Abstract
BACKGROUND:
Managing expenditures on pharmaceuticals is important for health systems to sustain universal access to necessary medicines. We sought to estimate the size and sources of differences in expenditures on primary care medications among high-income countries with universal health care systems.
METHODS:
We compared data on the 2015 volume and cost per day of primary care prescription drug therapies purchased in 10 high-income countries with various systems of universal health care coverage (7 from Europe, in addition to Australia, Canada and New Zealand). We measured total per capita expenditure on 6 categories of primary care prescription drugs: hypertension treatments, pain medications, lipid-lowering medicines, noninsulin diabetes treatments, gastrointestinal preparations and antidepressants. We quantified the contributions of 5 drivers of the observed differences in per capita expenditures.
RESULTS:
Across countries, the average annual per capita expenditure on the primary care medicines studied varied by more than 600%: from $23 in New Zealand to $171 in Switzerland. The volume of therapies purchased varied by 41%: from 198 days per capita in Norway to 279 days per capita in Germany. Most of the differences in average expenditures per capita were driven by a combination of differences in the average mix of drugs selected within therapeutic categories and differences in the prices paid for medicines prescribed.
INTERPRETATION:
Significant international differences in average expenditures on primary care medications are driven primarily by factors that contribute to the average daily cost of therapy, rather than differences in the volume of therapy used. Average expenditures were lower among single-payer financing systems that appeared to promote lower prices and the selection of lower-cost treatment options.
As a result of increased availability, use and cost of prescription drugs, maintaining the affordability of medicines has been a challenge worldwide. Among the 20 Organisation for Economic Co-operation and Development (OECD) countries with gross domestic product per capita of more than Can$45 000, average per capita expenditures on pharmaceuticals increased by about one-third in the past 10 years and more than doubled in the past 20 years.1 Despite cost pressures in all such countries, some have achieved greater control of pharmaceutical spending than others: in 2014, total pharmaceutical expenditures per capita were less than $600 in 6 of these OECD countries and more than $900 in 5 of them — an interquartile range (IQR) of more than 50%.1 It is unclear what factors drive such differences in per capita pharmaceutical expenditures: utilization levels, product choices, prices or a combination of such factors.
The purpose of this study was to quantify the levels and drivers of international differences in spending per capita on primary care pharmaceuticals — those routinely prescribed by primary care providers — within a selection of high-income countries with universal health care systems. Although much attention has recently been paid to the rising cost of specialty pharmaceuticals, health care systems must also manage the cost of more commonly used primary care prescription medicines. These pharmaceuticals are expected to account for the majority of global pharmaceutical expenditure for the foreseeable future because of their widespread use.2 We analyze the drivers of international differences in expenditure on 6 large categories of primary care medicines using economic methods developed to compute multiple cost drivers in the pharmaceutical sector.
Methods
Study design and setting
This is a comparative economic analysis of market research data from calendar-year 2015 for 10 selected, high-income countries with a variety of systems of universal health care coverage: Canada, Australia, New Zealand, Norway, Sweden, the United Kingdom, France, Germany, the Netherlands and Switzerland. Summary information about populations, economies and pharmaceutical policies in the 10 countries is provided in Table 1.
