Abstract
The pathologic crosstalk between the heart and kidney is known as cardiorenal syndrome (CRS). While the specific mechanisms underlying this crosstalk remain poorly understood, CRS is associated with exacerbated dysfunction of either or both organs and reduced survival. Maladaptive fibrotic remodeling is a key component of both heart and kidney failure pathogenesis and progression.
G-protein coupled receptor (GPCR) signaling is a crucial regulator of cardiovascular and renal function. Chronic/pathologic GPCR signaling elicits the interaction of the G-protein Gβγ subunit with GPCR kinase 2 (GRK2), targeting the receptor for internalization, scaffolding to pathologic signals, and receptor degradation. Targeting this pathologic Gβγ-GRK2 interaction has been suggested as a possible strategy for the treatment of HF. In the current review, we discuss recent updates in understanding the role of GPCR-Gβγ-GRK2 signaling as a crucial mediator of maladaptive organ remodeling detected in HF and kidney dysfunction, with specific attention to small molecule-mediated inhibition of pathologic Gβγ-GRK2 interactions. Further, we explore the potential of GPCR-Gβγ-GRK2 signaling as a possible therapeutic target for cardiorenal pathologies.
Keywords: Heart Failure, Kidney injury, Cardiorenal syndrome, Fibrosis, Signal Transduction
1. Introduction
Cardiovascular diseases (CVD) involve heart and blood vessels and include coronary artery disease (CAD), stroke, hypertension, congenital heart disease, cardiomyopathy, etc. [1]. CVD is the leading cause of death worldwide that accounts for more than 17.3 million deaths per year [2]. In 2013, CVD represented about one of every three deaths in America. Over 85 million Americans are living with some type of CVD or the after-effects of stroke. Projected costs of CVD including the cost of health care services, medications and loss of productivity totals more than $600 billion in 2015, and it is expected to grow to more than $1200 billion by 2030 [3].
Heart failure (HF) is a chronic, progressive condition in which the heart muscle is unable to pump enough blood to meet the body’s metabolic demands. HF arises as the final manifestation of many CVDs such as coronary artery disease, congenital malformations and hypertension. About 5.7 million adults in the United States are affected by this debilitating disease [3]; HF treatment costs the nation an estimated $30.7 billion each year [4]. Notwithstanding significant advances in HF treatment and management realized with β-adrenergic receptor (β-AR) blockers, angiotensin receptor blockers, angiotensin converting enzyme (ACE) inhibitors, aldosterone inhibitors, and diuretics, conventional pharmacological therapies only impede the progression and death due to HF, but do not cure it causatively [5]. Taking into consideration the steady growth of aging and diabetic populations, deeper understanding of the molecular and cellular processes that contribute to the disease pathogenesis, along with development of innovative therapeutic strategies allowing the causative cure of HF, are indispensable.
Multiple pathophysiological mechanisms contribute to HF development and progression, including neurohumoral activation [6], G-protein-coupled receptor desensitization and down-regulation [7], [8] and [9], and extracellular matrix (ECM) mediated pathologic remodeling [10]. Moreover, cardiac pathologies in HF are frequently accompanied by worsening renal function, which is known to be a strong predictor of increased mortality in HF patients [11] and [12]; this is defined as Cardiorenal syndrome (CRS) type II. In the present review, we discuss recent advances in exploring GPCR signaling as a possible therapeutic target in cardiac disease and as a potential link between failing heart and kidney, with the particular emphasis on small molecule targeting of G-protein βγ subunit - GPCR kinase 2 (Gβγ-GRK2) components of GPCR signaling.
2. G-protein-coupled receptor signaling
G-protein-coupled receptors (GPCRs), also known as seven-transmembrane domain receptors, represent a conserved family of receptors that sense molecules outside the cell that activate intracellular signal transduction pathways and consecutive cellular responses. GPCRs are integral proteins comprised of an extracellular N-terminus, seven transmembrane (7-TM) α-helixes (TM-1 to TM-7) connected by three intracellular (IL-1 to IL-3) and three extracellular loops (EL-1 to EL-3), and an intracellular C-terminus [13]. Ligand binding to an extracellular active site of the receptor induces a conformational change in the GPCR which allows for coupling with heterotrimeric guanine-nucleotide regulatory proteins (G-proteins) [14]. G-proteins are heterotrimers of α, β and γ subunits known as Gα, Gβ and Gγ, respectively. The heterotrimeric G-proteins are rendered inactive when reversibly bound to Guanosine diphosphate (GDP) but active when bound to Guanosine triphosphate (GTP) [15]. Receptor activation facilitates the exchange of GDP for GTP on Gα subunits that result in dissociation of the Gα from Gβγ subunits to mediate downstream signaling pathways [16]. Dissociated Gα subunits signal via activation of an effector molecule, such as adenyl cyclase (AC) or phospholipase C β (PLCβ) to produce second messengers such as cyclic adenosine 3′, 5′ monophosphate (cAMP), diacylglycerol (DAG), or inositol 1, 4, 5-triphosphate (IP3), respectively. These second messengers modulate a variety of downstream processes, particularly regulation of contractility, hypertrophy, and apoptosis in the heart [15]. Gα proteins are classified into the families Gαs, Gαi, Gαq, and Gα11/12 [15] with respect to downstream signaling molecules and modulated physiological processes. Dissociated Gβγ subunits target a wide range of signaling pathways involved in receptor desensitization and down-regulation, ion channel activation, enzyme activity modulation, cell division, transcription and cellular organelle function [17], [18], [19] and [20].
GPCRs respond to extracellular signaling mediated by an extensive amount of agonists such as hormones, proteins and lipids, and participate in a comprehensive variety of physiological processes [21]. In particular, GPCRs play an important role in local and systemic regulation of cardiac function. Specifically, cardiac β-adrenergic receptors (β-ARs) are prominent regulators of cardiovascular chronotropy and inotropy [22] and [23]. Furthermore, GPCRs mediate a variety of functions in the kidney, and inappropriate activation and regulation of GPCRs may lead to kidney disease [24]. In this review, we focus on Gβγ-mediated signaling as a crucial component of HF pathogenesis and as a potential therapeutic target in cardiorenal pathologies.
3. β-Adrenergic receptor signaling in healthy and diseased heart
As mentioned above, cardiac β-ARs represent crucial regulators of cardiac contractile function. In response to sympathetic nervous system (SNS) activity released via mediators, catecholamines (CA) epinephrine (Epi, also named adrenaline) and norepinephrine (NEpi, also named noradrenaline), β-ARs modulate the rate and force of myocardial contractions [8]. There are three β-AR subtypes identified in mammalian hearts: β1, β2, and β3-ARs [25]. Both β1- and β2-ARs are coupled to the downstream excitatory Gαs protein, which generally results in the activation of adenylyl cyclase (AC) and the generation of cyclic AMP (cAMP), eliciting positive chronotropic and inotropic responses. Upon chronic stimulation,β2-ARs also couple to the inhibitory Gαi protein, which has been reported to exert a cardioprotective effect during cardiac injury [26].
In healthy human myocardium, the predominant β-ARs subtypes are the β1- and β2-ARs, which are present in an approximate 80:20 ratio, respectively with only a relatively minor contribution of β3-ARs [27]. Under physiological conditions, β-ARs account for regulation of both heart rate and contractility [14, 28]. In HF pathogenesis, excess SNS activation and subsequent catecholamine overdrive is initiated as an adaptation to compensate for decreased heart rate and cardiac contractility and to maintain mean arterial pressure (MAP) [29]. Initially, the elevated SNS activity increases heart rate and contractility through β-AR stimulation. However, maladaptive effects of the elevated SNS activity including myocardial ischemia, pathologic hypertrophy, arrhythmogenicity, myocardial necrosis and apoptosis contribute substantially to disease progression [22], [30], [31] and [32]. This maladaptive response results partially from down-regulation and desensitization of cardiac β-ARs due to chronic CA stimulation [15]. In failing hearts, heightened CA β-AR stimulation induces selective down-regulation of β1-ARs and consequent alteration of the β1-AR to β2-AR ratio from an 80:20 distribution to a ratio of 60:40 [27, 33]; the remaining β1-ARs and β2-ARs in failing hearts prevail in a desensitized condition [30].
Cardiac β-AR signaling regulation involves activation-dependent and -independent mechanisms of desensitization [8]. Homologous, agonist-mediated, activation-dependent desensitization is accomplished by an active form of a G-protein-coupled receptor kinase (GRK) that is translocated to the adrenergic receptor after binding with the activated membrane-associated Gβγ subunit to phosphorylate the agonist-occupied receptor [34]. An alternative, activation-independent pathway, known as heterologous desensitization, is accomplished through the activity of a downstream signaling product of β-AR activation or other GPCR signaling events. In both cases, phosphorylated β-AR is bound by β-arrestin molecules which block the access of heterotrimeric G proteins to the receptor thereby uncoupling it and attenuating βAR signaling in the heart [35, 36].
