Abstract
Background
PCSK9 rs505151 and rs11591147 polymorphisms are identified as gain- and loss-of-function mutations, respectively. The effects of these polymorphisms on serum lipid levels and cardiovascular risk remain to be elucidated.
Methods
In this meta-analysis, we explored the association of PCSK9 rs505151 and rs11591147 polymorphisms with serum lipid levels and cardiovascular risk by calculating the standardized mean difference (SMD) and odds ratios (OR) with 95% confidence intervals (CI).
Results
Pooled results analyzed under a dominant genetic model indicated that the PCSK9 rs505151 G allele was related to higher levels of triglycerides (SMD: 0.14, 95% CI: 0.02 to 0.26, P = 0.021, I2 = 0) and low-density lipoproteins cholesterol (LDL-C) (SMD: 0.17, 95% CI: 0.00 to 0.35, P = 0.046, I2 = 75.9%) and increased cardiovascular risk (OR: 1.50, 95% CI: 1.19 to 1.89, P = 0.0006, I2 = 48%). The rs11591147 T allele was significantly associated with lower levels of total cholesterol (TC) and LDL-C (TC, SMD: -0.45, 95% CI: -0.57 to −0.32, P = 0.000, I2 = 0; LDL-C, SMD: -0.44, 95% CI: -0.55 to −0.33, P = 0.000, I2 = 0) and decreased cardiovascular risk (OR: 0.77, 95% CI: 0.60 to 0.98, P = 0.031, I2 = 59.9) in Caucasians.
Conclusions
This study indicates that the variant G allele of PCSK9 rs505151 confers increased triglyceride (TG) and LDL-C levels, as well as increased cardiovascular risk. Conversely, the variant T allele of rs11591147 protects carriers from cardiovascular disease susceptibility and lower TC and LDL-C levels in Caucasians. These findings provide useful information for researchers interested in the fields of PCSK9 genetics and cardiovascular risk prediction not only for designing future studies, but also for clinical and public health applications.
Keywords: Proprotein convertase subtilisin/kexin type 9, Polymorphisms, Cardiovascular risk, Lipids, Meta-analysis
Background
Cardiovascular disease (CVD) is the leading cause of death and contributes substantially to the heavy disease burden worldwide [1]. It is a complex and multifactorial disease caused by the interaction of vascular risk factors, as well as environmental and genetic factors. An elevated level of serum low-density lipoprotein cholesterol (LDL-C), which is the most common and clinically relevant dyslipidemia, has been well established as a major risk factor for cardiovascular disease [2]. Genetic susceptibility to cardiovascular disease and dyslipidemia has been researched extensively and there is mounting evidence demonstrating that genetic variants are associated with CVD and dyslipidemia.
Proprotein convertase subtilisin/kexin type 9 (PCSK9), which was identified as the ninth member of the proprotein convertase family in 2003, plays a key role in lipid metabolism, and has emerged as an important modulator of cardiovascular health [3]. Insights into the physiological function of PCSK9 were derived initially from the recognition of functional mutations in the PCSK9 gene that cause an autosomal dominant form of hypercholesterolemia (ADH). Extensive research into the function of PCSK9 has since been conducted. To date, the best characterized property of PCSK9 is its ability to enhance the intracellular degradation of the LDL receptor (LDLR), which mediates approximately 70% of LDL-C clearance. Secreted PCSK9 in the circulation effectively binds to the LDLR on the surface of hepatocytes, thereby targeting the LDLR for lysosomal degradation and preventing recycling to the hepatocyte surface, thus leading to considerable elevation in LDL-C levels [4].
The PCSK9 gene is located on the small arm of chromosome 1p32.3 and comprises 12 exons and 11 introns [5]. It is highly polymorphic, with a total of 163 mutations identified so far. These mutations and polymorphisms are distributed in all PCSK9 domains. Although the PCSK9 gene has been found to cause only 2% of ADH; its numerous nonsynonymous variants are functionally relevant in cholesterol regulation and result in considerable changes in blood cholesterol levels in the general population much more than do LDLR or APOB polymorphisms, which are the other two common genes that cause ADH [6]. These functional variants are classified as two categories: gain-of-function (GOF) mutations associated with hypercholesterolemia phenotype and loss-of-function (LOF) mutations, which cause hypocholesterolemia [7]. PCSK9 rs505151 (−23968A > G, E670G) is a common GOF-mutation, this SNP is located in exon 12, and results in an amino acid substitution from glutamate to glycine at position 670 [8]. The PCSK9 rs11591147 (137G > T, R46L) variant contains a replacement mutation (arginine to leucine at position 46) located in exon 1. This relatively rare variant is considered to be a LOF-mutation of PCSK9 [9]. Numerous studies in different ethnic group have been performed to investigate the impact of the rs505151 and rs11591147 variants on plasma lipid homeostasis and associations with the incidence of cardiovascular risk; however, the findings to date are inconsistent. Variants are unequally distributed in different ethnic group and their impact vary in different populations. Therefore, we conducted the current meta-analysis of all eligible studies to provide robust evidence of the associations of rs505151 and rs11591147 variation with lipid traits and susceptibility to CVD.
Methods
Search strategy, study selection and data extraction
The current meta-analysis was performed according to the principles proposed by the Human Genome Epidemiology Network (HuGeNet) HuGE Review Handbook of Genetic Association Studies [10, 11].
Studies dealing with the associations of the two SNPs (rs505151 and rs11591147) with plasma lipids levels and risk of cardiovascular disease in humans were considered eligible. Relevant studies were searched in PubMed, Chinese National Knowledge Infrastructure and WANFANG database. The search work was last updated on September 1, 2016. The following three groups of keywords we performed by searching MEDLINE (via the PubMed gateway): “proprotein convertase subtilisin/kexin type 9” OR PCSK9 OR “neural apoptosis-regulated convertase 1” OR NARC1, polymorphism OR SNP OR “single nucleotide polymorphism” OR variant OR variation OR mutation, lipid OR dyslipidemia OR “coronary heart disease” OR “myocardial infarction” OR “coronary artery disease” OR “ischemic heart disease” OR “acute coronary syndrome” OR “CAD” OR “CHD”. References from the retrieved articles and previous meta-analysis were searched manually for additional qualified studies.
The studies eligible for the meta-analysis must meet all the following inclusion criteria: (i) case-control or cohort studies; (ii) contained rs505151 and/or rs11591147 genotype data; (iii) adequate data for calculating the standardized mean difference (SMD) and odds ratios (ORs) and correspond 95% confidence intervals (CIs). Exclusion criteria were as follows: (i) studies did not provide sufficient data to extract the information we needed; (ii) case report, review, meta-analysis, cell line and animal experiment studies; (iii) repeated publication about the same population.
Two investigators (Qiu and Li) screened all the records and extracted data independently, the third investigator (Zhang) was involved in discussing to avoid bias when there were disagreements between Qiu and Li. Following information was extracted from each of the eligible studies: first author, year of publication, ethnic groups of the patients, type of study, sample size, genotyping method, age, sex, minor allelic frequency (MAF); Hardy-Weinberg equilibrium (HWE).
