Significance
The results of this study help resolve an important open question regarding visual learning: Can the human brain acquire face classification skill late in life, or is such learning limited only to a critical period early in development? Working with a group of congenitally blind children in whom we were able to surgically initiate sight, we tracked their face/nonface categorization ability over several months. The data reveal that although the newly sighted children do not possess innately specified face schemas, they are able to learn this distinction to a high degree of proficiency through natural visual experience. These findings have implications for visual learning, brain plasticity, and prognoses for late treatments of blindness.
Keywords: face classification, blindness, visual learning, sight restoration, plasticity
Abstract
It is unknown whether the ability to visually distinguish between faces and nonfaces is subject to a critical period during development. Would a congenitally blind child who gains sight several years after birth be able to acquire this skill? This question has remained unanswered because of the rarity of cases of late sight onset. We had the opportunity to work with five early-blind individuals who gained sight late in childhood after treatment for dense bilateral cataracts. We tested their ability to categorize patterns as faces, using natural images that spanned a spectrum of face semblance. The results show that newly sighted individuals are unable to distinguish between faces and nonfaces immediately after sight onset, but improve markedly in the following months. These results demonstrate preserved plasticity for acquiring face/nonface categorization ability even late in life, and set the stage for investigating the informational and neural basis of this skill acquisition.
In our natural environment, few patterns can rival the ecological significance of faces and the skills we exhibit for processing them. We are adept at detecting faces in challenging viewing conditions, against complex backgrounds, and across large image transformations and degradations (1–4). Such proficiency is evident very soon after birth. Infants just a few weeks old have been found to look preferentially at face-like patterns over nonface ones of similar complexity (5–11).
The consistent and early manifestation of these skills suggests they may be subject to a stereotypical timeline of development, strongly tied to maturational and experience-driven processes in the first few weeks of life (12, 13). To investigate whether or not the development of these skills is time-bound, we need to examine whether their timeline can be significantly delayed beyond the normal window of deployment. This harkens to the classical notions of a critical period in visual development (14, 15). Several findings suggest that low-level aspects of visual function, such as high-resolution vision and binocular depth perception, are subject to a critical period, and visual deprivation during this period renders impossible their acquisition later in life (14,16–19). Do critical periods apply similarly to higher-level visual tasks such as face perception? This question has remained open thus far, largely because classic studies of critical periods were conducted with nonhuman animals in which assessments of high-level skills are operationally difficult.
Human data on this issue are scant, given the rarity of cases of early blindness that are treated late in life. Individual case studies suggest early visual deprivation impairs object as well as face recognition abilities (20–24). Furthermore, such deprivation has been shown to compromise electrophysiological components believed to be associated with face perception (25). Even relatively short periods of deprivation ranging in duration from 2 to 6 mo after birth have been shown to have significant detrimental consequences on face recognition skills. Maurer and colleagues (26–29) have examined visual performance of teenagers who, as infants, had suffered from congenital cataracts for a few months. The researchers found that this short period of early deprivation had a long-term effect; specifically, the children as adolescents exhibited impairments relative to controls in their ability to discriminate between faces on the basis of configural cues (the precise spacing between eyes, nose, and mouth). These results are consistent with the notion that precocial abilities are more likely to be associated with a critical period during which abnormalities in environmental input will have permanent adverse effects on their subsequent development (30).
Given the long-lasting effect on face perception skills of even short episodes of visual deprivation, protracted periods of blindness, lasting several years rather than just a few months, would be expected to have profound consequences on face perception abilities. Arguably, the most basic of these skills is the categorization of visual patterns as faces and nonfaces, the earliest-manifesting face-perception ability in infants (5–11, 31), which presumably serves as scaffolding for additional face-related proficiencies. Our goal here is to investigate whether this skill can be acquired despite several years of early-onset visual deprivation (i.e., in blind children who had been left untreated for several years before receiving sight surgeries).