4. Gβγ-GRK2 signaling manipulation as a strategy to treat cardiac disease
4.1. GRK2: structure, subcellular localization and function in the heart
GRK2 (aka β-adrenergic receptor kinase, βARK) belongs to a family of serine/threonine kinases that share common structural and functional features. Seven mammalian GRKs that have been characterized so far are classified into three subfamilies according to their sequence and structural similarity: (1) the rhodopsin kinase subfamily (GRK1 and GRK7); (2) the βARK subfamily (GRK2 and GRK3); and (3) the GRK4-like subfamily (GRK4, GRK5, GRK6) [37]. Within the cardiovascular system, GRKs 2, 3 and 5 are known to be expressed and play a role in GPCR phosphorylation [38], with GRK2 as a predominant GRK isoform in the heart [39].
GRKs are characterized by a tri-domain structure, with the conserved central catalytic domain and two flanking domains variable in structure in different GRK subfamilies [40]. GRK2’s amino (N)-terminal domain that is responsible for receptor recognition and activity regulation contains a regulator of G protein signaling (RGS) homology (RH) domain that has been demonstrated to interact with Gαq proteins [41]. The carboxyl (C)-terminal domain of GRK2 determines membrane targeting and subcellular localization of the enzyme. This domain contains a pleckstrin homology (PH) domain that binds Gβγ subunits [42]. Under basal conditions, GRK2 is distributed primarily in the cytoplasm. Upon GPCR activation, GRK2 is translocated to the plasma membrane via binding with the activated Gβγ subunits. GRK2-mediated phosphorylation of the GPCR causes β-arrestin recruitment to the receptor and consequent inhibition of dissociated G-proteins from coupling to the receptor/β-arrestin complex and further attenuation of downstream signaling [43]. Moreover, β-arrestin-bound receptors are targeted for clathrin-coated pits in the cell membrane that are internalized and either degraded in intracellular lysosomes or recycled back to the cell surface [44].
Apart from the classical mechanism of modulating GPCR signaling in the heart and extracardiac tissues, GRK2 may have other functions independent of GPCR phosphorylation. Recently emerging data suggest the concept of an extensive “GRK2 interactome” that refers to GRK2 interactions with other intracellular proteins such as α-actinin, clathrin, calmodulin, caveolin, tubulin, Akt, HDAC6 and ERK1/2 [39] and [45]. Investigation of GRK2 functions beyond GPCR desensitization and down-regulation may provide new insights in understanding its role in disease pathogenesis. In the current review, we highlight recent updates relevant for GPCR- Gβγ signaling in HF modulation.
Understanding of the in vivo function of GRK2, particularly its role in cardiovascular system function and development, emerged from gene knockout studies. In 1996, Jaber et al demonstrated lethality of GRK2 homozygous knockout (KO) in mouse models [46]. These animals exhibited hypoplasia of the ventricular myocardium and a 70% decrease in ejection fraction and died by gestational day 15.5, presumably owing to HF. Further studies demonstrated that specific deletion of GRK2 in murine embryonic cardiomyocytes utilizing Cre recombinase expressed under the control of the Nkx2.5 promoter did not cause any apparent developmental abnormalities, suggesting that embryonic lethality of GRK2−/− mice might result from extracardiac or non-cardiomyocyte effects [47].
Cardiomyocytes from adult global heterozygous GRK2 KO mice exhibited significantly enhanced cardiac contractile function compared to wild-type cells [48]. Cardiac-specific overexpression of GRK2 following myocardial ischemia/reperfusion injury (I/R) [49] caused reduced β-adrenergic signaling mediated cardiac contractility and function along with increased apoptosis [50]. These observations demonstrated that cardiac contractile function can be modulated by the level of GRK2 activity. To further evaluate the role of GRK2 in adult cardiac function, two conditional models of GRK2 ablation were generated: αMHC-Cre/GRK2flox/flox for targeted KO of GRK2 specifically in cardiomyocytes in the constitutive way (at birth) and αMHCMerCreMer/GRK2flox/flox for tamoxifen-induced cardic deletion [51]. Both models resulted in positive outcomes following cardiomyocyte-restricted GRK2 ablation after myocardial infarction (MI); the αMHC-Cre/GRK2flox/flox mice exhibited prevention of HF development post-MI and the αMHCMerCreMer/GRK2flox/flox mice demonstrated improved cardiac function and induced positive reverse remodeling following MI. Moreover, cardiomyocyte GRK2 KO in post-MI mice showed reduced mortality levels. Cardioprotective effects demonstrated by αMHCMerCreMer/GRK2flox/flox mice were significantly better compared to the results when the β-blocker metoprolol was used for treatment of post-MI wild-type (WT) mice over the same time period [51].
A recent study conducted by Woodall and colleagues aimed to determine the implications of GRK2 deletion in cardiac fibroblasts (CFs) prior to myocardial injury [52]. CFs significantly contribute to multiple aspects of cardiac function and disease [53], particularly via modulation of fibrotic remodeling in the heart following injury [10], [54] and [55]. To examine the consequences of GRK2 loss in CFs for cardiac function, authors used tamoxifen-inducible collagen1α2-CreER(T)/GRK2fl/fl mice (GRK2 fKO mice). GRK2 ablation was achieved weeks prior to in vivo acute myocardial ischemia/reperfusion (I/R) injury [56]. GRK2 fKO mice subjected to myocardial I/R injury exhibited advantageous effects of pre-injury GRK2 ablation in CFs including decreased infarct size, fibrosis and apoptosis, reduced neutrophil extravasation and tumor necrosis factor α (TNFα) expression and secretion along with restored cardiac function. Collectively, GRK2 gene knockout studies highlighted the beneficial effects of GRK2 ablation in cardiomyocytes and CFs after myocardial injury and elicited a proposal that GRK2 should be considered as a therapeutic target for HF treatment.
4.2. GRK2 expression in cardiac disease
The first link between GRK2 and β-adrenergic receptor signaling desensitization and down-regulation was established in 1993, when Ungerer et al demonstrated significant elevation of GRK2 in explanted failing human hearts at mRNA, protein and activity levels [33]. These observations initiated a series of studies performed on animal models or human tissue that aimed to delineate the role of GRK2 in cardiac disease [57]. Particularly, cardiac overexpression of GRK2 was demonstrated to be capable of direct HF induction in experimental animal models; moreover, mice overexpressing GRK2 exhibited decreased isoproterenol (Iso)-stimulated left ventricular contractility in vivo, diminished myocardial AC activity, and reduced functional coupling of β-ARs [58]. Furthermore, GRK2 expression and activity were found to be elevated in human cardiac tissue and in circulating lymphocytes, demonstrating direct correlation with the severity of HF [59] and [60]. More recent studies showed that the changes of GRK2 levels in peripheral lymphocytes mirror changes in the salutary LVAD-supported failing human heart and that these changes correlate strongly with cardiac function such that lower levels of GRK2 are associated with improved β-adrenergic signaling and myocardial function in mechanically supported failing hearts and transplanted human hearts [61] and [62]. The recent study conducted by Rengo and colleagues involved over 200 patients with HF and demonstrated that GRK2 lymphocyte level has a prognostic value for outcomes and mortality in HF patients, thus supporting the hypothesis that GRK2 levels in blood can be used as a biomarker in HF [63].
Overall, the aforementioned studies suggest consideration of GRK2 as a therapeutic target for cardiac disease and as a potential biomarker for heart function [64], [65] and [66].
4.3. Recombinant proteins as a way to inhibit Gβγ-GRK2 signaling
Excess cardiac Gβγ-mediated signaling leading to chronic β-AR desensitization and down-regulation is a crucial component of HF pathophysiology [67]. Thus, several approaches, including genetic manipulations and pharmacological targeting, have been explored to interdict pathologic Gβγ-GRK2 signaling.
The first reported approach utilized a recombinant carboxyl (C)-terminal fragment of GRK2 comprised of 194 amino acids encoding the Gβγ binding domain (βARKct) as a presumed inhibitor of Gβγ-GRK2 interactions. In 1994, βARKct was expressed in COS-7 cells where it attenuated Gβγ mediated signaling with unaffected Gα mediated signaling, indicating its ability to discriminate between Gα and Gβγ pathways [68]. Subsequently, in 1995 Koch et al demonstrated enhanced baseline cardiac contractility in vivo with or without Iso stimulation in transgenic mice with cardiac-specific GRK2 overexpression [58]. Additionally, βARKct was demonstrated to normalize β-adrenergic signaling and cardiac function in hybrid transgenic mice with cardiac-specific concomitant overexpression of both GRK2 and βARKct [69].
Further studies demonstrated salutary effects of βARKct on the recovery of failing myocytes function [70], [71] and [72] and prevention of cardiac dysfunction [73]. Oligonucleotide microarray left ventricular (LV) gene expression analysis performed in normal, failing and βARKct overexpressing (“rescued”) cardiac samples revealed the ability of βARKct to normalize gene expression changes associated with HF [74]. More recent studies performed in large animal HF models revealed preservation and amelioration of cardiac function along with normalization of CA signaling owing to stable myocardial βARKct gene delivery [75] and [76], suggesting that inhibition of Gβγ-GRK2 interactions with recombinant viral-delivered βARKct peptide is a promising therapy for HF treatment [66].
