Abstract
Survival data are presented for a fecal strain of Escherichia coli exposed to three concentrations of chlorine dioxide at four temperatures. Chick's first-order reaction equation is generalized to a pseudo nth-order model. Nonlinear least squares curve-fitting of the survival data to the nth order model was performed on an analogue computer. The data were observed to follow fractional order kinetics with respect to survival concentration, with an apparent activation energy of 12,000 cal/mole. Initial experiments support the thesis that the mechanism of chlorine dioxide kill occurs via disruption of protein synthesis.
Full text
PDF

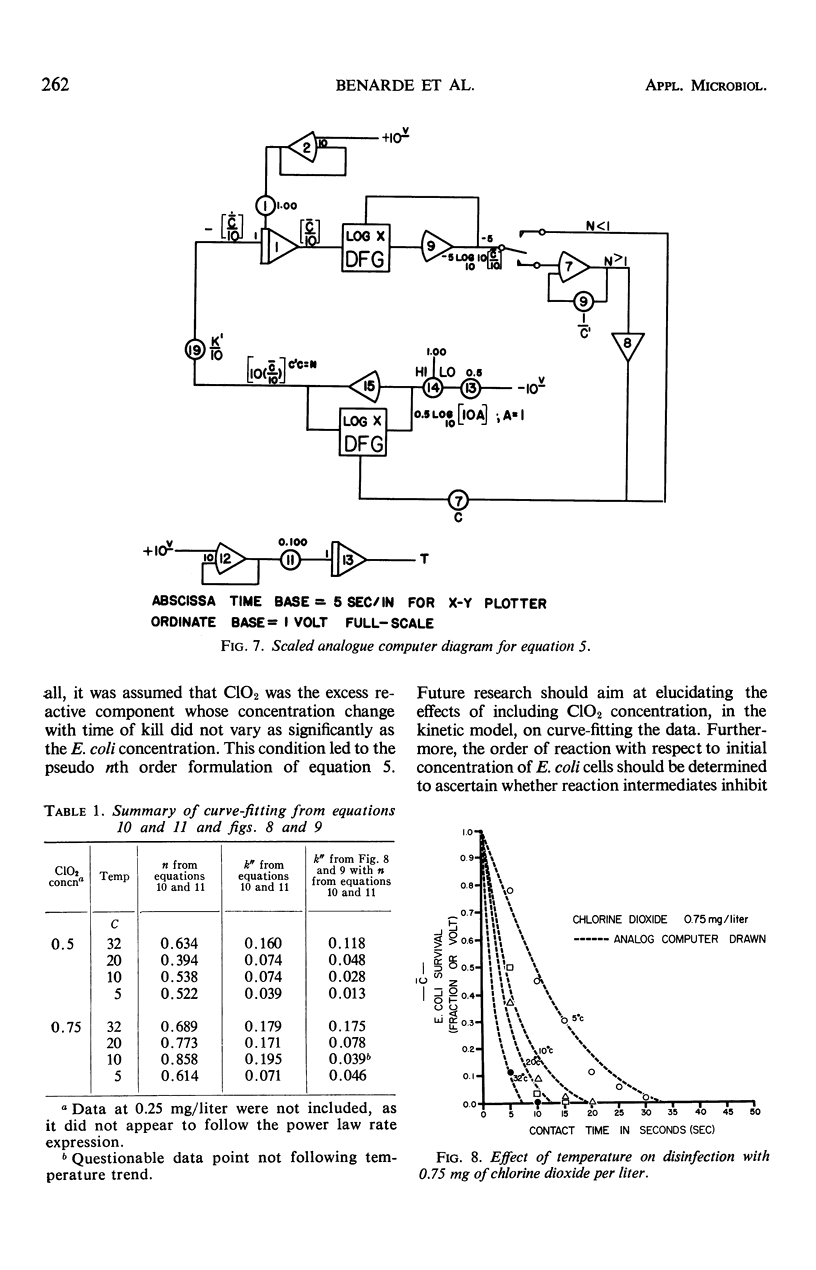
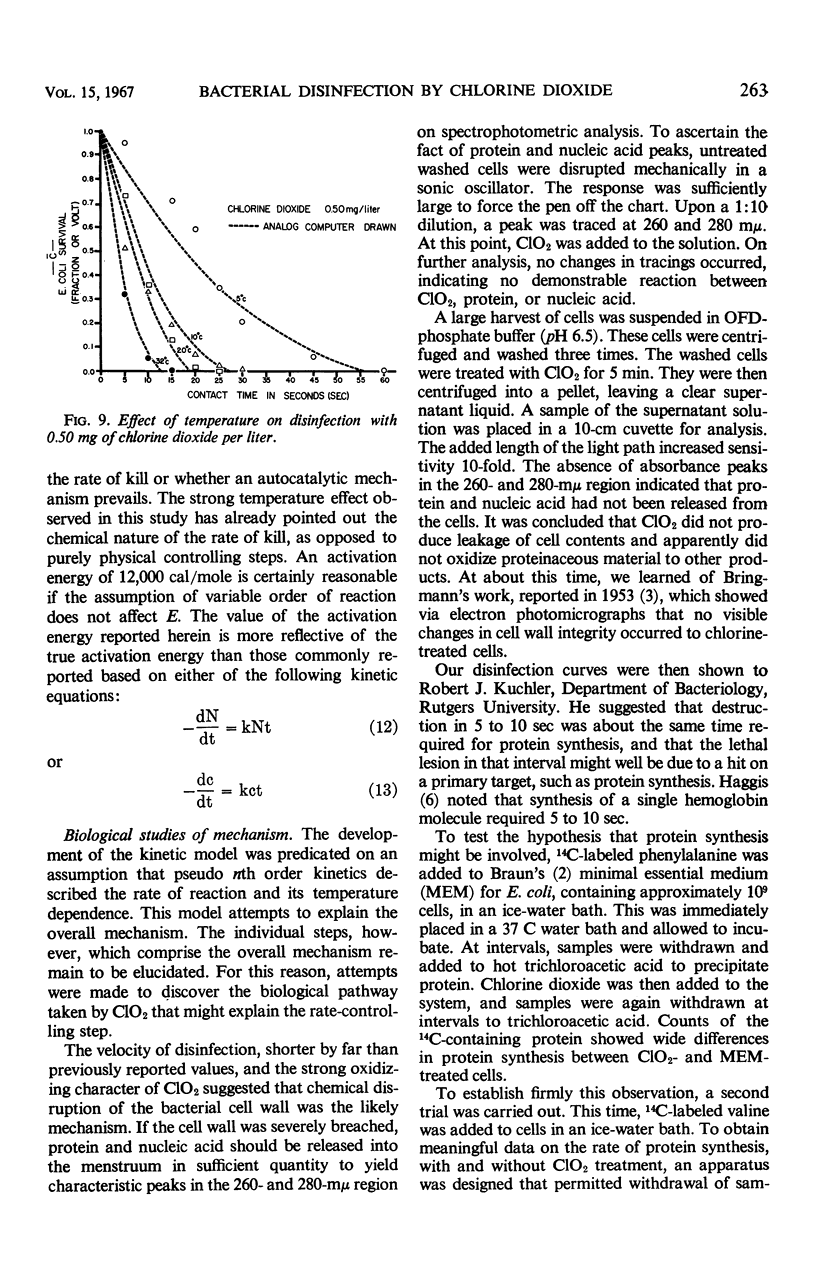
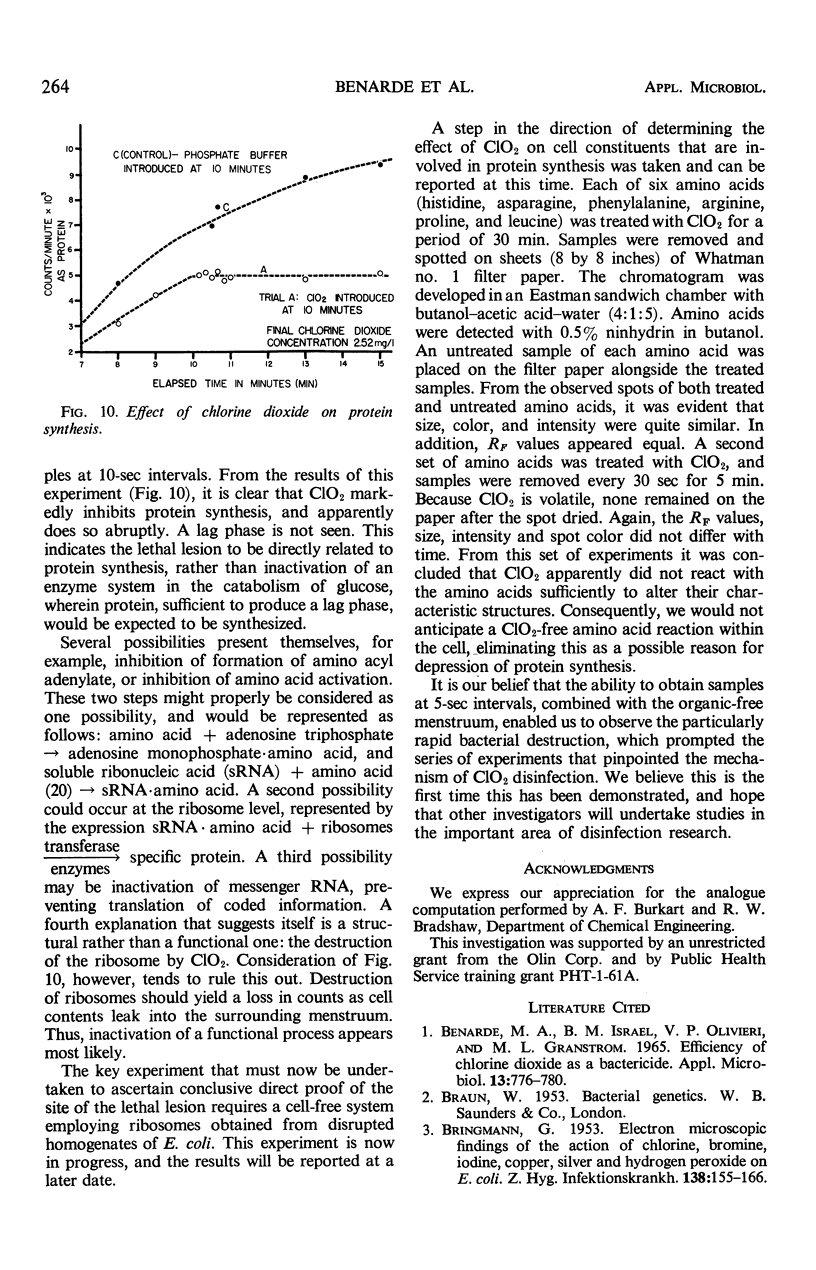

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINGMANN G. Elektronenmikroskopische Befunde zur Wirkung von Chlor, Brom, Jod, Kupfer, Silber und Wasserstoff-superoxyd auf E. coli. Z Hyg Infektionskr. 1953;138(2):155–166. [PubMed] [Google Scholar]
- Benarde M. A., Israel B. M., Olivieri V. P., Granstrom M. L. Efficiency of chlorine dioxide as a bactericide. Appl Microbiol. 1965 Sep;13(5):776–780. doi: 10.1128/am.13.5.776-780.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox W. E., Stumpf P. K., Green D. E., Auerbach V. H. The Inhibition of Sulfhydryl Enzymes as the Basis of the Bactericidal Action of Chlorine. J Bacteriol. 1948 Apr;55(4):451–458. [PMC free article] [PubMed] [Google Scholar]