Abstract
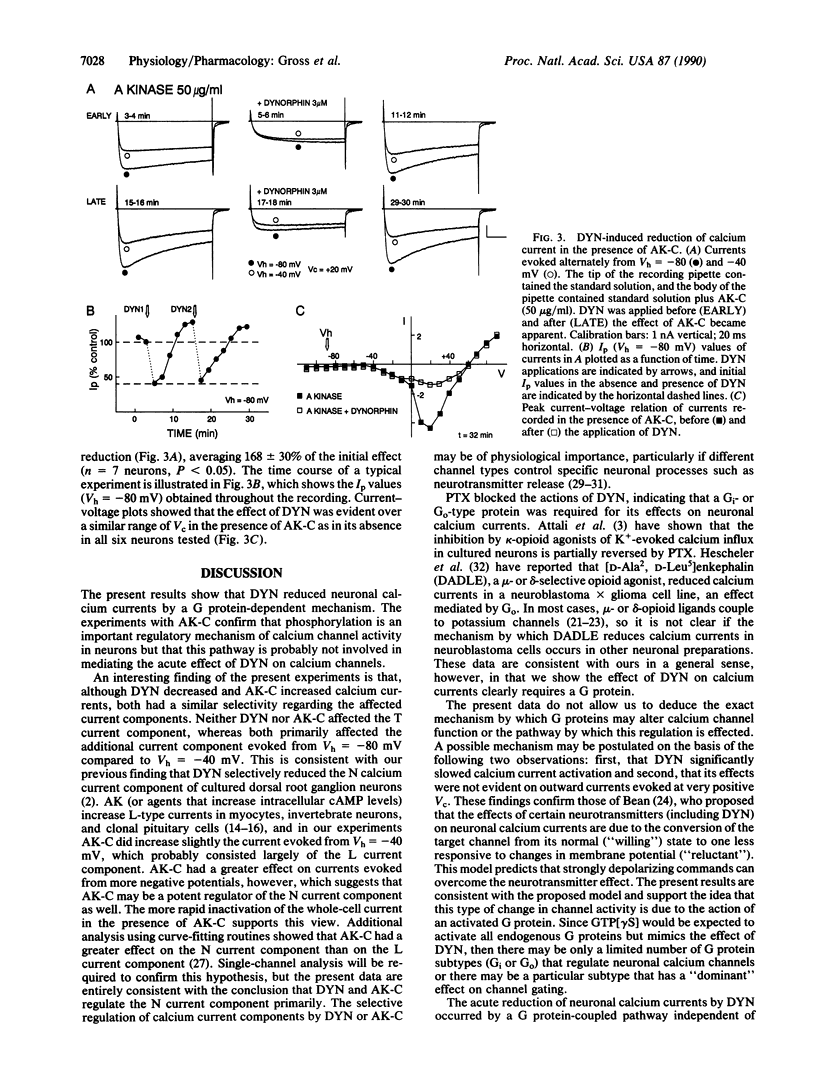
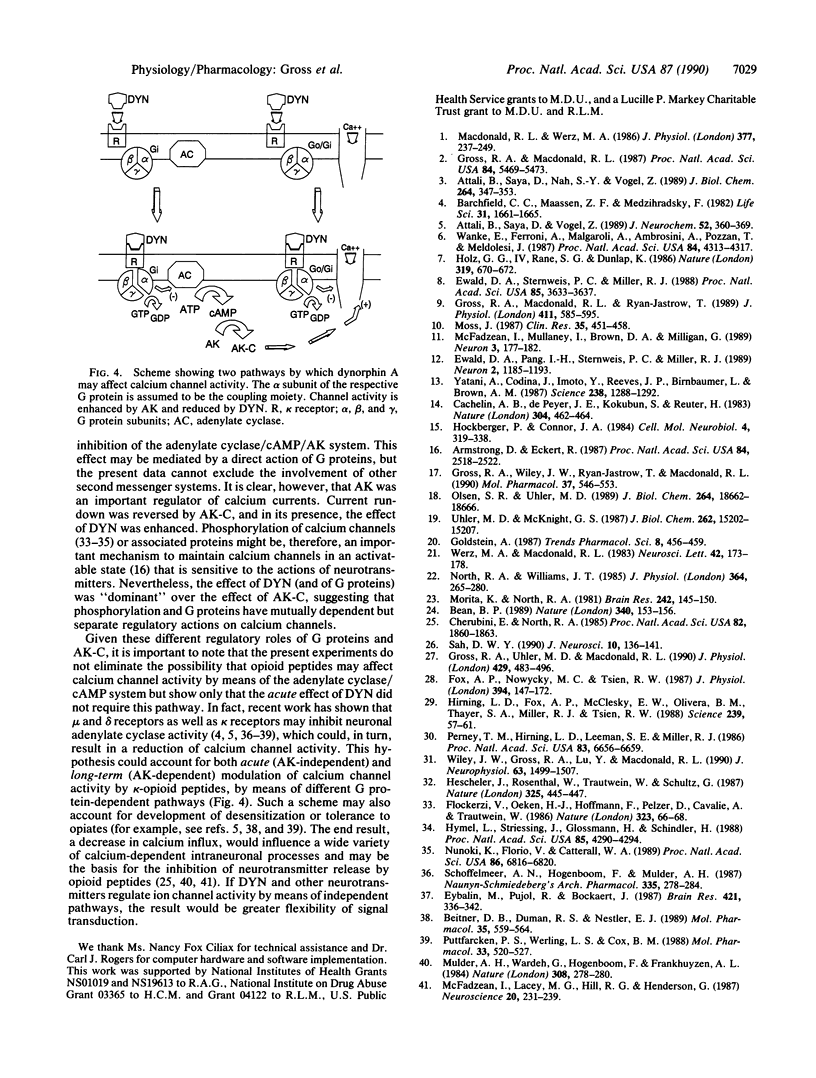
The kappa-selective opioid peptide dynorphin A (DYN) inhibits neuronal adenylate cyclase activity and reduces neuronal voltage-dependent calcium currents. It is not yet known, however, whether the regulation of calcium channel activity is dependent on or independent of the adenylate cyclase/cAMP system. We used the whole-cell variation of the patch clamp technique to show that DYN reversibly reduced, in a naloxone-sensitive manner, calcium currents in acutely dissociated rat nodose ganglion neurons. DYN slowed the rate of current activation and had a greater effect on currents evoked from relatively negative holding potentials. These actions were mimicked by guanosine 5'-[gamma-thio]triphosphate, which activates GTP-binding proteins (G proteins), and were blocked by pretreatment with pertussis toxin, which inactivates Gi- and Go-type G proteins. In contrast, calcium currents recorded in the presence of the catalytic subunit of the cAMP-dependent protein kinase (AK-C), included in the recording pipette, increased in magnitude throughout the recording. DYN was applied to neurons before and after the effect of AK-C became apparent; the reduction of calcium currents by DYN was greater in the presence of AK-C than in its absence. We conclude that the acute reduction of neuronal calcium currents by DYN occurred by means of activation of pertussis toxin-sensitive Gi- or Go-type G proteins. The persistence of the action of DYN in the presence of AK-C indicates, however, that this effect was independent of a reduction of the activity of the adenylate cyclase/cAMP system and suggests in addition that phosphorylated channels may be preferentially inhibited by DYN.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali B., Saya D., Nah S. Y., Vogel Z. Kappa opiate agonists inhibit Ca2+ influx in rat spinal cord-dorsal root ganglion cocultures. Involvement of a GTP-binding protein. J Biol Chem. 1989 Jan 5;264(1):347–353. [PubMed] [Google Scholar]
- Attali B., Saya D., Vogel Z. Kappa-opiate agonists inhibit adenylate cyclase and produce heterologous desensitization in rat spinal cord. J Neurochem. 1989 Feb;52(2):360–369. doi: 10.1111/j.1471-4159.1989.tb09130.x. [DOI] [PubMed] [Google Scholar]
- Barchfeld C. C., Maassen Z. F., Medzihradsky F. Receptor-related interactions of opiates with PGE-induced adenylate cyclase in brain. Life Sci. 1982 Oct 18;31(16-17):1661–1665. doi: 10.1016/0024-3205(82)90180-1. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Beitner D. B., Duman R. S., Nestler E. J. A novel action of morphine in the rat locus coeruleus: persistent decrease in adenylate cyclase. Mol Pharmacol. 1989 May;35(5):559–564. [PubMed] [Google Scholar]