4.4. Small molecule interdiction of Gβγ-GRK2 signaling
βARKct inhibition of Gβγ-GRK2 interactions has demonstrated salutary effects on cardiac function in both acute and chronic models of HF, however, viral-based gene delivery remains a daunting therapeutic approach. In that perspective, small molecule inhibitors that could be administered systemically may represent an alternative approach to attenuate pathologic components of Gβγ-GRK2 signaling or interactions [64] and [66].
One of the described approaches to small molecule pharmacological inhibition of GRK2 signaling is paroxetine, the selective serotonin reuptake inhibitor, identified by Thal et al in 2012 [77]. This antidepressant drug binds to the active site of GRK2 and stabilizes the kinase domain, thereby inhibiting the downstream signaling. This study demonstrated increased contractility in isolated cardiomyocytes in the presence of paroxetine. Further, paroxetine was tested in vivo in a mouse model of MI. Schumacher et al recently showed that paroxetine treatment initiated two weeks post-MI results in improved cardiac function, limited ventricular remodeling and normalized SNS overdrive along with myocardial β-adrenergic system [78]. Thus, direct GRK2 pharmacological inhibition demonstrated salutary effects on HF progression.
Another potential strategy to interdict Gβγ-GRK2 pathologic signaling is targeting Gβγ subunit and inhibiting its protein-protein interactions [5] and [66]. Hence, Bonacci et al in 2006 performed a virtual screening of 1990 compounds from the National Cancer Institute (NCI) chemical library to identify small molecules capable of binding Gβγ protein interaction domain mentioned above [79]. Eighty-five identified compounds were further tested in an enzyme-linked immunosorbent assay (ELISA) for their ability to compete with a phage-displaying SIRK peptide derivative (SIGK) [80] for binding to Gβγ subunit. Among several tested compounds, one termed M119 (cyclohexanecarboxylic acid [2-(4,5,6-trihydroxy-3-oxo-3H-xanthen-9-yl)-(9Cl)]) demonstrated high apparent affinity for the Gβγ subunit and inhibited Gβγ-SIGK binding in vitro. Moreover, pretreatment of differentiated HL-60 leukocytes with M119 resulted in interference with Gβγ binding to GRK2 and consequent inhibition of GRK2 translocation to the membrane, along with suppression of PLCβ2/3 and PI3Kγ activation by Gβγ. Thus, the small molecule M119 confirmed its ability to interfere with Gβγ-mediated signaling downstream of GPCRs.
Since Gβγ subunits are known to modulate a majority of signaling pathways, the inhibitor that selectively influences a particular subset of Gβγ interactions is needed for Gβγ-GRK2 targeting [28]. Thus, M119 was examined in vivo for efficacy and specificity. It has been demonstrated that inhibition of PLCβ3 that is activated by Gβγ subunits is associated with enhanced morphine-induced antinociception [81]. Co-administration of M119 with μ-opioid receptor agonist morphine resulted in substantial increase of morphine-dependent antinociception in wild-type mice due to M119-induced inhibition of Gβγ-PLCβ3 interactions, whereas M119 alone had no effects on antinociception [79]. Also, M119 demonstrated no effects on morphine-dependent antinociception in PLCβ3−/− mice. Further studies showed that M119 increases analgetic potencies of morphine or μ-selective peptide, whereas it does not have any significant influence on analgesia induced by κ- or δ-opioid receptor agonists [82], corroborating the suggestion that M119 acts as a specific inhibitor of a particular subset of Gβγ-mediated signaling. Accordingly, we have conducted various studies to evaluate the potential of small molecule inhibition of Gβγ-GRK2 associations in different animal HF models.
4.5. Gβγ inhibitory treatment in acute and advanced heart failure models
Taking into account the aforementioned role of β-AR-dependent Gβγ-GRK2 signaling in cardiac disease pathogenesis and the proven efficiency of small molecule inhibitor M119 in selective interdiction of Gβγ-GRK2 interactions, we explored the effects of Gβγ signaling small molecule disruption in myocardial cells and in murine models of HF [83]. In this study, M119 or its highly homologous, more chemically stable analogue gallein was utilized to inhibit Gβγ-GRK2 interactions. M119 pretreatment followed by administration of the β-AR agonist Iso significantly enhanced AC activity and consequent cAMP generation in isolated cardiomyocytes from adult wild-type mice. Moreover, M119 increased the rate of cardiomyocyte contraction alone and in combination with Iso. Importantly, the β-AR antagonist propranolol abolished the effect of M119 and Iso on cardiomyocyte contractility, confirming the selectivity of the compound for β-AR-Gβγ signaling. In addition, both M119 and gallein demonstrated the ability to reduce GRK2 recruitment to the membrane of cardiomyocytes induced by Iso treatment.
To examine cardiac-specific effects of Gβγ inhibitory treatment initiated at the onset of HF, an acute pharmacologic murine model of HF [84] was implemented. Chronic β-AR stimulation by Iso delivered via implantable miniosmotic pumps was started simultaneously with systemic administration of M119 or vehicle and continued for 7 days. M119 treatment mitigated HF progression; particularly, M119-treated mice maintained essentially normal cardiac function and showed significantly reduced cardiac hypertrophy along with decreased level of interstitial and perivascular fibrosis, compared to vehicle-treated animals. Considering these data, Gβγ small molecule inhibition was administered after the onset of HF, in a transgenic mouse model of established HF generated by cardiac restricted calsequestrin (CSQ) overexpression [85]. Importantly, the CSQ transgenic mouse model recapitulates essential hallmarks of HF, including pathologic β-AR signaling [83]. One month of daily gallein administration resulted in prevention of HF progression, especially in normalized echocardiographic parameters, reduced pathologic cardiac hypertrophy and diminished expression of HF molecular markers. Moreover, M119 and gallein significantly reduced pathologically increased cardiac GRK2 protein level in Iso-pumped and CSQ animals, respectively. Overall, this study demonstrated salutary effects of Gβγ small molecule inhibitory treatment on both manifestation and progression of HF.
Beneficial effects of small molecular inhibitors observed in acute pharmacological and transgenic mouse models of HF elicited further interest to investigation of cardiac and systemic effects of Gβγ signaling inhibition. Consequently, Kamal and colleagues in 2014 examined outcomes of utilizing the small molecule Gβγ inhibitor gallein in a transverse aortic constriction (TAC) mouse model of pressure-overload induced cardiac hypertrophy and HF [86]. The TAC surgical model, firstly validated by Rockman et al [87], is considered a relatively clinically relevant model of HF [88] and [89]. In this study, vehicle-treated mice developed the decline in cardiac function at 8 weeks post-TAC with the concomitant worsening at 12 weeks post-TAC. Daily gallein administration for eight weeks was initiated after the establishment of HF (four weeks post-TAC). This treatment regimen, initiated after the onset of HF, alleviated cardiac dysfunction and hypertrophy along with significantly enhanced survival in the gallein-treated group compared to the vehicle-treated group. Preservation of cardiac function was accompanied by the recovery of β-AR density and reduction of GRK2 gene expression and membrane translocation. Furthermore, membrane recruitment of phosphoinositide 3-kinase γ (PI3Kγ), which GPCR-induced activation was implicated in maladaptive cardiac hypertrophy and dysregulated β-AR function [28], [90], [91], [92] and [93], was reduced in gallein-treated mice compared with vehicle-treated mice. Gallein treatment also resulted in attenuated progression of cardiac hypertrophy and reduced myocardial fibrosis. Interestingly, ameliorated cardiac remodeling was accompanied by decreased phosphorylation of cardiac Akt (aka protein kinase B) and its downstream signal GSK-3β. As mentioned above, PI3Kγ-mediated GPCR dependent Akt activation and subsequent GSK-3β Ser-9 phosphorylation lead to cardiac hypertrophy [92], [94] and [95]. Of note, authors attributed the significantly reduced expression of the fetal genes atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) detected in gallein-treated TAC mice to elevated levels of nonphosphorylated GSK-3β that was suggested to negatively regulate transcription and protein translation of the hypertrophic genes [96]. Other detected beneficial effects of gallein treatment on TAC HF mice were attenuated cardiac inflammatory cytokine expression and reduced myocardial apoptosis. Collectively, these data corroborate the suggestion that Gβγ inhibitory treatment preserves cardiac function and halts HF progression in different small animal models of HF, in part via downstream inhibitory effects on cardiac fibrosis, hypertrophic gene expression and inflammation along with promotion of cell survival.
As discussed previously, compensatory sympathetic nervous system activation and subsequent systemic release of catecholamines (CA) from the adrenal gland medullary chromaffin cells increase the rate and the intensity of cardiac contractions in response to diminished cardiac output of the failing heart [97]. Adrenal chromaffin cell α2-ARs that belong to GPCR family are essential regulators of sympathetic outflow in HF, providing the feedback inhibition for CA release [32] and [98]. Lymperopoulos and colleagues showed that, similar to the dysregulation of β-AR signaling in failing hearts, the adrenal α2-ARs undergo desensitization and down-regulation in response to catecholamine overdrive and concomitant adrenal GRK2 upregulation, thus contributing to HF pathogenesis [99]. Further studies revealed that delivery of adenoviral vectors containing GRK2 to adrenal glands resulted in enhancement of plasma CA levels and failure of adrenal α2-ARs to inhibit CA secretion, whereas GRK2 inhibition using βARKct or chromaffin cell specific GRK2 gene deletion recovers adrenal α2-AR function, reduces CA release and attenuates HF progression [31], [99] and [100]. More recently, Jafferjee et al demonstrated that CA treatment of rat pheochromocytoma-derived or primary chromaffin cells results in GRK2 gene transcription upregulation and subsequent enhancement of α2-AR desensitization and down-regulation accompanied by elevated CA biosynthesis and release [101].
