Abstract
Background: In the Antarctic, only two species of Chironomidae occur naturally—the wingless midge, Belgica antarctica, and the winged midge, Parochlus steinenii. B. antarctica is an extremophile with unusual adaptations. The larvae of B. antarctica are desiccation- and freeze-tolerant and the adults are wingless. Recently, the compact genome of B. antarctica was reported and it is the first Antarctic eukaryote to be sequenced. Although P. steinenii occurs naturally in the Antarctic with B. antarctica, the larvae of P. steinenii are cold-tolerant but not freeze-tolerant and the adults are winged. Differences in adaptations in the Antarctic midges are interesting in terms of evolutionary processes within an extreme environment. Herein, we provide the genome of another Antarctic midge to help elucidate the evolution of these species.
Results: The draft genome of P. steinenii had a total size of 138 Mbp, comprising 9513 contigs with an N50 contig size of 34,110 bp, and a GC content of 32.2%. Overall, 13,468 genes were predicted using the MAKER annotation pipeline, and gene ontology classified 10,801 (80.2%) predicted genes to a function. Compared with the assembled genome architecture of B. antarctica, that of P. steinenii was approximately 50 Mbp longer with 6.2-fold more repeat sequences, whereas gene regions were as similarly compact as in B. antarctica.
Conclusions: We present an annotated draft genome of the Antarctic midge, P. steinenii. The genomes of P. steinenii and B. antarctica will aid in the elucidation of evolution in harsh environments and provide new resources for functional genomic analyses of the order Diptera.
Keywords: Parochlus steinenii, Cold-tolerant, Complete mitochondrial genome, Antarctic winged midge
Data description
Sequencing
Parochlus steinenii specimens [1–3] were collected from King George Island, West Antarctica (62° 14΄ S, 58° 47΄ W) during 2014 and 2015. Twenty adults were used for genome sequencing, regardless of gender. Genomic DNA was extracted using a DNeasy Tissue Kit (Qiagen, Valencia, CA, USA). For genome sequencing and assembly using ALLPATHS-LG [4], two types of libraries were prepared. One was a fragment library, which was a paired-end type with an insert size of 400 bp (PE400), whereas others were jumping libraries, which were mate-pair types with insert sizes of 3 kbp (MP3K) and 5 kbp (MP5K). The paired-end library was sequenced with the MiSeq platform (Illumina, San Diego, CA, USA) using a read-length configuration of 2 × 300 bp, and the mate-pair libraries were sequenced with the HiSeq platform (Illumina, San Diego, CA, USA) using a read-length configuration of 2 × 150 bp (see Table 1). Library preparation and sequencing were performed according to the manufacturer's instructions.
Table 1.
Sequencing libraries and respective yield used for genome assembly of Parochlus steinenii
| Insert | Library | Total read | ||||
|---|---|---|---|---|---|---|
| Library | Mode | size | type | Reads | lengths (Gbp) | Source |
| PE400 | 2 × 300 | 400 | paired-end | 51,892,430 | 15.6 | Genomic DNA |
| MP3K | 2 × 150 | 3000 | mate-pair | 170,887,140 | 25.6 | Genomic DNA |
| MP5K | 2 × 150 | 5000 | mate-pair | 157,622,418 | 23.6 | Genomic DNA |
| PE300A | 2 × 150 | 300 | paired-end | 27,663,170 | 3.5 | RNA |
| PE300B | 2 × 150 | 300 | paired-end | 27,782,288 | 3.5 | RNA |
| PE300C | 2 × 150 | 300 | paired-end | 30,806,804 | 3.9 | RNA |
For gene annotation with RNA evidence, total RNA was extracted from the whole bodies of ten adults in three different groups using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions. Three paired-end libraries with an insert size of 300 bp (PE300) were constructed using the TruSeq Stranded mRNA Library Prep Kit (Illumina, San Diego, CA, USA) and sequenced with the HiSeq platform (Illumina, San Diego, CA, USA) using a read-length configuration of 2 × 150 bp (Table 1).
Before assembly using ALLPATHS-LG, the paired-end reads resulting from the fragment library were trimmed using the fastq_quality_trimmer in the FASTX-Toolkit (Ver. 0.0.11) [5] with the parameters “-t 30”, “-l 200” and “-Q 33”. Paired sequences from the trimmed Illumina reads were then selected. Finally, after quality trimming, yields for the fragment library totaled 14.8 giga base pairs (Gbp).
Tree-type libraries were constructed in this study, as shown in Table 1. A PE400 library was constructed as a fragment library for ALLPATHS-LG. Mate-pair libraries (MP3K and MP5K) were also constructed for ALLPATHS-LG assembly. Three PE300 libraries (PE300A, PE300B, and PE300C) were constructed from RNA for gene annotation.
