Abstract
Objective
Delayed antimicrobial therapy in sepsis is associated with increased hospital mortality, but the impact of antimicrobial timing on long-term outcomes is unknown. We tested the hypothesis that hourly delays to antimicrobial therapy are associated with one-year mortality in pediatric severe sepsis.
Design
Retrospective observational study.
Setting
Quaternary academic pediatric intensive care unit (PICU) from February 1, 2012 to June 30, 2013.
Patients
One hundred sixty patients aged ≤21 years treated for severe sepsis.
Interventions
None.
Measurements and Main Results
We tested the association of hourly delays from sepsis recognition to antimicrobial administration with one-year mortality using multivariable Cox and logistic regression. Overall one-year mortality was 24% (39 patients), of whom 46% died after index PICU discharge. Median time from sepsis recognition to antimicrobial therapy was 137 min (IQR 65-287). After adjusting for severity of illness and comorbid conditions, hourly delays up to 3 hours were not associated with one-year mortality. However, increased one-year mortality was evident in patients who received antimicrobials ≤1 hour (aOR 3.8, 95% CI 1.2, 11.7) or >3 hours (aOR 3.5, 95% CI 1.3, 9.8) compared to patients who received antimicrobials within 1-3 hours from sepsis recognition. For the subset of patients who survived index PICU admission, antimicrobial therapy ≤1 hour was also associated with increased one-year mortality (aOR 5.5, 95% CI 1.1, 27.4), while antimicrobial therapy >3 hours was not associated with one-year mortality (aOR 2.2, 95% CI 0.5, 11.0).
Conclusions
Hourly delays to antimicrobial therapy, up to three hours, were not associated with one-year mortality in pediatric severe sepsis in this study. The finding that antimicrobial therapy ≤1 hour from sepsis recognition was associated with increased one-year mortality should be regarded as hypothesis-generating for future studies.
Keywords: timing, critically ill children, long-term mortality
INTRODUCTION
Pediatric sepsis remains a significant healthcare problem worldwide, accounting for over 75,000 annual hospitalizations in the United States, with an estimated 8.9% mortality rate and an associated annual cost of $4.8 billion (1). For the subset of critically ill children requiring treatment for severe sepsis in an intensive care unit, hospital mortality rates as high as 25% have been reported (2, 3). The Surviving Sepsis Campaign is a global initiative aimed at improving the management and outcomes of sepsis. One of the core elements of its resuscitation bundle is to administer empiric antimicrobial therapy within one hour of sepsis recognition (4). This recommendation is based on adult studies demonstrating an association between progressive hourly delays in antimicrobial administration and increased mortality at hospital discharge (5-10). We recently reported similar findings for pediatric patients with severe sepsis, with significantly increased mortality rate at intensive care unit discharge in children with a three hour or greater delay in empiric antimicrobial administration (11).
Despite data demonstrating increased hospital mortality with delayed antimicrobial administration, there are limited data about the association of delayed antimicrobial therapy with long-term mortality. There is growing recognition that inflammatory changes present during the acute phase of sepsis have enduring deleterious effects on health following hospital discharge (12). Studies in both adult and pediatric sepsis survivors have shown that function is impaired and mortality outpaces non-septic patients well beyond hospital discharge (12-14). Moreover, early resuscitative therapies that mitigate deleterious inflammatory, immune, and microvascular phenomena in the acute phase of sepsis have been shown to improve long-term outcomes (15, 16). The enduring effects of acute sepsis therapies have also been increasingly emphasized in critical care research. For example, human recombinant activated protein C initially showed improvement in short-term mortality that did not persist when patients were subsequently followed beyond hospital discharge, but was removed from the market when subsequent studies showed no improvement in long-term mortality (17, 18). Understanding the long-term impact of acute therapies—both routine and investigational—can help to identify modifiable factors to improve morbidity and mortality beyond the intensive care unit and hospital discharge. We therefore sought to determine if delayed antimicrobial administration is associated with increased one-year mortality in critically ill pediatric patients treated for severe sepsis.
MATERIALS AND METHODS
Study Design
We conducted a retrospective observational study of patients treated for severe sepsis, including septic shock, at The Children’s Hospital of Philadelphia pediatric intensive care unit (PICU), an academic quaternary care medical center. Data were collected from an electronic health record-based sepsis registry (11). The study was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia under a waiver of informed consent.
Study Population
Patients eligible for analysis were identified as having severe sepsis on a daily PICU sepsis screening algorithm between February 2012 and June 2013. The screening algorithm consisted of a detailed checklist for presence of systemic inflammatory response syndrome (SIRS) criteria, potential infections, and organ dysfunctions at any point in the preceding 24 hrs. Although screening was performed once daily, the retrospective review ensured that patients were identified as having severe sepsis if they met criteria at any point in the 24 hours prior to PICU admission or throughout their PICU course. The screen was completed first by the bedside nurse and then discussed with the clinical team as part of morning rounds. Three investigators (MH, SLW, JCF) subsequently reviewed each positive screen to ensure that patients met criteria for severe sepsis as defined by the International Pediatric Sepsis Consensus Conference (19). Consensus criteria define severe sepsis as 1) two or more age-based SIRS criteria, 2) confirmed or suspected invasive infection, and 3) cardiovascular dysfunction, acute respiratory distress syndrome, or at least two new organ system dysfunctions. Septic shock was defined as the subset of patients with severe sepsis due to cardiovascular dysfunction (19). Patients with confirmed severe sepsis were then entered into an electronic health record-based sepsis registry.