Taking into account the aforementioned role of adrenal α2-ARs in regulation of SNS activity in HF, we recently assessed effects of small molecule Gβγ signaling disruption on the adrenal gland in pathogenesis of pressure-overload induced HF [86]. Significantly decreased CA production and release, alleviated adrenal medulla hypertrophy and restored α2-AR feedback inhibition was observed 12 weeks post-TAC in the gallein-treated group compared to the vehicle-treated group. Additionally, cultured in vitro adrenal glands from gallein-treated mice exhibited significantly decreased levels of basal CA secretion. Moreover, the effects of gallein on CA generation and GRK2 expression were examined in cultured human pheochromocytoma tissue, a tumor characterized by increased CA production. Gallein treatment significantly reduced CA production in cultured pheochromocytoma slices as reflected by lowered expression of tyrosine hydroxylase, an enzyme that catalyzes the rate limiting step in CA synthesis [102], and chromogranin A, a neurokine that is synthesized and co-secreted with vesicular CA [103]. Importantly, authors observed downregulation of both GRK2 protein level and membrane translocation in cultured pheochromocytoma slices treated with gallein. Taken together, this study provides deeper insights into understanding of pathological mechanisms contributing to HF progression. In addition, the study suggested small molecule Gβγ inhibition as a potential systemic therapy that attenuates HF progression due to simultaneous inhibition of cardiac and adrenal Gβγ-GRK2 interactions [86] and [64] (see overview of approaches in Figure 1).
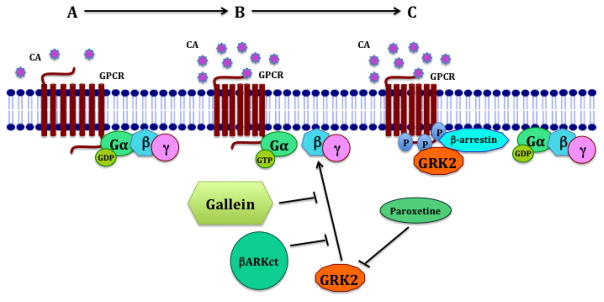
Figure 1. Novel strategies to target Gβγ-GRK2 interactions.
A: GPCR ligand binding, G-protein subunits dissociation, downstream signaling activation; B: SNS overactivation, GRK2 recruitment, GPCR phosphorylation, conformational change in GPCR; C: β-arrestin binding, G-proteins uncoupling, GPCR desensitization and downregulation, GPCR signaling attenuation. The uncoupled GPCR undergoes endocytosis and either degradation in proteasomes or recycling. Paroxetine stabilizes the kinase domain of GRK2 and thus inhibits its ability to phosphorylate GPCRs. βARKct is a recombinant peptide that represents the Gβγ binding domain of GRK2 and blocks Gβγ-GRK2 interactions. The small molecule gallein competitively binds with Gβγ subunits and inhibits its protein-protein interactions, thus attenuating downstream Gβγ-GRK2 signaling.
In view of the correlation between the elevated GRK2 activity in circulating lymphocytes and the severity of HF demonstrated in the recent studies [61], [62] and [63], further investigation will be needed to determine potential effects of Gβγ-GRK2 inhibitory treatment on GPCR-Gβγ-GRK2 signaling in circulating immune system cells. As Lehmann and colleagues showed, small molecule inhibition of Gβγ-dependent signaling with M119 and gallein blocks chemotaxis and neutrophil migration in vitro and suppresses neutrophil-dependent inflammation in a murine carrageenan-induced paw edema model [104]. Moreover, recent data demonstrated that GRK2 modulates T- and B-lymphocytes migration from circulatory fluids into lymphoid tissues and within the spleen via desensitization of sphingosine-1-phosphate receptor-1 (S1PR1) that is necessary for sphingosine-1-phosphate (S1P) gradient dependent movement of lymphocytes [105] and [106]. To address the role of GRK2 in immune cell migration, the authors utilized CD4-Cre/GRK2f/− and Mb1-Cre/GRK2f/− mice for GRK2 ablation in T and B cell populations, respectively. GRK2-deficient T and B cells displayed resistance to S1PR1 ligand-mediated desensitization and impaired ability to enter lymph nodes compared to the control WT cells. Further studies will be required to define the influence of Gβγ-GRK2 signaling small molecule disruption on circulating immune cell behavior as well as signaling pathways affected by Gβγ-GRK2 inhibition in immune cells.
As recently reviewed, GRK2 has been found to possess non-canonical activities including regulation of molecular pathways essential for cardiac physiology and metabolism such as the insulin signaling [107] and [108]. Particularly, Lucas et al demonstrated the interconnection between GRK2 dosage and cardiac insulin sensitivity [109]. Authors characterized insulin signaling pathway, cardiac hypertrophy and gene expression patterns in hemizygous-GRK2 (GRK2+/−) mice compared to wild-type control animals. GRK2+/− mice revealed improved cardiac insulin sensitivity, non-pathologic hypertrophy and significant upregulation of genes known to play a protective role in CVDs along with decreased expression of genes associated with pathological cardiac hypertrophy and devastating diseases such as diabetes and obesity. Considering the role of GRK2 in systemic insulin resistance and obesity [110], authors further investigated whether GRK2 is upregulated in cardiac tissue in adult obese or high fat diet (HFD) fed mice and found significantly increased GRK2 protein levels in both conditions. Recent research demonstrated the role of GRK2 in obesity-related cardiac remodeling and lipid accumulation [111]. Moreover, genetic ablation of GRK2 resulted in reversed glucose tolerance and global insulin sensitivity, prevention of further body weight gain, increased fatty acid metabolism and attenuated lipid accumulation and inflammation in the liver in HFD-induced mouse model of obesity and insulin resistance [112]. Importantly, recent studies have also demonstrated a key role for GRK2 in mitochondrial metabolism [113]. Overall, aforementioned studies provided a new insight into the molecular mechanisms of worsening cardiac function in CVD comorbidities and highlighted the role of GRK2 signaling in these pathologic processes. In that perspective, small molecule inhibition of GPCR-Gβγ-GRK2 signaling might represent a promising approach for treatment CVD co-morbidities.
5. GPCR-Gβγ-GRK2 signaling in cardiorenal pathologies
The kidney performs essential regulatory roles in the body, including waste excretion, homeostasis maintenance, fluid volume and blood pressure regulation, as well as hormone secretion. GPCRs are widely expressed in the kidney, exemplified by arginine vasopressin receptor (AVP), dopamine-1 receptor (D1-R), angiotensin II receptors and endothelin (ET)-1 receptors [24]. GPCRs are involved in regulation of numerous kidney functions including water and electrolyte transport in renal tubules, maintenance of acid-base balance and renal blood flow and filtration [24] and [65]. Dysregulation of GPCR signaling is associated with severe kidney and systemic disorders such as renal fibrosis [114], [115] and [116], acute kidney injury (AKI) [117] and [118], hypertension [119], [120] and [121], and chronic kidney disease (CKD) [122].
Combined heart and kidney disorders, characterized by pathological interactions (“crosstalk”) between affected organs, are defined as cardiorenal syndrome (CRS) [123]. Different approaches have been applied to characterization and classification of CRS [124]; according to a classification proposed by Ronco et al in 2008, CRS is discriminated into five types with respect to the acute or chronic pathogenesis and the initiating event [123]. While the specific mechanisms behind this pathologic crosstalk between heart and kidney remain poorly understood, CRS is associated with exacerbated dysfunction of either or both organs and reduced survival [125] and [126]. Essentially, kidney maladaptive remodeling and impaired function serve as a strong predictor of mortality in HF patients [11] and [12]. Thus, investigation of mechanisms of pathologic crosstalk between failing heart and kidney may contribute to development of novel therapeutic strategies for HF, kidney dysfunction and CRS.
Considering the role of GPCR signaling in normal physiology and pathology of both heart and kidney, we recently scrutinized the role of GPCR-Gβγ-GRK2 in CRS type 2 (CRS2), which is characterized as a chronic heart failure (CHF) accompanied by the development of CKD [123] and [127]. The study proposed that elevated activity of SNS and endothelin (ET) system causes desensitization and down-regulation of renal GPCRs owing to pathologic Gβγ-GRK2 interactions, resembling the dysregulation of β-ARs observed in HF. To recapitulate the clinical features of CRS2 progression, a non-ischemic TAC mouse model of pressure-overload induced HF was utilized. To determine the role of Gβγ-GRK2 signaling in kidney dysfunction besides the crosstalk with the heart, a direct bilateral ischemia reperfusion (I/R) acute kidney injury (AKI) model was also implemented [128]. Development of CKD secondary to TAC was reflected by elevated serum creatinine levels, emerged morphological and molecular signs of tubular damage and increased focal tubulo-interstitial and perivascular fibrosis in the kidneys at 12 weeks post-TAC. Development of CKD in the chronic phase of HF is consistent with clinically observed consequence of CRS2 progression. Importantly, observed maladaptive changes were accompanied by elevated levels of ET-1 along with increased protein expression and membrane localization of ET receptors (ETA and ETB), that corroborates the proposed role of ET system in CRS. Furthermore, authors detected the elevation of Gβγ-GRK2 signaling in kidneys at 12 weeks post-TAC; essentially, upregulation of both ET and Gβγ-GRK2 signaling was attenuated by small molecule Gβγ inhibitor gallein treatment. Gallein pretreatment of mice subjected to AKI revealed protective effects of small molecule Gβγ-GRK2 inhibition on kidney function. Importantly, GRK2, ET-1 and ETA gene expression was elevated in kidneys of both CHF and AKI I/R mice.
