Key Points
Question
Does a sedation strategy with dexmedetomidine compared with no dexmedetomidine improve ventilator-free days and mortality among patients with sepsis requiring ventilation?
Findings
In this randomized clinical trial that included 201 adults, treatment with dexmedetomidine compared with treatment without dexmedetomidine did not significantly improve ventilator-free days (20 days vs 18 days) or 28-day mortality (23% vs 31%; hazard ratio, 0.69).
Meaning
Treatment with dexmedetomidine in patients with sepsis did not improve either ventilator-free days or 28-day mortality.
Abstract
Importance
Dexmedetomidine provides sedation for patients undergoing ventilation; however, its effects on mortality and ventilator-free days have not been well studied among patients with sepsis.
Objectives
To examine whether a sedation strategy with dexmedetomidine can improve clinical outcomes in patients with sepsis undergoing ventilation.
Design, Setting, and Participants
Open-label, multicenter randomized clinical trial conducted at 8 intensive care units in Japan from February 2013 until January 2016 among 201 consecutive adult patients with sepsis requiring mechanical ventilation for at least 24 hours.
Interventions
Patients were randomized to receive either sedation with dexmedetomidine (n = 100) or sedation without dexmedetomidine (control group; n = 101). Other agents used in both groups were fentanyl, propofol, and midazolam.
Main Outcomes and Measures
The co–primary outcomes were mortality and ventilator-free days (over a 28-day duration). Sequential Organ Failure Assessment score (days 1, 2, 4, 6, 8), sedation control, occurrence of delirium and coma, intensive care unit stay duration, renal function, inflammation, and nutrition state were assessed as secondary outcomes.
Results
Of the 203 screened patients, 201 were randomized. The mean age was 69 years (SD, 14 years); 63% were male. Mortality at 28 days was not significantly different in the dexmedetomidine group vs the control group (19 patients [22.8%] vs 28 patients [30.8%]; hazard ratio, 0.69; 95% CI, 0.38-1.22; P = .20). Ventilator-free days over 28 days were not significantly different between groups (dexmedetomidine group: median, 20 [interquartile range, 5-24] days; control group: median, 18 [interquartile range, 0.5-23] days; P = .20). The dexmedetomidine group had a significantly higher rate of well-controlled sedation during mechanical ventilation (range, 17%-58% vs 20%-39%; P = .01); other outcomes were not significantly different between groups. Adverse events occurred in 8 (8%) and 3 (3%) patients in the dexmedetomidine and control groups, respectively.
Conclusions and Relevance
Among patients requiring mechanical ventilation, the use of dexmedetomidine compared with no dexmedetomidine did not result in statistically significant improvement in mortality or ventilator-free days. However, the study may have been underpowered for mortality, and additional research may be needed to evaluate this further.
Trial Registration
clinicaltrials.gov Identifier: NCT01760967
This randomized clinical trial compares the effects of sedation with vs without dexmedetomidine on mortality and ventilator-free days in patients with sepsis.
Introduction
Dexmedetomidine, a highly selective α2-adrenergic agonist, is a unique sedative agent compared with γ-aminobutyric acid receptor agonists. Dexmedetomidine can improve patients’ ability to communicate pain compared with midazolam and propofol. Therefore, dexmedetomidine is likely to be useful for light sedation. The analgesic effects of dexmedetomidine or the reduction of other deliriogenic sedatives may lessen both agitation and delirium.
Furthermore, several studies have shown that dexmedetomidine can suppress inflammatory reactions and protect organs in both animals and humans. Particularly, the Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction (MENDS) randomized clinical trial showed in a subgroup analysis that patients with sepsis treated with dexmedetomidine had an improved 28-day mortality rate compared with those receiving lorazepam.
Therefore, it was hypothesized that dexmedetomidine would improve patient-oriented outcomes among patients with sepsis. The Dexmedetomidine for Sepsis in Intensive Care Unit (ICU) Randomized Evaluation (DESIRE) trial was conducted to evaluate whether a sedation strategy with dexmedetomidine would improve mortality and ventilator-free days during the 28 days after the initiation of ventilation among ICU patients with sepsis.
