Abstract
Purpose
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide. Although improvements in surgical techniques, chemotherapy and radiation delivery, and supportive care have improved quality of life for patients with HNSCC, regional and distant recurrence remain common. Recent evidence suggests that cancer stem-like cells (CSCs) play a significant role in recurrence and chemo-resistant. We previously observed that c-Fos was highly up-regulated in the HNSCC sphere forming cells. Consequences of c-Fos upregulation for the biology of HNSCC-CSCs are poorly understood. In this study, we investigated the role of c-Fos in renewal of stemness of HNSCC and tumor growth.
Experimental Design and Results
We generated stable HNSCC cell lines ectopically expressing the c-Fos gene. Exogenous expression of c-Fos in non-tumorigenic MDA1386Tu cells makes these cells tumorigenic in nude mice. Further, subcutaneous transplantation of c-Fos overexpressing Cal27 cells (tumorigenic) into immunocompromised mice enhanced tumor growth as compared to parental cells. Mechanistic investigations demonstrated that c-Fos overexpression enhanced the epithelial mesenchymal transition (EMT) state and expression of CSC markers (Nanog, c-Myc, Sox2 and Notch1). Ectopic expression of c-Fos in HNSCC cells also display increased number of sphere formation. We further observed that overexpression of c-Fos increased the expression of pERK and cyclin D1 in HNSCC cells.
Conclusion
Together, our results strongly suggest a novel role of c-Fos as a regulator of EMT and cancer stem cell reprogramming in HNSCC cells, which may hold potential as a CSC-directed therapeutic approach to improve HNSCC treatment.
Keywords: Head and neck squamous cell carcinoma, c-Fos, Cancer stem-like cells, EMT
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most prevalent cancer in the world. Overall survival rate has not improved in the past couple of decades, despite significant improvements in surgical procedures, radiotherapy, and chemotherapy. Thus, gaining insights into the signaling pathways that control the high incidence of local recurrence and distant metastases will be instrumental for developing new therapeutic modalities for the management of HNSCC. Solid tumors contain a small population of cancer stem-like cells (CSCs) in several types of cancers including HNSCC, which plays a critical role in the resistance to therapy, facilitating in cell growth and metastasis. However, the pathogenesis and biological significance of CSCs in HNSCC has not been fully understood.
We have recently observed that c-Fos was up-regulated in HNSCC-sphere forming cells as compared to parental cells (1). c-Fos is a proto-oncogene and present in various types of cancers including HNSCC (2-5). c-Fos encodes a nuclear DNA binding protein domain that forms a dimer with the gene product of c-Jun and ultimately forms the transcription factor activating protein 1 (AP-1). As a member of AP-1 family, c-Fos protein is associated primarily in signal transduction, cell differentiation, and proliferation (6). However, the downstream signaling pathways induced by c-Fos activation and their role in tumorigenesis and metastasis remains to be understood.
Transgenic mice overexpressing the c-Fos proto-oncogene postnatally develop osteosarcomas with 100% penetrance (7). Additionally, overexpression of c-Fos has been shown to promote drug resistance phenotype (8). c-Fos can cause a loss of cell polarity and epithelial-mesenchymal transition, leading to invasive and metastatic growth in mammary epithelial cells (9). AP-1 complex may play a crucial role in the maintenance of colon cancer stem cells (10). Clinical data suggested that c-Fos may be associated with lymph node metastasis in oral cancer (11). Our previous results (1) and these observations in the literature prompted us to investigate the role of c-Fos in cancer stemness of HNSCC cells. In this study, we observed that c-Fos expression in HNSCC cell lines augments cell proliferation, enhances CSC marker gene expression and promote tumor growth. Our results strongly demonstrated that c-Fos plays an important role in tumor progression and promotes cancer stemness in HNSCC.
Materials and Methods
RNA-seq analysis
RNA was isolated from CD133+ and CD133- cells and RNA-seq was done using Washington University ICTS Core Services. Briefly, ribosomal RNA was removed by a hybridization method using Ribo-ZERO kits (EpiCentre). mRNA was then fragmented and reverse transcribed to yield double stranded cDNA. cDNA was blunt ended, had an A base added to the 3’ ends and then had Illumina sequencing adapters ligated to the ends. Ligated fragments were then amplified for 12 cycles using primers incorporating unique index tags. Fragments were sequenced on an Illumina HiSeq-2500 using single reads extending 50 bases.
Cell lines and plasmid DNAs
Head and neck squamous cell carcinoma cell line (Cal27) were purchased from the American Type Culture Collection (ATCC) and non-tumorigenic HNSCC MDA1386Tu cells were obtained from Jeff Myers (MD Anderson Cancer Center), and maintained in gDMEM or RPMI-1640 (Sigma) medium supplemented with 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin in a humidified CO2 incubator. c-Fos-Myc-DDK plasmid DNA or control plasmid DNA in mammalian expression vector (Origene) was transfected into Cal27 and MDA1386Tu cell lines, selected with puromycin, pooled for establishment of a stable cell line. c-Fos shRNA in non-replicating lentivirus was purchased from Origene. A-Fos (dominant negative) plasmid DNA was obtained from Addgene.
Cell proliferation assay
Cells (Cal27 control, Cal27-c-Fos, MDA1386Tu control and MDA1386Tu-c-Fos) were seeded in triplicates, and cells were trypsinized at 24, 48 and 72 h. All cells were counted by Trypan blue exclusion method using a haemocytometer.
Sphere (oralspheres) formation assay
For sphere formation, Cal27 control, Cal27-c-Fos, MDA1386Tu control and MDA1386Tu-c-Fos cells (5000 cells/well) were seeded in single cells suspension on ultralow attachment plates (Corning) as described previously (1). After 10 days of incubation, numbers of oralspheres (>75 μm diameter) were counted using inverted microscope (Leica).