In contextualizing our work amid the earlier studies of sight recovery after treatment for blindness, a few points deserve note. First, investigations of sight onset in adulthood or late childhood have hitherto not involved the systematic longitudinal assessments of face perception necessary to quantify and track the acquisition of face classification abilities after treatment (20–24, 32, 33). Second, studies of children who gained sight after a few months of blindness show that although they exhibit subtle deficits in face identification (26, 29, 34), there are no indications that their basic ability to classify patterns as faces is compromised. Indeed, Mondloch et al. (35) have reported that infants a few months old rapidly exhibit preferences for patterns with canonical face geometry over nonface ones after treatment for congenital cataracts. This leads to the inference that the acquisition of face/nonface classification ability is resilient to short-duration deprivation. However, this does not allow any conclusions regarding what the consequences of extended blindness might be when the duration of deprivation exceeds putative visual critical periods in humans (36). The study we describe here helps address this issue by exploring the consequences of childhood blindness left untreated for several years.
This work is based on a study of five children in India (two girls/three boys, ages ranging from 9 to 17 y at treatment) with an unusual visual history. All had early-onset (by 1 y of age) dense bilateral cataracts and had not received any medical treatment for blindness until they were identified as candidates for surgery through outreach activities undertaken as part of Project Prakash (37, 38). Assessment of cataract onset was based on parental reports and on the presence of nystagmus, which is known to be induced by profound visual impairment early in life, typically within 4 mo of birth (39). The children were provided surgeries, which involved cataract extraction and intraocular lens implantation. Their visual progress was followed longitudinally thereafter. This enabled us to investigate their visual skills immediately after sight onset, and any changes therein over time. Although we attempted to assess subjects at regular and identical intervals, this was not always possible because of the logistical challenges of patients and their families traveling to Delhi from remote villages, and the time and space constraints of the hospital.
Preoperative acuity was measured as perception of light, hand motion, or the farthest distance at which subjects could correctly count fingers. Acuity postsurgery was measured using Landolt C optotypes presented one at a time on an iPad at a test distance of 40 cm. If the subject’s vision was still too poor to perform the Landolt C test, then acuity was measured using the preoperative method. Pre- and postoperative visual acuities, as well as individual subject characteristics, are included in Table S1.
Table S1.
Patient information
| Subject | Sex | Age at treatment, y | Detection of blindness | Pretreatment acuity | Posttreatment acuity (logMAR) |
| S1 | Female | 9 | By 1 y | HM | 1 mo: 2.1 |
| 7 mo: 1.3 | |||||
| 20 mo: 1.2 | |||||
| S2 | Female | 11 | At birth | FC at 30 cm | 2 d: 1.6 |
| 6 d: 1.4 | |||||
| 1 mo: 1.4 | |||||
| 7 mo: 1 | |||||
| 13 mo: 1.3 | |||||
| 19 mo: 1.1 | |||||
| S3 | Male | 17 | At birth | HM | 5 mo: 1 |
| 17 mo: 0.93 | |||||
| 23 mo: 0.93 | |||||
| S4 | Male | 12 | By 1 y | FC at 50 cm | 6 mo: 1.3 |
| 30 mo: 1.5 | |||||
| S5 | Male | 11 | By 6 mo | HM | 4 mo: 1.5 |
| 10 mo: 1.1 | |||||
| 16 mo: 1 | |||||
| 22 mo: 1.3 |
FC, finger counting; HM, hand movements; MAR, minimum angle of resolution.
In pursuing this experimental approach, it is important to point out that although data on face learning in newly sighted children can yield insights regarding vulnerability of such acquisition to early deprivation, they should not be treated as proxies for infancy data to infer the nature of processes underlying the acquisition of these abilities in normal development.
Our control group comprised 30 normally sighted children with a mean age of 9.97 y (eight girls/22 boys, ages ranging from 8 to 11 y). They were tested while wearing blur goggles simulating Snellen visual acuities of 20/300, 20/600, and 20/800 (nonoverlapping groups of 10 children at each blur level). Blurring was achieved by attaching Bangerter Occlusion foils (40) to clear safety goggles. Assessing the performance of normally sighted subjects while they were wearing blurring goggles enabled us to titrate the effects of reduced acuity comparable to the Prakash children’s postoperative outcomes, separate from nonoptical factors on face classification performance.