Methods
Study Design
The DESIRE trial was an investigator-initiated, multicenter, open-label randomized clinical trial conducted among patients who had sepsis requiring mechanical ventilation for more than 24 hours. The trial was designed to assess the effects of a sedation strategy with dexmedetomidine on mortality and ventilator-free days during the 28-day period after initiation of ventilation. The trial was conducted in 8 ICUs in Japan. The first patient was enrolled in February 2013 and the trial was completed in January 2016. This study was approved by the institutional review boards of Wakayama Medical University and each participating institution. Written informed consent was obtained from patients or patients’ families before randomization. Patients did not receive a stipend for participation in this trial. The study protocol is available in Supplement 1.
Patients
Patients who were aged 20 years or older, had sepsis, and needed mechanical ventilation for at least 24 hours (at the discretion of ICU physicians) were eligible for the study. Mechanical ventilation included both invasive and noninvasive ventilation (ie, ventilation with a nasal mask, face mask, head mask, or cuirass). Sepsis was defined as systemic inflammatory response syndrome due to infection. Because fluid resuscitation and antisepsis treatments are necessary for acute pancreatitis (similar to an infection), cases of acute pancreatitis were included as an infection whereas cases of burns or heatstroke were not. Soft tissue infection including necrotizing fasciitis and Fournier gangrene were considered to take unusually longer treatment for debridement in the ICU. Patients were excluded if they (1) had severe chronic liver disease (Child-Pugh grade B or C); (2) had acute myocardial infarction or severe heart failure (New York Heart Association functional class 4); (3) had drug dependence or alcoholism or a psychological illness or severe cognitive dysfunction; or (4) were pregnant or lactating or were allergic to dexmedetomidine. Detailed inclusion and exclusion criteria are provided in the trial protocol (Supplement 1).
Randomization and Intervention
Eligible patients were randomly assigned to receive either the sedation strategy with dexmedetomidine or the sedation strategy without dexmedetomidine (control group). Registration and data management were conducted using an electronic data capturing system. Randomization was conducted by permuted block randomization stratified by study center, presence of emergency surgery, chronic obstructive pulmonary disease, and soft tissue infection. The block size was 4 but physicians and investigators were not notified of this during the study.
Patients in the dexmedetomidine group received dexmedetomidine and analgesia continuously, and other sedatives were added as needed. Patients in the control group received sedative drugs such as propofol, midazolam, and analgesia without dexmedetomidine. The targets of sedation depth were a Richmond Agitation-Sedation Scale (RASS) score of 0 (calm) during the day and a RASS score of −2 (lightly sedated) during the night in both groups. Sedation was maintained throughout the duration of mechanical ventilation or as needed. The sedation protocols were also in compliance with the Clinical Practice Guidelines for the Sustained Use of Sedatives and Analgesics in the Critically Ill Adult. The detailed sedation protocol is available in eFigure 1 in Supplement 2.
The treatment protocol for sepsis was based on the Guidelines for the Management of Sepsis by the Japanese Society of Intensive Care Medicine. Enteral feeding was provided according to our protocols.
Primary and Secondary Outcomes
The co–primary outcomes were 28-day mortality and ventilator-free days. In the original protocol, we planned to assess the duration of ventilation as a co–primary outcome. Because duration of mechanical ventilation was highly influenced by mortality, the number of 28-day ventilator-free days was set as the co–primary end point instead on May 2, 2015, before obtaining outcome data. Ventilator-free days were calculated in the first 28 days or the days alive minus days under mechanical ventilation after randomization. Patients were weaned from mechanical ventilation with a spontaneous breathing trial procedure if they met the predefined criteria, including improvement of the underlying illness, a partial pressure of arterial oxygen of more than 60 mm Hg with a fraction of inspired oxygen of less than 0.5 and a positive end-expiratory pressure of less than 8 cm H2O, a normal partial pressure of carbon dioxide, and sufficient spontaneous inspiration. The full ventilator-weaning criteria are described in the trial protocol (Supplement 1). Secondary outcomes included length of ICU stay and length of hospital stay (days); RASS score; Confusion Assessment Method for ICU Patients (CAM-ICU) score; Sequential Organ Failure Assessment (SOFA) score; disseminated intravascular coagulation score (as defined by the Japanese Association for Acute Medicine); a score comprising Mini-Mental State Examination (MMSE) score at hospital discharge, serum urea nitrogen and creatinine levels, estimated glomerular filtration rate, daily urinary output, requirement for renal replacement therapy, C-reactive protein and procalcitonin levels, daily energy intake by enteral nutrition, and prealbumin level. The RASS is a distinctive scale for the degree of sedation or agitation; thus, it cannot be evaluated with means or medians. Well-controlled sedation was defined as an RASS score between −3 and +1 throughout 1 day spent in the ICU and was defined as (rate of controlled sedation) = (patient’s number of days with well-controlled sedation)/(total number of patients in the ICU), calculated for each day. Coma was defined as an RASS score between −4 and −5 throughout 1 day in the ICU. The rate of days with well-controlled sedation and days free from delirium or coma were set as post hoc analyses. Predefined adverse events included any arrhythmias and myocardial ischemia such as acute coronary syndrome during sedation.
