Abstract
BACKGROUND
Prophylactic exclusion of the left atrial appendage (LAA) is often performed during cardiac surgery ostensibly to reduce the risk of stroke. However, the clinical impact of LAA closure in humans remains inconclusive.
METHODS
Of 10 633 adults who underwent coronary artery bypass grafting and valve surgery between January 2000 and December 2005, 9792 patients with complete baseline characteristics, surgery procedure, and follow-up data were included in this analysis. A propensity score–matching analysis based on 28 pretreatment covariates was performed and 461 matching pairs were derived and analyzed to estimate the association of LAA closure with early postoperative atrial fibrillation (POAF) (atrial fibrillation ≤30 days of surgery), ischemic stroke, and mortality.
RESULTS
In the propensity-matched cohort, the overall incidence of POAF was 53.9%. In this group, the rate of early POAF among the patients who underwent LAA closure was 68.6% versus 31.9% for those who did not undergo the procedure (P<0.001). LAA closure was independently associated with an increased risk of early POAF (adjusted odds ratio, 3.88; 95% confidence interval, 2.89–5.20), but did not significantly influence the risk of stroke (adjusted hazard ratio, 1.07; 95% confidence interval, 0.72–1.58) or mortality (adjusted hazard ratio, 0.92; 95% confidence interval, 0.75–1.13).
CONCLUSIONS
After adjustment for treatment allocation bias, LAA closure during routine cardiac surgery was significantly associated with an increased risk of early POAF, but it did not influence the risk of stroke or mortality. It remains uncertain whether prophylactic exclusion of the LAA is warranted for stroke prevention during non–atrial fibrillation-related cardiac surgery.
Atrial fibrillation (AF) is the most prevalent sustained cardiac rhythm disorder seen in clinical practice, affecting 2.3 million adults in the United States alone.1 It is estimated that AF may account for 15% of all ischemic strokes2 and increases the incidence of embolic stroke 5-fold.2 AF-associated strokes impart worse prognosis than those occurring in the absence of AF.3,4 There is substantial evidence that the left atrial appendage (LAA) is an important source of thrombi in patients with AF and underlying heart disease.5–7 In a systematic review of 23 separate studies of patients with nonvalvular and valvular AF in which the LAA was examined by autopsy, transesophageal echocardiography, or direct intraoperative inspection, intracardiac thrombus was identified in 13% of cases, and ≈90% of atrial thrombi in nonvalvular AF may arise from the LAA.5 Consequently, prophylactic exclusion of the LAA from the systemic circulation during cardiac surgery has been proposed as a means of reducing the risk of future thromboembolic events in patients with AF.8 Although exclusion of the LAA as a potential source of thrombi from the systemic circulation seems to be a logical alternative to conventional anticoagulation therapy in patients with AF,5,9,10 this proposition has not yet been proven conclusively. Because the LAA is a more distensible chamber than the left atrium (LA), it plays an important role as a decompression chamber for the LA and spares the LA from acute rise in pressure.11 Previous animal studies have shown that exclusion of the LAA may impair hemodynamic response to volume or pressure overload.12 Animal experiments have also demonstrated that eliminating access to the LAA results in an increase in the mean dynamic stiffness constant of the LA diastolic pressure volume relationship.12,13 These findings suggest that, after LAA closure, for similar abrupt increases in LA volume, reduced LA compliance may result in a larger increase in LA pressure and pulmonary vein stretch and endothelial dysfunction, thereby promoting the development of early postoperative atrial fibrillation (POAF) and thrombogenesis. We hypothesize that LAA closure could paradoxically increase the risk of early POAF following cardiac surgery. The objective of the present study was to examine the association between LAA closure and the risk of early POAF, ischemic stroke, and mortality after routine non–AF-related cardiac surgery.
METHODS
Study Population
We analyzed prospectively collected data on a cohort of 10 633 adults from the Mayo Clinic Cardiovascular Surgery Database who underwent coronary artery bypass grafting (CABG) and valve surgery between January 1, 2000, and December 31, 2005, to investigate the association between LAA closure and the risk of POAF, stroke, and mortality after routine non–AF-related cardiac surgery. Exclusion criteria were concurrent or prior history of maze procedure and pulmonary vein isolation (n=841). A total of 9792 patients were enrolled. The study was approved by the Mayo Clinic Institutional Review Board, and written informed consent requirement was waived.
Surgical Techniques
LAA closure was performed through a median sternotomy using cardiopulmonary bypass. When left atrial appendage closure was performed as part of a mitral valve operation, the ostium of the left atrial appendage was usually sutured in 2 layers of polypropylene suture from inside the left atrium. When LAA closure was performed as part of other cardiac procedures, the LAA was amputated and its opening was sutured in 2 layers of polypropylene suture from the outside of the heart. In a minority of cases, the LAA was stapled or suture closed from inside the LA depending on the patient tissue quality. During the period of 2000 to 2005, 1 staff surgeon at our hospital routinely performed LAA ligation during mitral valve surgery. Otherwise, LAA closure was performed primarily in patients with AF or LAA thrombus. CABG procedures (95%) were routinely performed under cardiopulmonary bypass with cardioplegic arrest.
Definition of Postoperative Atrial Fibrillation
POAF was defined as AF occurring within 30 days after cardiac surgery, based on documentation of AF episodes lasting ≥30 seconds on continuous telemetry throughout hospitalization: ECGs, Holter, or event monitors.
Postoperative Care
All postoperative patients were routinely admitted to the surgical intensive care unit and monitored with continuous telemetry throughout the hospitalization. A 12-lead ECG was obtained immediately after each operation, at the time a patient developed POAF, and just before hospital discharge. POAF episodes were typically treated with rate control, antiarrhythmic drug therapy, or electric cardioversion if the rhythm failed to terminate.
Anticoagulation was initiated in patients with POAF persisting for >48 hours. Postoperative use of antithrombotic therapy was documented. However, the decision regarding long-term anticoagulation following LAA closure was left to the discretion of the treating provider.
Outcome Ascertainment
Exposure variables were derived from the Mayo Clinic Surgical Database, which contains detailed basic demographic information, surgical procedure, including complications and reoperation, and preoperative medication records for all patients who underwent a surgical procedure at the institution. Patients were followed up postoperatively for a median of 9.1 years (maximum, 14.6 years) by means of standardized biannual questionnaires and telephone interviews by trained interviewers. If the patient died during follow-up, the closest surviving relative and the patient’s physician were surveyed to determine whether the patient had the outcome of early POAF (AF ≤30 days after surgery), ischemic stroke, or death. Covariate data and ascertainment of AF and stroke outcomes were also obtained by review of the medical record of each patient and direct examination of the ECG, Holter, or event monitor. If surgery was repeated any time after the indexed cardiac surgical procedure, the patient’s data were censored at the time of the second operation. Survival status was obtained from review of medical records and query of the Social Security Death Index using the patient’s social security number.
