Abstract
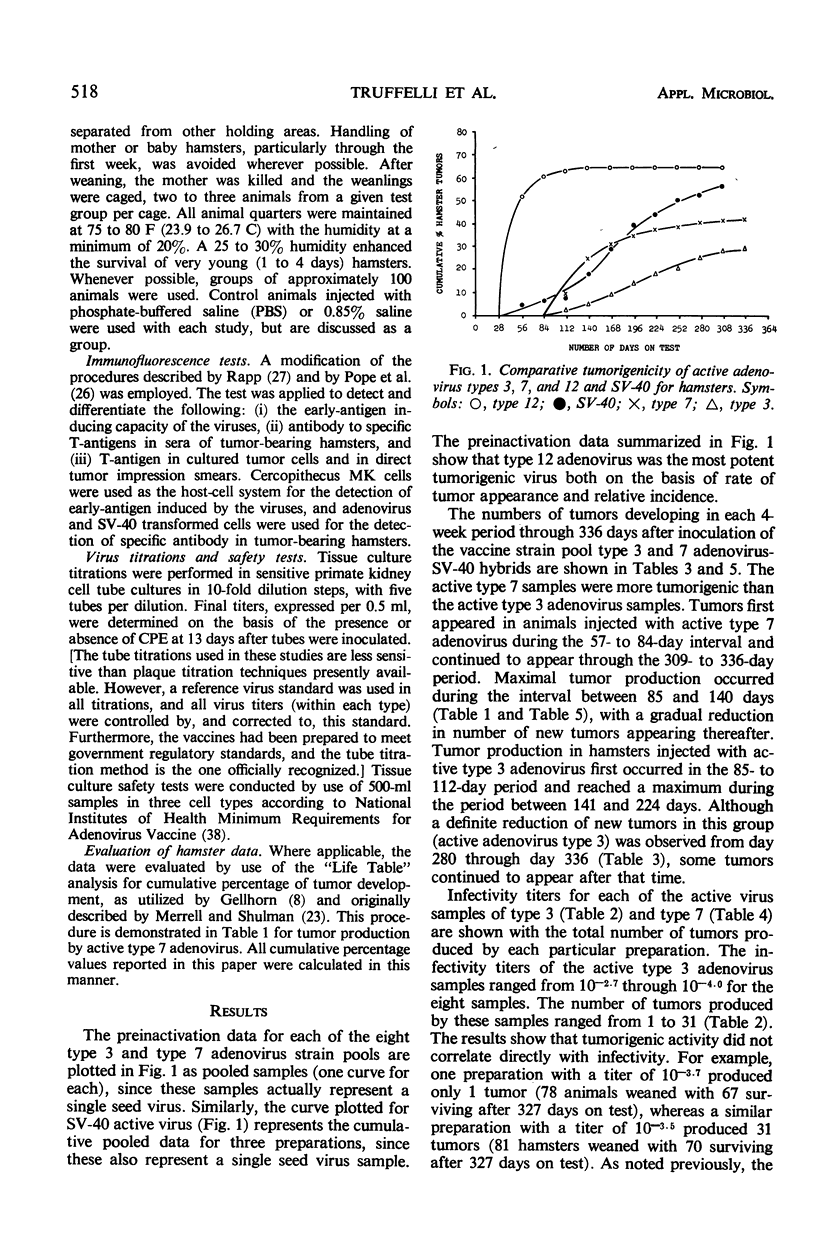
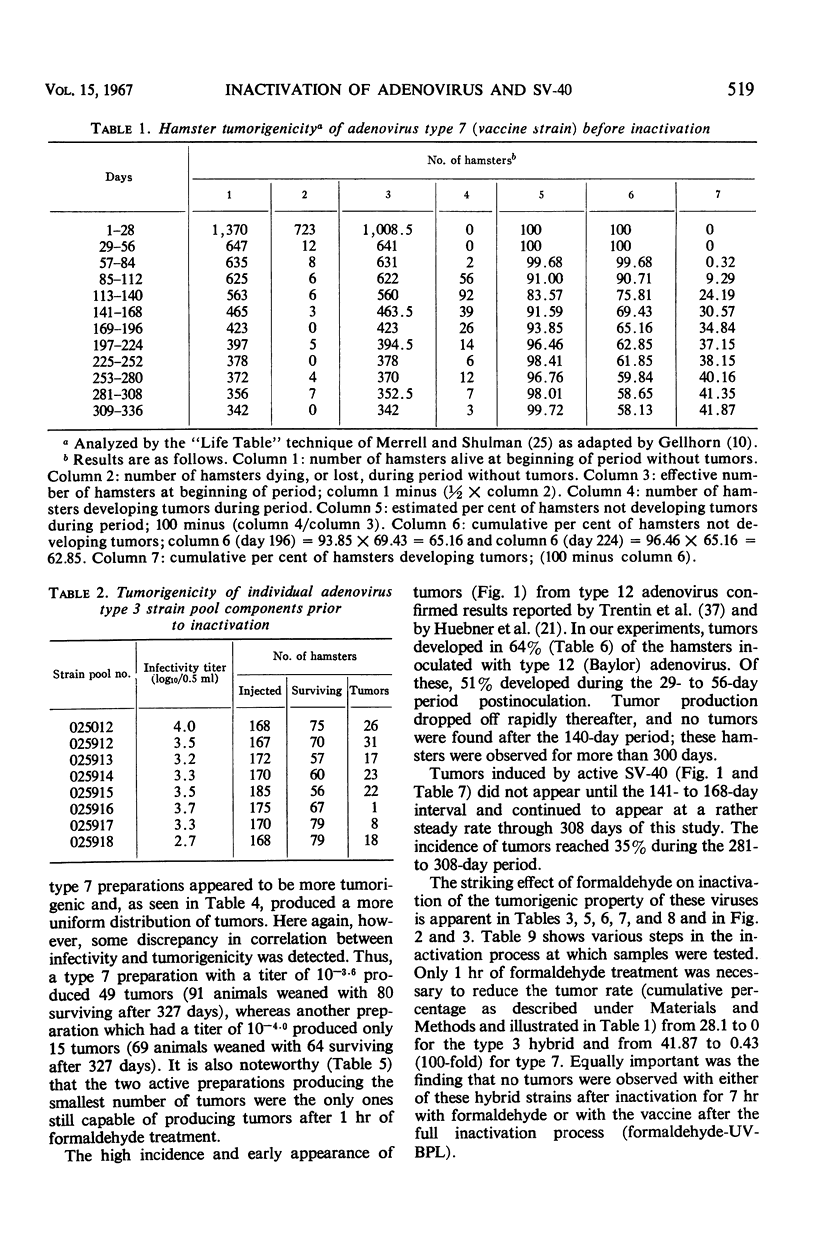
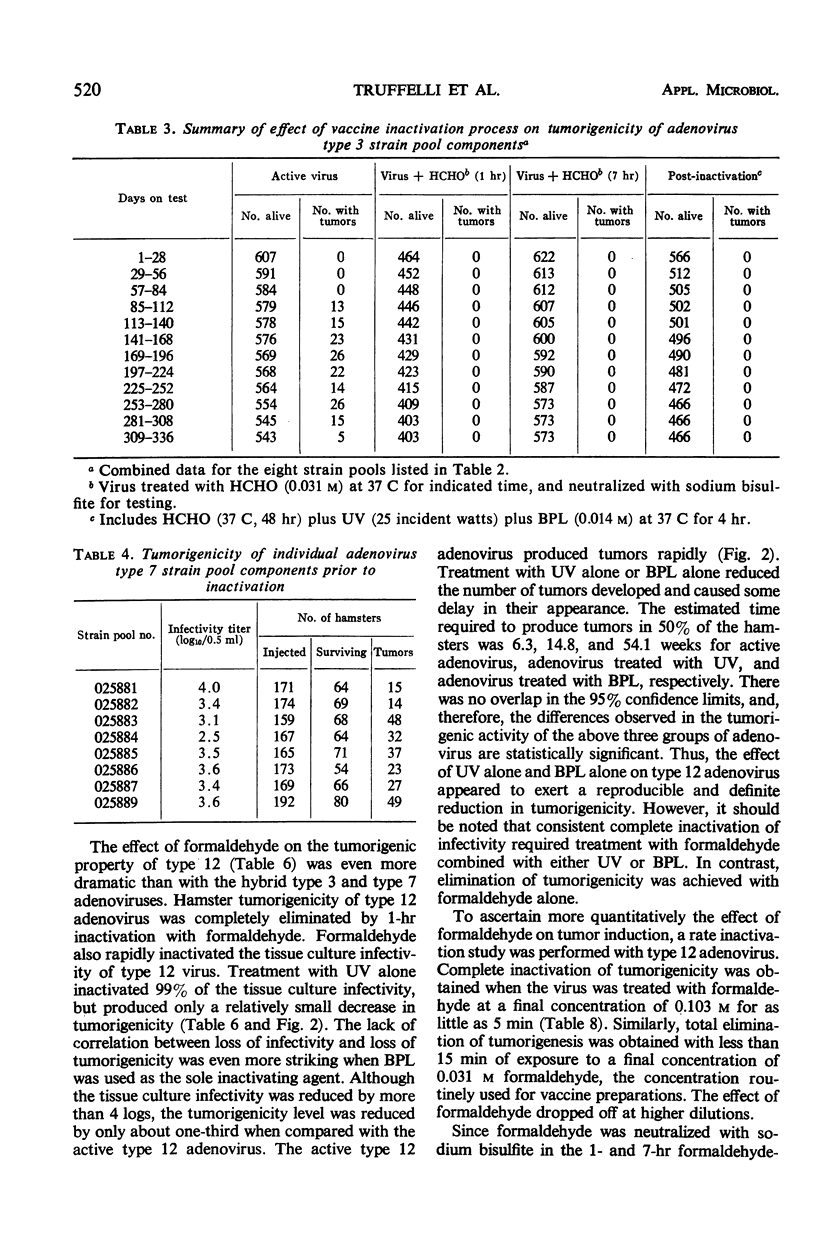
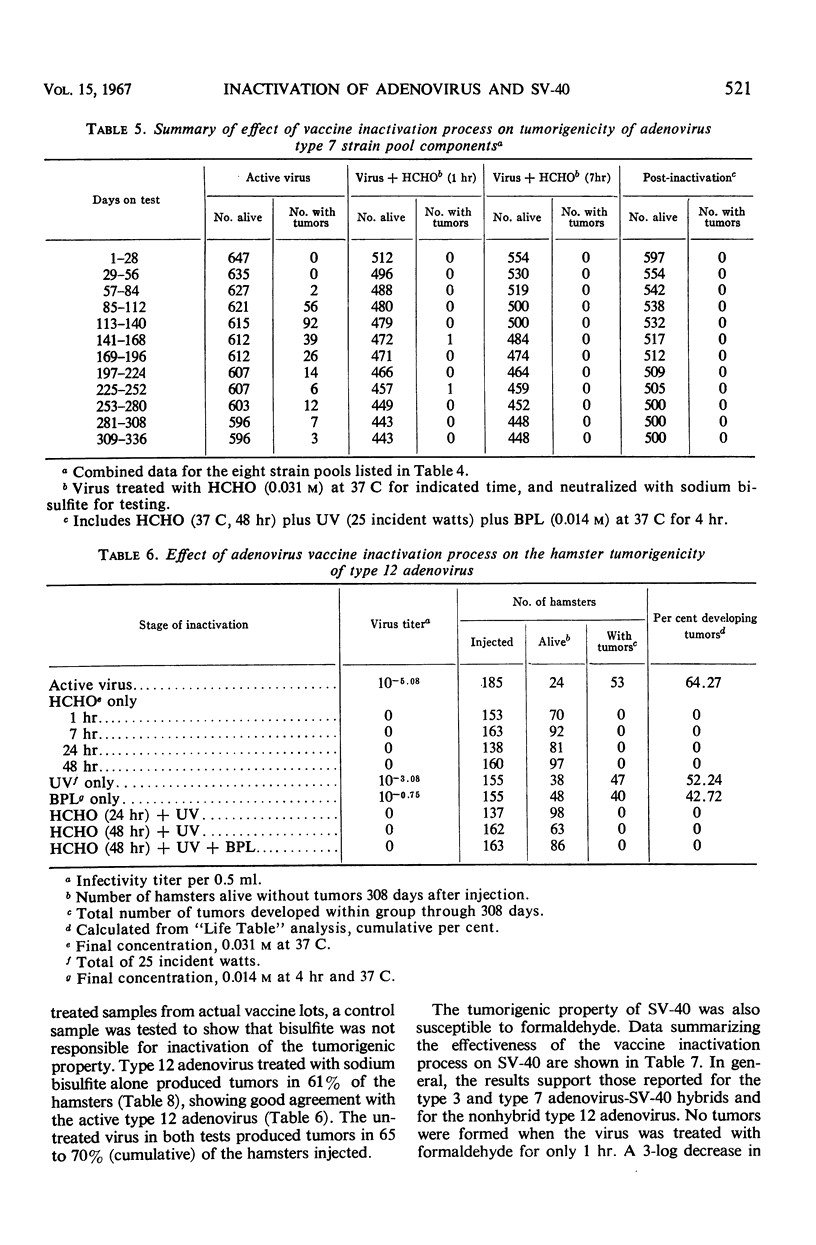
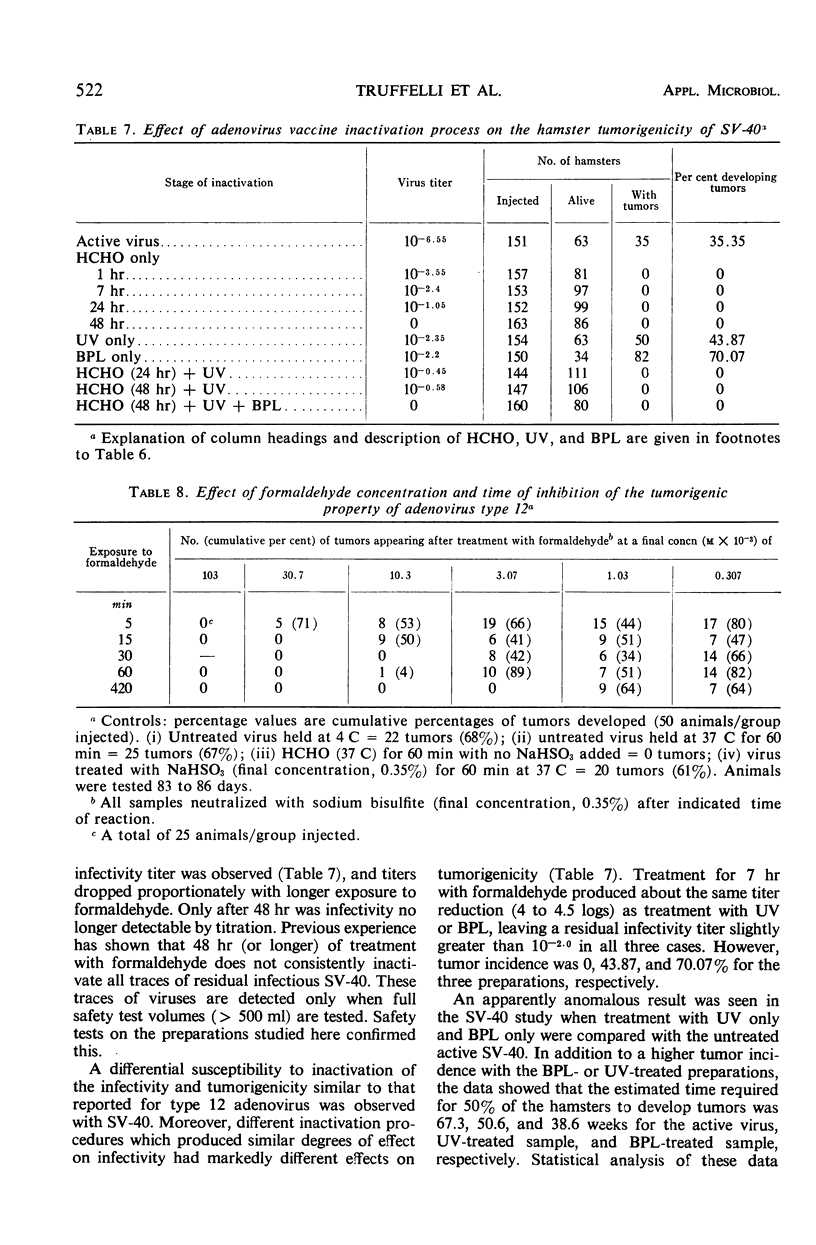
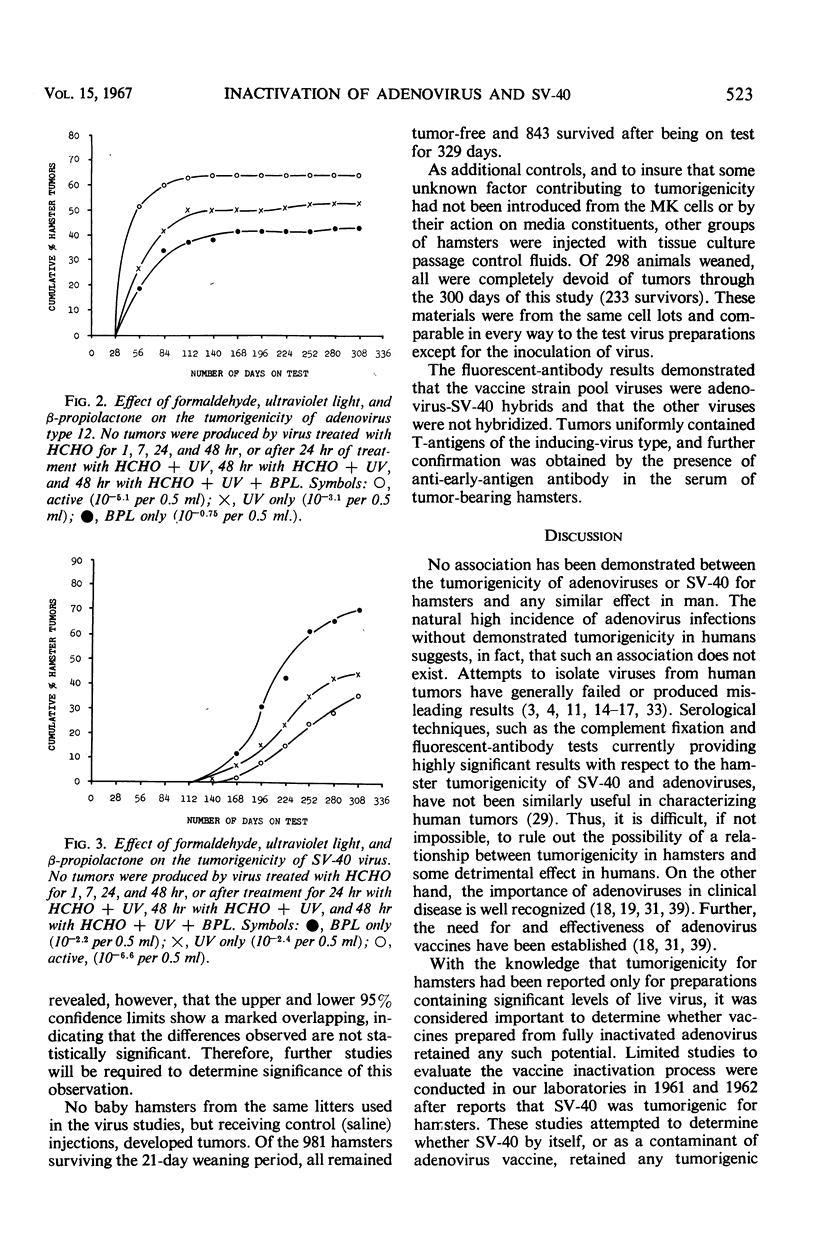
The effectiveness of the adenovirus vaccine inactivation process in destroying the tumorigenic potential for hamsters of adenoviruses, simian virus 40 (SV-40), and adenovirus-SV-40 hybrids was studied. Baby hamsters injected with untreated virus and with samples subjected to the complete inactivation process and to portions of the process were observed for tumor development for periods in excess of 300 days. Over 20,000 hamsters were injected. From 1 to 7 hr of exposure to formaldehyde at a concentration of 0.031 m at 37 C was sufficient to destroy the tumorigenicity observed in the nontreated preparations. Since the inactivation process included 48 hr of exposure at 37 C to 0.031 m formaldehyde plus treatment with ultraviolet (UV) and with β-propiolactone (BPL), it was concluded that the process has a large margin of safety. Adenovirus isolates free from tumorigenic potential are difficult, if not impossible, to obtain. Therefore, a proven inactivation process appears to