The stimulus set we used for assessing face/nonface categorization has been described in detail in Meng et al. (41), and has proven effective for exploring face classification in behavioral studies, as well as in those using neuroimaging and electrophysiological tools (42). Briefly, this set comprises 300 natural images that span a range of facial semblance from nonfaces to genuine faces. These images include true hits and false positives from a computational face detection system developed at Carnegie Mellon University by Rowley et al. (43). Based on human and computationally derived ratings of face semblance, the stimuli are divided into five equal-cardinality nonoverlapping bins (NF0, nonfaces; NFL, low face semblance; NFM, medium face semblance; NFH, high face semblance; and F, faces). The five bins do not differ from each other in the spectral composition and mean luminance of their constituent images. The bin of genuine faces comprises front-facing images from both sexes under different lighting conditions. Fig. 1 shows a few example images from the stimulus set.
Fig. 1.
Sample stimuli used in our studies. Rows are arranged in order of increasing face semblance, with genuine faces at the bottom. The full stimulus set comprised 300 images and was divided into five equal-size groups labeled NF0 (randomly selected nonface images), NFL, NFM, NFH [false alarms of a computational face detection system (43) with low, medium, and high face semblance, as determined by human raters and computational metrics (41)], and F (genuine face images).
Results
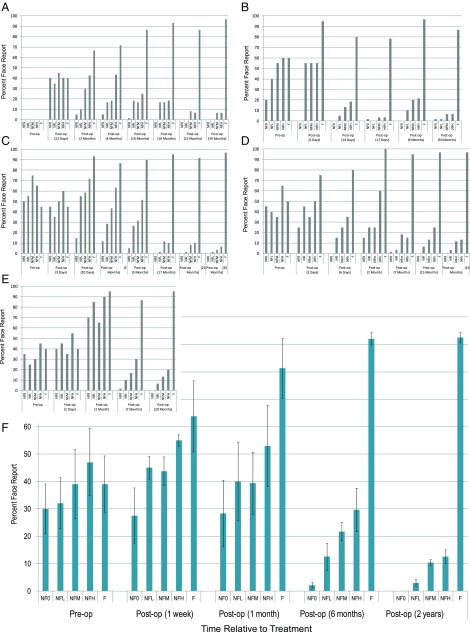
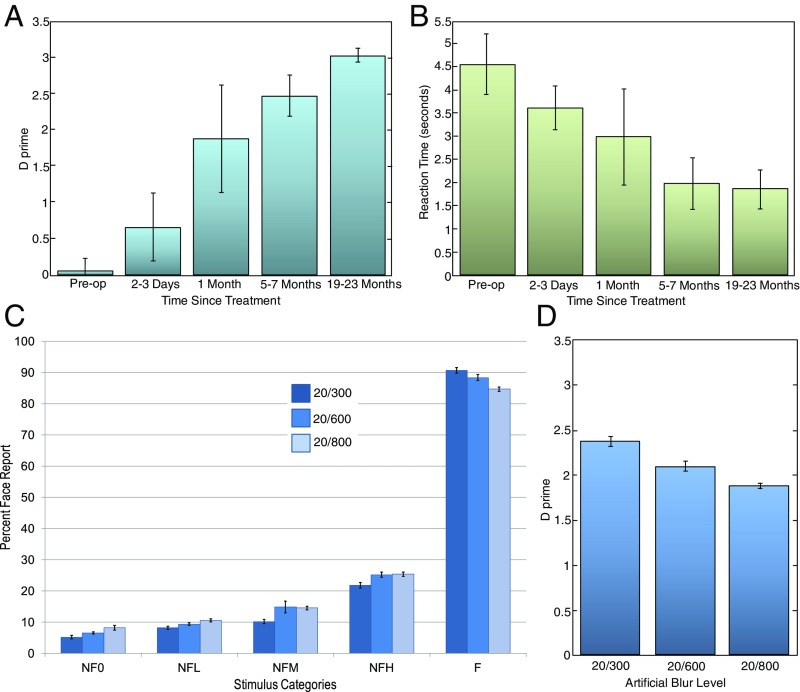
Fig. 2 A–E shows longitudinal data for children in our experimental group. The measure plotted is the percentage of trials for each image category that subjects classified as faces. Note that for nonface images, these trials correspond to false alarms, and for face images, these trials correspond to hits. Fig. 2F shows the average response across the five subjects, and Fig. 3A displays the corresponding discrimination-sensitivity (d′) values. Although there are significant individual differences in performance, some commonalities are apparent. Unsurprisingly, given their profound visual impairment, all children were incapable of discriminating faces from nonfaces preoperatively. Importantly, surgery did not immediately instantiate discrimination; performance was poor when tested within a few days after sight onset.