Inclusion criteria for this study included 1) entry into sepsis registry for confirmed severe sepsis, 2) treatment for severe sepsis, including septic shock, in the PICU, and 3) age less than or equal to 21 years at time of sepsis recognition. While treatment for sepsis may have started in the emergency department (ED), operating room, or inpatient ward, only patients ultimately admitted to the PICU were included. Patients were excluded if initial sepsis recognition and treatment occurred at an outside institution because timing of initial interventions could not be consistently determined. For patients with more than one sepsis episode during the study period, only the first episode was included in the analysis. A portion of the patients in this study were previously analyzed for short-term mortality (11) but all analyses presented here are novel.
Data Collection
Clinical data, including demographics, comorbid conditions, time and location of sepsis recognition, microbiology and other laboratory results, and time of antimicrobial and intravenous fluid administration, were obtained from the sepsis registry. The accuracy of the automated data abstraction process has been previously described (11, 20, 21) but the time to antimicrobial administration (the primary exposure) was manually confirmed. Other data collection was supplemented by manual review when needed (e.g., radiograph results). We used the Pediatric Complex Chronic Conditions (CCC) scheme to categorize comorbid conditions (22). Severity of illness was measured using the Pediatric Risk of Mortality (PRISM) III score and the Pediatric Index of Mortality (PIM) 2 (23, 24), which were obtained from the Virtual PICU Systems (VPS) database. Definitions for source of infection were adapted from published criteria (25).
Time of sepsis recognition was defined as triage time for patients presenting to the ED or time of the first ‘sepsis-related’ intervention documented in the medical record for patients initially treated for sepsis in the PICU, inpatient ward, or operating room, as previously described (11, 26). Sepsis-related interventions included an electronic order for antimicrobial therapy, blood culture collection, fluid bolus, or transfer to the PICU (11, 26). Time of sepsis recognition was used in the analysis because it represents a pragmatic timeframe that has meaning for clinicians and mirrors the Surviving Sepsis Campaign recommendation to administer empiric antimicrobial therapy within one hour of sepsis recognition (4, 11).
Bacterial and fungal infection were diagnosed as part of routine clinical practice using microbial culture assays with samples collected from suspected sources of infection, i.e., blood, tracheal aspirate, stool, CSF, urine, and wound. Viral infections were diagnosed using polymerase chain reaction assays with samples collected from suspected sources of infection. Antimicrobial administration was considered appropriate if there was microbiology data confirming in vitro activity of the selected antimicrobial against the identified causative organism(s). When microbiology data were not available or the only pathogen identified was a virus, antimicrobials were considered appropriate if they were consistent with local guidelines for empiric treatment of the most likely source of infection (7, 9, 11, 27). However, in cases of influenza without identification of a concurrent bacterial and/or fungal pathogen, oseltamivir was included as appropriate treatment. We separately measured time from sepsis recognition to administration of initial antimicrobial therapy and to first appropriate antimicrobial therapy.
Outcomes
The primary outcome measure was all-cause mortality at 365 days following the day of sepsis recognition (one-year mortality). Vital status at one year was determined using death classification recorded in the hospital’s electronic medical record, which captures both in-hospital and community deaths. Survivors were considered confirmed if they were not listed as deceased in the medical record and there was at least one documented patient encounter after 365 days from sepsis recognition. Patients for whom survivorship could not be confirmed using the hospital’s electronic medical record were considered alive at 365 days from sepsis recognition for the primary analysis.
Statistical Analysis
Statistical analysis was performed using STATA (Version 13.1, College Station, TX). Descriptive data are presented as medians with interquartile range (IQR) for continuous variables and frequencies with percentages for categorical variables. We used multivariable Cox regression to compare hazard ratios (HR) for one-year mortality for all patients receiving antimicrobials at pre-defined hourly time cutoffs (≤1 hour versus >1 hour, ≤2 versus >2 hours, and ≤3 versus >3 hours) from time of sepsis recognition to both initial and first appropriate antimicrobial administration. We a priori identified the following covariates as potential confounders based on biological plausibility, data availability, and prior studies: age, sex, at least one CCC, number of CCCs, malignancy, history of stem cell transplant, PIM2, PRISM III, maximum lactate level on day one of sepsis, and appropriateness of initial antimicrobials (7, 9, 27). Covariates were individually tested in separate bivariable models that included exposure (i.e. time to antimicrobial administration), the outcome, and the covariate. Only those covariates that changed the base model HR by 10% or greater were considered to be true confounders and included in the final multivariable model (28). Both unadjusted and adjusted HRs with 95% confidence intervals (CI) are presented. We performed a sub-analysis limited to patients with identified bacterial and/or fungal pathogens on microbiologic assays, and excluded the patients with no organism or only viral organism identified who may not have benefitted from antimicrobials, regardless of timing.