Statistical analysis
The deviations from the HWE for the PCSK9 rs505151 and rs11591147 genotype distributions were assessed by Fisher’s exact test. A p < 0.05 for the test was considered deviated from the HWE. The pooled SMD with 95% confidence interval (CI) was applied to calculate the differences of plasma lipid levels between different genotypes. The OR and corresponding 95% CI were used to evaluate the strength of the association between the polymorphisms of two SNPs and cardiovascular risk. Dominant genetic model was conducted to assess the genetic associations, the reasons for the choice are as follows: (i) PCSK9 rs505151 and rs11591147 polymorphisms are rare in human and low MAF were presented in candidate gene studies, on the premise that the difference between carrying one and two copies of the genetic variant is likely to have less effect on the effect size (OR and SMD), perhaps a dominate mode is most reasonable. (ii) For some studies, only dominant genetic data were available; (iii) one model does not require adjustment for multiple hypotheses (which is necessary when different models are used); however, dominant model is commonly used in genetic association synopses.
Between-study heterogeneity was assessed by the chi-square-based Q test and I2 statistics [12]. A p < 0.10 for the Q test was considered statistically significant. For I2, which describes the proportion variation in point estimates that is due to variance rather than within-study error, the value of I2 ranged from 0 to100% indicates different degree of heterogeneity (0 to 25%: no heterogeneity; 25 to 50%: moderate heterogeneity; 50 to 75%: large heterogeneity; and 75 to 100%: extreme heterogeneity). Meta-regression, meta-sensitivity and subgroup analysis were conducted to explore the sources of heterogeneity when p < 0.10 for the Q test. SMD, ORs and corresponding 95%CI were calculated by performing fixed effect meta-analysis when the heterogeneity was under the moderate degree or not exist; in otherwise, the analysis model reduced to a random effect meta-analysis. The choice of this model was suggested mainly by the heterogeneity mostly expected in genetic association studies. Meanwhile, the potential bias was assessed by statistical evaluation with Begg’s rank correlation [13] and Egger’s linear regression tests [14] while the numbers of single studies reached three or more. For each variant, a meta-analysis was performed if at least two independent studies were available.
The α level of significance was set at 0.05, except for the Q-test (0.10).
All statistical analyses were performed with with STATA/SE.12.0 (StataCorp, College station, Texas, USA), Review Manager Version 5.3 (The Nordic Cochrane Center, Copenhagen, Denmark).
Assessment of cumulative evidence
The Venice criteria was applied to assess the credibility of each nominally statistically significant association identified by meta-analysis [15]. Three categories were defined according to the amount of evidence, extent of replication and protection from bias, and also generates a composite assessment of ‘strong’, ‘moderate’ or ‘weak’ epidemiological credibility.
Amount of evidence, mainly based on the study sample size, was graded by the sum of subjects carrying the variant allele (total number of cases and controls), category “A” corresponds to a sample size over 1000, “B” and “C” correspond to 100–1000 and <100, respectively.
Replication of genetic associations were depended upon the between-study inconsistency defined by I2, where I2 < 25% was considered as “A”, 25–50% and >50% were identified as “B” and “C”, respectively.
Protection from bias was graded as “A” if there was no notable bias or may not affect the presence of the association; in category “B”, bias could be present or there was considerable missing information on the generation of evidence; in category “C”, demonstrable bias that can affect the presence or absence of the association.
Followed the three letters stated above, evidence was categorized as “strong” (A grades only), “weak” (one or more C grades) or “moderate” (all other combinations).
The quality of summary evidence for no association was also assessed in the meta-analysis. We calculated the power instead of the sample size; the other aspects including replication and protection from bias were also accounted for according to the Venice criteria [15]. The power was identified as “A” if the power were ≥90%, “B” and “C” correspond to 80–90% and <80%, respectively [16]. Evidence was categorized as “strong” (A grade only), “weak” (one or more C grades) or “moderate” (all other combinations).
Results
Characteristics of eligible studies
A total of 32 studies met the inclusion criteria and were included in the final meta-analysis, the process of study selection were shown in Fig. 1. The number of studies which were included in meta-analysis ranged from 2 to 20. With regard to PCKS9 rs505151 polymorphism, 15 articles comprising 14,451 subjects were identified from the initial search corresponded to the plasma lipid levels, 3373 (23%) were Asians and 11,078 (77%) were Caucasians. The MAF ranged greatly from 2.1%to 14.7%. Genotype distributions in four studies deviated from HWE and two studies did not provide sufficient data to evaluate genotype distributions. There were 12 case-control articles including 11,203 subjects related to the association between rs505151 and cardiovascular disease risk. The constituent ratios of Asians and Caucasians were 23% and 77% respectively. In this group, the MAF varied from 3.8% to 7.5%, and it lower than the reported frequency (10%). Among them, only one study of the genotypes distribution in controls deviated from HWE, and one study was failed to assess the genotype distributions. We identified 8 eligible articles encompassing 17,090 subjects to study the association between PCSK9 rs11591147 variation and plasma lipid levels, most cases were Caucasians (98%), MAF of this group higher than the reported frequency (0.6%) and it varied from 1.6% to 25.3%. Except four articles that could not to be assessed the distribution of genotypes, it did not deviated from HWE in the rest 6 articles. Four case-control studies and three cohort studies revealed the association between PCSK9 rs11591147 polymorphism and cardiovascular disease risk, a total of 60,677 subjects included in this group and all of them were Caucasians. The MAF ranged from 1% to 1.7%. The distribution of genotypes in controls of these studies did not deviated from HWE, except one study that could not to be evaluated. The details of characteristics of each individual study were demonstrated in Table 1 and Table 2.
Fig. 1.
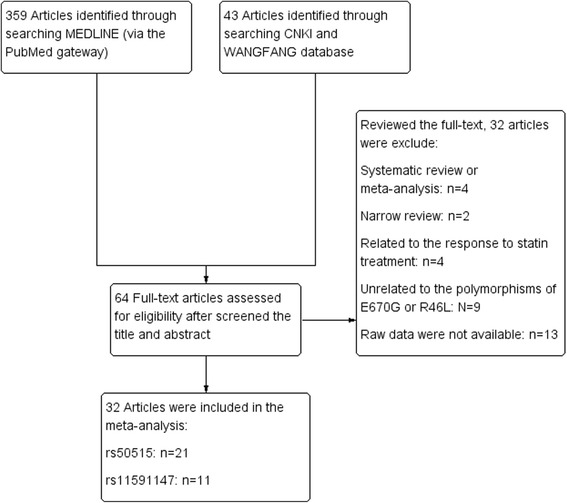
Flow diagram of the study selection process
Table 1.