Statistical Analyses
To estimate the sample size, we assumed the 28-day survival to be 80% in the dexmedetomidine group and 60% in the control group based on the results of the MENDS randomized controlled trial, which showed that the 28-day survival was 84% among the group receiving dexmedetomidine and 59% among the control group. The study needed 172 patients to achieve 80% power with a 2-sided α level of .05. A 15% dropout or withdrawal rate was estimated and 200 patients were planned for enrollment.
Clinical outcomes were analyzed according to the intention-to-treat principle. All time-to-event data were censored at 28 days. Missing data were analyzed without imputation, but there were no missing data of observations during the ICU stay. Continuous variables were presented as means with standard deviations or medians with interquartile ranges (IQRs), and categorical variables were shown as numbers and percentages.
Because there were 2 co–primary end points, both comparisons had to meet significance to determine the efficacy of treatment. The cumulative incidence was estimated by the Kaplan-Meier method, and differences between the groups were assessed by the log-rank test. We used the Cox proportional hazards model to estimate the hazard ratio (HR) and 95% confidence interval of the dexmedetomidine group compared with the control group. Proportional hazard assumptions for the variables were assessed on the plots of log(time) vs log(−log [survival]) stratified by the variables, and the assumptions were verified to be acceptable. To compare other clinical outcomes between the dexmedetomidine group and the control group, χ2 tests or Fisher exact tests were used for categorical variables and t tests or Wilcoxon rank sum tests for continuous variables based on the distribution.
To examine the effect of dexmedetomidine on sedation control and the occurrence of delirium and coma, a generalized linear model (GENMOD procedure with logit function) was used to account for repeated measurements in the same patient. We included the status of patients in the dependent variable and treatment allocation in the independent variable with a repeated variable of patient.
The Cox proportional hazard models were constructed in prespecified subgroups: age (≥65 vs <65 years old), Acute Physiology and Chronic Health Evaluation II (APACHE II) scores (≥23 vs <23), shock on admission (presence vs absence), and site of infection (thorax, abdomen, etc). Shock was defined as a circulatory SOFA score of 3 or higher. The threshold of APACHE II score was determined by the median value. To estimate P values for interaction, Cox proportional hazards models with variables of outcomes, subgroup factor, and the interaction variables in addition to allocation factors were constructed. We also constructed the same analyses including study center as a stratification variable because management of sepsis might be different between centers. Because this stratification variable was not predetermined, the analyses adjusted for study center were considered post hoc sensitivity analyses.
All statistical analyses were conducted by an independent statistician who was masked to treatment group allocation. All statistical analyses were performed using JMP, version 11.2.0 (SAS Institute Inc), and SAS, version 9.4 (SAS Institute Inc). A 2-sided P<.05 was considered statistically significant. The statistical analysis plan for the study is available in Supplement 3.
