Significance
Small spaces in nanoreactors may have big implications in chemistry, because the chemical nature of molecules and reactions within the nanospaces can be changed significantly due to the nanoconfinement effect. Two-dimensional (2D) nanoreactor formed under 2D materials can provide a well-defined model system to explore the confined catalysis. We demonstrate a general tendency for weakened surface adsorption under the confinement of graphene overlayer, illustrating the feasible modulation of surface reactions by placing a 2D cover on top of the surface. The developed concept “catalysis under cover” can be applied to reactions between two opposite 2D walls interacting with each other through van der Waals force, which helps to design high-performance nanocatalysts interfacing with 2D material overlayers.
Keywords: confined catalysis, two-dimensional materials, density functional theory, oxygen reduction reaction, graphene
Abstract
Confined microenvironments formed in heterogeneous catalysts have recently been recognized as equally important as catalytically active sites. Understanding the fundamentals of confined catalysis has become an important topic in heterogeneous catalysis. Well-defined 2D space between a catalyst surface and a 2D material overlayer provides an ideal microenvironment to explore the confined catalysis experimentally and theoretically. Using density functional theory calculations, we reveal that adsorption of atoms and molecules on a Pt(111) surface always has been weakened under monolayer graphene, which is attributed to the geometric constraint and confinement field in the 2D space between the graphene overlayer and the Pt(111) surface. A similar result has been found on Pt(110) and Pt(100) surfaces covered with graphene. The microenvironment created by coating a catalyst surface with 2D material overlayer can be used to modulate surface reactivity, which has been illustrated by optimizing oxygen reduction reaction activity on Pt(111) covered by various 2D materials. We demonstrate a concept of confined catalysis under 2D cover based on a weak van der Waals interaction between 2D material overlayers and underlying catalyst surfaces.
In heterogeneous catalysis, active sites have long been regarded as one of the most important concepts (1–3), and, recently, the heterogeneous catalysis community has started to recognize that microenvironment around the active site is equally important (4–9). The confined microenvironment on a heterogeneous catalyst can help to stabilize active sites and modulate chemistry at the sites, which has a significant effect on catalytic performance, similar to the role of spheroproteins in an enzyme (10). Hence, understanding fundamentals of confined catalysis has become an important topic in heterogeneous catalysis (11, 12).
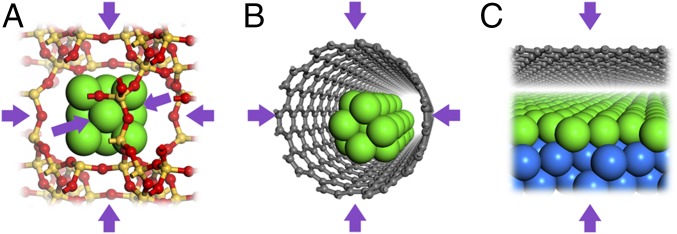
Reactions in zero-dimensional (0D) nanocavities of microporous and mesoporous materials such as zeolites and metal−organic frameworks (MOFs) have shown enhanced catalytic performance (6, 9, 13–15). Theoretical investigations reveal that the spatially confined environment increases the molecular orbital energy and changes activity of the confined molecules, which have been recognized as the nest effect and quantum confinement effect (15, 16). In past decades, some experimental studies have demonstrated that carbon nanotubes (CNTs) strongly affect catalytic reactions occurring inside the nanotubes (7, 17, 18), and confined catalysis in 1D nanocavities has been theoretically addressed by studying molecule adsorption on metal clusters encapsulated in CNTs (19). As illustrated in Fig. 1, both 0D nanocavities in zeolites and 1D nanocavities in CNTs are spatially confined in three dimensions and two dimensions, respectively, in which simple and well-defined model systems are not feasible for both theoretical and experimental studies. Moreover, structural and compositional complexity in zeolites, MOFs, and curved nanotubes makes it challenging to understand the confinement effect at microscopic level.
Fig. 1.
Schemes for confined catalysis within various microenvironments including (A) 0D pores of zeolites, (B) 1D channels of CNTs, and (C) 2D space under 2D materials. Catalytic sites (in green color) and reaction molecules are confined in three dimensions, two dimensions, and one dimension (marked with arrows), respectively, in the above three systems.
Compared with the 0D and 1D systems, 2D confined microenvironments are much more structurally well defined (Fig. 1). The planar microenvironment walls can be appropriately modeled in theoretical calculations (20, 21) and investigated well by advanced characterization techniques, particularly surface science methods (22–26). The newly emerging 2D materials such as graphene (Gr) and hexagonal boron nitride (h-BN) have been facilely supported on solid surfaces via surface deposition or transfer methods (27). Many experiments provide solid evidence that 2D spaces under the 2D material overlayers can act as stable nanoreactors for molecule adsorption and chemical reactions, which provide intriguing confined spaces for catalysis (22, 25, 28–31). A few sophisticated theoretical studies have been conducted to understand reactions under graphene covers in a quantitative way (32, 33). Overall, a 2D microenvironment formed under 2D material can provide an ideal and practical model system to explore the fundamentals of confined catalysis.