Characteristics of studies related to the associations of rs50515 and rs11591147 with lipids levels
| Author | Year | Ethnicity | Type of study | Sample size | SNP | Genotyping method | Age(year) | Sex(M/F) | MAF (%) | HWE |
|---|---|---|---|---|---|---|---|---|---|---|
| He, X M [27] | 2016 | Asians | C-C | 233 | rs505151 | PCR-RFLP | 63.95 ± 12.35 | 130/103 | 13.9 | 0.015 |
| Jeenduang, N1 [28] | 2015 | Asians | Cohort | 307 | rs505151 | PCR-RFLP | NA | 97/210 | 1.95 | 0.727 |
| Jeenduang, N2 [28] | 2015 | Asians | Cohort | 188 | rs505151 | PCR-RFLP | NA | 38/150 | 1.06 | 0.883 |
| Yuqian, Mo [29] | 2015 | Asians | C-C | 100 | rs505151 | TaqMan | 56.4 ± 11.7 | 58/42 | 7.19 | 0.000 |
| Anderson, J M1 [30] | 2014 | Caucasians | C-C | 171 | rs505151 | PCR-RFLP | 47 ± 7 | 108/55 | 12.6 | NA |
| Anderson, J M2 [30] | 2014 | Caucasians | C-C | 163 | rs505151 | PCR-RFLP | 55 ± 10 | 127/44 | 14.7 | NA |
| Slimani, A [31] | 2014 | Caucasians | C-C | 258 | rs505151 | PCR-RFLP | Case:55 (52–65) Control:61 (55–67) | 212/46 | 0.88 | 0.000 |
| Mayne, J [32] | 2013 | Caucasians | Cohort | 207 | rs505151 | PCR-RFLP | NA | NA | 0.036 | 0.591 |
| Aung, L H1 [33] | 2013 | Asians | Cohort | 567 | rs505151 | PCR-RFLP | 45.13 ± 13.35 | 456/111 | 0.021 | 0.60 |
| Aung, L H2 [33] | 2013 | Asians | Cohort | 785 | rs505151 | PCR-RFLP | 47.36 ± 14.34 | 573/212 | 0.026 | 0.453 |
| Meng yanhui [34] | 2011 | Asians | Cohort | 165 | rs505151 | PCR-RFLP | 66.49 ± 9.92 | 102/63 | 0.058 | 0.430 |
| Zeng jian [35] | 2011 | Asians | C-C | 212 | rs505151 | PCR-RFLP | NA | NA | 0.123 | 0.020 |
| Norata, G D [36] | 2010 | Caucasians | Cohort | 1541 | rs505151 | Taqman | 54.71 ± 10.97 | 621/923 | 0.024 | 0.32 |
| Hsu, L A1 [37] | 2009 | Asians | C-C | 202 | rs505151 | PCR-RFLP | 55.6 ± 10.5 | 171/31 | 0.046 | 0.513 |
| Hsu, L A2 [37] | 2009 | Asians | C-C | 614 | rs505151 | PCR-RFLP | 45.9 ± 10.4 | 325/289 | 0.060 | 0.381 |
| Polisecki, E [38] | 2008 | Caucasians | Cohort | 5416 | rs505151 | Taqman | 75.64 ± 3.38 | 2620/2795 | 0.030 | 0.072 |
| Scartezini, M [39] | 2007 | Caucasians | Cohort | 2444 | rs505151 | PCR-RFLP | 50–61 | 2444(M) | 0.065 | 0.788 |
| Evans, D [40] | 2006 | Caucasians | Cohort | 506 | rs505151 | PCR-RFLP | 44 ± 14 | 239/267 | 0.050 | 0.111 |
| Chen, SN [41] | 2005 | Caucasians | Cohort | 372 | rs505151 | PCR-RFLP | 58.8 ± 7.7 | 310/62 | 0.074 | 0.000 |
| Jeenduang, N1 [28] | 2015 | Asians | Cohort | 97 | rs11591147 | PCR-RFLP | NA | 97(M) | 0.031 | 0.753 |
| Jeenduang, N2 [28] | 2015 | Asians | Cohort | 209 | rs11591147 | PCR-RFLP | NA | NA | 0.019 | 0.778 |
| Bonnefond, A [42] | 2015 | Caucasians | C-C | 4319 | rs11591147 | Metabochip | 46.7 ± 10.0 | 2272/2246 | 0.020 | 0.179 |
| Saavedra, YG [43] | 2014 | Caucasians | Cohort | 560 | rs11591147 | PCR-RFLP | 37.0 ± 13.9 | 329/231 | 0.016 | 0.699 |
| Guella, I [44] | 2010 | Caucasians | c-c | 3453 | rs11591147 | Taqman | 39.6 ± 4.9 | 3039/414 | NA | NA |
| Strom, T B [45] | 2010 | Caucasians | Cohort | 1130 | rs11591147 | PCR-RFLP | 34 ± 13.3 | 536/594 | NA | NA |
| Huang, C C [46] | 2009 | Caucasians | Cohort | 1828 | rs11591147 | Taqman | 25.5 ± 3.4 | 852/916 | NA | NA |
| Humphries, S E [47] | 2009 | Caucasians | Cohort | 81 | rs11591147 | PCR-RFLP | 56 ± 3.6 | 81(M) | 0.253 | 0.840 |
| Polisecki, E [38] | 2008 | Caucasians | Cohort | 5413 | rs11591147 | Taqman | 75.64 ± 3.38 | 2619/2794 | 0.018 | 0.069 |
Abbreviations: C-C case-control, M male, F female, MAF minor allelic frequencies, HWE Hardy-Weinberg equilibrium, NA not assess
Table 2.
Characteristics of the studies related to the associations of rs50515 and rs11591147 with cardiovascular risk
| Author | Year | Ethnicity | Subgroup | Type of study | Sample size | SNP | Genotyping method | Age(year) | Sex (M/F) | MAF (%) | HWE (p) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| He, X M [27] | 2016 | Asians | CHD | C-C | 472 | rs505151 | PCR-RFLP | 62.80 ± 12.76 | 251/221 | 0.056 | 0.07 |
| Yuqian, Mo [29] | 2015 | Asians | CHD | C-C | 200 | rs505151 | Taqman | 55.55 ± 10.98 | 114/86 | 0.040 | 0.677 |
| Yangchun [48] | 2015 | Asians | MI | C-C | 342 | rs505151 | PCR-RELP | 58.38 ± 6.45 | 200/144 | 0.038 | 0.589 |
| Slimani, A1 [31] | 2014 | Asians | CHD | C-C | 258 | rs505151 | PCR-RFLP | Case:55 (52–65) Control:61 (55–67) | 212/46 | 0.068 | 0.552 |
| Slimani, A3 [31] | 2014 | Caucasians | IS | C-C | 346 | rs505151 | PCR-RELP | Case:66 (54.5–76.5) Control:49 (45–55) | 237/109 | 0.073 | 0.812 |
| Lei, Jing [49] | 2014 | Caucasians | IS | C-C | 756 | rs505151 | SNaPshot | 61.91 ± 112 | 425/331 | 0.056 | 0.925 |
| Meng yanhui [34] | 2011 | Asians | CHD | C-C | 345 | rs505151 | PCR-RFLP | 65.37 ± 13.06 | 200/180 | 0.039 | 0.587 |
| Zeng jian [35] | 2011 | Asians | CHD | C-C | 396 | rs505151 | PCR-RFLP | NA | NA | 0.057 | 0.055 |
| Guella, I [44] | 2010 | Caucasians | MI | C-C | 4643 | rs505151 | PCR-RELP | 36.69 ± 4.92 | 3317/420 | NA | NA |
| Hsu, L A [37] | 2009 | Asians | CHD | C-C | 816 | rs505151 | PCR-RFLP | 48.30 ± 11.23 | 496/320 | 0.060 | 0.381 |
| Scartezini, M [39] | 2007 | Caucasians | CHD | C-C | 2065 | rs505151 | PCR-RFLP | 56.16 ± 3.41 | 2065(M) | 0.033 | 0.465 |
| Abboud, S [50] | 2007 | Caucasians | IS | C-C | 564 | rs505151 | TaqMan | Case(53.5) Control(70.3) | NA | 0.075 | 0.012 |
| Saavedra, Y G3 [43] | 2014 | Caucasians | Cohort | Cohort | 560 | rs11591147 | PCR-RFLP | 37.0 ± 13.9 | 329/231 | 0.016 | 0.699 |
| Benn, M1 [21] | 2010 | Caucasians | C-C | C-C | 10,032 | rs11591147 | Taqman | 58.0 (44–690 | 4514/5518 | 0.012 | 0.676 |
| Benn, M2 [21] | 2010 | Caucasians | C-C | C-C | 26,013 | rs11591147 | Taqman | 59.8 (51–69) | 12746/13267 | 0.014 | 0.334 |
| Benn, M3 [21] | 2010 | Caucasians | C-C | C-C | 9654 | rs11591147 | Taqman | 60.0 (51–69) | 5599/4055 | 0.012(total) | 0.748 |
| Guella, I [44] | 2010 | Caucasians | C-C | C-C | 4733 | rs11591147 | Taqman | 39.6 ± 4.9 | 3039/414 | NA | NA |
| Huang, C C [46] | 2009 | Caucasians | Cohort | Cohort | 1828 | rs11591147 | Taqman | 25.52 ± 3.41 | 882/946 | 0.170 | 0.505 |
| Polisecki, E1 [38] | 2008 | Caucasians | Cohort | Cohort | 5413 | rs11591147 | Taqman | 75.64 ± 3.38 | 2619/2794 | 0.018 | 0.840 |
| Scartezini, M [39] | 2007 | Caucasians | C-C | C-C | 2444 | rs11591147 | PCR | 50–61 | 2444(M) | 0.010 | 0.074 |
Abbreviations: CHD coronary heart disease, MI myocardial infarction, IS ischemic stroke, C-C case-control, M male, F female, MAF minor allelic frequencies, HWE Hardy-Weinberg equilibrium, NA not assess
Quantitative synthesis of data
Associations of PCSK9 rs505151 and rs11591147 polymorphisms with plasma lipid levels and blood pressure
As for PCSK9 rs505151, pooled results under dominant genetic model (AG + GG VS AA) indicated that G allele carriers had higher TG levels in Asians (SMD: 0.14, 95% CI: 0.02 to 0.26, P = 0.021, I2 = 0) and higher LDL-C levels (SMD: 0.17, 95% CI: 0.00 to 0.35, P = 0.046, I2 = 75.9%. Fig. 2), the grade of evidence were identified as “moderate” and “weak”, respectively. No Statistical association was found between PCSK9 rs505151 variant and TC, HDL-C, diastolic blood pressure (DBP) and systolic blood pressure (SBP) Table 3.