Table 1:
Summary of pharmaceutical coverage and general approach to drug price regulation in study countries
| Country | Population, thousands | % of population ≥ 65 yr | GDP per capita, Can$ PPP | Drug expenditure per capita, $* | Dominant source of prescription drug financing (%) | General approach to controlling brand-name drug prices | General approach to controlling generic drug prices |
|---|---|---|---|---|---|---|---|
| Australia | 23 778 | 15 | 58 880 | 535 | Public (71) | Cost-effectiveness considerations for national formulary | Statutory price disclosure and reductions |
| Canada | 35 852 | 16.1 | 55 992 | 824 | Public (39) | Mix of statutory regulation and voluntary price negotiations by some payers | Statutory reductions relative to brand-name drug price |
| France | 66 415 | 18.4 | 49 450 | 679† | Social insurance (70†) | Statutory regulation based on internal and external reference pricing | Statutory reductions relative to brand-name drug price |
| Germany | 81 198 | 21 | 58 586 | 815 | Social insurance (88) | Mix of statutory regulation and voluntary price negotiations | Internal reference-based reimbursement pricing |
| Netherlands | 16 901 | 17.8 | 61 195 | 494† | Social insurance (80†) | Mix of statutory regulation and voluntary price negotiations | Mix of statutory regulation and preferred provider contracts with insurers |
| New Zealand | 4596 | 14.7 | 47 047 | 365‡ | Public (74) | Negotiated supply contracts for national formulary | Competitive tendering of national supply contracts |
| Norway | 5167 | 16.1 | 81 497 | 522 | Public (57) | Mix of statutory regulation and voluntary price negotiations | Statutory reductions relative to brand-name drug price |
| Sweden | 9747 | 19.6 | 58 221 | 407 | Public (72) | Cost-effectiveness considerations for national formulary | Statutory reductions relative to brand-name drug price |
| Switzerland | 8238 | 17.8 | 74 112 | 694 | Social insurance (84) | Statutory regulation based on internal and external reference pricing | Statutory reductions relative to brand-name drug price |
| United Kingdom | 64 875 | 17.7 | 50 601 | 598† | Public (66†) | Mix of statutory profit regulation and negotiated patient access schemes | Statutory price disclosure and reductions |
Note: GDP = gross domestic product, PPP = purchasing power parity.
2015 or closest year.
Select figures are for total pharmaceutical expenditures, including expenditures on nonprescription medicines.
Latest figure (2007) for New Zealand projected to 2015 based on average growth rates in other countries.
Source: Organisation for Economic Co-operation and Development Health Data 2016.1
We chose these countries because they are all high-income countries offering universal health coverage, and all were part of a large study involving countries that have participated in the Commonwealth Fund’s International Health Policy surveys.3 We did not include the United States in this analysis because it is an outlier in 2 important ways: first, it has not yet achieved universal health coverage; second, it has such exceptionally high pharmaceutical expenditures that its inclusion in this analysis would skew comparisons among the 10 more comparable countries studied here.4
Residents of all countries except Canada were eligible for universal health coverage that included universal coverage of outpatient prescription drugs. Residents in Canada were eligible for universal public health insurance for medical and hospital care (including inpatient prescription drugs) but may or may not have had access to either public or private coverage for prescriptions used in the community setting, depending on their age, occupation, income and province of residence.5 The systems of drug coverage in Australia, New Zealand, Norway, Sweden and the UK can be described as universal public systems. In these countries, prescription medicines are financed predominantly by government, and either government or an arm’s-length public body manages the selection and the price-setting of medicines to be covered by the universal system of public financing. Prescription drugs in France, Germany, the Netherlands and Switzerland are financed through multiple-payer, social insurance systems with various statutory policies governing minimum coverage and pricing for brand-name and generic drugs.
Data sources
This study draws on data sets maintained by QuintilesIMS. Estimates of the dollar value and volume of sales of medicines in each country were obtained from the IMS MIDAS sales database.6 This sales database captures more than 95% of the value of the global pharmaceutical market, either at the point of sale to retail and hospital pharmacies or at the point of dispensing to consumers, exclusive of confidential rebates from manufacturers of brand-name products. All national sales values were converted to a constant currency (Canadian dollars) by applying a constant exchange rate (first quarter of 2016).
The MIDAS sales database tracks sales volumes in terms of standard units of a medicine and kilograms of the primary active ingredient. IMS converted these figures into standardized days of therapy using average daily doses for each medicine as computed from MIDAS Prescribing Insights, which draws on information collected from a panel of office-based physicians in each country. Although variations are small, estimated average daily doses of medicines differ by country, reflecting differences in local prescribing behaviours.