Statistical Analysis
Continuous data were expressed as mean±standard deviation. To allow for possible skewness, data were compared by using the Wilcoxon rank-sum test. Categorical variables were presented as counts with percentages and compared using the χ2 or Fisher exact test, where indicated. To reduce the potential for imbalance in baseline covariates, propensity score matching was used to control for differences in patients’ baseline characteristics. Each patient who underwent LAA closure was matched to a patient who underwent no LAA closure on the basis of his or her propensity score without replacement, using the greedy matching protocol with a fixed caliper width of 0.020. After matching, we assessed balance within the matched pairs using the standardized differences in covariate means and proportions. The absolute standardized differences for all baseline variables were <10%, indicating acceptable balance. To allow isolation of the effect of the major exposure (LAA closure) on POAF, multivariable logistic regression models were used to estimate odds ratios and 95% confidence intervals (CIs) for the association of LAA closure relative to POAF, with adjustment to control for simultaneous and interactive effects of potential residual confounders. Model selection was conducted using backward selection procedure with a retention criterion of P<0.05 to identify predictors of each outcome. All baseline covariates, with the exception of LAA closure, were entered into the model, and then LAA closure was added into the final model. We used Cox proportional hazards regression models to test for the association of LAA closure with the hazard of ischemic stroke and, separately, long-term mortality, and to estimate the corresponding relative hazards. This was done both with and without adjusting for other variables (univariable and multivariable). Cumulative stroke- and mortality-free event rates as a function over time were obtained by the Kaplan-Meier method, and event-free survival curves were compared using the log-rank test. The assumption of proportional hazard for the final models was checked with the use of scaled Schoenfeld residuals. For associations, we used a 2-sided test of significance at the P<0.05 level. All analyses were performed using SAS version 9.4M3 (SAS Institute Inc) and R v3.1 (R Development Core Team, 2016).
RESULTS
Baseline Characteristics
The baseline characteristics and surgical procedure details of the 9792 patients enrolled are shown in Table 1. The mean (standard deviation) age of the study cohort was 65.5 (13.7) years and 68.1% were male. LAA closure was performed by excision in 11 patients (2.3%) and by ligation (oversewing or stapling) in 458 patients (97.7%) during isolated CABG (4%) and valve or combined CABG+valve or other (96%) surgery.
Table 1.
Baseline Characteristics of the 9792 Study Patients and Propensity Score Matching
| Entire Cohort | Propensity Matched Cohort | |||||
|---|---|---|---|---|---|---|
| No LAAc | LAAc | P Value | No LAAc | LAAc | P Value | |
| N | 9323 | 469 | 461 | 461 | ||
| Demographics | ||||||
| Age, y | 65.6±13.7 | 67.6±12.8 | 0.002 | 67.6±13.5 | 67.4±12.7 | 0.68 |
| Sex | 0.001 | 0.54 | ||||
| Female, n(%) | 2940 (32) | 181 (39) | 1269 (33) | 178 (39) | ||
| Male, n(%) | 6383 (68) | 288 (61) | 292 (63) | 283 (61) | ||
| BMI, mean, kg/m2 | 28.8±5.7 | 27.1±5.6 | <0.001 | 27.2±5.6 | 27.2±5.7 | 0.85 |
| Creatinine, mg/dL | 1.27±0.59 | 1.25±0.52 | 0.52 | 1.28±0.57 | 1.25±0.53 | 0.71 |
| LVEF, mean±SD | 56.5±13.8 | 58.4± 12.3 | 0.01 | 58.3±13.6 | 58.4±12.4 | 0.60 |
| History variables, n(%) | ||||||
| Hypertension | 6293 (67) | 275 (59) | <0.001 | 279 (61) | 270 (59) | 0.55 |
| Diabetes mellitus | 2150 (23) | 65 (14) | <0.001 | 76 (16) | 62 (13) | 0.20 |
| Dyslipidemia | 6818 (73) | 275 (59) | <0.001 | 277 (60) | 270 (59) | 0.64 |
| History of AF | 1334 (14) | 225 (48) | <0.001 | 207 (45) | 217 (47) | 0.51 |
| Prior stroke | 587 (6) | 33 (7) | 0.52 | 32 (7) | 33 (7) | 0.90 |
| Previous MI | 2295 (25) | 55 (12) | <0.001 | 58 (13) | 54 (12) | 0.69 |
| CHF | 1617 (17) | 131 (28) | <0.001 | 134 (29) | 123 (27) | 0.42 |
| Smoker | 5469 (59) | 223 (48) | <0.001 | 210 (46) | 220 (48) | 0.51 |
| Peripheral arterial disease | 1245 (13) | 34 (7) | <0.001 | 25 (5) | 34 (7) | 0.23 |
| Chronic lung disease | 1338 (14) | 70 (15) | 0.73 | 62 (13) | 66 (14) | 0.70 |
| Prior surgery | 1470 (16) | 35 (7) | <0.001 | 35 (8) | 35 (8) | 1.00 |
| Preoperative medications; n (%) | ||||||
| β-Blocker | 5562 (60) | 218 (46) | <0.001 | 201 (44) | 216 (47) | 0.32 |
| Antiarrhythmics | 523 (6) | 37 (8) | 0.04 | 41 (9) | 36 (8) | 0.55 |
| Calcium channel blocker | 2083 (22) | 90 (19) | 0.11 | 89 (19) | 88 (19) | 0.93 |
| Statin | 4740 (51) | 141 (30) | <0.001 | 222 (48) | 211 (46) | 0.47 |
| ACE-I or AR8 | 3942 (42) | 217 (46) | 0.07 | 141 (31) | 139 (30) | 0.89 |
| Digoxin, n (%) | 865 (9) | 111 (24) | <0.001 | 102 (22) | 105 (23) | 0.81 |
| Surgical data | ||||||
| Isolated CABG n (%) | 3922 (42) | 21 (4) | <0.001 | 10 (2) | 21 (5) | 0.05 |
| Aortic valve surgery (any, n (%)) | 2399 (26) | 54 (12) | 0.002 | 51 (11) | 54 (12) | 0.76 |
| Mitral valve surgery (any), n (%) | 1226 (13) | 295 (63) | <0.001 | 287 (62) | 287 (62) | 1.00 |
| Tricuspid valve surgery (any), n (%) | 147 (2) | 9 (2) | 0.56 | 14 (3) | 9 (2) | 0.29 |
| Pulmonary valve surgery (any), n (%) | 9 (0.1) | 0 (0.0) | 0.50 | 0 (0.0) | 0 (0.0) | – |
| Combined CABG+valve or other, n (%) | 1620 (17) | 90 (19) | 0.31 | 99 (21) | 90 (20) | 0.46 |
| Perfusion time, min | 79.1±47.9 | 79.1±39.6 | 0.51 | 74.3±43.3 | 79.2±39.7 | 0.08 |
ACE-I indicates angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CHF, congestive heart failure; LAAc, left atrial appendage closure; LVEF, left ventricular ejection fraction; and MI, myocardial infraction.
Matched Cohort
Propensity score matching matched 461 patients 1:1 between LAA closure (n=469) and no LAA closure (n=9323) patients based on similar propensity scores. Specific components used to estimate the propensity score along with a description of their distributions are shown in Table 1. Baseline characteristics of propensity-matched pairs stratified by LAA closure exposure status were almost identical. After matching, the LAA closure group principally comprised patients who underwent LAA occlusion by ligation or stapling (n=457; 99.1%).