Overall, these data suggest the role of Gβγ-GRK2 interactions in both acute and chronic kidney injury and the potential mechanism underlying pathologic crosstalk between heart and kidney in CRS2. Moreover, the study provides mechanistic insight into fibrotic tissue remodeling, demonstrating the role of Gβγ signaling in mouse embryonic fibroblasts (MEFs) endothelin-1 induced activation and migration [127] and [129].
A study performed by White et al aimed to explore the role of Gβγ subunits in kidney remodeling after AKI I/R injury [130]. In this study, rats were treated with supraphysiologic doses of gallein (30 mg/kg or 100 mg/kg) daily for three days post-I/R I/R with minimal benefit [131]. We recently reported a gallein dose-response study in which maximal efficacy and minimal toxicity were observed at 10 mg/kg/d [86], with high-dose toxicity possibly attributable to effects on cell cycle progression [45] and [132] and cell division [19] and [20]. Further, the different treatment schemes between our studies may produce divergent outcomes. As described above, our study with a much lower (i.e. physiologic) dose of gallein pretreatment was indeed renoprotective [128].
Taking into consideration the bidirectional nature of the crosstalk between heart and kidney, Polhemus and colleagues recently examined whether catheter-based renal denervation (RDN) that is thought to reduce blood pressure owing to the disruption of sympathetic signaling [133] and [134] possesses cardioprotective effects [135]. To model the heart injury, authors subjected spontaneously hypertensive rats (SHR) representing a model of established hypertension to myocardial I/R at 4 weeks after either bilateral radiofrequency-RDN (RF-RDN) or Sham-RDN. Rats treated with RF-RDN exhibited a significant reduction in myocardial infarct size and substantially improved left ventricular function 24 hours following the I/R. Moreover, RF-RDN treated rats demonstrated the attenuation of myocardial oxidative stress and elevation of cytoprotective nitric oxide (NO) signaling [136] compared to the Sham-RDN treated group. Importantly, RF-RDN treatment caused the reduction in myocardial GRK2 gene expression level, particularly the decrease in GRK2 Ser670 phosphorylated protein level.
Phosphorylation at residue Ser670 of GRK2 results in the activation of downstream mitochondrial cell death pathways [137]. Interestingly, neither significant cardioprotective effects nor alterations in myocardial GRK2 signaling were detected in normotensive rats following RF-RDN treatment. These findings highlighted the importance of CA signaling for the communication between heart and kidney, suggesting involvement of GRK2 signaling in these interactions (see overview of cardiorenal crosstalk in Figure 2). Future studies will provide more mechanistic insights into GPCR-Gβγ-GRK2 signaling modulation in cardiorenal crosstalk, as well as the role of GPCR-Gβγ-GRK2 signaling in the development and progression of heart disorders concomitant to the kidney injury. A summary of current animal models for CVD and CRS utilized in studies outlining the role of GPCR-Gβγ-GRK2 signaling pathway in these pathologies is provided in Table 1.
Figure 2. The proposed role of GPCR-Gβγ-GRK2 signaling in pathologic crosstalk between organs.
Decreased myocardial contractile function and reduced cardiac output in HF are initially compensated by increased activity of SNS released by CA produced in the adrenal gland. However, eventually CA overdrive elicits maladaptive effects including myocardial β-AR dysregulation via elevated Gβγ-GRK2 signaling. Moreover, Gβγ-GRK2 desensitizes adrenal α2-ARs, thus creating and maintaining the vicious cycle of SNS overactivation that leads to further progression of HF. Our recent study demonstrated elevated activity of GPCR-GRK2 signaling in acute kidney injury detected in a pressure-overload induced murine model of HF 12 weeks after TAC. Future experiments will explore and validate the role of GPCR-Gβγ-GRK2 signaling in pathologic cardiorenal crosstalk.
Table 1.
Current approaches for GPCR-Gβγ-GRK2 signaling manipulation and animal models for studying its role in the development and progression of CVD and CRS.
| GPCR-Gβγ-GRK2 signaling manipulation | Summary of effects | Model name and reference |
|---|---|---|
GRK2 knockout:
|
|
|
GRK2 overexpression:
|
|
|
Recombinant protein inhibition of Gβγ-GRK2 signaling:
|
|
|
Small molecule interdiction of Gβγ-GRK2 signaling:
|
|
|
6. Conclusions
GPCR signaling modulates a wide array of physiologic processes throughout the organism, consequently its dysregulation causes maladaptive responses in different organ systems, including the cardiovascular and renal systems. Owing to the increasing clinical significance of disorders involving multiple organ systems, investigation of molecular pathways that mediate pathological crosstalk between affected organs may hold therapeutic promise. Further exploration of inhibiting the GPCR-Gβγ-GRK2 signaling pathway might lead to the development of novel approaches for HF, kidney injury and CRS treatment.
Highlights.
GPCR signaling is a crucial regulator of cardiac and renal function;
Dysregulation of GPCR-Gβγ-GRK2 signaling is associated with severe cardiac and renal disorders;
Gβγ-GRK2 small molecule inhibitory treatment elicited beneficial effects in acute and advanced heart failure models;
Gβγ-GRK2 signaling interdiction resulted in advantageous outcomes in acute kidney injury and in kidney injury concomitant with cardiac injury;
GPCR-Gβγ-GRK2 signaling represents a potential mechanism underlying the crosstalk between heart and kidney in cardiorenal pathologies.
Acknowledgments
This work was supported in part by: NIH 1R01HL129772-01, 1R01HL132551-01, NIH 1R01HL134321-01, NIH 1R01HL133695-01 (BCB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mendis S, Puska P, Norrving B World Health Organization., World Heart Federation., World Stroke Organization. Global atlas on cardiovascular disease prevention and control. World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization; Geneva: 2011. [Google Scholar]
- 2.GBDC Mortality. Causes of Death, Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.M Writing Group; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB C. American Heart Association Statistics, S. Stroke Statistics. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ C. American Heart Association Advocacy Coordinating, C. Stroke, R. Council on Cardiovascular, Intervention, C. Council on Clinical, E. Council on, Prevention, A. Council on, Thrombosis, B. Vascular, C, Council on, C. Critical, Perioperative, Resuscitation, N. Council on Cardiovascular, D. Council on the Kidney in Cardiovascular, S. Council on Cardiovascular, Anesthesia, C. Interdisciplinary Council on Quality of, R. . Outcomes, Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 5.Bernardo BC, Blaxall BC. From Bench to Bedside: New Approaches to Therapeutic Discovery for Heart Failure. Heart lung & circulation. 2016;25:425–434. doi: 10.1016/j.hlc.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brum PC, Rolim NP, Bacurau AV, Medeiros A. Neurohumoral activation in heart failure: the role of adrenergic receptors. Anais da Academia Brasileira de Ciencias. 2006;78:485–503. doi: 10.1590/s0001-37652006000300009. [DOI] [PubMed] [Google Scholar]
- 7.Tilley DG, Rockman HA. Role of beta-adrenergic receptor signaling and desensitization in heart failure: new concepts and prospects for treatment. Expert review of cardiovascular therapy. 2006;4:417–432. doi: 10.1586/14779072.4.3.417. [DOI] [PubMed] [Google Scholar]
- 8.Madamanchi A. Beta-adrenergic receptor signaling in cardiac function and heart failure. McGill journal of medicine : MJM : an international forum for the advancement of medical sciences by students. 2007;10:99–104. [PMC free article] [PubMed] [Google Scholar]
- 9.Santulli G, Iaccarino G. Adrenergic signaling in heart failure and cardiovascular aging. Maturitas. 2016 doi: 10.1016/j.maturitas.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valiente-Alandi I, Schafer AE, Blaxall BC. Extracellular matrix-mediated cellular communication in the heart. Journal of molecular and cellular cardiology. 2016;91:228–237. doi: 10.1016/j.yjmcc.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smilde TD, Hillege HL, Navis G, Boomsma F, de Zeeuw D, van Veldhuisen DJ. Impaired renal function in patients with ischemic and nonischemic chronic heart failure: association with neurohormonal activation and survival. American heart journal. 2004;148:165–172. doi: 10.1016/j.ahj.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 13.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 14.Salazar NC, Chen J, Rockman HA. Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochimica et biophysica acta. 2007;1768:1006–1018. doi: 10.1016/j.bbamem.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 16.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 17.Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. The Journal of biological chemistry. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 18.Krapivinsky G, Krapivinsky L, Wickman K, Clapham DE. G beta gamma binds directly to the G protein-gated K+ channel, IKACh. The Journal of biological chemistry. 1995;270:29059–29062. doi: 10.1074/jbc.270.49.29059. [DOI] [PubMed] [Google Scholar]
