&E staining (a,b,c,d) and E-CADHERIN expression (e, f) (100x) done by immunohistochemistry in a well- (a,d,e) and poorly-differentiated (b,d,f) CSCCs. There is strong E-CADHERIN expression in the epithelial cells of the well-differentiated CSCC (e) (100x). In d (400x), there are typical epithelial cells in the upper portion of the picture (ӿ) that correspond to zones with strong staining to E-CADHERIN (f, upper portion of the spot), and tumoral spindle cells in the lower one (ӿ ӿ) that correspond to zones without expression of E-CADHERIN (f, lower portion of the spot). This zone illustrates the tumour heterogeneity and the epithelial to mesenchymal transition process. H) Upper figure: miR-203 expression is significantly absent when P63 is present. Lower figure: miR-205 and P63 coexpressed in the same tumours in a statistically significant manner. P values were obtained by the Pearson’s Chi-square test.
We then evaluated the expression patterns of miR-205 and miR-203 in CSCC and found them to be reciprocally exclusive (Figure 2B and Supplementary Table S4). MiR-203 was more frequently expressed, to a moderate or intense degree, in differentiated rather than in undifferentiated areas (P = 0.014), whereas miR-205 was more frequently expressed at high levels in undifferentiated areas (P < 0.0001) in the invasion front (P = 0.0008) and in zones with perineural invasion (Figures 2C–F, and Supplementary Table S4). Interestingly, we observed that tumour cells, inside a blood vessel and forming a thrombus, sometimes overexpressed miR-205 (Figure 2F).
In addition, miR-205 was, in general, more frequently expressed in tumours with an infiltrative growth pattern (P = 0.003), perineural invasion (P = 0.016), and in thicker tumours (P = 0.023) - all pathological tumour traits associated with a poor prognosis 32, 33, 42. By contrast, miR-203 expression was not associated with these same tumour traits (Supplementary Table S5A).
Regarding the expression of these miRNAs in the invasion front, it was determined that miR-205 was more frequently expressed in tumours with aggressive traits, such as infiltrative growth pattern and perineural invasion. However, miR-203 was significantly less expressed in the invasion front of such aggressive tumours (Supplementary Table S5B). In conclusion, miR-205 and miR-203 tended to exhibit mutually exclusive expression patterns in human CSCC.
Reciprocally exclusive associations between miR-205 and miR-203 and P63
It has been reported that miR-203 inhibits P63, leading to cell differentiation and the repression of stemness 21, 23, whereas miR-205 represses E-CADHERIN and expands into the stem cell compartment 41. Thus, we evaluated the associations of miR-203 and miR-205 with P63 and E-CADHERIN as grading markers in epithelial differentiation. As expected, P63 was more frequently expressed in poorly differentiated tumours, and it was significantly more common in undifferentiated areas, whereas E-CADHERIN expression was more intense in well-differentiated tumours, and less common in undifferentiated areas (Figures 2C, 2D and 2G).
P63 was inversely correlated to miR-203 expression, and most of the tumours with a remarkable P63 expression did not show miR-203 (P = 0.009). Conversely, the majority of tumours with P63 expression showed expression of miR-205 (P = 0.0001). We did not observe a statistically significant association between the expressions of miR-205 and E-CADHERIN in the tumours of our cohort (Figure 2H and Supplementary Table S5C). In conclusion, miR-203 and miR-205 showed a mutually exclusive P63 protein distribution in human CSCC.
Prediction of CSCC prognosis based on miRNAs expression and tumour traits
We later evaluated the association between events of poor prognosis and miR-205 and miR-203 expression. MiR-205 expression was associated with local CSCC recurrence (P = 0.038), and with the general events related to a poor clinical evolution (P = 0.011). We could not find an association between the expression of miR-203 with specific events of poor prognosis (Table 1B).