Surgical Complications
Matched Population
After matching, apart from hospital length of stay (7 [6–11] versus 6 [6–10] days; P=0.047) particularly in association with POAF versus no POAF (median 8 [6–13] days versus 7 [5–10] days, P<0.001), the rate of postoperative complications, including the incidence of postoperative surgical reexploration for bleeding (3% versus 4%; P=0.72), pneumonia (3% versus 3%; P=1.00), acute renal failure (4% versus 4%; P=0.87), did not differ between patients who had LAA closure and those who did not.
Early POAF
Among the 9792 subjects in the study, 3299 (33.7%) developed early POAF during 30-day follow-up. In the propensity-matched cohort, the overall incidence of POAF was 53.9%.
Matched Population
The rate of early POAF was 68.6% among the matched patients who underwent LAA closure versus 31.9% for those who did not undergo LAA closure; P<0.001. Patients who underwent LAA closure were more likely to develop POAF than those who did not undergo the procedure (unadjusted odds ratio, 3.37; 95% CI, 2.57–4.42) (Table 2, right). After adjusting for a large pool of potential confounders, multivariable logistic regression analysis showed that LAA closure was independently associated with an increased risk of POAF after cardiac surgery (odds ratio, 3.88; 95% CI, 2.89–5.20). Additional risk factors were age and prior history of AF. Antiarrhythmic drug use was independently protective against POAF (Table 3, right).
Table 2.
Univariable Logistic Regression Analysis for Predicting Early Postoperative Atrial Fibrillation After LAA Closure
| Covariates | Unmatched Cohort | Propensity-Matched Cohort | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Demographics | ||||
| Age | 1.05 (1.04–1.05) | <0.001 | 1.05 (1.03–1.06) | <0.001 |
| Female, sex | 1.01 (0.92–1.10) | 0.91 | 1.23 (0.94–1.60) | 0.14 |
| BMI | 1.00 (0.99, 1.01) | 0.77 | 1.01 (0.98–1.03) | 0.69 |
| Creatinine | 1.08 (1.01–1.16) | 0.03 | 1.10 (0.85–1.39) | 0.50 |
| LVEF | 0.99 (0.99–1.00) | 0.01 | 0.99 (0.98–1.00) | 0.20 |
| History variables | ||||
| Hypertension | 1.26 (1.15–1.37) | <0.001 | 1.39 (1.07–1.81) | 0.02 |
| Diabetes mellitus | 0.96 (0.87–1.07) | 0.47 | 0.99 (0.69–1.42) | 0.94 |
| Dyslipidemia | 1.02 (0.93–1.12) | 0.73 | 1.04 (0.80–1.35) | 0.77 |
| History of AF | 1.71 (1.53–1.91) | <0.001 | 2.38 (1.82–3.11) | <0.001 |
| Prior stroke | 1.24 (1.04–1.46) | 0.01 | 1.74 (1.02–2.95) | 0.04 |
| Previous MI | 1.09 (0.99–1.20) | 0.09 | 1.21 (0.81–1.81) | 0.35 |
| Congestive heart failure | 1.40 (1.25–1.55) | <0.001 | 1.20 (0.90–1.61) | 0.21 |
| Smoker | 1.01 (0.93–1.10) | 0.85 | 1.16 (0.89–1.50) | 0.28 |
| Peripheral arterial disease | 0.98 (0.87–1.11) | 0.76 | 1.18 (0.69–2.00) | 0.55 |
| Chronic lung disease | 1.22 (1.09–1.38) | 0.001 | 1.20 (0.82–1.75) | 0.34 |
| Prior surgery | 0.77 (0.69–0.87) | <0.001 | 0.90 (0.55–1.46) | 0.66 |
| Preoperative medications | ||||
| β-Blocker | 1.09 (1.00–1.19) | 0.05 | 1.33 (1.03–1.73) | 0.03 |
| Antiarrhythmics | 0.93 (0.77–1.11) | 0.42 | 0.58 (0.36–0.93) | 0.02 |
| Calcium channel blocker | 1.16 (1.05–1.28) | 0.003 | 1.52 (1.09–2.13) | 0.01 |
| Statin | 1.16 (1.06–1.26) | 0.001 | 1.08 (0.84–1.41) | 0.54 |
| ACE-I or ARB | 0.98 (0.90–1.06) | 0.57 | 1.16 (0.87–1.54) | 0.31 |
| Digoxin | 1.32 (1.15–1.51) | <0.001 | 1.24 (0.91–1.69} | 0.18 |
| Surgical data | ||||
| LAA closure | 4.62 (3.79–5.64) | <0.001 | 3.37 (2.57–4.42) | <0.001 |
| Isolated CABG | 0.78 (0.71–0.85) | <0.001 | 2.89 (1.18–4.20) | 0.01 |
| Aortic valve surgery | 1.22 (1.11–1.34) | <0.001 | 1.16 (0.77–1.75) | 0.48 |
| Mitral valve surgery | 1.55 (1.39–1.74) | <0.001 | 0.84 (0.64–1.10) | 0.20 |
| Tricuspid valve surgery | 0.75 (0.52–1.06) | 0.10 | 1.11 (0.48–2.57) | 0.80 |
| Pulmonary valve surgery | 0.56 (0.12–2.71) | 0.47 | – | – |
| Combined CABG+valve or other | 0.79 (0.70–0.88) | <0.001 | 0.95 (0.69–1.31) | 0.76 |
| Perfusion time, min | 1.002 (1.001–1.003) | <0.001 | 1.001 (0.998–1.005) | 0.38 |
ACE-I indicates angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CI, confidence interval; LAA, left atrial appendage; LVEF, left ventricular ejection fraction; MI, myocardial infarction; and OR, odds ratio.
Table 3.
Backward Multivariable Logistic Regression Model for Predicting Postoperative Atrial Fibrillation After LAA Closure
| Covariates | Unmatched Cohort | Propensity-Matched Cohort | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| LAA closure | 3.73 (3.00–4.64) | <0.001 | 3.88 (2.89–5.20) | <0.001 |
| Age | 1.05 (1.04–1.05) | <0.001 | 1.04 (1.03–1.06) | <0.001 |
| History of AF | 1.04 (1.04–1.05) | <0.001 | 2.11 (1.55–2.88) | <0.001 |
| β-Blocker | 1.19 (1.08–1.31) | 0.001 | ||
| Antiarrhythmics | 0.42 (0.25–0.71) | 0.001 | ||
| Aortic valve surgery | 1.40 (1.25–1.57) | <0.001 | ||
| Mitral valve surgery | 1.74 (1.51–2.00) | <0.001 | ||
| Combined CABG + valve or other | 1.30 (1.13–1.50) | <0.001 | ||
| C-statistic | 0.68 | 0.75 | ||
AF indicates atrial fibrillation; CABG, coronary artery bypass grafting; CI, confidence interval; LAA, left atrial appendage; and OR, odds ratio.
