Abstract
We report the construction of a tetracycline inducible expression vector that allows regulated gene expression in the enteric pathogen Vibrio cholerae. The expression vector, named pXB300, contains the tetracycline regulatory elements from Tn10, a multiple cloning site downstream of the tetA promoter and operator sequences, a ColE1 origin of replication, a β-lactamase resistance gene for positive selection, and the hok/sok addiction system for selection in the absence of antibiotic. The function of the tetracycline expression system was demonstrated by cloning lacZ under control of the tetA promoter and quantifying β-galactosidase expression in Escherichia coli and V. cholerae. The utility for pXB300 was documented by complementation of V. cholerae virulence mutants during growth under virulence inducing conditions. The results showed that pXB300 allowed high-level expression of recombinant genes with linear induction in response to the exogenous concentration of the inducer anhydrotetracycline. We further show that pXB300 was reliably maintained in V. cholerae during growth in the absence of antibiotic selection.
Keywords: Vibrio cholerae, anhydrotetracycline, ToxR regulon, expression vector
1. Introduction
Vibrio cholerae is an important human pathogen that is responsible for as many as 11 million cases of the acute intestine disease cholera each year (Ali et al., 2012). V. cholerae resides in aquatic environments, from which people acquire cholera by ingestion of V. cholerae contaminated food or water (Kaper et al., 1995). Once ingested, V. cholerae activates a complex hierarchical regulatory cascade that results in the production of virulence factors, like cholera toxin (CT), that are essential for disease (reviewed in (Reidl and Klose, 2002)). CT is an enterotoxin that is responsible for the hallmark secretory diarrhea that is associated with cholera. CT production is positively regulated by the ToxR regulon in response to environmental cues. Expression of the ToxR regulon is initiated by AphA and AphB activation of tcpP expression. TcpP then binds with ToxR at the toxT promoter and activates toxT expression. ToxT then functions as the terminal regulator in the ToxR regulon and positively regulates the expression of a number of virulence genes, including ctxAB which encode for CT.
Plasmids have played an important role in defining and characterizing the function of genes that contribute to V. cholerae virulence. One of the primary uses of plasmid vectors in V. cholerae has been to facilitate recombinant gene expression. The ability to regulate the expression of a gene has been advantageous for both virulence studies and physiological studies; including complementation studies, phenotypic characterization of the effects of gene expression (or depletion), and expression of deleterious genes. The pBAD series of expression vectors have been widely used for regulated gene expression in V. cholerae (Guzman et al., 1995). The pBAD expression vectors are based on the E. coli arabinose regulatory system and modulate recombinant gene expression in response to exogenous arabinose (reviewed in (Schleif, 2010)). While pBAD vectors have proven useful under many growth conditions, the use of arabinose as an inducer can be problematic in some situations due to its dependence on cell transport systems for uptake, its metabolic breakdown which occurs in some vibrios (Amaral et al., 2014), the susceptibility of the arabinose regulated expression system to catabolite repression (Casadaban, 1976), and the undesirable effect of arabinose on endogenous gene expression in V. cholerae and other Vibrios (Ali et al., 2005; Visick et al., 2013).
In this report we generated the expression vector pXB300 for use in V. cholerae. pXB300 utilized the backbone of pBAD18, but replaced the arabinose regulatory system with the tetracycline regulatory elements from the Escherichia coli Tn10 tetracycline (Tet) resistance operon (Hillen and Berens, 1994). We further incorporated a multiple cloning site downstream of the tetA promoter/operator to facilitate DNA cloning. The utility of pXB300 was demonstrated by characterizing the effects of inducer concentration on the expression of β-galactosidase in E. coli and V. cholerae. The results showed inducer concentration-dependent gene expression in both backgrounds. We showed a linear relationship between inducer concentration and gene expression and that pXB300 can be used under virulence gene inducting conditions to complement V. cholerae ToxR regulon mutants. pXB300 was also found to be stable in the absence of antibiotic selection.
2. Materials and methods
2.1 Bacterial strains and culture conditions
Bacterial strains used in this study are listed in Table 1. E. coli EC100Dpir was used for cloning experiments. V. cholerae strains used in this study were derivatives of O1 El Tor strain N16961 (Cameron et al., 2008; Heidelberg et al., 2000; Thelin and Taylor, 1996). V. cholerae strain JB58 (N16961-ΔlacZ SmR) was used as the wild-type (WT) strain for all studies. Bacterial strains were grown at 37°C in Luria-Bertani (LB) broth or on LB agar. In vitro induction of the ToxR regulon was achieved by growth under AKI conditions (i.e. virulence inducing conditions) as described previously (Danese and Silhavy, 1997). Bacterial stocks were stored at −80°C in LB broth containing 25% glycerol. Carbenicillin (Cb) and streptomycin (Sm) were used at 100 μg/mL as needed. Culture media was purchased from Difco (Lawrence, KS) and chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Table 1.