Drug categories included in the analysis
We studied the use and cost of 6 therapeutic categories of primary care prescription drugs based on primary indication: hypertension treatments; pain medicines, including nonsteroidal anti-inflammatory drugs and opioids; lipid-lowering medicines; noninsulin diabetes treatments; gastrointestinal preparations (primarily drugs for ulcers and heartburn); and antidepressants. Pharmacologic classes included in each therapeutic category are provided in Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.161481/-/DC1.
We selected these primary care categories of medicines because they are widely used, typically purchased at retail pharmacies (as opposed to hospitals) and usually sold in solid dosage forms (as opposed to liquids or injections, for example). Focusing on market research data for drug classes that satisfy these criteria improves the accuracy of how the use and cost of the medicines are measured and of the accuracy of the analytic methods described below.
We focused our analysis on pharmaceutical products from each therapeutic category classified as prescription-only medicines in most markets. We therefore excluded pharmaceuticals commonly sold over the counter (e.g., acetaminophen for pain). We also excluded herbal therapies (e.g., caraway for digestive problems).
A total of 1035 different products were included in the study, identified by active ingredient(s) and whether they were brand-name or generic drugs. These products were assigned into hierarchical, mutually exclusive groupings using the World Health Organization’s Anatomic Therapeutic Chemical (ATC) classification system. In total, the data set contained 614 different drugs (merging brand-name and generic versions of the same active ingredients together) that fell into 81 pharmacologic classes within the 6 broad therapeutic categories.
Drivers of variation in expenditure
For each therapeutic category, we quantified differences in prescription drug expenditure between each country and the average of the remaining 9 countries combined. The comparison with averages in the other countries rather than with the 10-country average ensures that the data from each country are not in both the numerator and the denominator of comparisons.
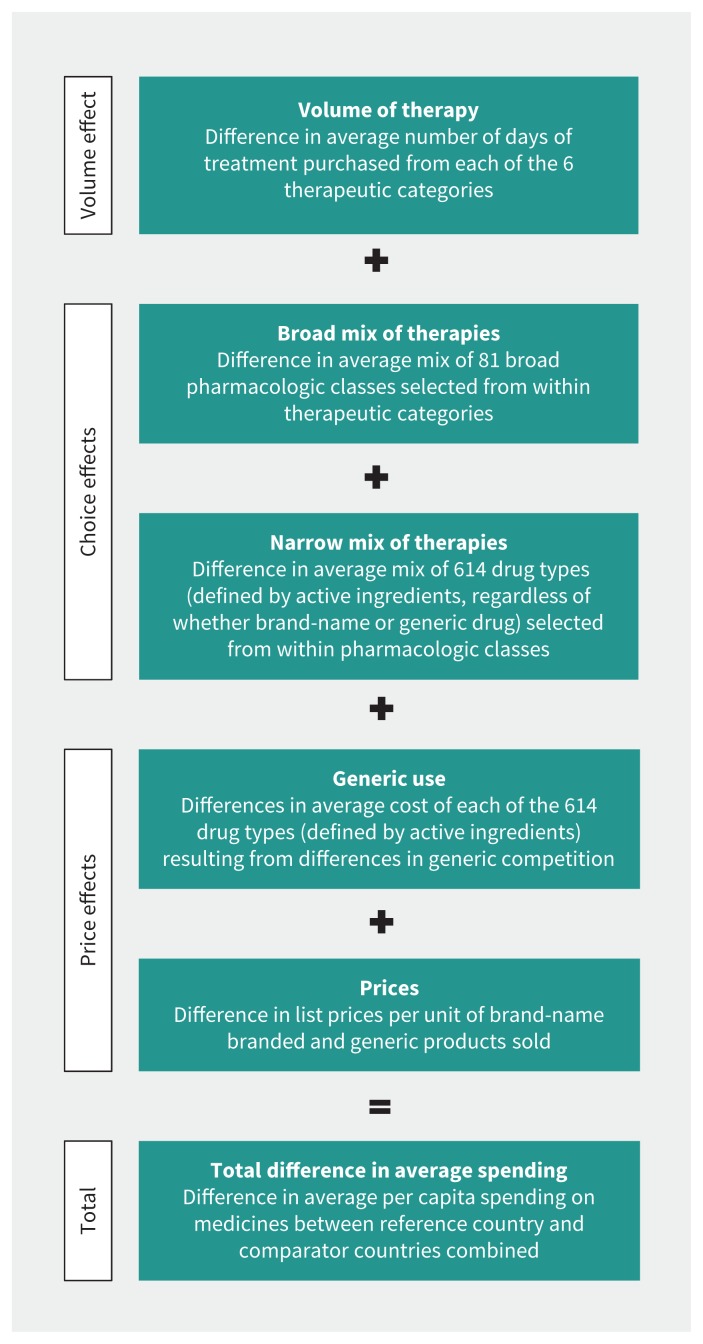
We quantified sources of international differences in prescription drug expenditures per capita per year using methods developed to compute multiple cost drivers in the pharmaceutical sector.7–9 We estimated the effects of 5 sources of difference in expenditure that fall into 3 broad categories: volume, choice effects and price effects (Figure 1).