Herein, we choose graphene/Pt(111) surface as a model system to study confined catalysis via density functional theory (DFT) calculations. A general tendency for weakened adsorption of atoms and molecules in the 2D space between graphene and Pt surfaces has been clearly demonstrated, and catalytic reactions such as oxygen reduction reactions (ORR) occurring in the 2D microenvironment can be effectively tuned. We reveal that both the geometric constraint and the confinement field imposed by the 2D cover result in the observed confinement phenomena. More importantly, the confined catalysis concept can be applied to reactions between two opposite walls interacting with each other through weak van der Waals (vdW) force.
Theoretical Models and Methods
A √7 × √7 Pt(111) supercell with 3 × 3 graphene overlayer was used for calculations (SI Appendix, Fig. S1). Adsorption energy (Ead) is characterized by the binding strength of atoms or molecules with the surface,
where Etotal represents total energies of Gr/Pt(111) or Pt(111) surface with adsorbed atoms or molecules and Esurface represents the total energies of bare Gr/Pt(111) or Pt(111) surfaces; μ is chemical potential of free molecules or atoms; and n is number of molecules or atoms adsorbed within one supercell. For example, n = 1 corresponds to 1/7 monolayer (ML) coverage.
Adsorption energies were calculated on a bare Pt(111) surface and the Pt(111) surface with Gr cover, respectively. The adsorption energy difference can be used to explain confinement effect inside the 2D nanospace, which is defined as confinement energy (Econ) (19),
where Ead (w/ Gr) and Ead (w/o Gr) are the binding energies of adsorbates on Pt(111) surface with and without graphene cover, respectively (SI Appendix, Fig. S1). The vdW corrections have been introduced to take into consideration the weak interaction between graphene overlayers and metal surfaces. A number of vdW corrections, including vdW-D2 (34), vdW-D3 (35), and vdW-DF opt-B88 (36, 37), have been attempted (SI Appendix, Table S1). Among them, vdW-D2 was used for all of the following calculations (for more computational details, see SI Appendix).
Results and Discussion
Confinement Energy Induced by 2D Cover.
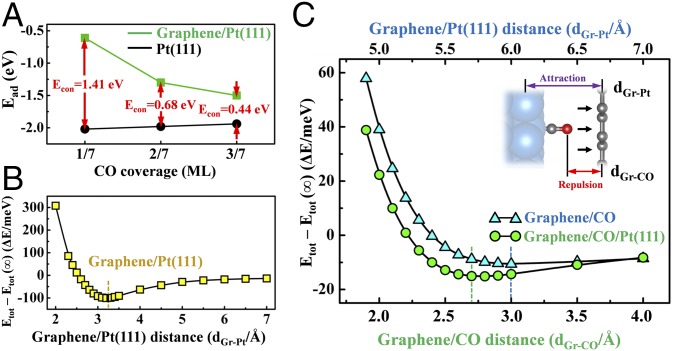
Take 3/7 ML CO on Pt(111) as an example: The calculated Ead(CO) decreases from −1.94 eV for CO on the bare Pt(111) surface to −1.50 eV in case of CO adsorption between Gr and Pt(111). Accordingly, Econ(CO) is derived to be 0.44 eV (Fig. 2A). Surface science measurements show that CO desorbs from Pt(111) surface around 420 K (38), whereas CO desorption occurs on the Gr/Pt(111) surface at 360 K (22), which is equivalent to an adsorption energy difference of around 0.3 eV. DFT calculations and surface science experiment results are consistent with each other, indicating that the corresponding DFT parameters are reliable in this study. With CO coverages at 1/7 ML and 2/7 ML, the adsorption energies were calculated in the same way, and Econ(CO) is derived to be 1.41 eV and 0.68 eV, respectively (Fig. 2A). We find that the Econ values for all studied cases are positive, suggesting that the Gr overlayer has strongly weakened CO adsorption on the Pt(111) surface. The weakened CO adsorption has been found on Pt(110) and Pt(100) surfaces with Gr overlayers (SI Appendix, Table S2), indicating that the confinement effect is not dependent on the specific metal facets. We have also examined CO adsorption energies on different sites. The confinement energies increase from 1.28 eV, to 1.33 eV, to 1.41 eV for hollow, to bridge, to top sites, suggesting that CO molecules adsorbed at a higher position above the surface are more strongly influenced by the Gr confinement effect (SI Appendix, Table S3).
Fig. 2.
Confinement effect of graphene on CO interaction with Pt(111) and the confinement energy. (A) Adsorption energies of CO on Pt(111) (black) and Gr/Pt(111) (green) surfaces and the derived confinement energies (Econ) with CO coverage at 1/7, 2/7, and 3/7 ML. (B) Total energy differences of the Gr/Pt(111) system (averaged to each C atom of Gr) at each specific distance between Gr and Pt(111) surface (dGr-Pt) relative to infinity (d∞). The equilibrium distance is 3.2 Å. (C) Total energy differences of the Gr/CO and Gr/CO/Pt(111) systems (averaged to each C atom of Gr) at each specific distance between Gr and CO (dGr-CO) relative to infinity. The equilibrium distances are 2.7 and 3.0 Å in the Gr/CO and Gr/CO/Pt(111) systems, respectively. (Inset) Interaction between Gr layer, CO molecule, and Pt slab.
Geometric Constraint in 2D Microenvironment.