Genome assembly
Before assembly, we estimated the genome size and heterozygosity using a k-mer analysis with sequencing reads. Jellyfish (Ver. 1.1.10) [6] and GenomeScope [7, 8] software were used. The 17-mers were counted in the reads from the PE400 library and the resulting histogram of 17-mer occurrence was used as a query for GenomeScope [8]. The estimated genome size was 143.8 mega base pairs (Mbp) and the estimated heterozygosity was 0.613%.
Assembly was performed using ALLPATHS-LG for both the fragment library (400 bp) and the jumping libraries (3 kbp and 5 kbp) [4]. This was performed on a 96-processor workstation with Intel Xeon X7460 2.66 GHz processors, 1 TB of RAM, and default parameters. For better assembly in ALLPATHS-LG, a larger k-mer size was used with one longer read generated from the paired-end library [4]. As a result, the paired-end reads from the fragment library were designed to overlap, and the insert size of the paired-end library was slightly less than twice the read size [4]. In this assembly, 93.8% of the paired-end reads from the fragment library overlapped and merged into one longer read. The resulting assembly had a total size of 138 Mbp, comprising 9513 contigs with an N50 contig size of 34,110 bp and an N50 scaffold size of 168 kbp (Table 2). The GC content was 32.2% and the assembly revealed contig coverage of approximately 89 × total read length from the fragment library. A total of 57.2% of the 3-kbp jumping library and 33.1% of the 5-kbp jumping library were used to improve scaffolding. If more jumping libraries or long jumping libraries (with insert size larger than 20 kbp) were used, the scaffolding might improve the assembly. The assembled genome size was similar to the predicted genome size (143.8 Mbp). We also validated this assembly using CEGMA [9] and BUSCO [10]. CEGMA evaluation showed that the gene completeness of this assembly was 85.08%, and BUSCO analysis using arthropod databases showed 67.2% completeness (Tables 3 and 4). If partially matched genes were considered, 92.34% and 87.5% of the genes were identified in CEGMA and BUSCO, respectively (Tables 3 and 4).
Table 2.
Global statistics of the Parochlus steinenii genome assembly
| Assembly results | Number | N50 (kbp)a | Size (Mbp) |
|---|---|---|---|
| Contig | 9513 | 34.1 | 130.6 |
| Scaffold | 4151 | 168.1 | 138.0 |
| Annotation | Number | Total length (kbp) | Percentage of the assembled genome |
| Genes | 13,468 | 36,239.1 | 26.3 |
| Coding regions (Coding regions in B. antarctica) | 13,468 (11,005) | 17,967.6 (17,518.0) | 13.0 (19.6) |
| Introns (Introns in B. antarctica) | 69,960 (43,577) | 24,191.6 (15,494.9) | 17.5 (17.2) |
| Repeats (Repeats in B. antarctica) | 37,507 (10,084) | 2252.6 (361.4) | 1.6 (0.40) |
Minimum sequence length in which half of the assembled bases were found
Table 3.
CEGMA analysis of two Antarctic midges
| CEG | Complete | Percentage | Total | Average | Percentage | |
|---|---|---|---|---|---|---|
| set | proteins | complete | observed | copy number | of orthologs | |
| P. steinenii | Complete | 211 | 85.08 | 247 | 1.17 | 14.22 |
| Partial | 229 | 92.34 | 283 | 1.24 | 19.65 | |
| B. antarctica | Complete | 241 | 97.18 | 283 | 1.17 | 12.03 |
| Partial | 247 | 99.6 | 311 | 1.26 | 18.18 |
CEG core eukaryotic gene
Table 4.
BUSCO analysis of two Antarctic midges
| Genome assembly | Gene set | |||
|---|---|---|---|---|
| P. steinenii | B. antarctica | P. steinenii | B. antarctica | |
| Complete BUSCOs (%) | 1798 (67.2) | 2310 (86.4) | 1890 (70.7) | 2316 (86.6) |
| Complete and single-copy BUSCOs (%) | 1648 (61.6) | 2170 (81.1) | 1620 (60.6) | 2074 (77.5) |
| Complete and duplicated BUSCOs (%) | 150 (5.6) | 140 (5.2) | 270 (10.1) | 242 (9.0) |
| Fragmented BUSCOs (%) | 543 (20.3) | 270 (10.1) | 343 (12.8) | 137 (5.1) |
| Missing BUSCOs (%) | 334 (12.5) | 95 (0.04) | 442 (16.5) | 222 (8.3) |
| Total BUSCO groups searched | 2675 (100) | |||
The statistics for gene annotation for B. antarctica were from a reanalysis for comparison of the percentage of the genome created, based on the assembled genome size. From a previous report [11], the assembled genome size of B. antarctica was 89.6 Mbp.
