Abstract
Cancer stem cells (CSCs), or tumor-initiating cells, comprise a subset of tumor cells with demonstrated ability for tumor growth, invasion, metastasis, and resistance to chemotherapy and radiation. Targeting of CSCs remains an attractive yet elusive therapeutic option, with the goal of increasing specificity and effectiveness in tumor eradication, as well as decreasing off-target or systemic toxicity. Research into further characterization and targeted therapy toward head and neck CSCs is an active and rapidly evolving field. This review discusses the current state of research into therapy against head and neck CSCs and future directions for targeted therapy.
Keywords: HNSCC, tumor initiating cells, treatment, characteristics, cancer biology, cancer genes
Introduction
Head and neck cancer is the sixth most common cancer worldwide, with over 600,000 new cases diagnosed yearly (Jemal et al. 2011). Most head and neck cancers are squamous cell carcinomas (HNSCCs), with tumor sites including the nasopharynx, oropharynx, oral cavity, hypopharynx, and larynx. These cancers carry a poor prognosis, with low 5-y survival rates for late-stage tumors and with minimal improvement in survival trends (Pulte and Brenner 2010). Current standardized treatment for HNSCC relies on a combination of surgery, radiation, and chemotherapy, with no significant change in treatment regimens for decades.
Two principal models of tumor progression exist: the stochastic model and the cancer stem cell (CSC) or tumor-initiating cell model. In the stochastic model, any tumor cell carries the potential to create new tumor, invade, and metastasize. Under the CSC model, only a subset of these cells (the CSCs) has the ability to establish a heterogeneous tumor population, self-renew, invade, and metastasize (Reya et al. 2001). These cells were identified in head and neck cancer in 2007 (Prince et al. 2007) and demonstrated to have CSC hallmarks of self-renewal and tumor neogenesis. Using CD44 as a CSC marker, Prince et al. (2007) demonstrated that as few as 500 CD44-positive cells could regenerate a tumor, whereas a larger number of CD44-negative cells could not. Further evidence of the importance of these CSCs in cancer progression was demonstrated with the ability of head and neck CD44-positive cells (but not CD44-negative cells) to generate tumors and drive metastasis (Davis et al. 2010; Chinn et al. 2015). Since then, there has been increasing evidence and understanding that CSCs play an important role in tumor progression, metastasis, and resistance to current therapy.
Identifying and specifically targeting head and neck CSCs is appealing from multiple aspects. Current therapy regimens carry significant morbidity, from disfigurement and functional changes from surgery, to systemic toxicity from chemotherapy, to radiation-induced side effects from radiotherapy. Moreover, due to a variety of intrinsic mechanisms, CSCs are often resistant to traditional chemotherapy and radiation. These cells can survive such treatments and repopulate tumors with chemoradioresistant cells. Specifically targeting head and neck CSCs provides a potential means of improved cancer outcomes while addressing organ preservation and reducing off-target toxicity. In this review, we describe advances in current research and future challenges toward developing therapeutic targeting of head and neck CSCs.
CSC Characteristics
CSCs are defined as cells within a tumor with the ability to re-create the original tumor population, drive tumor proliferation, and self-renew (Reya et al. 2001). These cells have been distinguished from the remaining tumor cell population via a number of intrinsic characteristics, including cell marker overexpression, chemotherapy and radiation resistance, and an increased ability to invade and metastasize (Table).
Table.
Characteristics and Genes Involved in Cancer Stem Cells (CSCs).
| CSC Characteristic | Genes Involved |
|---|---|
| Cell surface markers | CD44 |
| CD10 | |
| CD98 | |
| CD271 | |
| CD166 | |
| Cell differentiation | WNT |
| CTNNB1 | |
| NOTCH1 | |
| SHH | |
| Cell proliferation | JAK |
| STAT3 | |
| AKT1 | |
| MAPK | |
| ERK | |
| Embryonic transcription factors | SOX2 |
| OCT4 (POU5F1) | |
| NANOG | |
| Epithelial to mesenchymal transition | TGFB1 |
| SNAI1 | |
| TWIST1 | |
| BMI1 | |
| CTGF | |
| SHH | |
| Chemoradiation resistance | ABCG |
| MDR1 | |
| CHEK1/2 | |
| GRP78 | |
| SOD2 | |
| CAT | |
| Antiapoptotic mechanisms | BCL2 |
| IAP | |
| Hypoxic microenvironment | HIF1A |
| VEGF |
CSCs have specific phenotypes and genetic profiles that separate them from the remaining tumor cell population. Key CSC characteristics and corresponding involved genes are listed.
Cell Markers
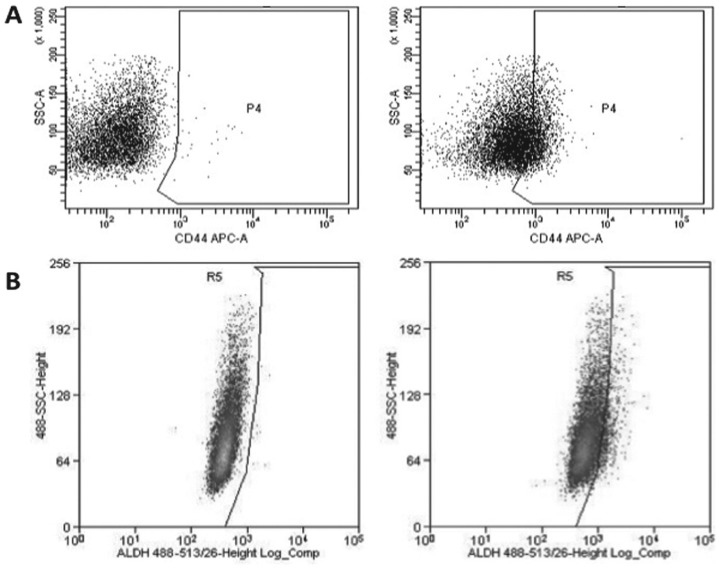
In HNSCC, a cell subpopulation with characteristics of CSCs was first isolated in 2007 (Prince et al. 2007). These cells were noted to have increased expression of the cell surface marker CD44 relative to non-CSCs (Fig. 1A). The identification and isolation of head and neck CSCs has evolved since this initial study, with the development of multiple new methods to isolate head and neck CSCs for study. In addition to CD44, markers frequently used to identify CSCs include CD133 and aldehyde dehydrogenase (ALDH) (Fig. 1B) (Zhou et al. 2007; Clay et al. 2010). Recently, additional potential CSC surface markers have been identified, including CD10 (Fukusumi et al. 2014), CD98 (Martens-de Kemp et al. 2013), CD271 (Murillo-Sauca et al. 2014), CD166 (Yan et al. 2013), and ABCG2 (Wan et al. 2010; Table). A separate method of isolation and identification of CSCs has been through the increased ability of CSCs to efflux Hoechst 33342 dye (due to increased activity of CSC membrane transporters). Such cells have been identified as a side population of CSCs (Tabor et al. 2011).