To investigate vdW interaction between Gr ML and Pt slab, the total energy differences of the Gr/Pt(111) system at each specific distance between Gr and Pt(111) surface (dGr-Pt) relative to infinity (d∞) [ΔE = Etotal – Etotal (∞)] were calculated. As shown in Fig. 2B, dependence of ΔE on dGr-Pt represents a two-body potential, which is similar to the Lennard−Jones (L-J) type (39). As the Pt slab approaches the Gr layer from infinity, the total energy decreases because of attractive vdW interaction between the slab and the layer. The equilibrium distance is 3.2 Å, with vdW interaction energy at 0.1 eV/C atom. When the Pt slab moves much closer to the Gr surface, the strong repulsion results in a sharp increase in the total energy.
The same studies were conducted on the interaction between the Gr layer and CO molecule. The minimum total energy difference corresponds to dGr-CO at 3.0 Å (Fig. 2C). In the case of CO adsorption on Pt surface, we let the CO−Pt slab ensemble approach the Gr layer. In such a case, the equilibrium dGr-CO has been shortened to 2.7 Å (dGr-Pt = 5.7 Å; Fig. 2C). As illustrated by Fig. 2C, Inset, attractive vdW interaction between the Gr layer and Pt slab “pushes” the CO molecule much closer to the Gr surface. Thus, the interaction between Gr and CO is in the repulsion regime, and CO adsorption is destabilized in the confined space. It has been recently demonstrated that vdW forces between 2D materials can be so strong on a nanometer scale that huge pressure up to 1 GPa is built therein (40, 41). Therefore, we conclude that the vdW interaction between the opposite walls of the 2D microenvironment has induced strong geometric constraint on trapped interlayer molecules, which contributes to the confinement effect.
Confinement Field in 2D Microenvironment.
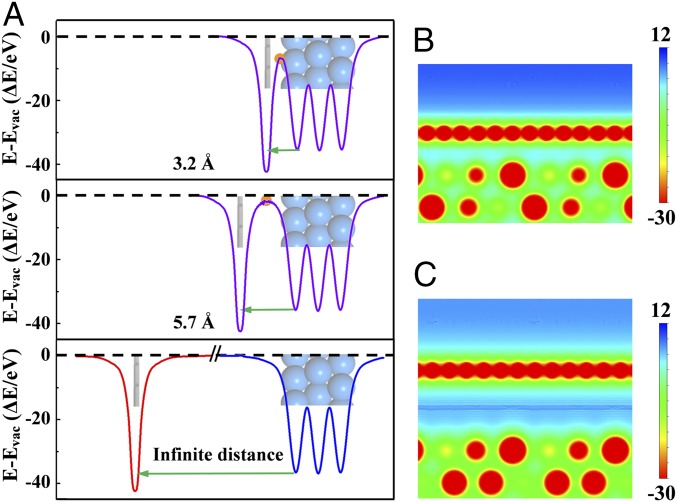
We further calculated potential distribution in the 2D space between the Gr layer and Pt(111) surface. Fig. 3A shows the 1D potential distributions along normal of the Gr and Pt(111) surfaces with dGr-Pt at 3.2 Å, 5.7 Å, and infinity, respectively. It can be seen that potential energy near the Pt(111) surface has been strongly modified by Gr. The 2D potential distribution images at the Gr/Pt(111) interfaces (Fig. 3 B and C) show more clearly that the confined space under Gr cover presents different potential energy compared with the bare Pt(111) surface. When choosing the maximum point of the potential distribution curve in the confined space as a descriptor, we find a gradual increase of the potential energy with increasing dGr-Pt (SI Appendix, Fig. S2). It should be noted that d-band states of Pt atoms at the Gr/Pt(111) interface remain intact in comparison with the bare Pt(111) surface (SI Appendix, Fig. S3) because the localized d-bands are not susceptible to the weak interaction. The potential distribution in the 2D space is more related to decay of delocalized sp electronic states into vacuum that are affected by interaction with the Gr layer.
Fig. 3.
Confinement field in the 2D confined spaces. (A) The 1D local potential distribution between Gr and Pt(111) surface with dGr-Pt at 3.2 Å (Top), 5.7 Å (Middle), and infinity (Bottom). The potential at vacuum is set to zero. (Insets) The corresponding schematic atomic structures in the real space. (B and C) The maximum points between Gr and Pt(111) surface are shown in orange dots. The 2D potential distribution (side view) at the Gr/Pt(111) interfaces with dGr-Pt at (B) 3.2 and (C) 5.7 Å.
In the case where an atom or molecule is adsorbed into the 2D space, Gr cover is lifted up away from the surface, which enlarges dGr-Pt, increases the potential energy, and consequently makes the system less stable. Placing a graphene cover on the metal surface provides an effective way to modify the surface potential, which can be continuously tuned by dGr-Pt. Such an effect is similar to applying an external electric field onto a solid electrode surface (42). From the perspective of quantum effect, the Gr cover acts as a constraint boundary for molecular orbitals of an adsorbate, which cannot relax farther away from the surface sufficiently compared to the case of adsorption on an open surface (15). Therefore, confining molecule adsorption under Gr implies an increase in energy levels of the molecular orbitals. Overall, the confinement effect in 2D microenvironment can be understood as a kind of confinement field imposed by the Gr cover, which destabilizes the surface adsorption.
Generalized Confinement Effect in 2D Microenvironment.
