ABSTRACT
Programmed death ligand 1 (PD-L1) expression represents a mechanism of immune escape by inhibiting T cell immunity. This study systematically evaluated the expression of PD-L1, spatial distribution of CD3+ immune cells and the relationship of both factors to survival in nasopharyngeal carcinoma (NPC) patients. A total of 209 NPC patients treated between 1991 and 2000 were included. Pairs of TMAs were immunohistochemically stained with PD-L1 and CD3. Survival analysis was evaluated according to PD-L1 status and the spatial distribution of CD3+ immune cells in the primary lesion microenvironment. PD-L1 staining was observed on tumor cells and tumor-infiltrating immune cells (TILs); however, PD-L1-positive immune cells were more common (98/209) than PD-L1-positive tumor cells (68/209). Limited numbers of intra-tumoral CD3+ T cells (median number: 20) were detected. Patients with higher CD3+ T cell infiltration, both intratumorally and peritumorally, had higher PD-L1 expression on tumor cells (both p < 0.001) and immune cells (p = 0.002 and p < 0.001, respectively). Increasing intratumoral CD3 infiltration was correlated with increased overall survival (OS) (p = 0.008) and disease-free survival (DFS) (p = 0.003). Nevertheless, patients with low levels of peritumoral TILs showed superior OS (p = 0.557) and DFS to those with higher levels of peritumoral TILs (p = 0.671). Moreover, type classification based on intratumoral CD3 infiltration and tumor cell PD-L1 expression was an independent prognostic factor for NPC patients. PD-L1 expression on tumor cells is a favorable prognosis factor in NPC patients with pre-existing intratumor-infiltrating lymphocytes.
KEYWORDS: Nasopharyngeal carcinoma, PD-L1, prognosis, tumor-infiltrating lymphocytes
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant cancer that arises from the epithelial surface of the nasopharynx, with the highest incidence in Guangdong Province.1 The anatomic structure of the nasopharynx and the nonspecific symptoms of NPC contribute to the late diagnosis of NPC patients.2 With the technical improvements attained in radiotherapy delivery, excellent local control can now be achieved.3 However, metastasis remains the leading cause of mortality in advanced stage disease.4 Therefore, novel and effective therapy for NPC is urgently needed in the clinic.
Recently, a series of randomized clinical trials showed that immune checkpoint inhibitors, particularly anti-programmed death-1 (PD-1) and anti-programmed death ligand 1 (PD-L1) antibodies, had remarkable clinical efficacy in numerous advanced solid cancers.5-9 Importantly, the treatment response to nivolumab, an anti-PD1 antibody, was correlated with the expression of PD-L1 in a subset analysis.9 This finding can help identify suitable patients for treatment, i.e., patients who will benefit from immunomodulatory agents. Four different types of tumor microenvironments have been proposed to exist based on the presence or absence of tumor-infiltrating lymphocytes (TILs) and PD-L1 expression to predict responders to immune checkpoint inhibitors.10 However, TIL presence alone, as a dichotomous variable, might be insufficient for prediction, as Tumeh et al. suggested that both the density and location of TILs and their interaction with a PD-L1-positive tumor microenvironment must be considered.11 Erdag et al. suggested a sub-stratified classification based on the spatial distribution of immune infiltration (immune contexture).12 Moreover, emerging clinical and preclinical data suggest that the expression of PD-L1 on tumors is not an oncogenic driver but rather a co-opted and maladaptive immune “shield” that protects the tumor from its immune microenvironment.13,14 However, the clinical significance of the spatial distribution of TILs and its relationship with PD-L1 expression in NPC has not been determined.
In the present study, we used pairs of tissue microarrays (TMAs), including 209 NPC specimens with a long follow-up at Sun Yat-Sen University (Guangdong Province), to investigate the expression of PD-L1 and TILs in NPC. We examined the expression of CD3+ lymphocytes that infiltrated the tumor nest (intratumoral lymphocytes) and the expression of CD3+ lymphocytes that infiltrated the surrounding stroma (peritumoral lymphocytes). We also determined whether the expression of PD-L1 and TILs impacted patient survival.
Materials and methods
Patients
The study was conducted retrospectively with a cohort of 209 patients with primary NPC that had been treated with either chemotherapy alone or radical radiotherapy with or without chemotherapy between January 1991 and August 2000 at the nasopharyngeal department of the Sun Yat-Sen University Cancer Center (Guangzhou, China). Tumor grade and stage were defined according to the 7th edition of the UICC Staging System. After treatment, patients were observed at least once every 3 mo during the first 3 y and then every 6 mo thereafter until death. Nasopharyngoscopy, magnetic resonance imaging of the head and neck, chest radiography, abdominal sonography, whole-body bone scan or positron emission tomography-computed tomography were routinely performed annually and when clinically indicated tumor relapse occurred.
TMA staining and evaluation
Tissue microarray (TMA) construction consisted of 209 FFPE tissue blocks that were used for histologic evaluation of TIL distribution and PD-L1 expression in the local microenvironment as measured by immunohistochemistry (IHC).15 All patients in this study have provided written informed consent before being subjected to tumor tissue samples. TMA sections were deparaffinized with xylene, rehydrated, microwaved in EDTA (1 mmol/L, pH = 9.0) antigen retrieval buffer and then boiled for 15 min in a microwave oven. After washing with PBS, endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide for 10 min, and then nonspecific staining was blocked with goat serum for 30 min. Slides were incubated with a rabbit anti-human PD-L1 mAb (1:200; E1L3N, Cell Signaling Technology) or anti-CD3 rabbit polyclonal antibody (1:100; Golden Bridge Biotech, Beijing, China) overnight in a humidified chamber at 4 °C and then incubated with horseradish peroxidase-conjugated secondary antibody (EnvisionTM Detection Kit, GK500705, Gene Tech) for 30 min after washing three times with PBS. 3, 3′-diaminobenzidine tetrahydrochloride was used to develop positive signals, and the sections were counter-stained with hematoxylin. The slides were then dehydrated, cleared, and evaluated.
