Abstract
Understanding the protective mechanism in the liver induced by recombinant vaccines against the pre-erythrocytic stages of malaria is important for vaccine development. Most studies in mice have focused on splenic and peripheral blood T cells and identified gamma interferon (IFN-γ)-producing CD8+ T cells as correlates of protection, which can be induced by prime-boost vaccination with recombinant poxviruses. Invariant natural killer T (Vα14iNKT) cells can also protect against liver stage malaria, when activated, and are abundant in the liver. Since poxviruses have nonspecific immunomodulating effects, which are incompletely understood, we investigated whether recombinant poxviruses affect the protective properties of hepatic Vα14iNKT cells and thus vaccine efficacy. We show that intradermal vaccination with recombinant poxviruses activated Vα14iNKT cells and NK cells in the livers of BALB/c mice while inducing IFN-γ- and tumor necrosis factor alpha (TNF-α)-producing pre-erythrocytic stage antigen-specific CD8+ T cells. Greater numbers of hepatic Vα14iNKT cells secreted interleukin-4 than IFN-γ. Vaccinated Vα14iNKT-cell-deficient mice had lower, but still protective levels of hepatic and splenic IFN-γ+ and TNF-α+ CD8+ T cells and better protection rates later after challenge with Plasmodium berghei sporozoites. Therefore, vaccine-activated hepatic Vα14iNKT cells help in generating specific T cells but are not required for protection induced by recombinant poxviruses. Furthermore, double-positive INF-γ+/TNF-α+ CD8+ T cells were enriched in protected livers, suggesting that cells expressing both of these cytokines may be most relevant for protection.
The malaria parasites, Plasmodium spp., are a leading cause of death and morbidity in tropical countries. In the face of the increasing drug resistance of these parasites, other effective interventions, such as vaccines, that target the pre-erythrocytic liver, blood, and transmission stages are urgently needed (33). Understanding the protective immune mechanisms in the liver may be crucial for the development of a pre-erythrocytic stage vaccine (14). Natural and vaccine-induced immunity to liver-stage malaria requires specific and vigorous cellular immunity mediated by gamma interferon (IFN-γ), as well as nonspecific interleukin-12 (IL-12), IFN-γ-producing natural killer (NK) cells, and γδ T cells (13, 14, 20, 42, 43). The best-defined and clinically applied correlates of protection are IFN-γ-producing pre-erythrocytic stage-specific CD4+ and CD8+ T cells, which trigger the intracellular nitric oxide pathway in hepatocytes infected by sporozoites (20, 43). Infection with radiation-attenuated sporozoites induces such protective T-cell responses, as does heterologous prime-boost immunization with recombinant DNA and viral vectors, encoding pre-erythrocytic-stage antigens, such as the circumsporozoite protein (CSP), a major sporozoite surface antigen (15, 43, 45). Attenuated recombinant poxviruses such as modified vaccinia virus Ankara (MVA) (32, 45) and the new fowlpox strain, FP9 (3), have been successfully used for heterologous T-cell boosting and confer substantial protection against malaria in animals, as well as in some volunteers in phase IIa clinical trials (3; unpublished data).
Vaccine-induced T-lymphocyte responses have mainly been described in peripheral blood and murine spleens but not in the liver, except for studies on sporozoite immunization (4, 28). Since the immunogenic properties of attenuated recombinant poxviruses are incompletely understood, we examined the influence of FP9 and MVA vaccination on liver lymphocytes in more detail after a recent study demonstrating the induction of hepatic IFN-γ-producing CD8+ T cells by these vaccines (3).
We investigated whether poxvirus vaccination induced not only IFN-γ-producing, but also TNF-α-producing CD8+ T cells in the murine liver and whether they correlated with protection against sporozoite challenge. The induction of TNF-α-producing T cells is of particular interest in malaria because TNF-α can inhibit both liver- and blood-stage parasites (23, 24, 29, 36) and synergizes with IFN-γ to induce nitric oxide and kill parasites (23, 24).
In addition to CSP-specific CD8+ T cells, we also examined nonspecific invariant natural killer T (Vα14iNKT) and NK cells, since little is known about the paraspecific effects of attenuated poxviruses (31). It is known from live or killed viruses that they can activate peripheral blood, splenic, and hepatic NK cells (12, 31, 41). Vα14iNKT cells are a CD4+ or CD4− CD8− NK cell receptor bearing subpopulation of T cells with restricted T-cell-receptor (TCR) repertoire expression. In mice this includes the invariant Vα14-Jα18/Vβ2/Vβ7/Vβ8.2 chains. Vα14iNKT cells are particularly abundant in the murine liver and can be rapidly activated similar to NK cells (10, 16). In contrast to NK cells, they can produce not only IFN-γ but also IL-4 and thus can promote Th1 or Th2 type-specific immunity, activating antigen-presenting cells, NK cells, B cells, and T cells (7, 10, 16, 42). Since the liver is also known to be a Th2-driving environment, which could hamper vaccine-induced Th1 immunity against liver-stage parasites (10, 28), we have studied here IL-4 and IFN-γ production by Vα14iNKT cells. IL-4, derived from conventional CD4+ T cells, can promote the generation of liver-stage-specific CD8+ T cells (8) but also suppress Th1 immunity (49). Vα14iNKT cells release cytokines upon TCR-mediated recognition of the synthetic glycolipid ligand alpha-galactosylceramide (α-GalCer), which binds to CD1d molecules on antigen-presenting cells (26). Triggered by α-GalCer, IFN-γ-producing Vα14iNKT cells confer very short-term protection and adjuvant vaccine-induced IFN-γ-producing specific T cells against murine liver-stage malaria (18, 19). We hypothesized that there might be a synergistic induction of Vα14iNKT, CD8+ T, and NK cells by poxvirus vaccination independently from α-GalCer and that this might influence protection. To test our hypotheses, we immunized wild-type (WT) BALB/c mice and Vα14iNKT-cell-deficient Jα18-chain knockout (KO; Jα18−/−) mice with malaria poxvirus vaccines and challenged them with infectious sporozoites.
This comparative analysis of hepatic and splenic antigen-specific CD8+ T cells, Vα14iNKT cells, and NK cells after malaria poxvirus vaccination and challenge provides novel information on liver immunity against pre-erythrocytic-stage malaria.
MATERIALS AND METHODS
Immunization of mice with malaria vaccines.
We used 6- to 12-week-old female WT BALB/c mice and Jα18−/− mice (formerly Jα281−/−, backcrossed more than 10 times to BALB/c background) (mainly female) mice, which lack Vα14iNKT cells, in these experiments. Mice were originally obtained from The Jackson Laboratory (Bar Harbor, Maine) and Chiba University (Chuoku, Chiba, Japan) (11) and were bred at the Oxford University animal facilities under specific-pathogen-free conditions. The mice were handled according to the Home Office Animals Act 1986 (Scientific Procedures) guidelines under project license PPL 30/1805. Construction of the MVAPbCSP vaccine encoding the P. berghei circumsporozoite protein (PbCSP) has been previously described (45). The attenuated fowlpox vaccine strain FP9 was produced by multiple passage of WT strain HP-1 as previously described (3, 30). For the empty control viruses (FP9/MVAlacZ), fowlpox vaccine strains were recombined with pEFL29 without an inserted antigen but contain the β-galactosidase gene under a late promoter (3). Recombinant viruses were administered intradermally bilaterally into the ears at a dose of 106 PFU, diluted in 50 μl of endotoxin-free Dulbecco’s phosphate-buffered saline (PBS; Sigma). Mice were primed with FP9PbCSP (F) and boosted 2 weeks later with MVAPbCSP (M). Some mice were prime-boosted with the control viruses FP9lacZ and MVAlacZ. Mice were sacrificed for immunogenicity assays 14 to 40 days postimmunization.
Challenge of mice with P. berghei sporozoites.