Fig. 2.
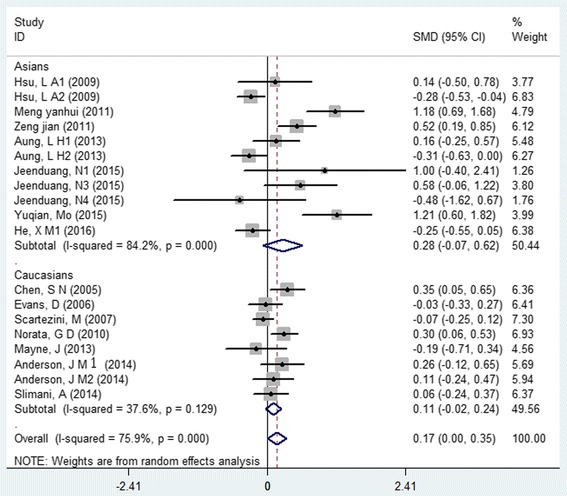
Forest plot of the association between PCSK9 rs505151 polymorphism and serum low-density cholesterol level
Table 3.
Associations of rs505151 and rs11591147 variants with serum lipids levels and blood pressure
| SNP | variant allele | Lipid traits or BP | Sub- group | No. of Study | Sample size | effect model | SMD | 95% CI | P | I2% | Phet | Begg ‘(P) | Egger’s (P) | Venice criteria | Level of evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs505151 | G | TC | Asians | 11 | 3318 | random | 0.16 | (−0.06,0.38) | 0.151 | 62.9 | 0.003 | 0.815 | 0.407 | BCA | weak |
| rs505151 | G | TC | Caucasians | 9 | 9664 | random | 0.03 | (−0.14,0.21) | 0.716 | 76.0 | 0.000 | 0.532 | 0.151 | BCA | weak |
| rs505151 | G | TC | Overall | 20 | 12,982 | random | 0.09 | (−0.04,0.22) | 0.190 | 68.5 | 0.000 | 0.846 | 0.601 | ACA | weak |
| rs505151 | G | TG | Asians | 12 | 3373 | fixed | 0.14 | (0.02,0.26) | 0.021 | 0.0 | 0.529 | 0.586 | 0.411 | BAA | Moderate |
| rs505151 | G | TG | Caucasians | 7 | 3183 | fixed | −0.09 | (−0.21,0.03) | 0.127 | 23.9 | 0.247 | 0.099 | 0.294 | BAA | Moderate |
| rs505151 | G | TG | Overall | 19 | 6556 | fixed | 0.02 | (−0.06,0.11) | 0.596 | 30.0 | 0.112 | 0.472 | 0.623 | BBA | Moderate |
| rs505151 | G | LDL-C | Asians | 11 | 3335 | random | 0.28 | (−0.07,0.62) | 0.113 | 84.2 | 0.000 | 0.312 | 0.190 | BCA | weak |
| rs505151 | G | LDL-C | Caucasians | 8 | 4248 | random | 0.11 | (−0.02,0.24) | 0.111 | 37.6 | 0.129 | 0.805 | 0.710 | BBA | Moderate |
| rs505151 | G | LDL-C | Overall | 19 | 7583 | random | 0.17 | (0.00,0.35) | 0.046 | 75.9 | 0.000 | 0.151 | 0.092 | BCA | weak |
| rs505151 | G | HDL-C | Asians | 11 | 3273 | fixed | −0.16 | (−0.19,0.06) | 0.340 | 44.1 | 0.065 | 0.245 | 0.560 | BBA | Moderate |
| rs505151 | G | HDL-C | Caucasians | 8 | 4248 | fixed | 0.02 | (−0.08,0.12) | 0.673 | 0.0 | 0.803 | 0.095 | 0.091 | BAA | Moderate |
| rs505151 | G | HDL-C | Overall | 19 | 7521 | fixed | −0.01 | (−0.09,0.07) | 0.792 | 18.7 | 0.230 | 0.103 | 0.154 | BAA | Moderate |
| rs505151 | G | DBP | Mixed | 8 | 5079 | fixed | −0.08 | (−0.21,0.05) | 0.224 | 0.0 | 0.692 | 0.805 | 0.810 | BAA | Moderate |
| rs505151 | G | SBP | Mixed | 8 | 5079 | fixed | −0.06 | (−0.19,0.06) | 0.332 | 7.0 | 0.376 | 0.805 | 0.643 | BAA | Moderate |
| rs11591147 | T | TC | Asians | 2 | 306 | random | −0.50 | (−1.44,0.44) | 0.299 | 66.5 | 0.084 | CCA | weak | ||
| rs11591147 | T | TC | Caucasians | 6 | 9496 | random | −0.45 | (−0.57,-0.32) | 0.000 | 0.0 | 0.936 | 0.851 | 0.913 | BAA | Moderate |
| rs11591147 | T | TC | Overall | 8 | 9802 | random | −0.45 | (−0.57,-0.33) | 0.000 | 0.0 | 0.733 | 1.000 | 0.869 | BAA | Moderate |
| rs11591147 | T | TG | Asians | 2 | 306 | fixed | −0.43 | (−0.97,0.11) | 0.119 | 0.0 | 0.447 | CAA | weak | ||
| rs11591147 | T | TG | Caucasians | 5 | 9254 | fixed | 0.00 | (−0.11,0.11) | 0.996 | 0.0 | 0.685 | 0.624 | 0.436 | BAA | Moderate |
| rs11591147 | T | TG | Overall | 7 | 9560 | fixed | −0.02 | (−0.13,0.09) | 0.747 | 0.0 | 0.522 | 0.176 | 0.102 | BAA | Moderate |
| rs11591147 | T | LDL-C | Asians | 2 | 306 | fixed | −0.53 | (−1.07,0.01) | 0.053 | 0.0 | 0.436 | ||||