Strains, plasmids and oligonucleotides.
| Strain: | Genotype: | Source: |
|---|---|---|
| Vibrio cholerae | ||
| JB58 | 01 El Tor strain N16961 ΔlacZ Streptomycin-resistant | (Bina et al., 2006) |
| JB460 | JB58 ΔtoxT | (Bina et al., 2003) |
| XBV153 | JB58 ΔaphA | This study |
| Escherichia coli | ||
| EC100Dpir+ | F− mcrA Δ(mrr-hsdRMS-mcrBC) Φ80dlacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara, leu)7697 galU galK λ− rpsL (SmR) nupG pir+ | Epicentre |
| SM10 pir+ | thi-1 thr leu tonA lacY supE recA∷RP4-2-4-Tc∷Mu KmR (λ pirR6K) | (Miller and Mekalanos, 1988) |
| Plasmid: | Description: | |
| pBAD24 | Expression plasmid, CbR, pBR322 origin of replication | (Guzman et al., 1995) |
| pEW1 | pXB300-aphA | This study |
| pJB510 | pBAD24∷toxT, CbR | (Bina et al., 2003) |
| pTL61T | Promoter probe vector containing lacZ; CbR | (Linn and St Pierre, 1990) |
| pWM91 | Allelic exchange vector, CbR | (Metcalf and Wanner, 1993) |
| pXB248 | pWM91 containing 1.9 kb aphA deletion construct | This study |
| pXB300 | TetR-regulated expression vector, pBR322 origin of replication, CbR | This study |
| pXB308 | pXB300-∷lacZ, CbR | This study |
| pXB320 | pXB300-∷toxT, CbR | This study |
| pXB324 | pBAD24-∷lacZ, CbR | This study |
| Oligonucleotides: | DNA sequence (5′ – 3′): | |
| toxT-F-BamHI | ATGGATCCTTCAGAGTAGAACGCAATGATTGG | |
| toxT-R-EcoRV | CTGATATCTAGGATCAAGTAAACGTATTCC | |
| aphA-F-SacI | CGGAGCTCTGGATTGAAGACATGTCATTACC | |
| aphA-R-BamHI | CCGGATCCTTTGGCTTGGCTTATGCCATCGC | |
| lacZ-F-SmaI | ATCATCGGAGCTCTCGAGTCAGCCC | |
| lacZ-R-SphI | AAGCATGCGGGGAGGCAGACAAGGTATAG | |
| aphA-F1-BamHI | TTGGATCCGGCAAGCTGCCATTGGGTTCCAGACCCG | |
| aphA-F2 | AACCGGGTACGATGCCGCTTATTACGCTAACCCAGCCGTG | |
| aphA-R1-SacI | TTGAGCTCCCGAGTATCTCAGAAGCGGCGGCGTGTG | |
| aphA-R2 | CGTAATAAGCGGCATCGTACCCGGTTGCATCGCGTGTGCTAAG |
2.2 Plasmid and mutant construction
pXB300 was constructed as depicted in Fig. 1. Briefly, a 1.4 kb fragment was designed in silico and synthesized by Life Technologies (Grand Island, NY) to generate p11AAZDYP. The synthesized fragment contained a 579 bp fragment of plasmid R1 (X05813.1) encoding the hok-sok addiction system linked to a 723 bp Tn10 fragment that encoded tetR and the tetA promoter/operator followed by a 115 bp fragment that encoded the pBBR1MCS (U02374.1) multiple cloning site. p11AAZDYP was digested with PacI restriction endonuclease and blunt ended by treatment with the Klenow fragment of DNA polymerase before being digested with SalI to release the 1.4 kb fragment containing the hok-sok-tetR-PtetAO1-O2-MCS DNA fragment. This 1.4 kb fragment was then ligated with the 3.28 kb fragment that was released from pBAD18 that had been digested with ClaI, treated with Klenow, before being digested with SalI. This ligation resulted in the generation of plasmid pXB300. The DNA sequence of pXB300 has been submitted to GeneBank and also available upon request.
Fig. 1.
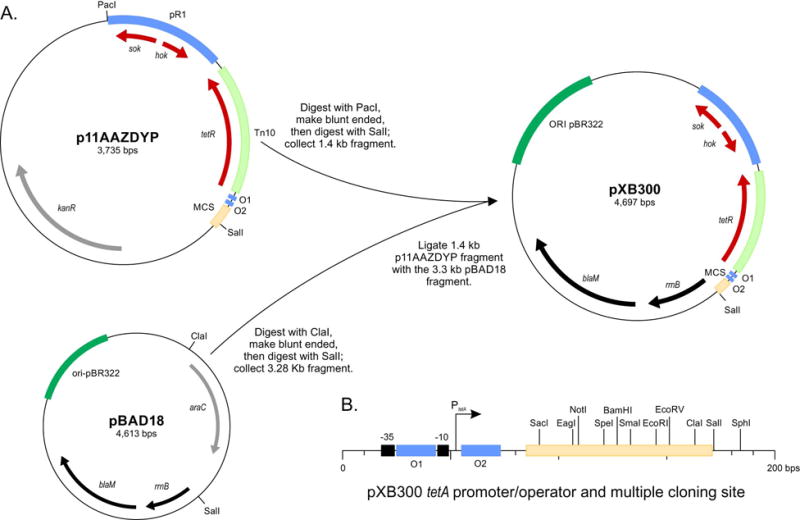
Schematic of pXB300 construction. (A.) The steps in the construction of pXB300 are indicated. p11AAZDYP was digested with PacI, treated with the Klenow fragment of DNA polymerase to blunt the ends, then digested with SalI. In a separate reaction, pBAD18 was digested with ClaI, treated with the Klenow fragment of DNA polymerase to blunt the ends, and then digested with SalI. The resulting 1.4 Kb fragment from p11AAZDYP was then ligated with the 3.3 kb pBAD18 fragment to generate pXB300. (B.) The pXB300 multiple cloning site region showing unique restrictions sites, the tetA promoter including the -35 and -10 promoter elements, and the TetR operator sequences (O1 and O2).