To predict the prognosis of CSCC, we built a logistic regression model in order to predict local recurrence, lymph nodal progression, and the existence of any event associated with a poor clinical evolution. As predictive variables, we used miR-203 and miR-205 expression, perineural invasion, the grade of differentiation (well-differentiated versus poorly-moderately differentiated), the growth pattern of invasion (infiltrative versus non-infiltrative), tumour thickness (more than 2 mm versus less than 2 mm), and tumour surface size (more than 20 mm versus less than 20 mm in the largest diameter).
Perineural invasion was the only independent variable associated with local recurrence (P = 0.017); and a low grade of differentiation was the only variable related to lymph nodal progression (P = 0.004). Interestingly, miR-205 expression was the only independent variable associated with the occurrence of any general outcome of poor clinical evolution (P = 0.021) (Table 2A). In summary, here we report that the logistic regression models are capable of predicting the clinical evolution of CSCC, based on miRNA expression and the pathological features of tumours.
Table 2. Definition of CSCC prognosis by multivativariate analyses.
A) Pathological features of CSCC associated with a poor clinical evolution obtained by logistic regression. B) Characteristics of the three clusters of CSCCs identified by the logistic biplot. The table shows the number and percentage of tumours within each cluster that showed the characteristics indicated.
| A. Logistic regression models of prognosis | |||||
|---|---|---|---|---|---|
| Variables | OR | CI 95% | P value | ||
| Local Recurrence | Perineural infiltration | 17.445 | 1.662–183.359 | 0.017 | |
| Nodal Progression | Grade of differentiation | 7.909 | 1.912–32.717 | 0.004 | |
| Events of Poor Clinical Evolution | miR-205 expression | 6.552 | 1.332–32.232 | 0.021 | |
| B. Clusters of prognosis identified by logistic biplot | |||||
|
Cluster 1 N=25(31.6%) |
Cluster 2 N=31 (39.2%) |
Cluster 3 N=23 (29.1%) |
P value | ||
| Events of Poor Clinical Evolution | 1 (4%) | 3 (9.7%) | 8 (34.8%) | 0.007 | |
|
miRNAs expression |
miRNA-203 | 15 (60%) | 11 (35.5%) | 3 (13%) | 0.007 |
| miRNA-205 | 3 (12%) | 15 (48.4%) | 21 (91.3%) | 0.0001 | |
|
Pathological Tumour Traits |
Infiltrative Growth Pattern | 12 (48%) | 4 (12.9%) | 22 (95.7%) | 0.002 |
|
Poor Grade of Differentiation |
2 (8%) | 25 (80.6%) | 13 (56.5%) | 0.043 | |
| Perineural Infiltration | 1 (4%) | 0 | 13(56.5%) | 0.0001 | |
| Desmoplasia | 1 (4%) | 0 | 13(56.5%) | 0.0001 | |
Identification of clusters of human CSCC with different prognoses
We then attempted to sort the 79 CSCCs from our study into clusters of different prognoses based on the multiple associations among the tumour traits and the expression of miR-203 and miR-205 using a logistic biplot 31. Thus, we included pathological variables involved in the prognosis of CSCC such as: the presence/absence of perineural invasion and desmoplasia, the degree of differentiation, the growth pattern of invasion, the tumour size and thickness 32, 34, 43, together with the expression of miR-203 and miR-205. We were able to discriminate three clusters of tumours, with different prognoses and with statistically significant differences, using all of the tumour characteristics considered (Table 2B and Supplementary Figure S1).
Cluster 1 comprised 25 CSCCs (31.6%), but only one developed a clinical outcome of poor prognosis. In this cluster, only three tumours (12%) showed miR-205 expression, but 15 (60%) expressed miR-203. In addition, this cluster contained several tumours with pathological traits of a good prognosis. In addition, there was only 1 tumour with perineural infiltration, 1 tumour with desmoplasia and 2 tumours with a poor grade of differentiation.