Figure 1.
CD44 and ALDH flow cytometry. Common methods to isolate head and neck cancer stem cells (CSCs) includes identifying high expression of CD44 (A) and ALDH (B). Flow cytometry of an oral cavity squamous cell carcinoma (SCC) cell line from the University of Michigan. Isotype control populations are on the left in each panel, and positive cells are in the boxed region in the right panels. In this example, 31% of cells are CD44 positive and 7% are ALDH positive. Multiple studies, as discussed in the article, show increased resistance to therapy for these cell populations.
Enhanced Growth, Proliferation, and Altered Cell Differentiation
Interestingly, head and neck CSCs express many of the same core genes as traditional stem cells, suggesting the potential for multipotency and self-renewal. These include the canonical embryonic stem cell transcription factors SOX2, OCT4 (POU5F1), and NANOG (Koo et al. 2014; Lee, Oh, et al. 2014). In addition, several key signaling pathways are dysregulated in CSCs in comparison to non-CSC tumor cells (Table). These include pathways for cell differentiation: NOTCH, WNT/CTNNB1 (Lee, Koo, et al. 2014), and SHH (Port et al. 2013). Other dysregulated pathways include cell growth and proliferation: JAK/STAT3, AKT, and MAPK/ERK. Overexpression of these signaling pathways activates downstream players in stem cell phenotypes (Lee, Oh, et al. 2014), leading to increased tumor growth and proliferation.
Epithelial to Mesenchymal Transition, Invasiveness, and Metastatic Potential
CSCs are involved in the epithelial to mesenchymal transition (EMT), in which HNSCCs lose their epithelial phenotype (with downregulation of E-cadherin) and adopt a more invasive mesenchymal form. This transition is thought to be a key mediator of CSC aggressiveness, as the previously differentiated CSCs lose polarity and invade the basement membrane, leading to tumor invasion and metastasis. WNT1, TGFB1, SNAI1, TWIST1, BMI1, and CTGF, among other genes, are key mediators of this EMT (Yang et al. 2012; Yu et al. 2013; Chang et al. 2013; Figure 2). As these genes are involved in squamous epithelial differentiation, their involvement is key to transitioning from epithelial to mesenchymal phenotypes.
Figure 2.
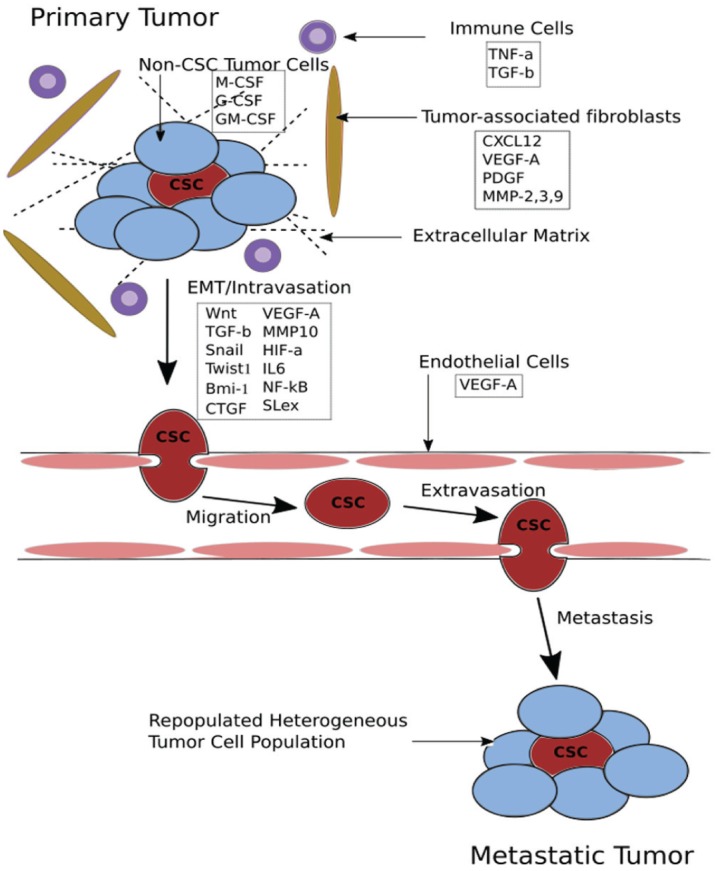
Cancer stem cells (CSCs) and tumor microenvironments. CSCs have significant interaction and cross-talk with the surrounding microenvironment with regard to the epithelial to mesenchymal transition (EMT), invasion, and metastasis. Multiple secreted factors from surrounding immune cells, fibroblasts, and endothelial cells signal CSC EMT and invasion.
Consequently, CSCs have increased metastatic capabilities. Head and neck CSCs have been demonstrated to maintain increased metastatic potential in both in vitro (Davis et al. 2010) and in vivo (Chinn et al. 2015) models. Sialyl Lewis X, a glycan that binds to endothelial cells, is upregulated in oral cavity CSCs, suggesting a mechanism for metastasis (Czerwinski et al. 2013). Knockdown of BMI1 decreases HNSCC metastatic rates, while overexpression of BMI1 increases metastatic rates (Yu et al. 2011).
