Abstract
Mycobacteria expressing recombinant antigens are already being developed as vaccines against both infections and tumors. Little is known about how dendritic cells might process such antigens. Two different mycobacterial species, the fast-growing Mycobacterium smegmatis and the slow-growing M. bovis M. bovis BCG, were engineered to express a model tumor antigen, the Kb-restricted dominant cytotoxic T-lymphocyte epitope OVA257-264. Recombinant M. bovis BCG but not recombinant M. smegmatis conferred protection to mice challenged with the B16-OVA tumor cell line. We went on to investigate whether the contrast in antitumor efficacy could be due to differences in how dendritic cells process antigen from the two mycobacterial strains for class I presentation. Both strains of mycobacteria caused phenotypic maturation of dendritic cells, but recombinant M. smegmatis infection led to a greater degree of dendritic cell maturation than recombinant M. bovis BCG infection. Antigen from recombinant M. smegmatis was processed and presented as OVA257-264 on Kb molecules by the dendritic cell line DC2.4 but not by bone marrow-derived dendritic cells (BMDC) or splenic dendritic cells. In contrast, antigen from recombinant M. bovis BCG was presented by all three dendritic cell types as long as the mycobacteria were viable. Such presentation was dependent on proteasome function and nascent major histocompatibility complex (MHC) class I molecules in DC2.4 cells but independent of the proteasome and transporter associated with antigen processings (TAP) in BMDC and splenic dendritic cells. These data demonstrate for the first time that antigen vectored by the slow-growing M. bovis BCG but not that vectored by fast-growing, readily destroyed M. smegmatis is processed and presented on MHC class I by in vitro-generated dendritic cells, which has implications for recombinant microbial vaccine development.
Mycobacterium bovis BCG, the only vaccine available against tuberculosis (22), has also been used successfully as a vaccine against leprosy (29) and, for over 20 years, as a local immunotherapy against superficial bladder cancer (2). Mycobacterium smegmatis is a related organism which has potential advantages as a recombinant vaccine over M. bovis BCG, as it is nonpathogenic and frequently commensal in humans (36, 42). Indeed, emulsified M. smegmatis and M. bovis BCG have comparable antitumor activity in a tumor immunotherapy model (56). The recent realization that mycobacteria are promising vehicles for a new generation of recombinant vaccines lends urgency to investigating the relative value of different strains in that role.
Despite their close relationship, there are several key differences between M. bovis BCG and M. smegmatis. M. bovis BCG is derived from the intracellular pathogen M. bovis and survives in host cells for months (31). In contrast, M. smegmatis is rapidly destroyed within infected cells and is nonpathogenic. Highly pathogenic mycobacteria such as M. tuberculosis can survive and replicate within cells because they inhibit phagosome maturation and hence their own degradation (11). Although the exact mechanism is not yet fully understood (47), it is known that such mycobacteria prevent acidification of the phagosome (13) and subsequent formation of a phagolysosome. M. bovis BCG shares many characteristics with M. tuberculosis and induces biochemical markers of maturation arrest (53, 54). On the other hand, M. smegmatis does not arrest phagosome maturation and is degraded rapidly by phagolysosomal proteases, which probably accounts for its lack of pathogenicity (28, 54).
Since the development of mycobacterium-Escherichia coli shuttle plasmids (23), there has been much interest in the generation of recombinant mycobacteria as vaccines against infectious disease, such as tuberculosis and human immunodeficiency virus. Furthermore, a recent study has also shown that recombinant M. bovis BCG can also protect against tumor challenge in model systems (15). The authors demonstrated that if they vaccinated mice with 102 or 104 M. bovis BCG secreting OVA230-359 and then challenged at day 30, 120, or 150 with B16-OVA, a significant delay in tumor growth was observed.
It is generally accepted that the generation of effective antitumor and anti-infective immune responses requires antigen-presenting cells to prime cytotoxic T lymphocytes and T-helper cells by processing antigen and presenting it on MHC class I and class II molecules, respectively. Mice immunized with recombinant M. bovis BCG expressing human immunodeficiency virus antigens (1) and β-galactosidase (49) generate specific cytotoxic T lymphocytes, but the mechanisms underlying antigen-presenting cell processing and presentation of antigen from recombinant mycobacteria have not been elucidated.
Macrophages are well recognized as antigen-presenting cells which host mycobacteria (43). Several lines of evidence suggest that dendritic cells might be equally important processors of mycobacterial antigens. Dendritic cells are a major host cell for mycobacteria and undergo levels of infection similar to those of macrophages (25). Dendritic cells are highly specialized antigen-presenting cells and uniquely potent primers of naïve T-cell responses (4, 20). However, exactly how antigens secreted from mycobacteria reach MHC class I presentation pathways by dendritic cells has not been defined.
Antigen-presenting cell processing pathways for MHC class I and class II presentation were long thought to be distinct (24). The “classical” MHC class I pathway involves degradation of cytosolic proteins by the proteasome into MHC class I binding epitopes, whereas antigens from exogenous sources were presented on MHC class II after phagocytosis. These peptides are transported into the endoplasmic reticulum, where they bind MHC class I molecules by the transporter associated with antigen processing (TAP) (38). However, it has recently been discovered that phagocytic cells can present antigens from exogenous sources, such as extracellular bacteria (e.g., Escherichia coli) not only on MHC class II molecules, but also on MHC class I.
The amino acid position 257 to 264 epitope (SIINFEKL) of the model antigen OVA, expressed as a fusion protein in E. coli and Salmonella enterica serovar Typhimurium, is presented on cell surface MHC class I in macrophages (41), dendritic cells (45, 50) and neutrophils (44). In macrophages, processing of SIINFEKL from E. coli occurs via both the classical pathway and a TAP-independent, proteasome-independent pathway, i.e., without requiring nascent MHC class I molecules but involving the cysteine proteases which are more often employed for MHC class II processing (7). Neutrophils also appear to use a proteasome-independent pathway (44). In contrast, dendritic cells process SIINFEKL from E. coli via a proteasome-dependent, TAP-dependent pathway (50).
Two alternative pathways have thus been proposed for nonclassical MHC class I presentation in which antigen is either degraded in vacuolar compartments such as the phagolyosome and bound to trafficking MHC class I molecules or regurgitated from the cell whereby it competes for binding to MHC class I molecules at the cell surface (6, 10, 19, 46). The intracellular bacterium Listeria monocytogenes escapes the phagosome and enters the cytosol by secretion of the pore-forming protein listeriolysin O (LLO), which allows its proteins access to the classical MHC I pathway (6).
We chose to study the capacity of two very different mycobacteria, M. bovis BCG and M. smegmatis, to generate a CD8-restricted T-cell response to recombinant antigen and the routes by which this antigen is presented to CD8+ T cells. The B16-OVA tumor model was employed to assess CD8 T-cell responses after vaccination, as this also enabled the use of the well-characterized MHC class I binding epitope OVA257-264 to study antigen presentation (27, 35). Our results demonstrate for the first time that dendritic cells present recombinant antigen from slow-growing mycobacteria on MHC class I and that the nonpathogenic bacterium M. smegmatis is a poor candidate for a CD8-restricted vaccine, reflecting its inability to gain access to the MHC class I processing pathway in dendritic cells.
MATERIALS AND METHODS
Generation of recombinant mycobacteria.