Western blot analysis and antibodies
Cell lysates were analyzed by SDS-PAGE and transferred onto 0.45 μM nitrocellulose membrane (Bio-Rad). Membranes were blocked using 5% low fat dry milk in TBST and probed with the respective primary antibodies. Proteins were detected using ECL Western Blotting Substrate (Thermo Scientific) and autoradiography. The protein loading was normalized using antibody to β-actin or GAPDH. The following antibodies were used in this study: Nanog, Vimentin, c-Met c-Myc, Sox2, Notch 1, pERK, ERK, c-Fos (Cell Signaling Technologies), cytokeratin19, cyclin D1 and β-actin (Santa Cruz Biotechnology).
Immunohistochemistry
The paraffin-embedded sections (5-μm thick) were deparaffinized and stained using antibody against cytokaretin 19 and vimentin, followed by 3,3′-diaminobenzidine staining in clinical histology laboratory. We used mouse monoclonal antibodies for Cytokeratin 19 (A53-B/A2.26) from Cell Marque (CA) and Vimentin (V9) from Ventana (AR). The slides were evaluated by a pathologist, Dr. Nancy Phillips.
Wound healing assay
Cells were grown to confluency and then scratched using a pipette tip. Two wounds were made for each sample. Migration distance of cells were photographed and measured at 0 and 24 hours. Photographs were captured at 0 and 24 h from more than three randomly selected fields using inverted microscope (Leica).
Luciferase reporter assay
MDA1386Tu control and MDA1386Tu-c-Fos cells were transfected with pGL2 basic vector (Promega) containing full-length VEGF promoter sequence (−2361 to +298 bp or -350 to +298 bp) relative to the transcription start site). After 48 h of transfection, luciferase assay was performed using a GloMax (Promega).
In vivo studies
Animal experiments were performed according to the NIH guidelines, following a protocol approved by the Institutional Animal Care and Use Committee of Saint Louis University. Nude mice (6 week old females) were purchased from Charles River, and housed in a specific pathogen free animal facility at the Saint Louis University. Cal27 control, Cal27-c-Fos, MDA1386Tu control and MDA1386Tu-c-Fos cells were resuspended in 100 μl serum free medium, mixed with 40% BD-Matrigel (BD Bioscience) and implanted (2×106 /site) subcutaneously into the flank (right flanks with control cells and left flanks with c-Fos overexpressing cells) of each mouse (n=5). We also implanted higher number of MDA1386Tu control and MDA1386Tu-c-Fos cells (1×107) similarly in 3 nude mice. Tumor volume was measured using digital caliper till the end of experiments. Tumor volume was calculated according to the formula L × W2 × 0.5 (L = length; W = width; all parameters in millimeters). After sacrificing, a portion of the tumor was snap-frozen and stored at -80 °C for biochemical analysis. Some portion of the tumors were fixed and used for H & E staining and immunohistochemistry.
Statistical analysis
Results were expressed as the mean ± standard deviation (SD), and statistical analyses were performed using two-tailed paired or unpaired Student t test in GraphPad Prism 6 (GraphPad, La Jolla, CA). A p value of <0.05 was considered statistically significant.
Results
c-Fos is overexpressed in oralspheres
We have shown previously that c-Fos expression is ~20 fold higher in oralspheres as compared to parental OSC19 cells (1). Early oncogene c-Fos plays a pivotal role in cell growth regulation in association with c-Jun by forming AP-1 complex (12). c-Fos is also involved in signal transduction and cell proliferation in cancer cells (6). CD133, a stemness marker, is highly expressed in the oral sphere as compared to parental cells (1). CD133 is known to be highly up-regulated not only in various types of cancers cells but also in cancer stem cells including HNSCC cancer (13-15). We further performed RNA-seq analysis in CD133+ and compared with CD133- Cal27 cells for identification of genes involved in stemness. Our RNA-seq analysis data suggested that several genes are differentially expressed including a significant upregulation of FosB in CD133+ cells (Table 1). Among all the members of c-Fos family, only c-Fos and FosB shared structural similarities such as transactivation motifs present in the C-terminal and N-terminal parts of these proteins, and are directly associated with transcriptional activation (16). Further, AP-1 transcriptional complexes containing other members of this family such as Fra-1, Fra-2 are less potent transcriptionally than complexes containing c-Fos or FosB (17). We previously observed that c-Fos was highly upregulated in the oralspheres as compared to parental cells (1). However, in our array data we did not observe the upregulation of other Fos family members.
Table 1.
Differentially expressed genes
| Highly up-regulated genes | ||||
|---|---|---|---|---|
| Gene ID | Symbol | Fold change | P-value | FDR |
| 125740 | FOSB | 382.842 | 2.99E-85 | 1.73E-80 |
| 118503 | TNFAIP3 | 195.041 | 252E-44 | 5.83E-40 |
| 169429 | CXCL8 | 178.842 | 8.67E-41 | 1.67E-36 |
| 114315 | HES1 | 168.761 | 1.38E-38 | 2.28E-34 |
| 128422 | KRT17 | 157.761 | 3.49E-36 | 5.04E-32 |
| 143537 | ADAM15 | 141.341 | 1.35E-32 | 1.74E-28 |
| 143398 | PIP5K1A | 126.338 | 2.59E-29 | 2.99E-25 |
| 137497 | NUMA1 | 104.068 | 1.95E-24 | 1.88E-24 |
| 118515 | SGK1 | 102.12 | 5.23E-24 | 4.64E-20 |
| 124788 | ATXN1 | 99.408 | 2.05E-23 | 1.69E-19 |
| Highly downregulated genes | ||||
| Gene ID | Symbol | Fold change | P-value | FDR |
| 150779 | TIMM8B | 11.899 | 5.62E-04 | 5.00E-02 |
| 168036 | CTNNB1 | 11.911 | 5.58E-04 | 4.97E-02 |
| 176095 | IP6K1 | 12.046 | 5.19E-04 | 4.72E-02 |
| 8710 | PKD1 | 12.358 | 4.39E-04 | 4.15E-02 |
| 146678 | IGFBP1 | 13.156 | 2.87E-04 | 3.00E-02 |
| 143514 | TP53BP2 | 13.509 | 2.37E-04 | 2.62E-02 |
| 198001 | IRAK4 | 14.154 | 1.68E-04 | 2.04E-02 |
| 99875 | MKNK2 | 14.175 | 1.67E-04 | 2.02E-02 |
| 184545 | DUSP8 | 15.185 | 9.75E-05 | 1.41E-02 |
| 33327 | GAB2 | 15.261 | 9.37E-05 | 1.39E-02 |
Overexpression of c-Fos enhance tumor growth in vivo
Next, we examined whether overexpression of c-Fos in HNSCC cells has an effect in tumor growth. We chose non-tumorigenic MDA1386Tu cells and exogenously expressed c-Fos (MDA1386Tu-c-Fos). Additionally, we used tumorigenic Cal27 cells and stable transfected expressed c-Fos (Cal27-c-Fos). Overexpression of c-Fos in HNSCC cells were verified by Western blot analysis (Fig. 1A). c-Fos overexpressing Cal27 and MDA1386Tu and their respective control cells were injected into the flanks of each nude mice. We implanted control and c-Fos overexpressing HNSCC cells in the same mouse to avoid animal to animal variation. We observed that tumor size was significantly larger in those flanks of mice injected with c-Fos overexpressing cells as compared to control Cal27 cells (Fig. 1B). Histopathological analysis demonstrated squamous carcinoma appearance in all tumors of Cal27 control or Cal27-c-Fos implanted cells (data not shown).