Because our a priori planned analyses revealed increased one-year mortality for patients administered antimicrobials ≤1 hour and >3 hours, we performed a post hoc analysis using trichotomous time cutoffs of ≤1 hour, 1-3 hours, and >3 hours. We used logistic regression analyses for the trichotomous analyses since this exposure failed to maintain the proportionality hazards assumption required in Cox regression. To further determine the impact of antimicrobial timing on one-year mortality separate from death at PICU discharge, we also performed an analysis limited only to patients who survived the index PICU admission. These post hoc analyses were adjusted using the same confounders identified in our primary analysis. Kaplan-Meier survival analyses with log-rank testing were used to further compare mortality by both hourly time cutoffs and the trichotomous time exposure variable. Two sensitivity analyses were performed to test the potential impact of outcome misclassification by 1) excluding all patients with unconfirmed one-year vital status and 2) recoding all patients with unconfirmed one-year vital status (classified as survivors in the primary analysis) as nonsurvivors. Performance of all regression models is reported as the area under the receiver operating characteristic (AUROC) curve. Statistical significance was defined as a two-sided p-value <0.05.
RESULTS
One hundred sixty patients met all inclusion criteria, including 35 with severe sepsis and 125 with septic shock. Patient characteristics are presented in Table 1. Median time to administration of initial antimicrobial was 137 minutes (IQR 65-287) and median time to administration of first appropriate antimicrobial was 169 minutes (IQR 81-488). Overall one-year mortality was 24% (39 patients), which included 21 patients who died in the PICU and 18 patients who died after the index PICU discharge. Vital status at one year was confirmed in 141 of 160 (88%) patients.
Table 1.
Characteristics of Study Cohort.
| Variablea | Value |
|---|---|
| Age, yr | 6.3 (1.5-13.8) |
|
Sex, n (%)
Male Female |
88 (55) 72 (45) |
|
Race, n (%)
White Black Asian Unknown |
76 (48) 53 (33) 4 (3) 27 (16) |
|
Comorbid Conditions, n (%)
None One Two Three or more Oncologic comorbid condition, n (%) Stem cell transplant, n (%) |
69 (43) 62 (39) 18 (11) 11 (7) 33 (21) 6 (4) |
|
Location of Sepsis Recognition, n (%)
Emergency Department Inpatient Ward Pediatric Intensive Care Unit Periop Complex |
83 (52) 44 (28) 31 (19) 2 (1) |
| Pediatric Index of Mortality 2 | 1.8 (0.9-4.5) |
| Pediatric Risk of Mortality III score | 8 (3-15) |
|
Baseline laboratory valuesb
White blood cell count (1,000/μL) Hemoglobin (g/dL) Platelets (1,000/μL) Creatinine (mg/dL) International normalized ratio Lactate (mmol/L) |
8.3 (3.8-14.3) 10.2 (8.8-12) 211 (76-340) 0.3 (0.2-0.5) 1.24 (1.1-1.4) 1.8 (1.2-2.7) |
|
Compliance with initial resuscitation goals, n (%)
Blood culture drawn before antimicrobial Initial Antimicrobial ≤ 1hr Initial Antimicrobial ≤ 3hr Appropriate antimicrobial ≤ 1hr Appropriate antimicrobial ≤ 3hr Initial fluid bolus ≤ 20 min Initial fluid bolus ≤ 60 min Lactate measured |
129 (81) 35 (22) 98 (61) 24 (15) 84 (52.5) 33 (20) 60 (37.5) 143 (89) |
|
Sepsis related therapies
Central venous catheter, n (%) Required vasoactive infusion, n (%) Required mechanical ventilation, n (%) Required extracorporeal membrane oxygenation, n (%) |
107 (67) 101 (63) 70 (44) 2 (<1) |
Median values (interquartile range), unless indicated.
Values at or closest to time of sepsis recognition.
Total patients n=160.
The site of infection was identified in 82% of patients (Table 2) and a pathogen was identified in 72% (see Table, Supplemental Digital Content 1, which describes identified pathogens). The most common site of infection was the respiratory tract. Fifty-three percent of patients had bacterial infections. The most commonly isolated gram positive organism was methicillin-resistant Staphylococcus aureus, and the most commonly identified gram negative organism was Pseudomonas species. Forty-one percent of patients had viral infections. The most common viral organism identified was rhinovirus. Eighteen percent of patients had polymicrobial infections, defined as identification of more than one bacterial and/or viral pathogens in a single patient. Compared to patients for whom a bacterial and/or fungal pathogen was isolated, patients with identified viral organisms trended toward younger age, lower PIM-2 scores, and lower mortality. However, these differences were not statistically significant (see Table, Supplemental Digital Content 2, which describes patient characteristics by viral-only sepsis versus bacterial and/or fungal sepsis). Seventy-eight percent of the initial antimicrobials administered was considered appropriate.
Table 2.
Site of Infection.
| Source | No. of Patients (%) |
|---|---|
| Respiratory | 67 (42) |
| Bacteremia | 21 (13) |
| Gastrointestinal | 16 (10) |
| Genitourinary | 10 (6) |
| CNS | 8 (5) |
| Wound | 5 (3) |
| Skin | 3 (2) |
| Endocarditis | 1 (1) |
| Unknown | 29 (18) |
The hazard ratios for one-year mortality at progressive hourly time cutoffs between sepsis recognition and initial antimicrobial administration are shown in Table 3. Unadjusted analysis showed increased risk of one-year mortality for patients receiving antimicrobials >3 hours from sepsis recognition (HR 2.01, CI 1.07, 3.78). The survival curves for patients who received initial antimicrobial therapy either ≤3 or >3 hours following sepsis recognition is shown in Figure 1.