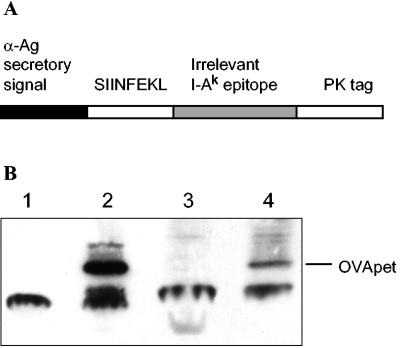
Mycobacterium bovis BCG Pasteur strain (American Type Culture Collection) and M. smegmatis (American Type Culture Collection) were grown in Middlebrook 7H9 (Difco) supplemented with 0.1% Tween 80, 0.2% glycerol, and 10% oleic acid dextrose catalase (Difco) or on solid 7H11 (Difco) supplemented with 0.5% glycerol and 10% oleic acid dextrose catalase. The mycobacterial- Escherichia coli shuttle vector pMOD8 was a gift from M. O'Donnell (Department of Urology, University of Iowa, Iowa City, Iowa). The polyepitope OVApet was constructed by PCR amplification of the oligonucleotide (Cancer Research UK peptide synthesis laboratory) 5′-CGGGATCCGCCACCATGAGTATAATCAACTTTGAAAAACTGAACACCGATGGGAGTACCGACTACGGAATCCTACAGATCAACAGCCGCGGCAAGCCCATCCCCAACCCCCTGCTGGGCCTGGACTCCACCTCTAGATAGGAATTCCG-3′; 20-bp primers were synthesized complementary to the 5′ and 3′ ends of the oligonucleotide and OVApet was amplified by PCR with Deep vent DNA polymerase (New England Biolabs). The cDNA was cloned into pMOD8 by BamHI and EcoRI restriction sites downstream of the alpha antigen secretory sequence under the control of the constitutive HSP60 promoter. The 148-bp polyepitope contains the H2-Kb OVA257-264 epitope (SIINFEKL) followed by an I-Ak46-61 epitope from the irrelevant hen egg lysozyme and a PK antibody tag (Fig. 1).
FIG. 1.
M. bovis BCG and M. smegmatis were engineered to secrete a polyepitope, OVApet. A polyepitope, OVApet, was constructed to express the OVA257-264 H2-Kb binding epitope downstream of the alpha antigen secretory signal from M. bovis BCG, which ensured it was secreted out of the bacteria (A). Lysates of wild-type M. bovis BCG (1), M. bovis BCG-OVApet (2), M. smegmatis (3) and M. smegmatis-OVApet (4) were run on a sodium dodecyl sulfate-16% reducing polyacrylamide gel, blotted onto a nitrocellulose membrane, and probed with an anti-PK monoclonal antibody followed by a biotinylated secondary antibody and then streptavidin-horseradish peroxidase. Expression of OVApet was detected by enhanced chemiluminescence (Fig. 1B). α-Ag, alpha antigen.
pMOD8-OVApet plasmid DNA was sequenced and electroporated into M. bovis BCG and M. smegmatis (39). Recombinant clones were selected for by growth on 7H11 agar (30 μg/ml kanamycin). Single colonies were picked into 7H9 medium as above containing 30 μg/ml kanamycin and grown by rolling for 3 to 4 weeks (M. bovis BCG) or by shaking at 100 rpm for 2 to 3 days (M. smegmatis) until an optical density at 600 nm was approximately equal to 1. Clones were stored at −80°C in phosphate-buffered saline (PBS)-15% glycerol and tested for expression of OVApet by Western blotting with an anti-PK monoclonal antibody (Serotec). CFU of the frozen clones were determined by plating serial dilutions on 7H11 plates containing kanamycin and counting resultant colonies.
Mice and immunizations.
Female C57BL/6 mice aged 6 to 8 weeks old were obtained from Harlan-UK Ltd., Oxon, United Kingdom. TAP1−/− mice were a gift from Caetano Reis e Sousa (Cancer Research UK London Institute. London, United Kingdom).
Mycobacteria were thawed, diluted in PBS as appropriate, and 100 μl (M. bovis BCG-OVApet) or 200 μl (M. smegmatis-OVApet) was injected subcutaneously into the left inguinal region on days 0, 14, and 28. Eight mice in each group were injected with 1.5 × 108, 1.5 × 107 or 1.5× 106 M. bovis BCG-OVApet or 1.5 × 107 wild-type M. bovis BCG. Five mice in each group were injected with 108, 107 or 106 M. smegmatis-OVApet or 107 wild-type M. smegmatis. On day 42, 2 × 105 B16-OVA cells (a gift from Edith Lord, University of Rochester, Rochester, N.Y.) were injected subcutaneously in PBS into the contralateral flank. Tumor growth was measured every 2 days with calipers, and mice were sacrificed when tumor size reached 1.44 cm2. Kaplan-Meier curves of survival were constructed with SAS, and statistical significance was determined by log rank tests.
Maintenance of cell lines.
The dendritic cell line DC2.4 was a gift from K. Rock (Dana-Farber Cancer Institute, Boston, Mass.) and was maintained in RPMI 1640 (Gibco-BRL) supplemented with 10% heat-inactivated fetal calf serum (Harlan Sera-labs), 2 mM l-glutamine, and 0.05 mM 2-mercaptoethanol. The B3Z hybridoma, which secretes interleukin (IL)-2 upon ligation of its Kb-SIINFEKL-specific receptor (27), was a gift from N. Shastri (Department of Molecular and Cell Biology, University of California, Berkeley). It was maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 1 mM sodium pyruvate, and nonessential amino acids (Gibco-BRL). Cells were screened for mycoplasma contamination by two assays, extranuclear DNA determination with Vero African green monkey indicator cells and a stab culture technique for atypical bacterial colonies on mycoplasma agar, and were universally negative.
Generation of bone marrow-derived dendritic cells and splenic dendritic cells.
Bone marrow was removed from the femur and tibia of C57BL/6 and TAP1−/− females by flushing into RPMI. To enrich for dendritic cell precursors, cells were incubated with biotinylated antibodies to B220, I-Ab, Gr1, CD3ɛ, and CD4 (BD-Pharmingen), washed, incubated with streptavidin microbeads, and passed through a magnetic cell separation column (Miltenyi-Biotech). Eluted cells were cultured at 37°C in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, penicillin and streptomycin, and 10 ng/ml murine recombinant granulocyte-macrophage colony-stimulating factor, and 100 U/ml murine recombinant IL-4 (both from Biosource International). Two thirds of the medium was replaced on day 2, and on day 4 the medium was replaced with fresh antibiotic-free medium. Bone marrow-derived dendritic cells (BMDC) were used for experiments on days 6 to 8, at which point about 70% were CD11c+ by flow cytometry. Single-cell splenocyte suspensions were obtained by digestion of spleens from C57BL/6 and TAP1−/− females in 66.67 Wünsch U/ml Liberase CI (Roche) and 1% (wt/vol) DNase I (Roche) for 30 min at 37°C. Dendritic cells were then purified by positive magnetic selection of single-cell suspensions with CD11c+ microbeads (Miltenyi Biotech), which produced a CD11c+ purity by fluorescence-activated cell sorting analysis of about 80% CD11c. Splenic dendritic cells were used immediately.
Phenotype of bacterially infected dendritic cells.
DC2.4 and BMDC (106) were plated in 24-well plates and infected with M. bovis BCG-OVApet or M. smegmatis-OVApet for 2 h at an multiplicity of infection of 10. Amikacin was added to a final concentration of 200 μg/ml for a further 2 h to kill any remaining extracellular bacteria. The medium was then changed for antibiotic-free medium, and the dendritic cells were incubated for 16 h overnight at 37°C.
Dendritic cells (105) were washed in PBS containing 1% fetal calf serum and 0.1% sodium azide, incubated for 15 min with a CD16/CD32 Fcγ III/II receptor antibody (BD-Pharmingen) to block nonspecific antibody binding and then incubated with phycoerythrin-conjugated antibodies to CD40, CD54, CD80, CD86, H2-Kb and I-Ab (BD-Pharmingen) or the relevant isotype control for 30 min at 4°C. Cells were washed and 104 events were acquired on a FACscalibur flow cytometer. (Becton Dickinson). Analysis was performed with Cell Quest Pro software (Becton Dickinson).
Antigen presentation assays.