Results
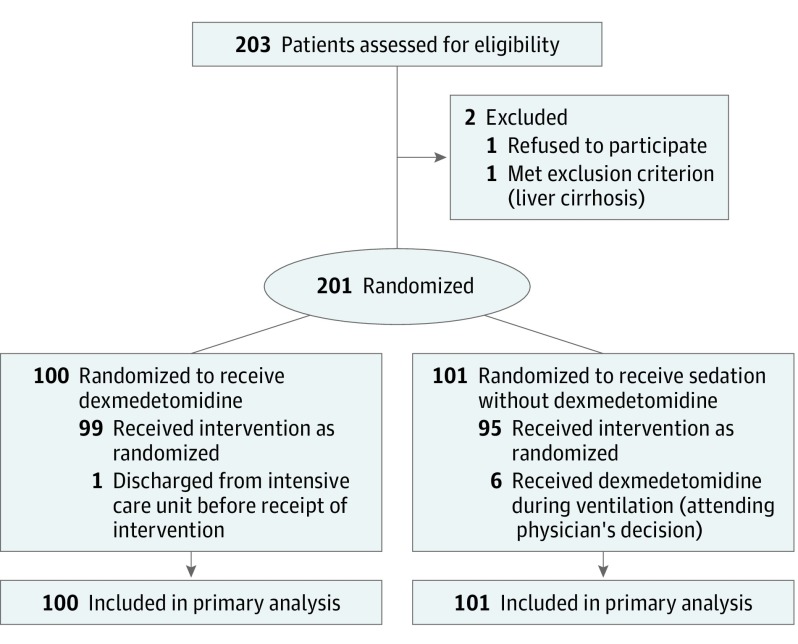
A total of 203 ventilated patients with sepsis were initially enrolled and 2 patients were excluded (1 declined to participate and 1 had liver cirrhosis). Two hundred one patients were randomized either into the dexmedetomidine group (n = 100) or the control group (n = 101) (Figure 1). The patients’ backgrounds, severity of illness, comorbidities, and site of infection were similar between groups (Table 1).
Figure 1. Flow of Participants in the Dexmedetomidine for Sepsis in Intensive Care Unit Randomized Evaluation Trial.
Table 1. Participant Characteristics.
| Characteristics | Dexmedetomidine Group (n = 100) |
Control Group (n = 101) |
|---|---|---|
| Age, mean (SD), y | 68 (14.9) | 69 (13.6) |
| Male, No. (%) | 63 (63) | 64 (63) |
| Body weight, mean (SD), kg | 55 (12.5) | 58 (15.3) |
| COPD, No. (%) | 8 (8) | 9 (9) |
| Soft tissue infection, No. (%) | 8 (8) | 10 (10) |
| Emergency surgery, No. (%) | 37 (37) | 36 (36) |
| Renal replacement therapy, No. (%) | 22 (22) | 20 (20) |
| APACHE II score, median (IQR)a | 23 (18-29) | 22 (16-29.5) |
| SOFA scores, median (IQR)b | ||
| Overall score | 8 (6-11) | 9 (5-11) |
| Respiratory score | 2 (1-3) | 2 (1-3) |
| Circulatory score | 3 (2-4) | 3 (1.5-4) |
| Renal score | 1 (0-2) | 1 (0-3) |
| Hepatic score | 0 (0-1) | 0 (0-1) |
| Neurological score | 1 (0-2) | 0 (0-3) |
| Coagulation score | 0 (0-2) | 1 (0-2) |
| Shock, No. (%)c | 71 (71) | 68 (67) |
| Lactate level, median (IQR), mmol/Ld | 3.7 (2.1-5.4) | 3.3 (1.8-5.4) |
| C-reactive protein, median (IQR), mg/dL | 13.8 (6.0-25.7) | 16.8 (6.4-25.8) |
| Procalcitonin, median (IQR), ng/mL | 15.2 (2.8-44.9) | 14.6 (1.5-81.5) |
| Comorbidities, No. (%) | ||
| Immunocompromised | 16 (16) | 15 (15) |
| Chronic hemodialysis | 7 (7) | 7 (7) |
| Chronic respiratory disorder | 4 (4) | 5 (5) |
| Chronic heart failure | 2 (2) | 2 (2) |
| Liver insufficiency | 1 (1) | 1 (1) |
| Site of infection, No. (%) | ||
| Abdomen | 39 (39) | 35 (35) |
| Thorax | 39 (39) | 33 (33) |
| Urinary tract | 6 (6) | 10 (10) |
| Pancreatitis | 3 (3) | 9 (9) |
| Skin and soft tissue | 6 (6) | 7 (7) |
| Central nervous system | 1 (1) | 1 (1) |
| Others | 6 (6) | 6 (6) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
The APACHE II score ranges from 0 to 71, with higher scores indicating more severe disease.
