ABSTRACT
As its name suggests, the host receptor herpesvirus entry mediator (HVEM) facilitates herpes simplex virus (HSV) entry through interactions with a viral envelope glycoprotein. HVEM also bridges several signaling networks, binding ligands from both tumor necrosis factor (TNF) and immunoglobulin (Ig) superfamilies with diverse, and often opposing, outcomes. While HVEM was first identified as a viral entry receptor for HSV, it is only recently that HVEM has emerged as an important host factor in immunopathogenesis of ocular HSV type 1 (HSV-1) infection. Surprisingly, HVEM exacerbates disease development in the eye independently of entry. HVEM signaling has been shown to play a variety of roles in modulating immune responses to HSV and other pathogens, and there is increasing evidence that these effects are responsible for HVEM-mediated pathogenesis in the eye. Here, we review the dual branches of HVEM function during HSV infection: entry and immunomodulation. HVEM is broadly expressed; intersects two important immunologic signaling networks; and impacts autoimmunity, infection, and inflammation. We hope that by understanding the complex range of effects mediated by this receptor, we can offer insights applicable to a wide variety of disease states.
KEYWORDS: HVEM, herpes simplex virus, herpes stromal keratitis
INTRODUCTION
Herpes simplex virus 1 (HSV-1) infects the majority of the world's population by adulthood and is responsible for the vast majority of ocular herpesvirus infections (1–3). In humans and mice, HSV-1 establishes lifelong latency in the trigeminal ganglia (TG) (4, 5). Reactivations of HSV-1 in the TG can lead to anterograde movement of the virus along the ophthalmic branch of the trigeminal nerve, resulting in recurrent infection of virtually all the superficial tissues of the eye, including the cornea, conjunctiva, and eyelid (6–8). Ocular herpesvirus infections can lead to epithelial ulceration of the cornea, uveitis, and retinitis but most commonly cause herpes stromal keratitis, or HSK (9). HSK is characterized by chronic inflammation of the corneal stroma, leading to corneal thickening, opacification, scarring, and, potentially, blindness (10, 11). While reactivation accounts for the majority of human disease, most murine studies of HSK model primary infection as mice do not efficiently or reliably reactivate from latency (6).
In the primary murine model of HSK, actively replicating HSV-1 in the cornea is detectable by plaque assay for up to 5 to 6 days postinfection (dpi) (9, 12). Viral replication initiates HSK, as UV-inactivated or replication-deficient mutant HSV fail to induce HSK in BALB/c mice (13). However, HSK is an inflammatory disease, brought about by host infiltrates, particularly CD4+ T cells and polymorphonuclear cells (PMN), that invade the murine cornea several days after replicating virus has been cleared and that persist chronically (14–18). A dizzying array of cytokines, chemokines, and immune cell types have been implicated in the pathogenesis of HSK, the temporal and functional relationships of which have been expertly reviewed elsewhere (11, 15, 18–20). Here, we focus on a single host factor, herpesvirus entry mediator (HVEM, also called tumor necrosis factor [TNF] superfamily member receptor 14 [Tnfrsf14]), originally identified as a viral entry receptor. HVEM has a multitude of roles in mucosal responses to a variety of pathogens (21–25) and has been found to influence HSV pathogenesis in the murine eye during most stages of infection, including entry/acute viral replication, early/innate responses, chronic inflammation, and even viral latency. Results from our laboratory indicate that HVEM functions as a proinflammatory factor in murine herpes keratitis and call into question its importance as an entry receptor during ocular infection. However, the role of HVEM in immunopathogenesis of murine HSK is still emerging, and its importance during human ocular herpesvirus infections remains unexplored.
HVEM IS A VIRAL ENTRY RECEPTOR
Entry mechanics of HSV.
Herpesviruses are large, enveloped, double-stranded DNA viruses (26). Glycoproteins studding the viral envelope are essential for entry into host cells, a complex process requiring at least four separate glycoproteins, including glycoprotein B (gB), gH/gL, and gD (27). Fusion between viral and host membranes requires binding of gD to a host surface receptor (27). Several surface proteins have been identified as gD receptor targets, including nectin-2, HVEM, and nectin-1, although nectin-1 is considered the most biologically relevant entry receptor for HSV (28–31). Nectin-1 is involved in cell-cell adhesion and is a member of the immunoglobulin (Ig) superfamily (32), while HVEM is a TNF receptor superfamily member (28).
The crystal structures of gD bound to both nectin-1 and HVEM have been solved (33, 34). Binding of HVEM or nectin-1 occurs on distinct regions of gD dimers (35), although binding by either ligand triggers similar conformational changes in the structure of the viral glycoprotein, exposing the C-terminal pro-fusion domain (36). HSV entry is a complex process that has been reviewed elsewhere (27), but a highly simplified description of the mechanism is as follows: glycoprotein D interacts with gB trimers bound to a gB-specific receptor and to the heterodimer gH-gL, which bridges receptor binding with gB activation and fusion (27, 37). The sum of these interactions is the insertion of the gB fusion loops into the cellular plasma membrane (or, in some circumstances, into the endosomal membrane) and refolding of the gB trimer into a postfusion form (29). Subsequent mixing of viral and host membranes and the formation of a fusion pore through which the viral capsid and tegument proteins can be deposited complete the entry process (38).
While HVEM and nectin-1 exhibit similar kinetics and affinities of binding to gD in vitro (37, 39), early in vivo studies showed a greater importance for nectin-1 in the pathogenesis of HSV, especially in terms of invasion of and spread throughout the nervous system in intravaginal and intracranial models of HSV-2 infection (40, 41). Remarkably, nectin-1 knockout (KO) mice inoculated with HSV-2 directly into the hippocampus do not develop encephalitis, despite the presence of HVEM in the brain; they also lack demonstrable virus by immunofluorescence (40). Although HVEM is largely dispensable for HSV-2 infection of the brain (40), vagina (41), or eye (42), our laboratory has shown that HVEM promotes HSV-1 pathogenesis specifically in the setting of murine ocular infection (43, 44). In order to understand why this receptor is required for pathogenesis of ocular herpetic infections, both the entry and immunomodulatory functions of HVEM must be explored.
HVEM-mediated entry in the eye.
HVEM expression has been evaluated in a number of ocular tissues. Results of studies of cultured human cell lines by real-time PCR (RT-PCR) and immunofluorescence or flow cytometry indicate that retinal pigment epithelial (RPE) cells (45), corneal fibroblasts (46), trabecular meshwork cells (47), and conjunctival and corneal epithelial cells (48, 49) express HVEM mRNA and membrane-bound protein. Use of HVEM-blocking antibodies (47, 49) or HVEM small interfering RNA (siRNA) knockdown (48) reduces viral entry of some, but not all, of these cell types in vitro, suggesting that HVEM is the entry receptor in use. However, widespread expression of nectin-1 and the sufficiency of this receptor for in vivo infection of the murine cornea and TG preclude the notion that HVEM is the primary receptor in the eye (43, 50–52). Our experiments performed with a well-characterized HSV-1 mutant, HSV-1 (17)gDΔ7-15, which is restricted to nectin-1 entry through targeted deletion of the HVEM-specific binding region of gD (29, 31, 34, 35, 39, 53, 54), confirmed that HVEM is not the primary entry receptor in the cornea: infection with the gDΔ7-15 mutant produces titers, clinical disease, and inflammatory cytokine levels equivalent to those seen with the HVEM-competent virus control (44). Furthermore, recent analysis of whole murine corneas by flow cytometry indicated that HVEM expression on epithelial or endothelial cells is limited in vivo (55).
This is not to say that HVEM is unimportant during ocular HSV-1 infection. Despite the presence of a suitable alternate entry receptor, nectin-1, in the cornea, HSV-1 infection of Tnfrsf14−/− (HVEM KO) mice results in lower viral loads in eye swabs, with subsequent loss of titer in the TG, brain, and periocular skin (POS), and reduced rates of reactivation from the TG (42, 43). Along with this replication defect, HVEM KO mice are protected from systemic clinical symptoms of HSV-1, including lesion development and neurologic morbidity (42, 43), as well as from cornea-specific pathology, including loss of sensitivity to mechanical pressure (55), inflammatory cytokine release, and stromal leukocytic infiltration (44). Entry-independent contributions of HVEM during HSV-1 pathogenesis likely stem from tuning of immune responses by HVEM during inflammation. While we are convinced that HVEM contributes to inflammation and immunopathology during murine HSK, the precise mechanisms underlying this process remain largely unknown. Evidence gleaned from other disease models/organs in which HVEM signaling has been more thoroughly explored provides clues about how HVEM may promote inflammation in the eye.
HVEM ALTERS HOST IMMUNE RESPONSES TO HSV
HVEM signaling in the host.
HVEM, also designated CD270, is a bidirectional receptor that can bind ligands of both the TNF and Ig superfamilies (Fig. 1), producing a wide diversity of outcomes (25). Outside of the eye, HVEM expression is broad: although its expression fluctuates throughout maturation, HVEM is found on most types of leukocytes, including T and B cells, dendritic cells (DCs), natural killer (NK) cells, and myeloid cells (56). HVEM is also highly expressed by nervous tissue and gut and lung epithelia (23, 57).