Figure 1:
Framework for determining the drivers of differences in expenditure on the 6 therapeutic categories of primary care prescription drugs studied.
Volume relates to the average amounts of prescription drug therapy received by a population. It depicts how much of the difference in expenditure per capita between a given country and the remaining comparator countries is the result of differences in the average number of days of therapy purchased per capita. The remaining cost drivers estimate the sources of differences in costs per day of therapy.
Choice effects describe the differences in the average daily cost of treatment between a given country and the remaining comparator countries that stem from differences in the average mix of medicines selected from within therapeutic categories, holding the relative prices of treatment options constant. Other things being equal, prescribing higher-cost therapeutic options will result in higher overall spending.
Among choice effects, the “broad mix” cost driver reflects the cost impact of decisions concerning the pharmacologic classes (third level of the ATC coding system) selected from within each broad therapeutic category. This includes such choices as whether to use plain β-blockers (ATC code C07A), angiotensin-converting-enzyme (ACE) inhibitors (C09A) or other drug types for hypertension treatment.
The “narrow mix” cost driver reflects the cost impact of the selection of specific active ingredients within a particular chemical or pharmacologic class. This includes such choices as whether to use enalapril or ramipril within the class of ACE inhibitors; it is, in effect, a choice among a “narrower” range of therapeutic options than is captured by the “broad mix” cost driver.
Price effects are factors that influence the average unit cost of treatments without altering the quantity or type of active ingredient used. These cost drivers include “generic use,” which captures the average difference in unit costs between a given country and the remaining comparator countries that stem from differences in the rate of generic use when multisource products are prescribed. Price effects also include simple differences between prices in a given country and those in the remaining comparator countries, as measured by a sales-weighted average of differences in the unit prices of brand-name or generic versions of a product.
All cost drivers are computed based on a series of Fisher ideal price and quantity indexes that have the desirable property of “adding up” in the sense that they explain the total difference in spending between jurisdictions without residuals. The methods are described in detail elsewhere.7–9
For ease of interpretation, we used logarithms to convert the multiplicative results of the analysis into additive percent changes for each component. The additive cost drivers reported here will add up exactly to the 3 respective main cost-driver subtotals, and those subtotals will add up to the total differences observed.
Statistical and sensitivity analyses
We used nonstochastic measures; standard tests of statistical significance therefore do not apply. In advance of computations, we decided that differences of less than 5% in absolute value would not be considered significant.
We tested the sensitivity of results to the measure of daily doses versus kilograms of ingredient or physical units of drug products. Results were qualitatively similar, so we present the analyses based on the most intuitive measure of quantity: estimated days of therapy purchased.
Results
Table 2 lists the levels and drivers of differences in estimated 2015 expenditure per capita on all 6 therapeutic categories of primary care prescription drugs in each country compared with the other 9 comparator countries combined. The levels of expenditure per capita on these classes of medicines ranged from $23 in New Zealand to $171 in Switzerland (IQR $56–$104). The population-weighted average expenditures per capita on these primary care medicines were $77 in the 5 countries with universal public systems of prescription drug financing, $99 in the 4 countries with universal social insurance for prescription medicines, and $158 for Canada, where financing is a nonuniversal mix of private and public financing.