Resistance to Chemotherapy and Radiation
Chemotherapy and radiation resistance is a key characteristic of CSCs and of great clinical concern as these cell populations are able to overcome these therapies and repopulate the tumor with aggressive, chemoradioresistant cells. Chemotherapy resistance is generated in CSCs in part due to an upregulation of membranous drug efflux proteins (ABCG, MDR1) and regulatory genes involved in drug processing (Nör et al. 2014). Reactive oxygen species (ROS) are depleted in CSCs, contributing to CSC resistance to chemotherapy by means of decreased toxic oxidized intermediates. The importance of low ROS levels in CSCs is highlighted by studies in which restoration of ROS to normal levels is associated with a loss of CSC-like properties and increased sensitivity to cisplatin in HNSCC (Chang et al. 2014).
Resistance to radiation is another crucial CSC phenotypic characteristic and one that significantly contributes to treatment challenges. These cells have increased activity of DNA damage repair pathways (particularly the genes CHEK1 and CHEK2), thus conferring added protection in response to chemotherapy and radiation therapy through enhanced DNA editing. Specifically, CHEK1 and CHEK2 are able to activate DNA repair genes and act as cell cycle checkpoint genes (Wang et al. 2013; Bertrand et al. 2014). Similarly to CSC resistance to chemotherapy, low levels of ROS in CSCs decrease the ability of radiation-induced free radicals to cause DNA damage.
Antiapoptotic Mechanisms
Chemotherapy and radiation therapy in part act on targeted cells by inducing apoptosis. In CSCs, however, apoptotic mechanisms are decreased, and these cells are highly resistant to apoptosis. In support of these findings, head and neck CSCs express higher levels of antiapoptotic genes (BCL2 and IAP gene families) (Chikamatsu et al. 2012), resulting in increased cell survival.
Epigenetic Changes in CSCs
We are beginning to characterize unique epigenetic signatures of head and neck CSCs. These cells contain high proportions of oncogenic microRNAs (miRNAs) and a decreased expression of tumor suppressor miRNAs. As a result, these miRNAs increase oncogene expression, inhibit tumor suppressor gene expression, contribute to therapeutic resistance, initiate cell reprogramming, and promote EMT (Sun X et al. 2014). Altered DNA methylation patterns in CSCs, corresponding with altered miRNA expression levels, suggest unique oncogenic methylation profiles in CSCs (Wiklund et al. 2011). Histone modifications may also play a key epigenetic role in regulating CSC expression patterns. Recent studies into histone deacetylase inhibitors in head and neck CSCs suggest a role of histone deacetylases in maintaining CSC expression phenotypes (Chikamatsu et al. 2013).
CSC Niches and Tumor Microenvironment
The surrounding tumor microenvironments contribute to CSC activity and phenotypes, as significant cross-talk exists between the CSC and surrounding stromal cells (Fig. 2). CSCs exist in specific perivascular niches and microenvironments enriched to enhance cell growth and survival (Ritchie and Nör 2013; Plaks et al. 2015). Endothelial, immune, fibroblast, and non-CSC tumor cell signaling in this milieu plays an important role in CSC propagation and survival. Non-CSC tumor cells secrete stimulatory factors (macrophage colony-stimulating factor [CSF], granulocyte CSF, and granulocyte macrophage CSF) to attract immune cells, which in turn promote CSC survival and EMT (Fig. 2). Tumor-associated fibroblasts secrete vascular endothelial growth factor (VEGF) to promote angiogenesis, for extracellular matrix remodeling, and CXCL12 to attract inflammatory cells (Plaks et al. 2015). Endothelial cells, as well, produce VEGF, which promotes CSC proliferation. The CXCL12–CXCR4 axis generated in this tumor microenvironment is of importance in CSC migration, attachment, and morphology (Faber et al. 2013). Interestingly, increased hypoxia in this microenvironment has also been associated with increased CSC survival. Hypoxia induces upregulation of hypoxia-inducing factor 1α (HIF-1α), a transcription factor that increases production of VEGF (Kung et al. 2000), as well as key CSC regulators Twist1 and Bmi-1.
CSC Therapeutic Paradigms
Directing therapies specifically against CSCs has become an area of increased interest. The potential for such targeted therapy is 2-fold: increased therapeutic efficacy against CSCs may result in improved cure rates and survival (Fig. 3), while reducing toxicities from conventional therapy and preserving surrounding tissue integrity. Current investigational therapeutic paradigms are wide-ranging, including targeting CSC markers and pathways, increasing CSC sensitivity to chemotherapy and radiation, employing epigenetic modulators, using immunotherapy agents, and modifying the CSC microenvironment. We discuss progress in each of these areas below.
Figure 3.
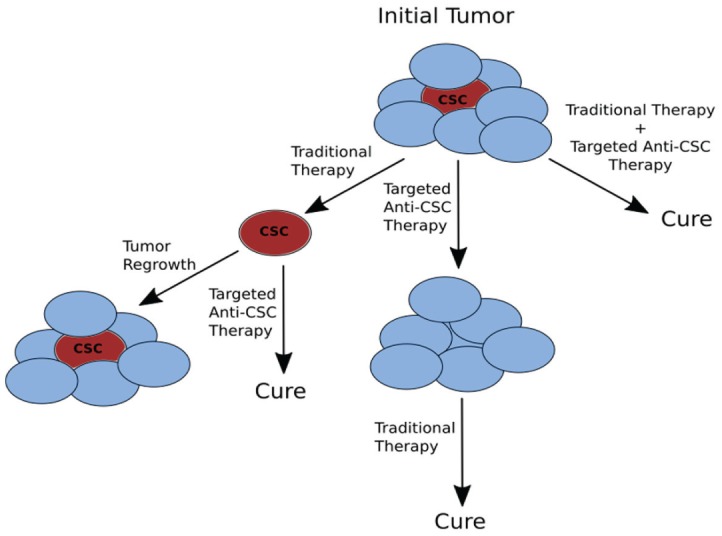
Treatment paradigms for cancer stem cells (CSCs). CSCs may play a part in tumor recurrence and resistance to standard therapy. Employing anti-CSC therapy potentially can be performed in combination with traditional therapy (surgery, chemotherapy, and radiation) or as neoadjuvant or adjuvant treatments.