We plated 105 dendritic cells in 100 μl in 96-well plates. Dendritic cells were infected with an equal volume of mycobacteria at multiplicities of infection of 1, 10, 50, and 100 for 2 h and treated with amikacin as above. After several washes in PBS, 105 B3Z were added to the wells in 200 μl of medium and incubated at 37°C for 16 h. Supernatants were stored at 4°C or −20°C until IL-2 was measured. In inhibitor experiments, dendritic cells were treated for 3 h with 10 μM lactacystin (Calbiochem) in T25 flasks at a cell density of 106/ml. Cells treated with brefeldin A were incubated with 2 μg/ml brefeldin A in a 100-μl volume in 96-well plates for 30 min and then diluted to 1 μg/ml by addition of an equal volume of medium containing mycobacteria. As brefeldin A is reversible after 5 h, dendritic cells were fixed in 4% paraformaldehyde for 10 min at room temperature, washed in PBS, quenched in 0.1 M glycine, and washed twice in PBS before addition of B3Z to prevent further processing in the absence of brefeldin A. Statistical analysis of the effects of TAP, lactacystin, and brefeldin A on antigen presentation were performed with the paired Student t test.
Measurement of IL-2 in supernatants by ELISA.
We coated 96-well flat-bottomed Maxisorb immunoplates (Nunc) with 100 ng of anti-mouse IL-2 capture antibody (BD-Pharmingen) in 0.1 M NaHCO3 at 4°C overnight, washed in PBS with 0.05% Tween 20, and blocked with 10% heat-inactivated fetal calf serum in PBS. Supernatants from dendritic cells, B3Z antigen presentation assays were added at a 1:5 dilution in medium in a final volume of 100 μl. Recombinant murine IL-2 (R&D Systems) was used as a standard.
Detection of OVApet protein in bacterial supernatants.
M. bovis BCG, M. bovis BCG-OVApet, M. smegmatis, and M. smegmatis OVApet were thawed from −80°C and resuspended at 8 × 107 bacteria per ml. M. bovis BCG-OVApet and M. smegmatis OVApet were also heat killed at 80°C for 20 min; 8 × 107 bacteria were incubated at 37°C for 4 h, and 200 μl of supernatant was added to 96-well flat-bottomed Maxisorb immunoplates. One in two serial dilutions were made of 100 μl of supernatant into 100 μl of 0.1 M NaHCO3 coating buffer and 100 μl of coating buffer was then added to the undiluted supernatants.
OVApet peptide was used as a standard at 1 in 2 consecutive serial dilutions in coating buffer from a concentration of 1 μM to 0.2 pM. All conditions were performed in triplicate. Samples were incubated at 4°C overnight and then blocked with 10% heat-inactivated fetal calf serum in PBS and incubated with a 1:1,000 anti-PK monoclonal antibody in blocking buffer (Serotec) for 1 h at room temperature. Following further washes, plates were incubated with a 1:500 dilution of anti-mouse immunoglobulin G-horseradish peroxidase (Serotec) and then incubated in the dark with tetramethylbenzidine peroxidase E1A substrate for approximately 5 to 10 min at room temperature. The colorimetric reaction was stopped by the addition of 50 μl of 1 M H2SO4 (BDH). The absorbance at 450 nm was then read, and a standard curve was constructed with the OVApet peptide standards to determine sample OVApet concentrations.
RESULTS
Differential tumor protection mediated by M. bovis BCG-OVApet and M. smegmatis-OVApet.
A polyepitope containing the H2-Kb epitope SIINFEKL downstream of the alpha antigen secretory signal of M. bovis BCG under the control of the hsp60 promoter was constructed (Fig. 1A). A PK tag was fused to the distal portion of the protein for identification. The SIINFEKL epitope was flanked on its N terminus by the anti-antigen signal sequence and on its C terminus by an irrelevant I-Ak binding epitope so to generate a Kb-binding epitope, precise cleavage must occur at both the N and C termini of SIINFEKL.
Recombinant clones of M. bovis BCG and M. smegmatis were examined by nonquantitative Western blotting and confirmed to contain the OVApet polyepitope by Western blot (Fig. 1B). Secretion of OVApet peptide from M. bovis BCG-OVApet and M. smegmatis-OVApet during a 4-h period was quantified by enzyme-linked immunosorbent assay (ELISA) and M. bovis BCG-OVApet was shown to secrete 25 to 150 pM OVApet per 107 bacteria, whereas less than 0.2 pM was detected from M. smegmatis-OVApet.
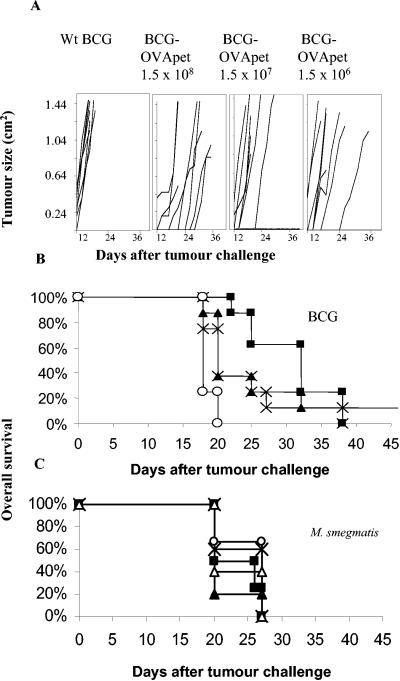
Mice were vaccinated with either M. bovis BCG-OVApet or M. smegmatis-OVApet and challenged with B16-OVA (Fig. 2). All doses of M. bovis BCG-OVApet significantly delayed onset of tumor growth compared to wild-type M. bovis BCG [P = 0.0001 (1.5 × 108), P = 0.008 (1.5 × 107), or P = 0.0028 (1.5 × 106)] (Fig. 2A). Median survival of controls was 18 days (95% confidence interval, 18 to 20 days) compared to 32 days (95% confidence interval, 25 to 38 days)/20 days (95% confidence interval, 20 to 32 days)/20 days (95% confidence interval, 18 to 27 days) for mice vaccinated with 1.5 × 108, 1.5 × 107 or 1.5 × 106 CFU of M. bovis BCG-OVApet, respectively (Fig. 2B). There was no significant difference between the three doses of M. bovis BCG-OVApet (P = 0.249), but there was a significant increase in survival time for these three doses of M. bovis BCG-OVApet over wild-type M. bovis BCG (P = 0.0001). In contrast, M. smegmatis-OVApet did not afford protection against tumor challenge irrespective of dose (Fig. 2C) (P = 0.36).
FIG. 2.
M. bovis BCG-OVApet protects against B16-OVA challenge but M. smegmatis-OVApet does not. C57BL/6 mice were vaccinated with 1.5 × 108 (▪), 1.5 × 107 (▴), or 1.5× 106 (×) M. bovis BCG-OVApet or 1.5 × 107 wild-type M. bovis BCG (○) in PBS subcutaneously in the inguinal region on day 0, 14, and 28. At day 42, mice were challenged with 2 × 105 B16-OVA tumor cells subcutaneously in the opposite flank, and progression in tumor growth was measured every 2 days. Tumor growth curves for each individual mouse are shown (A). Kaplan-Meier curves for eight mice per group are shown (B). C57BL/6 mice were vaccinated with 108 (▪), 107 (▴), or 106 (×) M. smegmatis-OVApet or 107 wild-type M. smegmatis (○) in PBS subcutaneously in the inguinal region on days 0, 14, and 28. At day 42, mice were challenged with 2 × 105 B16-OVA tumor cells subcutaneously in the opposite flank, and progression in tumor growth was measured every 2 days. Kaplan-Meier curves for five mice per group are shown (C).
Mice vaccinated with recombinant mycobacteria were also examined 2 weeks after the last vaccination for antigen-specific T-cell responses. Following a week of restimulation with irradiated EG7OVA cells, specific lysis of SIINFEKL-pulsed RMA-S cells was observed in two of five, five of five, and one of five mice given 1.5 × 108, 1.5 × 107 or 1.5 × 106 CFU of M. bovis BCG-OVApet, respectively, whereas no lysis was seen in mice given 108, 107, or 106 CFU of M. smegmatis-OVApet or wild-type bacteria (data not shown).
Subsequent experiments addressed the effects of the respective mycobacteria on dendritic cells and how their secreted antigen gains access to the MHC class I of dendritic cells.
Both recombinant M. bovis BCG and M. smegmatis efficiently mature dendritic cells.