Ischemic Stroke
Matched Population
During a median follow-up of 9.1 years (maximum, 14.6 years), 65 patients (7.1%) developed ischemic stroke. Forty percent (n=26) of the stroke events occurred in the first year, and 12% (n=8) occurred in the first 30 days after surgery. The rate of stroke in the first 30 days postoperatively was similar among patients who underwent LAA closure and those who did not (0.9% versus 1.0%, P=0.22). Likewise, the 1- and 5-year rates of stroke were not different between patients who underwent LAA closure and those who did not (2.7% versus 4.6%, P=0.17) and (9.6% versus 10.7%; P=0.87), respectively. Patients who underwent LAA closure were as likely to develop stroke as those who did not undergo LAA closure (unadjusted hazard ratio, 1.08; 95% CI, 0.74–1.60) (Table 4). In stepwise multivariable proportional hazards regression analysis, after controlling for a large pool of potential confounders, LAA closure did not influence the risk of ischemic stroke after cardiac surgery (hazard ratio, 1.07; 95% CI, 0.72–1.58) (Table 5). Kaplan-Meier analysis showed no difference in the probability of stroke-free survival during follow-up with LAA closure versus no LAA closure (Figure 1).
Table 4.
Univariable Cox Regression Analysis for Predicting Stroke After LAA Closure
| Covariates | Unmatched Cohort | Propensity-Matched Cohort | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P Value | |
| Demographics | ||||
| Age | 1.02 (1.01–1.02) | <0.001 | 1.00 (0.99–1.02) | 0.76 |
| Female, sex | 1.12 (1.00–1.26) | 0.06 | 0.78 (0.52–1.17) | 0.23 |
| BMI | 0.99 (0.98–1.00) | 0.02 | 1.00 (0.96–1.04) | 0.89 |
| Creatinine | 1.12 (1.03–1.21) | 0.005 | 1.14 (0.86–1.50) | 0.37 |
| LVEF | 1.00 (0.99–1.00) | 0.21 | 1.01 (1.00–1.03) | 0.11 |
| History variables | ||||
| Hypertension | 1.41 (1.24–1.61) | <0.001 | 1.19 (0.80–1.77) | 0.40 |
| Diabetes mellitus | 1.21 (1.07–1.38) | 0.003 | 0.93 (0.54–1.60) | 0.78 |
| Dyslipidemia | 1.22 (1.06–1.40) | 0.005 | 1.05 (0.70–1.56) | 0.82 |
| History of AF | 1.14 (0.98–1.32) | 0.09 | 1.25 (0.85–1.83) | 0.27 |
| Prior stroke | 3.51 (3.01–4.08) | <0.001 | 3.91 (2.37–6.44) | <0.001 |
| Previous MI | 1.31 (1.16–1.48) | <0.001 | 0.92 (0.49–1.71) | 0.78 |
| Congestive heart failure | 1.08 (0.93–1.25) | 0.32 | 0.69 (0.43–1.12) | 0.13 |
| Smoker | 1.00 (0.89–1.12) | 0.98 | 1.07 (0.73–1.58) | 0.73 |
| Peripheral arterial disease | 1.58 (1.37–1.83) | <0.001 | 1.28 (0.65–2.55) | 0.47 |
| Chronic lung disease | 0.99 (0.84–1.16) | 0.90 | 0.60 (0.30–1.19) | 0.14 |
| Prior surgery | 1.16 (0.99–1.35) | 0.06 | 0.88 (0.41–1.91) | 0.75 |
| Preoperative medications | ||||
| β-Blocker | 1.13 (1.00–1.27) | 0.04 | 0.73 (0.49–109) | 0.12 |
| Antiarrhythmics | 1.02 (0.79–1.31) | 0.91 | 0.88 (0.44–1.73) | 0.70 |
| Calcium channel blocker | 1.30 (1.14–1.48) | <0.001 | 1.32 (0.83–2.11) | 0.24 |
| Statin | 1.26 (1.13–1.41) | <0.001 | 1.22 (0.83–1.80) | 0.31 |
| ACE-I or ARB | 1.19 (1.06–1.33) | 0.003 | 1.37 (0.91–2.04) | 0.13 |
| Digoxin | 1.14 (0.95–1.36) | 0.17 | 1.02 (0.64–1.63) | 0.93 |
| Surgical data | ||||
| LAA closure | 0.99 (0.75–1.29) | 0.93 | 1.08 (0.74–1.60) | 0.69 |
| Isolated CABG | 1.11 (0.99–1.24) | 0.08 | 1.95 (0.85–4.47) | 0.12 |
| Aortic valve surgery | 1.03 (0.91–1.17) | 0.61 | 0.83 (0.45–1.51) | 0.54 |
| Mitral valve surgery | 0.91 (0.77–1.08) | 0.27 | 1.18 (0.78–1.77) | 0.43 |
| Tricuspid valve surgery | 0.30 (0.13–0.73) | 0.008 | 0.33 (0.05–2.34) | 0.27 |
| Pulmonary valve surgery | 0.36 (0.12–1.10) | 0.07 | – | – |
| Combined CABG+valve or other | 0.92 (0.78–1.09) | 0.33 | 0.67 (0.37–1.19) | 0.17 |
| Perfusion time, min | 1.000 (0.999–1.001) | 0.81 | 0.999 (0.994–1.004) | 0.64 |
ACE-I indicates angiotensin-converting enzyme inhibitor: AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CI, confidence interval; HR, hazard ratio; LAA, left atrial appendage; LVEF, left ventricular ejection fraction; and MI, myocardial infarction.
Table 5.
Backward Multivariable Cox Proportional Hazard Model for Predicting Stroke After LAA Closure
| Covariates | Unmatched Cohort | Propensity-Matched Cohort | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| LAA closure | 1.02 (0.78–1.34) | 0.89 | 1.07 (0.72–1.58) | 0.74 |
| Age | 1.01 (1.01–1.02) | <0.001 | ||
| Prior stroke | 3.19 (2.73–3.71) | <0.001 | 3.77 (2.28–6.23) | <0.001 |
| Previous MI | 1.17 (1.03–1.32) | 0.02 | ||
| Peripheral arterial disease | 1.33 (1.15–1.54) | <0.001 | ||
| Calcium channel blocker | 1.18 (1.04–1.34) | 0.01 | ||
| ACE-I or ARB | 1.16 (1.03–1.30) | 0.01 | ||
| C-Statistic | 0.62 | 0.58 | ||
ACE-I Indicates angiotensin-converting enzyme inhibitor AFB, angiotensin receptor blocker; CI, confidence interval; LAA, left atrial appendage; MI, myocardial infraction; and OR, odds ratio.
Figure 1.
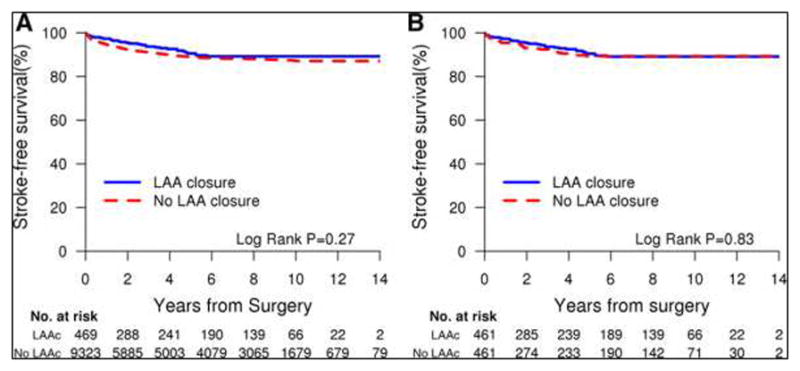
Kaplan-Meier analysis showing freedom from ischemic stroke in LAA closure group versus no LAA closure group.A, The survival curves show a significant difference in freedom from stroke in the group with LAA closure versus the group without LAA closure. B, Propensity score-adjusted Kaplan-Meier survival curves based on proportional hazard assumptions for patients who had LAA closure and those who did not have LAA closure show no significant difference in cumulative incidence of stroke between the 2 groups. LAA indicates left atrial appendage.