The confinement effect aroused in 2D microenvironments is not limited to the graphene cover. To confirm this point, a hypothetical experiment was performed by replacing the graphene layer with a layer of inert gas Ne atoms (SI Appendix, Fig. S4), and the dimension of 2D space was kept unchanged, i.e., dNe-Pt is the same as dGr-Pt. We see that the change of local potential distribution in the Ne-overlayer-confined space is similar to that in the graphene-confined space. Econ(CO) in the 2D microenvironment with dNe-Pt at 5.7 Å is calculated as 1.35 eV at 1/7 ML CO coverage, which is also similar to that under the graphene layer (1.41 eV). Considering that Ne atoms are almost chemically inert, the similar influence of the Ne layer on CO adsorption suggests that the confinement effects are closely related to the vdW interaction between two opposite walls of the confined space. Other noble gas atoms like He and Ar (SI Appendix, Fig. S4) have a similar effect on the confinement energy and surface potential distributions.
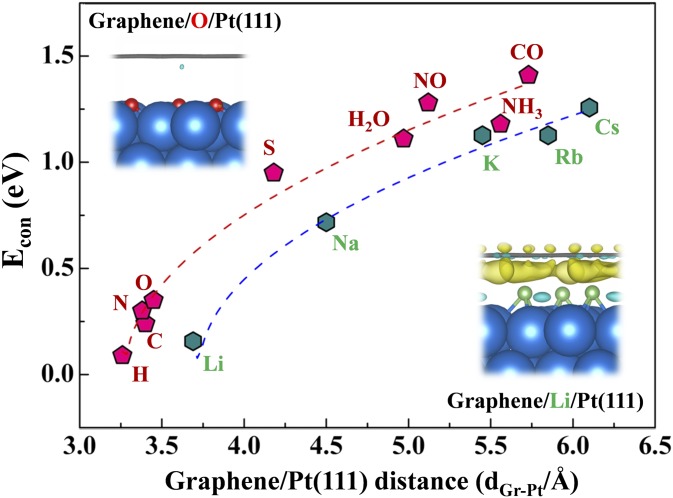
Econ in the 2D space between Gr and Pt(111) has been calculated for various adsorbates, including nonmetal atoms (H, C, N, O, and S), alkali metal atoms (Li, Na, K, Rb, and Cs), and small molecules (CO, NO, H2O, and NH3). Meanwhile, the equilibrium dGr-Pt with an adsorbate adsorbed between Gr and Pt was obtained as well (SI Appendix, Tables S4 and S5). As shown in Fig. 4, all Econ values with the adsorbate coverage at 1/7 ML are positive, which means that adsorption of all these adsorbates on the Pt surface is weakened in the confined space. The same tendency was observed at 3/7 ML coverage, but Econ at 1/7 ML coverage is higher than that at 3/7 ML coverage (SI Appendix, Fig. S5).
Fig. 4.
Econ for 1/7 ML nonmetal atoms and molecules (pink dots), as well as alkali metal atoms (green dots) in between Gr and Pt(111) with various dGr-Pt values. The pink dots and green dots are fitted in red and blue dashed lines, respectively. (Insets) Cross-section views of Gr/O/Pt(111) and Gr/Li/Pt(111) interfaces shown with electron density difference at the interface. ; Gr, gray sticks; Li, green balls; O, red balls; and Pt, blue balls. Light blue and yellow contours in the electron density difference are for electron depletion and electron accumulation, respectively. The isosurface levels are set to be 0.002 e/Bohr3.
For the same coverage, Econ increases almost monotonically with dGr-Pt. Econ data of nonmetal atoms and molecules can be fitted by the following equation (red dashed line in Fig. 4):
In the case of 1/7 ML coverage, the fitted θ is 1/7.03, d0 is 3.27 Å, and α is 0.49 with the coefficient of determination R2 = 0.97. The fitting of Econ data from 3/7 ML adsorbates on the Gr/Pt(111) surface also produces θ around 3/6.89, d0 around 3.48 Å, and α at 0.49 with R2 = 0.99 (SI Appendix, Fig. S5). Therefore, the fitted parameter of θ is strongly related to the adsorbate coverage, and d0 corresponds to the equilibrium distance between Gr cover and Pt(111) surface at the Gr/Pt(111) interface (3.2 Å). This root equation can be used to fit the calculated Econ data from the h-BN/Pt(111) surface as well (SI Appendix, Fig. S6).
Such a more quantitative analysis of Econ reveals two interesting points. First, Econ is dependent on the adsorbate coverage, and is higher at a lower coverage. The coverage-dependent Econ can be understood as that the confinement effect has been averaged by more adsorbed atoms or molecules at the higher coverage. Second, Econ is strongly determined by the size of the 2D confined space and, more specifically, the change of dGr-Pt, i.e., ΔdGr-Pt = dGr-Pt − d0. The stronger response of the confined microenvironment to the adsorption, the stronger the confinement effect induced, which is similar to the dynamic response of spheroproteins in an enzyme to reactions over the cofactor. The 2D confined space under the Gr overlayer provides a flexible microenvironment, in which the space thickness is dynamically adjusted with adsorbates inside. For example, intercalation of CO at Gr/metal interfaces produces a larger space than the H intercalation (25). This structural flexibility in the 2D confined system is in contrast to the 0D and 1D confined systems, which cannot adapt their pore sizes and tube diameters to reactions occurring inside (Fig. 1).