- 19.Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbe JC, Miller GJ, Hebert TE. The expanding roles of Gbetagamma subunits in G protein-coupled receptor signaling and drug action. Pharmacological reviews. 2013;65:545–577. doi: 10.1124/pr.111.005603. [DOI] [PubMed] [Google Scholar]
- 20.Khan SM, Sung JY, Hebert TE. Gbetagamma subunits-Different spaces, different faces. Pharmacol Res. 2016;111:434–441. doi: 10.1016/j.phrs.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Bjarnadottir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, Schioth HB. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. 2006;88:263–273. doi: 10.1016/j.ygeno.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Koch WJ, Lefkowitz RJ, Rockman HA. Functional consequences of altering myocardial adrenergic receptor signaling. Annual review of physiology. 2000;62:237–260. doi: 10.1146/annurev.physiol.62.1.237. [DOI] [PubMed] [Google Scholar]
- 23.Port JD, Bristow MR. Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. Journal of molecular and cellular cardiology. 2001;33:887–905. doi: 10.1006/jmcc.2001.1358. [DOI] [PubMed] [Google Scholar]
- 24.Weiss RH. G protein-coupled receptor signalling in the kidney. Cellular signalling. 1998;10:313–320. doi: 10.1016/s0898-6568(97)00137-x. [DOI] [PubMed] [Google Scholar]
- 25.Brodde OE, Bruck H, Leineweber K. Cardiac adrenoceptors: physiological and pathophysiological relevance. Journal of pharmacological sciences. 2006;100:323–337. doi: 10.1254/jphs.crj06001x. [DOI] [PubMed] [Google Scholar]
- 26.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circulation research. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 27.Bristow MR, Hershberger RE, Port JD, Minobe W, Rasmussen R. Beta 1- and beta 2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Molecular pharmacology. 1989;35:295–303. [PubMed] [Google Scholar]
- 28.Kamal FA, Smrcka AV, Blaxall BC. Taking the heart failure battle inside the cell: small molecule targeting of Gbetagamma subunits. Journal of molecular and cellular cardiology. 2011;51:462–467. doi: 10.1016/j.yjmcc.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2012;21:365–371. doi: 10.1016/j.carpath.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 31.Lymperopoulos A, Rengo G, Gao E, Ebert SN, Dorn GW, 2nd, Koch WJ. Reduction of sympathetic activity via adrenal-targeted GRK2 gene deletion attenuates heart failure progression and improves cardiac function after myocardial infarction. The Journal of biological chemistry. 2010;285:16378–16386. doi: 10.1074/jbc.M109.077859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lymperopoulos A, Rengo G, Koch WJ. Adrenal adrenoceptors in heart failure: fine-tuning cardiac stimulation. Trends in molecular medicine. 2007;13:503–511. doi: 10.1016/j.molmed.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 34.Freedman NJ, Liggett SB, Drachman DE, Pei G, Caron MG, Lefkowitz RJ. Phosphorylation and desensitization of the human beta 1-adrenergic receptor. Involvement of G protein-coupled receptor kinases and cAMP-dependent protein kinase. The Journal of biological chemistry. 1995;270:17953–17961. doi: 10.1074/jbc.270.30.17953. [DOI] [PubMed] [Google Scholar]
- 35.Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, Snyder SH, Caron MG, Lefkowitz RJ. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. The Journal of biological chemistry. 1992;267:17882–17890. [PubMed] [Google Scholar]
- 36.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 37.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annual review of biochemistry. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 38.Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circulation research. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 39.Huang ZM, Gao EH, Chuprun JK, Koch WJ. GRK2 in the Heart: A GPCR Kinase and Beyond. Antioxid Redox Sign. 2014;21:2032–2043. doi: 10.1089/ars.2014.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inglese J, Freedman NJ, Koch WJ, Lefkowitz RJ. Structure and mechanism of the G protein-coupled receptor kinases. The Journal of biological chemistry. 1993;268:23735–23738. [PubMed] [Google Scholar]
- 41.Day PW, Wedegaertner PB, Benovic JL. Analysis of G-protein-coupled receptor kinase RGS homology domains. Methods in enzymology. 2004;390:295–310. doi: 10.1016/S0076-6879(04)90019-5. [DOI] [PubMed] [Google Scholar]
- 42.Koch WJ, Inglese J, Stone WC, Lefkowitz RJ. The binding site for the beta gamma subunits of heterotrimeric G proteins on the beta-adrenergic receptor kinase. The Journal of biological chemistry. 1993;268:8256–8260. [PubMed] [Google Scholar]
- 43.Hausdorff WP, Caron MG, Lefkowitz RJ. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 44.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nature reviews Molecular cell biology. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 45.Penela P, Murga C, Ribas C, Lafarga V, Mayor F., Jr The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. British journal of pharmacology. 2010;160:821–832. doi: 10.1111/j.1476-5381.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW., 2nd Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circulation research. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 48.Rockman HA, Choi DJ, Akhter SA, Jaber M, Giros B, Lefkowitz RJ, Caron MG, Koch WJ. Control of myocardial contractile function by the level of beta-adrenergic receptor kinase 1 in gene-targeted mice. The Journal of biological chemistry. 1998;273:18180–18184. doi: 10.1074/jbc.273.29.18180. [DOI] [PubMed] [Google Scholar]
- 49.DeGeorge BR, Jr, Gao E, Boucher M, Vinge LE, Martini JS, Raake PW, Chuprun JK, Harris DM, Kim GW, Soltys S, Eckhart AD, Koch WJ. Targeted inhibition of cardiomyocyte Gi signaling enhances susceptibility to apoptotic cell death in response to ischemic stress. Circulation. 2008;117:1378–1387. doi: 10.1161/CIRCULATIONAHA.107.752618. [DOI] [PubMed] [Google Scholar]
- 50.Brinks H, Boucher M, Gao E, Chuprun JK, Pesant S, Raake PW, Huang ZM, Wang X, Qiu G, Gumpert A, Harris DM, Eckhart AD, Most P, Koch WJ. Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circulation research. 2010;107:1140–1149. doi: 10.1161/CIRCRESAHA.110.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR, Jr, Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW, 2nd, Koch WJ. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circulation research. 2008;103:413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodall MC, Woodall BP, Gao E, Yuan A, Koch WJ. Cardiac Fibroblast GRK2 Deletion Enhances Contractility and Remodeling Following Ischemia/Reperfusion Injury. Circulation research. 2016;119:1116–1127. doi: 10.1161/CIRCRESAHA.116.309538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furtado MB, Nim HT, Boyd SE, Rosenthal NA. View from the heart: cardiac fibroblasts in development, scarring and regeneration. Development. 2016;143:387–397. doi: 10.1242/dev.120576. [DOI] [PubMed] [Google Scholar]
- 54.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 55.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circulation research. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circulation research. 2010;107:1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sato PY, Chuprun JK, Schwartz M, Koch WJ. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiological reviews. 2015;95:377–404. doi: 10.1152/physrev.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 59.Blaxall BC, Tschannen-Moran BM, Milano CA, Koch WJ. Differential gene expression and genomic patient stratification following left ventricular assist device support. Journal of the American College of Cardiology. 2003;41:1096–1106. doi: 10.1016/s0735-1097(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 60.Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. European heart journal. 2005;26:1752–1758. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- 61.Hata JA, Williams ML, Schroder JN, Lima B, Keys JR, Blaxall BC, Petrofski JA, Jakoi A, Milano CA, Koch WJ. Lymphocyte levels of GRK2 (betaARK1) mirror changes in the LVAD-supported failing human heart: lower GRK2 associated with improved beta-adrenergic signaling after mechanical unloading. Journal of cardiac failure. 2006;12:360–368. doi: 10.1016/j.cardfail.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 62.Bonita RE, Raake PW, Otis NJ, Chuprun JK, Spivack T, Dasgupta A, Whellan DJ, Mather PJ, Koch WJ. Dynamic changes in lymphocyte GRK2 levels in cardiac transplant patients: a biomarker for left ventricular function. Clinical and translational science. 2010;3:14–18. doi: 10.1111/j.1752-8062.2010.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rengo G, Pagano G, Filardi PP, Femminella GD, Parisi V, Cannavo A, Liccardo D, Komici K, Gambino G, D’Amico ML, de Lucia C, Paolillo S, Trimarco B, Vitale DF, Ferrara N, Koch WJ, Leosco D. Prognostic Value of Lymphocyte G Protein-Coupled Receptor Kinase-2 Protein Levels in Patients With Heart Failure. Circulation research. 2016;118:1116–1124. doi: 10.1161/CIRCRESAHA.115.308207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belmonte SL, Blaxall BC. G protein coupled receptor kinases as therapeutic targets in cardiovascular disease. Circulation research. 2011;109:309–319. doi: 10.1161/CIRCRESAHA.110.231233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamal FA, Travers JG, Blaxall BC. G protein-coupled receptor kinases in cardiovascular disease: why “where” matters. Trends in cardiovascular medicine. 2012;22:213–219. doi: 10.1016/j.tcm.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 66.Hullmann J, Traynham CJ, Coleman RC, Koch WJ. The expanding GRK interactome: Implications in cardiovascular disease and potential for therapeutic development. Pharmacol Res. 2016;110:52–64. doi: 10.1016/j.phrs.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 68.Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. The Journal of biological chemistry. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 69.Akhter SA, Eckhart AD, Rockman HA, Shotwell K, Lefkowitz RJ, Koch WJ. In vivo inhibition of elevated myocardial beta-adrenergic receptor kinase activity in hybrid transgenic mice restores normal beta-adrenergic signaling and function. Circulation. 1999;100:648–653. doi: 10.1161/01.cir.100.6.648. [DOI] [PubMed] [Google Scholar]