- Cachelin A. B., de Peyer J. E., Kokubun S., Reuter H. Ca2+ channel modulation by 8-bromocyclic AMP in cultured heart cells. Nature. 1983 Aug 4;304(5925):462–464. doi: 10.1038/304462a0. [DOI] [PubMed] [Google Scholar]
- Cherubini E., North R. A. Mu and kappa opioids inhibit transmitter release by different mechanisms. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1860–1863. doi: 10.1073/pnas.82.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald D. A., Pang I. H., Sternweis P. C., Miller R. J. Differential G protein-mediated coupling of neurotransmitter receptors to Ca2+ channels in rat dorsal root ganglion neurons in vitro. Neuron. 1989 Feb;2(2):1185–1193. doi: 10.1016/0896-6273(89)90185-2. [DOI] [PubMed] [Google Scholar]
- Ewald D. A., Sternweis P. C., Miller R. J. Guanine nucleotide-binding protein Go-induced coupling of neuropeptide Y receptors to Ca2+ channels in sensory neurons. Proc Natl Acad Sci U S A. 1988 May;85(10):3633–3637. doi: 10.1073/pnas.85.10.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eybalin M., Pujol R., Bockaert J. Opioid receptors inhibit the adenylate cyclase in guinea pig cochleas. Brain Res. 1987 Sep 22;421(1-2):336–342. doi: 10.1016/0006-8993(87)91303-5. [DOI] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H. J., Hofmann F., Pelzer D., Cavalié A., Trautwein W. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986 Sep 4;323(6083):66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. A., Macdonald R. L. Dynorphin A selectively reduces a large transient (N-type) calcium current of mouse dorsal root ganglion neurons in cell culture. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5469–5473. doi: 10.1073/pnas.84.15.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. A., Macdonald R. L., Ryan-Jastrow T. 2-Chloroadenosine reduces the N calcium current of cultured mouse sensory neurones in a pertussis toxin-sensitive manner. J Physiol. 1989 Apr;411:585–595. doi: 10.1113/jphysiol.1989.sp017592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. A., Uhler M. D., Macdonald R. L. The cyclic AMP-dependent protein kinase catalytic subunit selectively enhances calcium currents in rat nodose neurones. J Physiol. 1990 Oct;429:483–496. doi: 10.1113/jphysiol.1990.sp018268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. A., Wiley J. W., Ryan-Jastrow T., Macdonald R. L. Regulation by GTP and its stable thiol derivatives of calcium current components in rat nodose ganglion neurons. Mol Pharmacol. 