CEGMA analysis (Table 3) was performed to validate the genome assembly of P. steinenii. The genome sequence of B. antarctica (JPYR00000000.1) from the National Center for Biotechnology Information (NCBI) was also analyzed for comparison.
BUSCO analysis was performed to validate genome assembly and gene annotation. For B. antarctica, the genome sequence (JPYR00000000.1) from NCBI and the gene set annotated in this study were used. Table 4 shows the numbers and percentages of BUSCO groups.
Repeat analysis and non-coding RNA
Interspersed repeats for P. steinenii were predicted using RepeatMasker (Ver. 3.3.0) [12] with a de novo repeat library. The de novo repeat library for P. steinenii was constructed using RepeatModeler (Ver. 1.0.3) [13], including the RECON (Ver. 1.07) [13] and RepeatScout (Ver. 1.0.5) [14] software, with default parameters. Tandem repeats, including simple repeats, satellites and low-complexity repeats, were predicted using TRF [15]. Putative tRNA genes were identified using tRNAscan-SE (Ver. 1.3.1) [16] with option “-H”. The repeat content for B. antarctica was re-estimated for comparison using RepeatMasker (Ver. 3.3.0) [12] with the Repbase library (Ver. 20140131) [17, 18]. The total coverage of repeat sequences in P. steinenii was approximately six times greater than that of repeat sequences in B. antarctica (Table 2), and the percentage of the genome was approximately three times higher than that of B. antarctica, based on the assembled genome size. Most statistics for repeats were higher in the P. steinenii library (Table 5). A total of 186 tRNAs were predicted through tRNAscan-SE [16] (Additional file 1: Table S1).
Table 5.
Repeat content in Antarctic midges
| P. steinenii | B. antarctica | |||
|---|---|---|---|---|
| Total | Number | Total | Number | |
| coverage (bp) | of sequences | coverage (bp) | of sequences | |
| Low complexity | 404,490 | 8661 | 290,095 | 8812 |
| Simple repeats | 1,105,449 | 26,336 | 40,475 | 1066 |
| Transposon elements | ||||
| Class I/LTR | 289,059 | 1075 | 945 | 13 |
| Class I/Non-LTR | 169,298 | 675 | 18,003 | 271 |
| Class II/DNA elements | 216,807 | 649 | 5247 | 83 |
| Small RNA | 67,503 | 111 | 6425 | 13 |
| Totals | 2,252,606 | 37,507 | 361,370 | 10,258 |
LTR long terminal repeat
Gene annotation
Gene annotation was accomplished using the MAKER annotation pipeline [19, 20]. RepeatMasker (Ver. 3.3.0) [12] was used to identify repetitive elements against a de novo repeat library, and the SNAP gene finder [21] was selected to perform ab initio gene prediction from the masked genome sequence. To find the best possible gene model for the given region, evidence of RNA and protein alignments were considered in MAKER2 [20]. Transcriptome assembly results were used for RNA evidence; the paired-end reads resulting from mRNA of the whole body of adults were trimmed using the fastq_quality_trimmer in the FASTX-Toolkit (Ver. 0.0.11) [5] with the parameters “-t 30”, “-l 80” and “-Q 33”, and they were assembled with CLC Genomics Workbench (Ver. 8.0.0) using default parameters. In all, 68,392 contigs, with an N50 contig size of 435 bp and an average contig size of 407 bp, were generated and used for RNA evidence. Protein sequences from six species, given in NCBI reference sequences, were used for protein evidence—Drosophila melanogaster (fruit fly, GCF_000001215.4), Ceratitis capitata (Mediterranean fruit fly, NC_000857.1), Bactrocera dorsalis (oriental fruit fly, NC_008748.1), Anopheles gambiae (African malaria mosquito, NZ_AAAB00000000.1), Aedes aegypti (yellow fever mosquito, AAGE00000000.2) and Culex quinquefasciatus (southern house mosquito, AAWU01000000). Alignment of transcriptome assembly with BLASTn and alignment of homologous protein information from tBLASTx were considered as evidence for annotation. To assess the annotated gene set, we ran a BUSCO analysis in the “OGS” mode for gene set completeness and identified 70.7% genes to be considered complete with the expanded gene set; 16.5% of the gene set was classified as missing [10].