Targeting Cell Surface Markers
CD44 has been a popular investigational target for directed therapy against CSCs. Hyaluronic acid (which selectively binds to CD44) has been used as an agent for delivering targeted treatments against CD44-positive cells, including hyaluronic acid–conjugated chemotherapeutics and hyaluronic acid–guided nanoparticles. Interestingly, studies have shown hyaluronic acid induces CD44 interaction with stem cell transcription factors Nanog, Oct-4, and Sox2 (Bourguignon et al. 2012). Further investigation will be needed to tease out the benefits of hyaluronic acid targeting without inducing further activation of CSCs. Anti-CD133 therapies have been investigated as targeted head and neck anti-CSC therapy. A study conjugating a bacterial toxin (cytolethal distending toxin) to an anti-human CD133 monoclonal antibody demonstrated inhibition of cell proliferation (Damek-Poprawa et al. 2011), whereas another study using a single-chain variable fragment targeting CD133 showed marked reduction in tumor proliferation in cell and mouse models (Waldron et al. 2011). Inhibition of CD271, as well, has been demonstrated in cell models to decrease tumor formation (Murillo-Sauca et al. 2014). Overall, targeting CSC surface markers remains an intriguing option for treatment. Their greatest efficacy may be in conjunction with other therapies as a delivery system.
Increasing CSC Sensitivity to Chemotherapy and Radiation
Adding new agents or targeted treatments in conjunction with standard cisplatin chemotherapy is a prime current research area. Salinomycin has been demonstrated to work in synergy with cisplatin and paclitaxel to increase apoptosis in head and neck CSCs (Kuo et al. 2012). Huang et al. demonstrated that small hairpin RNA knockdown of Nanog resulted in increased sensitivity to cisplatin (Huang et al. 2014). GRP78 is a multifunctional protein involved in cell survival and resistance to chemotherapy. Inhibition of GRP78 sensitized head and neck CSCs to both chemotherapy and radiation (Chiu et al. 2013). CSCs have been shown to have lower levels of ROS, which helps maintenance of stem-like properties and chemoresistance. Inhibition of ROS scavenging proteins (SOD2 and Catalase) leads to an increase in ROS and a subsequent increase in sensitivity to cisplatin (Chang et al. 2014).
A key means of CSC resistance to chemotherapy is an enhanced ability to efflux cytotoxic agents. As a result, cellular efflux proteins have been investigated as potential targets for sensitizing CSCs to current chemotherapy agents. Early studies in laryngeal cancer cell lines have shown a reduction of CSC proportion by verapamil, an inhibitor to the ABCG2 membrane transporter (Wan et al. 2010). Inhibitors to other members of the ABC transporter family, when applied to head and neck and CSC populations, lead to increased sensitivity to chemotherapy (Katayama et al. 2009).
Increasing CSC sensitivity to radiation is also being actively investigated. New studies have targeted the CHEK1/2 DNA damage repair genes and ATRA (a retinoid involved in cell terminal differentiation) in head and neck CSCs. These investigations show increased response to radiation in CSCs after inhibition of CHEK1/2 and application of ATRA (Bertrand et al. 2014). Inhibition of SHH/MTOR/RPS6KB1 pathways leads to increased radiosensitivity in CSCs, suggesting a role for these pathways and potential targetable options for increasing CSC radiosensitivity (Gan et al. 2014).
Epidermal growth factor receptor (EGFR) inhibition (with cetuximab) is increasingly being employed in advanced and recurrent HNSCC treatment protocols. Early studies suggest a potential role for EGFR targeted therapy specifically against head and neck CSCs. In nasopharyngeal carcinomas, EGFR acts via AKT and CTNNB1 pathways to drive CSC phenotypes (Ma et al. 2013). Activation of EGFR in head and neck CSCs increases expression of genes involved in CSC proliferation and growth (BMI1, OCT4, NANOG, CD44), and treatment of CSCs with EGFR inhibition results in decreased tumor growth and increased sensitivity to cisplatin (Ma et al. 2013).
Altering CSC Self-Renewal Pathways and Inducing CSC Differentiation
Downregulation of overexpressed stem cell pathways has been investigated as a potential therapeutic intervention option against CSCs. Inhibiting NANOG expression in head and neck CSCs results in decreased tumorigenesis and proliferation and increased sensitivity to cisplatin (Huang et al. 2014). The MET and WNT/CTNNB1 pathways have been targeted for inhibition, with a noted decrease in tumor growth and metastasis with a c-Met inhibitor (Sun S et al. 2014), as well as decreased EMT and stem cell–like phenotype with increased apoptosis with a Wnt antagonist (Warrier et al. 2014). Moreover, Wnt inhibitory factor 1 leads to reduced OCT4, MYC, and BMI1 expression (Ramachandran et al. 2014), consistent with data that the WNT pathway is key to cancer cell stemness. Shh inhibitors reduce EMT in HNSCC, and addition of Shh inhibitors to cetuximab increases cancer cell sensitivity to cetuximab (Ramachandran et al. 2014). Importantly, many agents targeting MET, WNT/CTNNB1, and SHH pathways are in early clinical trials in head and neck cancers. Further study into their specific activity against the CSC components of head and neck cancers will provide useful information on their viability as CSC-targeted agents.
Immunologic and Viral Therapy
Cancer immunology remains a complex but intriguing option for therapy. A key goal of cancer immunotherapy is priming the host’s immune system specifically against cancer cells and potentially the CSC subset of cancer cells. Tumor vaccines have significant potential for anti-CSC therapy. CSC lysates in head and neck cancers have been used as an antigen source for priming dendritic cells with the goal of activation of humoral and cell-mediated responses against the host tumor CSCs (Ning et al. 2012; Li et al. 2014). In addition, early studies have demonstrated that natural killer cells primed against head and neck cancers may preferentially target CSCs, offering another potential option for immunotherapy (Jewett et al. 2012). Modulating a host’s immune system to specifically target CSCs could potentially afford a very personalized and targeted therapeutic option for patients.