Table 3.
One-year mortality: Initial antimicrobials at hourly cutoff points
| Time to Initial Antimicrobial |
Number of Patients |
Mortality (%) |
Difference (%) |
Unadjusted HR (95%CI) |
Adjusted HRa
(95% CI) |
|---|---|---|---|---|---|
| ≤ 1 hour | 35 | 31 | Reference | Reference | |
| > 1 hour | 125 | 22 | −9 | 0.68 (0.34, 1.36) | 0.59 (0.29, 1.22)b |
| ≤ 2 hours | 71 | 24 | Reference | Reference | |
| > 2 hours | 89 | 25 | +1 | 1.03 (0.55, 1.95) | 0.86 (0.44, 1.67)c |
| ≤ 3 hours | 98 | 18 | Reference | Reference | |
| > 3 hours | 62 | 34 | +16 | 2.01 (1.07, 3.78) | 1.66 (0.85, 3.23)d |
Cox regression analysis of one-year mortality comparing initial antimicrobial therapy at hourly time cutoff points from severe sepsis recognition.
Adjusted for PIM2 and number of CCCs.
Area under the receiver operating characteristic (AUROC) curve is 0.73 (95% CI 0.64, 0.83).
AUROC curve is 0.72 (0.62, 0.83).
AUROC curve is 0.74 (0.65, 0.83).
HR=hazard ratio.
CI=confidence interval.
Total patients n=160.
Figure 1.
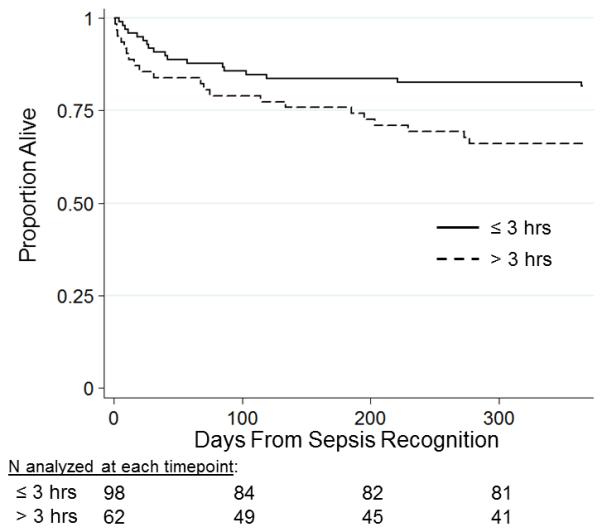
Survival analysis for time to initial antimicrobial therapy at 3 hour cutoff point Kaplan-Meier survival analysis comparing initial antimicrobial therapy at the 3 hour cutoff point from severe sepsis recognition. Total patients n=160. Log-rank test, p=0.03.
Multivariable modeling identified two confounders—PIM2 and number of CCCs—as changing the base model HR between time to antimicrobial administration and one-year mortality by 10% or greater. After adjusting for both PIM2 and number of CCCs, there was no significant association between hourly delays to initial antimicrobial administration and one-year mortality, including at the 3 hour time cutoff (Table 3). Hourly delays to administration of first appropriate antimicrobial were also not associated with one-year mortality at any of the hourly time cutoff points in either unadjusted or adjusted analyses (see Table, Supplemental Digital Content 3, which demonstrates association of time to first appropriate antimicrobials with one-year mortality and Figure, Supplemental Digital Content 4, which demonstrates the survival curves for patients who received first appropriate antimicrobial therapy either ≤3 or >3 hours following severe sepsis recognition). A sub-analysis of the 72 patients with identified bacterial and/or fungal pathogens demonstrated similar trends in association between hourly delays to initial antimicrobial therapy and one-year mortality when compared to the all-patient analysis, albeit with wider confidence intervals, reflecting reduced statistical power (see Table, Supplemental Digital Content 5, which demonstrates association of time to first antimicrobials with one-year mortality in patients with identified bacterial and/or fungal pathogen).
In the post-hoc trichotomous time cutoff analysis, patients who received initial antimicrobials ≤1 hour from sepsis recognition had the highest PIM2 illness severity, were more likely to receive fluids within 20 and 60 minutes, and had greater use of mechanical ventilation whereas patients who received initial antimicrobials >3 hours were less likely to be male and more likely to have sepsis recognition in an inpatient setting (see Table, Supplemental Digital Content 6, which describes characteristics of study cohort by time to initial antimicrobials by trichotomous cutoff points). PRISM scores were similar between the ≤1 hour and >3 hour groups, with both slightly higher than the 1-3 hour group.
Logistic regression analysis demonstrated a significant increased risk of one-year mortality for patients receiving initial antimicrobials both ≤1 hour or >3 hours from sepsis recognition compared to those in the 1-3 hour exposure group even after adjusting for PIM2 and number of CCCs (Table 4). Similar findings were noted with first appropriate antimicrobial administration for ≤1 hour and >3 hours, though only the ≤1 hour exposure reached statistical significance (see Table, Supplemental Digital Content 7, which demonstrates association of time to first appropriate antimicrobials at trichotomous time cutoff points with one-year mortality). Although oncologic comorbidity did not meet criteria as a confounder in the primary analysis and was not statistically different across trichotomous time cutoffs, there was a notably higher proportion with cancer in the ≤1 and >3 hours groups. When forced into the multivariable logistic regression model, the adjusted OR for one-year mortality decreased just below statistical significance to 3.1 (95% CI 0.98, 9.66) for ≤1 hour but remained significant for >3 hours (3.32, 95% CI 1.20, 9.12). For the subset of patients who survived to index PICU discharge, administration of initial antimicrobials ≤1 hour from sepsis recognition was significantly associated with one-year mortality while initial antimicrobial therapy >3 hours was not associated with one-year mortality after adjusting for PIM2 and number of CCCs (Table 5). The survival curves for patients who received initial antimicrobial therapy either ≤1 hour, 1-3 hours, or >3 hours following sepsis recognition is shown in Figure 2.