For tumor cells, the proportion of PD-L1-positive cells was estimated as the percentage of total tumor cells. Cases demonstrating ≥ 5% tumor cell expression were considered positive for this compartment, which is consistent with many other studies.16-19 For immune cells, variably sized aggregates were observed toward the periphery of the tumor mass, in stromal bands dissecting the tumor mass, as single cells scattered in stroma or within tumor-infiltrating immune cell aggregates. The percentage of PD-L1-positive immune cells occupying the tumor was scored as follows: IHC 0, < 1%; IHC 1, > 1% and < 5%, IHC 2, ≥ 5% and < 10%, and IHC 3, ≥ 10%. PD-L1 positivity was defined as ≥ 5% of cells per area being PD-L1 positive.20
IHC of CD3+ T cells populations within tumor were performed as reported previously.21 Slides were imaged at 4× and 20× using Vectra imaging software, and the number of CD3+ cells were enumerated from 20× fields using inForm analysis software (both from PerkinElmer). As CD3+ T cells are central to the adaptive immune environment, we quantified CD3+ intratumoral infiltration and associated peritumoral infiltration into the stroma using a representative scoring system. For limited infiltrating intratumoral CD3+ T cells, we counted and expressed the number of cells. For peritumoral CD3+ T cells, we calculated the percentage of CD3+ T cells that infiltrated the tumor stroma. The tissue sections were screened at low magnification (×100) and measured at ×400 magnification. The cut-off point was the median. All scoring was performed by pathologists (Mu-Yan Cai and Min Li) who were blinded to clinical outcomes.
Classification of tumor microenvironment based on TIL and PD-L1 expression
Four groups of NPC patients were classified according to tumor cells PD-L1 expression (TCs-PD-L1) status and the presence or absence of TILs as predictive biomarkers for anti-PD-1/PD-L1 antibodies10 as follows: type I (PD-L1+ TILshigh), type II (PD-L1− TILslow), type III (PD-L1+ TILslow) and type IV (PD-L1− TILshigh). Furthermore, we classified the immunological environment in the local lesion based on the combination of prognostic associated factors selected using the Kaplan–Meier method (intratumor CD3+ immune cells and TCs-PD-L1).
Statistical analysis
Statistical analyses were conducted using SPSS 17.0. Spearman's rank correlation analysis or the chi-square test was performed for the analysis of TILs and PD-L1 expression levels, as continuous or categorical variables, respectively, and their relationships with the clinicopathological features of patients. Survival curves were depicted using the Kaplan–Meier method and compared using the log-rank test. Overall survival (OS) of patients following treatment was calculated by considering all death events. Disease-free survival (DFS) of patients following treatment was calculated from the date of diagnosis to the date of the first recurrence/distant metastasis at any site, death from any cause, or the date of the last follow-up visit. In all analyses, p < 0.05 was considered statistically significant.
Results
Patient clinicopathological features
The clinicopathological characteristics of 209 NPC patients are presented in Table 1. The average age was 52 y, with a range of 20–75 y. There were 59 (28.2%) female patients and 150 (71.8%) male patients. Ten (4.8%) patients were diagnosed with stage I, 55 (26.3%) with stage II, 83 (39.7%) with stage III and 61 (29.2%) with stage IV. A total of 88 patients received only chemotherapy, and 121 patients were treated with radiotherapy with or without chemotherapy. Over a median follow-up time of 73 mo, 88 (42.1%) patients relapsed, and 79 (37.8%) died. The 5-y and 10-y DFS values were 64% and 54%, respectively, whereas the 5-y and 10-y OS values were 64% and 60%, respectively. All patients who had been treated with conventional radiotherapy were administered a dose of 1.8–2.2 Gy per fraction, with total doses ranging from 68 to 72 Gy.
Table 1.