Note that Econ values of alkali metals are slightly lower relative to other atoms and molecules (blue dashed line in Fig. 4). The difference was understood by analysis of electron density at the interfaces, taking Li and O atoms as examples. As shown by Fig. 4, Insets, electron transfer between Li and Gr cover is much stronger than that of O. The strong electronic interaction between adsorbate and Gr makes the adsorbate more stable to stay at the interface, which decreases Econ. The general tendency, that the 2D confinement effect induces weakened adsorption, relies on a premise that there is a weak interaction between adsorbates and the top cover.
Modulating Reactions Under 2D Cover.
In light of the Brønsted−Evans−Polanyi (BEP) relation, catalytic activity is related to the dissociative adsorption energy (Ediss) of a reactant on a catalyst surface, which presents a volcano curve (1, 43). A good catalyst should have a moderate Ediss, which is close to the apex of the volcano curve. Assuming that the BEP relation remains intact, in view of the feasible modulation of surface adsorption in 2D confined spaces, we attempt to take advantage of the confinement effect to modulate Ediss and the reaction activity. Our calculations show that Ediss values of various molecules at the interface between Gr and Pt(111) are smaller than those on bare Pt(111) surface (SI Appendix, Fig. S7), indicating that the dissociation process is more difficult with Gr cover. Therefore, Gr cover can eventually move Ediss toward the right side in the volcano curve such that the overall activity of reactions on the left side of the volcano curve will be improved whereas those on the right side will be suppressed (SI Appendix, Fig. S8).
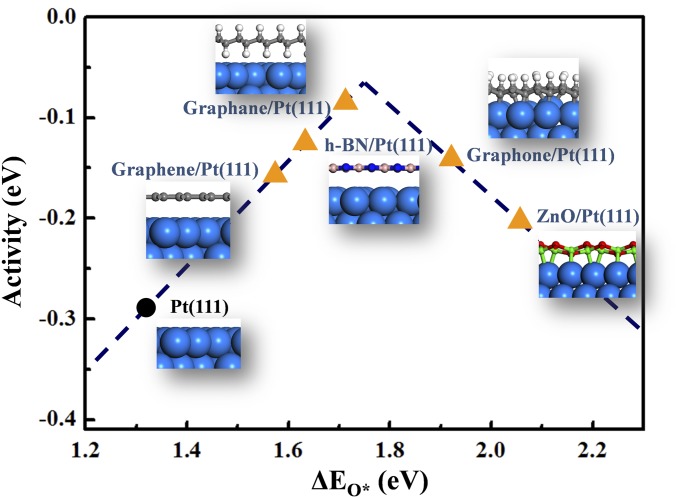
The confinement-induced modulation of surface reactivity is illustrated in Fig. 5 for Pt-catalyzed ORR under 2D covers. It has been well known that O binding energy is generally used as a descriptor for the ORR reaction activity (44). Although Pt is regarded as a good catalyst for this reaction, there is still much room to improve its performance, because Pt sits on the left side of the volcano curve (Fig. 5). Various routes to weaken the binding of O atoms on the Pt surface have been attempted, to promote the reactions (45, 46). According to the above-mentioned 2D confined catalysis concept, we suggest that the reactivity of Pt(111) toward ORR can be modulated by placing various 2D covers atop. ML graphene, h-BN, graphane (fully hydrogenated Gr) (47), graphone (half hydrogenated Gr) (48), and graphitic ZnO (g-ZnO) (49) were chosen to tune the surface adsorption. Binding energies of surface oxygen species (ΔEO*) on bare Pt(111) surface and the Pt(111) surface with different 2D covers were calculated, which decrease in the sequence of Pt(111) > Gr/Pt(111) > h-BN/Pt(111) > graphane/Pt(111) > graphone/Pt(111) > g-ZnO/Pt(111).
Fig. 5.
The volcano curve relation between ORR activity and binding energies of O atoms (ΔEO*) on Pt surface. (Insets) The interfacial structures. B, purple balls; C, gray balls; H, white balls; N, dark blue balls; O, red balls; Pt, light blue balls; and Zn, green balls.
Because the calculated binding energies of ΔEO* on different metals with vdW corrections are close to those without vdW corrections (SI Appendix, Fig. S9 and Table S6), the same volcano curve proposed by Nørskov et al. (42) was used here. As predicted in the volcano plot for the different 2D cover/Pt(111) surfaces (Fig. 5), covering the Pt(111) surface with graphene, h-BN, graphane, and graphone MLs all enhance the ORR activity. Among them, the graphane cover exhibits the largest enhancement, because ΔEO* has been tuned close to the apex of the volcano curve.
Discussion
The 2D material layers such as Gr, h-BN, and MoS2 have been prepared on many substrates (27). Despite ultrathin thickness, the 2D material sheets are quite stable under harsh conditions, e.g., there is no oxidation of graphene at 400 °C (50) and oxidation resistance of h-BN up to 850 °C in O2 (51). When exposed to gaseous or liquid environments, molecules or solutions can diffuse underneath the 2D covers via an intercalation process, confirming that 2D material/solid interfaces act as stable and practical nanoreactors (see references in ref. 25). Surface science experiments confirm that molecule adsorption in the 2D confined space has been significantly weakened (22, 26, 28, 29, 52), which is consistent with the theoretical prediction in the present work.