Derivatives of pXB300 expressing aphA and toxT were generated by PCR amplification of the respective genes using the aphA-F/aphA-R and toxT-F/toxT-R PCR primer pairs to amplify the respective genes from the N16961 ΔlacZ SmR (JB58) chromosome. The resulting PCR fragments were digested with restriction endonucleases (SacI and BamHI for aphA; BamHI and EcoRV for toxT) before being ligated into similarly digested pXB300 to generate pEW1 and pXB320, respectively. The E. coli lacZ gene was cloned under control of the tetA promoter in pXB300 and the arabinose regulated promoter in pBAD24 as follows. The lacZ-F/R PCR primer pairs were used to amplify the E. coli lacZ gene from pTL61T. The resulting PCR amplicon was digested with SmaI and SphI before being ligated with similarly digested pXB300 or pBAD24 to generate pXB308 (pXB300-lacZ) and pXB324 (pBAD24-lacZ). DNA sequencing was used to verify all plasmids.
The allelic exchange vector pWM91∷ΔaphA was generated by crossover PCR as previously described (Bina and Mekalanos, 2001; Bina et al., 2006; Bina et al., 2008; Imai et al., 1991). Briefly, the aphA-F1-BamHI/aphA-R2 and aphA-F2/aphA-R1-SacI primer pairs (Table 1) were used in separate PCR reactions with N16961 ΔlacZ SmR (JB58) genomic DNA as a template. The resulting ~1Kb PCR products were gel purified, pooled, and then used as the template for a second PCR reaction using the flanking aphA-F1-BamHI/aphA-R1-SacI PCR primers to generate the ~2 Kb aphA deletion fragment. The resulting ~2kb PCR amplicon was then purified, restricted with BamHI and SacI endonucleases before being ligated with similarly digested pWM91 to generate pXB248. The resulting plasmid was then used to delete aphA in JB58. Briefly, pXB208 was conjugated into JB58 and cointegrants were selected for Cb/Sm resistance. Cb/Sm resistant colonies were then plated onto LB agar without NaCl and containing 5% sucrose to select for the resolution of the integrated plasmid. Sucrose-resistant colonies were then screened for Cb sensitivity to confirm plasmid loss before deletion of aphA was confirmed by PCR using flanking primers.
2.3 β-galactosidase assay
The tested strains were grown in LB broth and culture aliquots were taken at the indicated time points to quantify β-galactosidase activity as previously described (Bina et al., 2013; Provenzano et al., 2000). All experiments were performed at least three times and the results averaged. The reported results are in Miller Units (MU) and were not background normalized. Statistical significance was determined using ANOVA with indicated post-test.
2.4 Plasmid stability determination
The stability of pXB300 in V. cholerae was determined as follows. N16961 ΔlacZ SmR (JB58) containing pXB300 was passaged daily for three days in LB broth with or without Cb. An aliquot of each culture was serially diluted and plated onto LB agar and LB agar + Cb each day to quantify colony forming units (CFU) per ml of culture. The ratio of pXB300 in the cell population at each time point was then estimated by dividing the Cb-resistant CFU/ml (i.e. pXB300 positive cells) by the total number of CFU/ml recovered from LB agar plates at each time point.
2.5 Cholera toxin production
CT production was quantified using a GM1 ganglioside ELISA as previously described (Bina et al., 2008; Taylor, 2012). Polyclonal CT antiserum that was kindly provided by John Mekalanos (Harvard Medical School, Boston, MA) and purified CT was purchased from Sigma and used as the standard.
3. Results and discussion
3.1 Construction of pXB300
Arabinose inducible pBAD plasmids have been widely used for ectopic gene expression in V. cholerae. However, the use of arabinose as an inducer is problematic in some situations. For example, arabinose has been shown to function as an environmental cue in V. cholerae and other Vibrio species (Ali et al., 2005; Visick et al., 2013). Arabinose also is affected by catabolite repression and is dependent upon cellular transporter for uptake (Casadaban, 1976; Schleif, 2010). We therefore set out to construct an expression vector that was suitable for use in V. cholerae while circumventing these potential problems.