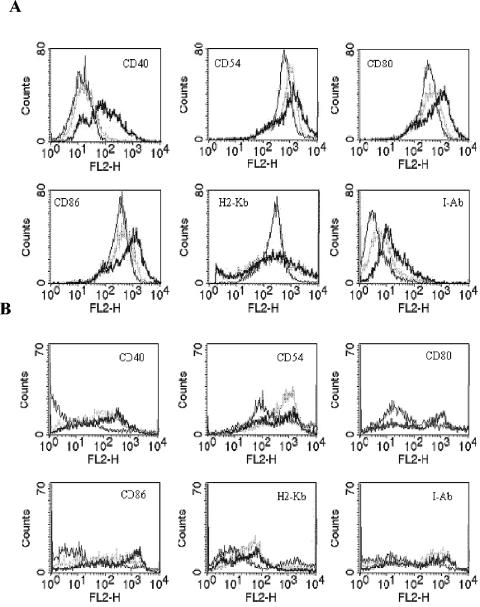
Dendritic cells took up either mycobacterial strain after 2 h of exposure (data not shown). Infection of both the bone marrow dendritic cell-derived cell line DC2.4 and BMDC with M. smegmatis-OVApet led to maturation of the dendritic cells as determined by upregulated surface expression of CD40, CD54, CD80, CD86, and MHC class II molecules (Fig. 3). However, although M. bovis BCG-OVApet led to maturation of BMDC, only a small increase in maturation markers on DC2.4 cells were observed following M. bovis BCG-OVApet infection. Maturation of BMDC was also less than that observed with M. smegmatis- OVApet. Maturation of splenic dendritic cells was not observed. However, splenic dendritic cells are very short lived, and 20 h after infection, 80 to 90% of the dendritic cells were apoptotic (data not shown). Both strains of mycobacteria upregulated MHC and costimulatory molecules in peritoneal macrophages, although expression levels were considerably lower than seen with dendritic cells (data not shown). Therefore, recombinant M. bovis BCG and M. smegmatis are capable of inducing maturation of murine dendritic cells, but M. smegmatis is a more potent inducer of maturation of dendritic cells than M. bovis BCG.
FIG. 3.
M. bovis BCG-OVApet and M. smegmatis-OVApet induce a mature dendritic cell phenotype. We infected 106 dendritic cells at a multiplicity of infection of 10 with M. bovis BCG-OVApet (grey hashed line) or M. smegmatis-OVApet (bold line) or left them uninfected (thin line) for 2 h. The remaining extracellular bacteria were killed with antibiotic treatment, and the dendritic cells were cultured for 16 h. Dendritic cells were incubated with phycoerythrin-conjugated antibodies to CD40, CD54, CD80, CD86, H2-Kb and I-Ab and analyzed by flow cytometry. DC2.4 cell surface expression (A) and BMDC cell surface expression (B) are shown.
Differential processing of MHC class I-restricted epitope when vectored by M. bovis BCG and M. smegmatis.
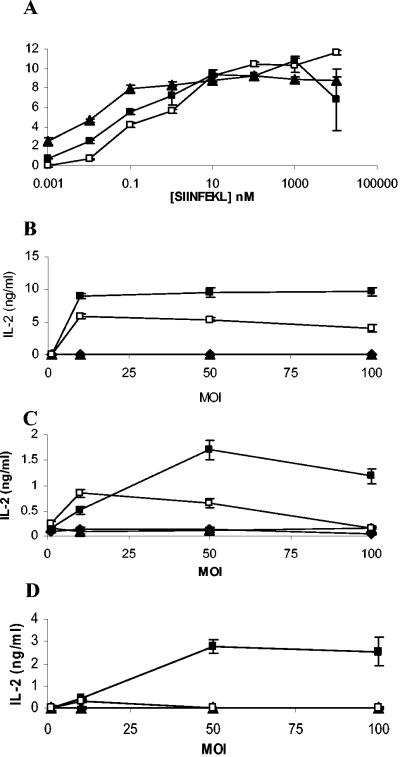
Having confirmed that dendritic cells undergo maturation when infected with M. bovis BCG or M. smegmatis, we investigated antigen processing of the Kb-binding epitope secreted by recombinant mycobacteria-OVApet. Control experiments where dendritic cells were pulsed with SIINFEKL peptide and used to stimulate the B3Z hybridoma showed that the three types of dendritic cells bound and presented SIINFEKL peptide with IL-2 secretion from the hybridoma, plateauing at 10 μM to 100 nM peptide. However, splenic dendritic cells were the most potent presenters of bound peptides, with half maximal stimulation of B3Z being obtained with only 10 pM peptide. BMDC required 500 pM peptide to stimulate half the B3Z cells and DC2.4 cells 1 nM peptide (Fig. 4. A). Comparisons were then made between the peptide and the presentation of SIINFEKL following infection of dendritic cells with recombinant mycobacteria.
FIG. 4.
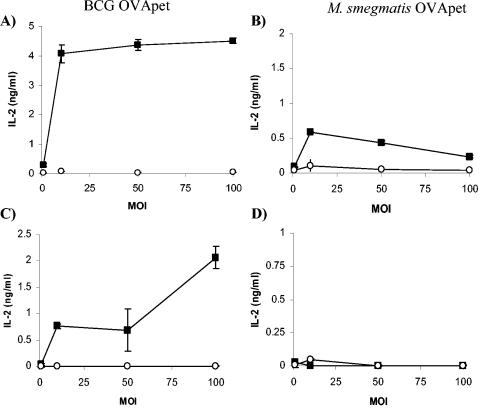
Antigen presentation from recombinant mycobacteria by dendritic cells. DC2.4 (□), BMDC (▪), and splenic dendritic cells (▴) were pulsed with increasing concentrations of SIINFEKL peptide for 2 h, washed, and then cultured with B3Z for 16 h. IL-2 production in supernatants was measured (A). Various dendritic cells were infected with increasing amounts of M. bovis BCG (▴), M. bovis BCG-OVApet (▪), M. smegmatis (▴), or M. smegmatis-OVApet (□) and cultured with the B3Z hybridoma for 16 h. Presentation of SIINFEKL on H2-Kb was measured by IL-2 secretion. The dendritic cell line DC2.4 (B), bone marrow-derived dendritic cells (C), and CD11c+ splenic dendritic cells (D) are shown.
The DC2.4 cell line processed SIINFEKL from both M. bovis BCG-OVApet and M. smegmatis-OVApet, although SIINFEKL was processed somewhat more efficiently from M. bovis BCG (Fig. 4B). BMDC also processed SIINFEKL from M. bovis BCG-OVApet, though much less efficiently than DC2.4 cells (Fig. 4C). Processing of SIINFEKL by BMDC following M. smegmatis-OVApet was only observed infection in 3/8 experiments. Splenic dendritic cells (CD11c+) processed SIINFEKL vectored by M. bovis BCG-OVApet (Fig. 4D). However, unlike DC2.4 and BMDC, splenic dendritic cells did not process and present SIINFEKL from M. smegmatis-OVApet.
Although SIINFEKL was processed and presented from M. smegmatis-OVApet, presentation was not always observed in dendritic cells generated directly from bone marrow precursor cells in vitro and never by splenic dendritic cells. Further controls which included comparison of live and nonviable M. bovis BCG-OVApet (Fig. 5A and C) and M. smegmatis-OVApet (Fig. 5B and D) showed that only epitopes secreted from live mycobacteria were processed and presented on MHC class I in both the DC2.4 cell line (Fig. 5A and B) and BMDC (Fig. 5C and D). There was no presentation of SIINFEKL from M. bovis BCG-OVApet or M. smegmatis-OVApet by peritoneal macrophages (thioglycolate elicited) or by the peritoneal macrophage cell line IC-21 (data not shown).
FIG. 5.
SIINFEKL from heat-killed M. bovis BCG-OVApet and M. smegmatis-OVApet is not presented on MHC class I. We infected 105 DC2.4 (A and B) or BMDC (C and D) in 96-well plates with live (solid squares) or heat-killed (open circles) M. bovis BCG-OVApet (A and C) or M. smegmatis-OVApet (B and D) at a multiplicity of infection of 1, 10, 50, or 100 for 2 h at 37°C. The remaining extracellular bacteria were killed by a 2-h treatment with 200 μg of amikacin per ml at 37°C. Dendritic cells were then washed in PBS and cocultured with 105 B3Z cells for 16 h at 37°C. Supernatants were assayed for IL-2 by solid-phase ELISA.