Viral targeting of cancers is another developing field with great promise. Lentiviruses may be employed as vectors in which specific genes may be inserted into targeted cells (Upreti et al. 2014). The introduction of cytotoxic, proapoptotic, or tumor suppressor genes into CSCs could provide targeted anti-CSC therapy. Another form of viral antitumor therapy is through the use of oncolytic viruses. These viruses can be engineered to specifically target cancer cells and may lead to cell death via intracellular viral replication or production of cytolethal compounds (Russell et al. 2012). The potential thus exists for specific targeting of head and neck CSCs. Oncolytic viral therapy, interestingly, although able to directly destroy CSCs, is thought to rely in part on activation of host immune responses (Pol et al. 2014). Thus, a combination of viral targeting and host immune response activation may provide enhanced therapeutic outcomes against CSCs.
Employing Epigenetic Modulators
The potential for epigenetics in modulating CSC phenotypes is high, with the ability to downregulate oncogenes and upregulate tumor suppressor genes in a novel fashion. miRNAs have been shown to confer resistance to radiation by downregulating proapoptotic genes in head and neck cancers (Shiiba et al. 2013). In other solid tumors, miRNAs regulating membrane transporters have been shown to restore sensitivity to chemotherapy drugs (Sun X et al. 2014). miR-145 has been demonstrated to be decreased in ALDH+/CD44+ head and neck CSCs. Introduction of miR-145 in these CSCs resulted in an inhibition of tumor progression via the SOX9/ADAM17 pathway (Yu et al. 2013). These miRNAs may be employed to decrease oncogene expression as well. Overall, miRNA modulation against CSC will be an intriguing field of study as we improve our understanding of these regulators. Further application of miRNA as well as other noncoding RNA will be of interest for further study.
Histone deacetylase inhibitors can alter the transcription of oncogenes in an epigenetic fashion and have been suggested to be useful in inducing apoptosis, cell cycle arrest, and reducing CD44 cell populations in HNSCC cell lines (Chikamatsu et al. 2013). With the importance of epigenetic regulation in maintaining CSC phenotypes, investigation into the modulation of these overlying epigenetic patterns will be of importance in future studies.
Modifying the Tumor Microenvironment
CSC niches are uniquely suited to drive cell survival and growth, as described above. Targeting this favorable microenvironment provides a potential therapeutic option. Antiangiogenic treatments may prove to be beneficial against head and neck CSCs, particularly because these cell populations are known to congregate in perivascular niches (Ritchie and Nör 2013). Targeted therapy against VEGF, particularly in combination with radiation, has shown some initial success against head and neck CSCs. Caution must be taken in application with anti-VEGF therapy, however, because reduction of VEGF can create a hypoxic environment in which CSC survival is favored (Conley et al. 2012).
Future Directions in CSC Therapy
Advancing Targeted Therapy in CSCs
Multiple targeted agents (primarily antibodies and small-molecule inhibitors) are in early clinical trials for HNSCC. As these agents are advanced, it will be important to study their effectiveness against head and neck CSC populations. Currently, the primary analyses for most of these trials are not toward studying effects on CSCs. Follow-up studies demonstrating a response or reduction in CSCs may portend to improved outcomes with these agents. Identifying pathways driving resistance to targeted therapy in CSCs and testing combinations of targeted agents will be key to enhancing CSC sensitivity to such agents.
Liposomal delivery systems can be targeted against CD44-positive cells, either with hyaluronic acid or RNA aptamers, to specifically deliver chemotherapeutic agents or other cytotoxic compounds (Dalla Pozza et al. 2013). Further studies refining the targeting of CSCs through cell surface markers and delivery of compounds to CSCs will be important in advancing targeted therapy. These delivery systems may offer the best means of introducing compounds that work to disrupt CSC internal genetic pathways.
Role of Human Papillomavirus in CSCs
The role of human papillomavirus (HPV) status in head and neck CSCs is an area of great interest. As HPV-positive tumors have a favorable prognosis, identifying the differences between HPV-positive and HPV-negative CSCs may highlight differences involved in tumor aggressiveness and response to therapy. Early studies have conflicting results in the proportion of CSCs in HPV-positive cancers compared with HPV-negative cancers, with some describing increased CSC proportions in HPV-positive cancers and others seeing the opposite (Tang et al. 2013; Zhang et al. 2014). Early studies show no difference in sensitivity to cisplatin between HPV-positive and HPV-negative CSCs (Tang et al. 2013). However, these studies remain limited and are preliminary. Despite these inconclusive early findings, studying HPV in CSCs may afford useful information in future more personalized and directed therapy.
Genomics in Head and Neck CSCs
Fully identifying the unique genomic signature of head and neck CSCs has not been fully explored. Important recent advances in genome-wide sequencing of HNSCCs have identified a wide array of mutations (Cancer Genome Atlas Network 2015). However, current next-generation sequencing studies have studied a primarily Caucasian, primarily HPV-negative population. In these specimens, mutations in genes canonically known to be associated with CSCs were not identified at a high rate. As our ability to sequence genomes more rapidly and with more fidelity improves, isolating CSCs for genomic study is becoming a viable option. Moreover, expression signatures of CSCs can be identified, particularly in comparison to non-CSC tumor cells. Whole-RNA transcriptome sequencing may provide insight into the unique genes and genetic pathways differentially expressed in CSCs versus non-CSC tumor cells. Although a significant amount of work has been performed identifying individual genes that are altered in CSCs (as described above), these genome-wide techniques afford a large-scale, unbiased analysis of the expression patterns of CSCs. As a result, new genes and genetic pathways could be uncovered as potential targets for future CSC therapy.