Laboratory-reared female Anopheles stephensi mosquitoes infected with P. berghei sporozoites (ANKA strain clone 234) were maintained at 21°C for 21 to 25 days after feeding on infected mice. Mosquito salivary glands were dissected and homogenized in 4°C RPMI 1640 media (Sigma) to release sporozoites (45). Groups of F/M-vaccinated mice (n = 3 to 17) were challenged and, as controls, naive mice (n = 3 to 9) were infected with 1,000 sporozoites (100 μl) by intravenous injection into the lateral tail vein, providing a stringent challenge (3). Blood-stage infection (patency) was monitored from days 3 to 13 postinfection or challenge for the presence of ring forms by using Giemsa-stained blood smears. Mice were either sacrificed 1 day or 6 to 13 days after challenge or maintained for rechallenge at 40 days after immunization.
Cell preparation.
Splenocytes were isolated by mechanical disruption of spleens, filtration, and red blood cell lysis in ammonium chloride buffer and prepared for enzyme-linked immunospot (ELISPOT) assays and fluorescence-activated cell sorter (FACS) analysis as previously described (45). Only lymphocytes with >98% viability were gated, and absolute numbers were counted on a CASY Cell Analyzer (Model TT; Schaerfe System GmbH, Reutlingen, Germany). For the isolation of hepatic mononuclear cells (MNC), livers were flushed in situ via the portal vein with 10 ml of PBS containing 10% fetal bovine serum. After mechanical disruption and filtration, pelleted cells were resuspended in 5 ml of buffered 80% Percoll (Amersham Pharmacia Biotech; supplemented with 0.5% bicarbonate [7%], 10% 10xRPMI, 1% fetal bovine serum) overlaid with 5 ml of 35% buffered Percoll and spun at room temperature at 500 × g without brake for 25 min. MNC from the interface were pelleted, washed, and resuspended in 1 ml of medium, and lymphocytes were counted as described above and further processed for ELISPOT assays or FACS analysis.
ELISPOT assays.
IFN-γ, tumor necrosis factor alpha (TNF-α), or IL-4 ex vivo ELISPOT assays on splenocytes and hepatic MNC were conducted as previously described for splenic IFN-γ-producing T cells (45). Briefly, ELISPOT plates (Millipore) were coated with 10 μg of anti-mouse IFN-γ antibody (Ab; clone R4-6A2), anti-TNF-α Ab (clone G281-2626), or anti-IL-4 Ab (clone BVD4-1D11)/ml (Becton Dickinson). Target cells (0.5 × 106 cells/well) were prepared by pulsing naive splenocytes with (i) the H-2Kd-restricted test peptide Pb9 (SYIPSAEKI), an immunodominant CD8 epitope from P. berghei CSP, which is only recognized by peptide Pb9-specific CD8+ T cells; (ii) α-GalCer (provided by Kirin Brewery, Ltd., Tokyo, Japan) to detect α-GalCer responsive Vα14iNKT cells; or (iii) an irrelevant control peptide. Splenocytes were applied at 0.5 × 106/well, and liver MNC at various cell numbers per milliliter (mean ± the standard deviation [SD]: [2.5 ± 2] × 106/ml; [1.2 ± 1] × 105/well), and responses as spot-forming cells (SFC) per million were calculated from duplicate wells after subtraction of negative control wells. After 18 to 24 h of incubation at 37°C under 5% CO2, the plates were washed once with distilled water and four times with PBS before overnight incubation with biotinylated anti-mouse IFN-γ Ab (clone XMG1.2), anti-TNF-α Ab (clone MP6-XT3), or anti-IL-4 Ab (clone BVD6-24G2) (Becton Dickinson). The assay was developed by further incubation with streptavidin-alkaline phosphatase polymer (Mabtech; Sigma), and spots were visualized by adding an alkaline phosphatase substrate solution (Bio-Rad). Spots were counted by using an AID ELISPOT counter (AID GmbH).
Cell staining and FACS analysis.
For detection of Vα14iNKT cells, splenocytes and hepatic MNC were four-color stained with CD1d/α-GalCer tetramer-phycoerythrin (PE), biotinylated anti-TCRβ chain Ab (clone H57-597) conjugated with peridinin chlorophyll protein (PerCP), anti-CD8a-allophycocyanin (APC) (Ly-2, clone 53-6.7), and anti-CD4-fluorescein isothiocyanate Ab (FITC; clone H129.19) (Becton Dickinson). A total of 15,000 to 70,000 gated lymphocytes were acquired. The CD1d/α-GalCer tetramer was generated by in vitro oxidative refolding chromatography as previously described (25). NK cells and conventional T cells were detected by four-color staining with anti-CD49b/Pan-NK cell Ab (clone DX5), biotinylated anti-TCRβ chain (clone H57-597) conjugated with streptavidin-PerCP, anti-CD8a-APC Ab (Ly-2, clone 53-6.7), and anti-CD4-FITC Ab (clone H129.19). A total of 10,000 lymphocytes were gated at analysis. Intracellular cytokine staining (ICS) was performed on approximately 2 × 106 splenocytes and 2 to 8 × 105 liver MNC pooled from two or four mice, stimulated with peptide Pb9, phytohemagglutinin, or an irrelevant peptide for 6 h. A Cytofix/Cytoperm Plus (with Golgi Plug) kit (Becton Dickinson) was applied according to the manufacturer by using anti-IFN-γ-FITC Ab (clone XMG1.2), biotinylated anti-TNF-α Ab (clone MP6-XT3) conjugated with streptavidin-PerCP, anti-CD8a-APC Ab (clone 53-6.7), and anti-CD3-PE Ab (clone 145-2C11) for staining. Isotype controls were performed with rat immunoglobulin G1 (IgG1)-PE (clone R3-34), rat IgG2a-FITC (clone R35-95), hamster IgG1-FITC (clone A19-3), hamster IgG2 (clone Ha4/8), and rat IgM (clone R4-22). A total of 30,000 to 200,000 gated lymphocytes were acquired. The PE-conjugated H-2Kd/Pb9 tetramer was generated as described previously (2) and applied, together with anti-B220-FITC Ab and anti-CD8β-Tricolor Ab (CalTag-Medsystems), in FACS buffer for incubation of cells for 20 to 30 min at 37°C. All samples were preincubated with Fc-block (anti-FcRγIII/II CD16/CD32 Ab, clone 2.4G2). After staining, all cells were fixed in PBS-2% paraformaldehyde and analyzed by a FACSCalibur instrument (Becton Dickinson) using CellQuest software (Becton Dickinson).
Statistical analysis.
The paired-sample and unpaired Student t test was used for analysis of normally distributed data. Differences in protection rates of P. berghei-infected mice were assessed by using 95% confidence intervals (CI) according to the method of Newcombe (34, 35). Statistical analysis was performed by using SPSS for Windows version 10 (SPSS, Inc.), Microsoft Excel 2001 or, for calculating the odds ratio (OR), QuickView 4.0/Statcalc.
RESULTS
Prime-boost vaccination induces IFN-γ- and TNF-α-producing pre-erythrocytic-stage antigen-specific CD8+ T cells in the liver and spleen.