1990 Apr;37(4):546–553. [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Hockberger P., Connor J. A. Alteration of calcium conductances and outward current by cyclic adenosine monophosphate (cAMP) in neurons of Limax maximus. Cell Mol Neurobiol. 1984 Dec;4(4):319–338. doi: 10.1007/BF00733595. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymel L., Striessnig J., Glossmann H., Schindler H. Purified skeletal muscle 1,4-dihydropyridine receptor forms phosphorylation-dependent oligomeric calcium channels in planar bilayers. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4290–4294. doi: 10.1073/pnas.85.12.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L., Werz M. A. Dynorphin A decreases voltage-dependent calcium conductance of mouse dorsal root ganglion neurones. J Physiol. 1986 Aug;377:237–249. doi: 10.1113/jphysiol.1986.sp016184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadzean I., Lacey M. G., Hill R. G., Henderson G. Kappa opioid receptor activation depresses excitatory synaptic input to rat locus coeruleus neurons in vitro. Neuroscience. 1987 Jan;20(1):231–239. doi: 10.1016/0306-4522(87)90015-7. [DOI] [PubMed] [Google Scholar]
- McFadzean I., Mullaney I., Brown D. A., Milligan G. Antibodies to the GTP binding protein, Go, antagonize noradrenaline-induced calcium current inhibition in NG108-15 hybrid cells. Neuron. 1989 Aug;3(2):177–182. doi: 10.1016/0896-6273(89)90030-5. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A. Opiate activation of potassium conductance in myenteric neurons: inhibition by calcium ion. Brain Res. 1982 Jun 17;242(1):145–150. doi: 10.1016/0006-8993(82)90504-2. [DOI] [PubMed] [Google Scholar]
- Moss J. Signal transduction by receptor-responsive guanyl nucleotide-binding proteins: modulation by bacterial toxin-catalyzed ADP-ribosylation. Clin Res. 1987 Sep;35(5):451–458. [PubMed] [Google Scholar]
- Mulder A. H., Wardeh G., Hogenboom F., Frankhuyzen A. L. Kappa- and delta-opioid receptor agonists differentially inhibit striatal dopamine and acetylcholine release. Nature. 1984 Mar 15;308(5956):278–280. doi: 10.1038/308278a0. [DOI] [PubMed] [Google Scholar]
- North R. A., Williams J. T. On the potassium conductance increased by opioids in rat locus coeruleus neurones. J Physiol. 1985 Jul;364:265–280. doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoki K., Florio V., Catterall W. A. Activation of purified calcium channels by stoichiometric protein phosphorylation. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6816–6820. doi: 10.1073/pnas.86.17.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen S. R., Uhler M. D. Affinity purification of the C alpha and C beta isoforms of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1989 Nov 5;264(31):18662–18666. [PubMed] [Google Scholar]