Addressing CSC Heterogeneity and Plasticity
Recent paradigms for CSCs address the possibility of heterogeneity within CSC populations, as well as plasticity in regards to which tumor cells express CSC phenotypes (Tang 2012). This will be an increasingly important issue as attempts are made to hone in on the specific CSC subpopulation. Multiple classes of CSCs may exist within a tumor, each responsive or resistant to different treatments and exhibiting varying degrees of aggressiveness and metastatic potential. Thus, successful therapy would require addressing each CSC subpopulation, potentially through different mechanisms.
CSC plasticity theories suggest that at different points, multiple tumor cells may exhibit a CSC phenotype. Thus, targeting and eliminating CSCs in one instance may not guarantee that subsequent tumor cells will not acquire a CSC phenotype. As such, continual surveillance for new CSCs will be important. Research into the underlying mechanisms driving CSC phenotypes (whether cell intrinsic or a product of microenvironment conditions) will be crucial to proactively identify and control factors that can lead to CSC neogenesis.
Combination Therapies
Employing combinations of targeted and traditional therapies will play an important role in future studies targeting CSCs. As CSCs are often highly resistant to established monotherapy and have several mechanisms for survival, disabling multiple survival mechanisms simultaneously may achieve a higher success rate in eliminating CSCs. As many of the earlier studies in CSC targeting have demonstrated, combination targeting can enhance CSCs to chemotherapy and radiation. Moreover, combination therapies can address different facets of head and neck CSC phenotypes, which could have synergistic effects on patient outcomes. For example, addressing factors contributing to EMT as well as radiation resistance could lead to decreased risk of metastasis and increased sensitivity to radiation, as well as an overall improved prognosis.
Clinical Trials
Ultimately, further exploration and clinical trials further characterizing the variety of therapies against head and neck CSC will be an important next phase in care. Interestingly, there are clinical trials under way targeting CD44 in a variety of tumors, including HNSCC (NCT01553851). Further trials targeting other CSC cell surface markers and combinations of therapies driven by targeting CSC surface markers will be important to study.
Conclusions
Head and neck CSCs remain a viable and intriguing option for targeted therapy. An increasing amount of literature suggests that CSCs play a key role in tumorigenesis, metastasis, and resistance to current treatment regimens. Intriguing targeted therapy options currently are being investigated. Despite current advances, however, much remains to be discovered, and a “magic bullet” to target and eliminate CSCs eludes us. Rather, further research studying a combination of therapies targeted against head and neck CSCs may provide significant advances.
Author Contributions
A.C. Birkeland, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; J.H. Owen, contributed to data analysis and interpretation, critically revised the manuscript; M.E. Prince, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
Andrew Birkeland is a Research Fellow funded on a T32 Advanced Research Training in Otolaryngology Program Training Grant (T32 DC005356) funded by the National Institutes of Health NIDCD (National Institute On Deafness And Other Communication Disorders).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bertrand G, Maalouf M, Boivin A, Battiston-Montagne P, Beuve M, Levy A, Jalade P, Fournier C, Ardail D, Magné N, et al. 2014. Targeting head and neck cancer stem cells to overcome resistance to photon and carbon ion radiation. Stem Cell Rev. 10(1):114–126. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Wong G, Earle C, Chen L. 2012. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem. 287(39):32800–32824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. 2015. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 517(7536):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Hsu WH, Wang CC, Chou CH, Kuo MY, Lin BR, Chen ST, Tai SK, Kuo ML, Yang MH. 2013. Connective tissue growth factor activates pluripotency genes and mesenchymal-epithelial transition in head and neck cancer cells. Cancer Res. 73(13):4147–4157. [DOI] [PubMed] [Google Scholar]
- Chang CW, Chen YS, Chou SH, Han CL, Chen YJ, Yang CC, Huang CY, Lo JF. 2014. Distinct subpopulations of head and neck cancer cells with different levels of intracellular reactive oxygen species exhibit diverse stemness, proliferation, and chemosensitivity. Cancer Res. 74(21):6291–6305. [DOI] [PubMed] [Google Scholar]
- Chikamatsu K, Ishii H, Murata T, Sakakura K, Shino M, Toyoda M, Takahashi K, Masuyama K. 2013. Alteration of cancer stem cell–like phenotype by histone deacetylase inhibitors in squamous cell carcinoma of the head and neck. Cancer Sci. 104(11):1468–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikamatsu K, Ishii H, Takahashi G, Okamoto A, Moriyama M, Sakakura K, Masuyama K. 2012. Resistance to apoptosis-inducing stimuli in CD44+ head and neck squamous cell carcinoma cells. Head Neck. 34(3):336–343. [DOI] [PubMed] [Google Scholar]
- Chinn SB, Darr OA, Owen JH, Bellile E, McHugh JB, Spector ME, Papagerakis SM, Chepeha DB, Bradford CR, Carey TE, et al. 2015. Cancer stem cells: mediators of tumorigenesis and metastasis in head and neck squamous cell carcinoma. Head Neck. 37(3):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CC, Lee LY, Li YC, Chen YJ, Lu YC, Li YL, Wang HM, Chang JT, Cheng AJ. 2013. Grp78 as a therapeutic target for refractory head-neck cancer with CD24(–)CD44(+) stemness phenotype. Cancer Gene Ther. 20(11):606–615. [DOI] [PubMed] [Google Scholar]
- Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME. 2010. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 32(9):1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley SJ, Gheordunescu E, Kakarala P, Newman B, Korkaya H, Heath AN, Clouthier SG, Wicha MS. 2012. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci U S A. 109(8):2784–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerwinski MJ, Desiderio V, Shkeir O, Papagerakis P, Lapadatescu MC, Owen JH, Athanassiou-Papaefthymiou M, Zheng L, Papaccio G, Prince ME, et al. 2013. In vitro evaluation of sialyl Lewis X relationship with head and neck cancer stem cells. Otolaryngol Head Neck Surg. 149(1):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Pozza E, Lerda C, Costanzo C, Donadelli M, Dando I, Zoratti E, Scupoli MT, Beghelli S, Scarpa A, Fattal E, et al. 2013. Targeting gemcitabine containing liposomes to CD44 expressing pancreatic adenocarcinoma cells causes an increase in the antitumoral activity. Biochim Biophys Acta. 1828(5):1396–1404. [DOI] [PubMed] [Google Scholar]
- Damek-Poprawa M, Volgina A, Korostoff J, Sollecito TP, Brose MS, O’Malley BW, Jr, Akintoye SO, DiRienzo JM. 2011. Targeted inhibition of CD133+ cells in oral cancer cell lines. J Dent Res. 90(5):638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SJ, Divi V, Owen JH, Bradford CR, Carey TE, Pagagerakis S, Prince ME. 2010. Metastatic potential of cancer stem cells in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 136(12):1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber A, Hoermann K, Stern-Straeter J, Schultz DJ, Goessler UR. 2013. Functional effects of SDF-1α on a CD44(+) CXCR4(+) squamous cell carcinoma cell lines as a model for interactions in the cancer stem cell niche. Oncol Rep. 29(2):579–584. [DOI] [PubMed] [Google Scholar]
- Fukusumi T, Ishii H, Konno M, Yasui T, Nakahara S, Takenaka Y, Yamamoto Y, Nishikawa S, Kano Y, Ogawa H, et al. 2014. CD10 as a novel marker of therapeutic resistance and cancer stem cells in head and neck squamous cell carcinoma. Br J Cancer. 111(3):506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan GN, Eagles J, Keysar SB, Wang G, Glogowska MJ, Altunbas C, Anderson RT, Le PN, Morton JJ, Frederick B, et al. 2014. Hedgehog signaling drives radioresistance and stroma-driven tumor repopulation in head and neck squamous cancers. Cancer Res. 74(23):7024–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CE, Yu CC, Hu FW, Chou MY, Tsai LL. 2014. Enhanced chemosensitivity by targeting Nanog in head and neck squamous cell carcinomas. Int J Mol Sci. 15(9):14935–14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. 2011. Global cancer statistics. CA Cancer J Clin. 61(2):69–90. [DOI] [PubMed] [Google Scholar]
- Jewett A, Tseng HC, Arasteh A, Saadat S, Christensen RE, Cacalano NA. 2012. Natural killer cells preferentially target cancer stem cells: role of monocytes in protection against NK cell mediated lysis of cancer stem cells. Curr Drug Deliv. 9(1):5–16. [DOI] [PubMed] [Google Scholar]
- Katayama R, Koike S, Sato S, Sugimoto Y, Truruo T, Fujitia N. 2009. Dofequidar fumarate sensitizes cancer stem-like side population cells to chemotherapeutic drugs by inhibiting ABCG2/BRCP-mediated drug export. Cancer Sci. 100(11):2060–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BS, Lee SH, Kim JM, Huang S, Kim SH, Rho YS, Bae WJ, Kang HJ, Kim YS, Moon JH, et al. 2014. Oct4 is a critical regulator of stemness in head and neck squamous cell carcinoma cells. Oncogene. 34(18):2317–2324. [DOI] [PubMed] [Google Scholar]
- Kung AL, Wang S, Kico JM, Kaelin WG, Livingston DM. 2000. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med. 6(12):1335–1340. [DOI] [PubMed] [Google Scholar]
- Kuo SZ, Blair KJ, Rahimy E, Kiang A, Abhold E, Fan JB, Wang-Rodriguez J, Altuna X, Ongkeko WM. 2012. Salinomycin induces cell death and differentiation in head and neck squamous cell carcinoma stem cells despite activation of epithelial-mesenchymal transition and Akt. BMC Cancer. 12:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Koo BS, Kim JM, Huang S, Rho YS, Bae WJ, Kang HJ, Kim YS, Moon JH, Lim YC. 2014. Wnt/β-catenin signaling maintains self-renewal and tumorigenicity of head and neck squamous cell carcinoma stem-like cells by activating Oct4. J Pathol. 234(1):99–107. [DOI] [PubMed] [Google Scholar]
- Lee SH, Oh SY, Do SI, Lee HJ, Kang HJ, Rho YS, Bae WJ, Lim YC. 2014. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br J Cancer. 111(11):2122–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Lu L, Tao H, Xue C, Teitz-Tennenbaum S, Owen JH, Moyer JS, Prince ME, Chang AE, Wicha MS. 2014. Generation of a novel dendritic-cell vaccine using melanoma and squamous cancer stem cells. J Vis Exp. (83):e50561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhang G, Miao XB, Deng XB, Wu Y, Liu Y, Jin ZR, Li XQ, Liu QZ, Sun DX, et al. 2013. Cancer stem-like cell properties are regulated by EGFR/AKT/β-catenin signaling and preferentially inhibited by gefitinib in nasopharyngeal carcinoma. FEBS J. 280(9):2027–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens-de Kemp SR, Brink A, Stigter-van Walsum M, Damen JM, Rustenburg F, Wu T, van Wieringen WN, Schuurhuis GJ, Braakhuis BJ, Slijper M, et al. 2013. CD98 marks a subpopulation of head and neck squamous cell carcinoma cells with stem cell properties. Stem Cell Res. 10(3):477–488. [DOI] [PubMed] [Google Scholar]