Figure 1.
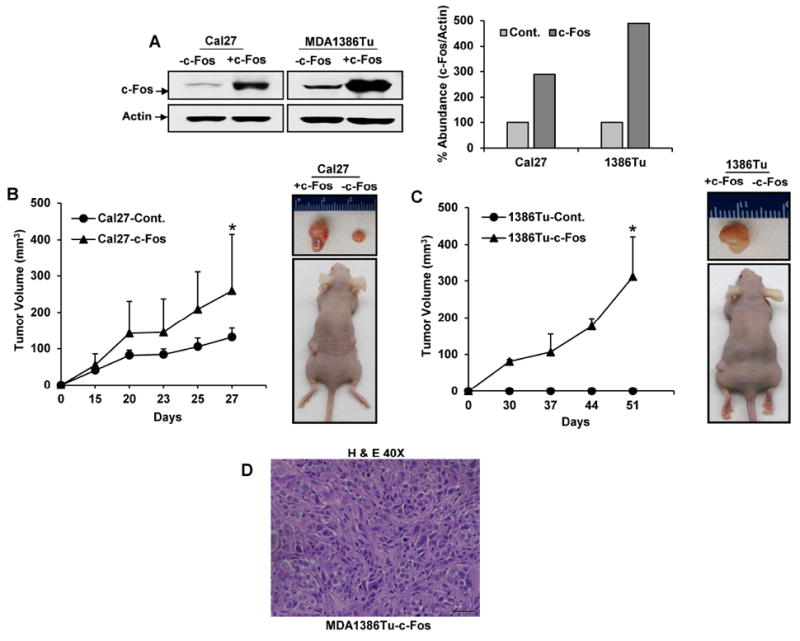
Exogenous expression of c-Fos augments tumor growth in xenograft mouse models. (A) Cal27-control, Cal27-c-Fos cells, MDA1386Tu-control and MDA1386Tu-c-Fos cells lysates were subjected to Western blot analysis using c-Fos antibody. The blots were reprobed with an antibody to actin for comparison of protein loading in each lane. Densitometric analysis of c-Fos were done by using Image J software and shown on the right. (B) Cal27 and (C) MDA1386Tu c-Fos overexpressing and their respective control cells were injected into the flanks of each nude mouse. Volume of tumor growth was measured as indicated times and presented as a mean ±SD. Small bar indicates standard error (*, p < 0.05). (D) Hematoxylin and eosin (H & E) stain image displayed spindle shaped cancer cells from MDA1386Tu-c-Fos overexpressing cells. Images are taken at 40X.
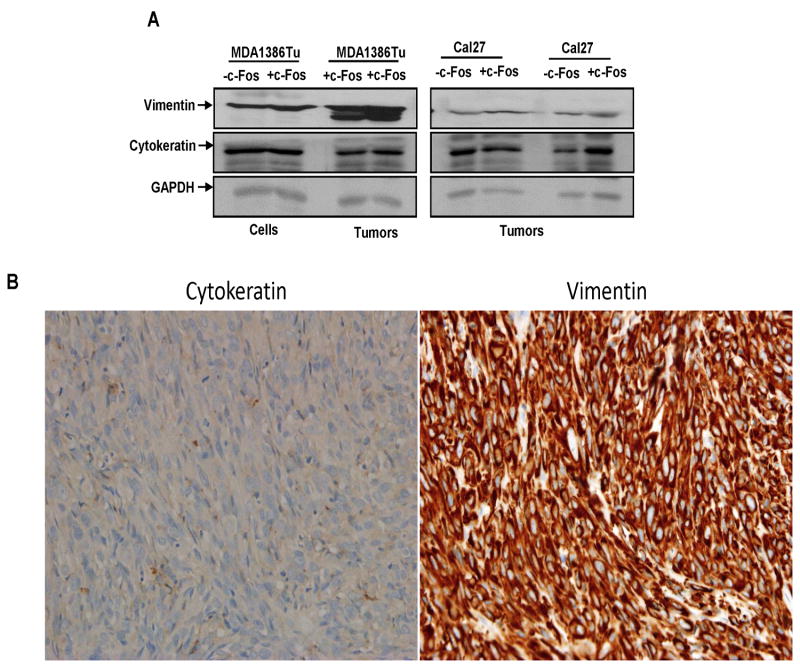
MDA1386Tu cells represented a previously untreated squamous cell carcinoma originating in the hypopharynx of a 72-year-man (T4N3M0). MDA1386Tu cells when implanted in the orthotopic nude mouse model did not form tumor till day 32 (18). In c-Fos overexpressing MDA1386Tu cells, we observed only one mouse display tumor from 2×106 injected cells. However, two out of three mice showed tumor growth from higher number (1×107) of implanted cells (Fig. 1C). We did not observe any tumor growth from control MDA1386Tu cells till the end of the experiments. Due to tumor condition, mice were sacrificed prematurely despite tumor volumes of ~400 mm3. Histopathological analysis demonstrated poorly differentiated with spindle cell appearance in all tumors (Fig. 1D). Spindle cell carcinoma of the HNSCC is unusual variants but present at the different sites (19). Since, we did not observe tumor from parental MDA1386Tu cells, we examined few markers from parental and c-Fos overexpressing cells along with the tumors. Our data suggested that vimentin (marker for spindle cells) and cytokeratin19 (marker for epithelial cells) are expressed at similar levels in Cal27 cells implanted xenograft tumors (Fig. 2A). Interestingly, we observed significantly higher expression of vimentin in tumor lysates from MDA1386Tu-c-Fos implanted cells (Fig. 2A). Immunohistochemistry data demonstrated a strong expression of vimentin, but weak expression of cytokeratin19 from MDA1386Tu-c-Fos implanted cells (Fig. 2B). Together, our results suggest that introduction of c-Fos in HNSCC cells enhances the tumor growth.