Long-Term Mortality
Matched Population
The 30-day mortality rate in the matched cohort was 3.9%, and did not differ between patients who underwent LAA closure and those who did not (2.5% versus 5.2%, P=0.12). During a median follow-up of 9.1 years (maximum, 14.6 years), 395 patients (43%) died. The mortality rate at the end of 5 years of follow-up among patients who underwent LAA closure in comparison with those who did not was 27.2% versus 26.6%, P=87. Patients who underwent LAA closure were as likely to die as those who did not undergo LAA closure (unadjusted hazard ratio, 0.92; 95% CI, 0.76–1.13) (Table 6). In multivariable proportional hazards regression analysis (Table 7), after controlling for a large pool of potential confounders, LAA closure did not influence the risk of death after cardiac surgery (hazard ratio, 0.92; 95% CI, 0.75–1.13). Kaplan-Meier analysis showed no excess mortality associated with LAA closure (Figure 2).
Table 6.
Univariable Cox Regression Analysis for Predicting Long-Term Mortality
| Covariates | Unmatched Cohort | Propensity-Matched cohort | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Demographics | ||||
| Age | 1.05 (1.05–1.05) | <0.001 | 1.07 (1.06–1.09) | <0.001 |
| Female, sex | 1.22 (1.15–1.30) | <0.001 | 1.14 (0.93–1.39) | 0.21 |
| BMI | 0.99 (0.98–0.99) | <0.001 | 1.00 (0.99–1.02) | 0.70 |
| Creatinine | 1.39 (1.35–1.43) | <0.001 | 1.43 (1.31–1.57) | <0.001 |
| LVEF | 0.98 (0.98–099) | <0.001 | 0.99 (0.97–0.99) | <0.001 |
| History variables | ||||
| Hypertension | 1.34 (1.25–1.43) | <0.001 | 1.39 (1.12–1.71) | 0.002 |
| Diabetes mellitus | 1.48 (1.38–1.58) | <0.001 | 2.01 (1.58–2.56) | <0.001 |
| Dyslipidemia | 0.78 (0.73–0.83) | <0.001 | 0.96 (0.78–1.18) | 0.70 |
| History of AF | 1.96 (1.82–2.10) | <0.001 | 1.84 (1.51–2.25) | <0.001 |
| Prior stroke | 1.64 (1.47–1.82) | <0.001 | 1.72 (1.22–2.44) | 0.002 |
| Previous MI | 1.33 (1.24–1.42) | <0.001 | 1.74 (1.33–2.28) | <0.001 |
| Congestive heart failure | 2.30 (2.15–2.46) | <0.001 | 2.73 (2.22–3.35) | <0.001 |
| Smoker | 1.14 (1.07–1.21) | <0.001 | 1.09 (0.89–1.33) | 0.41 |
| Peripheral arterial disease | 1.72 (1.59–1.86} | <0.001 | 1.74 (1.27–2.39) | 0.001 |
| Chronic lung disease | 1.90 (1.77–2.05) | <0.001 | 2.04 (1.59–2.62) | <0.001 |
| Prior surgery | 1.73 (1.61–1.87) | <0.001 | 1.75 (1.27–2.42) | 0.001 |
| Preoperative medications | ||||
| β-Blocker | 0.97 (0.91–1.03) | 0.30 | 1.09 (0.89–1.33) | 0.42 |
| Antiarrhythmics | 1.53 (1.36–1.72) | <0.001 | 0.95 (0.67–1.33) | 0.75 |
| Calcium channel blocker | 1.31 (1.22–1.40) | <0.001 | 1.13 (0.88–1.45) | 0.33 |
| Statin | 1.19 (1.12–1.26) | <0.001 | 1.16 (0.95–1.42) | 0.14 |
| ACE-I or ARB | 0.87 (0.82–0.92) | <0.001 | 0.96 (0.77–1.20) | 0.74 |
| Digoxin | 1.89 (1.74–2.06) | <0.001 | 1.57 (1.26–1.96) | <0.001 |
| Surgical data | ||||
| LAA closure | 1.12 (0.97–1.29) | 0.13 | 0.92 (0.76–1.13) | 0.43 |
| Isolated CABG | 0.71 (0.66–0.75) | <0.001 | 0.73 (0.41–1.30) | 0.28 |
| Aortic valve surgery | 1.15 (1.08–1.23) | <0.001 | 1.29 (0.99–1.68) | 0.06 |
| Mitral valve surgery | 0.90 (0.82–0.98) | 0.02 | 0.54 (0.44–0.66) | <0.001 |
| Tricuspid valve surgery | 2.09 (1.70–2.58) | <0.001 | 1.35 (0.78–2.34) | 0.29 |
| Pulmonary valve surgery | 0.78 (0.29–2.08) | 0.62 | – | – |
| Combined CABG+valve or other | 1.60 (1.48–1.73) | <0.001 | 2.09 (1.66–2.63) | <0.001 |
| Perfusion time, min | 1.003 (1.003–1.004) | <0.001 | 1.007 (1.005–1.009) | <0.001 |
ACE-I indicates angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CI, confidence interval; HR, hazard ratio; LAA. left atrial appendage; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Table 7.
Backward Multivariable Cox Proportional Hazard Model for Predicting Long-Term Mortality
| Covariates | Unmatched Cohort | Propensity-Matched Cohort | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| LAA closure | 0.94 (0.81–1.10) | 0.45 | 0.92 (0.75–1.13) | 0.43 |
| Age | 1.05 (1.04–1.05) | <0.001 | 1.07 (1.06–1.08) | <0.001 |
| Female, sex | 1.23 (1.15–1.32) | <0.001 | ||
| Creatinine | 1.33 (1.28–1.37) | <0.001 | 1.46 (1.29–1.65) | <0.001 |
| LVEF | 0.99 (0.98–0.99) | <0.001 | 0.99 (0.99–1.00) | 0.04 |
| Diabetes mellitus | 1.38 (1.28–1.47) | <0.001 | 1.49 (1.16–1.92) | 0.002 |
| Dyslipidemia | 0.79 (0.73–0.86) | <0.001 | ||
| History of AF | 1.17 (1.08–1.27) | <0.001 | ||
| Prior stroke | 1.36 (1.22–1.52) | <0.001 | ||
| Previous MI | 1.13 (1.05–1.21) | 0.002 | ||
| Congestive heart failure | 1.35 (1.25–1.46) | <0.001 | 1.71 (1.36–2.15) | <0.001 |
| Smoker | 1.23 (1.15–1.32) | <0.001 | ||
| Peripheral arterial disease | 1.40 (1.29–1.51) | <0.001 | ||
| Chronic lung disease | 1.47 (1.36–1.59) | <0.001 | 1.61 (1.25–2.06) | <0.001 |
| Prior surgery | 1.36 (1.26–1.47) | <0.001 | 1.44 (1.04–1.99) | 0.03 |
| Calcium channel blocker | 1.09 (1.02–1.17) | 0.01 | ||
| Statin | 0.93 (0.86–1.00) | 0.04 | ||
| Digoxin | 1.27 (1.16–1.40) | <0.001 | ||
| Aortic valve surgery | 1.17 (1.08–1.26) | <0.001 | ||
| Tricuspid valve surgery | 1.86 (1.49–2.32) | <0.001 | ||
| Combined CABG+valve or other | 1.67 (1.53–1.83) | <0.001 | 1.64 (1.29–2.07) | <0.001 |
| Perfusion time, min | 1.002 (1.002–1.003) | <0.001 | 1.005 (1.003–1.008) | <0.001 |
| C-Statistic | 0.73 | 0.76 | ||
AF, atrial fibrillation; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CI, confidence interval; HR, hazard ratio; LAA, left atrial appendage; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Figure 2.