| rs11591147 | T | LDL-C | Caucasians | 5 | 10,299 | fixed | −0.44 | (−0.55,-0.33) | 0.000 | 0.0 | 0.548 | 0.142 | 0.125 | BAA | Moderate |
| rs11591147 | T | LDL-C | Overall | 7 | 10,605 | fixed | −0.44 | (−0.55,-0.33) | 0.000 | 0.00.0 | 0.707 | 0.293 | 0.206 | BAA | Moderate |
| rs11591147 | T | HDL-C | Mixed | 7 | 9560 | fixed | 0.10 | (−0.01,0.21) | 0.074 | 35.4 | 0.158 | 0.453 | 0.026 | BBB | Moderate |
| rs11591147 | T | DBP | Mixed | 5 | 2797 | random | 3.60 | (−0.71,7.9) | 0.101 | 77.4 | 0.001 | 0.624 | 0.318 | CCA | weak |
| rs11591147 | T | SBP | Mixed | 5 | 2797 | random | 1.79 | (−2.78,6.37) | 0.442 | 45.6 | 0.118 | 0.624 | 0.306 | CBA | weak |
Abbreviations: SMD standardized mean difference, 95%C 95% confidence interval, TC cholesterol, TG triglyceride, LDL-C low-density lipoprotein cholesterol, HDL-C high density lipoprotein cholesterol, SBP systolic blood pressure, DBP diastolic blood pressure
Findings showed that T allele in rs11591147 was significantly associated with lower TC level and LDL-C level in Caucasians (TC, SMD: -0.45, 95% CI: -0.57 to −0.32, P = 0.000, I2 = 0; LDL-C, SMD: -0.44, 95% CI: -0.55 to −0.33, P = 0.000, I2 = 0, Fig. 3), the credibility of the both pooled findings were identified as “moderate”. We did not find any statistical significant association of the PCSK9 rs11591147 polymorphism with TG, HDL-C, DBP and SBP Table 3.
Fig. 3.
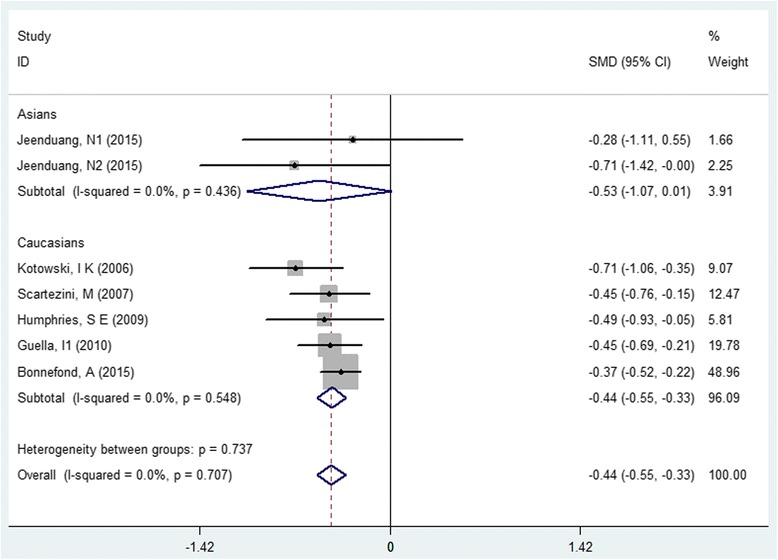
Forest plot of the association between PCSK9 rs11591147 polymorphism and serum low-density cholesterol level
Associations of the PCSK9 rs505151 and rs11591147 polymorphisms with cardiovascular risk
As shown in Fig. 4 and Table 4, the dominant model of G allele in PCSK9 rs505151 was significantly increased cardiovascular risk (OR:1.50, 95% CI: 1.19 to 1.89, P = 0.0006, I2 = 48%), the evidence was identified as “moderate”; subgroup meta-analysis, which stratified by ethnicity presented consistent results (Asians, OR:1.59, 95% CI: 1.12 to 2.27, P = 0.009, I 2 = 60; Caucasians, OR:1.31, 95% CI: 1.04 to 1.65, P = 0.02, I2 = 0). Conversely, the T allele of rs11591147 related to decreased cardiovascular risk in Caucasians (OR: 0.77, 95% CI: 0.60 to 0.98, P = 0.031, I2 = 59.9, Fig. 5), the evidence was graded as “weak” because of large between-study various (I2 = 63.2%).
Fig. 4.
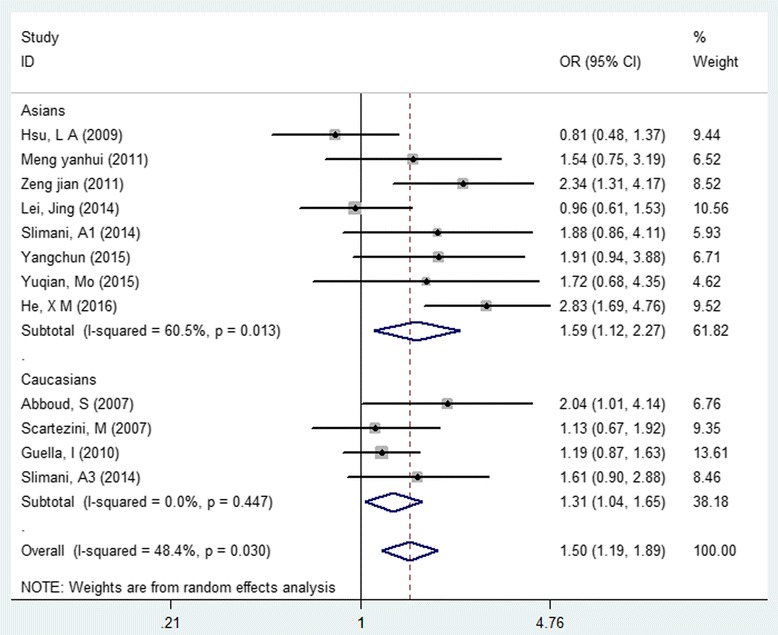
Forest plot of the association between PCSK9 rs505151 polymorphism and cardiovascular risk
Table 4.
Associations of rs505151 and rs11591147 variants with cardiovascular risk
| SNP | Variant allele | Subgroup | No. of Study | Sample size | effect model | OR | 95% CI | P value | I2% | Phet | Begg’ (P) | Egger’s (P) | Venice criteria | Level of evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs505151 | G | Asians | 8 | 1672 | random | 1.59 | (1.12,2.27) | 0.009 | 60 | 0.01 | 0.805 | 0.437 | BCA | Weak |
| rs505151 | G | Caucasians | 4 | 2369 | random | 1.31 | (1.04,1.65) | 0.02 | 0 | 0.45 | 0.042 | 0.229 | BAA | Moderate |
| rs505151 | G | Overall | 4041 | random | 1.50 | (1.19,1.89) | 0.0006 | 48 | 0.03 | 0.493 | 0.144 | BBA | Moderate | |
| rs11591147 | T | Caucasians | 9 | 66,090 | random | 0.77 | (0.60,0.98) | 0.031 | 59.9 | 0.015 | 0.216 | 0.165 | BCA | Weak |
Abbreviations: OR odds ratio, 95% CI 95% confidence interval
Fig. 5.