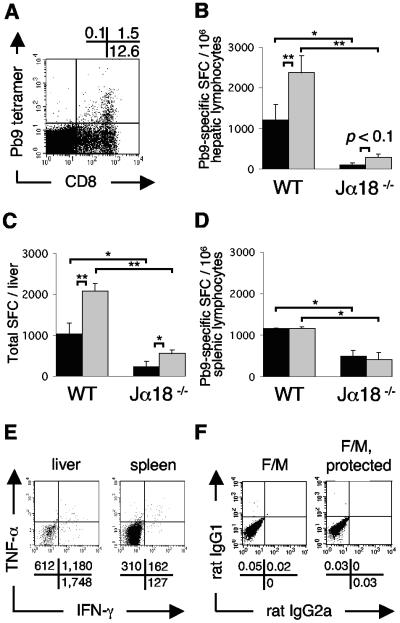
BALB/c mice were intradermally immunized against the P. berghei pre-erythrocytic-stage antigen CSP by heterologous prime-boost poxvirus vaccination with FP9PbCSP and MVAPbCSP (F/M). First, we determined the total number of CD8+ T cells specifically induced in the liver and spleen by staining with peptide Pb9-tetramer. About 10% of hepatic CD8+ T cells (Fig. 1A) were antigen specific. Next, we analyzed the levels of IFN-γ-producing peptide pb9-specific CD8+ T cells in ELISPOT assays. Vaccination clearly induced equal levels of IFN-γ-producing CD8+ T cells in livers (Fig. 1B and C) and spleens (Fig. 1D), reaching approximately 1,000 SFC/million lymphocytes. Further, we examined in parallel whether TNF-α-producing antigen-specific CD8+ T cells were generated alongside IFN-γ-producers, because the total number of peptide Pb9-specific tetramer+ T cells exceeded that of IFN-γ+/CD8+ T cells alone in ELISPOT assays (for example, 1,000 SFC/million hepatic lymphocytes corresponds to 0.5 to 1% tetramer+ CD8+ T cells). Vaccination also strongly induced TNF-α-producing peptide pb9-specific CD8+ T cells in livers (Fig. 1B and C) and spleens (Fig. 1D). The TNF-α secretors exceeded IFN-γ secretors in the liver (ratio of about 2:1) but not in the spleen. The nonspecific background production of both cytokines was very low. ICS confirmed that T cells and not macrophages secreted TNF-α and therefore accounted for the spots observed in ELISPOT assays (data not shown). ICS revealed that vaccination induced double-positive (IFN-γ+/TNF-α) CD8+ T cells, more in liver than in spleen, upon stimulation with peptide Pb9 (Fig. 1E); ICS isotype controls showed no or very little nonspecific staining (Fig. 1F), and controls with the irrelevant peptide or phytohemagglutinin were negative or positive, respectively (data not shown). In addition, in F/M-vaccinated WT mice no IL-4-producing CD8+ T cells were detected by peptide Pb9 ELISPOT assays in the liver or spleen above low naive background levels (data not shown).
FIG. 1.
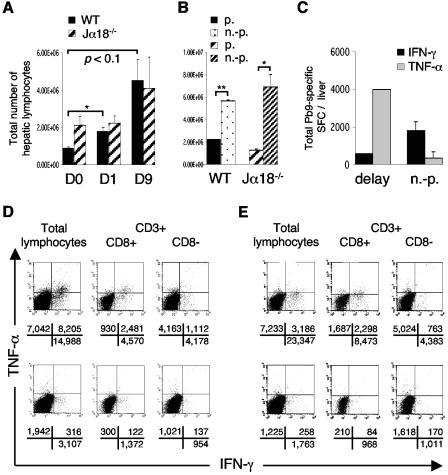
Prime-boost vaccination with FP9PbCSP/MVAPbCSP (F/M) induces peptide Pb9-specific IFN-γ-/TNF-α-producing CD8+ T cells, promoted by Vα14iNKT cells. (A) Representative Pb9 tetramer+/CD8+-T-cell population (gated first on lymphocytes and then on TCRβ+ cells) in the liver 14 days after intradermal vaccination of BALB/c mice. The percentages of positive cells in each quadrant are displayed above FACS analysis profiles. (B to D) IFN-γ-producing (▪) and TNF-α-producing (░⃞) peptide Pb9-specific CD8+ T cells in the livers (B and C) and spleens (D) of BALB/c WT mice in comparison with Vα14iNKT-cell-deficient Jα18−/− BALB/c mice (n = 4/group). SFC/million lymphocytes (B and D) and total SFC per liver (C) were detected by IFN-γ and TNF-α ELISPOT assays 20 days after F/M vaccination. Means ± the standard error of the mean (SEM) are shown; the statistical significance was determined by using the Student t test (**, P < 0.01; *, P < 0.05). The results of one representative experiment out of two performed are shown. (E) ICS of CD8+/CD3+ Pb9-stimulated pooled (n = 2) hepatic lymphocytes and splenocytes from WT mice 40 days after vaccination. The absolute numbers of positive cells per million lymphocytes are shown for the respective quadrants, as calculated from the events (gated on CD8+/CD3+) for the absolute numbers of CD8+ T cells in one million lymphocytes (according gated percentages of CD8+ T cells from all lymphocytes) are given below the FACS analysis profiles. (F) ICS isotype control dot plots for IFN-γ/TNF-α (rat IgG2a) and CD8 (rat IgG1) from hepatic MNC from pooled WT mice 25 days after F/M vaccination (n = 2) and for protected vaccinated WT mice (see Fig. 5D) 9 days after sporozoite challenge, at 40 days postvaccination (n = 2). The percentages of positive cells are shown for the respective quadrants.
Vα14iNKT cells promote the generation of IFN-γ- and TNF-α-producing pre-erythrocytic-stage antigen-specific CD8+ T cells in the liver and spleen.
We determined whether these specific T-cell responses were influenced by the presence of Vα14iNKT cells in the absence of the activating ligand αGalCer. F/M vaccination of Vα14iNKT-cell-deficient Jα18−/− mice induced significantly fewer IFN-γ-producing peptide Pb9-specific CD8+ T cells in livers and spleens compared to WT mice (Fig. 1B to D). In livers, lower frequencies were evident for the relative SFC per million cells (Fig. 1B), as well as for the total absolute number per liver (Fig. 1C). This reduction was also observed for splenic (Fig. 1D) and particularly strongly for hepatic (Fig. 1B and C) TNF-α-producing CD8+ T cells in the absence of Vα14iNKT cells. However, taken together, approximately 500 and 1,000 SFC/million lymphocytes were still induced in the liver and spleen, respectively. Further, in F/M-vaccinated Jα18−/− mice, similar to WT mice, no IL-4-producing CD8+ T cells were detected by peptide Pb9 ELISPOT assays in the liver or spleen above low naive background levels (data not shown).
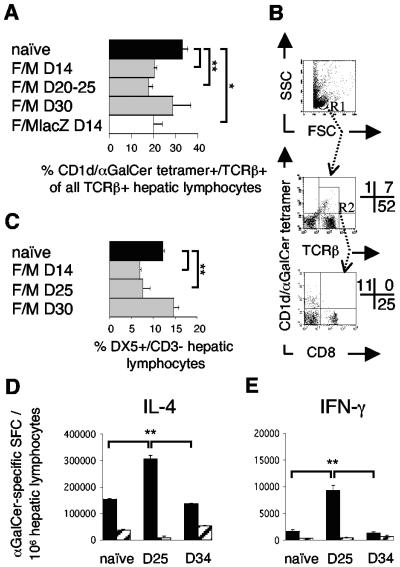
Prime-boost poxvirus vaccination activates α-GalCer responsive Vα14iNKT cells and NK cells in the liver.
Since Vα14iNKT cells promoted vaccine-induced CD8+ T cells in the absence of αGalCer, we tested whether attenuated poxviruses themselves exerted nonspecific influence on the hepatic and splenic populations of Vα14iNKT and NK cells after intradermal vaccination. To this end, we analyzed cell numbers and Vα14iNKT cell cytokine production. The frequencies of hepatic CD1d/α-GalCer tetramer+/TCRβ+ Vα14iNKT cells (percentage of all TCRβ+ cells) were reduced 14 and 20 to 25 days, but not 30 days, after the boost compared to naive mice (Fig. 2A and B). This reduction was also observed after administration of the non-CSP-expressing virus constructs (FP9lacZ/MVAlacZ) (Fig. 2A) and 14 days after single immunizations (data not shown). Splenic Vα14iNKT cells were not affected (data not shown). CD1d/α-GalCer tetramer+/TCRβ+ Vα14iNKT cells were CD8− (Fig. 2B). DX5+/CD3− NK cell frequencies were similarly reduced in the liver 14 and 25 days postvaccination (Fig. 2C), but not in spleens (3 to 5% of lymphocytes [data not shown]).
FIG. 2.