Table 2:
Effect of cost drivers on differences in estimated 2015 expenditure per capita on all 6 primary care therapeutic categories in each country compared with the other 9 comparator countries combined*
| Driver | Mixed financing, Canada | Universal public financing | Universal social insurance financing | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Australia | New Zealand | Norway | Sweden | UK | France | Germany | Netherlands | Switzerland | ||
| Volume | ||||||||||
|
| ||||||||||
| Domestic expenditure per capita, $ | 158 | 91 | 23 | 59 | 56 | 81 | 106 | 97 | 49 | 171 |
|
| ||||||||||
| Comparator expenditure per capita, $ | 91 | 99 | 99 | 99 | 100 | 103 | 96 | 99 | 101 | 96 |
|
| ||||||||||
| Difference in expenditure, % | 74 | −8 | −77 | −41 | −44 | −22 | 10 | −2 | −52 | 77 |
|
| ||||||||||
| Domestic days of therapy per capita | 260 | 222 | 228 | 198 | 243 | 267 | 236 | 279 | 238 | 202 |
|
| ||||||||||
| Comparator days of therapy per capita | 253 | 256 | 254 | 255 | 254 | 251 | 258 | 245 | 255 | 255 |
|
| ||||||||||
| Difference in days of therapy, % | 3 | −13 | −11 | −22 | −4 | 6 | −9 | 14 | −7 | −21 |
|
| ||||||||||
| Difference in cost per day of therapy, percentage point difference† | 71 | 6 | −66 | −19 | −40 | −28 | 18 | −16 | −45 | 98 |
|
| ||||||||||
| Choice effects | ||||||||||
|
| ||||||||||
| Broad mix of therapies, % | 11 | 14 | −10 | −3 | −16 | −11 | 4 | −12 | −11 | 11 |
|
| ||||||||||
| Narrow mix of therapies, % | 8 | 11 | −23 | −8 | −13 | −23 | 11 | −5 | −10 | 18 |
|
| ||||||||||
| Subtotal | 19 | 24 | −33 | −12 | −29 | −34 | 15 | −16 | −22 | 29 |
|
| ||||||||||
| Price effects | ||||||||||
|
| ||||||||||
| Generic use | −9 | 6 | −7 | 13 | 1 | 1 | 5 | −3 | −2 | 12 |
|
| ||||||||||
| Prices | 61 | −25 | −27 | −20 | −13 | 5 | −1 | 3 | −21 | 57 |
|
| ||||||||||
| Subtotal | 52 | −18 | −33 | −7 | −11 | 6 | 3 | 1 | −23 | 69 |
The comparator for each country is the population-weighted sum of all 9 other countries studied.
The percentage point differences for the drivers of cost per day of therapy depict how much higher or lower per capita expenditures are because of differences between the specific country’s utilization or pricing patterns and those of the other comparator countries. Some totals and subtotals will not add up exactly because of rounding.
The primary care medicines included in this study were used commonly but with differing frequency across the 10 countries. Measured in terms of average number of days of therapy purchased per capita, the total quantity of the 6 categories of primary care medicines purchased ranged from 198 days of therapy per capita in Norway to 279 days of therapy per capita in Germany (IQR 223–256). Medications indicated primarily for treating hypertension accounted for the largest share of days of primary care therapy in each country, ranging from 39% in Australia to 57% in Germany. Differences in the volume of hypertension therapy purchased also accounted for the majority of overall differences in primary care medication used across countries (details on each therapeutic category are provided in Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.161481/-/DC1.).
Average costs per day of therapy varied more significantly than the total volume of days of therapy purchased. Differences in costs per day of therapy across the countries studied were driven by a combination of differences in the average mix of drugs selected within therapeutic categories (choice effects) and differences in the prices paid for medicines prescribed (price effects).