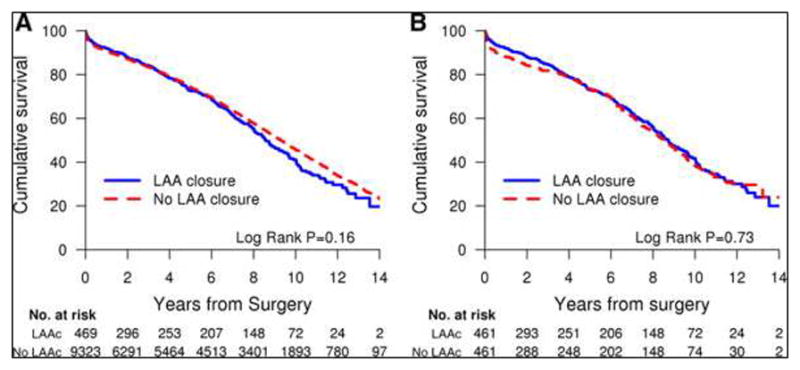
Kaplan-Meier survival curves of patients who had LAA closure during cardiac surgery versus controls. A, The survival curves show no significant difference in survival in the group with LAA closure versus the group without LAA closure. B, Propensity score-adjusted Kaplan-Meier survival curves show no significant difference in survival between patients who had LAA closure and those who did not. LAA indicates left atrial appendage.
DISCUSSION
Main Findings
To our knowledge, this is the largest study to date to investigate the clinical impact of LAA closure during routine non–AF-related cardiac surgery and the first to systematically evaluate the relationship of LAA closure to the development of POAF. In this large propensity score–matched cohort study, we found that LAA closure during routine non–AF-related cardiac surgery was associated with an increased risk of POAF irrespective of the type of surgery performed. LAA closure was associated with a nearly 4-fold increased risk of POAF and did not significantly influence the risk of subsequent ischemic stroke or mortality. This study also confirmed the safety and feasibility of the procedure. In the present study, the rate of surgical complications did not differ between patients who underwent LAA closure and those who did not. These findings strongly underscore the concept that the potential risks and benefits of LAA elimination during routine non–AF-related cardiac surgery should be carefully considered before a procedure is performed.
LAA Closure and POAF
POAF is a common complication after conventional cardiac surgery with cardiopulmonary bypass, with an incidence of ≈30% to 40%.14–16 In the present study, although the overall incidence of POAF in the propensity matched cohort was 53.9%, the rate of POAF among patients who underwent LAA closure was 68.6%, in comparison with 39.3% for patients who did not undergo LAA closure. Johnson and colleagues17 performed prophylactic LAA closure in 437 patients during open heart surgery. Similar to our study, exclusion of the LAA did not seem to add to the risk of surgery and was not associated with bleeding requiring reoperation. Although only 17 patients (4%) had preoperative AF, 149 patients (34.1%) developed POAF. Kim and colleagues18 retrospectively reviewed the charts of 2067 patients after cardiac surgery performed by a single cardiothoracic surgeon, of whom 631 underwent LAA ligation by surgical stapler; these investigators also reported an increase in the rate of POAF through postoperative day 30. These findings suggest the presence of an atrial arrhythmogenic state in the immediate postoperative period following LAA closure in response to atrial distension, increased filling pressure, inflammation, sympathovagal imbalance, favoring POAF.
Mechanism of POAF After LAA Closure
Previous studies have demonstrated an acute increase in left ventricular filling pressure in the early postoperative period after LAA closure because of a corresponding decrease in LA size and resultant LA pressure overload.19 Likewise, we postulate that the development of POAF following LAA closure is likely mediated by an acute decrease in LA compliance following LAA closure, resulting in LA and pulmonary vein stretch. The LAA is a compliant organ with great reservoir capacity that enables the LA to adapt to physiological and pathological conditions, particularly when challenged by acute volume expansion, thus protecting the pulmonary capillary system from encountering escalating pressures.12,13 In an experimental animal study on regional differences in LA distensibility, volume infusion resulted in a significantly greater increase in the reservoir function of the LAA than the body of the LA at each level of LA pressure.11 This suggests that regional differences in LA and LAA distensibility may play an important role in modulating the function of the LA, particularly the LA reservoir function in the presence of LA pressure and volume overload. Exclusion of the LAA profoundly decreases LA reservoir capacity and consequently decreases LA compliance. In the absence of adequate time for adaptation to additional blood volume after LAA occlusion, the LA functions acutely on a steep pressure-volume curve. Consequently, for any given volume, the LA pressure increases disproportionately, leading to LA and pulmonary vein stretch and activation of stretch-mediated ion channels, triggering POAF.20 Indeed, Tabata et al19 demonstrated that clamping of the LAA during cardiac surgery resulted in an elevation of LA pressure as evidenced by a corresponding increase in diastolic transmitral and pulmonary flow velocities. Tse et al21 also demonstrated that an acute increase in atrial pressure was associated with shortening of atrial refractoriness and a propensity for AF. LAA closure may therefore destroy the physiological compensatory mechanism for reservoir function of the LA, acutely inducing a profibrillatory substrate conducive to development of POAF by promoting heterogeneity in atrial refractoriness. Another potential mechanism for the acute increase in POAF is local atrial inflammation and sterile pericarditis.22 Local tissue and endothelial injury and inflammation can also create a prothrombotic milieu, which likely explains, in part, the increased rate of stroke in the immediate postoperative setting after LAA closure.