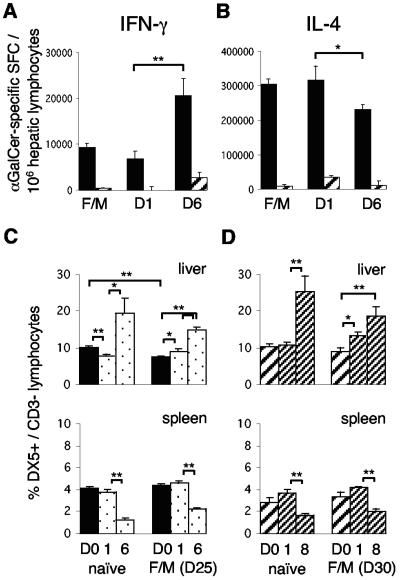
Systemic activation of Vα14iNKT and NK cells in the livers of BALB/c mice by intradermal F/M vaccination. (A) Hepatic CD1d/α-GalCer tetramer+/TCRβ+ Vα14iNKT cell frequencies (gated as shown in panel B) by FACS analysis 14 days (D14) to 30 days after vaccination with the malaria vaccines or with empty control viruses (FP9lacZ/MVAlacZ). Pooled data from eight experiments, each including naive mice (n = 3 to 4/group) are presented. (B) Representative FACS dot plots of Vα14iNKT cells on day 25 after F/M vaccination from panel A, with the percentages of positive cells displayed in quadrants. Gating first on lymphocytes (R1, upper dot plot) yielded CD1d/α-GalCer tetramer+/TCRβ+ cells (Vα14iNKT cells) in the upper right quadrant (middle dot plot). Vα14iNKT cell frequencies in panel A are calculated as a percentage of these double tetramer+/TCRβ+ cells from the sum of all TCRβ+ cells (upper and lower right quadrant, middle dot plot). A second gating on all TCRβ+ cells (R2, middle dot plot) demonstrates tetramer+ cells to be CD8− (lower dot plot). (C) Hepatic NK cell frequencies before and after vaccination as in panel A, as determined by FACS analysis. (D and E) Hepatic (▪) and splenic (░⃞) ex vivo IL-4 (D) and IFN-γ (E) Vα14iNKT-cell responses to α-GalCer, as measured by ELISPOT assay, from naive mice and mice 25 and 34 days after F/M vaccination (n = 4/group). The results from one representative experiment out of two performed are shown. Results are means ± the SEM; statistical analysis was done as described for Fig. 1.
Upon ex vivo stimulation with α-GalCer in ELISPOT assays, we found that hepatic Vα14iNKT cells were activated to produce IL-4 and IFN-γ within 25 days after F/M vaccination (Fig. 2D and E). The frequency of IL-4-producing liver Vα14iNKT cells was increased twofold (Fig. 2D), and that of IFN-γ producers was increased fivefold (Fig. 2E) in comparison to naive mice. Although the relative vaccine-induced activation of IFN-γ producers was more pronounced, IL-4-secreting liver Vα14iNKT cells greatly outnumbered IFN-γ secretors in both naive and immunized mice at a ratio of 75:1 and 30:1, respectively. In clear contrast to the liver, IL-4- and IFN-γ-producing α-GalCer responsive Vα14iNKT cells were much less frequent in the spleens of naive mice and not enhanced by vaccination. Splenic IL-4 producers were even reduced 25 days postvaccination, but they also outnumbered IFN-γ secretors in naive mice (90:1) and less so in immunized mice (20:1). At 34 days postvaccination, the cytokine production of hepatic Vα14iNKT cells had returned to naive levels (Fig. 2D and E).
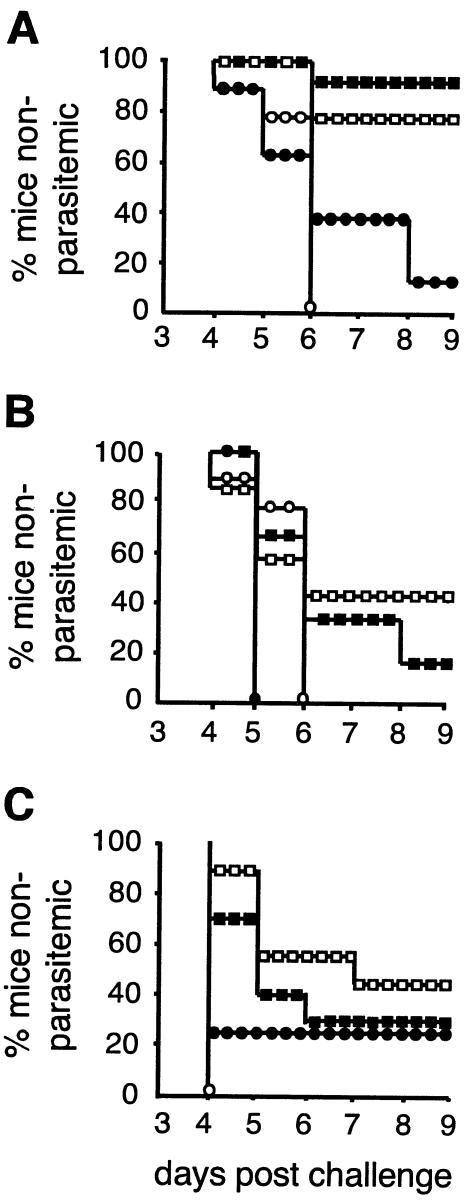
Vα14iNKT cells do not improve, but partially diminish, protection rates after sporozoite challenge.
F/M-vaccinated WT and Vα14iNKT-cell-deficient Jα18−/− mice were challenged with P. berghei sporozoites in order to evaluate whether such differences in the magnitude of specific T-cell responses between the strains and the absence or presence of poxvirus-activated Vα14iNKT cells affected protection rates. Challenges were performed 10, 20, and 40 days after vaccination to test the influence of short-lived vaccine-activated Vα14iNKT cells at days 10 and 20 (Fig. 2A) or nonactivated Vα14iNKT cells (day 30, Fig. 2A) on protection. After the first challenge at day 10, WT and Jα18−/− mice were similarly protected at a rate of 80 to 90% with no difference in the time to parasitemia compared to matched nonvaccinated controls (Fig. 3A). The protection rates dropped rapidly in both vaccinated strains to below 45% when challenged 20 days postimmunization (Fig. 3B). The level of protection was still significantly higher in vaccinated than in nonvaccinated Jα18−/− mice but not in WT mice. Protection rates also declined to below 45% after rechallenge at day 40 in mice of both strains previously protected by vaccination and were better in vaccinated Jα18−/− mice compared to pooled nonvaccinated KO controls (Fig. 3C).
FIG. 3.
Protection rates after sporozoite challenge of vaccinated BALB/c WT (▪) and Vα14iNKT-cell-deficient Jα18−/− (□) mice. (A) Challenge 10 days after F/M immunization or infection of nonimmunized control BALB/c WT (•) and Jα18−/− (○) mice by intravenous injection of 1,000 live sporozoites (n = 15 [▪], 17 [□], 8 [•], or 6 [○]). CIs significant for comparison of immunized versus control mice were as follows: WT mice (39.2 to 92.5%) and Jα18−/− mice (30.8 to 90.4%). (B) Challenge 20 days after immunization. Data were pooled from two experiments (n = 6 [▪], 7 [□], 6 [•], or 9 [○]). The CI values were significant only for immunized versus control Jα18−/− mice (2.5 to 75%). (C) Rechallenge of some of the mice, which were protected in panel A, at day 40 postimmunization (n = 10 [▪], 9 [□], 4 [•], or 3 [○]); there was a significant difference between vaccinated animals and nine pooled KO controls (5.1 to 73.3%). Parasitemia was monitored by peripheral blood films; protected mice were negative on day 13.