Fig. 2.
Timeline of emergence of face/nonface discrimination in newly sighted subjects. All values are in percentages of trials that were classified by subjects as a face. For NF0, NFL, NFM, and NFH, the bar represents false-alarm rate (proportion of nonfaces that are incorrectly classified as faces). For F, the bars represent the proportion of faces correctly classified (true positive/hit). The small plots with gray bars (A–E) show individual data, whereas F displays data averaged across all five subjects. op, operation.
Fig. 3.
(A) Sensitivity (d′) values corresponding to the subjects’ performance at various points. (B) Averaged response-time data of the experimental group on the face/nonface discrimination task as a function of time relative to surgery. (C and D) Influence of artificially reduced acuity (using Bangerter blur-foils) on the face/nonface discrimination performance of age-matched normally sighted control participants. (C) Performance in terms of percentage face reports for each of the five stimulus conditions across three levels of blur that match or are worse than the postoperative acuities of the experimental group. (D) Discrimination sensitivity (d′) values as a function of induced blur level. op, operation.
Although the newly sighted children’s performance starts out poorly, it improves steadily over time. Longitudinal data reveal that during a period of several months, children’s responses shift from exhibiting almost no biases across the stimulus groups to graded preferences, and eventually categorical discrimination. Kruskal-Wallis analysis of d′ values showed significant improvements in discrimination ability with time (χ2 = 12.03; P = 0.017).
Although we do not have true reaction time data (as subjects’ verbal responses were converted into button presses by the experimenter), the longitudinal trends in response times appear consistent with the observed accuracy improvements. Latency of responses progressively declined as discrimination accuracy increased (Fig. 3B). However, the latency reduction trend did not reach statistical significance (χ2 = 8.95; P = 0.062).
The compromised performance of the Prakash children during the sessions conducted soon after their surgeries cannot be attributed simply to their subpar postoperative acuities. Results from the control group of normally sighted children who performed the task while wearing blur foils are shown in Fig. 3 C and D. They demonstrate that although performance does decrease with blur (χ2 = 18.44; P < 0.001), even at the highest levels of blur we tested, the face/nonface distinction can be made with high accuracy.
Discussion
There are three key results that emerge from our studies of face classification with newly sighted children. First, this ability can be acquired late in life, well after its normal period of deployment. Second, the acquisition of above-chance face/nonface discrimination ability is rapid, requiring just a few weeks of visual experience. Third, the progression from no discrimination to categorical behavior involves intermediate stages of graded responses. These results bear on several important questions in the domain of visual face learning.
The finding that face/nonface classification abilities can be acquired even after a child has suffered several years of early-onset blindness suggests this visual skill may not be subject to a strict critical period. It is interesting to contrast this finding against the development of more basic visual functions, such as acuity. All the subjects exhibited reduced acuity after their surgeries, providing positive evidence for a critical period for this aspect of visual development. However, despite their subpar acuity, the newly sighted children in our experimental group were able to eventually perform the face/nonface discrimination task at a high level of proficiency. Taken together, acuity outcomes on the one hand and face discrimination performance on the other lead us to conclude that the notion of critical periods needs to be defined differently for different visual skills, rather than as a unitary construct applicable to all aspects of visual development (27, 44, 45). This more nuanced conceptualization of critical periods brings up several interesting questions for future studies. Key among them is: What determines the resilience or susceptibility of a particular visual function to early deprivation? The suggestion that the earliest manifesting visual proficiencies are most vulnerable to deprivation (30) needs to be reconsidered. The face/nonface discrimination ability is evident very early in the normal developmental timeline, yet our results suggest its acquisition is not precluded by early and extended visual deprivation. This may reflect either low susceptibility to a critical period or an extension of the critical period induced by visual deprivation (46–48).
The finding of a progressive improvement in face identification performance, rather than an abrupt onset of the ability, has bearing on the nature–nurture issue. If face detection were subserved by an innately available face schema that survived an extended period of deprivation, we would expect to see a high level of discrimination ability immediately after sight onset. Given that we see a ramping up of skill starting with chance-level performance, we are led to conclude that for our participants, visual experience may play a significant role in the acquisition of face classification ability. It is important to point out that this conclusion does not rule out a role for innate face schemas during infancy. Such schemas may be available early in life (35, 48), but may be extinguished during a long period of visual deprivation, leaving the visual system with no alternative but a learning-based strategy for acquiring a face concept later in childhood.