The regulatory elements from the Tn10 tetracycline resistance operon provide an alternative expression system that alleviates the concerns described above. The Tn10 tetracycline resistance locus encodes two divergently transcribed genes, tetA and tetR, which are expressed from overlapping promoters. TetA is a tetracycline efflux pump while TetR is a tetracycline-responsive transcriptional repressor. This system has evolved to be tightly regulated as overproduction of the TetA efflux pump is deleterious. In the absence of an inducer (e.g. tetracycline), TetR binds to the O1 and O2 operator sequences in the tetA-tetR promoter region (Fig 1B) and blocks tetA and tetR expression. Tetracycline, when present, binds with a high affinity to TetR and causes a conformational change which in turn results in TetR dissociation from the operator sequences and derepresses tetA expression. The regulatory elements of the tetracycline resistance locus have been widely adapted for regulated gene expression and have been shown to be tightly regulated and independent of catabolite repression. Uptake of tetracycline into bacterial cells occurs by diffusion, thus eliminating the requirement for active uptake systems for activity. Potential growth inhibition due to the inducer tetracycline can also be alleviated by use of low toxicity tetracycline derivatives like anhydrotetracycline (aTc) which has been shown to bind to TetR ~35-fold more strongly than tetracycline (Degenkolb et al., 1991; Gossen and Bujard, 1993).
The scheme for producing pXB300 is shown in Fig. 1A. The construction of pXB300 utilized the pBAD18 backbone; which contains the pBR322 origin of replication and is stably maintained in V. cholerae. We took advantage of unique restriction sites in pBAD18 to delete the arabinose regulatory region while retaining the origin of replication, rrnB transcriptional terminator, and the β-lactamase resistance gene (Fig. 1A). We then ligated this backbone to a 1.4 kb cassette that was designed in silico and synthesized by a commercial vendor (Life Technologies) to include the Tn10 tetracycline regulatory elements (i.e. the tetA/tetR promoters and tetR). The multiple cloning sites from pBBR1MCS (U02374.1) was placed downstream of the tetA promoter/operator sequence to facilitate gene cloning (Fig. 1B). We also included a 579 bp fragment from plasmid R1 (X05813.1) that contained the hok/sok plasmid addiction system to enhance plasmid stability in the absence of antibiotic selection.
3.2 Functional characterization of the tetracycline regulatory expression system in pXB300
The tetracycline resistance operon from Tn10 is regulated in response to the presence of tetracycline. Anhydrotetracycline is a low toxicity derivative of tetracycline which can be used to control gene expression from the TetR-regulated tetA promoter (Degenkolb et al., 1991; Oliva et al., 1992). To determine the limits for use of aTc in V. cholerae we determined the aTc MIC using gradient agar plates as previously described (Taylor et al., 2012). The results showed that the MIC for aTc for V. cholerae was 300 ng/ml while aTc MIC for E. coli was 3 ug/ml which is in agreement with previous findings (Oliva et al., 1992). The 10-fold difference in the aTc MIC between V. cholerae and E. coli is similar to the respective tetracycline MICs reported for each organism (Morris et al., 1985; Oliva et al., 1992).
To validate the function of the tetracycline regulatory system we cloned the E. coli lacZ gene into the multiple cloning site of pXB300. The lacZ gene encodes for β-galactosidase; an enzyme that is frequently used as a reporter for transcriptional studies. The resulting plasmid, pXB308, expressed lacZ from the tetracycline regulated tetA promoter in pXB300. Overnight broth cultures of E. coli EC100∷pXB308 and V. cholerae JB58∷pXB308 were then diluted 1:100 into fresh LB+Cb broth supplemented with varying concentrations of aTc. The cultures were then incubated with shaking at 37°C for two hours when β-galactosidase activity was assayed. The results showed an aTc concentration-dependent increase in β-galactosidase production in both E. coli and V. cholerae (Fig. 2). This confirmed that the tetracycline regulatory system in pXB300 functioned properly in both backgrounds. The apparent linear increase in β-galactosidase production with increasing aTc concentrations suggested that tetA promoter expression was titratable by aTc in both E. coli and V. cholerae. In E. coli β-galactosidase production increased linearly and reached a maximum level at ~40 ng/mL aTc. The results in V. cholerae were similar. Importantly, the concentration of aTc that was required to induce lacZ gene expression in V. cholerae was >10-fold lower than the aTc MIC. There was little lacZ expression in both strains in the absence of aTc, which is consistent with tight repression of the tetA promoter in the absence of inducer. The maximal level of β-galactosidase production was about two-fold higher in E. coli relative to V. cholerae. It is unclear whether this represents increased lacZ expression in E. coli or differences in lacZ abundance or stability in the two host backgrounds. Overall, these results confirmed that the tetracycline regulatory elements engineered into pXB300 functioned properly in V. cholerae and E. coli.
Fig. 2.
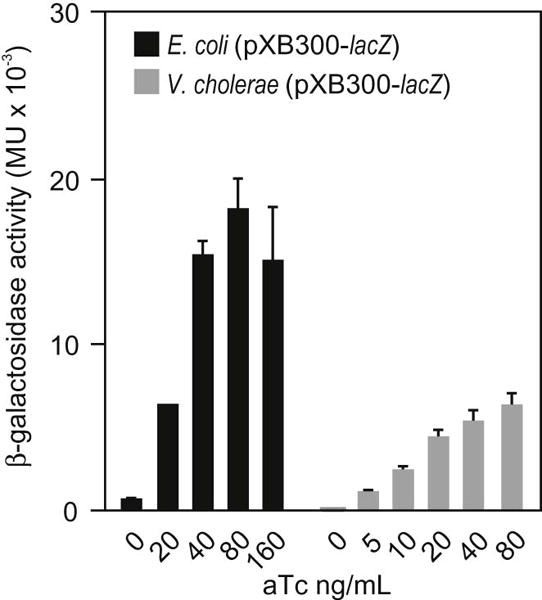
Effect of anhydrotetracycline (aTc) on lacZ expression in plasmid pXB308. E. coli and V. cholerae containing pXB308 (pXB300-lacZ) were diluted (1:100) from overnight cultures into LB broth supplemented with Cb and the indicated concentrations of aTc. The cultures were then incubated with shaking at 37°C for 2 hours before β-galactosidase activity was determined. The results are the average ± SD. of three experiments.