Figure 2.
Increased expression of vimentin from c-Fos overexpressing MDA1386Tu cells. (A) Lysates from Cal27, MDA1386Tu control and c-Fos expressing cells and tumors are subjected to Western blot analysis for vimentin and cytokeratin expression using specific antibodies. Blots are reprobed with an antibody to actin for comparison of protein loading in each lane. (B) Immunohistochemistry images showing expression of vimentin and cytokeratin from MDA1386Tu-c-Fos cells implanted tumors. Images are taken at 40x.
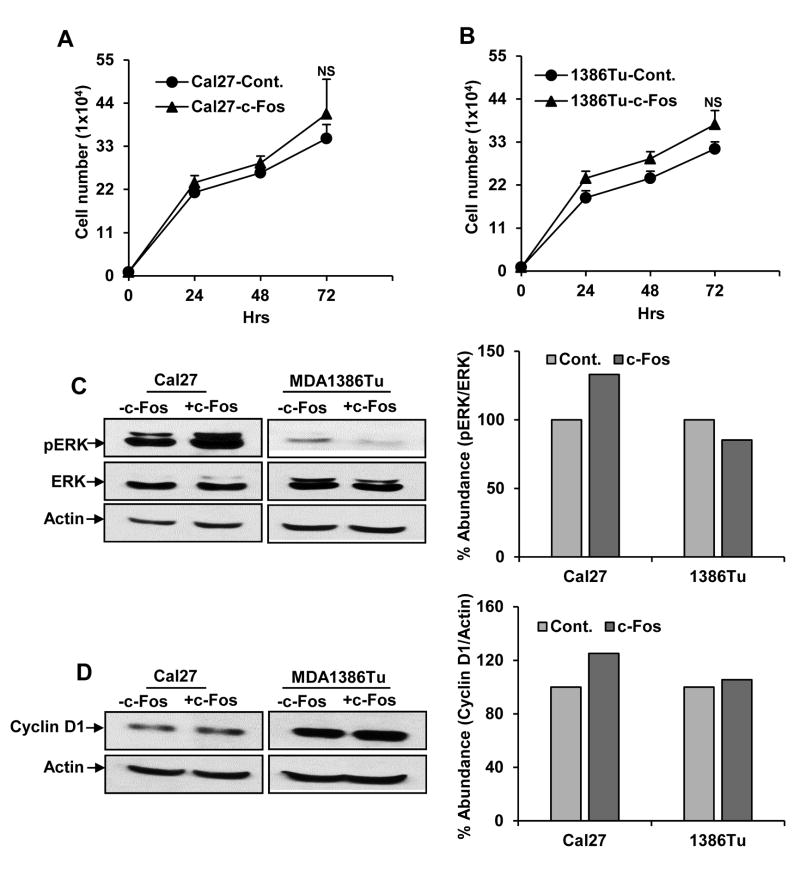
Overexpression of c-Fos does not enhance cell proliferation
One of the characteristic features of any tumor cells is uncontrolled proliferation of cells. c-Fos has a variety of functions in regulating cell proliferation, differentiation, and transformation (20). Therefore, we examined whether exogenous expression of c-Fos has an effect on cell proliferation. Our results suggested a slightly higher proliferation rate in c-Fos overexpressing HNSCC cells (Fig. 3A). Activated ERK is associated with cell growth and differentiation in various cancers including HNSCC (21, 22). ERK also regulates the expression of c-Fos (23). Studies also suggested that inhibition of ERK signaling, the size or number of the spheres can be reduced in various types of cancers (24, 25). We examined the protein level of pERK1/2 in c-Fos expressing cells by Western blot analysis. We observed that the protein level of pERK1/2 was increased only in Cal27 c-Fos overexpressing cells (Fig. 3B). No alternation was observed in the total ERK level in all the cells. Cyclin D1, a critical cell cycle regulatory protein, plays an essential role in regulation of cell proliferation of cancers cells (26). To explore whether c-Fos overexpression facilitated the cell proliferation through the modulation of cell cycle related protein, we examined the expression of cyclin D1 by Western blot analysis. Our results showed that overexpression of c-Fos in Cal27 cells enhance the expression of cyclin D1 as compared to control cells (Fig. 3C). Interestingly, we did not observe a significant change of these cell cycle regulatory proteins in MDA1386Tu cells. Taken together, our findings demonstrated that ectopic expression of c-Fos augments the cell proliferation of Cal27 cells and enhances pERK1/2 and cyclin D1 levels, but does not have an effect in MDA1386Tu cells.
Figure 3.
Exogenous expression of c-Fos alters cell proliferation and its related proteins. (A) Cal27-control and Cal27-c-Fos cells, and (B) MDA1386Tu-control and MDA1386Tu-c-Fos cells were plated in 35 mm plates in triplicates, and live cells were counted at indicated time points by using Trypan blue exclusion method. Small bar indicates standard error. (C) HNSCC cells lysates were subjected to Western blot analysis for pERK1/2 and ERK1/2 expression using specific antibodies. The blots were reprobed with an antibody to actin for comparison of protein loading in each lane. Densitometric analysis of pERK1/2 were done by using Image J software and shown on the right. (D) HNSCC cells lysates were subjected to Western blot analysis using antibody to cyclin D1. The blots were reprobed with an antibody to actin for comparison of protein loading in each lane. Densitometric analysis of c-Fos and cyclin D1 were done by using Image J software and shown on the right.