In deriving inferences from these data, we also have to keep in mind their limitations. First, for a variety of reasons, the newly sighted children cannot be thought of as proxies for normally developing newborns (37). Accordingly, the developmental trajectories we have observed here may not necessarily recapitulate those associated with normal visual development. This point is especially pertinent given the intersubject variability we see in our results. The variability, which is a common feature of many aspects of the vision of newly sighted children (18, 49), does not yet have a satisfactory explanation such as age at treatment. Second, we have considered only one of the many face perception tasks humans perform. We chose this task because face/nonface discrimination is arguably a fundamental precursor to other analyses of facial information. Nevertheless, it is quite possible that our findings about the developmental timelines and resilience to poor acuity in face classification may not apply directly to other face perception tasks such as identity or emotion judgments. Third, to the extent faces are expected to elicit an orienting response, it is possible that there might exist elevated sensitivity to face patterns in parafoveal or peripheral areas of the visual field. As our present study used free viewing, we cannot resolve this issue. However, future studies with a fixation requirement and tachistoscopic presentations of face/nonface stimuli in different retinal locations would allow us to probe face pattern sensitivity as a function of retinal location.
Even with these caveats, we believe these results give us insight into both visual plasticity late in childhood and the nature of learning processes that likely participate in the acquisition of face perception abilities in children who gain sight after several years of blindness. An especially interesting line of work for the future would involve correlating the changes in manifest face perception performance we have described here with changes in functional brain organization as observed through neuroimaging techniques. Doing so offers the tantalizing possibility of linking specific aspects of cortical reorganization with behavioral skills.
Materials and Methods
The study was approved by the Massachusetts Institute of Technology's IRB (Committee on the Use of Humans as Experimental Subjects). Informed consent was obtained from all participants. All subjects were implanted with an intraocular lens during surgery and were prescribed the best refractive correction after surgery. One hundred eighty false-alarm images were selected using the Pittsburgh Pattern Recognition face-detection algorithm (43). Sixty nonface images collected from natural scenes devoid of faces, and sixty genuine faces were also added to the stimulus set. Each image was made monochrome and normalized for scale and luminance. Elo ratings derived from 18 observers naive to the purpose of the experiment were used to divide the false-alarm images into high, medium, and low face-semblance groups.
During an experimental session, images from the entire stimulus set were shown in random order and, in a yes– no paradigm, the subject was asked to classify them as a face or a nonface. Subjects’ verbal responses were recorded on the computer by the experimenter by pressing one of two keys.
Images were displayed on an LCD monitor and viewed from an average distance of 40 cm. They subtended 15 degrees of visual angle horizontally. Presentations were self-timed, and the images stayed up until the subject had responded verbally. No feedback was provided during the experimental session. The protocol was run under Matlab control on a PC, and the responses of the subject, as well as the reaction time to generate a verbal response, were recorded. Each session lasted ∼30 min.
Acknowledgments
We thank all the children who participated in these studies, as well as members of the Project Prakash team who were instrumental in identifying and caring for the treatably blind children. We also thank Drs. Winrich Freiwald, Charles Nelson, Paul Quinn, and Doris Tsao for their comments on the manuscript. The research reported here was supported by the James McDonnell Foundation, the National Eye Institute (Grant R01EY020517), and the Nick Simons Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616050114/-/DCSupplemental.