The arabinose regulated pBAD series of expression vectors have been widely used for gene expression in V. cholerae (Guzman et al., 1995). We therefore compared expression from the aTc regulated tetA promoter in pXB300 to the PBAD promoter in pBAD24. This was accomplished by generating a dose response curve for lacZ expression in N16961 ΔlacZ SmR (JB58) containing pXB300-lacZ and pBAD24-lacZ using the respective inducers for each system (i.e. aTc for pXB300 and L-arabinose for pBAD24). In these experiments, V. cholerae JB58(pXB300-lacZ) and JB58(pBAD24-lacZ) were independently cultured in LB broth containing Cb and varying concentrations of inducer (Fig. 3A and 3B). To ensure that both expression systems were maximally induced, high concentrations of the arabinose or aTc inducer were included. The results showed a linear response of both promoters to their respective inducers at the lower concentrations. High concentrations of arabinose or aTc maximized lacZ expression from strain JB58(pXB300-lacZ) or JB58(pBAD24-lacZ), respectively. β-galactosidase production in JB58(pXB300-lacZ) reached a maximum of about 9,800 MU with 80 ng/ml of aTc. By contrast, β-galactosidase production in JB58(pBAD24-lacZ) reached a maximum of approximately 4,500 MU at an arabinose concentration of ~0.1%. As both plasmids contain the same origin of replication and lacZ allele, the fact that β-galactosidase production was ~2-fold higher in the pXB300-lacZ cultures relative to pBAD24-lacZ indicates that the tetA promoter exhibits a greater induction potential in V. cholerae relative to the arabinose promoter.
Fig. 3.
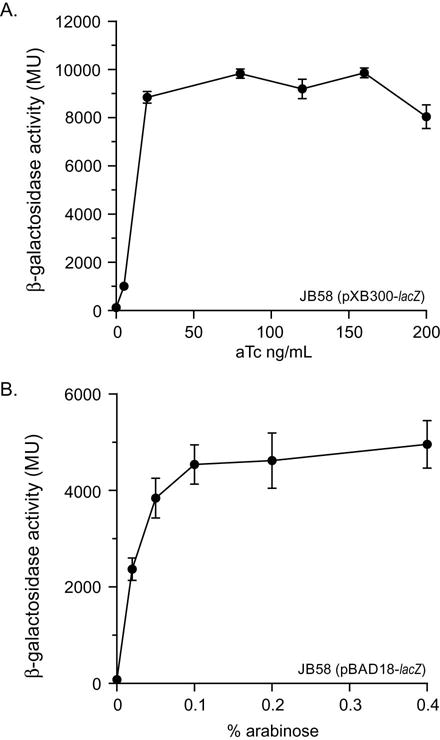
Comparison of lacZ expression from the PtetA and PBAD promoters in V. cholerae. (A) An overnight culture of V. cholerae JB58 (pXB300-lacZ) was diluted 1:100 into LB broth supplemented with Cb and the indicated concentrations of aTc. The cultures were then incubated at 37°C with shaking for 2.5 h before quantification of β-galactosidase. (B) An overnight culture of V. cholerae JB58 (pBAD18-lacZ) was diluted 1:100 into LB broth supplemented with Cb and the indicated concentrations of L-arabinose. The cultures were then incubated at 37°C with shaking for 2.5 h before quantification of β-galactosidase. The results are the average ± SD of three experiments.
3.4 Complementation V. cholerae aphA and toxT mutants for cholera toxin production
Production of the major V. cholerae virulence factors are under control of a hierarchical regulatory system called the ToxR regulon that responds to environmental cues in the intestinal tract. In vitro induction of the ToxR regulon in El Tor biotype strains can be achieved by using artificial in vitro conditions called AKI growth conditions. To validate the use of pXB300 in V. cholerae pathogenesis studies, we performed complementation experiments with aphA and toxT virulence mutants. We selected these two genes for analysis because both are members of the ToxR regulon, and thus are required for virulence factor production. AphA is a DNA binding protein that functions with AphB to activate expression of tcpP (Skorupski and Taylor, 1999). TcpP then binds with ToxR to the toxT promoter to activate its expression (Hase and Mekalanos, 1998). ToxT is the most downstream activator in the ToxR regulon and directly activates the expression of genes that are required for the production of CT and other virulence factors.