Presentation of SIINFEKL is only partly proteasome and TAP dependent.
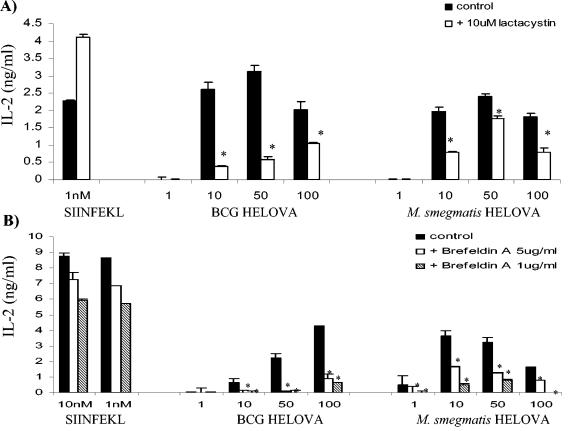
We next sought to determine the extent to which the classical MHC class I antigen-processing pathway was involved for processing antigen from M. bovis BCG and M. smegmatis by various dendritic cell types. To dissect the pathway by which the SIINFEKL epitope from recombinant mycobacteria is delivered to MHC class I, we used lactacystin, an irreversible proteasome inhibitor; brefeldin A, which blocks the export of newly synthesized MHC class I molecules from the trans-Golgi network; and TAP1-deficient (TAP1−/−) mice lacking a functional TAP transporter. After establishing that treatment with lactacystin did not diminish presentation of SIINFEKL, presentation from recombinant mycobacteria in dendritic cells treated with lactacystin was studied. Lactacystin reduced presentation of SIINFEKL by DC2.4 cells by 60% (M. bovis BCG-OVApet) and by 30% (M. smegmatis-OVApet) (Fig. 6A). A more pronounced reduction in SIINFEKL presentation was achieved by blocking export of nascent MHC class I molecules for M. bovis BCG-OVApet and M. smegmatis-OVApet (Fig. 6B).
FIG. 6.
Presentation of SIINFEKL from recombinant bacteria in DC2.4 cells is reduced by the proteasome inhibitor lactacystin and the inhibitor of retrograde transport brefeldin A. We infected 105 DC2.4 in 96-well plates with M. bovis BCG-OVApet or M. smegmatis-OVApet at a multiplicity of infection of 1, 10, 50, or 100 for 2 h at 37°C or pulsed them with SIINFEKL peptide. The remaining extracellular bacteria were killed by a 2-h treatment with 200 μg of amikacin per ml at 37°C. Dendritic cells were then washed in PBS and cocultured with 105 B3Z cells for 16 h at 37°C. Supernatants were assayed for IL-2 by solid-phase ELISA. In some instances dendritic cells were treated with 10 μM lactacystin for 3 h (A) or 1 or 5 μg of brefeldin A per ml for 30 min (B). Dendritic cells were fixed in 1% paraformaldehyde and washed extensively before addition of B3Z (B). A statistically significant decrease in presentation is indicated by * (P < 0.05, Student t test).
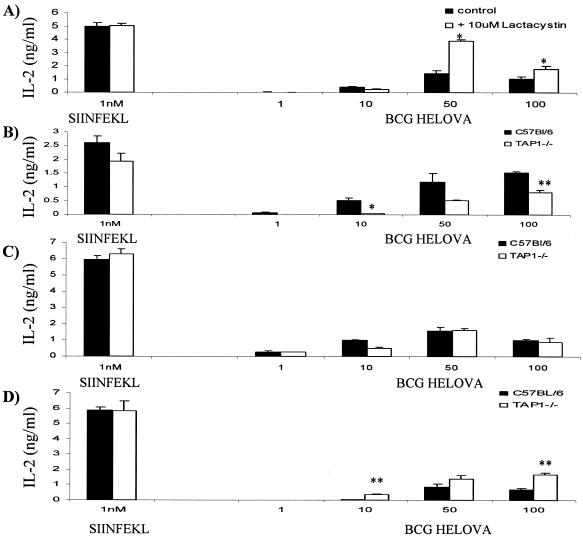
Lactacystin did not inhibit presentation of SIINFEKL from M. bovis BCG-OVApet by infected BMDC (Fig. 7A). Indeed, presentation increased following lactacystin treatment (two of four experiments) and was unaffected in the remainder. BMDC from TAP1−/− mice retained some ability to process and present SIINFEKL from M. bovis BCG-OVApet but with a significant decrease, but not abolishment, in presentation in two of four experiments (Fig. 7B). However, in two further experiments no effect on presentation was observed in BMDC from TAP1−/− mice (Fig. 7C).
FIG. 7.
MHC class I presentation of SIINFEKL is not inhibited by lactacystin and is partly TAP independent in BMDC and splenic dendritic cells. We treated 105 BMDC for 3 h with 10 μM lactacystin or left them untreated and then infected them in 96-well plates with M. bovis BCG-OVApet at a multiplicity of infection of 1, 10, 50, or 100 for 2 h at 37°C or pulsed them with SIINFEKL peptide. The remaining extracellular bacteria were killed by a 2-h treatment with 200 μg of amikacin per ml at 37°C. Dendritic cells were then washed in PBS and cocultured with 105 B3Z cells for 16 h at 37°C. Supernatants were assayed for IL-2 by solid-phase ELISA (A). We infected 105 BMDC (B and C) or splenic dendritic cells (D) from C57BL/6 or TAP1−/− mice with M. bovis BCG-OVApet or pulsed them with SIINFEKL peptide, and presentation of SIINFEKL to B3Z cells was determined as described above. A statistically significant decrease or increase in presentation following lactacystin treatment or the use of TAP1−/− dendritic cells is indicated by * (P < 0.05, Student t test).
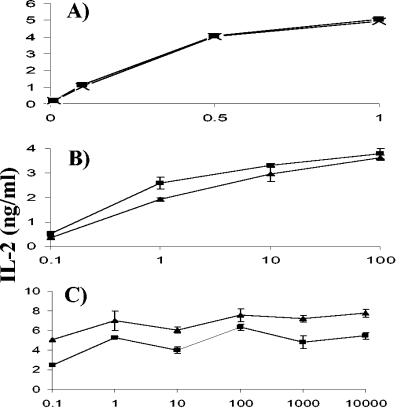
Control experiments indicated that SIINFEKL peptide was presented with nearly equal efficiency on BMDC that had been ptreated with lactacystin (Fig. 8A) or derived from TAP1−/− mice (Fig. 8B) compared to C57BL/6 mice. In splenic dendritic cells, presentation of SIINFEKL by M. bovis BCG-OVApet-infected cells was also independent of the TAP transporter complex. Indeed, presentation appeared greater on TAP1−/− CD11c+ splenic dendritic cells than on splenic dendritic cells from wild-type C57BL/6 mice (Fig. 7D). Control experiments in which splenic dendritic cells were pulsed with SIINFEKL peptide indicate that TAP1−/− dendritic cells may in fact have a higher affinity for SIINFEKL peptide than C57BL/6 dendritic cells (Fig. 8C).
FIG. 8.
Lactacystin-treated and TAP1−/− dendritic cells are able to present SIINFEKL peptide with similar efficiencies. We pulsed 105 C57BL/6 BMDC that had been treated with 10 uM lactacystin (▪) or not (×) with increasing concentrations of SIINFEKL for 2 h and washed and cocultured them with 105 B3Z for 16 h at 37°C. IL-2 secretion from B3Z in supernatants is shown (A). C57BL/6 BMDC (▪) and TAP1−/− BMDC (▴) were pulsed with increasing concentrations of SIINFEKL, washed, and cocultured with B3Z cells for 16 h. IL-2 secretion from B3Z in supernatants is shown (B). C57BL/6 splenic dendritic cells (▪) and TAP1−/− splenic dendritic cells (▴) were pulsed with increasing concentrations of SIINFEKL, washed, and cocultured with B3Z cells for 16 h. IL-2 secretion from B3Z in supernatants is shown (C).