Overexpression of c-Fos enhances the stemness in HNSCC cells
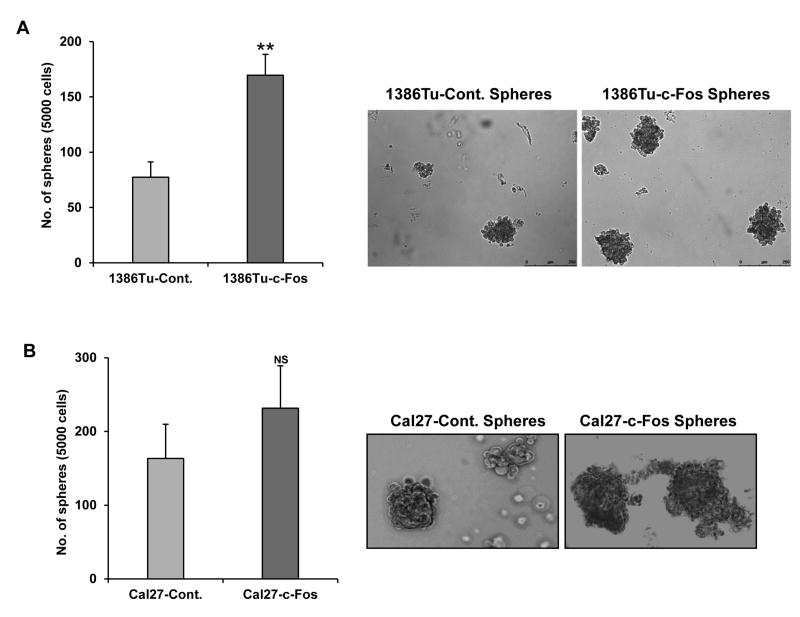
We initially observed higher expression of c-Fos in the HNSCC-sphere forming cells (Table 1). To better understand whether c-Fos plays any critical role in the stemness of non-tumorigenic MDA1386Tu cells, we investigated whether exogenous expression of c-Fos caused an alteration in the self-renewal capacity of oralspheres. Approximately 5000 cells of c-Fos overexpressing and control MDA1386Tu cells were seeded in ultra-low attachment plates for 10 days, and number of spheres were counted. We observed a significant increase in the numbers and size of oralspheres in c-Fos overexpressing cells as compared to control MDA1386Tu cells (Fig. 4A). A representative image is shown in right. c-Fos overexpression in Cal27 cells also enhanced number of oralspheres (Fig. 4B). Notably, oralspheres generated from c-Fos overexpressing cells were bigger in size as compared to those generated from control cells (Fig. 4, right panels). Further, we knocked down c-Fos in Cal27 cells using lentivirus mediated shRNA and knocked out c-Fos using CRISR/Cas9 system. However, in both cases we failed to generate a stable cell line. We have transfected A-Fos (dominant negative of c-Fos) into Cal27 cells. After 24 hr of transfection, cells were seeded in ultra-low attachment plates for 10 days for sphere formation assay. Our results indicated that A-Fos expression significantly inhibited oralspheres formation as compared to control cells (data not shown). Since the number of spheres was smaller than 75 μm diameter, it was not counted.
Figure 4.
Exogenous expression of c-Fos enhances sphere formation. (A) Equal number of Cal27-control, Cal27-c-Fos cells and (B) MDA1386Tu-control and MDA1386Tu-c-Fos cells were seeded in ultra-low attachment plates and incubated for 10 days. The number of spheres (>75 mm) were counted. The results are presented as means of three different experiments with standard deviations (*p < 0.05, **p < 0.01). Representative images of spheres are shown on the right.
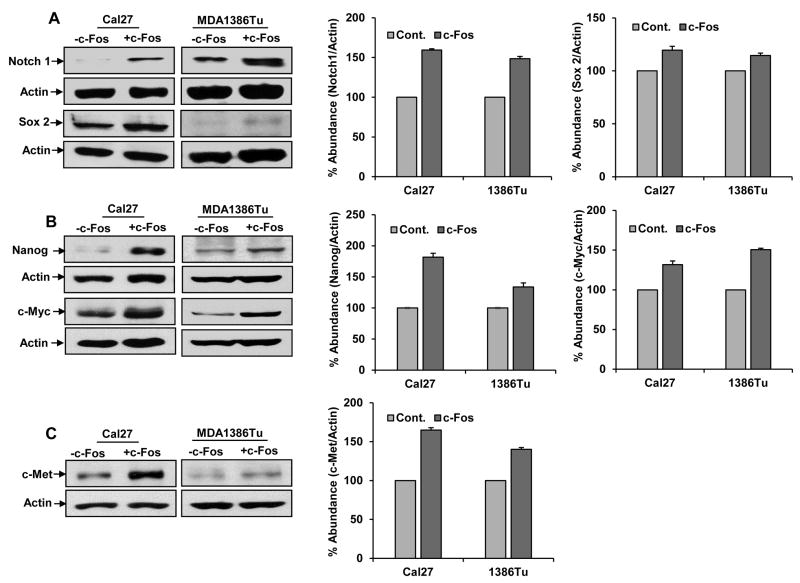
We next examined the expression of Nanog, Sox2, c-Myc and Notch 1 (stemness markers) in c-Fos overexpressing HNSCC cells. A significant upregulation of Notch1 protein was noted in both Cal27 and MDA1386Tu-c-Fos overexpressing cells (Fig. 5A). In our RNA-seq data, Hes1, downstream molecule of Notch1 was highly upregulated in CD133+ cells. We also observed upregulation of Hes1 in OSC19 oralspheres (1). A slight increase of Sox2 expression was noted from c-Fos overexpressing HNSCC cells (Fig. 5A). On the other hand, Nanog and c-Myc protein level was increased in c-Fos overexpressing MDA1386Tu or Cal27 cells compared with control cells (Fig. 5B). The c-Met pathway is aberrantly upregulated in HNSCC, and activates the same downstream signaling pathways. Further, c-Met expression was enhanced in c-Fos overexpressing HNSCC cells as compared to control cells (Fig. 5C).