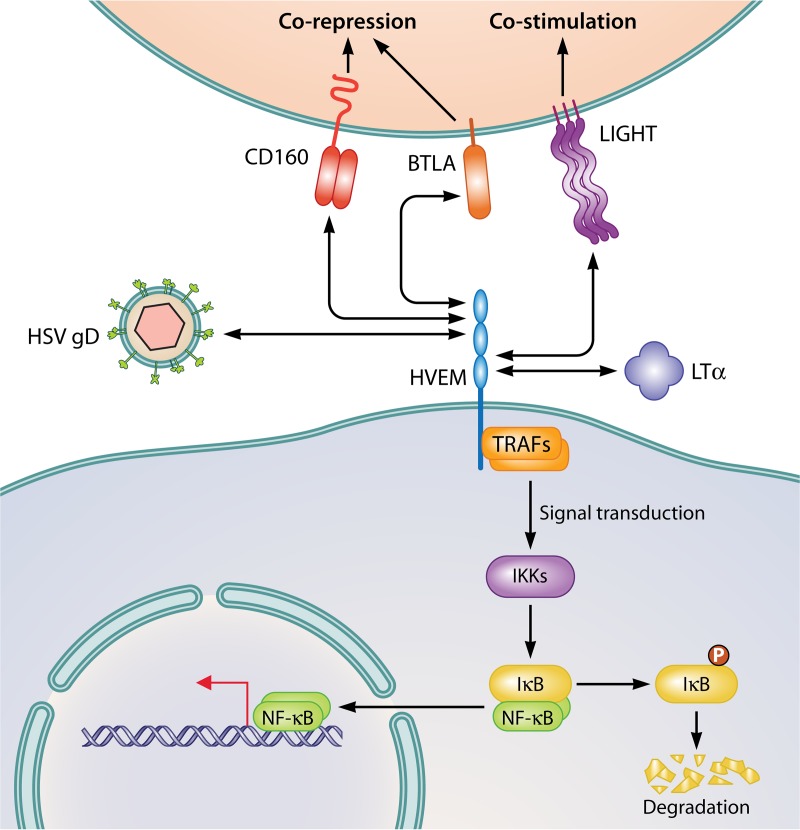
FIG 1.
HVEM signaling is bidirectional. HVEM is a TNF receptor superfamily member that can interact with Ig-like ligands (CD160 and BTLA), TNF ligands (LIGHT and LTα), and an HSV glycoprotein, gD. HVEM is expressed on a broad range of cell types, including T cells, B cells, DCs, NK cells, macrophages, PMN, neurons, and epithelial cells. BTLA and LIGHT are also found on most leukocytes, including B and T cells, granulocytes, NK cells, etc. CD160 is found on subsets of CD4+ and CD8+ T cells, NK cells, and intraepithelial lymphocytes (IELs). BTLA, CD160, and HSV gD bind cysteine-rich domain 1 (CRD1) of HVEM, while LIGHT and soluble LTα bind CRD2 and CRD3. Outcomes can vary from corepressive signals delivered through CD160 and BTLA in trans to costimulatory signals delivered through LIGHT. BTLA and LIGHT can also associate with HVEM in cis, forming heterotrimeric complexes, the conformation most commonly seen on resting T cells. Binding in cis holds HVEM in an inactive state, without NK-κB activation. In contrast, binding in trans of HVEM by any of its ligands leads to the activation of NF-κB signaling through recruitment of TRAF family members by the cytoplasmic portion of HVEM. Subsequent activation of IκB kinase/Iκα kinase (IKKβ/α), phosphorylation and degradation of the NF-κB chaperone IκB, and activation and nuclear translocation of NF-κB result in increased transcription of inflammatory factors and prosurvival signals within the HVEM-expressing cell.
Identified HVEM ligands include LTα (lymphotoxin α), LIGHT (lymphotoxin-related inducible ligand that competes for glycoprotein D binding to HVEM on T cells), BTLA (B and T lymphocyte attenuator), and CD160 (58–60). Immature DCs, monocytes, and activated T cells express membrane-bound or soluble forms of LTα and LIGHT (56). These trimeric TNF family ligands typically enhance activation or differentiation of a variety of immune cell types upon binding cysteine-rich domain 2 (CRD2) and CRD3 of HVEM (61–64). BTLA is an Ig superfamily member found on T and B cells, DCs, and myeloid cells (59). CD160 is a dimeric, glycosylphosphatidylinositol (GPI)-anchored protein with an Ig-like fold that is prototypically expressed by NK cells but is also found on subsets of CD4+ and CD8+ T cells (60). Both BTLA and CD160 communicate a corepressive signal upon binding HVEM CRD1 in most described cases (65–69), although CD160 is known to be activating in some circumstances, i.e., on NK cells, where it is required for interferon gamma (IFN-γ) production (70). However, the outcomes of HVEM signaling differ depending on whether the ligand is soluble or in the membrane-bound form, whether the interaction occurs in cis or in trans, and on the specific identity of the cells involved (25, 60, 71, 72). Viral gD also binds CRD1 and most directly competes with BTLA for HVEM binding, although there is evidence indicating that LIGHT and LTα interactions with HVEM may also be affected by the presence of gD (73). Because the TNF- and Ig-ligand domains occur on different faces of the receptor, combinations of HVEM and its ligands may result in ternary complexes (64, 65, 74–76).
As a receptor, HVEM engagement by LIGHT, CD160, BTLA, or viral gD recruits members of the TNF receptor-associated factor (TRAF) family to the cytoplasmic tail of HVEM and activates prosurvival nuclear factor κB (NF-κB) signaling (77, 78). NF-κB is a transcription factor normally sequestered in the cytoplasm by proteins, including IκB kinase/Iκα kinase (IKKβ/α); upon their degradation, NF-κB translocates to the nucleus to initiate transcription of DNA, cytokine production, and cell survival (79). HVEM activates prosurvival signaling through NF-κB even when its binding partner is a corepressive ligand, such as BTLA or CD160. For example, T cells receiving cosuppressive signals through BTLA cease proliferating, while Btla−/− T cells stimulated by soluble BTLA-Fc induce NF-κB signaling via HVEM and exhibit increased survival (78). NF-κB induced by the binding of gD to HVEM has antiapoptotic effects in vitro, and loss of NF-κB activation (and nuclear translocation), which is sensitive to blocking with anti-gD antibodies, reduces viral yield 80 to 90% and increases apoptosis (80–84). HSV may benefit from HVEM activation through NF-κB-mediated prosurvival signals that prevent apoptosis of infected cells (80, 83).
Understanding the intricacies of the HVEM signaling network is challenging given the number of possible ligands involved, the lack of predictability with respect to whether an interaction will ultimately be pro- or anti-inflammatory, and the possibility of competing, opposing signals arising from the same receptor-ligand interaction. There is increasing evidence that HVEM modulates aspects of the innate and adaptive immune responses to HSV in the murine cornea, although the clinical significance of HVEM signaling in human patients with HSK remains to be elucidated.
HVEM and innate immune responses.
One of the first indications that herpesviruses may alter early innate immune responses to infection via HVEM came from the murine vaginal model of genital HSV-2 infection. Mice infected with an HVEM-entry null HSV-2 strain (with the Δ7-15 deletion) have significantly higher levels of the cytokine interleukin-6 (IL-6) and chemokines CXCL9, CXCL10, and CCL4 in vaginal washes than do mice infected by HSV-2 that could engage HVEM (85). Infected HVEM KO corneas have decreased levels of several inflammatory cytokines, including IL-6 and CXCL10, compared to infected C57BL/6 (wild-type [WT]) controls early after infection (44). However, unlike in the vaginal model, the induction of these cytokines in the cornea is independent of HVEM-mediated entry of HSV-1 (44). IL-6 and CXCL10 are neutrophil and T cell chemoattractants, respectively, known to promote ocular HSV-1 pathogenesis (19, 86–89), and increased expression of these chemotactic factors may lead to heavier infiltration by myeloid and lymphoid cells in WT versus HVEM KO murine corneas (44). However, siRNA-mediated knockdown of HVEM from cultured telomere-immortalized human corneal epithelial cells decreased production of IFN-γ, MIP-1α (CCL3), and MIP-1β (CCL4) after HSV-1 challenge (90). This discrepancy may represent differences between murine and human cells, or may have arisen because in the in vitro system, HVEM stimulation occurred solely with HSV gD, while in vivo, binding by natural ligand(s) likely takes precedence (44).
The mechanism by which HVEM upregulates cytokine secretion in the murine cornea has not been defined, although it must involve one of the host HVEM ligands as gD binding is not required (44). Elegant studies of innate responses to bacteria in lung and gut mucosal epithelia have found a similar role for host HVEM signaling in the induction of IL-6 and other cytokines (22). In this model, CD160 on innate-like intraepithelial lymphocytes activates HVEM, which is highly expressed by the intestinal epithelium, resulting in NF-κB-mediated Stat3 activation and increased expression of genes and peptides related to epithelial immunity (22, 23). During HSK, the corneal epithelium secretes IL-6 and other cytokines in response to HSV-1 infection (91, 92). We performed experiments using adoptive transfer between WT and HVEM KO mice and found that HVEM on a radiation-resistant cell type(s), such as the corneal epithelium, is required for disease progression, while HVEM on a radiation-sensitive cell type(s), such as circulating immune cells, is dispensable (44). However, we were unable to detect substantial quantities of HVEM on the corneal epithelium (or stroma) in vivo either by IHC or flow cytometry, making it unlikely that these are the source of the HVEM-mediated cytokine production (55).