In these experiments, we compared the effect of varying concentrations of aTc on CT production in aphA and toxT deletion mutants that were respectively complemented with pXB300 expressing aphA or toxT. We introduced pXB300-aphA into V. cholerae strain XBV153 (ΔaphA), which contained an in-frame deletion of aphA. The resulting strains were then grown under AKI conditions in AKI broth with Cb and varying concentrations of aTc as indicated. Following overnight growth, CT production was assessed by a GM1-ELISA as described in the methods. The results showed that the ΔaphA mutant did not produce CT in the absence of inducer, whereas the WT strain produced 1300–1,500ng/ml/OD of CT. This confirmed, as expected, that aphA was required for virulence factor production. In the pXB300-aphA complemented strain there was a linear increase in CT production in the presence of aTc with CT production becoming saturated between 1 and 5 ng/ml aTc (Fig. 4A). At 5 ng/ml the amount of CT was marginally elevated relative to the WT control and the amount of CT did not change with increasing aTc. The complementation results from the ΔtoxT mutant mirrored those from the ΔaphA mutant. The ΔtoxT mutant JB460 containing pXB300∷toxT did not produce CT when grown under AKI conditions in the absence of aTc, but there was a linear increase in CT production with the addition of aTc, reaching a maximal level at ~10 ng/ml aTc.
Fig. 4.
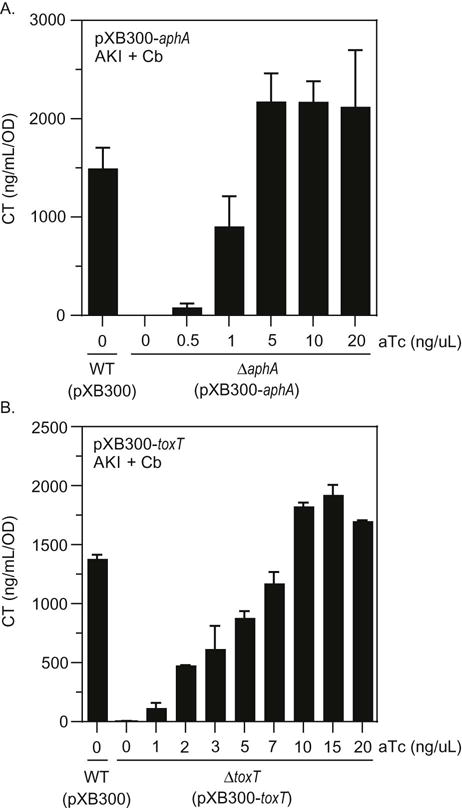
Complementation of V. cholerae virulence mutants for cholera toxin production. The V. cholerae ToxR regulon mutant strains were grown overnight under AKI conditions in AKI broth supplemented with the indicated concentrations of aTc. The culture supernatants were then used in the cholera toxin (CT) ELISA to quantify CT production as described in the methods. (A) V. cholerae strain XBV153 (ΔaphA) containing pEW1 (pXB300-aphA). (B) V. cholerae strain JB460 (ΔtoxT) containing pXB320 (pXB300-toxT). The results are representative of three experiments ± SD.
The above results confirmed that the tetA promoter is tightly repressed in V. cholerae in the absence of inducer under the in vitro virulence inducing AKI growth condition. This was evidenced by the lack of CT production in both the aphA and toxT complemented mutants in the absence of aTc. The results also show that there was a linear relationship between the amount of exogenous aTc added to the culture media and CT production. This confirmed that the tetA promoter that is driving expression of the recombinant genes (i.e. aphA and toxT) can be titrated by the amount of inducer. Taken together, the complementation results show that the tetA promoter is tightly repressed in the absence of inducer and confirm that pXB300 can be used for complementation and gene expression studies in V. cholerae during growth under AKI conditions.
3.3 pXB300 is stable in the absence of antibiotic selection in V. cholerae
The use of antibiotics for plasmid maintenance can be problematic under some instances. This can include drug studies where the presence of antibiotics can be antagonist or synergistic, or during in vivo studies where maintaining antibiotic selection is difficult. One approach to circumvent these problems is to incorporate a plasmid addiction system which functions to maintain plasmid selection in the absence of antibiotics. One of the best studied addiction systems is the hok/sok system from plasmid R1. Since the hok/sok system was previously shown to stabilize luciferase-expressing plasmids in V. cholerae (Morin and Kaper, 2009), we engineered the hok/sok system into pXB300 to provide stability in the absence of antibiotic selection. To test if the hok/sok addiction system works in V. cholerae, we analyzed the plasmid stability by growing V. cholerae carrying pXB300 in LB broth or LB supplemented with Cb for a period of 72 hrs. Culture aliquots were collected at the end of each 24 hr growth period and used to inoculate fresh LB broth cultures. A portion of the aliquot was also used to estimate the ratio of cells maintaining pXB300 by plating serial dilutions onto LB agar and LB agar supplemented with Cb. The results showed that the ratio of pXB300 positive cells (i.e. Cb resistant cells) relative to the total CFU on the nonselective LB plate was 0.8, 1.1 and 1.0 following at 24, 48 and 72 hrs. Similar ratios were obtained in the cultures grown in the presence of Cb. Thus, we concluded that pXB300 can be stably maintained in the absence of antibiotic selection.