Association between PD-L1 expression and clinicopathological features of nasopharyngeal carcinoma patients (N = 209)
| PD-L1 expression |
|||||||
|---|---|---|---|---|---|---|---|
| Feature | No. of patients | Tumor cellspos | Tumor cellsneg | p value | Immune cellspos | Immune cellsneg | p value |
| Gender | |||||||
| Female | 59 (28.2%) | 20 (33.9%) | 39 (66.1%) | 0.792 | 34 (34.7%) | 25 (65.3%) | 0.051 |
| Male | 150 (71.8%) | 48 (32%) | 102 (68%) | 64 (22.5%) | 86 (77.5%) | ||
| Age (years) | |||||||
| ≤ 45 | 89 (42.6%) | 30 (33.7%) | 59 (66.3%) | 0.755 | 48 (53.9%) | 41 (46.1%) | 0.079 |
| > 45 | 120 (57.4%) | 38 (31.7%) | 82 (68.3%) | 50 (41.7%) | 70 (58.3%) | ||
| T classification | |||||||
| T1 | 26 (12.5%) | 10 (38.5%) | 16 (61.5%) | 0.493 | 11 (42.3%) | 15 (57.7%) | 0.161 |
| T2 | 68 (32.5%) | 22 (32.4%) | 46 (67.6%) | 38 (55.9%) | 30 (44.1%) | ||
| T3 | 69 (33.0%) | 25 (36.2%) | 44 (63.8%) | 33 (47.8%) | 36 (52.2%) | ||
| T4 | 46 (22.0%) | 11 (23.9%) | 35 (76.1%) | 16 (34.8%) | 30 (65.2%) | ||
| N classification | |||||||
| N0 | 40 (19.1%) | 15 (37.5%) | 25 (62.5%) | 0.767 | 22 (55.0%) | 18 (45.0%) | 0.122 |
| N1 | 96 (45.9%) | 29 (30.2%) | 67 (69.8%) | 37 (38.5%) | 59 (61.5%) | ||
| N2 | 51 (24.4%) | 18 (35.3%) | 33 (64.7%) | 29 (56.9%) | 22 (43.1%) | ||
| N3 | 22 (10.5%) | 6 (27.3%) | 16 (72.7%) | 10 (45.5%) | 12 (54.5%) | ||
| M classification | |||||||
| M0 | 158 (75.6%) | 55 (34.8%) | 103 (65.2%) | 0.217 | 78 (49.4%) | 80 (50.6%) | 0.207 |
| M1 | 51 (24.4%) | 13 (25.5%) | 38 (74.5%) | 20 (39.2%) | 31 (60.8%) | ||
| Clinical stage | |||||||
| I | 10 (4.8%) | 6 (60.0%) | 4 (40.0%) | 0.034 | 5 (50.0%) | 5 (50.0%) | 0.195 |
| II | 55 (26.3%) | 15 (27.3%) | 40 (72.7%) | 26 (47.3%) | 29 (52.7%) | ||
| III | 83 (39.7%) | 33 (39.8%) | 50 (60.2%) | 45 (54.2%) | 38 (45.8%) | ||
| IV | 61 (29.2%) | 14 (23.0%) | 47 (77.0%) | 22 (36.1%) | 39 (63.9%) | ||
| Treatment | |||||||
| 1 | 42 (20.1%) | 11 (26.2%) | 31 (73.8%) | 0.154 | 15 (35.7%) | 27 (64.3%) | 0.091 |
| 2 | 88 (42.1%) | 25 (28.4%) | 63 (71.6%) | 39 (44.3%) | 49 (55.7%) | ||
| 3 | 79 (37.8%) | 32 (40.5%) | 47 (59.5%) | 44 (55.7%) | 35 (44.3%) | ||
| Histological differentiation | |||||||
| U | 54 (25.8%) | 21 (38.9%) | 33 (61.1%) | 0.247 | 30 (55.6%) | 24 (44.4%) | 0.138 |
| D | 155 (74.2%) | 47 (30.3%) | 108 (69.7%) | 68 (43.9%) | 87 (56.1%) | ||
| Peritumoral CD3 expression | |||||||
| Low | 105 (50.2%) | 15 (14.3%) | 90 (85.7%) | < 0.001 | 32 (69.5%) | 73 (30.5%) | < 0.001 |
| High | 104 (49.8%) | 53 (51.0%) | 51 (49.0%) | 66 (63.5%) | 38 (36.5%) | ||
| Intratumoral CD3 expression | |||||||
| Low | 105 (50.2%) | 18 (17.1%) | 87 (82.9%) | < 0.001 | 38 (36.2%) | 67 (63.8%) | 0.002 |
| High | 104 (49.8%) | 50 (48.1%) | 54 (51.9%) | 60 (57.7%) | 44 (42.3%) | ||
| Vital status | |||||||
| Alive | 130 (49.3%) | 49 (37.7%) | 81 (62.3%) | 0.041 | 65 (50.0%) | 65 (50.0%) | 0.248 |
| Dead | 79 (50.7%) | 19 (24.1%) | 60 (75.9%) | 33 (41.8%) | 46 (58.2%) | ||
PD-L1, Programmed death ligand 1.
Associations of CD3+ infiltration and PD-L1 expression with clinicopathological features
The IHC analysis of NPC tumor tissues showed that PD-L1 expression on NPC cells occurred predominantly in the cell membrane; variably strong staining was observed in the cytoplasm (Fig. 1A). CD3 expression in peritumoral and intratumoral areas showed spatial discordance in the primary lesion (Fig. 1B). Mixed immune infiltration was evident in the tumor (intratumoral region) and surrounding stroma (peritumoral region). The associations between PD-L1 (tumor cells and immune cells) expression and the clinicopathological features of the patients and CD3 (intratumorally and peritumorally) are presented in Table 1. PD-L1 expression on tumor cells (TCs-PD-L1) was associated with clinical stage, peritumoral CD3 expression, intratumoral CD3 expression and vital status, whereas no significant correlation was observed between TCs-PD-L1 and gender, age, T classification, N classification, M classification, treatment or histological differentiation. No significant correlation was observed between PD-L1 expression on immune cells (ICs-PD-L1) and any patient clinicopathological feature except peritumoral and intratumoral CD3 expression.
Figure 1.

Evaluation of CD3, PD-L1 protein and their associations in nasopharyngeal carcinoma patients. Tumor and immune cell PD-L1 protein (A) and intratumoral and peritumoral CD3+ lymphocytes (B). Correlation between intratumoral (C) or peritumoral CD3+ lymphocytes and TCs-PD-L1 (D), and correlation between intratumoral (E) or peritumoral CD3+ lymphocytes (F) and ICs- PD-L1 expression.