Flow cytometry of whole corneas from C57BL/6 mice indicates that the majority of HVEM is located on CD11b+ CD11c− Ly6C+ Ly6G− monocyte lineage cells in the acute phase and on PMN and CD4+ T cells in the chronic phase (55). The shift in HVEM-positive (HVEM+) populations likely reflects differences in the cellular composition of the ocular infiltrate over time rather than fluctuations in HVEM expression, although this was not directly tested in our study. Stromal macrophages (and DCs) incompletely turn over with irradiation (93); therefore, these could be the radiation-resistant HVEM+ cell type identified as mediating disease in adoptive-transfer experiments (44). Although other HVEM+ lineages may also contribute to pathogenesis, albeit in a subtler fashion, monocytes/macrophages are particularly intriguing to us in light of these findings.
Along with NK cells, which play an essential role in the regulation of early HSV replication (94), corneal and conjunctival macrophages are required for viral restriction in the first 48 h after infection (95–98). However, control of viral replication comes at a price: macrophages, along with corneal-infiltrating and lymph node-residing DCs (99), promote CD4+ T cell activation during ocular HSV-1 infection (98), although the roles of resident versus recruited macrophages in CD4+ T cell activation are poorly defined in HSK. Even without DCs, chronic-phase HSK (beyond day 7) still develops in HSV-infected mice, potentially through the activation of CD4+ T cells by closely associated corneal macrophages (99). Macrophage-associated cytokines such as MIP-1α and MIP-1β contribute to further corneal infiltration and damage (19). Macrophages and PMN also promote vascularization of the cornea, a required step in HSK development, through secretion of vascular endothelial growth factor receptor (VEGF) and matrix degradation enzymes (100, 101). All of these effects could be influenced by HVEM signaling on macrophages and neutrophils, potentially through HVEM activation operating directly or through interactions with its ligands.
LIGHT binding of HVEM on macrophages and neutrophils provides an activating signal, increasing phagocytic activity and production of inflammatory/antibacterial factors, including nitric oxide (NO), reactive oxygen species (ROS), IL-8, and TNF-α (102), via changes in intracellular calcium sequestration (103). Agonistic binding of HVEM on neutrophils also increases respiratory burst and degranulation, providing a further explanation for the increased bactericidal activity of PMN via HVEM (104). In vivo, secretion of type I interferon from splenic cells, especially macrophages, partially requires HVEM; in HVEM-deficient mice, loss of type I interferons reduces lymphocyte bystander activation and immunopathology after Listeria infection (105).
In contrast, HVEM-BLTA interactions reduce activation of innate cell populations, including macrophages, inflammatory monocytes, and PMN (24, 66, 67). During acute experimental sepsis, HVEM-BTLA interactions on innate populations worsen outcomes with respect to organ injury, bacterial burden, and mortality (106). Although both HVEM and BTLA are expressed on recruited myeloid cells in this model, BTLA-directed corepressive signals explain the finding of reduced myeloid activation and survival more convincingly than HVEM-directed prosurvival/NF-κB signals. Our laboratory is currently investigating the corneal expression of LIGHT and BTLA on resident and infiltrating cells during HSV-1 infection and what role these ligands play in HVEM-mediated disease.
HVEM and adaptive immune responses.
Helper Th1 CD4+ T cells are considered to represent the major immunopathologic cell type in HSK (9, 10, 15). Investigators have repeatedly shown that without functional CD4+ T cells, HSK does not develop (107–109). Corneal CD4+ T cells remain activated in the absence of replicating virus, likely through bystander activation (110), in which CD4+ T cells become nonspecifically activated due to the surrounding inflammatory milieu. Consistent with this hypothesis, CD4+ T cells do not have to be viral antigen specific to cause HSK, although virus-specific CD4+ T cells may initiate the process (110–114). While a variety of other mechanisms have been suggested, including auto-antigen unmasking (115) and viral molecular mimicry (116), these hypotheses fell out of favor after it was shown that the peptides proposed to produce autoreactive CD4+ T cells do not induce HSK in mice and are not recognized by T cells isolated from patients with HSK (114, 117).
Results of adoptive-transfer experiments performed in our laboratory with WT and HVEM KO mice indicate HVEM-mediated pathogenesis occurs when HVEM is present on radiation-resistant cells (44). Because they turn over with radiation and reconstitution, CD4+ T cells are not likely to be the HVEM+ cells responsible for pathology, at least in our adoptive-transfer model. However, interactions between HVEM (on other cell types) and HVEM ligands such as LIGHT, BTLA, or CD160 on CD4+ T cells could contribute to the development of HSK.
BTLA provides a corepressive signal on CD4+ T cells in cis or in trans (60, 66), but the majority of HVEM on naive T cells occurs in cis complexes with BTLA, with or without LIGHT as a part of the complex (118). In the cis complex, BTLA holds HVEM in an inactive state, preventing NF-κB activation (118). Absence of HVEM from CD4+ T cells may make BTLA and LIGHT available to interact with HVEM on other cell types, such as long-lived, resident macrophages, activating NF-κB signaling and inflammatory activity during HSK. This would be consistent with our finding that chimeras lacking HVEM from radiation-sensitive cells developed more severe disease than HVEM KO controls (44). BTLA levels have been reported to increase in the corneas of BALB/c HSV-1-infected mice, although the identity of BTLA+ corneal cells and the effect that endogenous BTLA expression has on pathogenesis were not thoroughly investigated (119). In that study, intravenous treatment with a recombinant BTLA-expressing plasmid prior to and during ocular HSV-1 infection reduced HSK symptoms, the overall number of corneal CD4+ T cells, and the proportion of the cells expressing IFN-γ, although no experiments were performed to determine which cell types absorbed the plasmid and how these effects were mediated (119).
Levels of a specialized set of anti-inflammatory CD4+ T cells, FoxP3+ regulatory T cells (Tregs), reportedly expand during murine HSV-1 infection through gD-HVEM interactions, leading to slightly diminished corneal pathology in HVEM KO corneas late after infection (120). While this instance of decreased HSK symptoms in HVEM KO corneas conflicts with data from a multitude of studies from our laboratory (42–44), differences in viral strain and inoculation dose could produce variability in findings. Consistent with this report, our laboratory found that at 14 dpi, a small but real population of the HVEM+ population was CD4+, although we did not assess FoxP3+ expression in these cells. Due to the complexity of HVEM signaling and to its widespread expression on nearly every leukocytic population implicated in the development of HSK, it would be surprising if only one HVEM-associated cell type or function influenced ocular herpes immunopathogenesis. While we are confident that HVEM-mediated inflammation is the more potent effect, it is plausible that HVEM could have contradictory roles and that HVEM on Tregs may provide some relief during HSK. Further investigation of this discrepancy is required.
While less important for HSK development, CD8+ T cells control viral spread into the nervous system (121, 122) and suppress viral reactivation from the TG (123). The latency state, characterized by suppression of all viral products except the long noncoding RNA latency-associated transcript (LAT), occurs in the TG in humans and mice (124). LAT is not required for latency (125, 126), although LAT(−) viruses establish latency and reactivate less efficiently (127). Most human cases of HSK result from reactivation of a latent infection rather than from primary infection (128). Unfortunately, significant gaps in our understanding of latency and reactivation persist because the vast majority of studies are performed in murine models of primary infection (129). However, it has been established that CD8+ memory T cells infiltrate latently infected TGs in humans and mice, residing in close association with neurons (130, 131). The close association of CD8+ T cells, which typically express a variety of HVEM ligands (69), and neurons of the TG, which express HVEM (132), raises the question of direct effects of HVEM signaling on HSV latency. HVEM KO mice have lower rates of latency and reactivation than WT mice, although the HVEM KO strain also has lower titers in the eye initially, likely leading to decreased seeding of the TG (43, 133). Recently, investigators reported that LAT upregulates HVEM expression in vivo and in vitro, potentially through binding of the HVEM promoter by small noncoding RNAs derived from LAT (133). Increased HVEM expression could alter immune responses to reactivation; alternatively, HVEM-mediated NF-κB activation could enhance survival of neurons undergoing reactivation. Because human disease is mostly caused by reactivation, our laboratory is actively pursuing murine models of recurrent disease (134) to study what contribution HVEM may make to this process.
HVEM SIGNALING IMPACTS A VARIETY OF HUMAN DISEASES, OFFERING TARGETS FOR THERAPY
We propose that, independently of viral entry, HVEM orchestrates an inflammatory response to HSV in the murine cornea that contributes to the extensive immune-mediated damage observed in HSK (Fig. 2). This finding has yet to be validated in human patients, a major limitation of this work. However, HVEM is implicated in a wide range of autoimmune, inflammatory, and infectious processes that impact an astonishing diversity of human syndromes (24). Because of this, the HVEM signaling network is a rich area of research for the discovery of new therapies.