provide the best assurance for obtaining adenovirus vaccines free from such potential. Data presented suggest that the tumorigenic property of the viruses studied might be independent of the infectivity of the preparation. The tumorigenic property was found to be highly susceptible to formaldehyde, but less sensitive to BPL or UV treatment. In contrast, treatment with UV or BPL decreased viral infectivity more readily than tumorigenicity. The three-stage inactivation process (formaldehyde, UV, and BPL) inactivated both tumorigenicity and infectivity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Di Mayorca G. Radiation target size of the lytic and the transforming ability of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):125–127. doi: 10.1073/pnas.54.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. Relative target sizes for the inactivation of the transforming and reproductive abilities of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):121–124. doi: 10.1073/pnas.54.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDDY B. E., BORMAN G. S., BERKELEY W. H., YOUNG R. D. Tumors induced in hamsters by injection of rhesus monkey kidney cell extracts. Proc Soc Exp Biol Med. 1961 May;107:191–197. doi: 10.3181/00379727-107-26576. [DOI] [PubMed] [Google Scholar]
- EDDY B. E., BORMAN G. S., GRUBBS G. E., YOUNG R. D. Identification of the oncogenic substance in rhesus monkey kidney cell culture as simian virus 40. Virology. 1962 May;17:65–75. doi: 10.1016/0042-6822(62)90082-x. [DOI] [PubMed] [Google Scholar]
- EDDY B. E. SIMIAN VIRUS 40 (SV-40): AN ONCOGENIC VIRUS. Prog Exp Tumor Res. 1964;4:1–26. doi: 10.1159/000385971. [DOI] [PubMed] [Google Scholar]
- GELLHORN A. The cocarcinogenic activity of cigarette tobacco tar. Cancer Res. 1958 Jun;18(5):510–517. [PubMed] [Google Scholar]
- GERBER P., HOTTLE G. A., GRUBBS R. E. Inactivation of vacuolating virus (SV-40) by formaldehyde. Proc Soc Exp Biol Med. 1961 Oct;108:205–209. [PubMed] [Google Scholar]
- GIRARDI A. J., HILLEMAN M. R., ZWICKEY R. E. TESTS IN HAMSTERS FOR ONCOGENIC QUALITY OF ORDINARY VIRUSES INCLUDING ADENOVIRUS TYPE 7. Proc Soc Exp Biol Med. 1964 Apr;115:1141–1150. doi: 10.3181/00379727-115-29138. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J., SLOTNICK V. B., HILLEMAN M. R. Search for virus in human malignancies. I. In vitro studies. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:776–785. doi: 10.3181/00379727-110-27648. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J., SWEET B. H., HILLEMAN M. R. Factors influencing tumor induction in hamsters by vacuolating virus, SV. Proc Soc Exp Biol Med. 1963 Mar;112:662–667. doi: 10.3181/00379727-112-28133. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J., SWEET B. H., SLOTNICK V. B., HILLEMAN M. R. Development of tumors in hamsters inoculated in the neonatal period with vacuolating virus, SV-40. Proc Soc Exp Biol Med. 1962 Mar;109:649–660. doi: 10.3181/00379727-109-27298. [DOI] [PubMed] [Google Scholar]
- GRACE J. T., Jr, MIRAND E. A., MILLIAN S. J., METZGAR R. S. Experimental studies of human tumors. Fed Proc. 1962 Jan-Feb;21:32–36. [PubMed] [Google Scholar]
- GRACE J. T., Jr Viruses and neoplasia. Surg Clin North Am. 1962 Apr;42:273–287. doi: 10.1016/s0039-6109(16)36630-0. [DOI] [PubMed] [Google Scholar]
- HILLEMAN M. R. Respiratory viruses and respiratory virus vaccines. Am Rev Respir Dis. 1963 Feb;87:165–180. doi: 10.1164/arrd.1963.87.2.165. [DOI] [PubMed] [Google Scholar]
- HOOK A. E., KUPSKY C. H., MCLEAN I. W., Jr, TIMM E. A. The nature of the formalin inactivation of poliomyelitis virus. J Immunol. 1956 Dec;77(6):444–452. [PubMed] [Google Scholar]