Blast2GO (Ver. 2.6.0) assigned preliminary functions for 13,468 genes, and gene ontology (GO) classified 10,801 (80.2%) of the predicted genes to a function using the BLASTp and InterproScan results [22]. GO annotation described the classified proteins as those required for biological processes (7434; 55.2%), molecular functions (9576; 71.1%) and cellular components (4871; 36.2%). Enzyme Commission (EC) numbers were obtained for 987 proteins.
Gene annotation for B. antarctica
To investigate the difference in gene content between P. steinenii and B. antarctica, we also annotated the genome of B. antarctica with the same methods used for P. steinenii. The reads in various experimental conditions for B. antarctica (SRR566981, SRR567289, SRR567164–SRR567167 and SRR567169–SRR567171) were downloaded from the NCBI Sequence Read Archive and we assembled them into 38,017 contigs, with an N50 contig size of 1799 bp and an average contig size of 913 bp, through CLC Genomics Workbench (Ver. 8.0.0). For RNA evidence, the resulting contigs were aligned to the genome sequence of B. antarctica with BLASTn in MAKER2. For protein evidence, we used the same protein sequence from the six species used for gene annotation in P. steinenii and predicted proteins of B. antarctica. From MAKER2, 11,005 genes were predicted in the B. antarctica genome. The annotated gene set in this analysis was assessed using BUSCO [10]. Gene set completeness was 86.6% including the expanded gene set, and 8.3% of the gene set was missing (Table 4).
Ortholog analysis
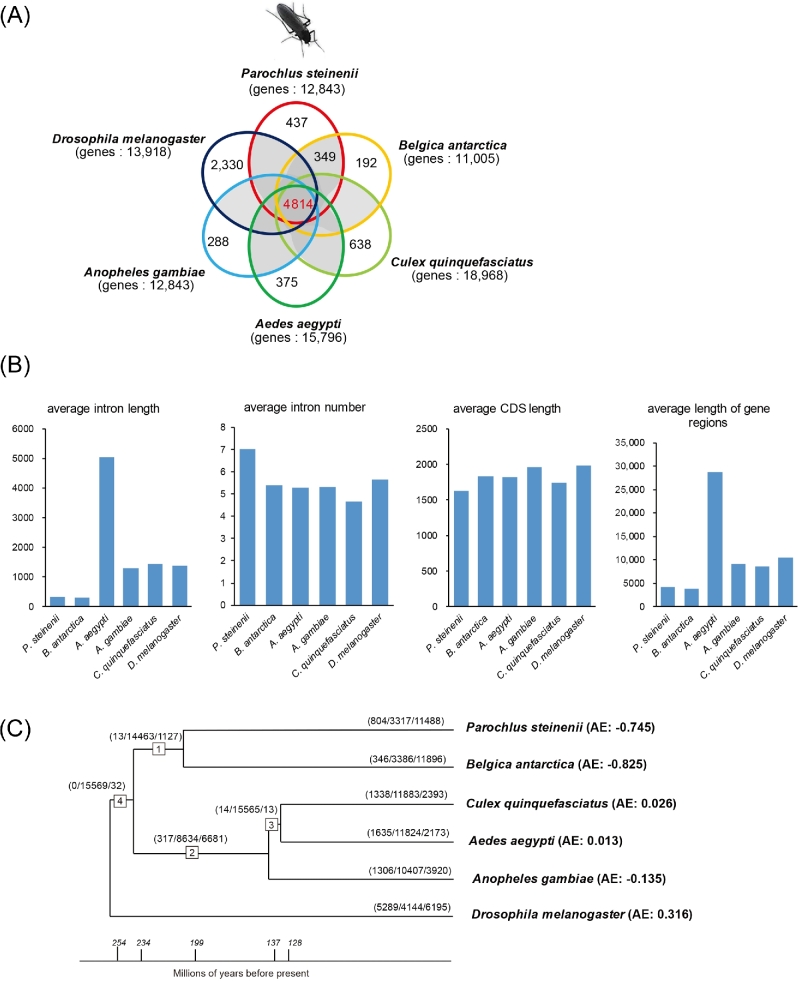
Orthologous groups were identified using OrthoMCL (Ver. 2.0.5) [23]. We used the standard parameters and options of OrthoMCL for all steps. In this analysis, coding sequences (CDS) from four genome assemblies (BDGP6 for D. melanogaster, AgamP4 for A. gambiae, AaegL3 for A. aegypti and CpipJ2 for C. quinquefasciatus) were collected from Ensemble Metazoa [24] and the CDS from MAKER2 were used for B. antarctica and P. steinenii. Total proteins were categorized into 15,633 groups—4814 orthologous groups were identified as common to all six insects, 437 groups specific to P. steinenii genes were not identified in any other species, and 349 groups were identified only in the two Antarctic midges (Fig. 1A and Additional file 1: Table S2).