DISCUSSION
We have shown that antigen delivered to professional antigen-presenting cells by recombinant M. bovis BCG is processed and presented on MHC class I molecules, whereas antigen from recombinant M. smegmatis is only processed and presented by dendritic cells sometimes and at much lower levels than that from recombinant M. bovis BCG. We have also shown that in a tumor challenge model, recombinant M. bovis BCG afforded protection whereas M. smegmatis did not. Additionally, SIINFEKL-specific cytotoxic T lymphocytes could be detected following in vitro restimulation after vaccination with M. bovis BCG-OVApet but not M. smegmatis-OVApet. M. bovis BCG-OVApet appears to secrete as much as 750-fold more OVA-pet than M. smegmatis-OVApet, which may impact significantly on the efficacy of the vaccine. We believe this is primarily through the resultant failure of dendritic cells, which are either infected with M. smegmatis-OVApet or have taken up OVApet from apoptotic infected macrophages and dendritic cells, to present peptide antigen on MHC class I and generate an antigen-specific CD8+ T-cell response.
Live wild-type M. smegmatis has potent nonspecific immunostimulatory properties and is as effective as live M. bovis BCG in a preclinical model of nonspecific cancer immunotherapy (data not shown). Indeed, we have shown that M. smegmatis induces a higher expression of costimulatory molecules on dendritic cells than does M. bovis BCG. It is possible that the increased maturation of dendritic cells observed with M. smegmatis is due to structural differences in the cell wall of fast- and slow-growing mycobacteria which may be related to pathogenicity (9, 14, 30). Although all three “types” of dendritic cells studied processed SIINFEKL from M. bovis BCG-OVApet, only the DC2.4 cell line processed antigen from recombinant M. smegmatis efficiently. Other differences between the three dendritic cell types were found with the DC2.4 cells processing SIINFEKL in a proteasome-dependent manner requiring newly synthesized MHC class I molecules, whereas processing in BMDC and splenic dendritic cells was independent of the proteasome and partly independent of TAP. These results highlight differences or limitations in the use of antigen-presenting cell lines for the study of antigen processing by dendritic cells.
Processing and presentation of SIINFEKL from M. bovis BCG required viable bacilli. This is in agreement with previous studies showing concurrent loading of macrophages with live M. tuberculosis and OVA protein which resulted in TAP-dependent processing of SIINFEKL (32, 37). This may suggest that M. tuberculosis secretes factors that facilitate antigen access to the cytosol. Slow-growing mycobacteria survive within cells, and pore forming in the phagosomal membrane may be an essential mechanism whereby nutrients from the cytosol are obtained or toxins released, as in the case of Listeria monocytogenes (40, 48). This may be one mechanism whereby antigen from M. bovis BCG-OVApet is presented more efficiently than from M. smegmatis-OVApet, as a fast-growing bacterium, rapidly destroyed in the phagolysosome, would not require access to the cytosol.
One possible reason for the lack of presentation of antigen from M. smegmatis in BMDC and splenic dendritic cells appears to be that no detectable secretion of OVApet is observed by ELISA compared to that from M. bovis BCG, as secreted antigen from mycobacteria has been shown to be the most immunogenic form (18, 21). However, it cannot be ruled out that M. smegmatis may upregulate its secretion of OVApet after uptake by dendritic cells, particularly by DC2.4 cells. The OVApet protein is under the control of the HSP60 promoter, and stresses within the phagosome may upregulate transcription and translation of OVApet and hence secretion, as shown for Mycobacterium avium LacZ (5). As proteasome-dependent processing is observed from M. smegmatis OVApet in DC2.4 cells, this indicates that either this route of presentation is very efficient in the cell line and extremely low levels of secreted OVApet are required for generation of SIINFEKL or that OVApet peptide does not need to be secreted from the bacteria for degradation to SIINFEKL by the proteasome.
Antigen processing of SIINFEKL from OVApet appeared to be partly independent of TAP in BMDC. This is in agreement with Neyrolles et al., who showed that processing by macrophages of the M. tuberculosis 19-kDa antigen from M. vaccae, M. smegmatis, and M. bovis BCG was independent of TAP (37). Presentation of a class I-restricted NP epitope, fused to the 19-kDa antigen, in fast-growing M. vaccae and M. smegmatis was found to be more efficient than in M. bovis BCG and correlated with enhanced phagosome acidification. Where our findings contrast with those of Neyrolles et al. is our presentation of SIINFEKL is greater by dendritic cells infected with recombinant M. bovis BCG and much less in those infected by recombinant M. smegmatis.
As an additional control, presentation from E. coli LLO OVA (45) was studied as a control, as the presence of LLO enables OVA to gain access to the cytosol. As expected, presentation from E. coli LLO OVA was abolished in TAP1−/− dendritic cells (data not shown). However, incomplete inhibition of presentation was seen following treatment with lactacystin in BMDC, although presentation was abolished in DC2.4 cells (data not shown). Although this suggests that 10 μM lactacystin was insufficient to completely inhibit proteasome function in BMDC, a reduction in presentation from E. coli LLO OVA was always seen. This is in contrast to presentation from M. bovis BCG-OVApet, which was increased in two of four experiments and unaffected in the remainder following lactacystin treatment of dendritic cells.
The presentation seen in BMDC and splenic dendritic cells is likely to be via alternative pathways (26) where antigen is degraded in the phagosome and binds to recycling MHC class I molecules in post-Golgi compartments or is regurgitated from the cell (46). In support of this Canaday et al. found that processing of M. tuberculosis antigens to CD8+ T cells was insensitive to brefeldin A (8). Indeed, it has recently been shown that parts of the phagosomal membrane appears to be derived from the membrane of the endoplasmic reticulum which may explain the presence of MHC class I molecules within the phagosome and alternative antigen processing (16). In contrast to the work presented here, presentation of SIINFEKL from E. coli and S. enterica serovar Typhimurium expressing the fusion protein Crl-OVA is TAP independent in macrophages but TAP dependent in dendritic cells (51, 55).
The fusion protein Crl-OVA contains the OVA257-264 epitope internal to the fusion protein, but if recombinant E. coli expressing full-length native OVA were used to infect macrophages, TAP-dependent processing was seen (55). The polyepitope in our study contained the OVA257-264 Kb-binding epitope in the absence of the native N and C termini, which normally flank this epitope. As the proteasome is largely responsible for correct cleavage of C-terminal amino acids from MHC class I epitopes (12, 33), substitution of flanking residues may alter the necessity for proteasomal cleavage and the resultant pathway and efficiency of presentation (34). However, as only the N-terminal flanking residue differs between OVApet and Crl-OVA, this indicates that both N- and C-terminal residues may affect proteasome-dependent processing in dendritic cells and macrophages. Alternatively, the different mechanisms that the bacteria use to inhibit phagolysosome fusion and gain access to the cytoplasm may affect presentation pathways (3).
The final part of our work explored the use of recombinant mycobacteria as in vivo antitumor vaccines. One study with recombinant mycobacteria as an antitumor vaccine warrants consideration in the context of our work. Recently, Dudani et al. (15) studied recombinant M. bovis BCG and showed a similar delay in tumor growth. Both their and our studies used a secretary signal to ensure secretion of the SIINFEKL-containing polypeptide into the phagosome, as this has been described as one of the best forms of antigen display for generation of immune responses (18, 21). However they used the intravenous route of M. bovis BCG administration, which, although it gives direct access of vaccine to the periphery, may not be appropriate in immunocompromised patients, as a tuberculoid type disease may result (17, 52). Subcutaneous and intradermal routes of vaccination, as used in our model, may represent a safer route for use of recombinant BCG as a tumor vaccine.