- Perney T. M., Hirning L. D., Leeman S. E., Miller R. J. Multiple calcium channels mediate neurotransmitter release from peripheral neurons. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6656–6659. doi: 10.1073/pnas.83.17.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttfarcken P. S., Werling L. L., Cox B. M. Effects of chronic morphine exposure on opioid inhibition of adenylyl cyclase in 7315c cell membranes: a useful model for the study of tolerance at mu opioid receptors. Mol Pharmacol. 1988 May;33(5):520–527. [PubMed] [Google Scholar]
- Sah D. W. Neurotransmitter modulation of calcium current in rat spinal cord neurons. J Neurosci. 1990 Jan;10(1):136–141. doi: 10.1523/JNEUROSCI.10-01-00136.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer A. N., Hogenboom F., Mulder A. H. Inhibition of dopamine-sensitive adenylate cyclase by opioids: possible involvement of physically associated mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Mar;335(3):278–284. doi: 10.1007/BF00172797. [DOI] [PubMed] [Google Scholar]
- Uhler M. D., McKnight G. S. Expression of cDNAs for two isoforms of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1987 Nov 5;262(31):15202–15207. [PubMed] [Google Scholar]
- Wanke E., Ferroni A., Malgaroli A., Ambrosini A., Pozzan T., Meldolesi J. Activation of a muscarinic receptor selectively inhibits a rapidly inactivated Ca2+ current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4313–4317. doi: 10.1073/pnas.84.12.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz M. A., MacDonald R. L. Opioid peptides selective for mu- and delta-opiate receptors reduce calcium-dependent action potential duration by increasing potassium conductance. Neurosci Lett. 1983 Dec 2;42(2):173–178. doi: 10.1016/0304-3940(83)90402-0. [DOI] [PubMed] [Google Scholar]
- Wiley J. W., Gross R. A., Lu Y. X., Macdonald R. L. Neuropeptide Y reduces calcium current and inhibits acetylcholine release in nodose neurons via a pertussis toxin-sensitive mechanism. J Neurophysiol. 1990 Jun;63(6):1499–1507. doi: 10.1152/jn.1990.63.6.1499. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Imoto Y., Reeves J. P., Birnbaumer L., Brown A. M. A G protein directly regulates mammalian cardiac calcium channels. Science. 1987 Nov 27;238(4831):1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]