- 70.Williams ML, Hata JA, Schroder J, Rampersaud E, Petrofski J, Jakoi A, Milano CA, Koch WJ. Targeted beta-adrenergic receptor kinase (betaARK1) inhibition by gene transfer in failing human hearts. Circulation. 2004;109:1590–1593. doi: 10.1161/01.CIR.0000125521.40985.28. [DOI] [PubMed] [Google Scholar]
- 71.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, Gao E, Dasgupta A, Rengo G, Remppis A, Katus HA, Eckhart AD, Rabinowitz JE, Koch WJ. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115:2506–2515. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- 73.Tevaearai HT, Walton GB, Keys JR, Koch WJ, Eckhart AD. Acute ischemic cardiac dysfunction is attenuated via gene transfer of a peptide inhibitor of the beta-adrenergic receptor kinase (betaARK1) The journal of gene medicine. 2005;7:1172–1177. doi: 10.1002/jgm.770. [DOI] [PubMed] [Google Scholar]
- 74.Blaxall BC, Spang R, Rockman HA, Koch WJ. Differential myocardial gene expression in the development and rescue of murine heart failure. Physiological genomics. 2003;15:105–114. doi: 10.1152/physiolgenomics.00087.2003. [DOI] [PubMed] [Google Scholar]
- 75.Swain JD, Fargnoli AS, Katz MG, Tomasulo CE, Sumaroka M, Richardville KC, Koch WJ, Rabinowitz JE, Bridges CR. MCARD-mediated gene transfer of GRK2 inhibitor in ovine model of acute myocardial infarction. Journal of cardiovascular translational research. 2013;6:253–262. doi: 10.1007/s12265-012-9418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, Pleger S, Mier W, Haberkorn U, Koch WJ, Katus HA, Most P, Muller OJ. AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. European heart journal. 2013;34:1437–1447. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, Chuprun JK, Song J, Gao E, Cheung JY, Sklar LA, Koch WJ, Tesmer JJ. Paroxetine is a direct inhibitor of g protein-coupled receptor kinase 2 and increases myocardial contractility. ACS chemical biology. 2012;7:1830–1839. doi: 10.1021/cb3003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schumacher SM, Gao E, Zhu W, Chen X, Chuprun JK, Feldman AM, Tesmer JJ, Koch WJ. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Science translational medicine. 2015;7:277ra231. doi: 10.1126/scitranslmed.aaa0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science. 2006;312:443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- 80.Scott JK, Huang SF, Gangadhar BP, Samoriski GM, Clapp P, Gross RA, Taussig R, Smrcka AV. Evidence that a protein-protein interaction ‘hot spot’ on heterotrimeric G protein betagamma subunits is used for recognition of a subclass of effectors. The EMBO journal. 2001;20:767–776. doi: 10.1093/emboj/20.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie W, Samoriski GM, McLaughlin JP, Romoser VA, Smrcka A, Hinkle PM, Bidlack JM, Gross RA, Jiang H, Wu D. Genetic alteration of phospholipase C beta3 expression modulates behavioral and cellular responses to mu opioids. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10385–10390. doi: 10.1073/pnas.96.18.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mathews JL, Smrcka AV, Bidlack JM. A novel Gbetagamma-subunit inhibitor selectively modulates mu-opioid-dependent antinociception and attenuates acute morphine-induced antinociceptive tolerance and dependence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12183–12189. doi: 10.1523/JNEUROSCI.2326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casey LM, Pistner AR, Belmonte SL, Migdalovich D, Stolpnik O, Nwakanma FE, Vorobiof G, Dunaevsky O, Matavel A, Lopes CM, Smrcka AV, Blaxall BC. Small molecule disruption of G beta gamma signaling inhibits the progression of heart failure. Circulation research. 2010;107:532–539. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade. Circulation. 1998;98:1783–1789. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- 85.Cho MC, Rapacciuolo A, Koch WJ, Kobayashi Y, Jones LR, Rockman HA. Defective beta-adrenergic receptor signaling precedes the development of dilated cardiomyopathy in transgenic mice with calsequestrin overexpression. The Journal of biological chemistry. 1999;274:22251–22256. doi: 10.1074/jbc.274.32.22251. [DOI] [PubMed] [Google Scholar]
- 86.Kamal FA, Mickelsen DM, Wegman KM, Travers JG, Moalem J, Hammes SR, Smrcka AV, Blaxall BC. Simultaneous adrenal and cardiac g-protein-coupled receptor-gbetagamma inhibition halts heart failure progression. Journal of the American College of Cardiology. 2014;63:2549–2557. doi: 10.1016/j.jacc.2014.02.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tarnavski O. Mouse surgical models in cardiovascular research. Methods in molecular biology. 2009;573:115–137. doi: 10.1007/978-1-60761-247-6_7. [DOI] [PubMed] [Google Scholar]
- 89.deAlmeida AC, van Oort RJ, Wehrens XH. Transverse aortic constriction in mice. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perrino C, Naga Prasad SV, Patel M, Wolf MJ, Rockman HA. Targeted inhibition of beta-adrenergic receptor kinase-1-associated phosphoinositide-3 kinase activity preserves beta-adrenergic receptor signaling and prolongs survival in heart failure induced by calsequestrin overexpression. Journal of the American College of Cardiology. 2005;45:1862–1870. doi: 10.1016/j.jacc.2005.02.062. [DOI] [PubMed] [Google Scholar]
- 91.Perrino C, Naga Prasad SV, Schroder JN, Hata JA, Milano C, Rockman HA. Restoration of beta-adrenergic receptor signaling and contractile function in heart failure by disruption of the betaARK1/phosphoinositide 3-kinase complex. Circulation. 2005;111:2579–2587. doi: 10.1161/CIRCULATIONAHA.104.508796. [DOI] [PubMed] [Google Scholar]
- 92.Naga Prasad SV, Esposito G, Mao L, Koch WJ, Rockman HA. Gbetagamma-dependent phosphoinositide 3-kinase activation in hearts with in vivo pressure overload hypertrophy. The Journal of biological chemistry. 2000;275:4693–4698. doi: 10.1074/jbc.275.7.4693. [DOI] [PubMed] [Google Scholar]
- 93.Naga Prasad SV, Jayatilleke A, Madamanchi A, Rockman HA. Protein kinase activity of phosphoinositide 3-kinase regulates beta-adrenergic receptor endocytosis. Nature cell biology. 2005;7:785–796. doi: 10.1038/ncb1278. [DOI] [PubMed] [Google Scholar]
- 94.Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, Hirsch E. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 95.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. Journal of molecular and cellular cardiology. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 96.Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, Gude N, Avitabile D, Alvarez R, Sundararaman B, Quijada P, Mason M, Konstandin MH, Malhowski A, Cheng Z, Khan M, McGregor M. Myocardial AKT: the omnipresent nexus. Physiological reviews. 2011;91:1023–1070. doi: 10.1152/physrev.00024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008;451:919–928. doi: 10.1038/nature06798. [DOI] [PubMed] [Google Scholar]
- 98.de Lucia C, Femminella GD, Gambino G, Pagano G, Allocca E, Rengo C, Silvestri C, Leosco D, Ferrara N, Rengo G. Adrenal adrenoceptors in heart failure. Frontiers in physiology. 2014;5:246. doi: 10.3389/fphys.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nature medicine. 2007;13:315–323. doi: 10.1038/nm1553. [DOI] [PubMed] [Google Scholar]
- 100.Lymperopoulos A, Rengo G, Zincarelli C, Soltys S, Koch WJ. Modulation of adrenal catecholamine secretion by in vivo gene transfer and manipulation of G protein-coupled receptor kinase-2 activity. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16:302–307. doi: 10.1038/sj.mt.6300371. [DOI] [PubMed] [Google Scholar]
- 101.Jafferjee M, Reyes Valero T, Marrero C, McCrink KA, Brill A, Lymperopoulos A. GRK2 Up-Regulation Creates a Positive Feedback Loop for Catecholamine Production in Chromaffin Cells. Molecular endocrinology. 2016;30:372–381. doi: 10.1210/me.2015-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagatsu T. Tyrosine hydroxylase: human isoforms, structure and regulation in physiology and pathology. Essays in biochemistry. 1995;30:15–35. [PubMed] [Google Scholar]