Higher CD3+ infiltration is associated with increased PD-L1 expression
Next, we evaluated the association between CD3+ lymphocyte spatial distribution and the expression of TCs-PD-L1 or ICs-PD-L1 in NPC. Patients with high levels of intratumoral TILs expressed higher TCs-PD-L1 and ICs-PD-L1 compared with those with weaker T cell infiltration into tumor nests (Table 1, p < 0.6001 and p = 0.002, respectively). A similar pattern was found between peritumoral TILs and PD-L1 expression both on tumor cells and tumor immune cells (Table 1, both p < 0.001). Similar results were obtained when intratumoral and peritumoral TIL levels were analyzed as continuous variables. Spearman analysis indicated associations between intratumoral/peritumoral TILs and TCs-PD-L1 levels (r = 0.477, p < 0.001; r = 0.440, p < 0.001; Fig. 1C and D; respectively). Moreover, a stronger association was detected between peritumoral TILs and ICs-PD-L1 (r = 0.311; p < 0.001; Fig. 1F) than between intratumoral TILs and ICs-PD-L1 (r = 0.211, p = 0.002; Fig. 1E).
Higher CD3+ infiltration and PD-L1 expression are associated with a favorable prognosis in NPC patients
Kaplan–Meier curves showed poorer OS (Fig. 2A) and DFS (Fig. 2B) for patients with CD3low TILs than for those with CD3high TILs (p = 0.183 and p = 0.138, respectively), but these differences were not statistically significant. Further analysis showed that patients with low intratumoral TILs demonstrated significantly worse OS (p = 0.008) and DFS (p = 0.003) than patients of the high intratumoral TIL group (Fig. 2C and D, respectively). The favorable outcome of the high intratumoral TIL group was also supported by Cox regression analysis (Table 2). Univariate analysis revealed lower risks of death (HR, 0.545; 95% CI, 0.346–0.859; p = 0.009) and disease relapse (HR, 0.522; 95% CI, 0.339–0.804; p = 0.003) for patients with high intratumoral TILs. By contrast, patients with low levels of peritumoral TILs showed superior OS (p = 0.557) and DFS (p = 0.671) compared with patients with high levels of peritumoral TILs (Fig. 2E and Fig. 2F, respectively). Cox regression analysis revealed no association between peritumoral TILs and the survival outcome of patients.
Figure 2.

Survival curves of different distributions of CD3+ TILs. Kaplan–Meier plots of OS (A) and DFS (B) according to the percentage of TILs. OS (C and E) and DFS (D and F) based on intratumoral and peritumoral CD3+ lymphocytes.
Table 2.
Cox proportional regression analysis for the prediction of nasopharyngeal patient OS and DFS
| Univariate analysis |
||||
|---|---|---|---|---|
| OS |
DFS |
|||
| Covariant | HR (95% CI) | p | HR (95% CI) | `p |
| Age (< 50 vs. ≥ 50) | 0.768 (0.494–1.194) | 0.241 | 0.742 (0.489–1.128) | 0.162 |
| Gender (Female vs. Male) | 0.863 (0.534–1.394) | 0.547 | 0.798 (0.509–1.251) | 0.326 |
| T classification (T1 vs. T2 vs. T3 vs.T4) | 1.774 (1.372–2.294) | < 0.001 | 1.765 (1.381–2.256) | < 0.001 |
| N classification (N0 vs. N1 vs. N2 vs. N3) | 1.660 (1.302–2.117) | < 0.001 | 1.686 (1.339–2.123) | < 0.001 |
| M classification (M0 vs. M1) | 2.783 (1.769–4.379) | < 0.001 | 2.660 (1.715–4.125) | < 0.001 |
| Clinical stage (I vs. II vs. III vs. IV) | 3.106 (1.679–5.746) | < 0.001 | 2.339 (1.742–3.141) | < 0.001 |
| Histological differentiation (U vs. D) | 1.727 (0.969–3.078) | 0.064 | 1.481 (0.882–2.489) | 0.138 |
| Treatment (1 vs. 2 vs. 3) | 0.571 (0.424–0.770) | < 0.001 | 0.565 (0.425–0.750) | < 0.001 |
| CD3 in total (High vs. Low) | 0.741 (0.475–1.156) | 0.186 | 0.730 (0.479–1.112) | 0.143 |
| Intratumoral CD3 (High vs. Low) | 0.545 (0.346–0.859) | 0.009 | 0.522 (0.339–0.804) | 0.003 |
| Peritumoral CD3 (High vs. Low) | 1.141 (0.734–1.774) | 0.559 | 1.094 (0.720–1.662) | 0.673 |
| ICs-PD-L1 (Postive vs. Negative) | 0.585 (0.309–1.106) | 0.099 | 0.571 (0.311–1.051) | 0.072 |
| TCs-PD-L1 (Postive vs. Negative) | 0.563 (0.335–0.945) | 0.030 | 0.597 (0.369–0.964) | 0.035 |
| Type in total CD3 (I vs. II vs. III vs. IV) | 1.143 (0.942–1.386) | 0.176 | 1.122 (0.934–1.348) | 0.220 |
| Type in intratumoral CD3 (I vs. II vs. III vs. IV) | 1.220 (1.008–1.478) | 0.041 | 1.180 (0.983–1.416) | 0.046 |
| Multivariate analysis |
||||
| OS |
DFS |
|||
| Covariant | HR (95% CI) | p | HR (95% CI) | p |
| T classification (T1 vs. T2 vs. T3 vs.T4) | 1.121 (0.820–1.531) | 0.475 | 1.113 (0.810–1.528) | 0.509 |
| N classification (N0 vs. N1 vs. N2 vs. N3) | 1.048 (0.772–1.422) | 0.763 | 0.982 (0.714–1.351) | 0.912 |
| M classification (M0 vs. M1) | 0.756 (0.377–1.516) | 0.431 | 0.673 (0.318–1.425) | 0.301 |
| Clinical stage (I vs. II vs. III vs. IV) | 2.412 (1.270–4.582) | 0.007 | 2.823 (1.417–5.625) | 0.003 |
| Treatment (1 vs. 2 vs. 3) | 0.447 (0.321–0.622) | < 0.001 | 0.444 (0.314–0.629) | < 0.001 |
| Intratumoral CD3 (High vs. Low) | 0.607 (0.371–0.995) | 0.084 | 0.624 (0.366–1.063) | 0.083 |
| TCs-PD-L1 (Postive vs. Negative) | 1.116 (0.647–1.924) | 0.693 | 1.037 (0.583–1.845) | 0.900 |
| Type in intratumoral CD3 (I vs. II vs. III vs. IV) | 1.387 (1.074–1.790) | 0.012 | 1.465 (1.113–1.928) | 0.006 |
TCs-PD-L1, Tumor Cells Programmed death ligand 1; ICs-PD-L1, Immune Cells Programmed death ligand 1.