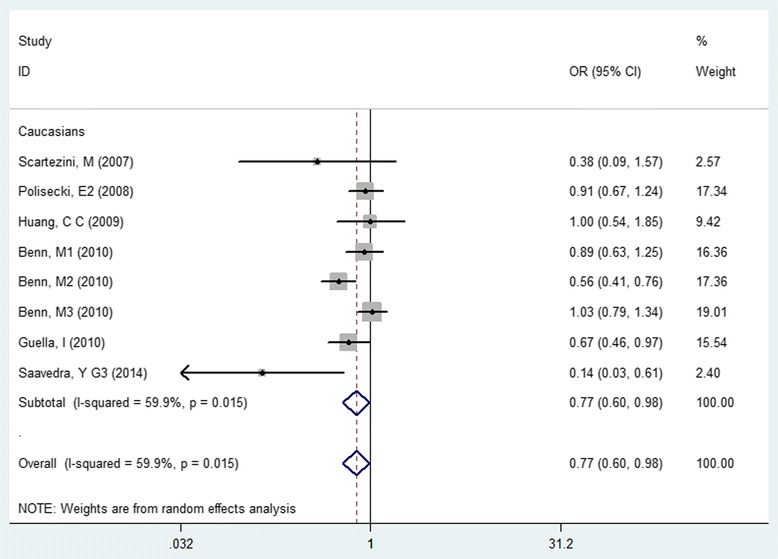
Forest plot of the association between PCSK9 rs11591147 polymorphism and cardiovascular risk
Heterogeneity and publication bias
Some heterogeneity was observed in the meta-analysis. Among these statistical Significant findings, the associations of PCSK9 rs505151 polymorphism with increased serum TG concentration and increased cardiovascular risk, the associations between PCSK9 rs11591147 polymorphism and decreased cardiovascular risk were based on heterogeneous data, which lowered the credibility of pooled evidence. Begg’s and Egger’s tests were applied to detect the potential publication bias, data showed there was no potential publication bias in all the comparisons except one meta-analysis about the association of rs11591147 polymorphism and plasma HDL-C level (P = 0.026 for Egger’s tests). More details were reported in Table 4.
Discussion
In the current study, we performed the most comprehensive meta-analysis of the associations of the rs505151 and rs11591147 functional mutations of PCSK9 with serum lipids level and cardiovascular risk that has been conducted to date. Synthetic results clearly showed an association between the G allele of PCSK9 rs505151 and increased serum LDL-C levels; this is the first time that the relationship between PCSK9 rs505151 variants and increased TG concentrations has been demonstrated. Furthermore, this single nucleotide polymorphism (SNP) was also shown to be related to an increased incidence of cardiovascular events. Conversely, the T allele of the PCSK9 rs11591147 variation was found to be associated with reduced serum TC and LDL-C levels and strongly related to a reduction in cardiovascular risk among the general Caucasian population. According to the Venice criteria [15], the credibility of all the nominally statistically significant associations was in the range “moderate” to “weak”, predominantly because of the observed between-study heterogeneity.
Previous meta-analyses have shown the association of PCSK9 rs505151 variants with increased serum TC and LDL-C levels, as well as increased cardiovascular risk [17–20]; however, the present meta-analysis revealed that this SNP was closely related to higher LDL-C and TG levels, but not with higher TC levels. The role of PCSK9 in cholesterol regulation is well-recognized; however, this study is the first to demonstrate the association between PCSK9 rs505151 variants and serum TG levels, although it remains to be determined whether this relationship is causal or a concomitant phenomenon. As for PCSK9 rs11591147, a meta-analysis has been reported that the PCSK9 46 L allele was associated with reductions in LDL-C and ischemic heart disease via pooled three independent studies in 2010 [21]. Given the discrepancies in the results reported in recent years, this meta-analysis was performed to explore the true associations of PCSK9 rs11591147 variants with lipid levels and cardiovascular risk; the consistency of the results of this meta-analysis further confirmed the robust relationship.
Since it was first reported in 2003, PCSK9 has attracted a lot of attention regarding its key role in lipid metabolism. PCSK9 enhances LDLR lysosomal degradation, resulting in reduced LDL-C clearance, thereby leading elevated serum LDL-C levels [22, 23]. Functional mutations of PCSK9 could have a real impact on serum lipids level and cardiovascular risk. The rs505151 and rs11591147 variants of PCSK9 are classified as GOF- and LOF-mutations, respectively. Notably, this study further confirmed the association of rs505151 with increased LDL-C levels and cardiovascular risk, while there was a strong association of the rs11591147 polymorphism with reduced LDL-C levels and cardiovascular risk. The PCSK9 gene is highly polymorphic, and functional variants affect the activity of the PCSK9 protein, resulting in lipid metabolism disturbances. Despite the less marked effect of a single SNP on the pathophysiological processing, adding genetic information to lipid management and cardiovascular risk prediction may be potentially useful in clinical practice. Pharmacogenetic studies have shown that GOF and LOF variants of PCSK9 are associated with worse and better responses to statin therapy, respectively [24, 25]. The most likely reason for this is that these functional variants of PCSK9 cause disturbances in cholesterol metabolism and lead to higher or lower cholesterol concentrations, respectively. Despite the current lack of genetic tests to guide statin therapy, the findings of this study could provide useful information for determining the optimal therapy. For instance, standard statin treatment fails to achieve cholesterol targets in some patients. In such cases, administration of a PCSK9 inhibitor would preferable to increasing the statin dose, regardless of knowledge of the patient’s genotype. Furthermore, combining identified variants, such as PCSK9 rs505151, into risk prediction models, may show some improvement in cardiovascular risk prediction for primary prevention. Unlike the traditional factors, genetic variants, if they can be identified, may be strong predictors with lifelong value in preventive management.
Some limitations of this meta-analysis should be noted. First, heterogeneity in the data may reduce the credibility of the pooled evidence. The main factors responsible for this heterogeneity were study design (cohort or case-control) and genotyping method (Taq Man or PCR-RFLP), which is a common problem in genetic meta-analyses. Therefore, the adoption of strict standards will be encouraged in performing clinical studies. Second, only one genetic model (dominant model) was applied in our analysis, as explained in the Materials and methods section. However, the use of different models would have increased the number of meta-analyses, with consequent inflation of the type I error [26]. Third, the size of the Asian population included in this meta-analysis of PCSK9 rs11591147 was small, and pooled results revealed heterogeneity in the Asian group, but not in the Caucasian group, indicating that more high-quality clinical studies in Asian populations are required. Fourth, most original studies included in the present meta-analysis used the combined cardiovascular event to estimate the cardiovascular risk, thus taking difficult to identify the risk of specific cardiovascular event. In the final, though we have provided evidence support the association between the two SNPs of PCSK9 (rs505151 and rs11591147) with lipid traits and cardiovascular events, we compromised estimates of heritability based on the current data. Accurate estimates of heritability will require more extensive examination of each identified SNP, especially in a scenario where variants are more likely to be causal for traits and disease.
Conclusions
This study provides evidence that the variant PCSK9 rs505151 allele confers increased TG and LDL-C levels on the carrier, as well as increased cardiovascular risk. Conversely, the variant rs11591147 allele protects against CVD susceptibility and is associated with lower TC and LDL-C levels. These findings could provide useful information for researchers interested in PCSK9 and cardiovascular risk prediction not only in the design of future studies, but also improved clinical and public health. However, further investigations are required to identify the biological function of the two PCSK9 SNPs and to distinguish direct or indirect influences of the variant alleles on cardiovascular risk.