Figure 5.
Exogenous expression of c-Fos enhances the expression stemness related markers. Cal27-control, Cal27-c-Fos, MDA1386Tu-control and MDA1386Tu-c-Fos cells lysates were subjected to Western blot analysis for (A) Notch1 and Sox2, (B) Nanog and c-Myc, and (C) c-Met expression using specific antibodies. The blots were reprobed with an antibody to actin for comparison of protein loading in each lane. Densitometric analysis were done by using Image J software and shown on the right.
Overexpression of c-Fos in MDA1386Tu cells enhances cell migration and VEGF expression
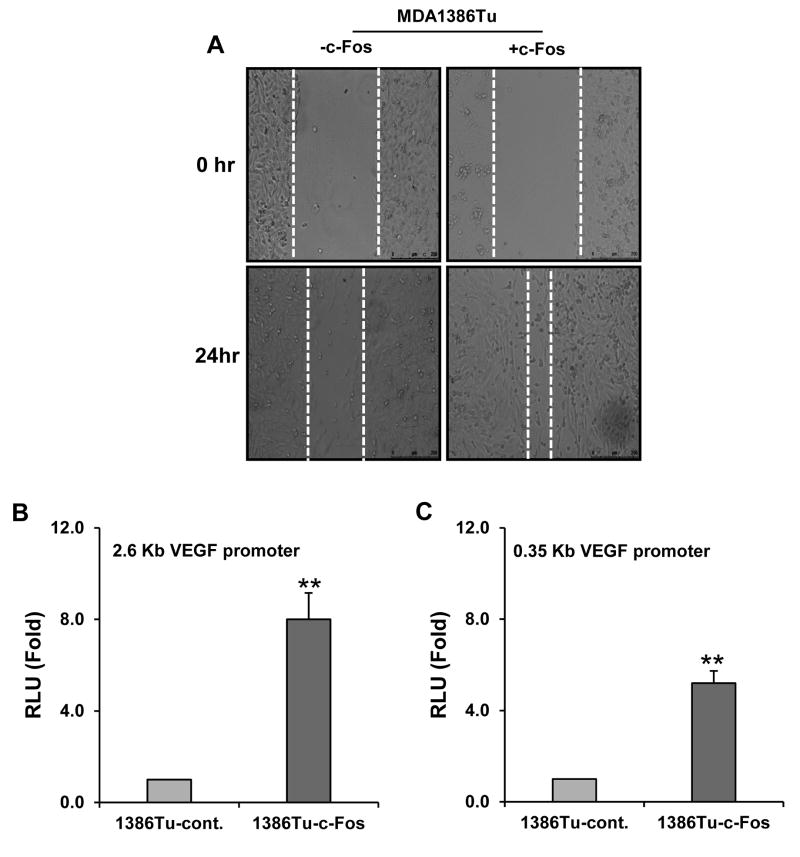
Various studies suggested a direct correlation between cell migration with cancer metastasis (27). MDA1386Tu cells expressing c-Fos were proficient in closing an artificial wound created over a confluent monolayer as compared to control cells (Fig. 6A). We also observed upregulation of vimentin in MDA1386Tu-c-Fos expressing cells (Fig. 2). However, an alteration at MMP2 expression was not noted. c-Fos has been involved in IL-6 and VEGF-A transcription in colon cancer (28). We transiently transfected the MDA1386Tu control or c-Fos expressing cells with 2.6-kb VEGF promoter-luciferase construct in pGL2 basic vector (29). We observed that VEGF-A promoter (2.6 kb) is highly increased in c-Fos overexpressing MDA1386Tu cells (Fig. 5B). Promoter bashing suggested that c-Fos binding is located at -621 position of VEGF-A promoter. When we used mutant promoter VEGF-A (0.35kb) construct, we observed significant reduction of promoter activity. However, conditioned media from c-Fos overexpressing MDA1386Tu cells did not support the tube formation. Together, this result suggests that c-Fos plays wound healing in HNSCC cells which in part may promote cell migration.
Figure 6.
Exogenous expression of c-Fos enhances the migration of cells and VEGF promoter activity. (A) MDA1386Tu control and MDA1386Tu-c-Fos cells were seeded at confluency. Representative images of wound healing (0 and 24 hours after scratch) in MDA1386Tu cells expressing control plasmid DNA or c-Fos are shown. Control MDA1386Tu and MDA1386Tu-c-Fos cells were transfected with 2.6 Kb (B) or 0.35 Kb (C) VEGF promoter sequences, and luciferase activities were measured. The results are presented as means of three different experiments with standard deviations (**p < 0.01).
Discussion
In this study, exogenous expression of c-Fos in non-tumorigenic HNSCC MDA1386Tu cells displays tumorigenic phenotype in immunodeficient mice. Histopathology study reveals spindle shaped cells and strong vimentin expression. We also observed that introduction of A-Fos significantly inhibits the sphere-forming ability of Cal27 cells. Further, we have shown that overexpression of c-Fos in HNSCC cells enhances CSC marker gene expression.
c-Fos is a member of the Fos family of transcription factors and associates with c-Jun to form heterodimeric activator protein-1 (AP-1) complexes which transcriptionally regulates several gene expression (12). c-Fos has been implicated in several cellular events including cell proliferation, genes associated with hypoxia, and angiogenesis. Recent study demonstrated that c-Fos enhances IL-6 and VEGF-A expression in colon cancer (28). We also observed exogenous expression of c-Fos enhances VEGF-A promoter activity, however, we do not observe an alteration of IL-6 transcription in our experimental system. Activation of ERK-c-Fos axis induces HIF-α in breast, gastric and hepatic cancer cells (30, 31). However, in our experimental system, c-Fos may not induce HIF-α, since VEGF-A promoter with HIF-α deleted sites are active (data not shown). c-Fos protein has been shown to act as a negative regulator of rat-1 fibroblasts (32). However, we have observed enhancement of cyclin D1 in Cal27 cells overexpressing c-Fos. Interestingly in MDA1386Tu cells, cyclin D1 and pERK1/2 expression remained unaltered with exogenous expression of c-Fos gene.