Table 4.
One-year mortality: Initial antimicrobials at trichotomous time cutoff points
| Time to Initial Antimicrobials |
Unadjusted OR (95% CI) |
Adjusted ORa
(95% CI) |
|---|---|---|
| 0 to ≤ 1 hour | 3.7 (1.2, 10.6) | 3.8 (1.2, 11.7) |
| 1 to ≤ 3 hours | Reference | Reference |
| > 3 hours | 4.1 (1.6, 10.5) | 3.5 (1.3, 9.8) |
Logistic regression analysis of one-year mortality comparing initial antimicrobial therapy at trichotomous time cutoff points from severe sepsis recognition. Area under the receiver operating characteristic (AUROC) curve for the adjusted logistic regression model is 0.76 (95% CI 0.67, 0.85).
Adjusted for PIM2 and number of CCCs.
OR=odds ratio.
CI=confidence interval.
Total patients n=160.
Table 5.
One-year mortality in PICU survivors: Initial antimicrobials at trichotomous time cutoff points
| Time to Initial Antimicrobials |
Unadjusted OR (95% CI) |
Adjusted ORa
(95% CI) |
|---|---|---|
| 0 to ≤ 1 hour | 4.7 (1.1, 20.2) | 5.5 (1.1, 27.4) |
| 1 to ≤ 3 hours | Reference | Reference |
| > 3 hours | 4.1 (1.0, 16.1) | 2.2 (0.5, 11.0) |
Logistic regression analysis of one-year mortality comparing initial antimicrobial therapy at trichotomous time cutoff points from severe sepsis recognition in PICU survivors. Area under the receiver operating characteristic (AUROC) curve for the adjusted logistic regression model is 0.74 (95% CI 0.64, 0.83).
Adjusted for PIM2 and number of CCCs.
OR=odds ratio.
CI=confidence interval.
Total patients n=139.
Figure 2.
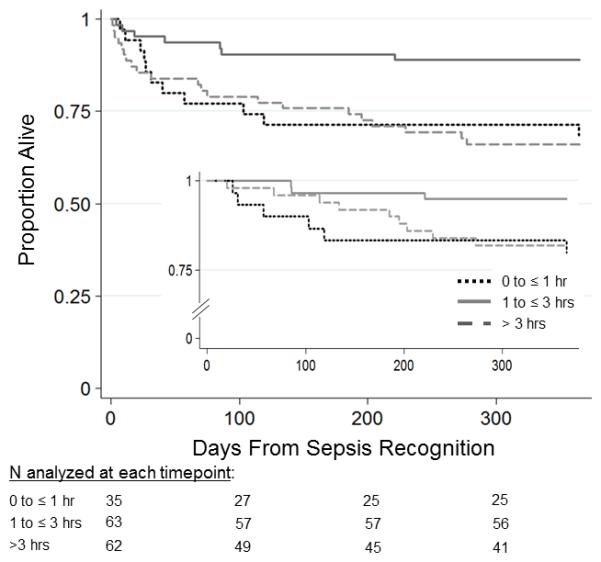
Survival analysis for time to initial antimicrobial therapy at trichotomous cutoff points. Kaplan-Meier survival analysis comparing initial antimicrobial therapy at trichotomous cutoff points from severe sepsis recognition. Total patients n=160. Log-rank test p=0.008.
Inset shows PICU survivors only, n=139. Log-rank test=0.06.
Median time to initial antimicrobial administration did not differ between patients with confirmed one-year vital status versus patients without confirmed one-year vital status (136 min, IQR 64-277 versus 167 min, IQR 67-372, p=0.74). Neither sensitivity analyses excluding the 19 patients with unconfirmed vital status or recoding patients with unconfirmed vital status as nonsurvivors instead of as survivors altered the findings from the primary analysis (see Table, Supplemental Digital Content 8, which demonstrates association of time to initial antimicrobials with one-year mortality limited to the 141 patients with confirmed one-year vital status; see Table, Supplemental Digital Content 9, which demonstrates association of time to initial antimicrobials with one-year mortality after recoding patients with unconfirmed one-year vital status as nonsurvivors).
DISCUSSION
In contrast to prior reports of increased hospital mortality with delayed antimicrobial therapy, in our primary a priori-planned analysis, we did not find an association between hourly delays to initial antimicrobial therapy and the longer-term outcome of one-year mortality in pediatric severe sepsis after adjusting for severity of illness and number of comorbid conditions. However, in a post hoc exploratory analysis, initial antimicrobial therapy administered very early (i.e. ≤1 hour) and after 3 hours from sepsis recognition were associated with increased one-year mortality when compared to administration within 1-3 hours.