The SOFA score ranges from 0 to 24, with higher scores indicating more severe disease.
Shock was defined as having 3 or more cardiovascular components of the SOFA score.
Serum lactate level was missing for 3 patients.
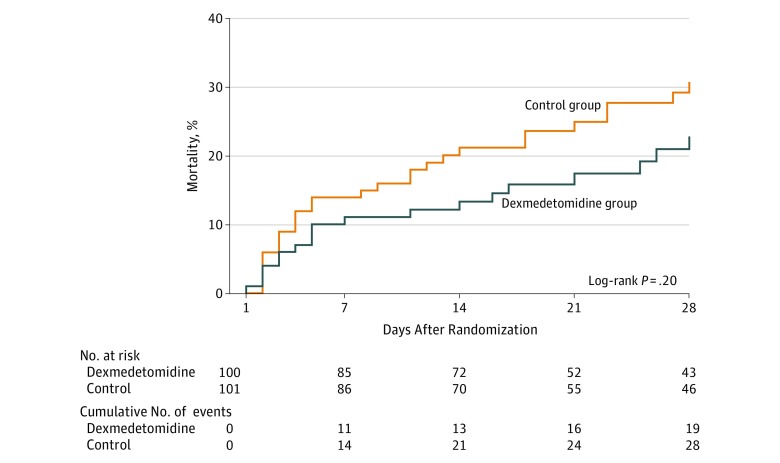
The cumulative incidence of death at 28 days was 22.8% (n=19) in the dexmedetomidine group and 30.8% (n=28) in the control group (P = .20) (Figure 2). The HR of the dexmedetomidine group was 0.69 (95% CI, 0.38-1.22; P = .20). The result of sensitivity analysis adjusting the center was similar (HR, 0.65; 95% CI, 0.36-1.16; P = .15). The median number of 28-day ventilator-free days in the dexmedetomidine group was 20 (IQR, 5-24) compared with 18 (IQR, 0.5-23) in the control group (P = .20). The number of ventilator days was 6 (IQR, 3-11) in the dexmedetomidine group and 6 (IQR, 3-11] in the control group (P = .64). Median length of ICU stay was 7 (IQR, 4-12) days in the dexmedetomidine group and 8 (IQR, 4-14) days in the control group (P = .43).
Figure 2. 28-Day Mortality Among the Dexmedetomidine and Control Groups.
Day 1 is defined as the first day of randomization into the trial.
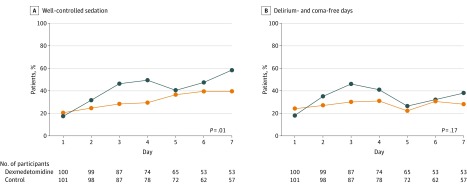
There were no significant differences in the daily total SOFA score between groups (Table 2). The rate of well-controlled sedation during ICU stay was significantly higher in the dexmedetomidine group than in the control group (range, 17%-58% vs 20%-39%; P = .01), while the rate of delirium- and coma-free days was not significantly different between groups (P = .17) (Figure 3). The frequency and dose of propofol and midazolam were lower in the dexmedetomidine group than in the control group, but the frequency and dose of fentanyl were not significantly different (eTable 1 in Supplement 2).
Table 2. Outcome Measurements and Adverse Events.
| Outcomes | Dexmedetomidine Group (n = 100) |
Control Group (n = 101) |
P Valuea |
|---|---|---|---|
| 28-Day mortality, No. (%) | 19 (19) | 28 (28) | .14 |
| Ventilator-free days, median (IQR) | 20 (5-24) | 18 (0.5-23) | .20 |
| Total daily SOFA score, median (IQR)b | |||
| Day 1 | 8 (6-11) | 9 (5-11) | .67 |
| Day 2 | 9 (7-11) | 9 (6-13) | .51 |
| Day 4 | 8 (5-11) | 8 (4.5-11.5) | .67 |
| Day 6 | 8 (5-10) | 7 (4-10) | .68 |
| Day 8 | 7 (5-9) | 5 (3-11) | .31 |
| Intensive care unit length of stay, median (IQR), d | 7 (4-12) | 8 (4-14) | .43 |
| Adverse events, No. (%) | |||
| Bradycardia | 7 (7) | 2 (2) | .10 |
| Acute coronary syndrome | 1 (1) | 1 (1) | >.99 |
Abbreviations: IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
The χ2 test was used for categorical variables and the Wilcoxon rank sum test for continuous variables.