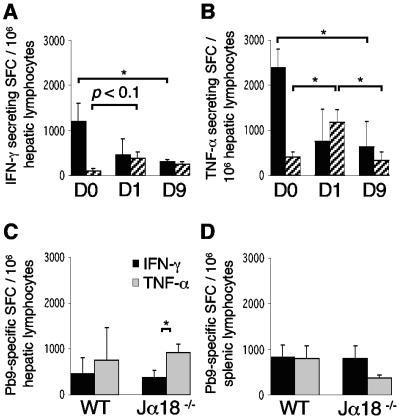
Kinetics of vaccine-induced hepatic IFN-γ+ CD8+ T cells correlate with TNF-α+ CD8+ T cells in response to sporozoite challenge and are influenced by Vα14iNKT cells.
Next, we tested whether hepatic IFN-γ- and TNF-α-producing CD8+ T cells responded to sporozoite challenge after vaccination dependent on Vα14iNKT cells. In both WT and Vα14iNKT-cell-deficient Jα18−/− mice the kinetics of IFN-γ-producing CD8+ T cells were similar to those of TNF-α producers in the early (day 1) and late (day 9) response to challenge, but were different between the strains, and were therefore influenced by Vα14iNKT cells (Fig. 4). In Vα14iNKT cell-deficient mice, hepatic TNF-α producers exceeded IFN-γ producers before (Fig. 1B and C) and after challenge (Fig. 4C), peaked 1 day after challenge at around 500 to 1,000 SFC/million, and declined to a level similar to that of WT mice after 1 week (Fig. 4A and B). The kinetics were different in WT mice because the levels of hepatic (Fig. 4A and B) and splenic (data not shown) IFN-γ-secreting (Fig. 4A) and TNF-α-secreting (Fig. 4B) CD8+ T cells markedly declined within 1 week; in some livers this occurred within 1 day after challenge. This reduction also implied a relative shift toward IFN-γ production (Fig. 4C). In the spleens, prechallenge differences (Fig. 1D) between WT and KO mice disappeared (Fig. 4D), but no significant reduction and therefore redistribution of these CD8+ T cells to the liver occurred.
FIG. 4.
Different kinetics of hepatic IFN-γ- and TNF-α-producing CD8+ T cells in vaccinated BALB/c WT (▪) and vaccinated Vα14iNKT-cell-deficient Jα18−/− (▨) mice after sporozoite challenge. (A and B) Peptide Pb9-specific CD8+-T-cell responses were measured 20 days after F/M immunization before challenge (day 0 [D0]) and 1 and 9 days after challenge (n = 4) in IFN-γ (A) and TNF-α (B) ELISPOT assays. (C and D) Comparison between these IFN-γ and TNF-α responses in the liver (C) and spleen (D) 1 day after challenge. The results in panels A and B include some data from Fig. 2. Values are means ± the SEM; statistical analysis was done as in Fig. 1. The results for one representative experiment out of two performed are shown.
Hepatic pre-erythrocytic-stage antigen-specific IFN-γ and TNF-α CD8+ T-cell responses are stronger in protected than parasitemic mice.
Since the kinetics of both IFN-γ and TNF-α-producers altered with challenge, we further investigated how this related to protection and patency. After challenge, lymphocytes infiltrated the liver (Fig. 5A), particularly strongly with patent infection in WT and Vα14iNKT-cell-deficient Jα18−/− mice (Fig. 5B). This influx was associated with increasing TNF-α+ and decreasing IFN-γ+/CD8+-T-cell numbers in mice with delayed parasitemia (partial protection) and was associated with the reverse in unprotected mice (Fig. 5C). At 13 days postchallenge IFN-γ+/CD8+ T cells predominated irrespective of protection status (Fig. 5D). Vaccination-induced double-positive (IFN-γ+/TNF-α+) CD8+ T cells were particularly enhanced—in absolute number and frequency—in livers of fully protected rechallenged WT mice (Fig. 5D; 2,481 versus 122), as well as Jα18−/− mice, compared to parasitemic mice (Fig. 5E; 2,298 versus 84) and compared to postvaccination levels (Fig. 1A and Fig. 5D; 2,481 versus 1,180 CD8+ T cells). These cells accounted for 30% of CD8+ T cells, whereas 60% produced IFN-γ and 10% produced TNF-α alone (Fig. 5D). Such populations of double-positive T cells were also observed in protected mice 9 days after one challenge (data not shown). After 1 week of parasitemia, the absolute number of single and double cytokine-producing CD8+ T cells was strongly reduced and, of those, only 7% expressed both cytokines, 77% expressed IFN-γ alone, and 17% expressed TNF-α alone (Fig. 5D). These data show that double-positive TNF-α+/IFN-γ+ CD8+ T cells in the liver were more strongly associated with protection than single-positive IFN-γ+ (OR = 6.56, P < 0.01) or TNF-α+ CD8+ T cells (OR = 6.11; P < 0.01). The same association was observed in Jα18−/− mice with lower frequencies of double IFN-γ+/TNF-α+ cells for total lymphocytes than CD3+/CD8+ or CD3+/CD8− T cells (Fig. 5E). The spleens of KO mice contained very few double-positive IFN-γ+/TNF-α+ CD8+ T cells after challenge, and in WT spleens they were decreased from postvaccination levels (Fig. 1E) independent of the protection status (data not shown). Furthermore, the livers of protected KO mice contained more single TNF-α+ and IFN-γ+ CD8+ T cells than those of protected WT mice (Fig. 5D and E).
FIG. 5.
Protection is associated with stronger hepatic peptide Pb9-specific IFN-γ and TNF-α CD8+-T-cell responses and weaker liver lymphocytosis than patent infection. (A) Absolute liver lymphocyte frequencies before (day 0 [D0]) and at days 1 and 9 days postchallenge of BALB/c WT and Jα18−/− mice (n = 4); challenge was performed 20 days after F/M vaccination. (B) Samples from panel A (day 9), separated into protected (p., n = 2) and unprotected (n.-p., n = 2) mice, including a delay in time to parasitemia as partial protection. (C) Examples of absolute peptide Pb9-specific liver ELISPOT responses at day 9 in WT mice from panel B, including a 2-day delay until day 8, with a read-out on the second parasitemic day, day 9. Means ± the SEM are shown; statistical analysis was done as in Fig. 1. (D and E) Pb9-stimulated pooled hepatic MNC from protected (n = 4, upper row) and unprotected (n = 4, bottom row) WT (D) and Jα18−/− (E) mice 13 days after rechallenge (rechallenge was performed at day 40 postvaccination). Absolute positive cells per million lymphocytes in the respective quadrants, calculated with percentages of gated CD8+/CD3+ and CD8−/CD3+ subpopulations, are demonstrated below the FACS analysis profiles.
Furthermore, nonspecific hepatic background production of IFN-γ and TNF-α was detected in ELISPOT assays and ICS in all infected, but particularly in protected mice, more in KO than in WT mice, when the total absolute frequencies were compared. Most of it derived from CD3− lymphocytes and some CD8− T cells (Fig. 5D and E).
ICS isotype controls of hepatic MNC from protected mice showed very low or no nonspecific staining of the cytokines (rat IgG1), CD8 (rat IgG2a) (Fig. 1F), or CD3 (hamster IgG1) (data not shown).
Sporozoite challenge affects hepatic Vα14iNKT cells and NK cells.
Since non-T-cell-related cytokine expression was pronounced in protected mice, we examined whether challenge influenced Vα14iNKT cells or NK cells. Significantly more hepatic Vα14iNKT cells secreted IFN-γ 6 days after challenge compared to 1 day after challenge and compared to mice that were vaccinated but not challenged (Fig. 6A) or to naive WT mice (Fig. 2E). At the same time, hepatic IL-4-producing Vα14iNKT cells decreased from a ratio of 30:1 (300,000:10,000) at 1 day postchallenge to 10:1 (200,000: 20,000) at 6 days (Fig. 6B). The total numbers of hepatic CD1d tetramer/α-GalCer+ or IL-4/IFN-γ-expressing splenic Vα14iNKT cells were not affected (data not shown). Concomitantly, DX5+/CD3− NK cells increased twofold in livers and conversely declined in spleens 6 days after challenge in vaccinated WT and Vα14iNKT-cell-deficient Jα18−/− mice, findings equal to those observed in naive infected mice. More NK cells appeared in the livers of naive and vaccinated Jα18−/− mice than in those of WT mice at the first day postchallenge (P = 0.023 and 0.001, respectively). In contrast to WT mice (Fig. 2C and 6C), immunization did not reduce the numbers of hepatic NK cells in Vα14iNKT-cell-deficient mice (Fig. 6D).