References
- 1.Lewis M, Edmonds A. Searching for faces in scrambled scenes. Vis Cogn. 2005;12:1309–1336. [Google Scholar]
- 2.Lewis MB, Ellis HD. How we detect a face: A survey of psychological evidence. Int J Imaging Syst Technol. 2003;13:3–7. [Google Scholar]
- 3.Tsao DY, Livingstone MS. Mechanisms of face perception. Annu Rev Neurosci. 2008;31:411–437. doi: 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fantz RL. Visual perception from birth as shown by pattern selectivity. Ann N Y Acad Sci. 1965;118:793–814. doi: 10.1111/j.1749-6632.1965.tb40152.x. [DOI] [PubMed] [Google Scholar]
- 6.Valenza E, Simion F, Cassia VM, Umiltà C. Face preference at birth. J Exp Psychol Hum Percept Perform. 1996;22:892–903. doi: 10.1037//0096-1523.22.4.892. [DOI] [PubMed] [Google Scholar]
- 7.Goren CC, Sarty M, Wu PY. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56:544–549. [PubMed] [Google Scholar]
- 8.Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 9.Kleiner KA, Banks MS. Stimulus energy does not account for 2-month-olds’ face preferences. J Exp Psychol Hum Percept Perform. 1987;13:594–600. doi: 10.1037//0096-1523.13.4.594. [DOI] [PubMed] [Google Scholar]
- 10.Mondloch CJ. Face perception during early infancy. Psychol Sci. 1999;10:419–422. [Google Scholar]
- 11.Farroni T, et al. Newborns’ preference for face-relevant stimuli: Effects of contrast polarity. Proc Natl Acad Sci USA. 2005;102:17245–17250. doi: 10.1073/pnas.0502205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slater A, Johnson MH, Morton J. Biology and Cognitive Development: The Case of Face Recognition. Blackwell; Oxford, UK: 1993. [Google Scholar]
- 13.Lewkowicz DJ. The biological implausibility of the nature-nurture dichotomy & what it means for the study of infancy. Infancy. 2011;16:331–367. doi: 10.1111/j.1532-7078.2011.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- 15.Daw NW, Fox K, Sato H, Czepita D. Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol. 1992;67:197–202. doi: 10.1152/jn.1992.67.1.197. [DOI] [PubMed] [Google Scholar]
- 16.Banks MS, Aslin RN, Letson RD. Sensitive period for the development of human binocular vision. Science. 1975;190:675–677. doi: 10.1126/science.1188363. [DOI] [PubMed] [Google Scholar]
- 17.Daw NW, Wyatt HJ. Kittens reared in a unidirectional environment: Evidence for a critical period. J Physiol. 1976;257:155–170. doi: 10.1113/jphysiol.1976.sp011361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganesh S, et al. Results of late surgical intervention in children with early-onset bilateral cataracts. Br J Ophthalmol. 2014;98:1424–1428. doi: 10.1136/bjophthalmol-2013-304475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timney B. The effects of early and late monocular deprivation on binocular depth perception in cats. Brain Res. 1983;283:235–243. doi: 10.1016/0165-3806(83)90180-3. [DOI] [PubMed] [Google Scholar]
- 20.von Senden M. 1960. Space and Sight: The Perception of Space and Shape in the Congenitally Blind Before and After Operation, Heath P (trans.) (Methuen, UK)
- 21.Fine I, et al. Long-term deprivation affects visual perception and cortex. Nat Neurosci. 2003;6:915–916. doi: 10.1038/nn1102. [DOI] [PubMed] [Google Scholar]
- 22.Gregory RL, Wallace JG. Recovery from early blindness a case study. Quat J Psych Monograph. 1963;2:1–44. [Google Scholar]
- 23.Sacks O. An Anthropologist on Mars. 1st Ed. Knopf; New York: 1995. To see and not see; pp. 108–152. [Google Scholar]
- 24.Valvo A. Sight Restoration After Long-term Blindness: The Problems and Behavior Patterns of Visual Rehabilitation. American Foundation for the Blind; New York: 1971. [Google Scholar]
- 25.Röder B, Ley P, Shenoy BH, Kekunnaya R, Bottari D. Sensitive periods for the functional specialization of the neural system for human face processing. Proc Natl Acad Sci USA. 2013;110:16760–16765. doi: 10.1073/pnas.1309963110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Grand R, Mondloch CJ, Maurer D, Brent HP. Neuroperception. Early visual experience and face processing. Nature. 2001;410:890. doi: 10.1038/35073749. [DOI] [PubMed] [Google Scholar]