Figure 1.
Genome-wide analysis of protein-coding genes in Parochlus steinenii. a Venn diagram displaying the overlap in orthologous genes of six insect species and the number of unique groups in each species. b The statistics of gene structures of the six insects. c Lineage-specific gene gains and losses among the six insects. The numbers in the boxes are identifiers for internal branches of the phylogeny. Numbers on each branch denote the number of gained, lost and stable genes, respectively. AE denotes the average expansion. The numbers on the bottom line denote the estimated divergence time of the corresponding tree nodes above, based on TimeTree
Gene structure of orthologous groups
B. antarctica showed a reduction in intron length with very low repeat sequences [11]. Therefore, we compared intron lengths of orthologous genes among the six insects to identify whether the intron lengths of the genes in P. steinenii were also reduced. We used the information from gene structures of the four genome assemblies (BDGP6 for D. melanogaster, AgamP4 for A. gambiae, AaegL3 for A. aegypti and CpipJ2 for C. quinquefasciatus) and the information from MAKER2 annotation of B. antarctica and P. steinenii. Among the six insects, the average intron length of B. antarctica (302 bp) was the smallest, although that of P. steinenii (319 bp) was similar (Fig. 1B). Despite a difference in the assembled genome size between B. antarctica and P. steinenii of approximately 50 Mbp, the average length of gene regions and CDS were also similar in the two. However, the average intron number in orthologous genes was higher in P. steinenii, which was the highest of all six insects (Fig. 1B).
GO enrichment test
We used AgriGO [25] to identify which GO terms of the 437 groups that were unique to P. steinenii were statistically overrepresented relative to the GO terms of all genes of P. steinenii. A total of 1352 genes comprised these 437 groups, and therein were 717 genes with GO terms. AgriGO is a web-based tool for GO analysis: we selected “Fisher's exact test” for the statistical test method and “Hochberg FDR” as the multiple test adjustment method. GO terms were tested with a significance level of p < 0.05. Complete hierarchies of GO terms for each gene were examined. GO enrichment analysis identified 49 GO terms as statistically overrepresented: 26 GO terms in biological processes, five in cellular components and 18 in molecular functions (Table 6). It is noteworthy that 14 of the 26 significant GO terms in biological processes were associated with the unfolded protein response (UPR). The UPR is a stress response that occurs in the lumen of the endoplasmic reticulum (ER) [26]. When unfolded or misfolded proteins are accumulated in the ER lumen under stress conditions, the UPR is activated to improve protein folding by increasing the production of chaperones [26]. Representative GO terms in biological processes related to the UPR were mRNA splicing via endonucleolytic cleavage and ligation (GO:0070054), response to unfolded protein (GO:0006986), and endoplasmic reticulum unfolded protein response (GO:0030968).
Table 6.
GO terms statistically overrepresented only in Parochlus steinenii-specific groups
| GO | GO | No. of | No. of | |||
|---|---|---|---|---|---|---|
| ID | tree | Term | genesa | genesb | p-value | FDR |
| GO:0006508 | P | proteolysis | 106 | 632 | 8.60E-13 | 2.60E-10 |
| GO:0006397 | P | mRNA processing | 32 | 120 | 6.80E-10 | 1.00E-07 |
| GO:0070054 | P | mRNA splicing, via endonucleolytic cleavage and ligation | 8 | 8 | 1.40E-09 | 1.40E-07 |
| GO:0016071 | P | mRNA metabolic process | 32 | 130 | 5.80E-09 | 4.50E-07 |
| GO:0000394 | P | RNA splicing, via endonucleolytic cleavage and ligation | 8 | 11 | 1.90E-07 | 1.10E-05 |
| GO:0006986 | P | response to unfolded protein | 6 | 7 | 1.50E-06 | 7.60E-05 |
| GO:0019538 | P | protein metabolic process | 173 | 1506 | 2.20E-06 | 9.40E-05 |
| GO:0051789 | P | response to protein stimulus | 6 | 8 | 5.50E-06 | 0.00021 |
| GO:0006950 | P | response to stress | 50 | 330 | 8.00E-06 | 0.00027 |
| GO:0006468 | P | protein amino acid phosphorylation | 42 | 272 | 2.40E-05 | 0.00074 |