FIG. 6.
Sporozoite challenge shifts the cytokine production of Vα14iNKT cells and influences NK cells in the liver. (A and B) IFN-γ (A) and IL-4 (B) Vα14iNKT cell responses to α-GalCer, measured by ELISPOT assay, in the livers (▪) and spleens (▨) of BALB/c WT mice, F/M vaccinated on day 25 (D25) and examined on days 1 and 6 after challenge (n = 4). This is the same experiment as in Fig. 1. (C and D) NK cell frequencies, as determined by FACS analysis, in the livers and spleens of WT (C) and Jα18−/− (D) mice. Mice were challenged 25 or 30 days after vaccination, or nonvaccinated mice were examined before infection (day 0, n = 8) or 1, 6, or 8 days postinfection (n = 4). Means ± the SEM are shown; statistical analysis was performed as in Fig. 1. Results from a representative experiment out of two performed are shown.
In summary, infection induced a Th1-directed shift of cytokine-producing Vα14iNKT cells. Immunization and challenge affected NK cells differently in the absence or presence of Vα14iNKT cells.
DISCUSSION
Recently, glycolipid-activated Vα14iNKT cells have been reported to play an adjuvant-like role in enhancing certain types of vaccination against liver-stage malaria (19), but it is unclear whether recombinant vaccines alone affect Vα14iNKT cells. There are very few kinetic studies of innate, non-macrophage-related responses after vaccination. For example, the killed influenza vaccine and combined mumps-measles-rubella vaccine enhance NK cell activity for 2 to 30 days (38, 41). The present study demonstrates that intradermal prime-boost vaccination with the attenuated, nonreplicating recombinant poxviruses FP9/MVAPbCSP systemically activates the already prevailing IL-4, as well as IFN-γ production of hepatic, but not splenic, Vα14iNKT cells in BALB/c mice and induces a temporary reduction in frequencies of Vα14iNKT and NK cells. The main mechanism for this reduction may be activation-induced cell death because hepatic Vα14iNKT cells, and in turn transactivated NK cells, are particularly prone to this, e.g., after in vivo stimulation with α-GalCer (37). In comparison, we found a longer and α-GalCer-independent activation of both populations ex vivo in response to αGalCer for approximately 25 days after immunization. This activation by αGalCer in ELISPOT assays (19) is unlikely to involve bystander activation of CD8+ or CD4+ T cells because αGalCer cannot indirectly stimulate more hepatic CD8+ T cells than peptide Pb9 itself (ca. 10,000 versus 1,000 SFC), a possibility also countered by the low numbers in CD8+/CD4+ T-cell- and NK-cell-rich spleens. Bystander activation was also not observed in a similar malaria vaccination model with nonseparated peptide-stimulated cells (9). Further, we detected no peptide Pb9-specific vaccine-induced IL-4-producing CD8+ T cells in the liver or spleen. Interestingly, IL-4-producing T cells predominate in human blood 14 days after subcutaneous measles virus vaccination (47), and repeated in vivo administrations of α-GalCer polarize splenic Vα14iNKT cells toward IL-4 (6). Here, two consecutive poxvirus vaccinations induced a proportional, but not a complete, Th1 shift in the liver because IL-4-producing Vα14iNKT cells prevailed. This supports the concept of the liver as a Th2-driving environment (10, 27) and might be relevant for vaccine design and studies with multiple poxvirus booster immunizations at short intervals, which have occasionally revealed reduced vaccine efficacy (3).
We assessed whether such activated Vα14iNKT cells and their absence in Jα18−/− mice could influence vaccine immunogenicity and protection. Nonactivated Vα14iNKT cells have no effect on sporozoite-induced memory responses, while α-GalCer-activated Vα14iNKT cells enhance protection mediated by IFN-γ CD8+ T-cell memory responses to recombinant Sindbis virus, adenovirus, and irradiated sporozoite malaria liver-stage vaccines (19). Our findings extend these data by demonstrating that not only IFN-γ-secreting but also TNF-α-secreting CSP (peptide Pb9)-specific CD8+ T cells were increased in the spleen and more so in the liver in a synergistic manner by Vα14iNKT cells independently of α-GalCer, because both were reduced in poxvirus-vaccinated Jα18−/− mice compared to WT mice. Sufficient backcrossing of the KO mice excludes the possibility that CD8+-T-cell numbers were influenced by differences in the genetic backgrounds of the lines (14). We propose that vaccine-activated IFN-γ- and IL-4-producing Vα14iNKT cells are responsible for this intrinsic adjuvant effect of poxvirus vaccines. IFN-γ and IL-4 can promote the generation of antigen-specific Th1 cells (8, 17, 22). Interestingly, levels of IL-4-secreting peripheral blood CD4+ T cells increase after each vaccination with attenuated sporozoites in volunteers, who show sustained protection (28).
As for protection, the overall protection rates after vaccination (vaccine efficacy) were lower here than in previous studies (45) using the intradermal route, which is currently used in most clinical trials. The intradermal route, as well as improved sporozoite isolation and infectivity, may contribute to this. However, the main efficacy results were that Vα14iNKT-cell-deficient and WT mice were similarly well protected to 90% at the earliest time point after vaccination, but at the later time points protection waned in >50% of mice of both strains, but vaccinated KO appeared to be better protected than vaccinated WT mice. This indicates, first, that nonspecific protective effects mediated by virus-activated Vα14iNKT cells can be ruled out. Parallel studies by our laboratory show that empty control viruses do not protect mice (3, 45), which also excludes such effects. Second, the intrinsic adjuvant effect of attenuated poxviruses mediated by Vα14iNKT cells is not strong enough to improve protection in WT mice despite an enlarged memory T-cell pool 3 weeks after vaccination, in contrast to α-GalCer-adjuvanted immunization (19). This supports other previous studies showing a dispensable physiological role of Vα14iNKT cells in protection against liver-stage malaria (18, 39, 43). The slightly better protection of KO mice might be due to the absence of IL-4-producing Vα14iNKT cells, promoting a Th1-polarized liver environment, indicated by higher numbers of NK cells as well as of CD8+ T and CD3− cells expressing IFN-γ or TNF-α intracellularly. In line with this, a shift toward protective IFN-γ responses in the liver occurs in naive infected IL-4 KO or IL-4 receptor KO BALB/c mice (40).
These results support the current view that IFN-γ-secreting CD8+ (and CD4+) T cells are the best-defined correlate of protection against liver-stage malaria. Furthermore, this is the first report of the induction of pre-erythrocytic-stage antigen-specific TNF-α-producing CD8+ T cells in the liver by vaccination, detected by ELISPOT assay and ICS in both liver and spleen. This induction is specific because vaccination with the empty control viruses does not yield peptide Pb9-specific cytokine producers (3), as also supported by the negative test controls. One previous study reports intracellularly stained TNF-α in splenic CD8+ memory T cells induced by malaria DNA recombinant vaccinia virus vaccines (46). TNF-α-producing antigen-specific CD8+ T cells have mainly been described for bacterial and viral infections (21, 48). Here, TNF-α-producing CD8+ T cells correlated with IFN-γ secretors after vaccination and in the early and late response to sporozoite challenge. Further, CD9+ T cells that produce both IFN-γ and TNF-α were associated with vaccine-induced protection, since they accumulated in greater numbers in livers of protected WT and Vα14iNKT-cell-deficient mice compared to unprotected mice and to postvaccination levels. Blood-stage parasites, accumulating in the livers of parasitemic mice, could conceivably account for the reduction in unprotected mice, due to some cross-reactivity with CSP (1) and the death of activated liver lymphocytes (10), but not for the shifts observed 1 day after challenge and in protected versus vaccinated mice. Therefore, we suggest that double-expressing, as well as single-expressing, T-cell populations should be further investigated as possible correlates of protection for liver-stage malaria. However, further studies are needed to prove their protective role. Interestingly, such IFN-γ+/TNF-α+ liver-stage-specific T cells were recently found to be enhanced in the blood of protected, vaccinated volunteers (S. Korten et al., unpublished data).