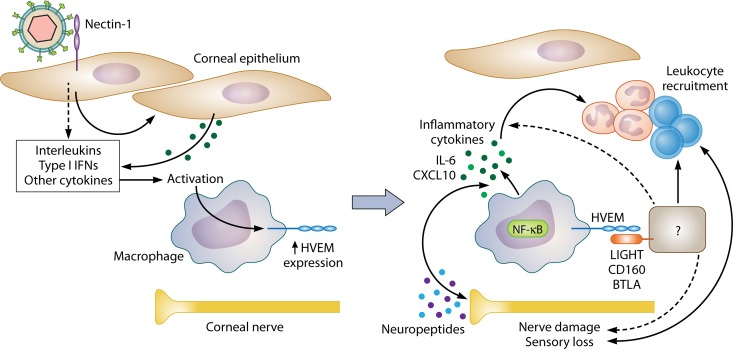
FIG 2.
Proposed mechanism of HVEM pathogenesis during ocular HSV-1 infection. (Left panel) Early after infection, the majority of corneal HVEM is localized to CD11b+ Ly6C+ monocytic lineages rather than epithelial or endothelial cells. We propose that these HVEM+ cells are corneal resident macrophages, as results of adoptive-transfer experiments indicate that radiation-resistant cells are sufficient for HVEM-mediated pathogenesis and that macrophages in the cornea turn over incompletely after irradiation. HSV-1 infects the corneal epithelium, causing secretion of type I interferons and other factors that activate corneal resident macrophages, which could induce HVEM expression on these cells early after infection. (Right panel) Because the gD-HVEM interaction is dispensable for pathogenesis, we hypothesize that HVEM on corneal macrophages interacts with a host ligand on an as-yet-unidentified cell type. This results in HVEM-dependent induction of inflammatory cytokines, loss of corneal sensitivity, and immune cell recruitment. It is not yet clear whether this stems from changes in the HVEM+ cells operating directly, such as through NF-κB activation, or through signaling on the HVEM ligand-bearing cell. Activated macrophages may damage neighboring corneal nerves, worsening inflammation through desiccation of the cornea, or nerves themselves may influence leukocytic recruitment through release of neuropeptides. The two processes likely influence each other, synergizing to create a cycle of chronic inflammation and corneal nerve damage.
Levels of soluble HVEM are elevated in the sera of patients with allergic asthma, rheumatoid arthritis (RA), and atopic dermatitis (135), but HVEM/LIGHT/BTLA signaling and therapeutic opportunities within that network have been most extensively studied in models of graft-versus-host disease (GVHD) (64, 74, 136–139). GVHD is an immunologic syndrome affecting transplant recipients in which engrafted donor T cells attack host tissues, causing rampant damage (140). Investigators have had success in decreasing symptoms of GVHD in mice by targeting both BTLA-HVEM and LIGHT-HVEM interactions with blocking antibodies (137–139). Blockade of LIGHT-HVEM signaling has also been shown to increase the rate of survival of solid allografts, including pancreatic islets and cardiac transplants (141, 142). Recently, lymphoma B cells from patients with mutations in the HVEM gene (TNFRSF14) were found to have increased alloantigen-presenting capacity in comparison to controls, corresponding to higher levels of GVHD in patients undergoing allogeneic hematopoietic stem cell transplantation (143).
In murine experimental autoimmune uveitis (EAU), a model of human autoimmune conditions with ocular manifestations such as Behçet disease and sarcoidosis, HVEM was shown to increase disease severity by inducing pathogenic Th1- and Th17-type T cell responses (144). Similarly to HVEM KOs, LIGHT and BTLA KOs are protected from severe disease during EAU, suggesting that these ligands, in combination with HVEM, promote pathogenesis in EAU (144). If LIGHT and/or BTLA are the ligands involved in HSK as well, targeting HVEM signaling with antibodies or small-molecule inhibitors could produce novel therapies applicable to a variety of ocular inflammatory conditions.
Our laboratory has recently discovered that pathological inflammation during murine HSK can be prevented with treatment with immune-modifying nanoparticles (IMPs). Inflammatory monocytes and other circulating inflammatory cells take up IMPs and subsequently undergo apoptosis in the spleen, with the effect of diminished tissue damage under a variety of inflammatory conditions (145, 146). In our primary murine HSK model, IMP treatment improved survival of BALB/c mice, which are highly susceptible to CNS involvement and mortality. It also improved corneal blink responses and decreased the levels of a variety of myeloid and lymphoid populations in the corneas of C57BL/6 mice during the chronic inflammatory phase (55). Although IMP treatment does not target HVEM signaling specifically, it achieves many of the same outcomes observed in the HVEM KO, likely by functioning downstream of HVEM, preventing HVEM-mediated recruitment of leukocytes to the cornea and the subsequent damage they cause.
CONCLUSIONS AND FUTURE DIRECTIONS
Based on previous findings and our most recent data, we propose that the presence of HVEM on corneal resident macrophages is critical for HSK development, operating either by interacting with HVEM ligands on other cells or through increased cytokine production, perhaps via NF-κB activation (Fig. 2). We hypothesize that the presence of HVEM on these cells leads to increased corneal nerve damage, immune cell recruitment, and overall severity of disease. Because resident macrophages and nerves are physically associated in the peripheral stroma (147), it is possible that HVEM-mediated secretion of damaging cytokines from macrophages hastens corneal nerve damage, decreasing blinking and desiccating the cornea (148). The presence of HVEM on other immune cell types, including CD4+ T cells and PMN, is not likely to be necessary for HSK development, in light of data from adoptive-transfer experiments, but may still be contributory. Resolving pathogenic versus protective functions of HVEM, i.e., on corneal resident macrophages compared to Tregs, is an important next step for the field, as this information is critical for targeted therapy design.
There is some evidence that HVEM contributes to latent infections as well (43, 133). Whether this is physiologically significant and how this effect comes about are intriguing lines of investigation for the future. Murine models of HSK are generally based on primary infection, because, unlike humans, mice do not undergo efficient spontaneous reactivations (129). The absence of data demonstrating how the immune responses that occur during HSK differ between a primary infection and a reactivation from latency represents a significant gap in our knowledge. Other areas in need of further study are the expression and role of the host HVEM ligands during HSV-mediated pathogenesis. Studies performed with the HVEM-entry-null Δ7-15 mutant indicated that the gD-HVEM interaction is not required for the inflammation HVEM causes; therefore, attention must be turned to the HVEM ligands BTLA, CD160, LIGHT, and LTα. To our knowledge, only BTLA expression has ever been examined in the cornea (119); expression of these molecules in the cornea is under active investigation by our laboratory. Beyond informing our mechanistic understanding of HVEM signaling during ocular herpetic infection, this information will also facilitate the testing of antibody or small-molecule therapies targeted to pathological HVEM signaling. Finally, the vast majority of studies reviewed here were performed in murine models, and studies with human tissue are needed to corroborate and expand these findings.
Both HSK signaling and HVEM signaling are complex processes involving nearly every type of leukocyte; consequently, untangling the role the HVEM plays during development of HSK has been and will continue to be a challenge. Advances made in investigation of the molecular signaling mechanisms of HVEM and its ligands will be extremely useful going forward, but the complications of bidirectional signaling interactions, with complementary or contradictory messages being delivered at the same time, remain. HVEM signaling warrants further investigation specifically in the context of immunomodulation in the eye, as our research clearly demonstrates a pathogenic and inflammatory role during ocular HSV infection. With luck, these discoveries will be translatable to new therapies for patients with this blinding condition.
ACKNOWLEDGMENTS
We sincerely thank Sarah Kopp and Nanette Susmarski for their invaluable assistance throughout these experiments; without their help, this work would not have been possible.
National Institutes of Health grants R01EY023977-01A1, CA021776, and EY021306 supported R.L. and R.G.E. R.G.E. was also supported by the Training Program in Vision (T32 EY025202).