The SOFA score ranges from 0 to 24, with higher scores indicating greater severity of organ failure.
Figure 3. Percentage of Patients With Well-Controlled Sedation and Delirium- and Coma-Free Days During ICU Stay Among the Dexmedetomidine and Control Groups.
To examine the effect of dexmedetomidine on sedation control and the occurrence of delirium and coma, a generalized linear model was used accounting for repeated measurements in the same patient. Well-controlled sedation was defined as a Richmond Agitation-Sedation Scale (RASS) score between −3 and +1 throughout 1 day spent in the intensive care unit (ICU) and was defined as (rate of controlled sedation) = (patient’s number of days with well-controlled sedation)/(total number of patients in the ICU), calculated for each day. Coma was defined as an RASS score between −4 and −5 throughout 1 day in the ICU. Day 1 is defined as the first day of randomization into the trial.
The subgroup analyses did not show any heterogeneity in the effect of dexmedetomidine on mortality in terms of age older than 65 years, presence of shock on admission, or site of infection (eFigure 2 in Supplement 2). In the subgroup with APACHE II scores of 23 or higher, the dexmedetomidine group was associated with lower hospital mortality (HR, 0.39; 95% CI, 0.16-0.91; P = .03).
Secondary outcomes included C-reactive protein, procalcitonin, and prealbumin levels and disseminated intravascular coagulation score. There was a significant difference only in C-reactive protein between the groups (eTable 2 in Supplement 2). The time taken to achieve a satisfactory MMSE score (or proportion of time spent with a satisfactory MMSE score) could not be reported because of insufficient data obtained.
The observed adverse events included bradycardia in 7 patients (7%) in the dexmedetomidine group and in 2 patients (2%) in the control group. Acute coronary syndrome was noted in 1 patient (1%) in each group, but other serious adverse events such as cardiac arrhythmia (ventricular fibrillation and sinus arrest) did not occur.
Discussion
In this randomized clinical trial to evaluate the effect of dexmedetomidine on clinical outcomes in patients with sepsis under ventilation and to attempt to verify the results of subgroup analysis in the MENDS trial, administration of dexmedetomidine in patients with sepsis did not improve mortality or ventilator-free days but did achieve better control of light sedation.
The difference in results between this trial and the MENDS study may be attributable to a number of factors. First, the previous study was a subgroup analysis of patients with sepsis in the MENDS trial in which ventilated ICU patients were randomized to receive dexmedetomidine or lorazepam; second, this trial was a randomized clinical trial of patients with sepsis undergoing ventilation and receiving vs not receiving dexmedetomidine; third, the administered dexmedetomidine dosage in the trial was less than the usual dosage in other countries because of the lower dosage limit covered by Japanese medical insurance; and fourth, the participants in this study were older (mean age of 68.8 vs 59.0 years) and their APACHE II scores were lower (mean of 23.0 vs 29.5) than in the MENDS subgroup analysis.
The study may have identified a clinically important benefit of dexmedetomidine—an 8% reduction in 28-day mortality—that did not demonstrate statistical significance. The trial was prospectively powered to detect a 20% difference in 28-day survival based on the results of the MENDs subgroup analysis. Physicians may consider an 8% difference in 28-day mortality to be clinically significant but this study was underpowered to detect this difference.
Dexmedetomidine reduces prevalence of delirium in those with prolonged ICU sedation compared with midazolam, and delirium is an independent predictor of mortality in patients undergoing mechanical ventilation. Dexmedetomidine has been associated with improved patient communication with nursing staff and a reduction in delirium compared with a γ-aminobutyric acid agonist. The establishment of a systemic sedation protocol and early physical therapy including a ventilator-weaning protocol in the ICU can improve delirium and prognosis. In the current study, with systematic protocolized light sedation, dexmedetomidine treatment was associated with better sedation but not with delirium reduction in patients with sepsis. The systematic sedation protocol might have been more effective than drug choice for reducing delirium.