We have observed Notch1 expression is enhanced in c-Fos expressing HNSCC cells. Notch1 mutations occur in approximately 15% of patients with HNSCC, implicating a critical role of NOTCH signaling pathways in HNSCC tumors (33). In fact Notch1 targets genes are also increased on c-Fos overexpressing cells. It will be important to understand how c-Fos is enhancing Notch 1 expression in future study. c-Fos binds to the promoter region of the FZD8 gene in patients derived cancer stem like cells, which is regulated by c-Met (34). The ubiquitous expression of tyrosine kinase, such as EGFR and/or c-Met, is higher in HNSCC tumors, however, the clinical response using these tyrosine kinase inhibitors is limited due to resistance (35). The c-Met pathway is aberrantly upregulated in HNSCC, and activates the same downstream signaling pathways. We have shown previously that c-Met expression can be limited in HNSCC cells by treating them with a natural product, bitter melon (36). However, the effect of c-Fos on c-Met expression is not fully understood. Our results suggested that exogenous expression of c-Fos in Cal27 and MDA1386Tu cells upregulates c-Met expression. We also observed upregulation of vimentin in c-Fos expressing cells. In fact, CSC markers are also enhanced on both Cal27 and MDA1386Tu cells, although cell type specificity is noted.
Overexpression of c-Fos was noted in primary oral squamous cell carcinoma (5). The molecular mechanism of the effect of c-Fos in HNSCC is incompletely understood. This study provides a promising mechanism for the first time directly linking c-Fos with Notch1 to HNSCC for promotion of tumor growth, which is worthy for further investigation. Little is known about the role of c-Fos in cancer stemness. We observed that c-Fos overexpression in HNSCC enhances Nanog and c-myc expression, the key molecules for cancer stemness. We have also observed that VEGF, one of the downstream targets of c-Fos, is upregulated in c-Fos overexpressing HNSCC. The differential gene expression analysis in future will help for understanding potential signaling pathways for c-Fos mediated cancer cell stemness. In summary, we demonstrated a novel role of c-Fos protein in HNSCC cells. We have shown that c-Fos overexpression in non-tumorigenic cell line make the cells tumorigenic and enhance the EMT/CSC marker genes.
Translational Relevance.
Head and neck squamous cell carcinoma (HNSCC) has a high mortality rate despite advancement in chemotherapy, radiotherapy, surgical procedure and targeted therapy. Emerging evidence implicated that recurrence and metastasis from these tumors occur from cancer like stem cells, although the mechanism remains unknown. Our study revealed the role of c-Fos in the promotion of cancer stem like cell properties in head and neck cancer. Overexpression of c-Fos in non-tumorigenic HNSCC cells exhibits tumorigenic phenotype, increases stemness markers and increases oralsphere formation. Taken together our data indicates that c-Fos plays a crucial role in driving the stemness properties in HNSCC and may be a good therapeutic target.
Acknowledgments
We thank Saumitro Pal for VEGF-promoter constructs. This work was supported by research grant R01 DE024942 from the National Institutes of Health, Saint Louis University Cancer Center Seed Grant, and NIH CTSA Grant # UL1 TR000448 for RNA-seq analysis. We also thank other member of Ray lab for the suggestions in this work.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Shrivastava S, Steele R, Sowadski M, Crawford SE, Varvares M, Ray RB. Identification of molecular signature of head and neck cancer stem-like cells. Sci Rep. 2015;15(5):7819. doi: 10.1038/srep07819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 3.Bakin AV, Curran T. Role of DNA 5-methylcytosine transferase in cell transformation by fos. Science. 1999;283:387–390. doi: 10.1126/science.283.5400.387. [DOI] [PubMed] [Google Scholar]
- 4.Hu E, Mueller E, Oliviero S, Papaioannou VE, Johnson R, Spiegelman BM. Targeted disruption of the c-fos gene demonstrates c-fos dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994;13:3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong C, Ye DX, Zhang WB, Pan HY, Zhang ZY, Zhang L. Overexpression of c-fos promotes cell invasion and migration via CD44 pathway in oral squamous cell carcinoma. J Oral Pathol Med. 2015;44:353–60. doi: 10.1111/jop.12296. [DOI] [PubMed] [Google Scholar]
- 6.Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer. 2005;41:2449–2461. doi: 10.1016/j.ejca.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Grigoriadis AE, Schellander K, Wang ZQ, Wagner EF. Osteoblasts are target cells for transformation in c-fos transgenic mice. J Cell Biol. 1993;122:685–701. doi: 10.1083/jcb.122.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Luo A, Li S, Zhang W, Chen H, Li Y, et al. Inhibitor of Differentiation/DNA Binding 1 (ID1) Inhibits Etoposide-induced Apoptosis in a c-Jun/c-Fos-dependent Manner. J Biol Chem. 2016;25:6831–42. doi: 10.1074/jbc.M115.704361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fialka I, Schwarz H, Reichmann E, Oft M, Busslinger M, Beug H. The estrogen-dependent c-JunER protein causes a reversible loss of mammary epithelial cell polarity involving a destabilization of adherens junctions. J Cell Biol. 1996;132:1115–1132. doi: 10.1083/jcb.132.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apostolou P, Toloudi M, Ioannou E, Chatziioannou M, Kourtidou E, Vlachou I, et al. AP 1 Gene Expression Levels May Be Correlated with Changes in Gene Expression of some Stemness Factors in Colon Carcinomas. J Signal Transduct. 2013;2013:497383. doi: 10.1155/2013/497383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Xu X, Xu F, Meng Y, Sun C, Shi L, Zhao E. Combined Expression of c-jun, c-fos,and p53 Improves Estimation of Prognosis in Oral Squamous CellCarcinoma. Cancer Invest. 2016;24:1–8. doi: 10.1080/07357907.2016.1217422. [DOI] [PubMed] [Google Scholar]