- 103.Cotesta D, Caliumi C, Alo P, Petramala L, Reale MG, Masciangelo R, Signore A, Cianci R, D’Erasmo E, Letizia C. High plasma levels of human chromogranin A and adrenomedullin in patients with pheochromocytoma. Tumori. 2005;91:53–58. doi: 10.1177/030089160509100110. [DOI] [PubMed] [Google Scholar]
- 104.Lehmann DM, Seneviratne AM, Smrcka AV. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Molecular pharmacology. 2008;73:410–418. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 106.Arnon TI, Xu Y, Lo C, Pham T, An J, Coughlin S, Dorn GW, Cyster JG. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science. 2011;333:1898–1903. doi: 10.1126/science.1208248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mayor F, Jr, Lucas E, Jurado-Pueyo M, Garcia-Guerra L, Nieto-Vazquez I, Vila-Bedmar R, Fernandez-Veledo S, Murga C. G Protein-coupled receptor kinase 2 (GRK2): A novel modulator of insulin resistance. Arch Physiol Biochem. 2011;117:125–130. doi: 10.3109/13813455.2011.584693. [DOI] [PubMed] [Google Scholar]
- 108.Woodall MC, Ciccarelli M, Woodall BP, Koch WJ. G protein-coupled receptor kinase 2: a link between myocardial contractile function and cardiac metabolism. Circulation research. 2014;114:1661–1670. doi: 10.1161/CIRCRESAHA.114.300513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lucas E, Jurado-Pueyo M, Fortuno MA, Fernandez-Veledo S, Vila-Bedmar R, Jimenez-Borreguero LJ, Lazcano JJ, Gao E, Gomez-Ambrosi J, Fruhbeck G, Koch WJ, Diez J, Mayor F, Jr, Murga C. Downregulation of G protein-coupled receptor kinase 2 levels enhances cardiac insulin sensitivity and switches on cardioprotective gene expression patterns. Biochimica et biophysica acta. 2014;1842:2448–2456. doi: 10.1016/j.bbadis.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 110.Garcia-Guerra L, Nieto-Vazquez I, Vila-Bedmar R, Jurado-Pueyo M, Zalba G, Diez J, Murga C, Fernandez-Veledo S, Mayor F, Jr, Lorenzo M. G protein-coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes. 2010;59:2407–2417. doi: 10.2337/db10-0771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Lucas E, Vila-Bedmar R, Arcones AC, Cruces-Sande M, Cachofeiro V, Mayor F, Jr, Murga C. Obesity-induced cardiac lipid accumulation in adult mice is modulated by G protein-coupled receptor kinase 2 levels. Cardiovasc Diabetol. 2016;15:155. doi: 10.1186/s12933-016-0474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vila-Bedmar R, Cruces-Sande M, Lucas E, Willemen HL, Heijnen CJ, Kavelaars A, Mayor F, Jr, Murga C. Reversal of diet-induced obesity and insulin resistance by inducible genetic ablation of GRK2. Sci Signal. 2015;8:ra73. doi: 10.1126/scisignal.aaa4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sato PY, Chuprun JK, Ibetti J, Cannavo A, Drosatos K, Elrod JW, Koch WJ. GRK2 compromises cardiomyocyte mitochondrial function by diminishing fatty acid-mediated oxygen consumption and increasing superoxide levels. Journal of molecular and cellular cardiology. 2015;89:360–364. doi: 10.1016/j.yjmcc.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hocher B, Thone-Reineke C, Rohmeiss P, Schmager F, Slowinski T, Burst V, Siegmund F, Quertermous T, Bauer C, Neumayer HH, Schleuning WD, Theuring F. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. The Journal of clinical investigation. 1997;99:1380–1389. doi: 10.1172/JCI119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kohan DE. Endothelins in the normal and diseased kidney. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1997;29:2–26. doi: 10.1016/s0272-6386(97)90004-4. [DOI] [PubMed] [Google Scholar]
- 116.Shi-Wen X, Denton CP, Dashwood MR, Holmes AM, Bou-Gharios G, Pearson JD, Black CM, Abraham DJ. Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. The Journal of investigative dermatology. 2001;116:417–425. doi: 10.1046/j.1523-1747.2001.01256.x. [DOI] [PubMed] [Google Scholar]
- 117.Dhaun N, Webb DJ. The road from AKI to CKD: the role of endothelin. Kidney international. 2013;84:637–638. doi: 10.1038/ki.2013.158. [DOI] [PubMed] [Google Scholar]
- 118.Forbes JM, Jandeleit-Dahm K, Allen TJ, Hewitson TD, Becker GJ, Jones CL. Endothelin and endothelin A/B receptors are increased after ischaemic acute renal failure. Experimental nephrology. 2001;9:309–316. doi: 10.1159/000052626. [DOI] [PubMed] [Google Scholar]
- 119.Shindo T, Kurihara H, Maemura K, Kurihara Y, Ueda O, Suzuki H, Kuwaki T, Ju KH, Wang Y, Ebihara A, Nishimatsu H, Moriyama N, Fukuda M, Akimoto Y, Hirano H, Morita H, Kumada M, Yazaki Y, Nagai R, Kimura K. Renal damage and salt-dependent hypertension in aged transgenic mice overexpressing endothelin-1. Journal of molecular medicine. 2002;80:105–116. doi: 10.1007/s00109-001-0284-4. [DOI] [PubMed] [Google Scholar]
- 120.Sidhu A, Vachvanichsanong P, Jose PA, Felder RA. Persistent defective coupling of dopamine-1 receptors to G proteins after solubilization from kidney proximal tubules of hypertensive rats. The Journal of clinical investigation. 1992;89:789–793. doi: 10.1172/JCI115657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Uh M, White BH, Sidhu A. Alteration of association of agonist-activated renal D1(A) dopamine receptors with G proteins in proximal tubules of the spontaneously hypertensive rat. Journal of hypertension. 1998;16:1307–1313. doi: 10.1097/00004872-199816090-00012. [DOI] [PubMed] [Google Scholar]
- 122.Andress DL, Coll B, Pritchett Y, Brennan J, Molitch M, Kohan DE. Clinical efficacy of the selective endothelin A receptor antagonist, atrasentan, in patients with diabetes and chronic kidney disease (CKD) Life sciences. 2012;91:739–742. doi: 10.1016/j.lfs.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 123.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. Journal of the American College of Cardiology. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 124.Braam B, Joles JA, Danishwar AH, Gaillard CA. Cardiorenal syndrome--current understanding and future perspectives. Nature reviews Nephrology. 2014;10:48–55. doi: 10.1038/nrneph.2013.250. [DOI] [PubMed] [Google Scholar]
- 125.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 126.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. Journal of cardiac failure. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 127.Kamal FA, Travers JG, Schafer AE, Ma Q, Devarajan P, Blaxall BC. G Protein-Coupled Receptor-G-Protein betagamma-Subunit Signaling Mediates Renal Dysfunction and Fibrosis in Heart Failure. Journal of the American Society of Nephrology : JASN. 2016 doi: 10.1681/ASN.2015080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Singh AP, Junemann A, Muthuraman A, Jaggi AS, Singh N, Grover K, Dhawan R. Animal models of acute renal failure. Pharmacological reports : PR. 2012;64:31–44. doi: 10.1016/s1734-1140(12)70728-4. [DOI] [PubMed] [Google Scholar]
- 129.Neuhofer W, Pittrow D. Role of endothelin and endothelin receptor antagonists in renal disease. European journal of clinical investigation. 2006;36(Suppl 3):78–88. doi: 10.1111/j.1365-2362.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 130.White SM, North LM, Haines E, Goldberg M, Sullivan LM, Pressly JD, Weber DS, Park F, Regner KR. G-protein betagamma subunit dimers modulate kidney repair after ischemia-reperfusion injury in rats. Molecular pharmacology. 2014;86:369–377. doi: 10.1124/mol.114.092346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Regner KR, Nozu K, Lanier SM, Blumer JB, Avner ED, Sweeney WE, Jr, Park F. Loss of activator of G-protein signaling 3 impairs renal tubular regeneration following acute kidney injury in rodents. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:1844–1855. doi: 10.1096/fj.10-169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Penela P, Rivas V, Salcedo A, Mayor F., Jr G protein-coupled receptor kinase 2 (GRK2) modulation and cell cycle progression. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1118–1123. doi: 10.1073/pnas.0905778107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 134.Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT, Malaiapan Y, Papademetriou V. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. European heart journal. 2013;34:2132–2140. doi: 10.1093/eurheartj/eht197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Polhemus DJ, Gao J, Scarborough AL, Trivedi R, McDonough KH, Goodchild TT, Smart F, Kapusta DR, Lefer DJ. Radiofrequency Renal Denervation Protects the Ischemic Heart via Inhibition of GRK2 and Increased Nitric Oxide Signaling. Circulation research. 2016;119:470–480. doi: 10.1161/CIRCRESAHA.115.308278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Archiv : European journal of physiology. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 137.Chen M, Sato PY, Chuprun JK, Peroutka RJ, Otis NJ, Ibetti J, Pan S, Sheu SS, Gao E, Koch WJ. Prodeath signaling of G protein-coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal-regulated kinase-dependent heat shock protein 90-mediated mitochondrial targeting. Circulation research. 2013;112:1121–1134. doi: 10.1161/CIRCRESAHA.112.300754. [DOI] [PMC free article] [PubMed] [Google Scholar]