The Surviving Sepsis Campaign recommendation to administer empiric antimicrobial therapy within one hour of sepsis recognition is largely based on an adult study by Kumar et al, which showed an increase of 7.6% in hospital mortality with each hourly delay in antimicrobial administration from the onset of hypotension (4, 9). Subsequent data in adult sepsis have been conflicting, with some studies reporting increased while others suggest no change in mortality with progressive time to antimicrobial therapy (5-8, 16, 27, 29). In a recent meta-analysis of 11 studies, Sterling et al. found no significant association between delays in antimicrobial therapy and hospital mortality (29). These inconsistent data in the literature call into question the optimal timing for antimicrobial therapy and the appropriateness of using antimicrobial administration within one hour as a quality metric (27, 29).
In pediatric sepsis, we recently demonstrated that delayed antimicrobial therapy beyond 3 hours from sepsis recognition was associated with increased PICU mortality (11). However, in our current study, we could not demonstrate a durable association of delayed antimicrobial therapy with the longer term outcome of one-year mortality in our primary a priori planned analysis using the common reference group of <1 hour. A recent study by van Paridon et al. similarly did not find a significant association between time to antimicrobial therapy and one-year mortality in 79 pediatric patients treated for severe sepsis (30). A notable caveat in our study, however, was that illness severity, oncologic comorbidities, and one-year mortality were higher in the group receiving antimicrobials within 1 hour compared to 1-3 hours. When antimicrobial administration was referenced to faster administration times excluding the <1 hour group, the odds of death at one year increased by 3.5 times for antimicrobial delays >3 hours.
Our study was spurred by the growing evidence that adult and pediatric patients with severe sepsis have increased risk of long-term morbidity and mortality (12, 14, 16). A study by Quartin et al. demonstrated that adult sepsis survivors had a higher risk of death for the ensuing five years compared to non-septic patients, even after adjusting for underlying comorbidities (14). In children, Czaja et al. found a significant increase in mortality beyond 28 days from the acute sepsis episode compared to the general pediatric population in Washington state (13). Yet, despite these epidemiologic data, the mechanisms through which an acute septic (or other critical illness) episode adversely impacts long-term health through the post-intensive care syndrome are not clear (31). One compelling hypothesis supposes that persistent immune dysregulation may contribute to long-term complications of sepsis. Studies have demonstrated increased rates of recurrent infections in adult survivors of sepsis (32), and murine models of sepsis have associated changes in immunomodulatory cytokines and immune cells with increased long-term susceptibility to infection and mortality (33). Other possible mechanisms for post-intensive care syndrome include physical debilitation and psychological disturbances that increase the risk of subsequent life-threatening events (31), though these roles in pediatrics are not clear.
An unexpected finding in our current study was the association between antimicrobial therapy administered ≤1 hour from sepsis recognition and one-year mortality. We did not observe this negative effect on short-term mortality in a previous analysis, although that study had limited power to detect a difference due to a smaller sample size and lower rate of hospital versus one-year mortality (11). The biologic reason for why very early antimicrobial therapy may increase longer-term mortality risk is unclear, although we can hypothesize a number of explanations. One possibility is that prioritization of early antimicrobial administration ≤1 hour from sepsis recognition occurred at the expense of other interventions, such as early adequate fluid resuscitation. Antimicrobial therapy with subsequent bacterial lysis, endotoxin release, and cytokine production in patients yet to be adequately fluid resuscitated may have harmful effects. This scenario was suggested in the rodent model by Zurovsky et al, which showed increased mortality in hypovolemic rats injected with endotoxin compared to euvolemic rats (34). However, in our study, patients who received antimicrobials ≤1 hour were also more likely to receive rapid initial fluid resuscitation.
Another possible explanation is that early antimicrobials were preferentially administered to oncologic patients or to the more severely ill patients due to more fulminant disease. While we attempted to control for confounding by severity of illness using PIM2, our reliance on illness severity scores that are calculated at time of PICU presentation may not reflect initial severity of illness prior to the PICU. We also tested comorbid conditions as possible confounders. The association between antimicrobial therapy ≤1 hour from sepsis recognition and one-year mortality remained after adjusting for number of CCCs. Although forcing oncologic comorbidity into the model decreased the adjusted OR slightly with a 95% confidence interval that included one, the overall trend remained strongly in favor of increased mortality in the ≤1 hour group. We cannot exclude, however, that there may be unmeasured confounders that resulted in increased one-year mortality in patients who received antimicrobials ≤1 hour from sepsis recognition.
It is also possible that since time to antimicrobial administration was anchored to sepsis recognition rather than onset, very early (i.e., <1 hour) antimicrobial administration may have been an artifact of a delay in recognition that could have contributed to poor outcomes in some cases. Such a scenario may also have masked a potential adverse effect of prolonged (i.e., >3 hours) antimicrobial administration in our primary analysis when using <1 hour as a reference group. Although we did demonstrate an increased risk of one-year mortality with delayed antimicrobials >3 hours when compared to patients with administration within 1-3 hours, this finding should be interpreted cautiously given the post-hoc nature of this analysis cannot exclude unmeasured confounding, statistical chance, or the possibility that this association was largely driven by patients who died prior to PICU discharge.