| GO:0080135 | P | regulation of cellular response to stress | 9 | 24 | 4.80E-05 | 0.0013 |
| GO:0006396 | P | RNA processing | 34 | 210 | 5.00E-05 | 0.0013 |
| GO:0051347 | P | positive regulation of transferase activity | 8 | 22 | 0.00016 | 0.0031 |
| GO:0033674 | P | positive regulation of kinase activity | 8 | 22 | 0.00016 | 0.0031 |
| GO:0045860 | P | positive regulation of protein kinase activity | 8 | 22 | 0.00016 | 0.0031 |
| GO:0034620 | P | cellular response to unfolded protein | 4 | 5 | 0.00017 | 0.0031 |
| GO:0030968 | P | endoplasmic reticulum unfolded protein response | 4 | 5 | 0.00017 | 0.0031 |
| GO:0042246 | P | tissue regeneration | 6 | 13 | 0.00024 | 0.0041 |
| GO:0031099 | P | regeneration | 6 | 14 | 0.00039 | 0.0063 |
| GO:0071445 | P | cellular response to protein stimulus | 4 | 6 | 0.00049 | 0.0071 |
| GO:0071216 | P | cellular response to biotic stimulus | 4 | 6 | 0.00049 | 0.0071 |
| GO:0034976 | P | response to endoplasmic reticulum stress | 4 | 7 | 0.0011 | 0.015 |
| GO:0061053 | P | somite development | 3 | 4 | 0.0018 | 0.024 |
| GO:0006984 | P | ER-nuclear signaling pathway | 4 | 8 | 0.002 | 0.026 |
| GO:0006379 | P | mRNA cleavage | 4 | 9 | 0.0034 | 0.041 |
| GO:0016310 | P | phosphorylation | 49 | 421 | 0.0041 | 0.049 |
| GO:0031463 | C | Cul3-RING ubiquitin ligase complex | 5 | 5 | 2.90E-06 | 0.00019 |
| GO:0031461 | C | cullin-RING ubiquitin ligase complex | 5 | 12 | 0.0014 | 0.047 |
| GO:0005789 | C | endoplasmic reticulum membrane | 11 | 55 | 0.0032 | 0.063 |
| GO:0042175 | C | nuclear envelope–endoplasmic reticulum network | 11 | 57 | 0.0042 | 0.063 |
| GO:0044432 | C | endoplasmic reticulum part | 11 | 58 | 0.0049 | 0.063 |
| GO:0004252 | F | serine-type endopeptidase activity | 76 | 292 | 3.70E-20 | 5.50E-18 |
| GO:0004540 | F | ribonuclease activity | 30 | 54 | 1.90E-19 | 1.40E-17 |
| GO:0008236 | F | serine-type peptidase activity | 76 | 318 | 6.90E-18 | 2.50E-16 |
| GO:0017171 | F | serine hydrolase activity | 76 | 318 | 6.90E-18 | 2.50E-16 |
| GO:0004175 | F | endopeptidase activity | 84 | 416 | 5.70E-15 | 1.70E-13 |
| GO:0070011 | F | peptidase activity, acting on L-amino acid peptides | 103 | 570 | 1.60E-14 | 4.00E-13 |
| GO:0008233 | F | peptidase activity | 103 | 595 | 2.40E-13 | 5.10E-12 |
| GO:0004518 | F | nuclease activity | 30 | 102 | 1.70E-10 | 3.10E-09 |
| GO:0031072 | F | heat shock protein binding | 10 | 17 | 1.00E-07 | 1.60E-06 |
| GO:0004672 | F | protein kinase activity | 47 | 300 | 5.90E-06 | 8.70E-05 |
| GO:0008234 | F | cysteine-type peptidase activity | 15 | 59 | 3.70E-05 | 0.00049 |
| GO:0016787 | F | hydrolase activity | 171 | 1580 | 5.00E-05 | 0.00061 |
| GO:0016773 | F | phosphotransferase activity, alcohol group as acceptor | 49 | 363 | 0.00018 | 0.002 |
| GO:0042802 | F | identical protein binding | 10 | 38 | 0.00052 | 0.0055 |
| GO:0031625 | F | ubiquitin protein ligase binding | 5 | 12 | 0.0014 | 0.014 |
| GO:0005515 | F | protein binding | 229 | 2357 | 0.0015 | 0.014 |
| GO:0016301 | F | kinase activity | 48 | 405 | 0.0032 | 0.027 |
| GO:0003676 | F | nucleic acid binding | 144 | 1469 | 0.0055 | 0.045 |
A total of 49 GO terms were statistically overrepresented: 26 in biological processes (P), five in cellular components (C) and 18 in molecular functions (F) were identified as significant by GO enrichment analysis. Fisher's exact test was performed and the resulting p-values were adjusted using the Hochberg FDR for multiple comparisons. GO terms associated with the unfolded protein response are shown in bold font
ER endoplasmic reticulum, FDR false discovery rate, GO gene ontology
The number of genes with GO terms in the P. steinenii-specific groups
The number of genes with GO terms in P. steinenii's entire gene set
Likelihood analysis of gene gain and loss