The results presented in Table 2 indicate that pXB300 is stable in the absence of antibiotic selection. To further expand on these results we tested whether these results would translate to the expression of a recombinant gene in the presence of aTc. We hypothesized that the expression of a recombinant gene, like lacZ, would represent a metabolic burden to the cell, which would select for loss of the plasmid in the absence of antibiotic selection. We therefore cultured N16961 ΔlacZ SmR (JB58) bearing pXB300-lacZ (pXB308) in LB broth with and without Cb in the presence of varying aTc concentrations. Following 24 hrs of growth, we assayed for β-galactosidase activity in each of the cultures. The results of this assay showed that there was no significant difference in β-galactosidase activity between cultures grown in LB and LB supplemented with Cb (Fig. 5). This provides additional evidence to show that pXB308 is stably maintained in the absence of antibiotic selection in V. cholerae.
Table 2.
Maintenance of pXB300 in Vibrio cholerae.
| Culture medium | CFU Ratio (Lb+Cb/Lb) | |
|---|---|---|
| 1st passage (24h) | LB + Cb | 1.2 |
| LB | 0.8 | |
| 2nd passage (48h) | LB + Cb | 0.7 |
| LB | 1.1 | |
| 3rd passage (72h) | LB + Cb | 1.0 |
| LB | 1.0 |
Fig. 5.
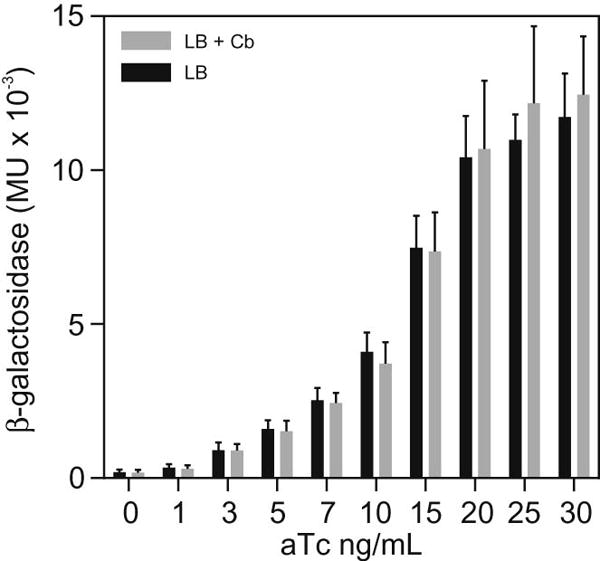
β-galactosidase production in V. cholerae JB58(pXB300-lacZ) grown in LB with or without antibiotics. V. cholerae JB58(pXB300-lacZ) was cultured at 37°C with shaking in LB broth or LB+Cb and the indicated concentrations of aTc. Aliquots from the cultures were collected at 24 hours and β-galactosidase production was quantified as described in the methods. The results are the average ± SD of three experiments.
3.5 Summary and conclusions
Herein we report the construction of pXB300, a new tetracycline regulated expression vector for use in V. cholerae. This vector contains the pBR322 ColE1 origin of replication and the hok/sok addiction system to stabilize the plasmid in the absence of antibiotic selection. The ColE1 origin of replication is widely in bacterial research which suggests that pXB300 could be used in a broad range of bacteria. Tetracycline-dependent gene expression is mediated by inclusion of tetR and the tetR/tetA promoter/operator elements from Tn10. A MCS was placed downstream from the TetR-regulated tetA promoter to facilitate the cloning of target sequences under control of the tetracycline regulated tetA promoter. Expression from the tetA promoter was shown to be titratable by exogenous aTc with maximum expression achieved at aTc levels well below the concentration which inhibited bacterial growth. The tetA promoter was shown to be tightly repressed in V. cholerae in the absence of effector. Using lacZ as a reporter, the maximal level of expression from the tetA promoter in V. cholerae was about two-fold higher than what was observed for the E. coli arabinose promoter in pBAD18, indicating a larger induction potential in the tet regulatory system relative to the arabinose regulatory system.
In conclusion, pXB300 expands the set of genetic tools that can be used for the genetic manipulation of V. cholerae. The collective results suggest that pXB300 will be a useful vector for regulated gene expression in V. cholerae and likely other bacterial genera. pXB300 provides an alternative to pBAD plasmid series for use in situations where arabinose may be problematic.
Research Highlights.