Moreover, it was hypothesized that dexmedetomidine can improve prognosis by suppressing the hypersympathetic condition in sepsis. High plasma catecholamine levels seem to be harmful to the human body in critically ill patients. Consequently, a β1-adrenoceptor blockade reduces mortality in patients with sepsis. In another study, heart rate control with esmolol, a typical β1-adrenoceptor blockade, was shown to reduce norepinephrine requirements, resulting in an improvement in 28-day survival. Moreover, even the smallest dose of dexmedetomidine suppresses both endogenous norepinephrine and epinephrine in a healthy human body. From these reports, it was considered that dexmedetomidine and β1-adrenoceptor blockade could have a similar effect on improving mortality in patients with sepsis.
A new definition of sepsis was announced in February 2016. Owing to the inclusion criteria used in this study, the study enrolled patients with more severe sepsis than specified in the new definition because patients with sepsis who needed mechanical ventilation were included. Therefore, the findings should be applicable to patients with sepsis as defined by the new definition.
There are several limitations in this study. First, this was an open-label study and the end points were assessed by physicians at discharge. However, mortality was less likely influenced by physicians’ judgment, and the criteria for weaning from mechanical ventilation were set prior to the study. The event adjudication committee also verified assessment and the use of sedative drugs or other interventions for sepsis. The RASS and CAM-ICU were assessed by nurses who were not blinded to the allocation, but they assessed patients based on a manual irrespective of the study. Therefore, the influence of unblinding should be small. Second, the sample size was not large enough to detect the 8% difference in 28-day mortality as a result. We estimated the sample size based on the MENDS subgroup trial, which showed 16% and 41% mortality (a 25% difference). Third, although long-term outcomes are important in this area of research, only short-term outcomes were analyzed in this study. Further studies with long observation periods are warranted.
In this study, only 203 patients were screened and 201 enrolled. The number of patients screened but not enrolled was small for a general clinical trial. In Japan, clinicians ascertain the inclusion and exclusion criteria and decide to offer enrollment. Therefore, patients were screened and enrolled at the same time; there was 1 case excluded for liver cirrhosis before allocation.
Conclusions
Among patients requiring mechanical ventilation, the use of dexmedetomidine compared with no dexmedetomidine did not result in statistically significant improvement in mortality or ventilator-free days. However, the study may have been underpowered for mortality, and additional research may be needed to evaluate this further.
Trial Protocol
eTable 1. Sedative and Opioid Usage and Dosing in the First Week
eTable 2. Secondary Outcome Measurements
eFigure 1. Sedation and Analgesia Protocol
eFigure 2. Results of Subgroup Analyses on Mortality
Statistical Analysis Plan
Abbreviations List
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- CAM-ICU
Confusion Assessment Method for Intensive Care Unit Patients
- MMSE
Mini-Mental State Examination
- RASS
Richmond Agitation-Sedation Scale
- SOFA
Sequential Organ Failure Assessment
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Jakob SM, Ruokonen E, Grounds RM, et al. ; Dexmedetomidine for Long-Term Sedation Investigators . Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151-1160. [DOI] [PubMed] [Google Scholar]
- 2.Reade MC, Eastwood GM, Bellomo R, et al. ; DAHLIA Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group . Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. 2016;315(14):1460-1468. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32(6):1322-1326. [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi T, Kurita A, Kobayashi K, Yamamoto K, Inaba H. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth. 2008;22(3):221-228. [DOI] [PubMed] [Google Scholar]
- 5.Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17(5):576-584. [DOI] [PubMed] [Google Scholar]
- 6.Frumento RJ, Logginidou HG, Wahlander S, Wagener G, Playford HR, Sladen RN. Dexmedetomidine infusion is associated with enhanced renal function after thoracic surgery [retraction published in J Clin Anesth. 2013;25(5):432]. J Clin Anesth. 2006;18(6):422-426. [DOI] [PubMed] [Google Scholar]