REFERENCES
- 1.Roberts CM, Pfister JR, Spear SJ. 2003. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis 30:797–800. doi: 10.1097/01.OLQ.0000092387.58746.C7. [DOI] [PubMed] [Google Scholar]
- 2.Tran T, Druce JD, Catton MC, Kelly H, Birch CJ. 2004. Changing epidemiology of genital herpes simplex virus infection in Melbourne, Australia, between 1980 and 2003. Sex Transm Infect 80:277–279. doi: 10.1136/sti.2004.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liesegang TJ. 1989. Epidemiology of ocular herpes simplex. Natural history in Rochester, Minn, 1950 through 1982. Arch Ophthalmol 107:1160–1165. [DOI] [PubMed] [Google Scholar]
- 4.Baringer JR, Swoveland P. 1973. Recovery of herpes-simplex virus frorm human trigeminal ganglions. N Engl J Med 288:648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- 5.Cohrs RJ, Laguardia JL, Gilden D. 2005. Distribution of latent herpes simplex virus type-1 and varicella zoster virus DNA in human trigeminal ganglia. Virus Genes 31:223–227. doi: 10.1007/s11262-005-1799-5. [DOI] [PubMed] [Google Scholar]
- 6.Margolis TP, Elfman FL, Leib D, Pakpour N, Apakupakul K, Imai Y, Voytek C. 2007. Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. J Virol 81:11069–11074. doi: 10.1128/JVI.00243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roizman B, Whitley RJ. 2013. An inquiry into the molecular basis of HSV latency and reactivation. Annu Rev Microbiol 67:355–374. doi: 10.1146/annurev-micro-092412-155654. [DOI] [PubMed] [Google Scholar]
- 8.Summers BC, Margolis TP, Leib DA. 2001. Herpes simplex virus type 1 corneal infection results in periocular disease by zosteriform spread. J Virol 75:5069–5075. doi: 10.1128/JVI.75.11.5069-5075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswas PS, Rouse BT. 2005. Early events in HSV keratitis–setting the stage for a blinding disease. Microbes Infect 7:799–810. doi: 10.1016/j.micinf.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Streilein J, Reza Dana M, Ksander B. 1997. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunology Today 18:443–449. doi: 10.1016/S0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- 11.Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL. 2013. Herpes keratitis. Prog Retin Eye Res 32:88–101. doi: 10.1016/j.preteyeres.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farooq AV, Shukla D. 2011. Corneal latency and transmission of herpes simplex virus-1. Future Virol 6:101–108. doi: 10.2217/fvl.10.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babu JS, Thomas J, Kanangat S, Morrison LA, Knipe DM, Rouse BT. 1996. Viral replication is required for induction of ocular immunopathology by herpes simplex virus. J Virol 70:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagoo P, Chan G, Larkin DFP, George AJT. 2004. Inflammatory cytokines induce apoptosis of corneal endothelium through nitric oxide. Invest Ophthalmol Vis Sci 45:3964–3973. doi: 10.1167/iovs.04-0439. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande S, Banerjee K, Biswas PS, Rouse BT. 2004. Herpetic eye disease: immunopathogenesis and therapeutic measures. Expert Rev Mol Med 6:1–14. doi: 10.1017/S1462399404007604. [DOI] [PubMed] [Google Scholar]
- 16.Thomas J, Kanangat S, Rouse BT. 1998. Herpes simplex virus replication-induced expression of chemokines and proinflammatory cytokines in the eye: implications in herpetic stromal keratitis. J Interferon Cytokine Res 18:681–690. doi: 10.1089/jir.1998.18.681. [DOI] [PubMed] [Google Scholar]
- 17.Araki-Sasaki K, Tanaka T, Ebisuno Y, Kanda H, Umemoto E, Hayashi K, Miyazaki M. 2006. Dynamic expression of chemokines and the infiltration of inflammatory cells in the HSV-infected cornea and its associated tissues. Ocul Immunol Inflamm 14:257–266. doi: 10.1080/09273940600943581. [DOI] [PubMed] [Google Scholar]
- 18.Giménez F, Suryawanshi A, Rouse BT. 2013. Pathogenesis of herpes stromal keratitis–a focus on corneal neovascularization. Prog Retin Eye Res 33:1–9. doi: 10.1016/j.preteyeres.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr DJ, Tomanek L. 2006. Herpes simplex virus and the chemokines that mediate the inflammation. Curr Top Microbiol Immunol 303:47–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouse BT, Sehrawat S. 2010. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol 10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shui JW, Kronenberg M. 2014. HVEM is a TNF receptor with multiple regulatory roles in the mucosal immune system. Immune Netw 14:67–72. doi: 10.4110/in.2014.14.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shui JW, Larange A, Kim G, Vela JL, Zahner S, Cheroutre H, Kronenberg M. 2012. HVEM signalling at mucosal barriers provides host defence against pathogenic bacteria. Nature 488:222–225. doi: 10.1038/nature11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shui JW, Kronenberg M. 2013. HVEM: an unusual TNF receptor family member important for mucosal innate immune responses to microbes. Gut Microbes 4:146–151. doi: 10.4161/gmic.23443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shui JW, Steinberg MW, Kronenberg M. 2011. Regulation of inflammation, autoimmunity, and infection immunity by HVEM-BTLA signaling. J Leukoc Biol 89:517–523. doi: 10.1189/jlb.0910528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware CF, Sedy JR. 2011. TNF superfamily networks: bidirectional and interference pathways of the herpesvirus entry mediator (TNFSF14). Curr Opin Immunol 23:627–631. doi: 10.1016/j.coi.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chayavichitsilp P, Buckwalter JV, Krakowski AC, Friedlander SF. 2009. Herpes simplex. Pediatr Rev 30:119–129. doi: 10.1542/pir.30-4-119. [DOI] [PubMed] [Google Scholar]
- 27.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. 2011. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat Rev Microbiol 9:369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery RI, Warner MS, Lum BJ, Spear PG. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427–436. doi: 10.1016/S0092-8674(00)81363-X. [DOI] [PubMed] [Google Scholar]
- 29.Spear PG, Manoj S, Yoon M, Jogger CR, Zago A, Myscofski D. 2006. Different receptors binding to distinct interfaces on herpes simplex virus gD can trigger events leading to cell fusion and viral entry. Virology 344:17–24. doi: 10.1016/j.virol.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Lopez M, Cocchi F, Menotti L, Avitabile E, Dubreuil P, Campadelli-Fiume G. 2000. Nectin2α (PRR2α or HveB) and nectin2δ are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J Virol 74:1267–1274. doi: 10.1128/JVI.74.3.1267-1274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon M, Spear PG. 2004. Random mutagenesis of the gene encoding a viral ligand for multiple cell entry receptors to obtain viral mutants altered for receptor usage. Proc Natl Acad Sci U S A 101:17252–17257. doi: 10.1073/pnas.0407892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krummenacher C, Baribaud I, Sanzo JF, Cohen GH, Eisenberg RJ. 2002. Effects of herpes simplex virus on structure and function of nectin-1/HveC. J Virol 76:2424–2433. doi: 10.1128/jvi.76.5.2424-2433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. 2011. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog 7:e1002277. doi: 10.1371/journal.ppat.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Eisenberg RJ, Cohen GH. 2002. Structure-based analysis of the herpes simplex virus glycoprotein D binding site present on herpesvirus entry mediator HveA (HVEM). J Virol 76:10894–10904. doi: 10.1128/JVI.76.21.10894-10904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Cohen GH, Eisenberg RJ. 2003. Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD-HveA/HVEM binding interface. J Virol 77:8127–8140. doi: 10.1128/JVI.77.14.8127-8140.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazear E, Whitbeck JC, Zuo Y, Carfi A, Cohen GH, Eisenberg RJ, Krummenacher C. 2014. Induction of conformational changes at the N-terminus of herpes simplex virus glycoprotein D upon binding to HVEM and nectin-1. Virology 448:185–195. doi: 10.1016/j.virol.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krummenacher C, Supekar VM, Whitbeck JC, Lazear E, Connolly SA, Eisenberg RJ, Cohen GH, Wiley DC, Carfi A. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J 24:4144–4153. doi: 10.1038/sj.emboj.7600875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carfí A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ, Wiley DC. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell 8:169–179. doi: 10.1016/S1097-2765(01)00298-2. [DOI] [PubMed] [Google Scholar]