Dudani et al. also showed that a large proportion of the cytotoxic T lymphocyte response to SIINFEKL was lost in CD4-deficient mice vaccinated with M. bovis BCG-OVA. However, although the M. bovis BCG-OVA construct consisted of OVA230-359, encompassing both the Kb-binding and the I-Ab epitope 323 to 339, tumor protection in CD4-deficient mice was not studied. Although we were able to demonstrate only small increases in SIINFEKL-specific lysis in 5 to 10% in mice vaccinated with M. bovis BCG-OVApet but no increase in mice vaccinated with M. smegmatis-OVApet, no SIINFEKL-tetramer-specific CD8+ T cells were observed following restimulation. However, the inclusion of IL-2 in the restimulation culture, as others have done, may have increased the expansion and detection of SIINFEKL-specific cytotoxic T lymphocytes (15). However, it must be noted that in contrast to the work of Dudani et al., our M. bovis BCG-OVApet was specifically designed to lack specific MHC class II epitopes (from OVA or tumor), thus excluding a role for vaccine-induced specific CD4+ reactivity.
In summary, it appears that antigen presentation from recombinant mycobacteria onto MHC class I by dendritic cells occurs by multiple pathways which may be both intra- and extracellular and which are most efficient when antigen is secreted from the bacteria. Fast-growing, nonpathogenic mycobacteria, like M. smegmatis, may not be suitable for antigen-specific anticancer vaccines in vivo as they are unable to present sufficient antigen on MHC I to stimulate an antigen-specific CD8-restricted T-cell response. Slow-growing mycobacteria such as M. bovis BCG, attenuated to be nonpathogenic yet retaining many of the features of pathogenic organisms, are attractive vaccine candidates as they can present their recombinant antigen to CD8+ T cells by several routes within dendritic cells.
Acknowledgments
This work was supported by Cancer Research UK (registered charity no. 1089464) and the University of Leeds.
We thank Mick Brown, Mark Chambers, Poulam Patel, Kristen Radford, and Georges Vassaux for critical comments during the preparation of the manuscript, S. Young and J. Porte for technical assistance and support, and Colin Johnston for help with statistical analysis. We thank Del Watling, Gary Martin, and Ron Raymond (Clare Hall Laboratories, Cancer Research UK) for help with in vivo studies and Caetano Reis e Sousa and Neil Rogers for provision of TAP1−/− mice and constructive comments. We are grateful to Kenneth Rock and the Dana Farber Cancer Institute for the DC2.4 cell line, Nilabh Shastri for the B3Z hybridoma, and Edith Lord for the B16-OVA cell line.
Editor: J. D. Clements
REFERENCES
- 1.Aldovini, A., and R. A. Young. 1991. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 351:479-482. [DOI] [PubMed] [Google Scholar]
- 2.Alexandroff, A. B., A. M. Jackson, M. A. O'Donnell, and K. James. 1999. BCG immunotherapy of bladder cancer: 20 years on. Lancet 353:1689-1694. [DOI] [PubMed] [Google Scholar]
- 3.Amer, A., and M. Swanson. 2002. A phagosome of one's own: a microbial guide to life in the macrophage. Curr. Opin. Microbiol. 7:78-84. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 5.Batoni, G., G. Maisetta, W. Florio, G. Freer, M. Campa, and S. Senesi. 1998. Analysis of the Mycobacterium bovis hsp60 promoter activity in recombinant Mycobacterium avium. FEMS Microbiol. Lett. 169:117-124. [DOI] [PubMed] [Google Scholar]
- 6.Busch, D. H., K. Kerksiek, and E. G. Pamer. 1999. Processing of Listeria monocytogenes antigens and the in vivo T-cell response to bacterial infection. Immunol. Rev. 172:163-169. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, D. J., T. Serwold, and N. Shastri. 2000. Bacterial proteins can be processed by macrophages in a transporter associated with antigen processing-independent, cysteine protease-dependent manner for presentation by MHC class I molecules. J. Immunol. 164:168-175. [DOI] [PubMed] [Google Scholar]
- 8.Canaday, D. H., C. Ziebold, E. H. Noss, K. A. Chervenak, C. V. Harding, and W. H. Boom. 1999. Activation of human CD8+ alpha beta TCR+ cells by Mycobacterium tuberculosis via an alternate class I MHC antigen-processing pathway. J. Immunol. 162:372-379. [PubMed] [Google Scholar]
- 9.Chatterjee, D., S. W. Hunter, M. McNeil, and P. J. Brennan. 1992. Lipoarabinomannan. Multiglycosylated form of the mycobacterial mannosylphosphatidylinositols. J. Biol. Chem. 267:6228-6233. [PubMed] [Google Scholar]
- 10.Cheadle, E. J., and A. M. Jackson. 2002. Bugs as drugs for cancer. Immunology 107:10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemens, D. L., and M. A. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craiu, A., T. Akopian, A. Goldberg, and K. L. Rock. 1997. Two distinct proteolytic processes in the generation of a major histocompatibility complex class I-presented peptide. Proc. Natl. Acad. Sci. USA 94:10850-10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowle, A. J., R. Dahl, E. Ross, and M. H. May. 1991. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect. Immun. 59:1823-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daffe, M., and P. Draper. 1998. The envelope layers of mycobacteria with reference to their pathogenicity. Adv. Microb. Physiol. 39:131-203. [DOI] [PubMed] [Google Scholar]
- 15.Dudani, R., Y. Chapdelaine, H. H. Faassen, D. K. Smith, H. Shen, L. Krishnan, and S. Sad. 2002. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T-cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J. Immunol. 168:5737-5745. [DOI] [PubMed] [Google Scholar]
- 16.Gagnon, E., S. Duclos, C. Rondeau, C. E. Chevet, P. H. Cameron, O. Steele-Mortimer, J. Paiement, J. J. Bergeron, and M. Desjardins. 2002. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110:119-131. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez, B., S. Moreno, R. Burdach, M. T. Valenzuela, A. Henriquez, M. I. Ramos, and R. U. Sorensen. 1989. Clinical presentation of Bacillus Calmette-Guerin infections in patients with immunodeficiency syndromes. Pediatr. Infect. Dis. J. 8:201-206. [PubMed] [Google Scholar]
- 18.Grode, L., M. Kursar, J. Fensterle, S. H. Kaufmann, and J. Hess. 2002. Cell mediated immunity induced by recombinant Mycobacterium bovis Bacille Calmette-Guerin strains against an intracellular bacterial pathogen: importance of antigen secretion or membrane targeted antigen display as lipoprotein for vaccine efficacy. J. Immunol. 168:1869-1876. [DOI] [PubMed] [Google Scholar]
- 19.Harding, C. V. 1995. Phagocytic processing of antigens for presentation by MHC molecules. Trends Cell Biol. 5:105-109. [DOI] [PubMed] [Google Scholar]
- 20.Hart, D. N. 1997. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90:3245-3287. [PubMed] [Google Scholar]
- 21.Himmelrich, H., R. Lo-Man, N. Winter, P. Guermonprez, C. Sedlik, M. Rojas, D. Monnaie, M. Gheorghiu, M. Lagranderie, M. Hofnung, B. Gicquel, J. M. Clement, and C. Leclerc. 2000. Immune responses induced by recombinant BCG strains according to level of production of a foreign antigen: malE. Vaccine 18:2636-2647. [DOI] [PubMed] [Google Scholar]
- 22.Huebner, R. E. 1996. BCG vaccination in the control of tuberculosis. Curr. Top. Microbiol. Immunol. 215:263-282. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs, W. R., Jr., M. Tuckman, and B. R. Bloom. 1987. Introduction of foreign DNA into mycobacteria using a shuttle phasmid. Nature 327:532-535. [DOI] [PubMed] [Google Scholar]
- 24.Janeway, C. A., P. Travers, and M. Walport, with the assistance of J. D. Capra. 1999. The immune system in health and disease, p. 124-133. In P. Austin and E. Lawrence (ed.), Immunobiology, 4th ed. Elsevier Science Ltd./Garland Publishing, London, England.