Local intrahepatic memory CD8+ T cells are thought to be most relevant for protection against liver-stage malaria (4). The protective threshold levels for antigen-specific hepatic CD8+ T cells that we present here are novel and might be helpful for further vaccine studies. For BALB/c WT spleens, the estimated level for protection is ca. 500 IFN-γ-secreting SFC/million splenocytes, which are specific for one peptide (44). Our data suggest a protective hepatic threshold level of 500 SFC/million lymphocytes specific for peptide Pb9 because this level was also achieved here in livers of KO mice for both cytokines together after immunization and for each cytokine 1 day after challenge of immunized KO mice. Infectious sporozoites induce a peak reactivity of hepatic effector memory CD8+ T cells within 1 to 6 h, whereas hepatic central memory CD8+ T cells are conscripted into effectors with some delay (4). This might apply to the different postchallenge kinetics of hepatic CD8+ T cells in WT and Vα14iNKT-cell-deficient mice. The livers of WT mice might have contained a greater effector CD8+-T-cell pool, that quickly responds and contracts 1 day after challenge, whereas in KO mice central memory CD8+ T cells had to be conscripted into effectors and therefore peaked 1 day after challenge. Splenic memory T cells were not significantly redistributed to the liver early after challenge, suggesting that hepatic memory T cells were the main effectors. Later redistributions from the spleen would have no protective effect, since CSP is not newly expressed by hepatic stages (5). Second, in the presence of Vα14iNKT cells, which rapidly activate NK cells (7), the cytokine cascade might be more potent and activate protective effector CD8+ T cells faster.
In conclusion, maximizing local hepatic or splenic memory CD8+-T-cell pools specific for one antigen alone does not automatically improve protection. Higher levels of IFN-γ producers can protect better, but this was shown for total, not single peptide specific, effector memory IFN-γ-producing CD8+-T-cell pools after vaccination with gamma-irradiated sporozoites (4). Therefore, our findings may prove generally valuable for evaluating vaccine efficacy and designing more effective vaccines against pre-erythrocytic-stage malaria.
Acknowledgments
This study was sponsored by the Wellcome Trust. S.K. was funded by the German Research Foundation (DFG). A.V.S.H. is a Wellcome Trust Principal Research Fellow.
We thank Vincenzo Cerundolo for assistance with the CD1d/α-GalCer tetramer, Kirin Brewery for providing αGalCer, Jacqui Mendoza and Volker Heussler for supplying P. berghei-infected mosquitoes, Britta Urban for technical assistance, and Michael Walther for statistical advice.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Ardeshir, F., R. F. Howard, S. Viriyakosol, O. Arad, and R. T. Reese. 1990. Cross-reactive asparagine-rich determinants shared between several blood-stage antigens of Plasmodium falciparum and the circumsporozoite protein. Mol. Biochem. Parasitol. 40:113-128. [DOI] [PubMed] [Google Scholar]
- 2.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R. J., C. M. Hannan, S. C. Gilbert, S. M. Laidlaw, E. G. Sheu, S. Korten, R. Sinden, G. A. Butcher, M. A. Skinner, and A. V. S. Hill. 2004. Enhanced CD8+ T-cell immune responses and protection elicited against Plasmodium berghei malaria by prime boost immunization regimes using a novel attenuated fowlpox virus. J. Immunol. 172:3094-3100. [DOI] [PubMed] [Google Scholar]
- 4.Berenzon, D., R. J. Schwenk, L. Letellier, M. Guebre-Xabier, J. Williams, and U. Krzych. 2003. Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells. J. Immunol. 171:2024-2034. [DOI] [PubMed] [Google Scholar]
- 5.Bodescot, M., O. Silvie, A. Siau, P. Refour, P. Pino, J. F. Franetich, L. Hannoun, R. Sauerwein, and D. Mazier. 2004. Transcription status of vaccine candidate genes of Plasmodium falciparum during the hepatic phase of its life cycle. Parasitol. Res. 92:449-452. [DOI] [PubMed] [Google Scholar]
- 6.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with alpha-galactosylceramide polarizes CD1-reactive NK T cells toward Th2 cytokine synthesis. Eur. J. Immunol. 29:2014-2025. [DOI] [PubMed] [Google Scholar]
- 7.Carnaud, C., D. Lee, O. Donnars, S. H. Park, A. Beavis, Y. Koezuka, and A. Bendelac. 1999. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163:4647-4650. [PubMed] [Google Scholar]
- 8.Carvalho, L. H., G. Sano, J. C. Hafalla, A. Morrot, M. A. Curotto de Lafaille, and F. Zavala. 2002. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 8:166-170. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho, L. H., J. C. Hafalla, and F. Zavala. 2001. ELISPOT assay to measure antigen-specific murine CD8+ T cell responses. J. Immunol. Methods 252:207-218. [DOI] [PubMed] [Google Scholar]
- 10.Crispe, I. N. 2003. Hepatic T cells and liver tolerance. Nat. Rev. Immunol. 3:51-62. [DOI] [PubMed] [Google Scholar]
- 11.Cui, J., T. Shin, T. Kawano, H. Sato, E. Kondo, I. Toura, Y. Kaneko, H. Koseki, M. Kanno, and M. Taniguchi. 1997. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278:1623-1626. [DOI] [PubMed] [Google Scholar]
- 12.Dokun, A. O., S. Kim, H. R. C. Smith, H.-S. P. Kang, D. T. Chu, and W. M. Yokoyama. 2001. Specific and nonspecific NK cell activation during virus infection. Nat. Immunol. 2:951-956. [DOI] [PubMed] [Google Scholar]
- 13.Doolan, D. L., and S. L. Hoffman. 1999. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J. Immunol. 163:884-892. [PubMed] [Google Scholar]
- 14.Doolan, D. L., and S. L. Hoffman. 2000. The complexity of protective immunity against liver-stage malaria. J. Immunol. 165:1453-1462. [DOI] [PubMed] [Google Scholar]
- 15.Dunachie, S. J., and A. V. Hill. 2003. Prime-boost strategies for malaria vaccine development. J. Exp. Biol. 206:3771-3779. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey, D. I., K. J. Hammond, L. D. Poulton, M. J. Smyth, and A. G. Baxter. 2000. NKT cells: facts, functions, and fallacies. Immunol. Today 21:573-583. [DOI] [PubMed] [Google Scholar]
- 17.Golding, B., M. Zaitseva, and H. Golding. 1994. The potential for recruiting immune responses toward type 1 or type 2 T-cell help. Am. J. Trop. Med. Hyg. 50:33-40. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Aseguinolaza, G., C. de Oliveira, M. Tomaska, S. Hong, O. Bruno-Romero, T. Nakayama, M. Taniguchi, A. Bendelac, L. Van Kaer, Y. Koezuka, and M. Tsuji. 2000. α-Galactosylceramide-activated Vα14 natural killer T cells mediate protection against murine malaria. Proc. Natl. Acad. Sci. USA 97:8461-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Aseguinolaza, G., L. Van Kaer, C. C. Bergmann, J. M. Wilson, J. Schmieg, M. Kronenberg, T. Nakayama, M. Taniguchi, Y. Koezuka, and M. Tsuji. 2002. Natural killer T-cell ligand α-galactosylceramide enhances protective immunity induced by malaria vaccines. J. Exp. Med. 195:617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green, S. J., S. Mellouk, S. L. Hoffman, M. S. Meltzer, and C. A. Nacy. 1990. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from l-arginine by macrophages and hepatocytes. Immunol. Lett. 25:15-19. [DOI] [PubMed] [Google Scholar]