The survival analysis highlighted the favorable outcome of patients overexpressing PD-L1 protein on tumors. Kaplan–Meier analysis (p = 0.027; Fig. 3A) and univariate Cox regression (HR, 0.563; 95% CI, 0.335–0.945; p = 0.030; Table 2) revealed an association between the overexpression of PD-L1 on tumor cells and superior OS outcome. Moreover, longer DFS intervals and a significantly lower risk for disease recurrence for patients with higher PD-L1 expression than for those with downregulated levels were revealed by Kaplan–Meier curves (p = 0.032; Fig. 3B) and univariate Cox regression analysis (HR, 0.597; 95% CI, 0.369–0.964; p = 0.035; Table 2). However, the association between ICs-PD-L1 and OS or DFS was not detected by the Kaplan–Meier curves (p = 0.278 and p = 0.290, respectively; Fig. 3C and D).
Figure 3.
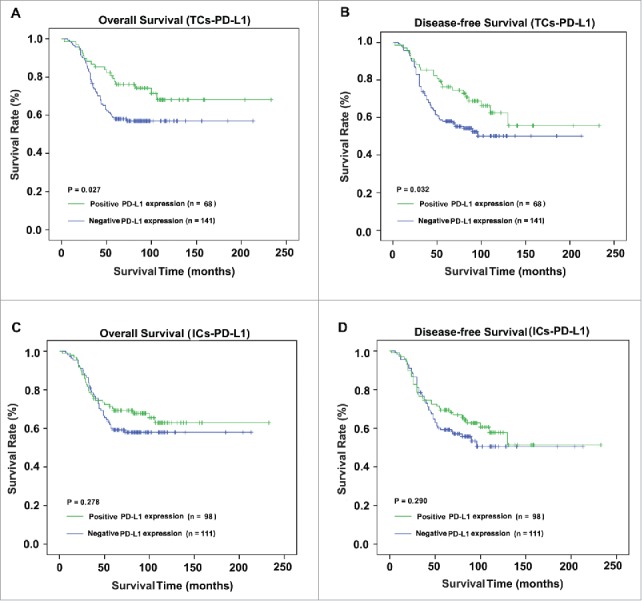
Survival curves of different distributions of PD-L1+ cells. The present study analyzed OS (A and C) and DFS (B and D) based on TCs-PD-L1 and ICs-PD-L1.
Multivariate Cox regression models, adjusted for T classification, N classification, M classification, clinical stage, treatment, intratumoral CD3 expression, TCs-PD-L1 and type based on TCs-PD-L1 and intratumoral CD3, were used to evaluate the independent prognostic factors of NPC patients (Table 2). Clinical stage (HR, 2.412; 95% CI, 1.270–4.582; p = 0.007), treatment (HR, 0.447; 95% CI, 0.321–0.622; p < 0.001) and type based on tumor PD-L1 and intratumoral CD3 expression (HR, 1.387; 95%CI, 1.074–1.790; p = 0.012) were independent predictors of superior OS. Similarly, multivariate Cox models showed independent correlations of clinical stage (HR, 2.823; 95% CI, 1.417–5.625; p = 0.003), treatment (HR, 0.444; 95% CI, 0.314–0.629; p < 0.001) and type based on tumor (HR, 1.465; 95%CI, 1.113–1.928; p = 0.006) with DFS interval of the patients following treatment.
Association of patient type with prognosis in NPC patients
Based on CD3+ lymphocytes (both intratumorally and peritumorally) and TCs-PD-L1, 52 (24.9%), 87 (41.6%), 18 (8.6%) and 52 (24.9%) patients were classified as having type I, II, III and IV tumor microenvironments, respectively. Significant differences in OS and DFS were not detected among the four types (p = 0.219 and p = 0.210, respectively; Fig. 4A and B, respectively). Further analysis was conducted to evaluate the difference in clinical significance based on the combination of prognostic associated factors (intratumor CD3+ immune cells and TCs-PD-L1). In this group, the mortality of type I, type II, type III and type IV was 9/50 (18%), 39/87 (44.8%), 10/18 (55.6%) and 21/54 (38.9%), respectively. Moreover, superior survival outcome was detected in type I (10-y OS: 78%; 10-y DFS: 72%) to that in type IV (10-y OS: 60%; 10-y DFS: 60%). Intriguingly, poorer survival outcome was detected in type II (10-y OS: 55%; 10-y DFS: 46%) than in type III (10-y OS: 40%; 10-y DFS: 36%) (Fig. 4C and D). Additionally, type based on intratumoral CD3 expression and TCs-PD-L1 was an independent factor for OS and DFS (p = 0.012 and p = 0.006, Table 2).