At the far end of cluster 1, cluster 3 comprised 23 CSCCs (29.1%) with the characteristics of a poor prognosis, and 8 tumours (34.8%) developed outcomes of poor clinical evolution. Interestingly, 21 (91.3%) tumours expressed miR-205, and only 3 (15%) expressed miR-203. This cluster contained several tumours with histopathological traits of poor prognosis; in addition, 22 (95%) showed an infiltrative growth pattern, 13 (56.5%), a poor grade of differentiation, and the same percentage of tumours with perineural infiltration and desmoplasia (Table 2B and Supplementary Figure S1).
In total, all clusters were well characterized by the percentage of tumours that expressed miR-205 and miR-203; such that a progressively increasing expression of miR-205 went from 12% of tumours in cluster 1 to 91% of tumours in cluster 4; whereas the trend of miR-203 expression was the opposite. This perfectly correlated with the percentage of cases with poor clinical evolution in each clusters, although the pathological tumour traits, including infiltrative growth pattern and poor grade of differentiation, showed a worse correlation among all three clusters (Table 2B and Supplementary Figure S1). Thus, although this study requires further validation using a new cohort of patients, in our cohort, the expression of these two miRNAs more accurately sorted tumours in terms of prognosis than the pathological features. In conclusion, this study illustrates the usefulness of miR-203 and miR-205 in predicting the prognosis of human CSCC.
DISCUSSION
Here we have evaluated miRNA expression and histopathological tumour features of human CSCC, and its association with local recurrence, lymph node dissemination and metastasis to predict clinical prognosis. MiR-205 expression in the primary tumour was associated with local recurrence (Tables 1B and 2A). We have also demonstrated the differential localization of miR-203 and miR-205 in human CSCC (Figures 2B–E), and have evaluated the associations of the expression pattern of miR-203 and miR-205 with pathological traits of CSCC. Overall, miR-203 and miR-205 were associated with different tumour traits in a mutually exclusive manner (Supplementary Tables S4 and S5). Thus, miR-205 was associated with clinical and histopathological variables of poor outcome.
Not much is known about the role of miR-205 in the pathogenesis of CSCC. It has been pointed out that miR-205 expression is higher in CSCC than in normal skin 15, in which it is restricted to basal layer progenitors. MiR-205 maintains epithelial proliferation during the development of skin 41, and the lack of expression of miR-205 inhibits the proliferation of cells of the basal layer. Although it has been described that miR-205 inhibits EMT through the inhibition of ZEB factors 39, we observed the expression of miR-205 in undifferentiated areas, zones of perineural invasion and along the invasion front in CSCC (Figures 2C–F and Supplementary Tables S4 and S5). This could be in agreement with the fact that a pure EMT is rarely observed in the invasion front of epithelial tumours of human origin. In fact, while a complete EMT is accepted as a critical step during embryogenesis, its participation in carcinoma metastasis is debated by several authors 44–46.
Here we observed a statistical association between miR-205 expression and local recurrence. Moreover, miR-205 expression was the only independent variable selected by a logistic regression model to define general events of poor evolution of CSCC (Table 2A). These facts, together with the expression of miR-205 in undifferentiated zones and in the invasion front suggest a protumoural role for miR-205 in the pathogenesis of CSCC. MiR-205 would probably help to maintain a poorly differentiated and more aggressive epithelial phenotype in the tumours, but at the same time would be an epithelial marker and would inhibit the whole mesenchymal transformation. It has been described that miR-205 could activate AKT in normal skin 41 and in carcinomas of other origins 47–49. This is also consistent with the inverse correlation between miR-205 and the positive correlation with P63, found in this study, and in agreement with previous works 22, 50, 51. Recently it was demonstrated that EMT occurs in parallel with AKT activation during CSCC invasion 52.
Despite the important role of miR-203 in keratinocyte homeostasis 21, its role in CSCC has not been previously studied. Here, we did not find statistical association with events of poor clinical evolution, but according to our data, miR-203 helps to identify a subgroup of CSCC with a better prognosis (Table 2B and Supplementary Figure 1). This could be in agreement with the fact that miR-203, which has an antitumoural effect in basal cell carcinomas 14, has been observed as being repressed in CSCC in mice 26, and also inhibits epithelial to mesenchymal transition 24. In conclusion, miR-203 could behave as a tumour suppressor in human CSCC, but additional studies are needed to clarify this possibility. An interesting aspect was the mutually exclusive pattern of expression between miR-203 and miR-205 in tumours, depending on the degree of differentiation, and in the invasion front (Figure 2 and Supplementary Tables S4 and S5).