- 40.Kopp SJ, Banisadr G, Glajch K, Maurer UE, Grunewald K, Miller RJ, Osten P, Spear PG. 2009. Infection of neurons and encephalitis after intracranial inoculation of herpes simplex virus requires the entry receptor nectin-1. Proc Natl Acad Sci U S A 106:17916–17920. doi: 10.1073/pnas.0908892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor JM, Lin E, Susmarksi N, Yoon M, Zago A, Ware CF, Pfeffer K, Miyoshi J, Takai Y, Spear PG. 2007. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe 12:19–28. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karaba AH, Kopp SJ, Longnecker R. 2012. Herpesvirus entry mediator is a serotype specific determinant of pathogenesis in ocular herpes. Proc Natl Acad Sci U S A 109:20649–20654. doi: 10.1073/pnas.1216967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karaba AH, Kopp SJ, Longnecker R. 2011. Herpesvirus entry mediator and nectin-1 mediate herpes simplex virus 1 infection of the murine cornea. J Virol 85:10041–10047. doi: 10.1128/JVI.05445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards RG, Kopp SJ, Karaba AH, Wilcox DR, Longnecker R. 2015. Herpesvirus entry mediator on radiation-resistant cell lineages promotes ocular herpes simplex virus 1 pathogenesis in an entry-independent manner. mBio 6:e01532-15. doi: 10.1128/mBio.01532-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiwari V, Oh MJ, Kovacs M, Shukla SY, Valyi-Nagy T, Shukla D. 2008. Role for nectin-1 in herpes simplex virus 1 entry and spread in human retinal pigment epithelial cells. FEBS J 275:5272–5285. doi: 10.1111/j.1742-4658.2008.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiwari V, Shukla SY, Yue BY, Shukla D. 2007. Herpes simplex virus type 2 entry into cultured human corneal fibroblasts is mediated by herpesvirus entry mediator. J Gen Virol 88:2106–2110. doi: 10.1099/vir.0.82830-0. [DOI] [PubMed] [Google Scholar]
- 47.Tiwari V, Clement C, Scanlan PM, Kowlessur D, Yue BY, Shukla D. 2005. A role for herpesvirus entry mediator as the receptor for herpes simplex virus 1 entry into primary human trabecular meshwork cells. J Virol 79:13173–13179. doi: 10.1128/JVI.79.20.13173-13179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akhtar J, Tiwari V, Oh MJ, Kovacs M, Jani A, Kovacs SK, Valyi-Nagy T, Shukla D. 2008. HVEM and nectin-1 are the major mediators of herpes simplex virus 1 (HSV-1) entry into human conjunctival epithelium. Invest Ophthalmol Vis Sci 49:4026–4035. doi: 10.1167/iovs.08-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah A, Farooq AV, Tiwari V, Kim M, Shukla D. 2010. HSV-1 infection of human corneal epithelial cells: receptor-mediated entry and trends of re-infection. Mol Vis 16:2476–2486. [PMC free article] [PubMed] [Google Scholar]
- 50.Farooq AV, Valyi-Nagy T, Shukla D. 2010. Mediators and mechanisms of herpes simplex virus entry into ocular cells. Curr Eye Res 35:445–450. doi: 10.3109/02713681003734841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shukla ND, Tiwari V, Valyi-Nagy K. 2012. Nectin-1-specific entry of herpes simplex virus 1 is sufficient for infection of the cornea and viral spread to the trigeminal ganglia. Mol Vis 18:2711–2716. [PMC free article] [PubMed] [Google Scholar]
- 52.Valyi-Nagy T, Sheth V, Clement C, Tiwari V, Scanlan P, Kavouras JH, Leach L, Guzman-Hartman G, Dermody TS, Shukla D. 2004. Herpes simplex virus entry receptor nectin-1 is widely expressed in the murine eye. Curr Eye Res 29:303–309. doi: 10.1080/02713680490516756. [DOI] [PubMed] [Google Scholar]
- 53.Yoon M, Zago A, Shukla D, Spear PG. 2003. Mutations in the N termini of herpes simplex virus type 1 and 2 gDs alter functional interactions with the entry/fusion receptors HVEM, nectin-2, and 3-O-sulfated heparan sulfate but not with nectin-1. J Virol 77:9221–9231. doi: 10.1128/JVI.77.17.9221-9231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manoj S, Jogger CR, Myscofski D, Yoon M, Spear PG. 2004. Mutations in herpes simplex virus glycoprotein D that prevent cell entry via nectins and alter cell tropism. Proc Natl Acad Sci U S A 101:12414–12421. doi: 10.1073/pnas.0404211101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edwards RG, Kopp SJ, Ifergan I, Shui JW, Kronenberg M, Miller SD, Longnecker R. 2017. Murine corneal inflammation and nerve damage after infection with HSV-1 are promoted by HVEM and ameliorated by immune-modifying nanoparticle therapy. Invest Ophthalmol Vis Sci 58:282–291. doi: 10.1167/iovs.16-20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy KM, Nelson CA, Sedy JR. 2006. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol 6:671–681. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 57.Kopp SJ, Karaba AH, Cohen LK, Banisadr G, Miller RJ, Muller WJ. 2013. Pathogenesis of neonatal herpes simplex 2 disease in a mouse model is dependent on entry receptor expression and route of inoculation. J Virol 87:474–481. doi: 10.1128/JVI.01849-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu G, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. 1998. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity 8:21–30. doi: 10.1016/S1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 59.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. 2005. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol 6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 60.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. 2008. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol 9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 61.Shi G, Luo H, Wan X, Salcedo TW, Zhang J, Wu J. 2002. Mouse T cells receive costimulatory signals from LIGHT, a TNF family member. Blood 100:3279–3286. doi: 10.1182/blood-2002-05-1404. [DOI] [PubMed] [Google Scholar]
- 62.Wan X, Zhang J, Luo H, Shi G, Kapnik E, Kim S, Kanakaraj P, Wu J. 2002. A TNF family member LIGHT transduces costimulatory signals into human T cells. J Immunol 169:6813–6821. doi: 10.4049/jimmunol.169.12.6813. [DOI] [PubMed] [Google Scholar]
- 63.Tamada K, Shimozaki K, Chapoval AI, Zhai Y, Su J, Chen SF, Hsieh SL, Nagata S, Ni J, Chen L. 2000. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol 164:4105–4110. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- 64.del Rio ML, Lucas CL, Buhler L, Rayat G, Rodriguez-Barbosa JI. 2010. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J Leukoc Biol 87:223–235. doi: 10.1189/jlb.0809590. [DOI] [PubMed] [Google Scholar]
- 65.Compaan DM, Gonzalez LC, Tom I, Loyet KM, Eaton D, Hymowitz SG. 2005. Attenuating lymphocyte activity: the crystal structure of the BTLA-HVEM complex. J Biol Chem 280:39553–39561. doi: 10.1074/jbc.M507629200. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Huchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. 2003. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 67.De Trez C, Schneider K, Potter K, Droin N, Fulton J, Norris PS, Ha SW, Fu YX, Murphy T, Murphy KM, Pfeffer K, Benedict CA, Ware CF. 2008. The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J Immunol 180:238–248. doi: 10.4049/jimmunol.180.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kojima R, Kajikawa M, Shiroishi M, Kuroki K, Maenaka K. 2011. Molecular basis for herpesvirus entry mediator recognition by the human immune inhibitory receptor CD160 and its relationship to the cosignaling molecules BTLA and LIGHT. J Mol Biol 413:762–772. doi: 10.1016/j.jmb.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 69.Cai G, Freeman GJ. 2009. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev 229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 70.Tu TC, Brown NK, Kim TJ, Wroblewska J, Yang X, Guo X, Lee SH, Kumar V, Lee KM, Fu YX. 2015. CD160 is essential for NK-mediated IFN-gamma production. J Exp Med 212:415–429. doi: 10.1084/jem.20131601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang M, Howard K, Winters A, Steavenson S, Anderson S, Smelt S, Doellgast G, Sheelo C, Stevens J, Kim H, Hamburger A, Sein A, Caughey DJ, Lee F, Hsu H, Siu G, Byrne FR. 2011. Monoclonal antibodies to B and T lymphocyte attenuator (BTLA) have no effect on in vitro B cell proliferation and act to inhibit in vitro T cell proliferation when presented in a cis, but not trans, format relative to the activating stimulus. Clin Exp Immunol 163:77–87. doi: 10.1111/j.1365-2249.2010.04259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Šedý JR, Bjordahl RL, Bekiaris V, Macauley MG, Ware BC, Norris PS, Lurain NS, Benedict CA, Ware CF. 2013. CD160 activation by herpesvirus entry mediator augments inflammatory cytokine production and cytolytic function by NK cells. J Immunol 191:828–836. doi: 10.4049/jimmunol.1300894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stiles KM, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C. 2010. Herpes simplex virus glycoprotein D interferes with binding of herpesvirus entry mediator to its ligands through downregulation and direct competition. J Virol 84:11646–11660. doi: 10.1128/JVI.01550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.del Rio ML, Schneider P, Fernandez-Renedo C, Perez-Simon JA, Rodriguez-Barbosa JI. 2013. LIGHT/HVEM/LTbetaR interaction as a target for the modulation of the allogeneic immune response in transplantation. Am J Transplant 13:541–551. doi: 10.1111/ajt.12089. [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez LC, Loyet KM, Calemine-Fenaux J, Chauhan V, Wranik B, Ouyang W, Eaton DL. 2005. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci U S A 102:1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheung TC, Humphreys IR, Potter KG, Norris PS, Shumway HM, Tran BR, Patterson G, Jean-Jacques R, Yoon M, Spear PG, Murphy KM, Lurain NS, Benedict CA, Ware CF. 2005. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proc Natl Acad Sci U S A 102:13218–13223. doi: 10.1073/pnas.0506172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marsters SA, Ayres TM, Skubatch M, Gray CL, Rothe M, Ashkenazi A. 1997. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP01. J Biol Chem 272:14029–14032. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 78.Cheung TC, Steinberg MW, Oborne LM, Macauley MG, Fukuyama S, Sanjo H, D'Souza C, Norris PS, Pfeffer K, Murphy KM, Kronenberg M, Spear PG, Ware CF. 2009. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci U S A 106:16535–16536. doi: 10.1073/pnas.0909295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilmore TD. 2006. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 80.Sciortino MT, Medici MA, Marino-Merlo F, Zaccaria D, Giuffre-Cuculletto M, Venuti A, Grelli S, Mastino A. 2008. Involvement of HVEM receptor in activation of nuclear factor kappaB by herpes simplex virus 1 glycoprotein D. Cell Microbiol 10:2297–2311. doi: 10.1111/j.1462-5822.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- 81.Teresa Sciortino M, Medici MA, Marino-Merlo F, Zaccaria D, Giuffre M, Venuti A, Grelli S, Mastino A. 2007. Signaling pathway used by HSV-1 to induce NF-kappaB activation: possible role of herpes virus entry receptor A. Ann N Y Acad Sci 1096:89–96. doi: 10.1196/annals.1397.074. [DOI] [PubMed] [Google Scholar]