Both gas-phase and aqueous phase reactions have been experimentally observed in the 2D spaces. More interestingly, the Gr or h-BN covers can promote reactions underneath the 2D overlayers. For example, CO oxidation on Pt(111) has been enhanced by covering the Pt surface with Gr and h-BN layers (22, 28), and metal corrosion has been accelerated in the presence of Gr overlayers (53, 54). Our calculation results for the feasible modulation of reactions under 2D covers can be solidified by recent reaction data. It should be noted that reactions occurring in the 2D confined spaces may be limited by surface diffusion under the 2D overlayers. To address this issue, we calculated diffusion barriers of CO on Pt(111) with and without Gr overlayer (SI Appendix, Fig. S10). It has been shown that molecule diffusion happens with similar barriers on the two surfaces, and diffusion under 2D covers may not be a limiting step.
Finally, the confined catalysis under 2D materials can be applied to supported nanocatalysts. Metal nanoparticles may be encapsulated by 2D materials, forming core−shell nanostructures in which the active core structures are well protected by the outer shells and catalyst stability is improved. Recent reports confirm that CO oxidation, methanation, and ORR reactions happen on the metal nanocatalysts covered with Gr or h-BN shells (55–58). Particularly, enhanced ORR activity has been observed on Pt nanocatalysts with Gr shells (57–59), which is consistent with our theory prediction illustrated in Fig. 5.
Conclusion
We show that 2D confined microenvironments created between 2D material overlayers and underlying metal surfaces can be used to explore confined catalysis. The vdW interaction between the opposite walls of the 2D microenvironment induces the geometric constraint for adsorbed atoms and molecules. Furthermore, the 2D cover modifies potential distribution at the metal surface, making surface adsorption less energetically stable. The geometric constraint and confinement field aroused in the 2D space result in a general tendency of intercalated species to adsorb on the metal surface weakly, which can be used to modulate surface reactivity and enhance ORR activity on Pt covered with various 2D materials. This concept can be applied to design novel nanocatalysts interfacing with 2D materials with improved performance.
Supplementary Material
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grants 21373208, 91545204, 21688102, and 21621063), the Ministry of Science and Technology of China (Grants 2016YFA0200200, 2013CB834603, and 2013CB933100), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant XDB17020200).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701280114/-/DCSupplemental.
References
- 1.Nørskov JK, et al. The nature of the active site in heterogeneous metal catalysis. Chem Soc Rev. 2008;37:2163–2171. doi: 10.1039/b800260f. [DOI] [PubMed] [Google Scholar]
- 2.Somorjai G, McCrea K, Zhu J. Active sites in heterogeneous catalysis: Development of molecular concepts and future challenges. Top Catal. 2002;18:157–166. [Google Scholar]
- 3.Thomas JM. The tortuous tale of the catalytically active site. Top Catal. 2006;38:3–5. [Google Scholar]
- 4.Schoenbaum CA, Schwartz DK, Medlin JW. Controlling the surface environment of heterogeneous catalysts using self-assembled monolayers. Acc Chem Res. 2014;47:1438–1445. doi: 10.1021/ar500029y. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz TJ, et al. Engineering catalyst microenvironments for metal-catalyzed hydrogenation of biologically derived platform chemicals. Angew Chem Int Ed Engl. 2014;53:12718–12722. doi: 10.1002/anie.201407615. [DOI] [PubMed] [Google Scholar]
- 6.Choi KM, Na K, Somorjai GA, Yaghi OM. Chemical environment control and enhanced catalytic performance of platinum nanoparticles embedded in nanocrystalline metal–organic frameworks. J Am Chem Soc. 2015;137:7810–7816. doi: 10.1021/jacs.5b03540. [DOI] [PubMed] [Google Scholar]
- 7.Pan X, Bao X. The effects of confinement inside carbon nanotubes on catalysis. Acc Chem Res. 2011;44:553–562. doi: 10.1021/ar100160t. [DOI] [PubMed] [Google Scholar]
- 8.Prieto G, et al. Hollow nano- and microstructures as catalysts. Chem Rev. 2016;116:14056–14119. doi: 10.1021/acs.chemrev.6b00374. [DOI] [PubMed] [Google Scholar]
- 9.Zhao M, et al. Metal-organic frameworks as selectivity regulators for hydrogenation reactions. Nature. 2016;539:76–80. doi: 10.1038/nature19763. [DOI] [PubMed] [Google Scholar]
- 10.Thomas JM, Williams RJ. Catalysis: Principles, progress, prospects. Philos Trans R Soc A Math Phys Eng Sci. 2005;363:765–791. doi: 10.1098/rsta.2004.1534. [DOI] [PubMed] [Google Scholar]