- 27.Lewis TL, Maurer D. Multiple sensitive periods in human visual development: Evidence from visually deprived children. Dev Psychobiol. 2005;46:163–183. doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- 28.Maurer D, Lewis TL, Mondloch CJ. Missing sights: Consequences for visual cognitive development. Trends Cogn Sci. 2005;9:144–151. doi: 10.1016/j.tics.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Maurer D, et al. Neural correlates of processing facial identity based on features versus their spacing. Neuropsychologia. 2007;45:1438–1451. doi: 10.1016/j.neuropsychologia.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Sengpiel F. The critical period. Curr Biol. 2007;17:R742–R743. doi: 10.1016/j.cub.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Quinn PC, Westerlund A, Nelson CA. Neural markers of categorization in 6-month-old infants. Psychol Sci. 2006;17:59–66. doi: 10.1111/j.1467-9280.2005.01665.x. [DOI] [PubMed] [Google Scholar]
- 32.McKyton A, Ben-Zion I, Doron R, Zohary E. The limits of shape recognition following late emergence from blindness. Curr Biol. 2015;25:2373–2378. doi: 10.1016/j.cub.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, et al. rapid integration of tactile and visual information by a newly sighted child. Curr Biol. 2016;26:1069–1074. doi: 10.1016/j.cub.2016.02.065. [DOI] [PubMed] [Google Scholar]
- 34.Grady CL, Mondloch CJ, Lewis TL, Maurer D. Early visual deprivation from congenital cataracts disrupts activity and functional connectivity in the face network. Neuropsychologia. 2014;57:122–139. doi: 10.1016/j.neuropsychologia.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Mondloch CJ, Lewis TL, Levin AV, Maurer D. Infant face preferences after binocular visual deprivation. Int J Behav Dev. 2013;37:148–153. [Google Scholar]
- 36.Daw N. Visual Development. 3rd Ed Springer; New York: 2014. [Google Scholar]
- 37.Sinha P, Held R. Sight restoration. F1000 Med Rep. 2012;4:17. doi: 10.3410/M4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha P. Once blind and now they see. Sci Am. 2013;309:48–55. doi: 10.1038/scientificamerican0713-48. [DOI] [PubMed] [Google Scholar]
- 39.Tusa RJ, Repka MX, Smith CB, Herdman SJ. Early visual deprivation results in persistent strabismus and nystagmus in monkeys. Invest Ophthalmol Vis Sci. 1991;32:134–141. [PubMed] [Google Scholar]
- 40.Odell NV, Leske DA, Hatt SR, Adams WE, Holmes JM. The effect of Bangerter filters on optotype acuity, Vernier acuity, and contrast sensitivity. J AAPOS. 2008;12:555–559. doi: 10.1016/j.jaapos.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng M, Cherian T, Singal G, Sinha P. Lateralization of face processing in the human brain. Proc Biol Sci. 2012;279:2052–2061. doi: 10.1098/rspb.2011.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moulson MC, Balas B, Nelson C, Sinha P. EEG correlates of categorical and graded face perception. Neuropsychologia. 2011;49:3847–3853. doi: 10.1016/j.neuropsychologia.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowley HA, Baluja S, Kanade T. 1995. Human Face Detection in Visual Scenes. Technology Report CMU-CS-95-158. (Carnegie Mellon Univ., Pittsburgh)
- 44.Harwerth RS, Smith EL, 3rd, Duncan GC, Crawford ML, von Noorden GK. Multiple sensitive periods in the development of the primate visual system. Science. 1986;232:235–238. doi: 10.1126/science.3952507. [DOI] [PubMed] [Google Scholar]
- 45.Levi DM, Li RW. Improving the performance of the amblyopic visual system. Philos Trans R Soc Lond B Biol Sci. 2009;364:399–407. doi: 10.1098/rstb.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cynader M, Berman N, Hein A. Recovery of function in cat visual cortex following prolonged deprivation. Exp Brain Res. 1976;25:139–156. doi: 10.1007/BF00234899. [DOI] [PubMed] [Google Scholar]
- 47.Hooks BM, Chen C. Critical periods in the visual system: Changing views for a model of experience-dependent plasticity. Neuron. 2007;56:312–326. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Sugita Y. Face perception in monkeys reared with no exposure to faces. Proc Natl Acad Sci USA. 2008;105:394–398. doi: 10.1073/pnas.0706079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalia A, et al. Development of pattern vision following early and extended blindness. Proc Natl Acad Sci USA. 2014;111:2035–2039. doi: 10.1073/pnas.1311041111. [DOI] [PMC free article] [PubMed] [Google Scholar]