Relative to comparator countries, the mix of therapies selected from within drug classes contributed to higher costs in Canada, Australia, France and Switzerland. In contrast, patients in New Zealand, Norway, Sweden, the UK, Germany and the Netherlands were prescribed lower-cost options from within the 6 therapeutic categories of primary care medicines. Holding other cost drivers constant, the choices of drugs prescribed to patients in New Zealand and the UK were enough to make the average costs per day of treatment in those countries about one-third less expensive than in the other countries.
Prices also added to international differences in average expenditure per capita. The list prices of medicines used in Canada were about 61% higher than the average list prices in the other countries; list prices in Switzerland were 57% higher than those in the comparator countries. When drugs were available in brand-name and generic versions, Canadians were more likely to receive generics than residents of the comparator countries combined. This reduced Canadian spending on the primary care drug classes by 9% relative to what it would have been if generic utilization patterns in Canada matched those of its comparators. In Switzerland, however, use of generics was lower than in its comparator countries, which resulted in an additional 12% increase in average costs per capita in that country compared with the other 9 countries combined.
Detailed results by therapeutic category are provided in Appendix 2. Differences in the per capita volume of therapy purchased in the countries studied had a greater effect on variations in average expenditure per capita for pain medications and for antidepressants than they did in other therapeutic categories. In the antidepressants category, therapeutic choices also had a significant effect on average costs per day of therapy in the countries studied, ranging from choices in the UK that cost 55% less than the average in comparator countries, to choices in Canada that cost 52% more than the average in comparator countries. In all 6 therapeutic categories studied, Switzerland and Canada had the highest list prices for the drugs used in their health care systems. They also had the highest average expenditure per capita on each of the 6 therapeutic categories of medicine studied.
Interpretation
Across 10 high-income countries with universal health care systems, the average expenditure per capita on 6 of the largest categories of primary care medicines varied by more than 600%. The volume of therapies purchased varied by only 41%, which meant that most of the differences in average expenditure per capita were driven by differences in the average mix of drugs selected within therapeutic categories and differences in the prices paid for medicines prescribed. In New Zealand, estimated costs per day of therapy were about one-third the level in comparator countries; in Switzerland, estimated costs per day were nearly double the level in comparator countries.
Averaged across the 6 classes of primary care medicines studied, the volume of therapy purchased in Canada was about the same as that in the comparator countries; however, Canadians spent an estimated $2.3 billion more than they would have in 2015 if these primary care treatments had had the same average cost per day in Canada as in the 9 comparator countries combined. About $600 million of this difference in expenditure was due to Canadians being prescribed costlier drug treatments from within these therapeutic categories, particularly in the categories of lipid-lowering treatments and antidepressants. A total of $1.7 billion of the difference was due to these primary care medicines having higher prices in Canada than in the comparator countries.
Our finding that international differences in expenditure were not primarily a function of differences in volumes of therapy used is comparable to a 2013 study of cross-national differences in pharmaceutical spending by Kanavos and colleagues,10 who concluded that the effects of differences in volumes of medicines purchased in the US versus comparator countries were smaller than the effects of differences in prices and uptake of new drugs.10 Our findings concerning the relative prices of drugs in the countries studied are also consistent with published price comparisons by Canada’s Patented Medicine Prices Review Board.4,11
Limitations
As with any analysis of international differences in the use and cost of pharmaceuticals, our study has some limitations. The data we obtained did not include information about confidential discounts on the price of brand-name drugs, which are now common in most of the countries studied.12 The relative prices of brand-name products in countries with extensive experience in negotiating confidential discounts, such as New Zealand,13 may be lower than we have estimated. Similarly, the relative price of brand-name products in countries without population-wide processes for negotiating rebates on pharmaceuticals, such as Canada,14 may be higher than estimated.