Figure 4.
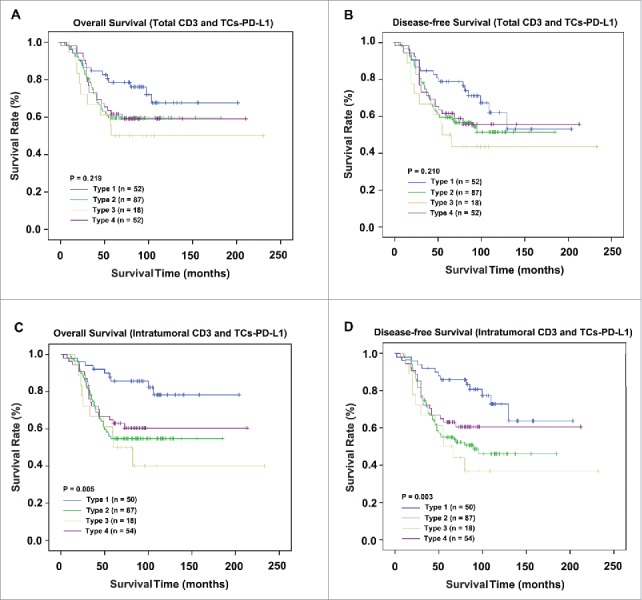
Comparison of survival according to staging system in 209 NPC patients. OS and DFS analyses according to the stage classification based on CD3+ lymphocytes and TCs-PD-L1 (A and B). Survival analysis based on intratumoral CD3+ lymphocytes and TCs-PD-L1 (C and D).
Discussion
PD-L1, which is expressed on immune cells and antigen-presenting cells including dendritic cells, macrophages and tumor cells, plays an important role in T cell tolerance by inhibiting naive and effector T cell responses.20 Currently, two mechanisms for the regulation of PD-L1 by tumor cells have been revealed: intrinsic immune resistance and adaptive resistance. Intrinsic resistance resulted in increased PD-L1 expression on tumor cells secondary to oncogenic signaling.13 It has been suggested that releasing the PD-1/PD-L1 checkpoint in pre-existing tumor antigen-specific T cells can lead to T cell proliferation, intratumoral infiltration and increased effector function.11 Adaptive immune resistance occurs when PD-L1 is upregulated in tumor cells in response to interferon gamma (IFNγ)-secreting CD8+ T cells.11,22,23 This mechanism is supported by the previous observations that PD-L1 expression is upregulated by T cell secretion of IFNγ and that patients with T-cell-rich tumors that express PD-L1 are likely to have better immune surveillance in laryngeal squamous cell carcinoma.16 Consistent with these observations, the present study detected a positive correlation between the spatial distribution of TILs and PD-L1 expression on surrounding immune cells and tumor cells. Patients with higher PD-L1 expression have higher CD3+ immune cells infiltration, reflecting the presence of endogenous antitumor immunity occurred in NPC patients, which explain why TCs-PD-L1 are likely to be a better survival factor.
The prognostic importance of tumor infiltration with CD3+ cells has been demonstrated in breast, esophageal, lung, ovarian, colon and anal cancers.24 In addition, Balermpas and colleagues reported that the intratumoral localization of TILs influences their prognostic impact.25 Tumeh et al. suggest that the presence of TILs is not a dichotomous variable and that the density and location of TILs and their interaction with the PD-L1-positive tumor microenvironment should be considered.11 Recent research has identified significant temporal and spatial discordance of PD-L1 and TIL expression between paired primary lesions and brain metastases in lung cancer.26 Advances in genetics have helped reveal the genetic complexity of melanoma and intratumoral heterogeneity.27,28 In addition, TCR sequencing has shown that differences in T cell receptor residents within these lesions are greater than the genetic differences between individual metastases.29 This discovery stimulates us to investigate whether the spatial discordance of PD-L1 and TILs in the primary lesion microenvironment has a different meaning.
Here, we evaluated the spatial discordance of PD-L1 (TCs and ICs) and CD3+ lymphocytes (intratumoral and peritumoral) in patients with NPC based on a large cohort (209 patients) with a long follow-up. In the present study, PD-L1 expression occurred at a lower level on tumor cells and immune cells than observed in previous reports as measured by IHC.30 This inconsistency may be attributed to study differences in detection antibodies, various cut-off values for positive and negative, or both. Limited numbers of intratumoral CD3+ T cells (median number: 20) were detected as measured by IHC in the present study. In many instances, effector T cells do not gain entry into the tumor bed because they are physically blocked by dense stroma or the tumor vasculature.10 Despite the presence of neo-antigens, there may be a lack of appropriate innate immune activation or chemokines required to promote T cell infiltration.10 TILs play an essential role in orchestrating the host immune response against cancer cells and may represent the antitumor immune response. A previous study found that a high number of TILs in melanoma tissues was associated with prolonged patient survival and reduced risk of metastasis.31 Although our analyses failed to show any significant association between the presence of TILs and the survival of NPC patients, patients with high intratumoral TILs had superior survival. Consistent with this latter result, a positive association was detected between TCs-PD-L1 and survival outcome. Intriguingly, the reverse result was obtained between survival and peritumoral TILs, although this association was not significant, which confirmed the necessity to differentiate the spatial distribution of TILs in the primary lesion. Although previous work found that the presence of intratumoral neutrophils was a poor prognostic factor for hepatocellular carcinoma after resection, intratumoral neutrophils were observed to frequently infiltrate the tumor stroma and remodel the barrier against cancer cell dissemination.32 These results suggested that pretumoral neutrophils might be a positive prognostic factor. Neutrophils can modulate the Th1/Th2 cytokine production of CD4+ T cells by the expression of lactoferrin33 or IL-634 and can enhance the suppressive activities of CD4+ suppressor T cells.35 Recently, a report found that a high density of macrophages in the peritumoral region was associated with vascular invasion and poor prognosis in HCC.36,37 In addition, co-operation exists between tumor-associated macrophages and both CD4+ T cells38 and CD8+ T cells.39 These observations reveal the existence of complex interaction mechanisms in the local microenvironment, which may be attributed to spatial differences. However, further experiments are needed to identify the molecular mechanisms.