- 21.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, L. R., F. L. Chen, Y. T. Chen, Y. M. Lin, and J. T. Kung. 2000. Potent induction of long-term CD8+ T cell memory by short-term IL-4 exposure during T cell receptor stimulation. Proc. Natl. Acad. Sci. USA 97:3406-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs, P., D. Radzioch, and M. M. Stevenson. 1996. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect. Immun. 64:535-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs, P., D. Radzioch, and M. M. Stevenson. 1996. In vivo regulation of nitric oxide production by tumor necrosis factor alpha and gamma interferon, but not by interleukin-4, during blood stage malaria in mice. Infect. Immun. 64:44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karadimitris, A., S. Gadola, M. Altamirano, D. Brown, A. Woolfson, P. Klenerman, J. L. Chen, Y. Koezuka, I. A. Roberts, D. A. Price, G. Dusheiko, C. Milstein, A. Fersht, L. Luzzatto, and V. Cerundolo. 2001. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc. Natl. Acad. Sci. USA 98:3294-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, H. Koseki, and M. Taniguchi. 1997. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278:1626-1629. [DOI] [PubMed] [Google Scholar]
- 27.Klugewitz, K., F. Blumenthal-Barby, A. Schrage, P. A. Knolle, A. Hamann, and I. N. Crispe. 2002. Immunomodulatory effects of the liver: deletion of activated CD4+ effector cells and suppression of IFN-gamma-producing cells after intravenous protein immunization. J. Immunol. 169:2407-2413. [DOI] [PubMed] [Google Scholar]
- 28.Krzych, U., R. Schwenk, M. Guebre-Xabier, P. Sun, D. Palmer, K. White, and I. Chalom. 2000. The role of intrahepatic lymphocytes in mediating protective immunity induced by attenuated Plasmodium berghei sporozoites. Immunol. Rev. 174:123-134. [DOI] [PubMed] [Google Scholar]
- 29.Lau, A. O., J. B. Sacci, Jr., and A. F. Azad. 2001. Host responses to Plasmodium yoelii hepatic stages: a paradigm in host-parasite interaction. J. Immunol. 166:1945-1950. [DOI] [PubMed] [Google Scholar]
- 30.Mayr, A., and K. Malicki. 1966. Attenuation of virulent fowl pox virus in tissue culture and characteristics of the attenuated virus. Zentbl. Veterinarmed. B. 13:1-13. [PubMed] [Google Scholar]
- 31.Mayr, A., M. Buttner, G. Wolf, H. Meyer, and C. Czerny. 1989. Experimental detection of the paraspecific effects of purified and inactivated poxviruses. Zentbl. Veterinarmed. 36:81-99. [PubMed] [Google Scholar]
- 32.McConkey, S. J., W. H. Reece, V. S. Moorthy, D. Webster, S. Dunachie, G. Butcher, J. M. Vuola, T. J. Blanchard, P. Gothard, K. Watkins, C. M. Hannan, S. Everaere, K. Brown, K. E. Kester, J. Cummings, J. Williams, D. G. Heppner, A. Pathan, K. Flanagan, N. Arulanantham, M. T. Roberts, M. Roy, G. L. Smith, J. Schneider, T. Peto, R. E. Sinden, S. C. Gilbert, and A. V. Hill. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 9:729-735. [DOI] [PubMed] [Google Scholar]
- 33.Miller, L. H., D. I. Baruch, K. Marsh, and O. K. Doumbo. 2002. The pathogenesis of malaria. Nature 415:673-679. [DOI] [PubMed] [Google Scholar]
- 34.Newcombe, R. G. 1998. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat. Med. 17:873-890. [DOI] [PubMed] [Google Scholar]
- 35.Newcombe, R. G. 1998. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med. 17:857-872. [DOI] [PubMed] [Google Scholar]
- 36.Nussler, A., S. Pied, J. Goma, L. Renia, F. Miltgen, G. E. Grau, and D. Mazier. 1991. TNF inhibits malaria hepatic stages in vitro via synthesis of IL-6. Int. Immunol. 3:317-321. [DOI] [PubMed] [Google Scholar]
- 37.Osman, Y., T. Kawamura, T. Naito, K. Takeda, L. Van Kaer, K. Okumura, and T. Abo. 2000. Activation of hepatic NKT cells and subsequent liver injury following administration of alpha-galactosylceramide. Eur. J. Immunol. 30:1919-1928. [DOI] [PubMed] [Google Scholar]
- 38.Pabst, H. F., D. W. Spady, M. M. Carson, H. T. Stelfox, J. A. Beeler, and M. P. Krezolek. 1997. Kinetics of immunologic responses after primary MMR vaccination. Vaccine 15:10-14. [DOI] [PubMed] [Google Scholar]
- 39.Romero, J. F., G. Eberl, H. R. MacDonald, and G. Corradin. 2001. CD1d-restricted NKT cells are dispensable for specific antibody responses and protective immunity against liver stage malaria infection in mice. Parasite Immunol. 23:267-269. [DOI] [PubMed] [Google Scholar]
- 40.Saeftel, M., A. Krueger, S. Arriens, V. Heussler, P. Racz, B. Fleischer, F. Brombacher, and A. Hoerauf. 2004. Mice deficient in interleukin-4 (IL-4) or IL-4 receptor alpha have higher resistance to sporozoite infection with Plasmodium berghei (ANKA) than do naive wild-type mice. Infect. Immun. 72:322-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schapiro, J. M., Y. Segev, L. Rannon, M. Alkan, and B. Rager-Zisman. 1990. Natural killer (NK) cell response after vaccination of volunteers with killed influenza vaccine. J. Med. Virol. 30:196-200. [DOI] [PubMed] [Google Scholar]
- 42.Scharton-Kersten, T. M., and A. Sher. 1997. Role of natural killer cells in innate resistance to protozoan infections. Curr. Opin. Immunol. 9:44-51. [DOI] [PubMed] [Google Scholar]
- 43.Schmieg, J., G. Gonzalez-Aseguinolaza, and M. Tsuji. 2003. The role of natural killer T cells and other T cell subsets against infection by the pre-erythrocytic stages of malaria parasites. Microbes Infect. 5:499-506. [DOI] [PubMed] [Google Scholar]
- 44.Schneider, J., S. C. Gilbert, C. M. Hannan, P. Degano, E. Prieur, E. G. Sheu, M. Plebanski, and A. V. Hill. 1999. Induction of CD8+ T cells using heterologous prime-boost immunization strategies. Immunol. Rev. 170:29-38. [DOI] [PubMed] [Google Scholar]
- 45.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 46.Sedegah, M., G. T. Brice, W. O. Rogers, D. L. Doolan, Y. Charoenvit, T. R. Jones, V. F. Majam, A. Belmonte, M. Lu, M. Belmonte, D. J. Carucci, and S. L. Hoffman. 2002. Persistence of protective immunity to malaria induced by DNA priming and poxvirus boosting: characterization of effector and memory CD8+-T-cell populations. Infect. Immun. 70:3493-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward, B. J., and D. E. Griffin. 1993. Changes in cytokine production after measles virus vaccination: predominant production of IL-4 suggests induction of a Th2 response. Clin. Immunol. Immunopathol. 67:171-177. [DOI] [PubMed] [Google Scholar]
- 48.Wizel, B., B. C. Starcher, B. Samten, Z. Chroneos, P. F. Barnes, J. Dzuris, Y. Higashimoto, E. Appella, and A. Sette. 2002. Multiple Chlamydia pneumoniae antigens prime CD8+ Tc1 responses that inhibit intracellular growth of this vacuolar pathogen. J. Immunol. 169:2524-2535. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, J., T. Bardos, Q. Shao, J. Tschopp, K. Mikecz, T. T. Glant, and A. Finnegan. 2003. IL-4 potentiates activated T cell apoptosis via an IL-2-dependent mechanism. J. Immunol. 170:3495-3503. [DOI] [PubMed] [Google Scholar]