-
➢
A tetracycline-regulated expression vector was constructed for Vibrio cholera
-
➢
The vector was based on the Tn10 tetracycline regulatory elements
-
➢
Heterologous gene expression was titratable with anhydrotetracycline
-
➢
The expression vector encoded a multiple cloning site and plasmid addiction system
-
➢
The expression vector represents a new tool for Vibrio cholerae genetic analysis
Acknowledgments
This work was supported by National Institutes of Health award R01AI091845. EAW was supported by NIH training grant T32 AI060525
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali A, et al. Sugars inhibit expression of the rugose phenotype of Vibrio cholerae. J Clin Microbiol. 2005;43:1426–9. doi: 10.1128/JCM.43.3.1426-1429.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, et al. The global burden of cholera. Bull World Health Organ. 2012;90:209–218A. doi: 10.2471/BLT.11.093427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral GR, et al. Genotype to phenotype: identification of diagnostic vibrio phenotypes using whole genome sequences. Int J Syst Evol Microbiol. 2014;64:357–65. doi: 10.1099/ijs.0.057927-0. [DOI] [PubMed] [Google Scholar]
- Bina J, et al. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc Natl Acad Sci U S A. 2003;100:2801–6. doi: 10.1073/pnas.2628026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bina JE, Mekalanos JJ. Vibrio cholerae tolC is required for bile resistance and colonization. Infect Immun. 2001;69:4681–5. doi: 10.1128/IAI.69.7.4681-4685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bina JE, et al. Characterization of the Vibrio cholerae vexAB and vexCD efflux systems. Arch Microbiol. 2006;186:171–81. doi: 10.1007/s00203-006-0133-5. [DOI] [PubMed] [Google Scholar]
- Bina XR, et al. Vibrio cholerae RND family efflux systems are required for antimicrobial resistance, optimal virulence factor production, and colonization of the infant mouse small intestine. Infect Immun. 2008;76:3595–605. doi: 10.1128/IAI.01620-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bina XR, et al. Vibrio cholerae ToxR downregulates virulence factor production in response to cyclo(Phe-Pro) MBio. 2013;4:e00366–13. doi: 10.1128/mBio.00366-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DE, et al. A defined transposon mutant library and its use in identifying motility genes in Vibrio cholerae. Proc Natl Acad Sci U S A. 2008;105:8736–41. doi: 10.1073/pnas.0803281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban MJ. Regulation of the regulatory gene for the arabinose pathway, araC. J Mol Biol. 1976;104:557–66. doi: 10.1016/0022-2836(76)90120-0. [DOI] [PubMed] [Google Scholar]
- Danese PN, Silhavy TJ. The sigma(E) and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 1997;11:1183–93. doi: 10.1101/gad.11.9.1183. [DOI] [PubMed] [Google Scholar]
- Degenkolb J, et al. Structural requirements of tetracycline-Tet repressor interaction: determination of equilibrium binding constants for tetracycline analogs with the Tet repressor. Antimicrob Agents Chemother. 1991;35:1591–5. doi: 10.1128/aac.35.8.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Anhydrotetracycline, a novel effector for tetracycline controlled gene expression systems in eukaryotic cells. Nucleic Acids Res. 1993;21:4411–2. doi: 10.1093/nar/21.18.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman LM, et al. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–30. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase CC, Mekalanos JJ. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A. 1998;95:730–4. doi: 10.1073/pnas.95.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberg JF, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–83. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W, Berens C. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu Rev Microbiol. 1994;48:345–69. doi: 10.1146/annurev.mi.48.100194.002021. [DOI] [PubMed] [Google Scholar]
- Imai Y, et al. A simple and rapid method for generating a deletion by PCR. Nucleic Acids Res. 1991;19:2785. doi: 10.1093/nar/19.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper JB, et al. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T, St Pierre R. Improved vector system for constructing transcriptional fusions that ensures independent translation of lacZ. J Bacteriol. 1990;172:1077–84. doi: 10.1128/jb.172.2.1077-1084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf WW, Wanner BL. Construction of new beta-glucuronidase cassettes for making transcriptional fusions and their use with new methods for allele replacement. Gene. 1993;129:17–25. doi: 10.1016/0378-1119(93)90691-u. [DOI] [PubMed] [Google Scholar]
- Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–83. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CE, Kaper JB. Use of stabilized luciferase-expressing plasmids to examine in vivo-induced promoters in the Vibrio cholerae vaccine strain CVD 103-HgR. FEMS Immunol Med Microbiol. 2009;57:69–79. doi: 10.1111/j.1574-695X.2009.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JG, Jr, et al. In vitro susceptibility of pathogenic Vibrio species to norfloxacin and six other antimicrobial agents. Antimicrob Agents Chemother. 1985;28:442–5. doi: 10.1128/aac.28.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva B, et al. Evidence that tetracycline analogs whose primary target is not the bacterial ribosome cause lysis of Escherichia coli. Antimicrob Agents Chemother. 1992;36:913–9. doi: 10.1128/aac.36.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano D, et al. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect Immun. 2000;68:1491–7. doi: 10.1128/iai.68.3.1491-1497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidl J, Klose KE. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol Rev. 2002;26:125–39. doi: 10.1111/j.1574-6976.2002.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Schleif R. AraC protein, regulation of the l-arabinose operon in Escherichia coli, and the light switch mechanism of AraC action. FEMS Microbiol Rev. 2010;34:779–96. doi: 10.1111/j.1574-6976.2010.00226.x. [DOI] [PubMed] [Google Scholar]
- Skorupski K, Taylor RK. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol Microbiol. 1999;31:763–71. doi: 10.1046/j.1365-2958.1999.01215.x. [DOI] [PubMed] [Google Scholar]
- Taylor DL, et al. Vibrio cholerae VexH encodes a multiple drug efflux pump that contributes to the production of cholera toxin and the toxin co-regulated pilus. PLoS One. 2012;7:e38208. doi: 10.1371/journal.pone.0038208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DL, Bina XR, Bina JE. Vibrio cholerae vexH Encodes a Multiple Drug Efflux Pump that Contributes to the Production of Cholera Toxin and the Toxin Co-Regulated Pilus. Plos One. 2012;7:e38208. doi: 10.1371/journal.pone.0038208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelin KH, Taylor RK. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun. 1996;64:2853–6. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL, et al. Arabinose induces pellicle formation by Vibrio fischeri. Appl Environ Microbiol. 2013;79:2069–80. doi: 10.1128/AEM.03526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