Acknowledgements
Excellent helps in the process of paper preparation which provided by X Zhao, LM Tan, XT Zeng, Q Zhou and J Zhu is greatly acknowledged.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This study was supported by the Natural Science Foundation of Hunan Province, China (Gant No. 2017JJ50), and the Traditional Chinese Medicine of Hunan province Program (Grant No. 201586).
Authors’ contributions
CF conceived and designed the research, acquired the data, performed data extraction and statistical analysis, drafted the manuscript. ZZ performed some statistical analysis. BJ, YF and YP carried out data extraction. YS and YP conducted data reviews. XH, PY and RF Chen made some conduct for language and writing assistance. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ADH
Autosomal dominant form of hypercholesterolemia
- CAD
Coronary artery disease
- CHD
Coronary heart disease
- CI
Confidence interval
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- GOF
Gain-of-function
- HDL-C
High density lipoprotein cholesterol
- HWE
Hardy-Weinberg equilibrium
- IS
ischemic stroke
- LDL-C
Low-density lipoprotein cholesterol
- LDLR
Low-density lipoprotein-receptor
- LOF
Loss-of-function
- MAF
Minor allelic frequency
- MI
and Myocardial infarction
- OR
odds ratio
- PCSK9
Proprotein convertase subtilisin/kexin type 9
- SBP
Systolic blood pressure
- SMD
Standardized mean difference
- TC
Total cholesterol
- TG
Triglyceride
Contributor Information
Chengfeng Qiu, Email: qiuchengfeng0721@163.com.
Pingyu Zeng, Email: zengpingyu@yahoo.com.
Xiaohui Li, Email: xiaohuili@csu.edu.cn.
Zhen Zhang, Email: js200_1986@163.com.
Bingjie Pan, Email: panbj3311@163.com.
Zhou Y. F. Peng, Email: zhaoxiang5566@sohu.com
Yapei Li, Email: sunshine0726@csu.edu.cn.
Yeshuo Ma, Email: Mayeshuo@sina.com.
Yiping Leng, Email: lyp0626@aliyun.com.
Ruifang Chen, Email: orx79927219@163.com.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Della-Morte D, Pacifici F, Rundek T. Genetic susceptibility to cerebrovascular disease. Curr Opin Lipidol. 2016;27:187–195. doi: 10.1097/MOL.0000000000000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urban D, Pöss J, Böhm M, Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol. 2013;62:1401–1408. doi: 10.1016/j.jacc.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Li JJ. Pcsk9: a key factor modulating atherosclerosis. J Atheroscler Thromb. 2015;22:221–230. doi: 10.5551/jat.27615. [DOI] [PubMed] [Google Scholar]
- 5.Seidah NG, Prat A. The proprotein convertases are potential targets in the treatment of dyslipidemia. J Mol Med (Berl) 2007;85:685–696. doi: 10.1007/s00109-007-0172-7. [DOI] [PubMed] [Google Scholar]
- 6.Na-Qiong Wu, Jian-Jun Li. PCSK9 gene mutations and low-density lipoprotein cholesterol. Clinica Chimica Acta. 2014;431:148–53. [DOI] [PubMed]
- 7.Schulz R, Schluter KD, Laufs U. Molecular and cellular function of the proprotein convertase subtilisin/kexin type 9 (pcsk9) Basic Res Cardiol. 2015;110:4. doi: 10.1007/s00395-015-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding K, Kullo IJ. Molecular population genetics of pcsk9: a signature of recent positive selection. Pharmacogenet Genomics. 2008;18:169–179. doi: 10.1097/FPC.0b013e3282f44d99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarasov K, Ekroos K, Suoniemi M, Kauhanen D, Sylvanne T, Hurme R. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and pcsk9 deficiency. J Clin Endocrinol Metab. 2014;99:E45–E52. doi: 10.1210/jc.2013-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sagoo GS, Little J, Higgins JP. Systematic reviews of genetic association studies. Human genome epidemiology network PLOS MED. 2009;6:e28. doi: 10.1371/journal.pmed.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoury MJ, Little J, Higgins J, Ioannidis JP, Gwinn M. Reporting of systematic reviews: the challenge of genetic association studies. PLoS Med. 2007;4:e211. doi: 10.1371/journal.pmed.0040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 14.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ioannidis JP, Boffetta P, Little J, O'Brien TR, Uitterlinden AG, Vineis P. Assessment of cumulative evidence on genetic associations: interim guidelines. Int J Epidemiol. 2008;37:120–132. doi: 10.1093/ije/dym159. [DOI] [PubMed] [Google Scholar]
- 16.Mocellin S, Verdi D, Pooley KA, Nitti D. Genetic variation and gastric cancer risk: a field synopsis and meta-analysis. Gut. 2015;64:1209–1219. doi: 10.1136/gutjnl-2015-309168. [DOI] [PubMed] [Google Scholar]
- 17.Cai G, Zhang B, Shi G, Weng W, Ma C, Song Y. The associations between proprotein convertase subtilisin/kexin type 9 e670g polymorphism and the risk of coronary artery disease and serum lipid levels: a meta-analysis. Lipids Health Dis. 2015;14:149. doi: 10.1186/s12944-015-0154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adi D, Xie X, Liu F, Ma YT, Abudoukelimu M, Wu Y. Relationships between genetic polymorphisms of e670g in pcsk9 gene and coronary artery disease: a meta-analysis. Int J Clin Exp Med. 2015;8:13251–13258. [PMC free article] [PubMed] [Google Scholar]
- 19.Au A, Griffiths LR, Cheng K, Wee Kooi C, Irene L, Keat WL. The influence of olr1 and pcsk9 gene polymorphisms on ischemic stroke: evidence from a meta-analysis. SCI REP-UK. 2015;5:18224. doi: 10.1038/srep18224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Yuan F, Liu P, Fei L, Huang Y, Xu L. Association between pcsk9 and ldlr gene polymorphisms with coronary heart disease: case-control study and meta-analysis. Clin Biochem. 2013;46:727–732. doi: 10.1016/j.clinbiochem.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg-Hansen A. Pcsk9 r46l, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol. 2010;55:2833–2842. doi: 10.1016/j.jacc.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 22.Strøm TB, Tveten K, Leren TP. Pcsk9 acts as a chaperone for the ldl receptor in the endoplasmic reticulum. Biochem J. 2014;457:99–105. doi: 10.1042/BJ20130930. [DOI] [PubMed] [Google Scholar]
- 23.Lagace TA. Pcsk9 and ldlr degradation. Curr Opin Lipidol. 2014;25:387–393. doi: 10.1097/MOL.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Q, Wei WQ, Chung CP, Levinson RT, Bastarache L, Denny JC. The effect of genetic variation in pcsk9 on the ldl-cholesterol response to statin therapy. Pharmacogenomics J. 2017;17:204–8. [DOI] [PMC free article] [PubMed]
- 25.Postmus I, Trompet S, Deshmukh HA, Barnes MR, Li X, Warren HR. Pharmacogenetic meta-analysis of genome-wide association studies of ldl cholesterol response to statins. Nat Commun. 2014;5:5068. doi: 10.1038/ncomms6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. JNCI Journal of the National Cancer Institute. 2004;96:434–442. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He XM, Chen L, Wang TS, Zhang YB, Luo JB, Feng XX. E670g polymorphism of pcsk9 gene of patients with coronary heart disease among han population in hainan and three provinces in the northeast of china. Asian Pac J Trop Med. 2016;9:172–176. doi: 10.1016/j.apjtm.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Jeenduang N, Porntadavity S, Wanmasae S. Combined pcsk9 and apoe polymorphisms are genetic risk factors associated with elevated plasma lipid levels in a thai population. Lipids. 2015;50:543–553. doi: 10.1007/s11745-015-4017-9. [DOI] [PubMed] [Google Scholar]
- 29.Yuqian M, Weiqi L, Yuru Z, Xiaoming Z. Dongguan han patients with coronary artery pcsk9 gene snp and its prognosis. International Journal of Laboratory Medicine. 2015;12:1725–7.