Four different types of tumor microenvironment have been proposed for predicting which patients will respond to checkpoint blockade based on the presence or absence of TILs and PD-L1 expression. In previous reports, the type I (PD-L1 positive with TILs driving adaptive immune resistance) tumor microenvironment was predicted to be largely responsive to checkpoint blockade.16,40 For the type II and type III tumor microenvironments, single agent checkpoint blockade would most likely not be successful because of the lack of pre-existing T cell infiltrates. Furthermore, combination therapy designed to bring T cells into tumors and then inhibit them from being turned off would be considered in this scenario.10,41 The type IV microenvironment is PD-L1 negative with TILs, indicating a role of other suppressor(s) in promoting immune tolerance; this type may also be amenable to the targeting of other immunosuppressive pathways.10 Malignant melanoma has been extensively studied, and high proportions of type I and type II microenvironments have been observed10; however, this information is yet to be defined in NPC. The present study showed the highest fraction of microenvironments in NPC to be type II, which suggests that certain chemotherapies, small molecule targeted therapies and radiotherapy, which all debulk tumors, might be more promising strategies for NPC patients. Radiotherapy to induce immunogenic cell death to liberate neo-antigens has been used to induce T cell responses in combination with anti-PD-1.42 Moreover, the survival curves of the present study were distinct and significantly different for the classification system based on intratumoral TILs and TCs-PD-L1 compared with those based on total TILs and TCs-PD-L1. Furthermore, this classification is a significant independent prognostic factor for OS and DFS in NPC patients. When T cells are present in sufficient number inside the tumor to induce the adaptive expression of PD-L1, patients are more likely to have a superior survival outcome. Our study highlights the complicated interactions that occur within the tumor microenvironment in the primary lesion and emphasizes the necessity of determining intratumoral TILs and TCs-PD-L1 in the NPC patient prognostic analysis. TCs-PD-L1 is associated with favorable prognosis in NPC patients who have pre-existing TILs. From a treatment standpoint, our findings provide a strong rationale to further evaluate the therapeutic potential of immunotherapy in NPC.
To our knowledge, the present study is the first study to comprehensively and specifically investigate the spatial distribution of TILs and PD-L1 in primary lesions and their clinical relevance in NPC. However, our study has several limitations. First, this study demonstrated independent survival factors for NPC patients involving long time spans and a heterogeneous radiotherapy technique. Second, we investigated only a small portion of the total tumor because we used TMAs.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Qian Zhu drafted the manuscript. Mu-Yan Cai and Chang-Long Chen collected the tissue specimens and patient information. Hao Hu conducted the IHC analysis. De-Sheng Weng, Jing-Jing Zhao and Huan-Xin Lin performed the statistical analyses. Mu-Yan Cai and Min Li conducted the IHC analysis. Ling Guo participated in designing the study and editing the manuscript. JianChuan Xia conceived the study and guided the entire project. All authors read and approved the final manuscript.
References
- 1.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomark Prev 2006; 15:1765-77; PMID:17035381; https://doi.org/ 10.1158/1055-9965.EPI-06-0353 [DOI] [PubMed] [Google Scholar]
- 2.Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 2011; 30:114-9; PMID:21272443; https://doi.org/ 10.5732/cjc.010.10377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suarez C, Rodrigo JP, Rinaldo A, Langendijk JA, Shaha AR, Ferlito A. Current treatment options for recurrent nasopharyngeal cancer. Eur Arch Oto-rhino-laryngol 2010; 267:1811-24; PMID:20865269; https://doi.org/ 10.1007/s00405-010-1385-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AW, Ng WT, Chan YH, Sze H, Chan C, Lam TH. The battle against nasopharyngeal cancer. Radiother Oncol 2012; 104:272-8; PMID:22938727; https://doi.org/ 10.1016/j.radonc.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; https://doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL et al.. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515:558-62; PMID:25428503; https://doi.org/ 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 7.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS et al.. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369:134-44; PMID:23724846; https://doi.org/ 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L et al.. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372:2018-28; PMID:25891174; https://doi.org/ 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; https://doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 2015; 75:2139-45; PMID:25977340; https://doi.org/ 10.1158/0008-5472.CAN-15-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V et al.. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515:568-71; PMID:25428505; https://doi.org/ 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL Jr.. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 2012; 72:1070-80; PMID:22266112; https://doi.org/ 10.1158/0008-5472.CAN-11-3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC et al.. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 2007; 13:84-8; PMID:17159987; https://doi.org/ 10.1038/nm1517 [DOI] [PubMed] [Google Scholar]