LAA Closure and Stroke
Although surgical LAA exclusion by either amputation or ligation is considered standard of care for stroke prevention in patients with AF undergoing mitral valve surgery, there are limited data to support the efficacy of this strategy. Theoretically, excluding the LAA from the systemic circulation should reduce the risk of embolization from stagnant flow and thrombus formation within the LAA cavity. However, previous studies have yielded mixed results. Almahameed et al23 published a case series of 136 patients undergoing LAA exclusion during mitral valve surgery and found a high incidence of thromboembolic events, particularly among patients who were not anticoagulated on hospital discharge. Several studies have raised concerns about residual communication between the LAA and LA in up to 50% of patients after surgical LAA ligation,24–28 potentially allowing thrombi to traverse this communication and predispose patients to subsequent thromboembolic sequelae. The single-center, randomized Left Atrial Appendage Occlusion Study of 77 patients undergoing concurrent CABG showed that occlusion of the LAA by suture or stapling without amputation was incomplete in 44% of cases. The perioperative thromboembolic event rate was 2.6%, with no additional stroke events after a mean (standard deviation) follow-up of 13 (7) months.24 This study was limited by its small sample size, and despite randomization, the LAA occlusion group had higher prevalence of AF (17% versus 8%) and prior stroke (17% versus 0%) than controls, suggestive of treatment allocation bias. Our study included a high-risk patient population with a wide spectrum of cardiovascular diseases. Moreover, we performed propensity score–matching analysis to reduce treatment allocation bias and increase precision in estimating LAA closure treatment effects.
LAA Closure and Long-Term Mortality
There are few reports on the impact of surgical LAA closure during routine cardiac surgery on survival. Previous studies in heterogeneous patient populations have reported mixed results on procedure outcomes. In a nonrandomized series of 37 patients with persistent AF who underwent a LAA closure as an adjunct to the Cox Maze III procedure with concomitant cardiac surgery in comparison with 66 controls who had heart surgery alone, Louagie et al.29 reported improved long-term survival at 5 years, although a proportion of patients in the control group also underwent LAA closure. However, no significant survival advantage was found with LAA exclusion versus no LAA exclusion in the nonablated control group (log-rank P=0.07). The study was limited by treatment selection bias and small sample size and heterogeneity in the patient population, making it difficult to draw conclusions about clinical benefit of LAA occlusion. To our knowledge, our study is the first propensity score–matched analysis to demonstrate that survival was not different between patients who underwent surgical exclusion of the LAA during non–AF-related cardiac surgery and those who did not. Our analyses were strengthened by including statistical approaches to address bias, with consistent results.
Clinical Implications
Failure of the fibrillating atrium to contract in patients with AF is believed to result in atrial stretch and dilatation, promoting stasis and thrombosis within the LAA, thus making this structure an attractive target for therapeutic intervention for stroke prevention. However, acutely after LAA closure, LA compliance is decreased, resulting in atrial and pulmonary vein stretch, thereby promoting POAF. In our study, 68.6% of the patients who underwent LAA closure developed POAF. Moreover, our data suggest that LAA ligation does not appear to be a viable stroke prevention strategy for patients with AF, which is in keeping with the literature with regard to the need for prospective studies to evaluate the efficacy and reliability of surgical LAA closure strategies in reducing the risk of stroke in patients with AF. It has been hypothesized that LAA excision may be an AF prevention strategy, but this benefit may be overwhelmed by inflammatory response postoperatively in the short term. Future studies are also needed to evaluate the long-term impact of LAA closure strategies.
It is unclear whether anticoagulant therapy can be safely discontinued without further data showing that the elimination of the LAA from the systemic circulation does indeed reduce the incidence of stroke in patients with AF. Prior studies have shown that the LAA closure is incomplete in>50% of patients,26 which raises the larger question of whether LAA occlusion is worse than no closure, given that reduced blood flow velocity in the LAA may enable more thrombus formation than in the fully patent situation.
In an effort to reduce the risk of postoperative stroke, anticoagulation should not be discontinued in these patients. Moreover, the risk of stroke in patients with AF as outlined in the components of the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, and prior stroke or transient ischemic attack–vascular disease/sex category) score reflect a systemic pathological state.30 Therefore, a local approach that controls only the LAA and not the cause of AF may not be sufficient to address such a complex systemic problem.
Strengths and Limitations
The major strength of this current study is its large sample size. Because of our study’s observational design, our data cannot prove causation, but it directs attention in a more logical direction. LAA closure treatment was not randomly assigned and therefore may have been subject to selection bias and uncontrolled confounding. To reduce this possibility, we took into account a range of pretreatment potential confounders through the use of a comprehensive propensity score model. We cannot rule out possible effects of residual confounding from an imbalance of unmeasured baseline covariates.
For example, off-pump CABG was performed more commonly in the non-LAA closure group, which may have impacted the rate of early POAF in this group. However, only a small proportion (<5%) of patients underwent this procedure. Likewise, aortic manipulation, a known contributing mechanism for postoperative stroke during CABG, was not specifically assessed as a variable in our propensity matching analysis. However, in a retrospective review of 8497 patients treated with isolated on-pump coronary artery bypass grafting from 1993 to 2010 from our surgical database, the rate of stroke was similar with single and partial occlusion aortic clamp techniques.31 Therefore, the results of this retrospective follow-up study should be interpreted with caution. It is also possible that the rate of stroke was underreported, but we anticipate that this problem would impact both groups equally. Another limitation is the lack of information on anticoagulation status during follow-up, which could have affected the rate of stroke. Long-term anticoagulation was left to the discretion of the treating provider, and we did not collect follow-up data on whether patients who have undergone LAA closure were continued on long-term anticoagulation. It is possible that physicians may be less inclined to continue anticoagulation in this patient population. Thus, underuse of anticoagulation within this group may have contributed to increased stroke occurrence. However, a group receiving more anticoagulation may have a higher incidence of major bleed, which would influence all-cause mortality numbers. No formal assessment of the completeness of LAA ligation was undertaken, which could have influenced the rate of POAF, stroke, and ultimately all-cause mortality. This study was also performed at a single center, and therefore its results may not be generalizable to other clinical settings.
CONCLUSIONS
LAA closure during routine non–AF-related cardiac surgery was independently associated with increased risk of early POAF and did not significantly influence the risk of stroke or long-term mortality. Our data raise the question of whether anticoagulant therapy can be safely discontinued after surgical exclusion of the LAA. We were unable to validate positive attributes of LAA exclusion. This study would suggest that a similar evaluation of all LAA occlusion techniques should be undertaken.
Clinical Perspective.
What Is New?
Atrial fibrillation (AF) is a highly prevalent condition and is associated with a 5-fold increase in the risk of stroke and a doubling in mortality.
Prophylactic exclusion of the left atrial appendage (LAA) from the systemic circulation during cardiac surgery has been proposed as a means of reducing the risk of future thromboembolic events in patients with AF, although data are mixed.
In this propensity score–matched cohort study, we demonstrated that LAA closure during non–AF related cardiac surgery was associated with an increased risk of postoperative AF and did not influence the risk of subsequent ischemic stroke or mortality.
What Are the Clinical Implications?
It is unclear whether anticoagulant therapy can be safely discontinued without further data showing that elimination of the LAA from the systemic circulation does indeed reduce the incidence of stroke in patients with AF.
Prior studies have shown that ligation of the LAA is incomplete in >50% of patients, raising the larger questions of whether LAA ligation is worse than no closure and whether surgical LAA occlusion should be performed by excision rather than ligation.
Future studies are needed to evaluate the long-term efficacy of LAA closure strategies in reducing the risk of stroke in patients with AF.
Acknowledgments
SOURCES OF FUNDING
Dr Melduni is supported by National Institutes of Health (NIH) K01 (HL 135288).
Footnotes
DISCLOSURES
None.