Finally, we identified three clusters of tumours with different prognoses by integrating the expression pattern of these miRNAs with clinical and pathological parameters. Although these clusters encompassed different pathological variables, they were well defined by the percentage of cases that expressed miR-203 and miR-205, and indeed, miR-203 and miR-205 sorted tumours in terms of prognosis more accurately than histopathological variables alone. Thus, these miRNAs could be used as markers of prognosis in CSCC. Finally, taking into account that miRNAs are master molecules that affect different processes in cellular homeostasis through the regulation of multiple proteins, miRNA-based targeted therapies might be more effective than those directed towards a single protein. Accordingly, they may become interesting potential therapeutic targets in CSCC.
Supplementary Material
Acknowledgments
We thank Dr. Balmain for the panel of skin cancer cell lines. JPL was partially supported by FEDER and MICINN (PLE2009-119, SAF2014-56989-R), Instituto de Salud Carlos III (PI07/0057, PI10/00328, PIE14/00066), Junta de Castilla y León (SA078A09, CSI034U13, CSI001U16), the “Eugenio Rodríguez Pascual”, the “Fundación Inbiomed” (Instituto Oncológico Obra Social de la Caja Guipozcoa-San Sebastian, Kutxa), IBSAL (IBY15/00003), and the “Fundación Sandra Ibarra de Solidaridad frente al Cáncer”. C R-C is funded by Q3718001E (2009-2010) y GRS 612/A/11 (2011-2012) and “the Fundación Eugenio Rodríguez Pascual”. AC was supported by FIS (PI07/0057) and MICINN (PLE2009-119). JHM was supported by the National Institutes of Health, a National Cancer Institute grant (R01 CA116481), and the Low-Dose Scientific Focus Area, Office of Biological & Environmental Research, US Department of Energy (DE-AC02-05CH11231). We thank Dr. Sánchez-García for useful comments of the manuscript and Nicholas Skinner and Emma Keck from the University of Salamanca for their help in English editing. We apologize to the many colleagues whose work could not be cited due to space restrictions. JA partially was supported by Gerencia Regional de Salud (Junta de Castilla y León), GRS 1342 / A / 16.
ABBREVIATIONS
- CSCC
Cutaneous Skin Cell Carcinoma
- miR
miRNA
Footnotes
COMPETING INTERESTS
The authors state no conflict of interest.
REFERENCES
- 1.Motley R, Kersey P, Lawrence C. Multiprofessional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. Br J Dermatol. 2002;146:18–25. doi: 10.1046/j.0007-0963.2001.04615.x. [DOI] [PubMed] [Google Scholar]
- 2.Preston DS, Stern RS. Nonmelanoma cancers of the skin. N Engl J Med. 1992;327:1649–1662. doi: 10.1056/NEJM199212033272307. [DOI] [PubMed] [Google Scholar]
- 3.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Housman TS, Feldman SR, Williford PM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48:425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 5.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 6.Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer--the role of sunlight. Adv Exp Med Biol. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- 7.Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 10.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Day E, Lal A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Res. 2010;12:201. doi: 10.1186/bcr2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang A, Landen NX, Meisgen F, et al. MicroRNA-31 is overexpressed in cutaneous squamous cell carcinoma and regulates cell motility and colony formation ability of tumor cells. PLoS One. 2014;9:e103206. doi: 10.1371/journal.pone.0103206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu N, Zhang L, Meisgen F, et al. MicroRNA-125b down-regulates matrix metallopeptidase 13 and inhibits cutaneous squamous cell carcinoma cell proliferation, migration, and invasion. J Biol Chem. 2012;287:29899–29908. doi: 10.1074/jbc.M112.391243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonkoly E, Loven J, Xu N, et al. MicroRNA-203 functions as a tumor suppressor in basal cell carcinoma. Oncogenesis. 2012;1:e3. doi: 10.1038/oncsis.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruegger C, Kempf W, Spoerri I, et al. MicroRNA expression differs in cutaneous squamous cell carcinomas and healthy skin of immunocompetent individuals. Exp Dermatol. 2013;22:426–428. doi: 10.1111/exd.12153. [DOI] [PubMed] [Google Scholar]