There are several limitations to this study. First, this was a retrospective study performed at a single center, limiting generalizability of the findings. Given the relatively small sample size in this study, we cannot exclude the possibility that we failed to detect a small harmful effect of delayed antimicrobials on one-year mortality. In addition, these findings are likely only relevant if a high proportion of initial antimicrobials administered are appropriate antimicrobials. Past studies suggest that time to first appropriate, not initial, antimicrobial therapy is the critical determinant of outcomes (7, 35). In our study, 78% of the initial antimicrobials were appropriate. Therefore, we felt that analysis with time to initial antimicrobials was biologically valid as well as clinically practical. In addition, analyses with times to first appropriate antimicrobials did not yield disparate results from analyses with times to initial antimicrobials. Second, given that the deleterious effects of sepsis begin with its onset rather than recognition, it would be ideal to time interventions and measure outcomes based on biologic onset. Using recognition as time zero could have led to misclassification of antimicrobial timing if there was a large delay from onset to recognition. However, it is not feasible to pinpoint the exact time of sepsis onset in our patients, and we believe that time of sepsis recognition is the most clinically relevant baseline. Third, we selected potential confounders based on biological plausibility, data availability, and published data, and restricted our selection to variables that occurred at the same time as or prior to the primary exposure of antimicrobial administration. Variables that occurred after this time frame, such as blood product transfusions, were excluded from analysis because of inability to differentiate them as confounders versus mediators of outcome. Fourth, because vital status at one year was based on data within the electronic medical record, it is possible that some non-survivors were not appropriately captured as deceased in our data collection. The risk for this misclassification bias was minimized by our ability to capture long-term data on 88% of our patients. Moreover, our primary findings were robust to sensitivity analyses both excluding patients with unconfirmed vital status as well as recoding all unconfirmed survivors as deceased at one year. Finally, the impact of time to antimicrobials on outcomes needs to be interpreted in the context of compliance with other resuscitation goals, which were generally low in this study. Thus, our findings may not be generalizable to settings where the overall bundle of care varies from the cohort included in this study.
Conclusion
Hourly delays to antimicrobial administration during the acute resuscitation of severe sepsis were not associated with one-year mortality in pediatric patients treated in the intensive care unit after controlling for comorbid conditions and severity of illness. An unexpected finding in our post hoc analysis was that one-year mortality was higher in patients who received antimicrobials ≤1 hour from sepsis recognition. The cause and significance of this finding is unclear and should be regarded as hypothesis-generating for future studies that investigate the most appropriate reference group to compare delays in antimicrobials. We continue to support current guidelines that antimicrobial therapy be administered ≤1 hour from sepsis recognition while awaiting results from larger, prospective studies with long-term outcomes.
Supplementary Material
Acknowledgments
Source of Funding:
Financial support was provided by the Endowed Chair, Department of Anesthesia and Critical Care, Division of Emergency Medicine, and the Office of the Chief Medical Office at The Children’s Hospital of Philadelphia, University of Pennsylvania Perelman School of Medicine. Dr. Weiss is also supported by NIGMS K23GM110496. Dr. Balamuth is also supported by NHLBI K12-HL109009.
Glossary
- PICU
Pediatric Intensive Care Unit
- IQR
interquartile range
- aOR
adjusted odds ratio
- CI
confidence interval
- SIRS
systemic inflammatory response syndrome
- ED
emergency department
- CCC
complex chronic conditions
- PRISM
Pediatric Risk of Mortality
- PIM
Pediatric Index of Mortality
- VPS
Virtual Pediatric Intensive Care Unit Systems
- HR
hazard ratio
Footnotes
This study was performed at The Children’s Hospital of Philadelphia.
Conflicts of Interest
For the remaining authors none were declared.
LIST OF SUPPLEMENTAL DIGITAL CONTENT
Supplemental Digital Content 1.docx
Supplemental Digital Content 2.docx
Supplemental Digital Content 3.docx
Supplemental Digital Content 4.tiff
Supplemental Digital Content 5.docx
Supplemental Digital Content 6.docx
Supplemental Digital Content 7.docx
Supplemental Digital Content 8.docx
Supplemental Digital Content 9.docx
Figure, Supplemental Digital Content 4. Survival analysis for time to first appropriate antimicrobial therapy at 3 hour cutoff point.
Kaplan-Meier survival analysis comparing first appropriate antimicrobial therapy at the 3 hour cutoff point from severe sepsis recognition. Total patients n=160. Log-rank test, p=0.26.