- HUEBNER R. J., CHANOCK R. M., RUBIN B. A., CASEY M. J. INDUCTION BY ADENOVIRUS TYPE 7 OF TUMORS IN HAMSTERS HAVING THE ANTIGENIC CHARACTERISTICS OF SV40 VIRUS. Proc Natl Acad Sci U S A. 1964 Dec;52:1333–1340. doi: 10.1073/pnas.52.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., LANE W. T. Oncogenic effects in hamsters of human adenovirus types 12 and 18. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2051–2058. doi: 10.1073/pnas.48.12.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Casey M. J., Chanock R. M., Schell K. Tumors induced in hamsters by a strain of adenovirus type 3: sharing of tumor antigens and "neoantigens" with those produced by adenovirus type 7 tumors. Proc Natl Acad Sci U S A. 1965 Aug;54(2):381–388. doi: 10.1073/pnas.54.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRELL M., SHULMAN L. E. Determination of prognosis in chronic disease, illustrated by systemic lupus erythematosus. J Chronic Dis. 1955 Jan;1(1):12–32. doi: 10.1016/0021-9681(55)90018-7. [DOI] [PubMed] [Google Scholar]
- McLEAN I. W., Jr, TAYLOR A. R. Experiences in the production of poliovirus vaccines. Prog Med Virol. 1958;1:122–164. [PubMed] [Google Scholar]