- 7.Aydin C, Bagcivan I, Gursoy S, Altun A, Topcu O, Koyuncu A. Altered spontaneous contractions of the ileum by anesthetic agents in rats exposed to peritonitis. World J Gastroenterol. 2009;15(13):1620-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandharipande PP, Sanders RD, Girard TD, et al. ; MENDS Investigators . Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14(2):R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bone RC, Balk RA, Cerra FB, et al. ; ACCP/SCCM Consensus Conference Committee . Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644-1655. [DOI] [PubMed] [Google Scholar]
- 10.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. [DOI] [PubMed] [Google Scholar]
- 11.Barr J, Fraser GL, Puntillo K, et al. ; American College of Critical Care Medicine . Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. [DOI] [PubMed] [Google Scholar]
- 12.Oda S, Aibiki M, Ikeda T, et al. ; Sepsis Registry Committee of the Japanese Society of Intensive Care Medicine . The Japanese guidelines for the management of sepsis. J Intensive Care. 2014;2(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent JL, de Mendonça A, Cantraine F, et al. ; Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine . Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26(11):1793-1800. [DOI] [PubMed] [Google Scholar]
- 14.Kushimoto S, Gando S, Saitoh D, et al. ; Japanese Association for Acute Medicine Disseminated Intravascular Coagulation Study Group . Clinical course and outcome of disseminated intravascular coagulation diagnosed by Japanese Association for Acute Medicine criteria: comparison between sepsis and trauma. Thromb Haemost. 2008;100(6):1099-1105. [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818-829. [PubMed] [Google Scholar]
- 16.Riker RR, Shehabi Y, Bokesch PM, et al. ; Safety and Efficacy of Dexmedetomidine Compared With Midazolam Study Group . Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489-499. [DOI] [PubMed] [Google Scholar]
- 17.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. [DOI] [PubMed] [Google Scholar]
- 18.Skrobik Y, Ahern S, Leblanc M, Marquis F, Awissi DK, Kavanagh BP. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111(2):451-463. [DOI] [PubMed] [Google Scholar]
- 19.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shehabi Y, Chan L, Kadiman S, et al. ; Sedation Practice in Intensive Care Evaluation Study Group Investigators . Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. 2013;39(5):910-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled Trial): a randomised controlled trial. Lancet. 2008;371(9607):126-134. [DOI] [PubMed] [Google Scholar]
- 22.Vasilevskis EE, Ely EW, Speroff T, Pun BT, Boehm L, Dittus RS. Reducing iatrogenic risks: ICU-acquired delirium and weakness—crossing the quality chasm. Chest. 2010;138(5):1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmittinger CA, Torgersen C, Luckner G, Schröder DC, Lorenz I, Dünser MW. Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med. 2012;38(6):950-958. [DOI] [PubMed] [Google Scholar]
- 24.Sander O, Welters ID, Foëx P, Sear JW. Impact of prolonged elevated heart rate on incidence of major cardiac events in critically ill patients with a high risk of cardiac complications. Crit Care Med. 2005;33(1):81-88. [DOI] [PubMed] [Google Scholar]
- 25.Schmittinger CA, Dünser MW, Torgersen C, et al. Histologic pathologies of the myocardium in septic shock: a prospective observational study. Shock. 2013;39(4):329-335. [DOI] [PubMed] [Google Scholar]
- 26.Stolk RF, van der Poll T, Angus DC, van der Hoeven JG, Pickkers P, Kox M. Potentially inadvertent immunomodulation: norepinephrine use in sepsis. Am J Respir Crit Care Med. 2016;194(5):550-558. [DOI] [PubMed] [Google Scholar]
- 27.Ackland GL, Yao ST, Rudiger A, et al. Cardioprotection, attenuated systemic inflammation, and survival benefit of β1-adrenoceptor blockade in severe sepsis in rats. Crit Care Med. 2010;38(2):388-394. [DOI] [PubMed] [Google Scholar]
- 28.Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310(16):1683-1691. [DOI] [PubMed] [Google Scholar]
- 29.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93(2):382-394. [DOI] [PubMed] [Google Scholar]
- 30.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Sedative and Opioid Usage and Dosing in the First Week
eTable 2. Secondary Outcome Measurements
eFigure 1. Sedation and Analgesia Protocol
eFigure 2. Results of Subgroup Analyses on Mortality
Statistical Analysis Plan