- 16.Dziunycz P, Iotzova-Weiss G, Eloranta JJ, et al. Squamous cell carcinoma of the skin shows a distinct microRNA profile modulated by UV radiation. J Invest Dermatol. 2010;130:2686–2689. doi: 10.1038/jid.2010.169. [DOI] [PubMed] [Google Scholar]
- 17.Navarro P, Gomez M, Pizarro A, et al. A role for the E-cadherin cell-cell adhesion molecule during tumor progression of mouse epidermal carcinogenesis. J Cell Biol. 1991;115:517–533. doi: 10.1083/jcb.115.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linardopoulos S, Street AJ, Quelle DE, et al. Deletion and altered regulation of p16INK4a and p15INK4b in undifferentiated mouse skin tumors. Cancer Res. 1995;55:5168–5172. [PubMed] [Google Scholar]
- 19.Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat Cell Biol. 2002;4:487–494. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- 20.Wong CE, Yu JS, Quigley DA, et al. Inflammation and Hras signaling control epithelial-mesenchymal transition during skin tumor progression. Genes Dev. 2013;27:670–682. doi: 10.1101/gad.210427.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lena AM, Shalom-Feuerstein R, Rivetti di Val Cervo P, et al. miR-203 represses 'stemness' by repressing DeltaNp63. Cell Death Differ. 2008;15:1187–1195. doi: 10.1038/cdd.2008.69. [DOI] [PubMed] [Google Scholar]
- 22.Tran MN, Choi W, Wszolek MF, et al. The p63 protein isoform DeltaNp63alpha inhibits epithelial-mesenchymal transition in human bladder cancer cells: role of MIR-205. J Biol Chem. 2012;288:3275–3288. doi: 10.1074/jbc.M112.408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi R, Poy MN, Stoffel M, et al. A skin microRNA promotes differentiation by repressing 'stemness'. Nature. 2008;452:225–229. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taube JH, Malouf GG, Lu E, et al. Epigenetic silencing of microRNA-203 is required for EMT and cancer stem cell properties. Sci Rep. 2013;3:2687. doi: 10.1038/srep02687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nissan X, Denis JA, Saidani M, et al. miR-203 modulates epithelial differentiation of human embryonic stem cells towards epidermal stratification. Dev Biol. 2011;356:506–515. doi: 10.1016/j.ydbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Gastaldi C, Bertero T, Xu N, et al. miR-193b/365a cluster controls progression of epidermal squamous cell carcinoma. Carcinogenesis. 2014;35:1110–1120. doi: 10.1093/carcin/bgt490. [DOI] [PubMed] [Google Scholar]
- 27.Viticchie G, Lena AM, Cianfarani F, et al. MicroRNA-203 contributes to skin re-epithelialization. Cell Death Dis. 2012;3:e435. doi: 10.1038/cddis.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gubian S SAaSP. ExiMiR: R functions for the normalization of Exiqon miRNA array data. R package version 2.10.0. 2012. [Google Scholar]
- 29.R-Development-Core-Team. R Foundation for Statistical Computing. Vienna, Austria: 2010. A language and environment for statistical computing. [Google Scholar]
- 30.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 31.Demey JR, Vicente-Villardon JL, Galindo-Villardon MP, et al. Identifying molecular markers associated with classification of genotypes by External Logistic Biplots. Bioinformatics. 2008;24:2832–2838. doi: 10.1093/bioinformatics/btn552. [DOI] [PubMed] [Google Scholar]