- O'CONOR G. T., RABSON A. S., MALMGREN R. A., BEREZESKY I. K., PAUL F. J. MORPHOLOGIC OBSERVATIONS OF GREEN MONKEY KIDNEY CELLS AFTER SINGLE AND DOUBLE INFECTION WITH ADENOVIRUS 12 AND SIMIAN VIRUS 40. J Natl Cancer Inst. 1965 May;34:679–693. [PubMed] [Google Scholar]
- OPPENHEIMER F., BENESI E., TAYLOR A. R. The ultraviolet irradiation of biological fluids in thin-flowing films. Am J Public Health Nations Health. 1959 Jul;49(7):903–923. doi: 10.2105/ajph.49.7.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. IMMUNOFLUORESCENT STUDIES OF ADENOVIRUS 12 TUMORS AND OF CELLS TRANSFORMED OR INFECTED BY ADENOVIRUSES. J Exp Med. 1964 Oct 1;120:577–588. doi: 10.1084/jem.120.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., MELNICK J. L. VIRUS-INDUCED INTRANUCLEAR ANTIGEN IN CELLS TRANSFORMED BY PAPOVAVIRUS SV40. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:1131–1135. doi: 10.3181/00379727-116-29472. [DOI] [PubMed] [Google Scholar]
- RAPP F., MELNICK J. L., BUTEL J. S., KITAHARA T. THE INCORPORATION OF SV40 MATERIAL INTO ADENOVIRUS 7 AS MEASURED BY INTRANUCLEAR SYNTHESIS OF SV40 TUMOR ANTIGEN. Proc Natl Acad Sci U S A. 1964 Dec;52:1348–1352. doi: 10.1073/pnas.52.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., MELNICK J. L., KITAHARA T., SHEPPARD R. SEARCH FOR VIRUS-INDUCED ANTIGENS IN HUMAN TUMORS USING THE SV40-HAMSTER SYSTEM AS A MODEL. Proc Soc Exp Biol Med. 1965 Mar;118:573–576. doi: 10.3181/00379727-118-29907. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., BAUM S. G. EVIDENCE FOR A POSSIBLE GENETIC HYBRID BETWEEN ADENOVIRUS TYPE 7 AND SV40 VIRUSES. Proc Natl Acad Sci U S A. 1964 Dec;52:1340–1347. doi: 10.1073/pnas.52.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWEET B. H., HILLEMAN M. R. The vacuolating virus, S.V. 40. Proc Soc Exp Biol Med. 1960 Nov;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- Sohier R., Chardonnet Y., Prunieras M. Adenoviruses. Status of current knowledge. Prog Med Virol. 1965;7:253–325. [PubMed] [Google Scholar]
- TAYLOR A. R., KAY W. W., MCLEAN I. W., Jr, OPPENHEIMER F., STIMPERT F. D. Effects of ultraviolet light on poliomyelitis virus. J Immunol. 1957 Jan;78(1):45–55. [PubMed] [Google Scholar]
- TAYLOR A. R., KAY W. W., TIMM E. A., HOOK A. E., McLEAN I. W., Jr Inactivation of poliomyelitis virus for the preparation of vaccines. I. Formalin and ultraviolet irradiation. J Immunol. 1957 Oct;79(4):265–275. [PubMed] [Google Scholar]
- TRENTIN J. J., YABE Y., TAYLOR G. The quest for human cancer viruses. Science. 1962 Sep 14;137(3533):835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]