- 30.Anderson JM, Cerda A, Hirata MH, Rodrigues AC, Dorea EL, Bernik MM. Influence of pcsk9 polymorphisms on plasma lipids and response to atorvastatin treatment in brazilian subjects. J CLIN LIPIDOL. 2014;8:256–264. doi: 10.1016/j.jacl.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Slimani A, Harira Y, Trabelsi I, Jomaa W, Maatouk F, Hamda KB. Effect of e670g polymorphism in pcsk9 gene on the risk and severity of coronary heart disease and ischemic stroke in a tunisian cohort. J Mol Neurosci. 2014;53:150–157. doi: 10.1007/s12031-014-0238-2. [DOI] [PubMed] [Google Scholar]
- 32.Mayne J, Ooi TC, Raymond A, Cousins M, Bernier L, Dewpura T. Differential effects of pcsk9 loss of function variants on serum lipid and pcsk9 levels in caucasian and african canadian populations. Lipids Health Dis. 2013;12:70. doi: 10.1186/1476-511X-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aung LH, Yin RX, Wu DF, Cao XL, Hu XJ, Miao L. Proprotein convertase subtilisin/kexin type 9 gene e670g polymorphism interacts with alcohol consumption to modulate serum lipid levels. Int J Med Sci. 2013;10:124–132. doi: 10.7150/ijms.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong-min MYL. A study on the correlation between e670g polymorphism of pcsk9 gene and coronary artery disease in the guangdong population. Journal of Tropical Medicine. 2011;11:137–140. [Google Scholar]
- 35.Jian Z, Zhi Z. Pcsk9 and dyslipidemia. Sichuan Medical Journal. 2007;01:22–4.
- 36.Norata GD, Garlaschelli K, Grigore L, Raselli S, Tramontana S, Meneghetti F. Effects of pcsk9 variants on common carotid artery intima media thickness and relation to apoe alleles. Atherosclerosis. 2010;208:177–182. doi: 10.1016/j.atherosclerosis.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Hsu LA, Teng MS, Ko YL, Chang CJ, Wu S, Wang CL. The pcsk9 gene e670g polymorphism affects low-density lipoprotein cholesterol levels but is not a risk factor for coronary artery disease in ethnic chinese in taiwan. Clin Chem Lab Med. 2009;47:154–158. doi: 10.1515/CCLM.2009.032. [DOI] [PubMed] [Google Scholar]
- 38.Polisecki E, Peter I, Robertson M, McMahon AD, Ford I, Packard C. Genetic variation at the pcsk9 locus moderately lowers low-density lipoprotein cholesterol levels, but does not significantly lower vascular disease risk in an elderly population. Atherosclerosis. 2008;200:95–101. doi: 10.1016/j.atherosclerosis.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scartezini M, Hubbart C, Whittall RA, Cooper JA, Neil AH, Humphries SE. The pcsk9 gene r46l variant is associated with lower plasma lipid levels and cardiovascular risk in healthy u.K. Men Clin Sci (Lond) 2007;113:435–441. doi: 10.1042/CS20070150. [DOI] [PubMed] [Google Scholar]
- 40.Evans D, Beil FU. The e670g snp in the pcsk9 gene is associated with polygenic hypercholesterolemia in men but not in women. BMC MED GENET. 2006;7:66. doi: 10.1186/1471-2350-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen SN, Ballantyne CM, Gotto AJ, Tan Y, Willerson JT, Marian AJ. A common pcsk9 haplotype, encompassing the e670g coding single nucleotide polymorphism, is a novel genetic marker for plasma low-density lipoprotein cholesterol levels and severity of coronary atherosclerosis. J Am Coll Cardiol. 2005;45:1611–1619. doi: 10.1016/j.jacc.2005.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonnefond A, Yengo L, Le May C, Fumeron F, Marre M, Balkau B. The loss-of-function pcsk9 p.r46l genetic variant does not alter glucose homeostasis. Diabetologia. 2015;58:2051–2055. doi: 10.1007/s00125-015-3659-8. [DOI] [PubMed] [Google Scholar]
- 43.Saavedra YG, Dufour R, Davignon J, Baass A. Pcsk9 r46l, lower ldl, and cardiovascular disease risk in familial hypercholesterolemia: a cross-sectional cohort study. Arterioscler Thromb Vasc Biol. 2014;34:2700–2705. doi: 10.1161/ATVBAHA.114.304406. [DOI] [PubMed] [Google Scholar]
- 44.Guella I, Asselta R, Ardissino D, Merlini PA, Peyvandi F, Kathiresan S. Effects of pcsk9 genetic variants on plasma ldl cholesterol levels and risk of premature myocardial infarction in the italian population. J Lipid Res. 2010;51:3342–3349. doi: 10.1194/jlr.M010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strom TB, Holla OL, Cameron J, Berge KE, Leren TP. Loss-of-function mutation r46l in the pcsk9 gene has little impact on the levels of total serum cholesterol in familial hypercholesterolemia heterozygotes. Clin Chim Acta. 2010;411:229–233. doi: 10.1016/j.cca.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Huang CC, Fornage M, Lloyd-Jones DM, Wei GS, Boerwinkle E, Liu K. Longitudinal association of pcsk9 sequence variations with low-density lipoprotein cholesterol levels: the coronary artery risk development in young adults study. Circ Cardiovasc Genet. 2009;2:354–361. doi: 10.1161/CIRCGENETICS.108.828467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humphries SE, Neely RD, Whittall RA, Troutt JS, Konrad RJ, Scartezini M. Healthy individuals carrying the pcsk9 p.r46l variant and familial hypercholesterolemia patients carrying pcsk9 p.d374y exhibit lower plasma concentrations of pcsk9. Clin Chem. 2009;55:2153–2161. doi: 10.1373/clinchem.2009.129759. [DOI] [PubMed] [Google Scholar]
- 48.Chun Y, Jun X, Jiming X, Yugang Y, Hua G, Xiang Y. A study on the correlation between e670g polymorphism of pcsk9 gene and acute myocardial infarction in the suwan (jiangsu and anhui) area population. Chinese Journal of Birth Health & Heredity. 2015;08:13–4.
- 49.Lei J. Correlation of pcsk9 gene polymorphism with cerebral ischemic stroke in xinjiang han and uygur populations. MED SCI MONITOR. 2014;20:1758–1767. doi: 10.12659/MSM.892091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abboud S, Karhunen PJ, Lutjohann D, Goebeler S, Luoto T, Friedrichs S. Proprotein convertase subtilisin/kexin type 9 (pcsk9) gene is a risk factor of large-vessel atherosclerosis stroke. PLoS One. 2007;2:e1043. doi: 10.1371/journal.pone.0001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