- 12.Shaulian Eitan, Karin Michael. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 13.Kumar D, Kumar S, Gorain M, Tomar D, Patil HS, Radharani NN, Kumar TV, Patil TV, Thulasiram HV, Kundu GC. Notch1-MAPK Signaling Axis Regulates CD133+ Cancer Stem Cell-Mediated Melanoma Growth and Angiogenesis. J Invest Dermatol. 2016 doi: 10.1016/j.jid.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Liu TJ, Sun BC, Zhao XL, Zhao XM, Sun T, Gu Q, et al. CD133+ cells with cancer stem cell characteristics associated with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 2013;32:544–53. doi: 10.1038/onc.2012.85. [DOI] [PubMed] [Google Scholar]
- 15.Major AG, Pitty LP, Farah CS. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int. 2013;2013:319489. doi: 10.1155/2013/319489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tkach V, Tulchinsky E, Lukanidin E, Vinson C, Bock E, Berezin V. Role of the Fos family members, c-Fos, Fra-1 and Fra-2, in the regulation of cell motility. Oncogene. 2003;22:5045–54. doi: 10.1038/sj.onc.1206570. [DOI] [PubMed] [Google Scholar]
- 17.Wisdom R, Verma IM. Proto-oncogene FosB: the amino terminus encodes a regulatory function required for transformation. Mol Cell Biol. 1993;13:2635–43. doi: 10.1128/mcb.13.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sano D, Xie TX, Ow TJ, Zhao M, Pickering CR, Zhou G, et al. Disruptive PT53 mutation is associated with aggressive disease characteristics in orthotopic murine model of oral tounge cancer. Clin Cancer Res. 2011;17:6658–70. doi: 10.1158/1078-0432.CCR-11-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viswanathan S, Rahman K, Pallavi S, Sachin J, Patil A, Chaturvedi P, et al. Sarcomatoid (spindle cell) carcinoma of the head and neck mucosal region: a clinocopathological review of 103 cases from a tertiary referral cancer center. Head Neck Pathol. 2010;4:265–75. doi: 10.1007/s12105-010-0204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eferl Robert, Wagner Erwin F. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 21.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogenactivated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 22.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 23.Monje P, Hernández-Losa J, Lyons RJ, Castellone MD, Gutkind JS. Regulation of the transcriptional activity of c-Fos by ERK. A novel role for the prolyl isomerase PIN1. J Biol Chem. 2005;280:35081–4. doi: 10.1074/jbc.C500353200. [DOI] [PubMed] [Google Scholar]
- 24.Ciccarelli C, Vulcano F, Milazzo L, Gravina GL, Marampon F, Macioce G, et al. Key role of MEK/ERK pathway in sustaining tumorigenicity and in vitro radioresistance of embryonalrhabdomyosarcoma stem-like cell population. Mol Cancer. 2016;15:16. doi: 10.1186/s12943-016-0501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S, Trevino J, Bora-Singhal N, Coppola D, Haura E, Altiok S, Chellappan SP. EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells innon-small cell lung cancer. Mol Cancer. 2012;11:73. doi: 10.1186/1476-4598-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casimiro MC, Crosariol M, Loro E, Li Z, Pestell RG. Cyclins and cell cycle control in cancer and disease. Genes Cancer. 2012;3:649–57. doi: 10.1177/1947601913479022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedl P, Wolf K. Tumour cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 28.Mar AC, Chu CH, Lee HJ, Chien CW, Cheng JJ, Yang SH, et al. Interleukin-1 receptor type 2 acts with c-fos to enhance the expression of interleukin-6 and vascular endothelial growth factor A in colon cancer cells and induce angiogenesis. J Biol Chem. 2015;290:22212–22224. doi: 10.1074/jbc.M115.644823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basu A, Datta D, Zurakowski D, Pal S. Altered VEGF mRNA stability following treatments with immunosuppressive agents: implications for cancer development. J Biol Chem. 2010;285:25196–25202. doi: 10.1074/jbc.M110.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rigiracciolo DC, Scarpelli A, Lappano R, Pisano A, Santolla MF, De Marco P, Cirillo F, Cappello AR, Dolce V, Belfiore A, Maggiolini M, De Francesco EM. Copper activates HIF-1α/GPER/VEGF signalling in cancer cells. Oncotarget. 2015;6:34158–77. doi: 10.18632/oncotarget.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao JH, Wang CH, Tong H, Wen SL, Huang ZY, Tang CW. Targeting inhibition of extracellular signal-regulated kinase kinase pathway with AZD6244 (ARRY-142886) suppresses growth and angiogenesis of gastric cancer. Sci Rep. 2015;5:16382. doi: 10.1038/srep16382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balsalobre A, Jolicoeur P. Fos proteins can act as negative regulators of cell growth independently of the fos transforming pathway. Oncogene. 1995;11:455–65. [PubMed] [Google Scholar]
- 33.Sun W, Gaykalova DA, Ochs MF, Mambo E, Arnaoutakis D, Liu Y, Loyo M, Agrawal N, Howard J, Li R, Ahn S, Fertig E, Sidransky D, Houghton J, Buddavarapu K, Sanford T, Choudhary A, Darden W, Adai A, Latham G, Bishop J, Sharma R, Westra WH, Hennessey P, Chung CH, Califano JA. Activation of the NOTCH Pathway in Head and Neck Cancer. Cancer Res. 2014;74:1091–104. doi: 10.1158/0008-5472.CAN-13-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun S, Liu S, Duan SZ, Zhang L, Zhou H, Hu Y, et al. Targeting the c-Met/FZD8 signalling axis eliminates patient-derived cancer stem like cells in head and neck squamous carcinomas. Cancer Res. 2014;74:7546–59. doi: 10.1158/0008-5472.CAN-14-0826. [DOI] [PubMed] [Google Scholar]
- 35.Sahu N, Grandis JR. New advances in molecular approaches to head and neck squamous cell carcinoma. Anticancer Drugs. 2011;22:656–664. doi: 10.1097/CAD.0b013e32834249ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajamoorthi A, Shrivastava S, Steele R, Nerurkar P, Gonzalez JG, Crawford S, et al. Bitter melon reduces head and neck squamous cell carcinoma growth by targeting c-Met signaling. PLoS One. 2013;17(8):e78006. doi: 10.1371/journal.pone.0078006. [DOI] [PMC free article] [PubMed] [Google Scholar]