REFERENCES
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med. 2013;14(7):686–93. doi: 10.1097/PCC.0b013e3182917fad. [DOI] [PubMed] [Google Scholar]
- 2.Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, Singhi SC, Erickson S, Roy JA, Bush JL, Nadkarni VM, Thomas NJ. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191(10):1147–57. doi: 10.1164/rccm.201412-2323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaramillo-Bustamante JC, Marin-Agudelo A, Fernandez-Laverde M, Bareno-Silva J. Epidemiology of sepsis in pediatric intensive care units: first Colombian multicenter study. Pediatr Crit Care Med. 2012;13(5):501–8. doi: 10.1097/PCC.0b013e31823c980f. [DOI] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, Perez XL, Sirvent JM. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009;180(9):861–6. doi: 10.1164/rccm.200812-1912OC. [DOI] [PubMed] [Google Scholar]
- 6.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, Artigas A, Schorr C, Levy MM. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–55. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 7.Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, Shofer FS, Goyal M. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045–53. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 8.Garnacho-Montero J, Aldabo-Pallas T, Garnacho-Montero C, Cayuela A, Jimenez R, Barroso S, Ortiz-Leyba C. Timing of adequate antibiotic therapy is a greater determinant of outcome than are TNF and IL-10 polymorphisms in patients with sepsis. Crit Care. 2006;10(4):R111. doi: 10.1186/cc4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D, Micek ST, Kollef MH. Time to Appropriate Antibiotic Therapy Is an Independent Determinant of Postinfection ICU and Hospital Lengths of Stay in Patients With Sepsis. Crit Care Med. 2015;43(10):2133–40. doi: 10.1097/CCM.0000000000001140. [DOI] [PubMed] [Google Scholar]
- 11.Weiss SL, Fitzgerald JC, Balamuth F, Alpern ER, Lavelle J, Chilutti M, Grundmeier R, Nadkarni VM, Thomas NJ. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42(11):2409–17. doi: 10.1097/CCM.0000000000000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–83. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 13.Czaja AS, Zimmerman JJ, Nathens AB. Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123(3):849–57. doi: 10.1542/peds.2008-0856. [DOI] [PubMed] [Google Scholar]
- 14.Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277(13):1058–63. [PubMed] [Google Scholar]
- 15.Puskarich MA, Marchick MR, Kline JA, Steuerwald MT, Jones AE. One year mortality of patients treated with an emergency department based early goal directed therapy protocol for severe sepsis and septic shock: a before and after study. Crit Care. 13(5):R167–2009. doi: 10.1186/cc8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varpula M, Karlsson S, Parviainen I, Ruokonen E, Pettila V. Community-acquired septic shock: early management and outcome in a nationwide study in Finland. Acta Anaesthesiol Scand. 2007;51(10):1320–6. doi: 10.1111/j.1399-6576.2007.01439.x. [DOI] [PubMed] [Google Scholar]
- 17.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ, Jr., C. W. E. i. S. S. s. g. Recombinant human protein: Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 18.Angus DC, Laterre PF, Helterbrand J, Ely EW, Ball DE, Garg R, Weissfeld LA, Bernard GR, Investigators P. The effect of drotrecogin alfa (activated) on long-term survival after severe sepsis. Crit Care Med. 2004;32(11):2199–206. doi: 10.1097/01.ccm.0000145228.62451.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 20.Grundmeier RW, Chilutti MR, Ostapenko S, Woodford AL, Wein N, Czaplicki DJ, Schilling DM, Fitzgerald JC, Weiss SL, Balamuth FB, Alpern ER, Lavelle JM. Defining the gold standard: Manual abstraction versus automated data extraction for a complex sepsis registry. Philadelphia, PA: 2013. [Google Scholar]
- 21.Prokosch HU, Ganslandt T. Perspectives for medical informatics. Reusing the electronic medical record for clinical research. Methods Inf Med. 2009;48(1):38–44. [PubMed] [Google Scholar]
- 22.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 23.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–52. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Slater A, Shann F, Pearson G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29(2):278–85. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 25.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Thiel SW, Asghar MF, Micek ST, Reichley RM, Doherty JA, Kollef MH. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med. 2009;37(3):819–24. doi: 10.1097/CCM.0b013e318196206b. [DOI] [PubMed] [Google Scholar]
- 27.Puskarich MA, Trzeciak S, Shapiro NI, Arnold RC, Horton JM, Studnek JR, Kline JA, Jones AE. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39(9):2066–71. doi: 10.1097/CCM.0b013e31821e87ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 29.Sterling SA, Miller WR, Pryor J, Puskarich MA, Jones AE. The Impact of Timing of Antibiotics on Outcomes in Severe Sepsis and Septic Shock: A Systematic Review and Meta-Analysis. Crit Care Med. 2015;43(9):1907–15. doi: 10.1097/CCM.0000000000001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Paridon BM, Sheppard C, Joffe AR. Timing of antibiotics, volume, and vasoactive infusions in children with sepsis admitted to intensive care. Crit Care. 2015;19:293. doi: 10.1186/s13054-015-1010-x. G. G. G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, Brady SL, Brodsky MB, Denehy L, Elliott D, Flatley C, Harabin AL, Jones C, Louis D, Meltzer W, Muldoon SR, Palmer JB, Perme C, Robinson M, Schmidt DM, Scruth E, Spill GR, Storey CP, Render M, Votto J, Harvey MA. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40(2):502–9. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 32.Wang T, Derhovanessian A, De Cruz S, Belperio JA, Deng JC, Hoo GS. Subsequent infections in survivors of sepsis: epidemiology and outcomes. J Intensive Care Med. 2014;29(2):87–95. doi: 10.1177/0885066612467162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamim CF, Hogaboam CM, Kunkel SL. The chronic consequences of severe sepsis. J Leukoc Biol. 2004;75(3):408–12. doi: 10.1189/jlb.0503214. [DOI] [PubMed] [Google Scholar]
- 34.Zurovsky Y, Barbiro E. Hypovolemia in rats increases mortality rates following endotoxin administration. Exp Toxicol Pathol. 2000;52(1):37–42. doi: 10.1016/S0940-2993(00)80013-5. [DOI] [PubMed] [Google Scholar]
- 35.Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med. 1998;244(5):379–86. doi: 10.1046/j.1365-2796.1998.00379.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