References
- 1.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147:1561–1564. [PubMed] [Google Scholar]
- 3.Steger C, Pratter A, Martinek-Bregel M, Avanzini M, Valentin A, Slany J, Stöllberger C. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke registry. Eur Heart J. 2004;25:1734–1740. doi: 10.1016/j.ehj.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 4.Jørgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke. 1996;27:1765–1769. doi: 10.1161/01.str.27.10.1765. [DOI] [PubMed] [Google Scholar]
- 5.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 6.Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;25:452–459. doi: 10.1016/0735-1097(94)00396-8. [DOI] [PubMed] [Google Scholar]
- 7.Ohyama H, Hosomi N, Takahashi T, Mizushige K, Osaka K, Kohno M, Koziol JA. Comparison of magnetic resonance imaging and transesophageal echocardiography in detection of thrombus in the left atrial appendage. Stroke. 2003;34:2436–2439. doi: 10.1161/01.STR.0000090350.73614.0F. [DOI] [PubMed] [Google Scholar]
- 8.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalra L, Yu G, Perez I, Lakhani A, Donaldson N. Prospective cohort study to determine if trial efficacy of anticoagulation for stroke prevention in atrial fibrillation translates into clinical effectiveness. BMJ. 2000;320:1236–1239. doi: 10.1136/bmj.320.7244.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz Ortiz M, Romo Peñas E, Franco Zapata MF, Mesa Rubio D, Anguita Sánchez M, López Granados A, Arizón del Prado JM, Vallés Belsué F. Oral anticoagulation in patients aged 75 years or older with chronic non-valvar atrial fibrillation: effectiveness and safety in daily clinical practice. Heart. 2005;91:1225–1226. doi: 10.1136/hrt.2004.050831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoit BD, Walsh RA. Regional atrial distensibility. Am J Physiol. 1992;262(5 pt 2):H1356–H1360. doi: 10.1152/ajpheart.1992.262.5.H1356. [DOI] [PubMed] [Google Scholar]
- 12.Hoit BD, Shao Y, Tsai LM, Patel R, Gabel M, Walsh RA. Altered left atrial compliance after atrial appendectomy. Influence on left atrial and ventricular filling. Circ Res. 1993;72:167–175. doi: 10.1161/01.res.72.1.167. [DOI] [PubMed] [Google Scholar]
- 13.Davis CA, 3rd, Rembert JC, Greenfield JC., Jr Compliance of left atrium with and without left atrium appendage. Am J Physiol. 1990;259(4 Pt 2):H1006–H1008. doi: 10.1152/ajpheart.1990.259.4.H1006. [DOI] [PubMed] [Google Scholar]
- 14.Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, Collins JJ, Jr, Cohn LH, Burstin HR. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390–397. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 15.Mathew JP, Parks R, Savino JS, Friedman AS, Koch C, Mangano DT, Browner WS. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA. 1996;276:300–306. [PubMed] [Google Scholar]
- 16.Melduni RM, Suri RM, Seward JB, Bailey KR, Ammash NM, Oh JK, Schaff HV, Gersh BJ. Diastolic dysfunction in patients undergoing cardiac surgery: a pathophysiological mechanism underlying the initiation of new-onset post-operative atrial fibrillation. J Am Coll Cardiol. 2011;58:953–961. doi: 10.1016/j.jacc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Johnson WD, Ganjoo AK, Stone CD, Srivyas RC, Howard M. The left atrial appendage: our most lethal human attachment! Surgical implications. Eur J Cardiothorac Surg. 2000;17:718–722. doi: 10.1016/s1010-7940(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 18.Kim R, Baumgartner N, Clements J. Routine left atrial appendage ligation during cardiac surgery may prevent postoperative atrial fibrillation-related cerebrovascular accident. J Thorac Cardiovasc Surg. 2013;145:582–589. doi: 10.1016/j.jtcvs.2012.10.016. discussion 589. [DOI] [PubMed] [Google Scholar]
- 19.Tabata T, Oki T, Yamada H, Iuchi A, Ito S, Hori T, Kitagawa T, Kato I, Kitahata H, Oshita S. Role of left atrial appendage in left atrial reservoir function as evaluated by left atrial appendage clamping during cardiac surgery. Am J Cardiol. 1998;81:327–332. doi: 10.1016/s0002-9149(97)00903-x. [DOI] [PubMed] [Google Scholar]
- 20.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 21.Tse HF, Pelosi F, Oral H, Knight BP, Strickberger SA, Morady F. Effects of simultaneous atrioventricular pacing on atrial refractoriness and atrial fibrillation inducibility: role of atrial mechanoelectrical feedback. J Cardiovasc Electrophysiol. 2001;12:43–50. doi: 10.1046/j.1540-8167.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 22.Ishii Y, Schuessler RB, Gaynor SL, Yamada K, Fu AS, Boineau JP, Damiano RJ., Jr Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111:2881–2888. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 23.Almahameed ST, Khan M, Zuzek RW, Juratli N, Belden WA, Asher CR, Novaro GM, Martin DO, Natale A. Left atrial appendage exclusion and the risk of thromboembolic events following mitral valve surgery. J Cardiovasc Electrophysiol. 2007;18:364–366. doi: 10.1111/j.1540-8167.2006.00755.x. [DOI] [PubMed] [Google Scholar]
- 24.Healey JS, Crystal E, Lamy A, Teoh K, Semelhago L, Hohnloser SH, Cybulsky I, Abouzahr L, Sawchuck C, Carroll S, Morillo C, Kleine P, Chu V, Lonn E, Connolly SJ. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005;150:288–293. doi: 10.1016/j.ahj.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 25.Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J Am Coll Cardiol. 2000;36:468–471. doi: 10.1016/s0735-1097(00)00765-8. [DOI] [PubMed] [Google Scholar]
- 26.Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008;52:924–929. doi: 10.1016/j.jacc.2008.03.067. [DOI] [PubMed] [Google Scholar]
- 27.García-Fernández MA, Pérez-David E, Quiles J, Peralta J, García-Rojas I, Bermejo J, Moreno M, Silva J. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol. 2003;42:1253–1258. doi: 10.1016/s0735-1097(03)00954-9. [DOI] [PubMed] [Google Scholar]
- 28.Aryana A, Singh SK, Singh SM, O’Neill PG, Bowers MR, Allen SL, Lewandowski SL, Vierra EC, d’Avila A. Association between incomplete surgical ligation of left atrial appendage and stroke and systemic embolization. Heart Rhythm. 2015;12:1431–1437. doi: 10.1016/j.hrthm.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Louagie Y, Buche M, Eucher P, Schoevaerdts JC, Gerard M, Jamart J, Blommaert D. Improved patient survival with concomitant Cox Maze III procedure compared with heart surgery alone. Ann Thorac Surg. 2009;87:440–446. doi: 10.1016/j.athoracsur2008.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 31.Araque JC, Greason KL, Li Z, Heins CN, Stulak JM, Daly RC, Joyce LD, Suri RM, Locker C, Schaff HV. On-pump coronary artery bypass graft operation: is one crossclamp application better than two? J Thorac Cardiovasc Surg. 2015;150:145–149. doi: 10.1016/j.jtcvs.2015.04.010. [DOI] [PubMed] [Google Scholar]