- 82.Patel A, Hanson J, McLean TI, Olgiate J, Hilton M, Miller WE, Bachenheimer SL. 1998. Herpes simplex virus type 1 induction of persistent NF-κB nuclear translocation increases the efficiency of viral replication. Virology 247:212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- 83.Sciortino MT, Medici MA, Marino-Merlo F, Zaccaria D, Giuffre-Cuculletto M, Venuti A, Grelli S, Bramanti P, Mastino A. 2008. Involvement of gD/HVEM interaction in NF-kB-dependent inhibition of apoptosis by HSV-1 gD. Biochem Pharmacol 76:1522–1532. doi: 10.1016/j.bcp.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 84.Goodkin ML, Ting AT, Blaho JA. 2003. NF-kB is required for apoptosis prevention during herpes simplex virus type 1 infection. J Virol 77:7261–7280. doi: 10.1128/JVI.77.13.7261-7280.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoon M, Kopp SJ, Taylor JM, Storti CS, Spear PG, Muller WJ. 2011. Functional interaction between herpes simplex virus type 2 gD and HVEM transiently dampens local chemokine production after murine mucosal infection. PLoS One 6:e16122. doi: 10.1371/journal.pone.0016122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Menten P, Wuyts A, Van Damme J. 2002. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev 13:455–481. [DOI] [PubMed] [Google Scholar]
- 87.Lundberg PS, Cantin EM. 2003. A potential role for CXCR3 chemokines in the response to ocular HSV infection. Curr Eye Res 26:137–150. doi: 10.1076/ceyr.26.3.137.14898. [DOI] [PubMed] [Google Scholar]
- 88.Inoue T, Inoue Y, Kosaki R, Inoue Y, Kohji N, Shimomura Y, Tano Y, Hayashi K. 2001. Immunohistological study of infiltated cells and cytokines in murine herpetic keratitis. Acta Ophthalmol Scand 79:484–487. doi: 10.1034/j.1600-0420.2001.790511.x. [DOI] [PubMed] [Google Scholar]
- 89.Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. 2002. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci 43:737–743. [PubMed] [Google Scholar]
- 90.Guo H, Pang K, Wei Y, Yi C, Wu X. 2015. Herpes virus entry mediator in human corneal epithelial cells modulates the production of inflammatory cytokines in response to HSV type 1 challenge. Ophthalmic Res 54:128–134. doi: 10.1159/000437209. [DOI] [PubMed] [Google Scholar]
- 91.Terasaka Y, Miyazaki D, Yakura K, Haruki T, Inoue Y. 2010. Induction of IL-6 in transcriptional networks in corneal epithelial cells after herpes simplex virus type 1 infection. Invest Ophthalmol Vis Sci 51:2441–2449. doi: 10.1167/iovs.09-4624. [DOI] [PubMed] [Google Scholar]
- 92.Li H, Zhang J, Kumar A, Zheng M, Atherton SS, Yu FS. 2006. Herpes simplex virus 1 infection induces the expression of proinflammatory cytokines, interferons and TLR7 in human corneal epithelial cells. Immunology 117:167–176. doi: 10.1111/j.1365-2567.2005.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chinnery HR, Humphries T, Clare A, Dixon AE, Howes K, Moran CB, Scott D, Zakrzewski M, Pearlman E, McMenamin PG. 2008. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunology 125:541–548. doi: 10.1111/j.1365-2567.2008.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frank GM, Buela KA, Maker DM, Harvey SA, Hendricks RL. 2012. Early responding dendritic cells direct the local NK response to control herpes simplex virus 1 infection within the cornea. J Immunol 188:1350–1359. doi: 10.4049/jimmunol.1101968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bauer D, Schmitz A, van Rooijen N, Steuhl K. 2002. Conjunctival macrophage-mediated influence of the local and systemic immune reponse after corneal herpes simplex virus-1 infection. Immunol 107:118–128. doi: 10.1046/j.1365-2567.2002.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bauer D, Mryzk S, van Rooijen N, Steuhl K, Heiligenhaus A. 2000. Macrophage-depletion influences the course of murine HSV-1 keratitis. Curr Eye Res 20:45–53. doi: 10.1076/0271-3683(200001)2011-HFT045. [DOI] [PubMed] [Google Scholar]
- 97.Mott K, Brick DJ, van Rooijen N, Ghiasi H. 2007. Macrophages are important determinants of acute ocular HSV-1 infection in immunized mice. Invest Ophthalmol Vis Sci 48:5605–5615. doi: 10.1167/iovs.07-0894. [DOI] [PubMed] [Google Scholar]
- 98.Cheng H, Tumpey TM, Staats HF, van Rooijen N, Oakes JE, Lausch RN. 2000. Role of macrophages in restricting herpes simplex virus type 1 growth after ocular infection. Invest Ophthalmol Vis Sci 41:1402–1409. [PubMed] [Google Scholar]
- 99.Buela KA, Hendricks RL. 2015. Cornea-infiltrating and lymph node dendritic cells contribute to CD4+ T cell expansion after herpes simplex virus-1 ocular infection. J Immunol 194:379–387. doi: 10.4049/jimmunol.1402326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Banerjee K, Biswas PS, Kim B, Lee S, Rouse BT. 2004. CXCR2-/- mice show enhanced susceptibility to herpetic stromal keratitis: a role for IL-6-induced neovascularization. J Immunol 172:1237–1245. doi: 10.4049/jimmunol.172.2.1237. [DOI] [PubMed] [Google Scholar]
- 101.Biswas PS, Banerjee K, Kinchington PR, Rouse BT. 2006. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Exp Eye Res 82:46–54. doi: 10.1016/j.exer.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 102.Heo SK, Ju SA, Lee SC, Park SM, Choe SY, Kwon B, Kwon BS, Kim BS. 2006. LIGHT enhances the bactericidal activity of human monocytes and neutrophils via HVEM. J Leukoc Biol 79:330–338. doi: 10.1189/jlb.1104694. [DOI] [PubMed] [Google Scholar]
- 103.Heo SK, Yoon MA, Lee SC, Ju SA, Choi JH, Suh PG, Kwon BS, Kim BS. 2007. HVEM signaling in monocytes is mediated by intracellular calcium mobilization. J Immunol 179:6305–6310. doi: 10.4049/jimmunol.179.9.6305. [DOI] [PubMed] [Google Scholar]
- 104.Haselmayer P, Tenzer S, Kwon BS, Jung G, Schild H, Radsak MP. 2006. Herpes virus entry mediator synergizes with Toll-like receptor mediated neutrophil inflammatory responses. Immunology 119:404–411. doi: 10.1111/j.1365-2567.2006.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lv M, Wu W, Zhang Y, Zhu M. 2015. Herpes virus entry mediator licenses Listeria infection induced immunopathology through control of type I interferon. Sci Rep 5:12954. doi: 10.1038/srep12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shubin NJ, Chung CS, Heffernan DS, Irwin LR, Monaghan SF, Ayala A. 2012. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J Leukoc Biol 92:593–603. doi: 10.1189/jlb.1211641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Metcalf JF, Hamilton DS, Reichert RW. 1979. Herpetic keratitis in athymic (nude) mice. Infect Immun 26:1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mercadal CM, Bouley DM, DeStepahno D, Rouse BT. 1993. Herpetic stromal keratitis in the reconstituted mouse model. J Virol 67:3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thomas J, Rouse BT. 1998. Immunopathology of herpetic stromal keratitis: discordance in CD4+ T cell function between euthymic host and reconstituted SCID recipients. J Immunol 160:3965–3970. [PubMed] [Google Scholar]
- 110.Deshpande S, Zheng M, Lee S, Banerjee K, Gangappa S, Kumaraguru U, Rouse BT. 2001. Bystander activation involving T lymphocytes in herpetic stromal keratitis. J Immunol 167:2902–2910. doi: 10.4049/jimmunol.167.5.2902. [DOI] [PubMed] [Google Scholar]
- 111.Gangappa S, Deshpande S, Rouse BT. 1999. Bystander activation of CD4(+) T cells can represent an exclusive means of immunopathology in a virus infection. Eur J Immunol 29:3674–3682. doi:. [DOI] [PubMed] [Google Scholar]
- 112.Gangappa S, Deshpande S, Rouse BT. 2000. Bystander activation of CD4+ T cells accounts for herpetic ocular lesions. Invest Ophthalmol Vis Sci 41:453–459. [PubMed] [Google Scholar]
- 113.Gangappa S, Babu JS, Thomas J, Daheshia M, Rouse BT. 1998. Virus-induced immunoinflammatory lesions in the absence of viral antigen recognition. J Immunol 161:4289–4300. [PubMed] [Google Scholar]
- 114.Verjans GM, Remeijer L, van Binnedijk RS, Cornelissen JG, Volker-Dieben HJ, Baarsma SG, Osterhaus AD. 1998. Indentification and characterization of herpes simplex virus-specific CD4+ T cells in corneas of herpetic stromal keratitis patients. J Infect Dis 177:484–488. doi: 10.1086/517382. [DOI] [PubMed] [Google Scholar]