We did not account for differences in morbidity of the populations in each country. Some of the differences in volume of therapies used and, potentially, the mix of drugs selected from within therapeutic categories may reflect differences in the average age and morbidity of the populations. Australia and New Zealand, for example, have slightly younger populations and may therefore not have as much demand for some of the medications included in this study. Although this is an unmeasured driver of potential differences in utilization — and is consistent with lower volumes of medicines used in countries with younger populations — differences in population age and health status among the countries studied here are relatively small by comparison with our findings concerning the differences in average cost per day of therapy received across those countries. Other factors — including international differences in pricing and reimbursement policies, prescribing guidelines and patterns, and patient expectations — are likely to play a substantial role in determining costs per day of therapy, even when differences in population age structure or health needs are taken into account.
Conclusion
Substantial international differences in average expenditures on primary care medications are driven primarily by factors that contribute to the average daily cost of therapy, rather than differences in the volume of therapy used. Average expenditures are lower among single-payer financing systems, which appear to promote lower prices and selection of lower-cost treatment options within therapeutic categories.
See related article at www.cmaj.ca/lookup/doi/10.1503/cmaj.170440
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Steven Morgan was responsible for project conception, the acquisition of funding and data, and the analysis of data. Christine Leopold assisted with the study design and the interpretation of results. Anita Wagner assisted with the study design, the acquisition of data and the interpretation of results. Steven Morgan drafted the manuscript, and Christine Leopold and Anita Wagner revised it for important intellectual content. All of the authors approved the final version to be published and agreed to act as guarantors of the work.
Funding: This work was supported in part by a research grant from the Commonwealth Fund [grant no. 20160646], a national, private foundation based in New York City. The views presented here are those of the authors and not necessarily those of the Commonwealth Fund, its directors, officers or staff. The funding agency had no role in study design, analysis or preparation of the paper.
References
- 1.OECD health statistics 2016. Paris: Organisation for Economic Co-operation and Development; 2016. [Google Scholar]
- 2.Global medicines use in 2020 — outlook and implications. Parsippany (NJ): IMS Health; 2015. [Google Scholar]
- 3.Osborn R, Moulds D, Squires D, et al. International survey of older adults finds shortcomings in access, coordination, and patient-centered care. New York: The Commonwealth Fund; 2014. [DOI] [PubMed] [Google Scholar]
- 4.Patented Medicine Prices Review Board: annual report 2014. Ottawa: Patented Medicine Prices Review Board; 2015. [Google Scholar]
- 5.Barnes S, Anderson L. Low earnings, unfilled prescriptions: employer-provided health benefit coverage in Canada. Toronto: Wellesley Institute; 2015. [Google Scholar]
- 6.IMS MIDAS — Global analysis made easy. London: IMS Health; 2012. [Google Scholar]
- 7.Morgan SG. Sources of variation in provincial drug spending. CMAJ 2004;170:329–30. [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan S. Drug spending in Canada: recent trends and causes. Med Care 2004; 42:635–42. [DOI] [PubMed] [Google Scholar]
- 9.The drivers of prescription drug expenditures — a methodological report. Ottawa: Patented Medicine Prices Review Board; 2013. [Google Scholar]
- 10.Kanavos P, Ferrario A, Vandoros S, et al. Higher US branded drug prices and spending compared to other countries may stem partly from quick uptake of new drugs. Health Aff 2013;32:753–61. [DOI] [PubMed] [Google Scholar]
- 11.Generic drugs in Canada, 2013. Ottawa: Patented Medicine Prices Review Board; 2014. [Google Scholar]
- 12.Morgan SG, Vogler S, Wagner AK. Payers’ experiences with confidential pharmaceutical price discounts: A survey of public and statutory health systems in North America, Europe, and Australasia. Health Policy 2017;121:354–62. [DOI] [PubMed] [Google Scholar]
- 13.Morgan SG, Hanley G, McMahon M, et al. Influencing drug prices through formulary-based policies: lessons from New Zealand. Healthc Policy 2007;3:121–40. [PMC free article] [PubMed] [Google Scholar]
- 14.Lexchin J. Drug pricing in Canada. In: Babar ZUD, editor. Pharmaceutical prices in the 21st century. Cham (Switzerland): Springer International Publishing; 2015:25–41. [Google Scholar]