To estimate the average gene expansion/contraction rate and to identify gene families that have undergone significant size changes through evolution [27, 28], we estimated differences in the size of 15,633 orthologs using the program CAFE3.0 [29]. A Newick description of a rooted and bifurcating phylogenetic tree was needed for this analysis. Therefore, we performed phylogenetic analyses among six insects with the protein-coding gene in the orthologous groups. We selected 4814 orthologous gene sets from the orthologous groups from OrthoMCL using the criterion of reciprocal best BLASTP hit. Protein-coding gene sequences were aligned using PRANK (Ver. 130820) under a codon model with the “-dna -codon” option [30], poor alignment sites were eliminated using Gblock (Ver. 0.91) under a codon model with the “-t = c” option [31], and the remaining alignment regions were concatenated for use in the phylogenetic analyses. The phylogenetic tree was constructed using the neighbor-joining method [32] in the MEGA (Ver. 6) program [33]. From the resulting phylogenetic tree, we prepared the ultrametric tree of the species, including branch lengths in units of time through TimeTree [34], for the analysis (Fig. 1C). We ran the program using p < 0.05, and estimated rates of birth (λ) and death (μ) were calculated using the program LambdaMu with the “-s” option. We calculated the number of gene gains and losses on each branch of the tree with the “-t” option. The average expansion (AE) sizes of the two Antarctic midges were lower than those of the other four insects (Fig. 1C), and D. melanogaster exhibited the highest AE score among the six. Using p < 0.0001 for the family-wide significance value, we expected approximately one significant result by chance and calculated the exact p-values for transitions over every branch. We called individual branches significant at p < 0.005 [35]. We identified three gene families that were significantly expanded in P. steinenii and two in B. antarctica, (Additional file 1: Table S3).
Availability of supporting data
Supporting data (sequence files for CDS, proteins, transcripts and the draft genome, and the general feature format for genes and repeats) are available in the GigaScience GigaDB database [36] and the raw data were deposited in the NCBI BioProject repository PRJNA284858 (SRX1976250–SRX1976255).
Additional file
Additional file 1: Table S1 tRNA in Parochlus steinenii. (DOCX 21 kb)
Additional file 1: Table S2 Shared orthologous gene clusters among six insects—Drosophila melanogaster, Anopheles gambiae, Aedes aegypti, Culex quinquefasciatus, Belgica antarctica and Parochlus steinenii
Additional file 1: Table S3 Gene families were significantly expanded in Antarctic midges
Abbreviations
CDS, coding sequence; Gbp, giga base pairs; GO, gene ontology; Mbp, mega base pairs.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SK, HGC and SCS designed the study. SK, WJ and HGC collected the samples and performed the experiments. SCS and JP analyzed the data. All authors participated in the writing of the manuscript.
Supplementary Material
Additional file 1: Table S1 tRNA in Parochlus steinenii. (DOCX 21 kb)
Additional file 1: Table S2 Shared orthologous gene clusters among six insects—Drosophila melanogaster, Anopheles gambiae, Aedes aegypti, Culex quinquefasciatus, Belgica antarctica and Parochlus steinenii
Additional file 1: Table S3 Gene families were significantly expanded in Antarctic midges
Acknowledgements
This study was supported by a Polar Genomics 101 Project grant (PE17080) and a Modeling responses of terrestrial organisms to environmental changes on King George Island grant (PE17090) funded by the Korea Polar Research Institute (KOPRI).
References
- 1. Convey P, Block W: Antarctic Diptera: ecology, physiology and distribution. Eur J Entomol. 1996; 93: 1–14. [Google Scholar]
- 2. EDWARDS M, USHER MB: The winged Antarctic midge Parochlus steinenii (Gerke)(Diptera: Chironomidae) in the South Shetland Islands. Biol J Linn Soc. 1985; 26(1): 83–93. [Google Scholar]
- 3. Shimada K, Ohyama Y, Pan C: Cold-hardiness of the Antarctic winged midge Parochlus steinenii during the active season at King George Island. Polar Biology. 1991; 11(5): 311–4. [Google Scholar]
- 4. Gnerre S, MacCallum I, Przybylski D, Ribeiro FJ, Burton JN, Walker BJ, Sharpe T, Hall G, Shea TP, Sykes S: High-quality draft assemblies of mammalian genomes from massively parallel sequence data. Proc Nat Acad Sci. 2011; 108(4): 1513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. FASTX-Toolkit. http://hannonlab.cshl.edu/fastx_toolkit. Accessed 22 November 2016.