- 11.Yang F, Deng D, Pan X, Fu Q, Bao X. Understanding nano effects in catalysis. Natl Sci Rev. 2015;2:183–201. [Google Scholar]
- 12.Fu Q, et al. Interface-confined ferrous centers for catalytic oxidation. Science. 2010;328:1141–1144. doi: 10.1126/science.1188267. [DOI] [PubMed] [Google Scholar]
- 13.Goel S, Zones SI, Iglesia E. Encapsulation of metal clusters within MFI via interzeolite transformations and direct hydrothermal syntheses and catalytic consequences of their confinement. J Am Chem Soc. 2014;136:15280–15290. doi: 10.1021/ja507956m. [DOI] [PubMed] [Google Scholar]
- 14.Mlinar AN, Shylesh S, Ho OC, Bell AT. Propene oligomerization using alkali metal-and nickel-exchanged mesoporous aluminosilicate catalysts. ACS Catal. 2014;4:337–343. [Google Scholar]
- 15.Sastre G, Corma A. The confinement effect in zeolites. J Mol Catal Chem. 2009;305:3–7. [Google Scholar]
- 16.Zicovich-Wilson C, Corma A, Viruela P. Electronic confinement of molecules in microscopic pores. A new concept which contributes to the explanation of the catalytic activity of zeolites. J Phys Chem. 1994;98:10863–10870. [Google Scholar]
- 17.Miners SA, Rance GA, Khlobystov AN. Chemical reactions confined within carbon nanotubes. Chem Soc Rev. 2016;45:4727–4746. doi: 10.1039/c6cs00090h. [DOI] [PubMed] [Google Scholar]
- 18.Serp P, Castillejos E. Catalysis in carbon nanotubes. ChemCatChem. 2010;2:41–47. [Google Scholar]
- 19.Xiao J, Pan X, Guo S, Ren P, Bao X. Toward fundamentals of confined catalysis in carbon nanotubes. J Am Chem Soc. 2015;137:477–482. doi: 10.1021/ja511498s. [DOI] [PubMed] [Google Scholar]
- 20.Martínez de la Hoz JM, Balbuena PB. Geometric and electronic confinement effects on catalysis. J Phys Chem C. 2011;115:21324–21333. [Google Scholar]
- 21.Doyle AD, Montoya JH, Vojvodic A. Improving oxygen electrochemistry through nanoscopic confinement. ChemCatChem. 2015;7:738–742. [Google Scholar]
- 22.Yao Y, et al. Graphene cover-promoted metal-catalyzed reactions. Proc Natl Acad Sci USA. 2014;111:17023–17028. doi: 10.1073/pnas.1416368111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emmez E, Yang B, Shaikhutdinov S, Freund H-J. Permeation of a single-layer SiO2 membrane and chemistry in confined space. J Phys Chem C. 2014;118:29034–29042. [Google Scholar]
- 24.Fu Q, Yang F, Bao X. Interface-confined oxide nanostructures for catalytic oxidation reactions. Acc Chem Res. 2013;46:1692–1701. doi: 10.1021/ar300249b. [DOI] [PubMed] [Google Scholar]
- 25.Fu Q, Bao X. Surface chemistry and catalysis confined under two-dimensional materials. Chem Soc Rev. 2017;46:1842–1874. doi: 10.1039/c6cs00424e. [DOI] [PubMed] [Google Scholar]
- 26.Brugger T, et al. Nanotexture switching of single-layer hexagonal boron nitride on rhodium by intercalation of hydrogen atoms. Angew Chem Int Ed Engl. 2010;49:6120–6124. doi: 10.1002/anie.201001064. [DOI] [PubMed] [Google Scholar]
- 27.Novoselov KS, Mishchenko A, Carvalho A, Castro Neto AH. 2D materials and van der Waals heterostructures. Science. 2016;353:aac9439. doi: 10.1126/science.aac9439. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, et al. Hexagonal boron nitride cover on Pt(111): A new route to tune molecule-metal interaction and metal-catalyzed reactions. Nano Lett. 2015;15:3616–3623. doi: 10.1021/acs.nanolett.5b01205. [DOI] [PubMed] [Google Scholar]
- 29.Grånäs E, et al. CO intercalation of graphene on Ir (111) in the millibar regime. J Phys Chem C. 2013;117:16438–16447. [Google Scholar]
- 30.Sutter P, Sadowski JT, Sutter EA. Chemistry under cover: Tuning metal-graphene interaction by reactive intercalation. J Am Chem Soc. 2010;132:8175–8179. doi: 10.1021/ja102398n. [DOI] [PubMed] [Google Scholar]
- 31.Deng D, et al. Catalysis with two-dimensional materials and their heterostructures. Nat Nanotechnol. 2016;11:218–230. doi: 10.1038/nnano.2015.340. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, et al. Enhancing the hydrogen activation reactivity of nonprecious metal substrates via confined catalysis underneath graphene. Nano Lett. 2016;16:6058–6063. doi: 10.1021/acs.nanolett.6b02052. [DOI] [PubMed] [Google Scholar]
- 33.Ferrighi L, Datteo M, Fazio G, Di Valentin C. Catalysis under cover: Enhanced reactivity at the interface between (doped) graphene and anatase TiO2. J Am Chem Soc. 2016;138:7365–7376. doi: 10.1021/jacs.6b02990. [DOI] [PubMed] [Google Scholar]
- 34.Grimme S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem. 2006;27:1787–1799. doi: 10.1002/jcc.20495. [DOI] [PubMed] [Google Scholar]