- 14.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; https://doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fountzilas E, Kotoula V, Angouridakis N, Karasmanis I, Wirtz RM, Eleftheraki AG, Veltrup E, Markou K, Nikolaou A, Pectasides D et al.. Identification and validation of a multigene predictor of recurrence in primary laryngeal cancer. PloS One 2013; 8:e70429; PMID:23950933; https://doi.org/ 10.1371/journal.pone.0070429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL et al.. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:22461641; https://doi.org/ 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansfield AS, Roden AC, Peikert T, Sheinin YM, Harrington SM, Krco CJ, Dong H, Kwon ED. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol 2014; 9:1036-40; PMID:24926549; https://doi.org/ 10.1097/JTO.0000000000000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res 2007; 13:709s-15s; PMID:17255298; https://doi.org/ 10.1158/1078-0432.CCR-06-1868 [DOI] [PubMed] [Google Scholar]
- 19.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H et al.. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006; 66:3381-5; PMID:16585157; https://doi.org/ 10.1158/0008-5472.CAN-05-4303 [DOI] [PubMed] [Google Scholar]
- 20.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN et al.. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563-7; PMID:25428504; https://doi.org/ 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Z, Jensen SM, Messenheimer DJ, Farhad M, Neuberger M, Bifulco CB, Fox BA. Multispectral imaging of T and B cells in murine spleen and tumor. J Immunol 2016; 196:3943-50; PMID:26994219; https://doi.org/ 10.4049/jimmunol.1502635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013; 5:200ra116; PMID:23986400; https://doi.org/ 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, Xu H, Meeker AK, Fan J, Cheadle C et al.. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res 2015; 21:3969-76; PMID:25944800; https://doi.org/ 10.1158/1078-0432.CCR-15-0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011; 105:93-103; PMID:21629244; https://doi.org/ 10.1038/bjc.2011.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balermpas P, Rodel F, Liberz R, Oppermann J, Wagenblast J, Ghanaati S, Harter PN, Mittelbronn M, Weiss C, Rödel C et al.. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br J Cancer 2014; 111:1509-18; PMID:25093488; https://doi.org/ 10.1038/bjc.2014.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansfield AS, Aubry MC, Moser JC, Harrington SM, Dronca RS, Park SS, Dong H. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol 2016; 27:1953-8; PMID:27502709; https://doi.org/ 10.1093/annonc/mdw289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanborn JZ, Chung J, Purdom E, Wang NJ, Kakavand H, Wilmott JS, Butler T, Thompson JF, Mann GJ, Haydu LE et al.. Phylogenetic analyses of melanoma reveal complex patterns of metastatic dissemination. Proc Natl Acad Sci U S A 2015; 112:10995-1000; PMID:26286987; https://doi.org/ 10.1073/pnas.1508074112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K et al.. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005; 11:2947-53; PMID:15837746; https://doi.org/ 10.1158/1078-0432.CCR-04-1469 [DOI] [PubMed] [Google Scholar]
- 29.Boddupalli CS, Bar N, Kadaveru K, Krauthammer M, Pornputtapong N, Mai Z, Ariyan S, Narayan D, Kluger H, Deng Y et al.. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight 2016; 1:e88955; PMID:28018970; https://doi.org/ 10.1172/jci.insight.88955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang W, Zhang J, Hong S, Zhan J, Chen N, Qin T, Tang Y, Zhang Y, Kang S, Zhou T et al.. EBV-driven LMP1 and IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget 2014; 5:12189-202; PMID:25361008; https://doi.org/ 10.18632/oncotarget.2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clemente CG, Mihm MC Jr., Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996; 77:1303-10; PMID:8608507; https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 32.Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS, Xu YF. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol 2011; 54:497-505; PMID:21112656; https://doi.org/ 10.1016/j.jhep.2010.07.044 [DOI] [PubMed] [Google Scholar]
- 33.Li KJ, Lu MC, Hsieh SC, Wu CH, Yu HS, Tsai CY, Yu CL. Release of surface-expressed lactoferrin from polymorphonuclear neutrophils after contact with CD4+ T cells and its modulation on Th1/Th2 cytokine production. J Leukoc Biol 2006; 80:350-8; PMID:16769765; https://doi.org/ 10.1189/jlb.1105668 [DOI] [PubMed] [Google Scholar]
- 34.Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, Bistoni F, Poli V. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with candida albicans. J Exp Med 1996; 183:1345-55; PMID:8666893; https://doi.org/ 10.1084/jem.183.4.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirohata S, Yanagida T, Yoshino Y, Miyashita H. Polymorphonuclear neutrophils enhance suppressive activities of anti-CD3-induced CD4+ suppressor T cells. Cell Immunol 1995; 160:270-7; PMID:7720089; https://doi.org/ 10.1016/0008-8749(95)80038-K [DOI] [PubMed] [Google Scholar]
- 36.Kuang DM, Wu Y, Chen N, Cheng J, Zhuang SM, Zheng L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood 2007; 110:587-95; PMID:17395778; https://doi.org/ 10.1182/blood-2007-01-068031 [DOI] [PubMed] [Google Scholar]
- 37.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 2009; 50:980-9; PMID:19329213; https://doi.org/ 10.1016/j.jhep.2008.12.033 [DOI] [PubMed] [Google Scholar]
- 38.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009; 16:91-102; PMID:19647220; https://doi.org/ 10.1016/j.ccr.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009; 206:1327-37; PMID:19451266; https://doi.org/ 10.1084/jem.20082173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20:5064-74; PMID:24714771; https://doi.org/ 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51-72; PMID:23157435; https://doi.org/ 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 42.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013; 123:2756-63; PMID:23863633; https://doi.org/ 10.1172/JCI69219 [DOI] [PMC free article] [PubMed] [Google Scholar]