- Murillo-Sauca O, Chung MK, Shin JH, Karamboulas C, Kwok S, Jung YH, Oakley R, Tysome JR, Farnebo LO, Kaplan MJ, et al. 2014. CD271 is a functional and targetable marker of tumor-initiating cells in head and neck squamous cell carcinoma. Oncotarget. 5(16):6854–6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning N, Pan Q, Zheng F, Teitz-Tennenbaum S, Egenti M, Yet J, Li M, Ginestier C, Wicha MS, Moyer JS, et al. 2012. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 72(7):1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nör C, Zhang Z, Warner KA, Bernardi L, Visioli F, Helman JI, Roesler R, Nör JE. 2014. Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia. 16(2):137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaks V, Kong N, Werb Z. 2015. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cancer Stem Cell. 16(3):225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol J, Bloy N, Obrist F, Eggermont A, Galon J, Cremer I, Erbs P, Limacher JM, Preville X, Zitvogel L, et al. 2014. Trial watch: oncolytic viruses for cancer therapy. Oncoimmunology. 3:e28694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port RJ, Pinheiro-Maia S, Hu C, Arrand JR, Wei W, Young LS, Dawson CW. 2013. Epstein-Barr virus induction of the Hedgehog signaling pathway imposes a stem cell phenotype on human epithelial cell. J Pathol. 231(3):367–377. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. 2007. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 104(3):973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulte D, Brenner H. 2010. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 15(9):994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran I, Ganapathy V, Gillies E, Fonseca I, Sureban SM, Houchen CW, Reis A, Queimado L. 2014. Wnt inhibitory factor 1 suppresses cancer stemness and induces cellular senescence. Cell Death Dis. 5:e1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. 2001. Stem cells, cancer, and cancer stem cells. Nature. 414(6859):105–111. [DOI] [PubMed] [Google Scholar]
- Ritchie KE, Nör JE. 2013. Perivascular stem cell niche in head and neck cancer. Cancer Lett. 338(1):41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SJ, Peng KW, Bell JC. 2012. Oncolytic virotherapy. Nat Biotechnol. 30(7):658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiiba M, Shinozuka K, Saito K, Fushimi K, Kasamatsu A, Ogawara K, Uzawa K, Ito H, Takiguchi Y, Tanzawa H. 2013. MicroRNA-125b regulates proliferation and radioresistance of oral squamous cell carcinoma. Br J Cancer. 108(9):1817–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Liu S, Duan SZ, Zhang L, Zhou H, Hu Y, Zhou X, Shi C, Zhou R, Zhang Z. 2014. Targeting the c-Met/FZD8 signaling axis eliminates patient-derived cancer stem-like cells in head and neck squamous carcinomas. Cancer Res. 74(24):7546–7559. [DOI] [PubMed] [Google Scholar]
- Sun X, Jiao X, Pestell TG, Fan C, Qin S, Mirabelli E, Ren H, Pestell RG. 2014. MicroRNAs and cancer stem cells: the sword and the shield. Oncogene. 33(42):4967–4977. [DOI] [PubMed] [Google Scholar]
- Tabor MH, Clay MR, Owen JH, Bradford CR, Carey TE, Wolf GT, Prince ME. 2011. Head and neck cancer stem cells: the side population. Laryngoscope. 121(3):527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AL, Owen JH, Hauff SJ, Park JJ, Papagerakis S, Bradford CR, Carey TE, Prince ME. 2013. Head and neck cancer stem cells: the effect of HPV—an in vitro and mouse study. Otolaryngol Head Neck Surg. 149(2):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DG. 2012. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 22(3):457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upreti D, Pathak A, Kung SK. 2014. Lentiviral vector-based therapy in head and neck cancer (Review). Oncol Lett. 7(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron NN, Kaufman DS, Oh S, Inde Z, Hexum MK, Ohlfest JR, Vallera DA. 2011. Targeting tumor-initiating cancer cells with dCD133KDEL shows impressive tumor reductions in a xenotransplant model of human head and neck cancer. Mol Cancer Ther. 10(10):1829–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan G, Zhou L, Xie M, Chen H, Tian J. 2010. Characterization of side population cells from laryngeal cancer cell lines. Head Neck. 32(10):1302–1309. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Wu SP, Liu JB, Shi YS, Huang X, Zhang QB, Yao KT. 2013. MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell–like population of nasopharyngeal carcinoma cells. Cancer Res. 73(3):1219–1231. [DOI] [PubMed] [Google Scholar]
- Warrier S, Bhuvanalakshmi G, Arfuso F, Rajan G, Millward M, Dharmarajan A. 2014. Cancer stem-like cells from head and neck cancers are chemosensitized by the Wnt antagonist, sFRP4, by inducing apoptosis, decreasing stemness, drug resistance and epithelial to mesenchymal transition. Cancer Gene Ther. 21(9):381–388. [DOI] [PubMed] [Google Scholar]
- Wiklund ED, Gao S, Hulf T, Sibbritt T, Nair S, Costea DE, Villadsen SB, Bakholdt V, Bramsen JB, Sørensen JA, et al. 2011. MicroRNA alterations and associated aberrant DNA methylation patterns across multiple sample types in oral squamous cell carcinoma. PLoS One. 6(11):e27840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Yang X, Wang L, Clark D, Zuo H, Ye D, Chen W, Zhang P. 2013. Plasma membrane proteomics of tumor spheres identify CD166 as a novel marker for cancer stem-like cells in head and neck squamous cell carcinoma. Mol Cell Proteomics. 12(11):3271–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Lan HY, Huang CH, Tai SK, Tzeng CH, Kao SY, Wu KJ, Hung MC, Yang MH. 2012. RAC1 activation mediates Twist1-induced cancer cell migration. Nat Cell Biol. 14(4):366–374. [DOI] [PubMed] [Google Scholar]
- Yu CC, Lo WL, Chen YW, Huang PI, Hsu HS, Tseng LM, Hung SC, Kao SY, Chang CJ, Chiou SH. 2011. Bmi-1 regulates snail expression and promotes metastasis ability in head and neck squamous cancer-derived ALDH1 positive cells. J Oncol. 2011:609259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CC, Tsai LL, Wang ML, Yu CH, Lo WL, Chang YC, Chiou GY, Chou MY, Chiou SH. 2013. miR145 targets the SOX9/ADAM17 axis to inhibit tumor-initiating cells and IL-6–mediated paracrine in head and neck cancer. Cancer Res. 73(11):3425–3440. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kumar B, Piao L, Xie X, Schmitt A, Arradaza N, Cippola M, Old M, Agrawal A, Ozer E, et al. 2014. Elevated intrinsic cancer stem cell population in human papillomavirus–associated head and neck squamous cell carcinoma. Cancer. 120(7):992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Wei X, Cheng L, Tian J, Jiang JJ. 2007. CD133, one of the markers of cancer stem cells in Hep-2 cell line. Laryngoscope. 117:455–460. [DOI] [PubMed] [Google Scholar]