- 35.Grimme S, Antony J, Ehrlich S, Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys. 2010;132:154104. doi: 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- 36.Klimeš J, Bowler DR, Michaelides A. Van der Waals density functionals applied to solids. Phys Rev B. 2011;83:195131. [Google Scholar]
- 37.Dion M, Rydberg H, Schröder E, Langreth DC, Lundqvist BI. van der Waals density functional for general geometries. Phys Rev Lett. 2004;92:246401. doi: 10.1103/PhysRevLett.92.246401. [DOI] [PubMed] [Google Scholar]
- 38.Yeo Y, Vattuone L, King D. Calorimetric heats for CO and oxygen adsorption and for the catalytic CO oxidation reaction on Pt {111} J Chem Phys. 1997;106:392–401. [Google Scholar]
- 39.Hansen J-P, Verlet L. Phase transitions of the Lennard-Jones system. Phys Rev. 1969;184:151. [Google Scholar]
- 40.Vasu KS, et al. Van der Waals pressure and its effect on trapped interlayer molecules. Nat Commun. 2016;7:12168. doi: 10.1038/ncomms12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Algara-Siller G, et al. Square ice in graphene nanocapillaries. Nature. 2015;519:443–445. doi: 10.1038/nature14295. [DOI] [PubMed] [Google Scholar]
- 42.Nørskov JK, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J Phys Chem B. 2004;108:17886–17892. doi: 10.1021/jp047349j. [DOI] [PubMed] [Google Scholar]
- 43.Michaelides A, et al. Identification of general linear relationships between activation energies and enthalpy changes for dissociation reactions at surfaces. J Am Chem Soc. 2003;125:3704–3705. doi: 10.1021/ja027366r. [DOI] [PubMed] [Google Scholar]
- 44.Rossmeisl J, Qu Z-W, Zhu H, Kroes G-J, Nørskov JK. Electrolysis of water on oxide surfaces. J Electroanal Chem. 2007;607:83–89. [Google Scholar]
- 45.Greeley J, et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat Chem. 2009;1:552–556. doi: 10.1038/nchem.367. [DOI] [PubMed] [Google Scholar]
- 46.Stamenkovic VR, et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science. 2007;315:493–497. doi: 10.1126/science.1135941. [DOI] [PubMed] [Google Scholar]
- 47.Elias DC, et al. Control of graphene’s properties by reversible hydrogenation: Evidence for graphane. Science. 2009;323:610–613. doi: 10.1126/science.1167130. [DOI] [PubMed] [Google Scholar]
- 48.Zhao W, et al. Reversible hydrogenation of graphene on ni(111)-synthesis of “graphone.”. Chemistry. 2015;21:3347–3358. doi: 10.1002/chem.201404938. [DOI] [PubMed] [Google Scholar]
- 49.Schott V, et al. Chemical activity of thin oxide layers: strong interactions with the support yield a new thin-film phase of ZnO. Angew Chem Int Ed Engl. 2013;52:11925–11929. doi: 10.1002/anie.201302315. [DOI] [PubMed] [Google Scholar]
- 50.Liu L, et al. Graphene oxidation: Thickness-dependent etching and strong chemical doping. Nano Lett. 2008;8:1965–1970. doi: 10.1021/nl0808684. [DOI] [PubMed] [Google Scholar]
- 51.Li LH, Cervenka J, Watanabe K, Taniguchi T, Chen Y. Strong oxidation resistance of atomically thin boron nitride nanosheets. ACS Nano. 2014;8:1457–1462. doi: 10.1021/nn500059s. [DOI] [PubMed] [Google Scholar]
- 52.Mu R, et al. Visualizing chemical reactions confined under graphene. Angew Chem Int Ed Engl. 2012;51:4856–4859. doi: 10.1002/anie.201200413. [DOI] [PubMed] [Google Scholar]
- 53.Schriver M, et al. Graphene as a long-term metal oxidation barrier: Worse than nothing. ACS Nano. 2013;7:5763–5768. doi: 10.1021/nn4014356. [DOI] [PubMed] [Google Scholar]
- 54.Zhou F, Li Z, Shenoy GJ, Li L, Liu H. Enhanced room-temperature corrosion of copper in the presence of graphene. ACS Nano. 2013;7:6939–6947. doi: 10.1021/nn402150t. [DOI] [PubMed] [Google Scholar]
- 55.Gao L, et al. Enhanced nickel-catalyzed methanation confined under hexagonal boron nitride shells. ACS Catal. 2016;6:6814–6822. [Google Scholar]
- 56.Sun M, et al. Catalysis under shell: Improved CO oxidation reaction confined in Pt@ h-BN core–shell nanoreactors. Nano Res. 2017;10:1403–1412. [Google Scholar]
- 57.Chung DY, et al. Highly durable and active PtFe nanocatalyst for electrochemical oxygen reduction reaction. J Am Chem Soc. 2015;137:15478–15485. doi: 10.1021/jacs.5b09653. [DOI] [PubMed] [Google Scholar]
- 58.Guo L, et al. Embedding Pt nanocrystals in N-doped porous carbon/carbon nanotubes toward highly stable electrocatalysts for the oxygen reduction reaction. ACS Catal. 2015;5:2903–2909. [Google Scholar]
- 59.Wu Z, Lv Y, Xia Y, Webley PA, Zhao D. Ordered mesoporous platinum@graphitic carbon embedded nanophase as a highly active, stable, and methanol-tolerant oxygen reduction electrocatalyst. J Am Chem Soc. 2012;134:2236–2245. doi: 10.1021/ja209753w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.