- 115.Banerjee K, Deshpande S, Zheng M, Kumaraguru U, Schoenberger SP, Rouse BT. 2002. Herpetic stromal keratitis in the absence of viral antigen recognition. Cell Immunol 219:108–118. doi: 10.1016/S0008-8749(02)00601-9. [DOI] [PubMed] [Google Scholar]
- 116.Zhao Z, Granucci F, Yeh L, Schaffer PA, Cantor H. 1998. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science 279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]
- 117.Deshpande SP, Lee S, Zheng M, Song B, Knipe D, Kapp JA, Rouse BT. 2001. Herpes simplex virus-induced keratitis: evaluation of the role of molecular mimicry in lesion pathogenesis. J Virol 75:3077–3088. doi: 10.1128/JVI.75.7.3077-3088.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheung TC, Oborne LM, Steinberg MW, Macauley MG, Fukuyama S, Sanjo H, D'Souza C, Norris PS, Pfeffer K, Murphy KM, Kronenberg M, Spear PG, Ware CF. 2009. T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding microenvironment. J Immunol 183:7286–7296. doi: 10.4049/jimmunol.0902490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xia L, Zhang S, Zhou J, Li Y. 2010. A crucial role for B and T lymphocyte attenuator in preventing the development of CD4+ T cell-mediated herpetic stromal keratitis. Mol Vis 16:2071–2083. [PMC free article] [PubMed] [Google Scholar]
- 120.Sharma S, Rajasagi NK, Veiga-Parga T, Rouse BT. 2014. Herpes virus entry mediator (HVEM) modulates proliferation and activation of regulatory T cells following HSV-1 infection. Microbes Infect 16:648–660. doi: 10.1016/j.micinf.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Banerjee K, Biswas PS, Kumaraguru U, Schoenberger SP, Rouse BT. 2004. Protective and pathological roles of virus-specific and bystander CD8+ T cells in herpetic stromal keratitis. J Immunol 173:7575–7583. doi: 10.4049/jimmunol.173.12.7575. [DOI] [PubMed] [Google Scholar]
- 122.Kastrukoff LF, Lau AS, Takei F, Smyth MJ, Jones CM, Clarke SR, Carbone FR. 2010. Redundancy in the immune system restricts the spread of HSV-1 in the central nervous system (CNS) of C57BL/6 mice. Virology 400:248–258. doi: 10.1016/j.virol.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 123.Held K, Derfuss T. 2011. Control of HSV-1 latency in human trigeminal ganglia–current overview. J Neurovirol 17:518–527. doi: 10.1007/s13365-011-0063-0. [DOI] [PubMed] [Google Scholar]
- 124.Nicoll MP, Proenca JT, Efstathiou S. 2012. The molecular basis of herpes simplex virus latency. FEMS Microbiol Rev 36:684–705. doi: 10.1111/j.1574-6976.2011.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Steiner I, Spivack JG, Lirette RP, Brown SM, MacLean AR, Subak-Sharpe JH, Fraser NW. 1989. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J 8:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Javier RT, Stevens JG, Dissette VB, Wagner EK. 1988. A herpes simplex virus transcript abundant in latently infected neurons is dispensable for establishment of latent state. Virology 166:254–257. doi: 10.1016/0042-6822(88)90169-9. [DOI] [PubMed] [Google Scholar]
- 127.Leib D, Hogard CL, Kosz-Vnenchak M, Hicks KA, Coen DM, Knipe DM, Schaffer PA. 1989. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol 63:2893–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Farooq AV, Shukla D. 2012. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol 57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stuart PM, Keadle TL. 2012. Recurrent herpetic stromal keratitis in mice: a model for studying human HSK. Clin Dev Immunol 2012:728480. doi: 10.1155/2012/728480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Derfuss T, Segerer S, Herberger S, Sinicina I, Hufner K, Ebelt K, Knaus HG, Steiner I, Meinl E, Dornmair K, Arbusow V, Strupp M, Brandt T, Theil D. 2007. Presence of HSV-1 immediate early genes and clonally expanded T-cells with a memory effector phenotype in human trigeminal ganglia. Brain Pathol 17:389–398. doi: 10.1111/j.1750-3639.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci U S A 99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kovacs SK, Tiwari V, Prandovszky E, Dosa S, Bacsa S, Valyi-Nagy K, Shukla D, Valyi-Nagy T. 2009. Expression of herpes virus entry mediator (HVEM) in the cornea and trigeminal ganglia of normal and HSV-1 infected mice. Curr Eye Res 34:896–904. doi: 10.3109/02713680903184250. [DOI] [PubMed] [Google Scholar]
- 133.Allen SJ, Rhode-Kurnow A, Mott KR, Jiang X, Carpenter D, Rodriguez-Barbosa JI, Jones C, Wechsler SL, Ware CF, Ghiasi H. 2014. Interactions between herpesvirus entry mediator (TNFRSF14) and latency-associated transcript during herpes simplex virus 1 latency. J Virol 88:1961–1971. doi: 10.1128/JVI.02467-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ramakrishna C, Ferraioli A, Calle A, Nguyen TK, Openshaw H, Lundberg PS, Lomonte P, Cantin EM. 2015. Establishment of HSV1 latency in immunodeficient mice facilitates efficient in vivo reactivation. PLoS Pathog 11:e1004730. doi: 10.1371/journal.ppat.1004730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jung HW, La SJ, Kim JY, Heo SK, Kim JY, Wang S, Kim KK, Lee KM, Cho HR, Lee HW, Kwon B, Kim BS, Kwon BS. 2003. High levels of soluble herpes virus entry mediator in sera of patients with allergic and autoimmune diseases. Exp Mol Med 35:501–508. doi: 10.1038/emm.2003.65. [DOI] [PubMed] [Google Scholar]
- 136.Sakoda Y, Park J, Zhao Y, Kuramasu A, Geng D, Liu Y, Davilla E, Tamada K. 10 January 2011. Dichotomous regulation of GVHD through bidirectional functions of the BTLA-HVEM pathway. Blood doi: 10.1182/blood-2010-08-301325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.del Rio ML, Jones ND, Buhler L, Norris P, Shintani Y, Ware CF, Rodriguez-Barbosa JI. 2012. Selective blockade of herpesvirus entry mediator-B and T lymphocyte attenuator pathway ameliorates acute graft-versus-host reaction. J Immunol 188:4885–4896. doi: 10.4049/jimmunol.1103698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Albring JC, Sandau MM, Rapaport AS, Edelson BT, Satpathy A, Mashayekhi M, Lathrop SK, Hsieh CS, Stelljes M, Colonna M, Murphy TL, Murphy KM. 2010. Targeting of B and T lymphocyte associated (BTLA) prevents graft-versus-host disease without global immunosuppression. J Exp Med 207:2551–2559. doi: 10.1084/jem.20102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu Y, Filies AS, Flies DB, Zhu G, Anand S, Flies SJ, Xu H, Anders RA, Hancock WW, Chen L, Tamada K. 19 December 2006. Selective targeting of the LIGHT-HVEM costimulatory system for the treatment of graft-versus-host disease. Blood doi: 10.1182/blood-2006-09-047332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gatza E, Choi SW. 2015. Approaches for the prevention of graft-versus-host disease following hematopoietic cell transplantation. Int J Hematol Oncol 4:113–126. doi: 10.2217/ijh.15.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fan K, Wang H, Wei H, Zhou Q, Kou G, Hou S, Qian Q, Dai J, Li B, Zhang Y, Zhu T, Guo Y. 2007. Blockade of LIGHT/HVEM and B7/CD28 signaling facilitates long-term islet graft survival with development of allospecific tolerance. Transplantation 84:746–754. doi: 10.1097/01.tp.0000280545.14489.df. [DOI] [PubMed] [Google Scholar]
- 142.Ye Q, Fraser CC, Gao W, Wang L, Busfield SJ, Wang C, Qiu Y, Coyle AJ, Gutierrez-Ramos JC, Hancock WW. 2002. Modulation of LIGHT-HVEM costimulation prolongs cardiac allograft survival. J Exp Med 195:795–800. doi: 10.1084/jem.20012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kotsiou E, Okosun J, Besley C, Iqbal S, Matthews J, Fitzgibbon J, Gribben JG, Davies JK. 2016. TNFRSF14 aberrations in follicular lymphoma increase clinically significant allogeneic T-cell responses. Blood 128:72–81. doi: 10.1182/blood-2015-10-679191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sakoda Y, Nagai T, Murata S, Mizuno Y, Kurosawa H, Shoda H, Morishige N, Yanai R, Sonoda KH, Tamada K. 2016. Pathogenic function of herpesvirus entry mediator in experimental autoimmune uveitis by induction of Th1- and Th17-type T cell responses. J Immunol 196:2947–2954. doi: 10.4049/jimmunol.1501742. [DOI] [PubMed] [Google Scholar]
- 145.Getts DR, Shea LD, Miller SD, King NJ. 2015. Harnessing nanoparticles for immune modulation. Trends Immunol 36:419–427. doi: 10.1016/j.it.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Getts DR, Terry RL, Getts MT, Deffrasnes C, Müller M, van Vreden C, Ashhurst TM, Chami B, McCarthy D, Wu H, Ma J, Martin A, Shae LD, Witting P, Kansas GS, Kühn J, Hafezi W, Campbell IL, Reilly D, Say J, Brown L, White MY, Cordwell SJ, Chadban SJ, Thorp EB, Bao S, Miller SD, King NJ. 2014. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med 6:219ra7. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seyed-Razavi Y, Chinnery HR, McMenamin PG. 2014. A novel association between resident tissue macrophages and nerves in the peripheral stroma of the murine cornea. Invest Ophthalmol Vis Sci 55:1313–1320. doi: 10.1167/iovs.13-12995. [DOI] [PubMed] [Google Scholar]
- 148.Chucair-Elliott AJ, Zheng M, Carr DJ. 2015. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Invest Ophthalmol Vis Sci 56:1097–1107. doi: 10.1167/iovs.14-15596. [DOI] [PMC free article] [PubMed] [Google Scholar]