- 32.Brantsch KD, Meisner C, Schonfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9:713–720. doi: 10.1016/S1470-2045(08)70178-5. [DOI] [PubMed] [Google Scholar]
- 33.Karia PS, Jambusaria-Pahlajani A, Harrington DP, et al. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women's Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2013;32:327–334. doi: 10.1200/JCO.2012.48.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cherpelis BS, Marcusen C, Lang PG. Prognostic factors for metastasis in squamous cell carcinoma of the skin. Dermatol Surg. 2002;28:268–273. doi: 10.1046/j.1524-4725.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 35.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2013;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Yu J, Peng H, Ruan Q, et al. MicroRNA-205 promotes keratinocyte migration via the lipid phosphatase SHIP2. FASEB J. 2010;24:3950–3959. doi: 10.1096/fj.10-157404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang N, Li Q, Feng NH, et al. miR-205 is frequently downregulated in prostate cancer and acts as a tumor suppressor by inhibiting tumor growth. Asian J Androl. 2013;15:735–741. doi: 10.1038/aja.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gregory PA, Bracken CP, Bert AG, et al. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 40.Wei T, Orfanidis K, Xu N, et al. The expression of microRNA-203 during human skin morphogenesis. Exp Dermatol. 2010;19:854–856. doi: 10.1111/j.1600-0625.2010.01118.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang D, Zhang Z, O'Loughlin E, et al. MicroRNA-205 controls neonatal expansion of skin stem cells by modulating the PI(3)K pathway. Nat Cell Biol. 2013;15:1153–1163. doi: 10.1038/ncb2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frierson HF, Jr, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol. 1986;17:346–354. doi: 10.1016/s0046-8177(86)80457-9. [DOI] [PubMed] [Google Scholar]
- 43.Veness MJ. High-risk cutaneous squamous cell carcinoma of the head and neck. J Biomed Biotechnol. 2007;2007:80572. doi: 10.1155/2007/80572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. discussion-1. [DOI] [PubMed] [Google Scholar]
- 45.Garber K. Epithelial-to-mesenchymal transition is important to metastasis, but questions remain. J Natl Cancer Inst. 2008;100:232–233. doi: 10.1093/jnci/djn032. 9. [DOI] [PubMed] [Google Scholar]
- 46.Ledford H. Cancer theory faces doubts. Nature. 2011;472:273. doi: 10.1038/472273a. [DOI] [PubMed] [Google Scholar]
- 47.Cai J, Fang L, Huang Y, et al. miR-205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non-small cell lung cancer. Cancer Res. 2013;73:5402–5415. doi: 10.1158/0008-5472.CAN-13-0297. [DOI] [PubMed] [Google Scholar]
- 48.Lei L, Huang Y, Gong W. miR-205 promotes the growth, metastasis and chemoresistance of NSCLC cells by targeting PTEN. Oncol Rep. 2013;30:2897–2902. doi: 10.3892/or.2013.2755. [DOI] [PubMed] [Google Scholar]
- 49.Qu C, Liang Z, Huang J, et al. MiR-205 determines the radioresistance of human nasopharyngeal carcinoma by directly targeting PTEN. Cell Cycle. 2012;11 doi: 10.4161/cc.11.4.19228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gandellini P, Folini M, Longoni N, et al. miR-205 Exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cepsilon. Cancer Res. 2009;69:2287–2295. doi: 10.1158/0008-5472.CAN-08-2894. [DOI] [PubMed] [Google Scholar]
- 51.Tucci P, Agostini M, Grespi F, et al. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci U S A. 2012;109:15312–15317. doi: 10.1073/pnas.1110977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barrette K, Van Kelst S, Wouters J, et al. Epithelial-Mesenchymal Transition during invasion of cutaneous Squamous Cell Carcinoma is paralleled by AKT activation. Br J Dermatol. 2014 doi: 10.1111/bjd.12967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.