- 25.Jiao, X., R. Lo-Man, P. Guermonprez, L. Fiette, E. Deriaud, S. Burgaud, B. Gicquel, N. Winter, and C. Leclerc. 2002. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J. Immunol. 168:1294-1301. [DOI] [PubMed] [Google Scholar]
- 26.Jondal, M., R. Schirmbeck, and J. Reimann. 1996. MHC class I-restricted CTL responses to exogenous antigens. Immunity 5:295-302. [DOI] [PubMed] [Google Scholar]
- 27.Karttunen, J., S. Sanderson, and N. Shastri. 1992. Detection of rare antigen-presenting cells by the LacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl. Acad. Sci. USA 89:6020-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuehnel, M. P., R. Goethe, A. Habermann, E. Mueller, M. Rohde, G. Griffiths, and P. Valentin-Weigand. 2001. Characterization of the intracellular survival of Mycobacterium avium ssp. paratuberculosis: phagosomal pH and fusogenicity in J774 macrophages compared with other mycobacteria. Cell. Microbiol. 3:551-566. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni, H. R., and S. P. Zodpey. 1999. Differential protective effect of bacillus Calmette-Guerin vaccine against multibacillary and paucibacillary leprosy in Nagpur, India. Public Health 113:311-313. [DOI] [PubMed] [Google Scholar]
- 30.Laneelle, G., and M. Daffe. 1991. Mycobacterial cell wall and pathogenicity, a lipodologist's view. Res. Microbiol. 142:433-437. [DOI] [PubMed] [Google Scholar]
- 31.Luo, Y., X. Chen, A. Szilvasi, and M. A. O'Donnell. 2000. Co-expression of interleukin-2 and green fluorescent protein reporter in mycobacteria: in vivo application for monitoring antimycobacterial immunity. Mol. Immunol. 37:527-536. [DOI] [PubMed] [Google Scholar]
- 32.Mazzaccaro, R. J., M. Gedde, E. R. Jensen, H. M. van Santen, H. L. Ploegh, K. L. Rock, and B. R. Bloom. 1996. Major histocompatibility class I presentation of soluble antigen facilitated by Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 93:11786-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mo, X. Y., P. Cascio, K. Lemerise, A. L. Goldberg, and K. Rock. 1999. Distinct proteolytic processes generate the C and N termini of MHC class I-binding peptides. J. Immunol. 163:5851-58599. [PubMed] [Google Scholar]
- 34.Mo, A. X., S. F. van Lelyveld, A. Craiu, and K. L. Rock. 2000. Sequences that flank subdominant and cryptic epitopes influence the proteolytic generation of MHC class I-presented peptides. J. Immunol. 164:4003-4010. [DOI] [PubMed] [Google Scholar]
- 35.Moore, M. W., Carbone, F. R., and Bevan, M. J. 1988. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 54:777-785. [DOI] [PubMed] [Google Scholar]
- 36.Newton, J. A., Jr., P. J. Weiss, W. A. Bowler, and E. C. Oldfield, III. 1993. Soft-tissue infection due to Mycobacterium smegmatis: report of two cases. Clin. Infect. Dis. 16:531-533. [DOI] [PubMed] [Google Scholar]
- 37.Neyrolles, O., K. Gould, M. P. Gares, S. Brett, R. Janssen, P. O'Gaora, J. L. Herrmann, M. C. Prevost, E. Perret, J. E. Thole, and D. Young. 2001. Lipoprotein access to MHC Class-I presentation during infection of murine macrophages with live mycobacteria. J. Immunol. 166:447-457. [DOI] [PubMed] [Google Scholar]
- 38.Pamer, E., and P. Cresswell. 1998. Mechanisms of MHC Class-I restricted antigen processing. Annu. Rev. Immunol. 16:323-358. [DOI] [PubMed] [Google Scholar]
- 39.Parish, T., and N. G. Stocker. 1998. Electroporation of mycobacteria. Methods Mol. Biol. 101:129-144. [DOI] [PubMed] [Google Scholar]
- 40.Paschen, A., K. E. Dittmar, R. Grenningloh, M. Rohde, D. Schadendorf, E. Domann, T. Chakraborty, and S. Weiss. 2000. Human dendritic cells infected by Listeria monocytogenes: induction of maturation, requirements for phagolysosomal escape and antigen presentation capacity. Eur. J. Immunol. 30:3447-3456. [DOI] [PubMed] [Google Scholar]
- 41.Pfeifer, J. D., M. J. Wick, R. L. Roberts, K. Findlay, S. J. Normark, and C. V. Harding. 1993. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature 361:359-362. [DOI] [PubMed] [Google Scholar]
- 42.Pierre-Audigier, C., E. Jouanguy, S. Lamhamedi, F. Altare, J. Rauzier, V. Vincent, D. Canioni, J. F. Emile, A. Fischer, S. Blanche, J. L. Gaillard, and J. L. Casanova. 1997. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon gamma receptor deficiency. Clin. Infect. Dis. 24:982-984. [DOI] [PubMed] [Google Scholar]
- 43.Pieters, J. 2001. Entry and survival of pathogenic mycobacteria in macrophages. Microbes Infect. 3:249-255. [DOI] [PubMed] [Google Scholar]
- 44.Potter, N. S., and C. V. Harding. 2001. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J. Immunol. 167:2538-2546. [DOI] [PubMed] [Google Scholar]
- 45.Radford, K. J., D. E. Higgins, S. Pasquini, E. J. Cheadle, L. Carta, A. M. Jackson, N. R. Lemoine, and G. Vassaux. 2002. A recombinant E. coli vaccine to promote MHC class I dependent antigen presentation: application to cancer immunotherapy. Gene Ther. 9:1455-1463. [DOI] [PubMed] [Google Scholar]
- 46.Rock, K. L. 1996. A new foreign policy: MHC class I molecules monitor the outside world. Immunol. Today 17:131-137. [DOI] [PubMed] [Google Scholar]
- 47.Russell, D. G. 2001. Mycobacterium tuberculosis: here today, and here tomorrow. Nat. Rev. Mol. Cell. Biol. 2:569-577. [DOI] [PubMed] [Google Scholar]
- 48.Schwab, J. C., C. J. Beckers, and K. A. Joiner. 1994. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. USA 91:509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stover, C. K., L. C. de, V., T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456-460. [DOI] [PubMed] [Google Scholar]
- 50.Svensson, M., and M. J. Wick. 1999. Classical MHC class I peptide presentation of a bacterial fusion protein by bone marrow-derived dendritic cells. Eur. J. Immunol. 29:180-188. [DOI] [PubMed] [Google Scholar]
- 51.Svensson, M., Stockinger, B., and M. J. Wick. 1997. Bone marrow-derived dendritic cells can process bacteria for MHC class I and MHC class II presentation to T cells. J. Immunol. 158:4229-4236. [PubMed] [Google Scholar]
- 52.Tan, L., G. Testa, and T. Yung. 1999. Diffuse alveolar damage in BCGosis: a rare complication of intravesical bacillus Calmette-Guerin therapy for transitional cell carcinoma. Pathology 31:55-56. [DOI] [PubMed] [Google Scholar]
- 53.Via, L. E., D. Deretic, R. J. Ulmer, N. S. Hibler, L. A. Huber, and V. Deretic. 1997. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272:13326-13331. [DOI] [PubMed] [Google Scholar]
- 54.Via, L. E., R. A. Fratti, M. McFalone, E. Pagan-Ramos, D. Deretic, and V. Deretic. 1998. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 111:897-905. [DOI] [PubMed] [Google Scholar]
- 55.Wick, M. J., and J. D. Pfeifer. 1996. Major histocompatibility complex class I presentation of ovalbumin peptide 257-264 from exogenous sources, protein context influences the degree of TAP-independent presentation. Eur. J. Immunol. 26:2790-2799. [DOI] [PubMed] [Google Scholar]
- 56.Yarkoni, E., and H. J. Rapp. 1980. Immunotherapy of experimental cancer by intralesional injection of emulsified nonliving mycobacteria: comparison of Mycobacterium bovis (BCG), Mycobacterium phlei, and Mycobacterium smegmatis. Infect. Immun. 28:887-892. [DOI] [PMC free article] [PubMed] [Google Scholar]