- 6. Marçais G, Kingsford C: A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 2011; 27(6): 764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. GenomeScope. http://qb.cshl.edu/genomescope. Accessed 22 November 2016.
- 8. Vurture GW, Sedlazeck FJ, Nattestad M, Underwood CJ, Fang H, Gurtowski J, Schatz M: GenomeScope: Fast reference-free genome profiling from short reads. bioRxiv. 2016; 075978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parra G, Bradnam K, Korf I: CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics. 2007; 23(9): 1061–7. [DOI] [PubMed] [Google Scholar]
- 10. Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM: BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015; btv351. [DOI] [PubMed] [Google Scholar]
- 11. Kelley JL, Peyton JT, Fiston-Lavier A-S, Teets NM, Yee M-C, Johnston JS, Bustamante CD, Lee RE, Denlinger DL: Compact genome of the Antarctic midge is likely an adaptation to an extreme environment. Nature Commun. 2014; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tarailo-Graovac M, Chen N: Using RepeatMasker to identify repetitive elements in genomic sequences. Current Protocols in Bioinformatics. 2009; 4.10.11–14.10.14. [DOI] [PubMed] [Google Scholar]
- 13. Bao Z, Eddy SR: Automated de novo identification of repeat sequence families in sequenced genomes. Genome Res. 2002; 12(8): 1269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Price AL, Jones NC, Pevzner PA: De novo identification of repeat families in large genomes. Bioinformatics. 2005; 21(Suppl 1):i351–8. [DOI] [PubMed] [Google Scholar]
- 15. Benson G: Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999; 27(2): 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lowe TM, Eddy SR: tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997; 25(5): 955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Repbase library. http://www.girinst.org/repbase/index.html. Accessed 22 November 2016.
- 18. Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J: Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005; 110(1-4): 462–7. [DOI] [PubMed] [Google Scholar]
- 19. Cantarel BL, Korf I, Robb SM, Parra G, Ross E, Moore B, Holt C, Alvarado AS, Yandell M: MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 2008; 18(1): 188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holt C, Yandell M: MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinformatics. 2011; 12(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Korf I: Gene finding in novel genomes. BMC Bioinformatics. 2004; 5(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M: Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005; 21(18): 3674–6. [DOI] [PubMed] [Google Scholar]
- 23. Li L, Stoeckert CJ, Roos DS: OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003; 13(9): 2178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ensemble Metazoa. http://metazoa.ensembl.org/index.html. Accessed 22 November 2016.
- 25. Du Z, Zhou X, Ling Y, Zhang Z, Su Z: agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010; gkq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Y, Brandizzi F: IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013; 23(11): 547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lynch M, Conery JS: The evolutionary fate and consequences of duplicate genes. Science. 2000; 290(5494): 1151–5. [DOI] [PubMed] [Google Scholar]
- 28. Hahn MW, De Bie T, Stajich JE, Nguyen C, Cristianini N: Estimating the tempo and mode of gene family evolution from comparative genomic data. Genome Res. 2005; 15(8): 1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Bie T, Cristianini N, Demuth JP, Hahn MW: CAFE: a computational tool for the study of gene family evolution. Bioinformatics. 2006; 22(10): 1269–71. [DOI] [PubMed] [Google Scholar]
- 30. Loytynoja A, Goldman N: An algorithm for progressive multiple alignment of sequences with insertions. Proc Natl Acad Sci U S A. 2005; 102(30): 10557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Talavera G, Castresana J: Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007; 56(4): 564–77. [DOI] [PubMed] [Google Scholar]
- 32. Saitou N, Nei M: The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987; 4(4): 406–25. [DOI] [PubMed] [Google Scholar]
- 33. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S: MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013; 30(12): 2725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hedges SB, Dudley J, Kumar S: TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics. 2006; 22(23): 2971–2. [DOI] [PubMed] [Google Scholar]
- 35. Hahn MW, Han MV, Han S-G. Gene family evolution across 12 Drosophila genomes. PLoS Genet. 2007; 3(11):e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim S, Oh M, Jung W, Park J, Choi H, Shin SC. Supporting data for "Genome sequencing of the winged midge, Parochlus steinenii, from the Antarctic Peninsula" GigaScience Database. 2016; http://dx.doi.org/10.5524/100256. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1 tRNA in Parochlus steinenii. (DOCX 21 kb)
Additional file 1: Table S2 Shared orthologous gene clusters among six insects—Drosophila melanogaster, Anopheles gambiae, Aedes aegypti, Culex quinquefasciatus, Belgica antarctica and Parochlus steinenii
Additional file 1: Table S3 Gene families were significantly expanded in Antarctic midges